Key Points
Question
Is there an association between meteorological conditions and/or air pollution and peritonsillar abscess formation?
Findings
In this nested case-control study of 3819 participants with peritonsillar abscesses, abscess formation was associated with high concentrations of nitrogen dioxide (NO2) and particulate matter 10 micrometers or less in diameter (PM10).
Meaning
The air pollutants of NO2 and PM10 may be risk factors for peritonsillar abscess formation.
This nested case-control study examines the association of meteorological conditions and air pollution with peritonsillar abscess formation in patients in Korea.
Abstract
Importance
Several studies reported an association between peritonsillar abscess formation and climate conditions, including seasonal changes; however, the results were inconsistent.
Objective
To evaluate the association between meteorological conditions and/or air pollution and peritonsillar abscess formation.
Design, Setting, and Participants
In this nested case-control study, 3819 participants with peritonsillar abscesses were matched (1:4) for age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia with 15 276 control participants. The Korean Health Insurance Review and Assessment Service-National Sample Cohort (HIRA-NSC) data from 2002 through 2013 were used.
Exposures and Main Outcomes and Measures
The meteorological data included the mean daily temperature (°C), highest daily temperature (°C), lowest daily temperature (°C), daily temperature difference (°C), relative humidity (%), spot atmospheric pressure (hPa), sulfur dioxide ([SO2], parts per million [ppm]), nitrogen dioxide (NO2, ppm), ozone (O3, ppm), carbon monoxide (CO, ppm), and particulate matter less than 10 μg (PM10, μg/m3) for the previous 14 days, 10 days, 7 days, 5 days, or 3 days before the matched index date. These factors were measured in 94 or 273 locations hourly. The crude and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) of meteorological data for peritonsillar abscess formation were analyzed using unconditional logistic regression analysis. Subgroup analyses were conducted according to age and sex.
Results
The male to female ratio of study participants was 1.43 (11 260 to 7835). Because the age groups were classified using 5-year intervals, the mean age could not be defined. The mean differences of NO2 and PM10 concentrations for the 14 days between peritonsillar abscess group and control group were 1.78 ppb (95% CI, 1.47-2.09) and 1.33 μg/m3 (95% CI, 0.67-1.99), respectively. The aORs of NO2 (0.1 ppm) and PM10 (10 μg/m3) during the 14 days prior to the index date for peritonsillar abscess formation were 12.8 (95% CI, 8.4-19.5) and 1.04 (95% CI, 1.02-1.06), respectively. The other meteorological conditions did not reach statistical significance.
Conclusions and Relevance
Peritonsillar abscess formation was associated with high concentrations of NO2 and PM10.
Introduction
Peritonsillar abscesses are characterized by a collection of pus between the tonsillar capsule and the pharyngeal constrictor muscle.1 The annual incidence of peritonsillar abscess formation is 30.1 per 100 000 in the United States2 and 9.7 per 100 000 in Northern Ireland,3 but the incidence of this disease has not yet been reported in Korea. The etiology of peritonsillar abscess formation has been suggested as a complication of acute tonsillitis4 and the blockage of the common duct from the Weber’s glands.5 However, the acute tonsillitis hypothesis gains an ascendancy over other hypothesis.6
We hypothesize that air pollution or meteorological conditions may affect peritonsillar abscess formation because they be associated with the incidence of upper respiratory infections.7,8,9,10 The tonsils are located in the upper respiratory tract and are in direct contact with the external environment. Moreover, air pollutants may influence the behavior of bacterial pathogens in the tonsils.11,12 However, to our knowledge, the association between air pollution and peritonsillar abscess formation has not been reported. When the PubMed and EMBASE databases were searched for studies using the keyword phrases “peritonsillar abscess” and “air pollution,” no articles were retrieved through September 2018. Several studies1,13,14 reported an association between peritonsillar abscess formation and climate conditions, including seasonal changes; however, the results were inconsistent.
We hypothesized that if meteorological conditions including air pollution do not affect peritonsillar abscess formation, the exposure to previous meteorological conditions (the conditions before the matched index date) would be similar between the peritonsillar abscess and control groups. To prove this hypothesis, the differences in meteorological conditions were analyzed between the peritonsillar abscess and control groups.
Methods
Participant Selection
The Ethics Committee of Hallym University (2017-I102) approved the use of these data. Written informed consent was exempted by the institutional review board owing to the retrospective nature of the study and the deidentification of all data used. We describe the Korean Health Insurance Review and Assessment Service-National Sample Cohort (HIRA-NSC), meteorological, and air pollution data in the supplemental information (eMethods in the Supplement). The general characteristics of the study participants are detailed in Table 1.
Table 1. General Characteristics of Participants.
| Characteristics | Total Participants, No. (%) | |
|---|---|---|
| Peritonsillar Abscess | Control Group | |
| Age, y | ||
| 0-4 | 8 (0.2) | 32 (0.2) |
| 5-9 | 33 (0.9) | 132 (0.9) |
| 10-14 | 101 (2.6) | 404 (2.6) |
| 15-19 | 374 (9.8) | 1496 (9.8) |
| 20-24 | 473 (12.4) | 1892 (12.4) |
| 25-29 | 493 (12.9) | 1972 (12.9) |
| 30-34 | 574 (15.0) | 2296 (15.0) |
| 35-39 | 464 (12.1) | 1856 (12.1) |
| 40-44 | 398 (10.4) | 1592 (10.4) |
| 45-49 | 303 (7.9) | 1212 (7.9) |
| 50-54 | 189 (4.9) | 756 (4.9) |
| 55-59 | 139 (3.6) | 556 (3.6) |
| 60-64 | 109 (2.9) | 436 (2.9) |
| 65-69 | 73 (1.9) | 292 (1.9) |
| 70-74 | 50 (1.3) | 200 (1.3) |
| 75-79 | 25 (0.7) | 100 (0.7) |
| 80-84 | 9 (0.2) | 36 (0.2) |
| ≥85 | 4 (0.1) | 16 (0.1) |
| Sex | ||
| Male | 2252 (59.0) | 9008 (59.0) |
| Female | 1567 (41.0) | 6268 (41.0) |
| Income | ||
| 1 (Lowest) | 40 (1.0) | 160 (1.0) |
| 2 | 241 (6.3) | 964 (6.3) |
| 3 | 259 (6.8) | 1036 (6.8) |
| 4 | 315 (8.2) | 1260 (8.2) |
| 5 | 333 (8.7) | 1332 (8.7) |
| 6 | 360 (9.4) | 1440 (9.4) |
| 7 | 430 (11.3) | 1720 (11.3) |
| 8 | 421 (11.0) | 1684 (11.0) |
| 9 | 454 (11.9) | 1816 (11.9) |
| 10 | 470 (12.3) | 1880 (12.3) |
| 11 (Highest) | 496 (13.0) | 1984 (13.0) |
| Region of residence | ||
| Urban | 1820 (47.7) | 7280 (47.7) |
| Rural | 1999 (52.3) | 7996 (52.3) |
| Hypertension | 548 (14.3) | 2192 (14.3) |
| Diabetes | 308 (8.1) | 1232 (8.1) |
| Dyslipidemia | 548 (14.3) | 2192 (14.3) |
| Mean daily temperature for 14 d, mean (SD), °C | 13.2 (9.6) | 13.4 (9.5) |
| Highest daily temperature for 14 d, mean (SD), °C | 18.4 (9.4) | 18.6 (9.3) |
| Lowest daily temperature for 14 d, mean (SD), °C | 8.8 (10.1) | 9.0 (9.9) |
| Daily temperature difference for 14 d, mean (SD), °C | 9.6 (2.4) | 9.5 (2.3) |
| Relative humidity for 14 d, mean (SD), % | 66.0 (10.5) | 66.1 (10.7) |
| Spot atmospheric pressure for 14 d, mean (SD), hPa | 1006.1 (7.5) | 1006.0 (7.7) |
| SO2 for 14 d, mean (SD), ppb | 5.6 (1.9) | 5.6 (2.0) |
| NO2 for 14 d, mean (SD), ppb | 26.0 (8.9) | 24.26 (8.6) |
| O3 for 14 d, mean (SD), ppb | 22.0 (8.5) | 22.8 (8.6) |
| CO for 14 d, mean (SD), ppb | 0.589 (0.191) | 0.588 (0.199) |
| PM10 for 14 d, mean (SD), μg/m3 | 54.7 (18.8) | 53.4 (18.5) |
Abbreviations: CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter less than 10 μg; ppb, parts per billion; ppm; SO2, sulfur dioxide.
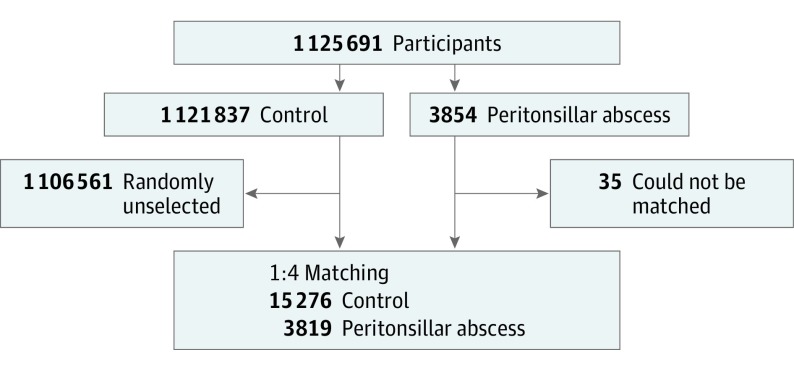
Of 1 125 691 patients with 114 369 638 medical claim codes, we included participants who were diagnosed with peritonsillar abscesses (n = 3854) from 2002 to 2013. Participants with peritonsillar abscesses were matched (1:4) with participants (control group) who were never diagnosed with peritonsillar abscesses from 2002 to 2013 among this cohort. The control group was selected from the total population (n = 1 121 837). The groups were matched for age group, sex, income group, region of residence, and past medical history (hypertension, diabetes, and dyslipidemia). When matching for the region of residence (urban/rural), participants who lived in urban areas were matched with control participants who lived in other urban areas. A similar principle was applied for rural areas. To prevent selection bias when selecting the matched participants, the control group participants were sorted using a random number order, and they were then selected from top to bottom. The matched control participants were assumed to be involved at the same time as each matched participant with peritonsillar abscess formation (index date). Therefore, participants in the control group who died before the index date were excluded. Participants with peritonsillar abscesses for whom we could not identify sufficient matching participants were excluded (n = 35). Finally, 1:4 matching resulted in the inclusion of 3819 participants with peritonsillar abscesses and 15 276 control participants (Figure).
Figure. Participant Enrollment Flow Diagram.
Of a total of 1 125 691 participants, 3819 of participants with peritonsillar abscesses were matched with 15 276 control participants by age group, sex, income group, region of residence, and past medical history. Those participants with peritonsillar abscess who could not be well matched were excluded. Then, the participants with peritonsillar abscesses and control participants were matched for meteorological data from 14, 10, 7, 5, and 3 days before the finding of peritonsillar abscess in the study group (index date) and the same data from the index date in the control group.
We analyzed the meteorological data for the previous 14, 10, 7, 5, and 3 days before peritonsillar abscess formation occurred (index date). In the matched control group of participants without peritonsillar abscesses, we used the same date (index date) used for matched participants with peritonsillar abscesses.
Variables
Independent Variables
The mean daily temperature (°C), highest daily temperature (°C), lowest daily temperature (°C), daily temperature difference (°C), relative humidity (%), spot atmospheric pressure (hPa), SO2 (parts per million [ppm]), nitrogen dioxide (NO2, ppm), ozone (O3, ppm), carbon monoxide (CO, ppm), and particulate matter less than 10 μg (PM10, μg/m3) for the previous 14, 10, 7, 5, and 3 days before the index date were defined as independent variables (eTable 1 in the Supplement).
Covariate Analysis
The age groups were classified using 5-year intervals (0-4, 5-9, 10-14, etc) and 85 years or older. A total of 18 age groups were designated. The income groups were initially divided into 41 classes (1 health aid class, 20 self-employment health insurance classes, and 20 employment health insurance classes). These groups were recategorized into 11 classes (class 1 [lowest income] to 11 [highest income]). The region of residence was divided into 16 areas according to administrative district. These regions were regrouped into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju) areas.
The medical histories of participants were evaluated using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. For accuracy of diagnosis, hypertension (I10 and I15), diabetes (E10-E49), and dyslipidemia (E78) were considered if the participants were treated 2 or more times.
Dependent Variable
Peritonsillar abscess formation was defined using the ICD-10 code J36. We only included participants who were treated with incision and drainage or aspiration (claim code: Q2320).
Statistical Analyses
A χ2 test was used to compare the general characteristics between the peritonsillar abscess and control groups. Independent t tests were used to compare the mean meteorological data for 14 days before the index date.
To analyze the odds ratios (ORs) of meteorological data for peritonsillar abscess formation, crude (simple) and adjusted (multiple) logistic regression were used, and 95% confidence intervals (CIs) were calculated. We described the selection of independent variables and the method of obtaining the final model in the eTables 1, 2, and 3 in the Supplement. We used the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to develop an appropriate model for the various durations of time before the index date (14, 10, 7, 5, and 3 days) and air pollution variables (NO2, O3, and PM10) (Table 2) (eTable 3 in the Supplement). Among the various models, we selected the appropriate model using AIC and BIC by the maximal likelihood function.
Table 2. Adjusted Odds Ratios (95% CI), Akaike Information Criterion, and Bayesian Information Criterion of the Pollution Matters in Logistic Regression for Peritonsillar Abscess.
| Pollution Matters in Each Modela | aOR (95% CI) | AIC | BIC |
|---|---|---|---|
| Model 1 | 18 989.35 | 19 060.07 | |
| NO2 for 14 d (0.1 ppm) | 12.77 (8.37-19.48) | ||
| Model 2 | 19 102.34 | 19 173.06 | |
| O3 for 14 d (0.1 ppm) | 0.33 (0.22-0.51) | ||
| Model 3 | 19 112.89 | 19 183.60 | |
| PM10 for 14 d (10 μg/m3) | 1.04 (1.02-1.06) | ||
| Model 4 | 18 991.33 | 19 069.91 | |
| NO2 for 14 d (0.1 ppm) | 12.59 (7.88-20.11) | ||
| O3 for 14 d (0.1 ppm) | 0.97 (0.61-1.54) | ||
| Model 5 | 18 982.23 | 19 060.80 | |
| NO2 for 14 d (0.1 ppm) | 19.79 (11.87-32.97) | ||
| PM10 for 14 d (10 μg/m3) | 0.97 (0.94-0.99) | ||
| Model 6 | 19 088.97 | 19 167.54 | |
| O3 for 14 d (0.1 ppm) | 0.33 (0.22-0.51) | ||
| PM10 for 14 d (10 μg/m3) | 1.04 (1.02-1.06) | ||
| Model 7 | 18 983.60 | 19 070.03 | |
| NO2 for 14 d (0.1 ppm) | 22.33 (12.34-40.40) | ||
| O3 for 14 d (0.1 ppm) | 1.22 (0.75-1.98) | ||
| PM10 for 14 d (10 μg/m3) | 0.96 (0.94-0.99) |
Abbreviations: AIC, Akaike Information Criterion; aOR, adjusted odds ratio; BIC, Bayesian Information Criterion; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter less than 10 μg; ppb, parts per billion; ppm, parts per million (= 1000 ppb).
Model 1: adjusted for general characteristics (age, sex, income, region, hypertension, diabetes, dyslipidemia) and NO2; Model 2: adjusted for general characteristics, and O3; Model 3: adjusted for general characteristics, and PM10; Model 4: adjusted for general characteristics, NO2, and O3; Model 5: adjusted for general characteristics, NO2, and PM10; Model 6: adjusted for general characteristics, O3, and PM10; Model 7: adjusted for general characteristics, NO2, O3, and PM10.
We used a single pollutant model for NO2, O3, and PM10, as well as a combined model. In our study, we analyzed each pollutant as an independent variable; age, sex, income, region, hypertension, diabetes, and dyslipidemia were adjusted as covariates, and peritonsillar abscess formation was considered the dependent variable.
For the subgroup analysis, we grouped the participants by age and sex (young [0-29 years], middle-aged [30-59 years], and elderly [≥60 years]; men and women) to confirm these associations among the different age and sex groups. We used the same final model for this analysis.
Two-tailed analyses were conducted, and P values less than .05 were considered to indicate significance. The results were analyzed using SPSS (version 22.0, IBM) and SAS (version 9.4; SAS Institute, Inc) statistical software.
Results
The male to female ratio of study participants was 1.43 (11 260 to 7835). Because the age groups were classified using 5-year intervals (Table 1), the mean age could not be defined. We analyzed the mean meteorological and air pollution measurements for 14 days before the index date. Only NO2, O3, and PM10 showed differences (Table 1). The mean differences of NO2, O3, and PM10 concentrations for the 14 days between peritonsillar abscess group and control group were 1.78 ppb (95% CI, 1.47 to 2.09), −0.78 ppb (95% CI, −1.08 to −0.47), and 1.33 μg/m3 (95% CI, 0.67 to 1.99), respectively. Age, sex, income level, region of residence, and medical history of hypertension, diabetes, and dyslipidemia were exactly matched between the peritonsillar abscess and control groups.
The adjusted OR (aOR) of NO2 (0.1 ppm) during the 14 days prior to the index date for peritonsillar abscess formation was 12.8 (95% CI, 8.4-19.5) (Table 2). The aOR of PM10 (10 μg/m3) during the 14 days prior to the index date for peritonsillar abscess formation was 1.04 (95% CI, 1.02-1.06). The mean daily temperature, highest daily temperature, lowest daily temperature, daily temperature difference, relative humidity, hPa, SO2, and CO values were not significantly different (eTable 1 in the Supplement).
We chose the final model from these variable models (models 1-7) (Table 2). We could not select both NO2 and O3/PM10 simultaneously owing to multicollinearity because these factors are closely related. We selected models 1 and 3 to include in the final model. We excluded O3 in the combined model because these factors are associated with each other (eTable 3 in the Supplement).
In the subgroup analyses, NO2 exposure (0.1 ppm) during the 14 days prior to the index date increased the risk of peritonsillar abscess formation in young men (aOR, 9.7; 95% CI, 4.0-23.5) and women (aOR, 4.3; 95% CI, 1.5-12.1), and middle-aged men (aOR, 17.7; 95% CI, 8.5-36.6) and women (aOR, 31.4; 95% CI, 12.3-80.3) (Table 3). However, these factors were not significant in the elderly men/women groups. These factors were significant in men aged 30 to 59 years in another subgroup analysis of PM10 (10 μg/m3) during the 14 days prior to the index date (eTable 4 in the Supplement).
Table 3. Adjusted Odds Ratios (95% CI) of Nitrogen Dioxide (NO2) for 14 Days (0.1 ppm) for Peritonsillar Abscess in Subgroup Analysis According to Age and Sex.
| Subgroup | Participants, No. | Peritonsillar Abscess, NO2, aOR (95% CI) |
|---|---|---|
| Total | 19 210 | 12.8 (8.4-19.5) |
| Age (0-29 y) | ||
| Men | 4235 | 9.7 (4.0-23.5) |
| Women | 3175 | 4.3 (1.5-12.1) |
| Age (30-59 y) | ||
| Men | 6380 | 17.7 (8.5-36.6) |
| Women | 3955 | 31.4 (12.3-80.3) |
| Age (≥60 y) | ||
| Men | 645 | 11.1 (1.0-127.4) |
| Women | 705 | 4.2 (0.4-46.9) |
Abbreviation: aOR, adjusted odds ratio.
Discussion
The aORs for NO2 and PM10 exposure were higher in the peritonsillar abscess group than in the control group. Other meteorological factors including temperature, humidity, atmospheric pressure, and the air pollutants SO2 and CO were not significantly associated with peritonsillar abscess. All age and sex subgroups except those 60 years or older showed consistent findings. Because the occurrence of peritonsillar abscess in the 60 years or older groups were relatively lower than other age groups, the statistical power did not reach the significance level.
The toxicity of NO2 might affect the occurrence of peritonsillar abscess formation because it is a reactive compound that can initiate free radical reactions. It may react with unsaturated fatty acids and induce auto-oxidation of organic compounds.15 It has been noted that NO2 can induce decreased lung function and bronchial constriction.16 Furthermore, NO2 may also provoke an inflammatory response. This results in an increase in macrophages and lymphocytes and a reduction of the phagocytic activity of macrophages.17 In animal studies, damage to the pleural lining has been shown occur at concentrations of NO2 that are marginally higher than those in ambient air.16 Sunyer et al18 reported that the increased risk of daily mortality associated with NO2 was marginally higher for those older than 69 years compared with individuals of all ages.
Particular matter (PM) may also be associated with the occurrence of peritonsillar abscess formation. It has been shown that PM is a mutagenic material that can induce oxidative stress, inflammation, and tissue damage and increase susceptibility to infection,19 and PM may directly effect bacterial pathogens.12 In a previous study, black carbon (the major component of PM) was shown to alter the biofilm structure of Staphylococcus pneumoniae and Staphylococcus aureus, induce changes in proteolytic degradation of biofilms, and alter the tolerance of biofilms to multiple antibiotics.20 Exposure to PM may also affect host immunity.21 Importantly, PM may activate the proinflammatory response mediated by Toll-like receptor (TLR) activation, modifying the ability of TLRs to respond to other ligands and thus change the nature of the inflammatory response.21
In this study, we found a negative association between O3 and peritonsillar abscess formation (Table 2, model 2) (eTable 1 in the Supplement). This result may be a consequence of a negative association between NO2 and O3 (eTable 3 in the Supplement) because O3 can be metabolized from NO2.22 The dominant effect of NO2 might cover the toxic effects of O3.
In this study, meteorological conditions (temperature, humidity, and atmospheric pressure) were not associated with peritonsillar abscess formation. To our knowledge, the association of meteorological conditions with peritonsillar abscess formation have not been previously discussed. The incidence of peritonsillar abscess formation was calculated in previous studies, but climate data were not considered.1,13,23,24,25 In addition, these results were inconsistent. Only 1 study used monthly climate data. They found a moderate positive correlation between the incidence of peritonsillar abscess formation and temperature (Pearson correlation = 0.61, P = .04).14 In this study, we used personal climate data for each participant, which is a more sophisticated method than that used in other studies. However, we did not find any associations between climate data and peritonsillar abscess formation.
To our knowledge, this is the first study to evaluate the association between meteorological conditions including air pollution and peritonsillar abscess formation. The large, nationwide population database used in this study was designed to represent the entire Korean population.26 We analyzed the meteorological data for each participant in the peritonsillar abscess and control groups. The control group was randomly selected by matching for age, sex, income, region of residence, and medical history to avoid confounding effects. In addition, we used an adjusted regression model to minimize confounders.
Limitations
This study has several limitations. We did not have a record of possible confounders, such as history of smoking and alcohol consumption. Despite the large number of participants, statistical values did not reach significant levels in older adults because peritonsillar abscesses are more common in young adults.3 We could not analyze the PM2.5 data because this parameter was measured only after 2015 in Korea. Lack of these data could affect the results of other air pollutants, such as NO2. The degree of indoor air pollution, especially NO2, was not measured, which could have affected personal exposure. However, personal exposure is affected by outdoor concentrations,27 and in the absence of gas cooking, ambient concentrations of NO2 have a more substantial influence on personal exposure than indoor concentrations.28
Conclusions
The ORs of mean concentrations of NO2 and PM10 during the 14 days, 10 days, 7 days, 5 days, or 3 days before the matched index date were higher in the peritonsillar abscess group than in the control group. Other factors related to meteorological conditions and air pollution were not significantly different between the 2 groups.
eMethods
Meteorological Data
eTable 1. Crude odd ratios (95% confidence intervals) of the meteorological and pollution matter for peritonsillar abscess formation
eTable 2. Akaike Information Criterion and Bayesian Information Criterion of the pollution matter in crude logistic regression analysis for peritonsillar abscess formation
eTable 3. Correlation analysis of pollution matter
eTable 4. Adjusted odd ratios (95% confidence interval) of PM10 for 14 days (10 μg/m3) for peritonsillar abscess in subgroup analysis according to age and sex
References
- 1.Mazur E, Czerwińska E, Korona-Głowniak I, Grochowalska A, Kozioł-Montewka M. Epidemiology, clinical history and microbiology of peritonsillar abscess. Eur J Clin Microbiol Infect Dis. 2015;34(3):549-554. doi: 10.1007/s10096-014-2260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzon FS. Harris P. Mosher Award thesis. Peritonsillar abscess: incidence, current management practices, and a proposal for treatment guidelines. Laryngoscope Aug 1995;105(8 Pt 3 Suppl 74):1-17. [DOI] [PubMed] [Google Scholar]
- 3.Hanna BC, McMullan R, Gallagher G, Hedderwick S. The epidemiology of peritonsillar abscess disease in Northern Ireland. J Infect. 2006;52(4):247-253. doi: 10.1016/j.jinf.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Blair AB, Booth R, Baugh R. A unifying theory of tonsillitis, intratonsillar abscess and peritonsillar abscess. Am J Otolaryngol. 2015;36(4):517-520. doi: 10.1016/j.amjoto.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 5.Passy V. Pathogenesis of peritonsillar abscess. Laryngoscope. 1994;104(2):185-190. [DOI] [PubMed] [Google Scholar]
- 6.Klug TE, Rusan M, Fuursted K, Ovesen T. Peritonsillar abscess: complication of acute tonsillitis or Weber’s glands infection? Otolaryngol Head Neck Surg. 2016;155(2):199-207. doi: 10.1177/0194599816639551 [DOI] [PubMed] [Google Scholar]
- 7.Hajat S, Anderson HR, Atkinson RW, Haines A. Effects of air pollution on general practitioner consultations for upper respiratory diseases in London. Occup Environ Med. 2002;59(5):294-299. doi: 10.1136/oem.59.5.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellowes DS, Proctor IR. The incidence of the common cold in relation to certain meteorological parameters. Int J Biometeorol. 1973;17(2):193-203. doi: 10.1007/BF01809807 [DOI] [PubMed] [Google Scholar]
- 9.du Prel JB, Puppe W, Gröndahl B, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861-868. doi: 10.1086/605435 [DOI] [PubMed] [Google Scholar]
- 10.Lin YK, Chang CK, Chang SC, Chen PS, Lin C, Wang YC. Temperature, nitrogen dioxide, circulating respiratory viruses and acute upper respiratory infections among children in Taipei, Taiwan: a population-based study. Environ Res. 2013;120:109-118. doi: 10.1016/j.envres.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardas M, Gierek T, Paluch J, Pawłowska-Góral K, Pilch J, Markowski J. The influence of environmental pollution on the amount of glycosoaminoglycans in the tissue of palatine tonsils. Pathol Res Pract. 2002;198(6):425-427. doi: 10.1078/0344-0338-00276 [DOI] [PubMed] [Google Scholar]
- 12.Williams P. Particulate air pollution impacts directly on bacterial pathogen behaviour and infection. Environ Microbiol. 2017;19(10):3787-3788. doi: 10.1111/1462-2920.13790 [DOI] [PubMed] [Google Scholar]
- 13.Klug TE. Incidence and microbiology of peritonsillar abscess: the influence of season, age, and gender. Eur J Clin Microbiol Infect Dis. 2014;33(7):1163-1167. doi: 10.1007/s10096-014-2052-8 [DOI] [PubMed] [Google Scholar]
- 14.Freire GSM, Dos Santos JHZ, Rolón PA, Pinheiro GB, Sampaio ALL. Peritonsillar abscess: epidemiology and relationship with climate variations. J Laryngol Otol. 2017;131(7):627-630. doi: 10.1017/S0022215117000895 [DOI] [PubMed] [Google Scholar]
- 15.Department of Health Advisory Group on the Medical Aspects of Air Pollution Episodes. Third Report: Oxides of Nitrogen. December 1, 1993. https://webarchive.nationalarchives.gov.uk/20070403011328/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4102962. Accessed March 29, 2019.
- 16.Searl A. A Review of the Acute and Long Term Impacts of Exposure to Nitrogen Dioxide in the United Kingdom. Edinburgh: Institute of Occupational Medicine; 2004:1-196. [Google Scholar]
- 17.World Health Organization. Environmental Health Criteria 188, Nitrogen Oxides, second edition. http://www.inchem.org/documents/ehc/ehc/ehc188.htm. Accessed March 29, 2019.
- 18.Sunyer J, Castellsagué J, Sáez M, Tobias A, Antó JM. Air pollution and mortality in Barcelona. J Epidemiol Community Health. 1996;50(suppl 1):s76-s80. doi: 10.1136/jech.50.Suppl_1.s76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly FJ, Fussell JC. Linking ambient particulate matter pollution effects with oxidative biology and immune responses. Ann N Y Acad Sci. 2015;1340:84-94. doi: 10.1111/nyas.12720 [DOI] [PubMed] [Google Scholar]
- 20.Hussey SJK, Purves J, Allcock N, et al. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ Microbiol. 2017;19(5):1868-1880. doi: 10.1111/1462-2920.13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129(1):14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D-S, Jeong J, Ahn J. Characteristics in atmospheric chemistry between NO, NO2 and O-3 at an urban site during MAPS (Megacity Air Pollution Study)-Seoul, Korea. J Korean Soc Atmos Environ. 2016;32(4):422-434. doi: 10.5572/KOSAE.2016.32.4.422 [DOI] [Google Scholar]
- 23.Galioto NJ. Peritonsillar abscess. Am Fam Physician. 2008;77(2):199-202. [PubMed] [Google Scholar]
- 24.Segal N, El-Saied S, Puterman M. Peritonsillar abscess in children in the southern district of Israel. Int J Pediatr Otorhinolaryngol. 2009;73(8):1148-1150. doi: 10.1016/j.ijporl.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 25.Ong YK, Goh YH, Lee YL. Peritonsillar infections: local experience. Singapore Med J. 2004;45(3):105-109. [PubMed] [Google Scholar]
- 26.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. [DOI] [PubMed] [Google Scholar]
- 27.Garrett MH, Hooper MA, Hooper BM. Nitrogen dioxide in Australian homes: levels and sources. J Air Waste Manag Assoc. 1999;49(1):76-81. doi: 10.1080/10473289.1999.10463781 [DOI] [PubMed] [Google Scholar]
- 28.Rotko T, Kousa A, Alm S, Jantunen M. Exposures to nitrogen dioxide in EXPOLIS-Helsinki: microenvironment, behavioral and sociodemographic factors. J Expo Anal Environ Epidemiol. 2001;11(3):216-223. doi: 10.1038/sj.jea.7500162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Meteorological Data
eTable 1. Crude odd ratios (95% confidence intervals) of the meteorological and pollution matter for peritonsillar abscess formation
eTable 2. Akaike Information Criterion and Bayesian Information Criterion of the pollution matter in crude logistic regression analysis for peritonsillar abscess formation
eTable 3. Correlation analysis of pollution matter
eTable 4. Adjusted odd ratios (95% confidence interval) of PM10 for 14 days (10 μg/m3) for peritonsillar abscess in subgroup analysis according to age and sex