Abstract
Glycoconjugate mucin secretion from conjunctival goblet cells is tightly regulated by nerves and specialized pro-resolving mediators (SPMs) to maintain ocular surface health. Here we investigated the actions of the SPM resolvin E1 (RvE1) on cultured rat conjunctival goblet cell glycoconjugate secretion and intracellular [Ca2+] ([Ca2+]i) and the signaling pathways used by RvE1. Goblet cells were cultured from rat conjunctiva in RPMI medium. The amount of RvE1-stimulated glycoconjugate mucin secretion was determined using an enzyme-linked lectin assay with Ulex Europaeus Agglutinin 1 lectin. Cultured goblet cells were also incubated with the Ca2+ indicator dye fura 2/AM and [Ca2+]i was measured. Cultured goblet cells were incubated with inhibitors to phospholipase (PL-) C, D, and A2 signaling pathways. RvE1 stimulated glycoconjugate secretion in a concentration dependent manner and was inhibited with the Ca2+ chelator BAPTA. The response was also increased in a concentration manner when stimulated by RvE1. Inhibition of PLC, PLD, and PLA2, but not Ca2+/ calmodulin-dependent kinase blocked RvE1-stimulated increase in [Ca2+]i and glycoconjugate secretion. We conclude that under normal, physiological conditions RvE1 stimulates multiple pathways to increase glycoconjugate secretion and [Ca2+]i. RvE1 could be an important regulator of goblet cell glycoconjugate mucin secretion to maintain ocular surface health.
Keywords: Pro-resolving mediators, Inflammation, Allergy, Goblet cells, Conjunctiva
1. Introduction
The first defense a pathogen, allergen, or environmental pollutant encounters when challenging the eye is the tear film. The tear film is produced in part by the conjunctiva which is a mucous membrane that functions as a part of the innate immune system of the eye, and provides a critical barrier between the ocular surface and the environment. The conjunctiva is comprised of stratified epithelial cells, a basement membrane and stroma. Within the epithelial layer of the conjunctiva are goblet cells. Conjunctival goblet cells produce and secrete the high molecular weight glycoconjugate mucin MUC5AC, which protects the ocular surface by trapping pathogens, allergens, and environmental pollutants and removing them from the ocular surface by drainage through the nasolacrimal duct (Dartt and Masli, 2014; Jumblatt et al., 1999; Mantelli and Argueso, 2008).
In uncontrolled inflammatory diseases like dry eye disease and allergic conjunctivitis, mucin secretion is dysregulated (Mantelli and Argueso, 2008; Govindarajan and Gipson, 2010; Contreras-Ruiz et al., 2013; McGilligan et al., 2013). Patients with dry eye disease usually have decreased tear film mucin and may suffer from burning, itching and blurred vision. Studies also show substantially decreased quality of life for these patients (Uchino and Schaumberg, 2013). Patients with allergic conjunctivitis have increased tear film mucins, but similarly to patients with dry eye, complain of symptoms including itching, redness and tearing. Vernal keratoconjunctivitis, a very severe form of allergic conjunctivitis, may even lead to vision loss (La Rosa et al., 2013). Dry eye disease and allergic conjunctivitis both have dysregulated tear film mucin production and both are growing public health problems for which current treatments are limited (Gayton, 2009; Gomes, 2014).
Inflammation is crucial in order to remove pathogens, allergens, and environmental pollutants from the body. However, uncontrolled inflammation can occur without a pathogen, allergen, or tissue damage present, leading to unnecessary discomfort and tissue damage. In recent years, it has been established that there are lipid mediators which actively terminate the inflammation. These mediators are known as specialized pro-resolving mediators (SPMs) and consist of families termed resolvins, lipoxins, protectins and maresins (Serhan, 2014; Serhan and Chiang, 2013). The present study focuses on the SPM resolvin E1 (RvE1), which is produced from the omega-3 fatty acid eicosapentanoic acid (EPA) (Serhan et al., 2004). RvE1 induces intracellular signaling pathways through the ChemR23/ERV-1 receptor (Ohira et al., 2010). ChemR23 has been detected earlier by immunohistochemistry in rat conjunctival goblet cells (Dartt et al., 2011).
Numerous studies show that RvE1 reduces inflammation in the eye. We demonstrated that RvE1 blocks the pro-inflammatory leukotriene (LT) D4-stimulated increase in goblet cell secretion from cultured rat conjunctival goblet cells (Dartt et al., 2011). In a murine model of dry eye disease, topical application of RvE1 decreased inflammatory markers and increased the number of goblet cells and tear production (Li et al., 2010; de Paiva et al., 2012). In other studies, RvE1 decreased inflammation in the cornea (Lee et al., 2015; Rajasagi et al., 2011; Jin et al., 2009). The long acting RvE1 analog RX-10045 reduced post-operative complications after laser refractive surgery (Torricelli et al., 2014). Furthermore, multiple studies indicate that a dietary intake of omega-3 fatty acids including EPA has a beneficial effect on dry eye disease (Miljanovic et al., 2005; Viau et al., 2009). To date, RvE1 has been used in one clinical trial where an analog of RvE1 reduced symptoms in dry eye disease patients (Serhan et al., 2014). Studies on a molecular level, in animal models and a clinical trial implicate a role for RvE1 in terminating ocular surface inflammation.
Recent results from our group found that SPMs, not only play a part in terminating inflammation, but also have a physiological role in conjunctival goblet cells to maintain ocular surface health in the absence of disease. Amongst the SPMs that are effective in the conjunctiva are resolvin D1 (RvD1), aspirin-triggered RvD1 (AT-RvD1), and lipoxin A4 (LXA4). All these SPMs, on their own, increase the intracellular [Ca2+] ([Ca2+]i) and stimulate glycoconjugate secretion (Lippestad et al., 2017; Li et al., 2013; Hodges et al., 2017). Both RvD1 and LXA4 stimulate an increase in [Ca2+]i through activation of phospholipase (PLC), phospholipase D (PLD), and phospholipase A2 (PLA2) (Lippestad et al., 2017; Hodges et al., 2017).
Although numerous studies indicate that RvE1 can be a promising new treatment of ocular inflammatory diseases, the physiological functions of RvE1 in the eye to maintain health are unknown. In this study, we investigated the actions of RvE1 on glycoconjugate mucin secretion and [Ca2+]i from cultured conjunctival goblet cells and the signaling pathways used by RvE1 to do so.
2. Materials and methods
2.1. Materials
RPMI-1640 cell culture medium, penicillin/streptomycin and L-glutamine were purchased from Lonza (Walkerville, IL). Fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA). UEA-1 and histamine were obtained from Sigma–Aldrich (St. Louis, MO).
RvE1 and RvD1 were purchased from Cayman Chemical (Ann Arbor, MI) or obtained from the Serhan lab. RvE1 or RvD1, in ethanol, was stored at −80 °C, and diluted immediately before use in either RPMI medium or Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES, 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose (pH 7.40–7.45)) to the desired concentrations (10−8 M for RvD1 and 10−9 M for RvE1) and added to the cells.
Ro-318220, U73122 and U73343, KN92 and KN93 were purchased from Tocris Bioscience (Ellisville, MO). Histamine, carbachol (CCh), aristolochic acid (aris acid), BAPTA, 2-APB, 1-butanol (1-but) and t-butanol (t-but) were from Sigma-Aldrich (St Louis, MO). Fura-2/AM and BAPTA/AM were from Life Technologies (Grand Island, NY). Amplex Red was from Invitrogen (Grand Island, NY).
2.2. Animals
Male Sprague-Dawley rats (125–150 g) (Taconic Farms, Germantown, NY) were used for all the experiments. The rats were anesthetized for 1 min in CO2 before decapitation. The bulbar and forniceal conjunctiva were removed from both eyes. All experiments were in accordance with the National Institutes of Health guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). The animal protocol was approved by the Schepens Eye Research Institute Animal Care and Use Committee.
2.3. Cell culture
Goblet cells from male rats were grown in organ culture as described previously (Dartt et al., 2011; Lippestad et al., 2017; Li et al., 2013; Hodges et al., 2012a, 2016a, 2017; Shatos et al., 2001; Hayashi et al., 2012). The conjunctiva was cut into small pieces, and placed in 6 well plates containing RPMI 1640 medium supplemented with 10% FBS, 2 mM glutamine and 100 μg/ml penicillin-streptomycin. After 5–7 days, the explants were removed and the cells trypsinized. First passage goblet cells were used in all experiments. Cells were seeded in either glass bottomed culture dishes for Ca2+-experiments or 24 well plates for glycoconjugate secretion experiments. To confirm that goblet cells predominated in the cell culture, immunohistochemistry was conducted using the lectin UEA-1 (which recognized goblet cell secretory products) and an antibody to cytokeratin 7 (detects goblet cell body) (Dartt et al., 2011; Li et al., 2012, 2013; Hodges et al., 2012a, 2016a, 2017; Shatos et al., 2001; Hayashi et al., 2012). Ninety-five percent of cells cultured were goblet cells (data not shown).
2.4. Secretion
Cultured rat conjunctival goblet cells were trypsinized, passaged into 24 well plates, and grown to approximately 75% confluence. The cells were serum starved in serum free RPMI 1640 containing 0.5% bovine serum albumin (BSA) for 2 h before they were incubated with RvE1 (10−9 M-10−7 M) for 2 or 4 h. Histamine (10−5 M) and RvD1 (10−8 M) were used as controls. Basal conditions (e.g. no RvE1) included 0.04% ethanol, the highest concentration of ethanol present in any of the conditions. Ethanol had no significant effect on basal secretion (data not shown). In separate experiments, the cells were serum starved for 2 h before they were incubated with BAPTA/AM (10−5 M) for 30 min followed by RvE1 (10−9 M) or no additions for 2 h. U73122, PLC inhibitor, and the negative control, U73343, were added 15 min prior to RvE1-stimulation. 2-APB, an inositol 1,4,5-trisphosphate (IP3) receptor antagonist, Ro 31–8220, a protein kinase C (PKC) inhibitor, and aristolochic acid (aris), a PLA2 inhibitor were added 10 min before RvE1 stimulation for 2 h. 1-Butanol (1-but), a PLD inhibitor, and tertiary-but (t-but), the PLD negative control, were added 15 min before stimulation. KN93, a calcium/calmodulin-dependent protein kinase II (Ca2+/CaMK) inhibitor, or the inactive control KN92 were added 30 min before stimulation with RvE1 for 2 h.
The amount of goblet cell high molecular weight glycoconjugate secretion was measured using the lectin UEA-1 in an enzyme linked lectin assay (ELLA). UEA-1 binds to high molecular weight glycoproteins, including goblet cell mucin MUC5AC (Hodges et al., 2012a). The amount of lectin-detected glycoconjugates was measured, as described previously (Dartt et al., 2011; Li et al., 2013; Hodges et al., 2012a, 2016a, 2017; Hayashi et al., 2012). Glycoconjugate secretion is shown as fold increase above basal, which was set to 1.
2.5. Measurement of [Ca2+]i
Goblet cells were incubated for 1 h at 37 °C with KRB-HEPES containing 0.5% BSA, 0.5 μM fura-2/AM, 8 μM pluronic acid F127 and 250 μM sulfinpyrazone. Calcium measurements were made with a ratio imaging system (In Cyt Im2; Intracellular Imaging, Cincinnati, OH) using wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. For each experiment, at least five cells were selected and were followed for the entire experiment (approximately 2 min). Goblet cells were incubated with inhibitors as was done with glycoconjugate secretion before RvE1 was added. Thapsigargin, was added 15 min before stimulation. The data measuring [Ca2+]i are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]i was calculated by subtracting the average of the basal value, before addition of RvE1, from the peak [Ca2+]i.
2.6. Statistical analysis
Results are presented as average ± SEM. One way ANOVA with Tukey post-hoc test or Student’s t-test was used to perform statistical analysis, and p < 0.05 was considered statistically significant.
3. Results
3.1. RvE1 stimulates glycoconjugate secretion in rat conjunctival goblet cells
As the SPMs LXA4, RvD1 and AT-RvD1 each stimulate glycoconjugate secretion in rat goblet cells (Li et al., 2013; Hodges et al., 2017), we investigated if RvE1 can similarly regulate goblet cell glycoconjugate secretion. As both histamine and RvD1 stimulate glycoconjugate secretion from rat conjunctival goblet cells, these compounds were used as positive controls for this experiment (Lippestad et al., 2017; Hayashi et al., 2012). Goblet cells were stimulated for 2 h with RvE1 at 10−9–10−7 M, histamine (10−5 M), or RvD1 (10−8 M) and glycoconjugate secretion measured (Fig. 1A). When stimulating goblet cells with RvE1 at three different concentrations, all concentrations significantly increased glycoconjugate secretion by 2.6 ± 0.5 (p = 0.01), 2.1 ± 0.4 (p = 0.005), and 3.2 ± 0.8 (p = 0.002) fold at 10−9, 10−8, 10−7 M, respectively, above a basal value of 121.2 μg/ml. The controls, histamine and RvD1, significantly increased glycoconjugate secretion from basal in rat conjunctival goblet cells. Histamine 10−5 M increased glycoconjugate secretion by 1.8 ± 0.3 fold above basal (p = 0.05) and RvD1 10−8 M by 2.3 ± 0.5 fold above basal (p = 0.03).
Fig. 1. RvE1 increases glycoconjugate secretion in rat conjunctival goblet cells.
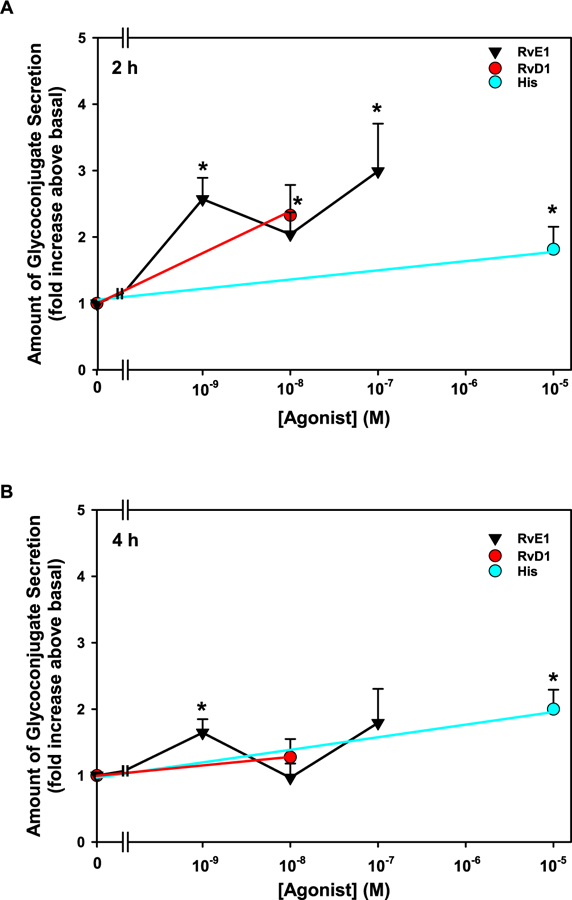
Cultured goblet cells were incubated with increasing concentrations of RvE1 (10−9-10−7 M), RvD1 (10−8 M), and histamine (his, 10−5 M) for 2 h (A) or 4 h (B). Glycoconjugate secretion was measured. Data are mean ± SEM from 4 independent experiments and shown as fold above basal, which set to 1. * indicates significant difference from basal.
Glycoconjugate secretion from rat conjunctival goblet cells was also measured 4 h after addition of RvE1, histamine, or RvD1. Only one concentration of RvE1, 10−9 M, significantly increase in glycoconjugate secretion compared to basal after 4 h, with an increase of 1.6 ± 0.2 (p = 0.02) fold above a basal of 445.9 μg/ml (Fig. 1B). Secretion stimulated by all other concentrations of RvE1 and RvD1 were decreased at 4 h compared to 2 h, though the values did not reach significance. In contrast, histamine-stimulated glycoconjugate secretion was unchanged from 2 to 4 h (p = 0.70). Thus, RvE1 stimulates conjunctival goblet cell high molecular weight glycoconjugate secretion, but is more effective at shorter time intervals of stimulation.
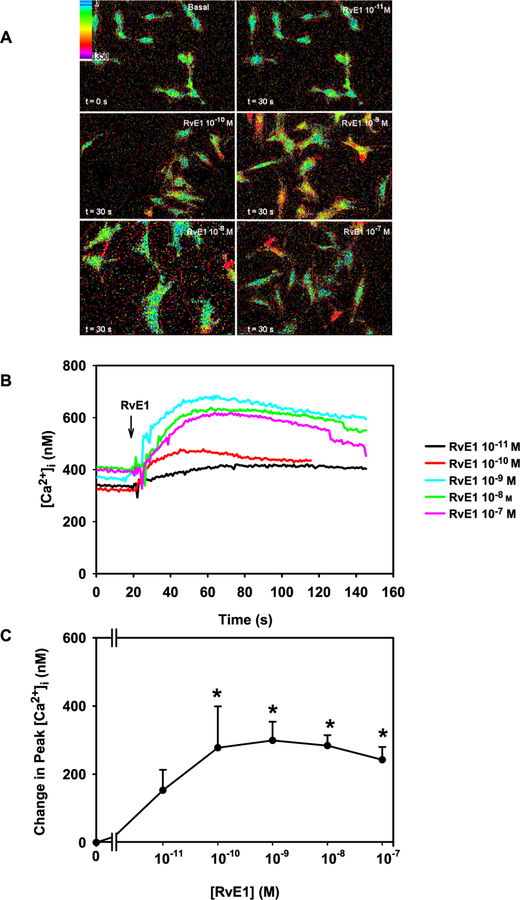
3.2. RvE1 increases [Ca2+]i in a concentration dependent manner in rat conjunctival goblet cells
In addition to increasing glycoconjugate secretion, RvD1, AT-RvD1 and LXA4 also increase [Ca2+]i in rat conjunctival goblet cells (Li et al., 2013; Hodges et al., 2017). To determine if RvE1 elevates [Ca2+]i in goblet cells, cultured cells were incubated with fura-2/AM, as described in 2.5 and stimulated with RvE1 10−11 M to 10−7 M. RvE1 increased [Ca2+]i in a concentration-dependent manner (Fig. 2A–C). Pseudo color images of cells stimulated with increasing concentrations of RvE1 are shown in Fig. 2A while [Ca2+]i over time is shown in Fig. 2B. RvE1 at 10−10–10−7 M increased [Ca2+]i significantly from basal. A peak increase in RvE1-stimulated [Ca2+]i in goblet cells was observed at RvE1 10−9 M, with a stimulation of 281.0 ± 53.3 nM (p = 0.002, Fig. 2C). The controls, RvD1 (10−8 M) and CCh (10−4 M) also significantly increased [Ca2+]i by 329.9 ± 129.9 nM (p = 0.01) and 181.5 ± 55.0 nM (p = 0.01), respectively (data not shown). Thus RvE1 increases the [Ca2+]i in conjunctival goblet cells.
Fig. 2. RvE1 elevates [Ca2+]i in rat conjunctival goblet cells.
Cultured goblet cells were incubated with fura-2/AM and stimulated with increasing concentrations of RvE1 (10−11-10−7 M). Representative pseudo color pictures of [Ca2+]i in goblet cells stimulated by RvE1 are shown in A. [Ca2+]i over time in response to RvE1 (10−11–10−7 M) is shown in B. Change in peak [Ca2+]i was calculated in response to increasing concentrations of RvE1 is shown in C. Data in B and C are from 6 independent experiments. Data in C are mean ± SEM. * indicates significant difference from basal. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. RvE1 stimulates glycoconjugate secretion by increasing [Ca2+]i in rat conjunctival goblet cells
[Ca2+]i is known to be a common stimulator of glycoconjugate mucin secretion in conjunctival goblet cells (Lippestad et al., 2017; Hodges et al., 2017; Li et al., 2012; Dartt et al., 2000). BAPTA/AM, an intracellular calcium chelator, was used to determinate whether RvE1 elevates [Ca2+]i to increase glycoconjugate secretion. Cultured goblet cells were incubated with BAPTA/AM (10−6-10−5 M) for 30 min before stimulation with RvE1 (10−9 M). In these experiments, BAPTA did not affect basal glycoconjugate secretion (data not shown). RvE1 increased glycoconjugate secretion to 1.8 ± 0.3 (p = 0.02) fold above basal (Fig. 3). BAPTA at 10−6 blocked RvE1-induced glycoconjugate secretion and was 1.3 ± 0.3 fold above basal. BAPTA at 10−5 M significantly decreased RvE1-stimulated secretion and was 1.1 ± 0.3 fold above basal (p = 0.01) (Fig. 3). Thus, RvE1 uses [Ca2+]i to stimulate high molecular weight glycoconjugate secretion from conjunctival goblet cells.
Fig. 3. Chelation of [Ca2+]i blocks RvE1-stimulated glycoconjugate secretion in rat conjunctival goblet cells.
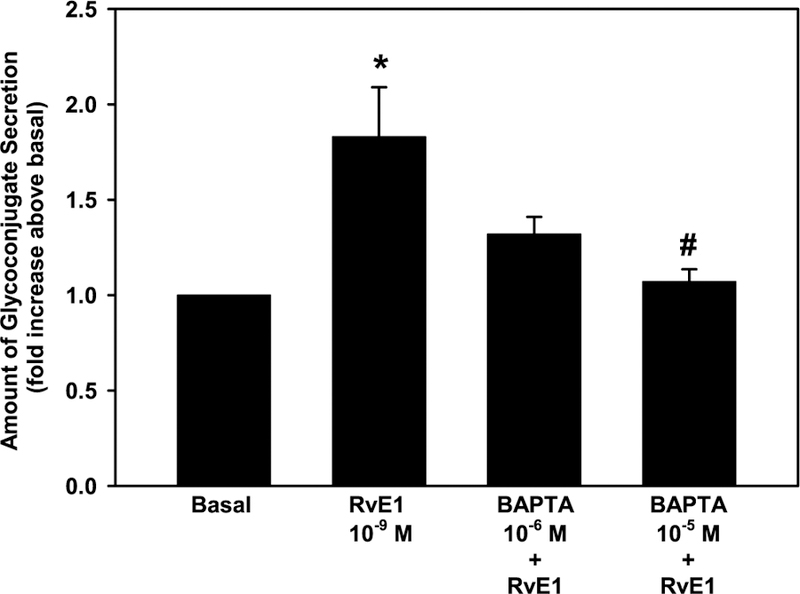
Cultured goblet cells were incubated with [Ca2+]i chelator BAPTA/AM (10−6-10−5 M) for 30 min before stimulation with RvE1 (10−9 M). Glycoconjugate secretion was measured. Data are mean ± SEM from 4 independent experiments and shown as fold above basal, which set to 1. * indicates significant difference from basal; # indicates significance from RvE1 alone.
3.4. RvE1 activates the PLC pathway to increase [Ca2+]i in cultured rat goblet cells
Our findings indicate that RvE1 stimulates goblet cells to secrete glycoconjugate mucin by increasing [Ca2+]i, thus we explored which signaling pathways RvE1 activates to increase [Ca2+]i. First, we studied the PLC pathway as activation of this pathway is well known to increase [Ca2+]i via production of IP3 (Berridge, 2009). Goblet cells were incubated with the PLC inhibitor U73122 (10−6 M) or the negative control U73343 (10−6 M) for 15 min prior to RvE1 (10−9 M) stimulation and [Ca2+]i measured. RvE1 significantly increased [Ca2+]i to a peak of 217.4 ± 67.7 nM (p = 0.03, Fig. 4A and B). RvE1-stimulated [Ca2+]i increase was 141.4 ± 15.1 nM with the active analog (Fig. 4A and B). The inactive analog U73343 affected RvE1-stimulated [Ca2+]i increase, decreasing it to 81.5 ± 52.6 nM. Neither U73122 nor U73343 significantly altered the basal value (p = 0.13 and 0.06, respectively).
Fig. 4. RvE1 activates the PLC pathway to increase [Ca2+]i in rat conjunctival goblet cells.
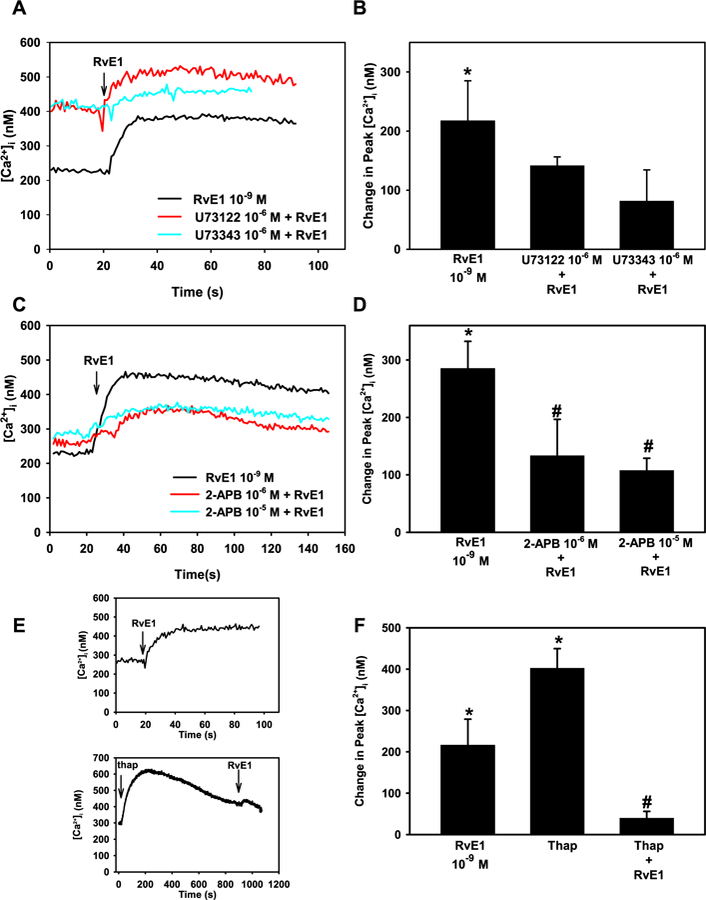
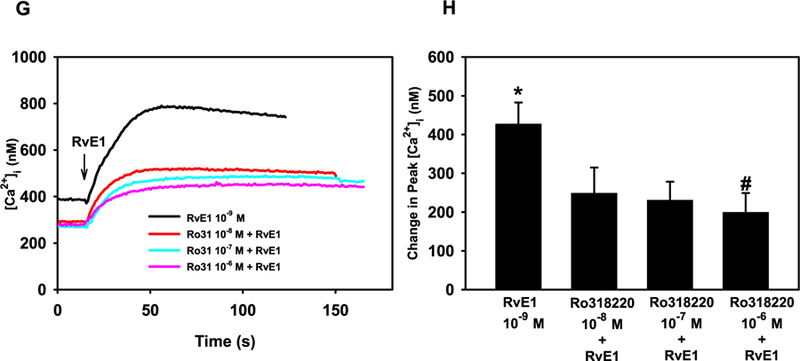
Cultured goblet cells were preincubated with the PLC inhibitor U73122 and its negative control U73343, both at 10−6 M, for 15 min (A and B), 2-APB (10−6 and 10−5 M) for 10 min (C and D), thapsagargin for 15 min (E and F) or Ro 31–8220 for 10 min (G and H). [Ca2+]i over time is shown in A, C, E, and G. Change in peak [Ca2+]i is shown in B, D, F, and H. Data are mean ± SEM from 3 (A and B), 7 (C and D), 6 (E and F) and 6 (G and H) independent experiments. * indicates significant difference from basal; # indicates significance from RvE1 alone.
Activation of PLC produces IP3 and diacylglycerol (DAG). IP3 binds to its receptors IP3RI, II, and III on the endoplasmic reticulum (ER), leading to release of Ca2+ into the cytosol (Berridge, 2009). Goblet cells were incubated for 10 min with 2-APB, which inhibits the IP3 receptors, and the [Ca2+]i increase was measured after stimulation with RvE1. RvE1 (10−9 M) alone significantly increased [Ca2+]i by 285.2 ± 47.2 nM (p = 5.76 × 10−5 M). RvE1-induced [Ca2+]i increase was significantly decreased to 133.2 ± 63.6 nM using 2-APB 10−6 M (Fig. 4C and D). Incubation with 2-APB 10−5 M also significantly decreased RvE1-stimulated increase in [Ca2+]i to 107.5 ± 21.5 nM (p = 0.01, Fig. 4C and D).
Thapsigargin inhibits Ca2+ uptake into the ER depleting the ER of Ca2+ by blocking the Ca2+/ATPase present in the ER that pumps Ca2+ into the ER (Luo et al., 2001). If RvE1 mobilizes Ca2+ from the ER to increase [Ca2+]i, pre-treatment with thapsigargin would decrease the RvE1-induced increase in [Ca2+]i. RvE1 significantly increased [Ca2+]i by 216.2 ± 62.7 nM (p = 0.01) (Fig. 4E and F). Thapsigargin (10−5 M) by itself increased [Ca2+]i by 402.1 ± 47.3 nM (p = 1.4 × 10−5), indicating the ER is depleted of Ca2+. When goblet cells were treated with thapsigargin before stimulation with RvE1, the RvE1-stimulated Ca2+ response was reduced to 39.7 ± 16.2 nM (p = 0.03).
DAG, generated from PLC, can activate protein kinase C (PKC). Alone, RvE1 (10−9 M) stimulated a peak increase in [Ca2+]i of 413.6 ± 64.1 nM (p = 7.3 × 10−5 M) (Fig. 4G and H). Preincubation of cells with Ro 31–8220 at all concentrations decreased the basal [Ca2+]i (Fig. 4G). When goblet cells were incubated with the PKC inhibitor Ro 31–8220, RvE1-stimulated increase in [Ca2+]i was significantly inhibited to 199.1 ± 50.4 nM (p = 0.03) by Ro 31–8220 10−6 M (Fig. 4G and H). Based on all these experiments, RvE1 appears to activate the PLC pathway producing IP3 and activating PKC to increase [Ca2+]i. in goblet cells.
3.5. RvE1 activates PLD to increase [Ca2+]i in cultured rat goblet cells
As other SPMs also use PLD to increase [Ca2+]i and glycoconjugate secretion (Lippestad et al., 2017; Hodges et al., 2017), we investigated the effects of RvE1 on PLD activation. Goblet cells were incubated with either the PLD inhibitor 1-but or the negative control t-but, both at 0.3%. In these experiments, RvE1 (10−9 M) alone significantly increased [Ca2+]i to a maximum of 366.5 ± 90.6 nM (p = 0.004) (Fig. 5A and B). Preincubation with 1-but significantly blocked RvE1-stimulated [Ca2+]i increase and was 63.6 ± 14.5 nM (p = 0.01), whereas t-but did not significantly alter the RvE1-stimulated [Ca2+]i increase (Fig. 5A and B). Thus, we conclude that RvE1 activates the PLD pathway in rat conjunctival goblet cells.
Fig. 5. RvE1 activates the PLD pathway to increase [Ca2+]i in rat conjunctival goblet cells.
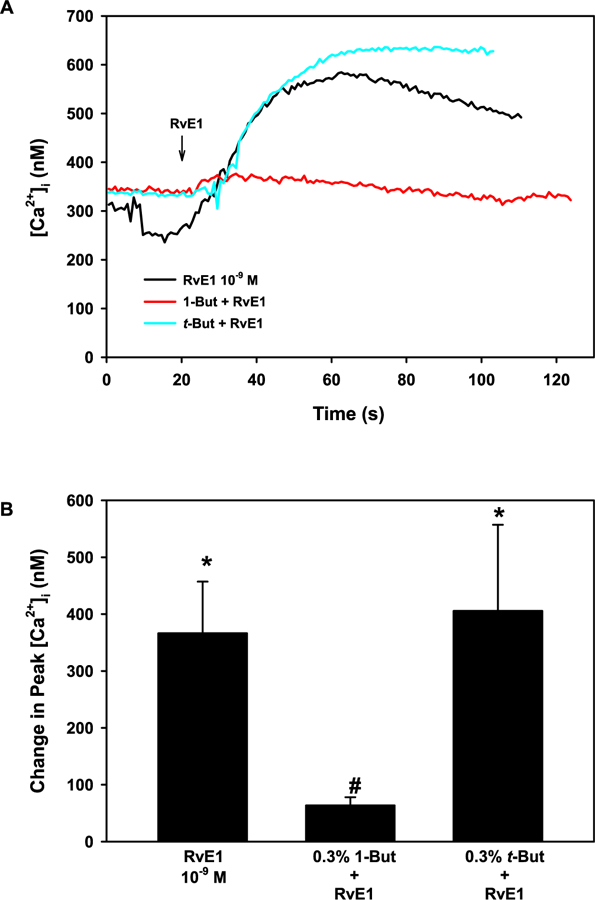
Cultured goblet cells were preincubated with the PLD inhibitor 1-butanol (1-but) and its negative control t-butanol (t-but), both at 0.3%, for 15 min. [Ca2+]i over time is shown in A. Change in peak [Ca2+]i is shown in B. Data are mean ± SEM from 5 independent experiments. * indicates significant difference from basal; # indicates significance from RvE1 alone.
3.6. RvE1 uses PLA2 to increase [Ca2+]i in cultured rat goblet cells
PLA2 can be activated by multiple mechanisms, including Ca2+ (Burke and Dennis, 2009). To determine whether PLA2 is activated by RvE1, aris acid at 10−6 or 10−5 M was used to inhibit PLA2. RvE1 (10−9 M) increased [Ca2+]i to a maximum of 184.5 ± 40.0 nM (p = 0.01) (Fig. 6A and B). Aris acid at 10−5 M significantly inhibited, RvE1-stimulated [Ca2+]i to 52.4 ± 16.6 nM (p = 0.04) (Fig. 6A and B). Thus, RvE1 appears to activate PLA2 to increase [Ca2+]i.
Fig. 6. RvE1 activates the PLA2 pathway to increase [Ca2+]i in rat conjunctival goblet cells.
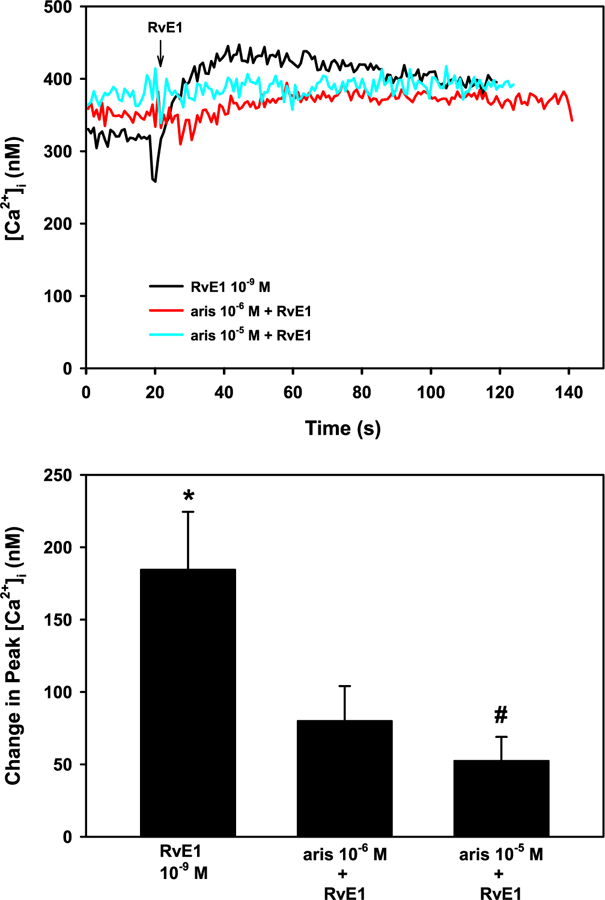
Cultured goblet cells were preincubated with the PLA2 inhibitor aristolochic acid (aris) at 10−6 and 10−5 M for 10 min. [Ca2+]i over time is shown in A. Change in peak [Ca2+]i is shown in B. Data are mean ± SEM from 4 independent experiments. * indicates significant difference from basal; # indicates significance from RvE1 alone.
3.7. RvE1 does not increase [Ca2+]i through Ca2+/CaMK in cultured rat goblet cells
As both RvD1-and LXA4-stimulated [Ca2+]i increase was inhibited by Ca2+/CaMK inhibitors, we wanted to examine if RvE1 also increases [Ca2+]i through Ca2+/CaMK (Lippestad et al., 2017; Hodges et al., 2016b). Goblet cells were incubated with the Ca2+/CaMK inhibitor KN93 or the inactive analog KN92 at 10−7 M for 30 min. RvE1 (10−9 M) significantly increased [Ca2+]i to a peak of 115.4 ± 16.8 nM (p = 0.001, Fig. 7A and B). Neither KN93 nor KN92 affected RvE1-stimulated [Ca2+]i increase, suggesting that RvE1 does not increase [Ca2+]i through Ca2+/CaMK (Fig. 7A and B).
Fig. 7. RvE1 does not activate the Ca2+/CaMK pathway to increase [Ca2+]i in rat conjunctival goblet cells.
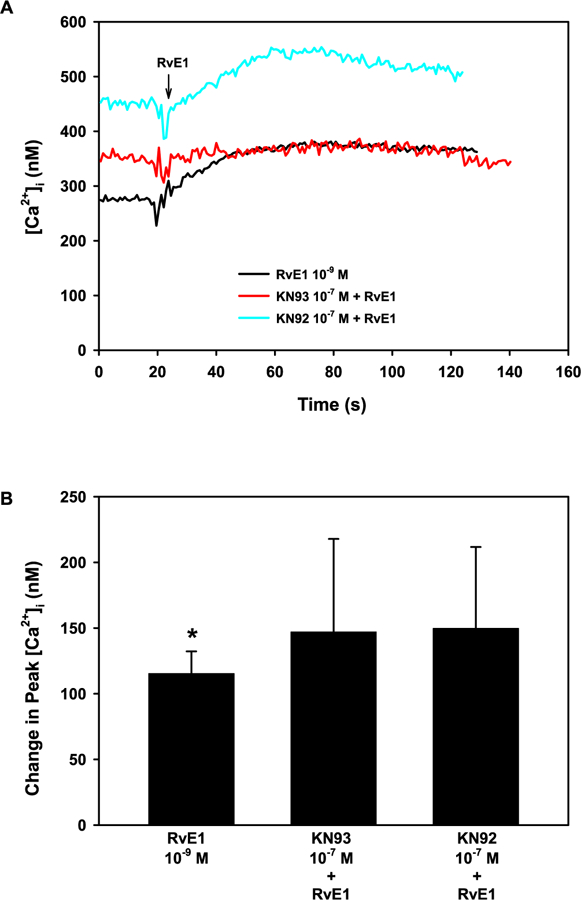
Cultured goblet cells were preincubated with the Ca2+/CaMK inhibitor KN93 and its negative control KN92, both at 10−7 M for 30 min. [Ca2+]i over time is shown in A. Change in peak [Ca2+]i is shown in B. Data are mean ± SEM from 4 independent experiments. * indicates significant difference from basal.
3.8. RvE1 uses PLC, PLD, and PLA2 but not Ca2+/CaMK to stimulate glycoconjugate secretion in rat conjunctival goblet cells
As RvE1-stimulated increase in [Ca2+]i was dependent on activation of the PLC, PLD, and PLA2 pathways, the effect of inhibitors of these pathways on glycoconjugate secretion was determined. Cells were preincubated with inhibitors as described in 2.4, followed by RvE1 (10−9 M). RvE1 alone significantly increased glycoconjugate secretion 2.1 ± 0.2 fold above basal. U73122, but not U73343, significantly inhibited RvE1 stimulated secretion to 1.1 ± 0.1 fold above basal (Fig. 8). 2-APB and Ro 31–8220 also significantly inhibited RvE1 stimulated secretion to 1.3 ± 0.1 and 1.1 ± 0.3 fold above basal, respectively. 1-Butanol, the inhibitor of PLD, but not the negative control t-but, significantly inhibited RvE1-stimulated secretion as did aris acid, a PLA2 inhibitor (Fig. 8). Inhibition of Ca2+/CaM-dependent kinase with KN93 did not alter RvE1-stimulated glycoconjugate secretion (Fig. 8).
Fig. 8. Inhibition of PLC, PLD, and PLA2, but not Ca2+/Cam Kinase, blocks RvE1-stimulated glycoconjugate secretion in rat conjunctival goblet cells.
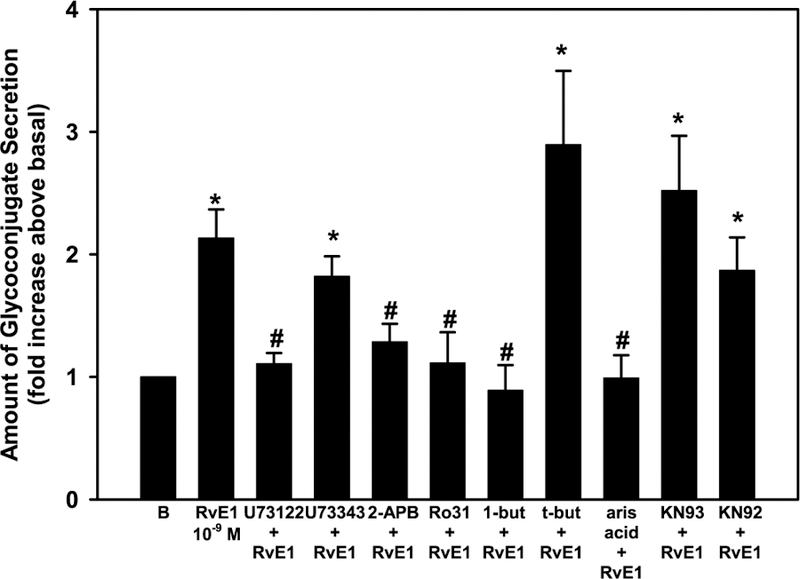
Cultured goblet cells were incubated with U73122, U73343, 2-APB, Ro 31–8220, 1-but, t-but, aris acid, KN93, or KN92 before stimulation with RvE1 (10−9 M). Glycoconjugate secretion was measured. Data are mean ± SEM from 4 independent experiments and shown as fold above basal, which set to 1. * indicates significant difference from basal; # indicates significance from RvE1 alone.
4. Discussion
Herein we found that RvE1 stimulates glycoconjugate secretion from conjunctival goblet cells and did so by increasing [Ca2+]i, and activation of the PLC, PLD, and PLA2 signaling pathways. The PLC downstream molecules IP3 and PKC were also activated by RvE1 (Fig. 9).
Fig. 9. Schemetic diagram of signaling pathways activated by RvE1.
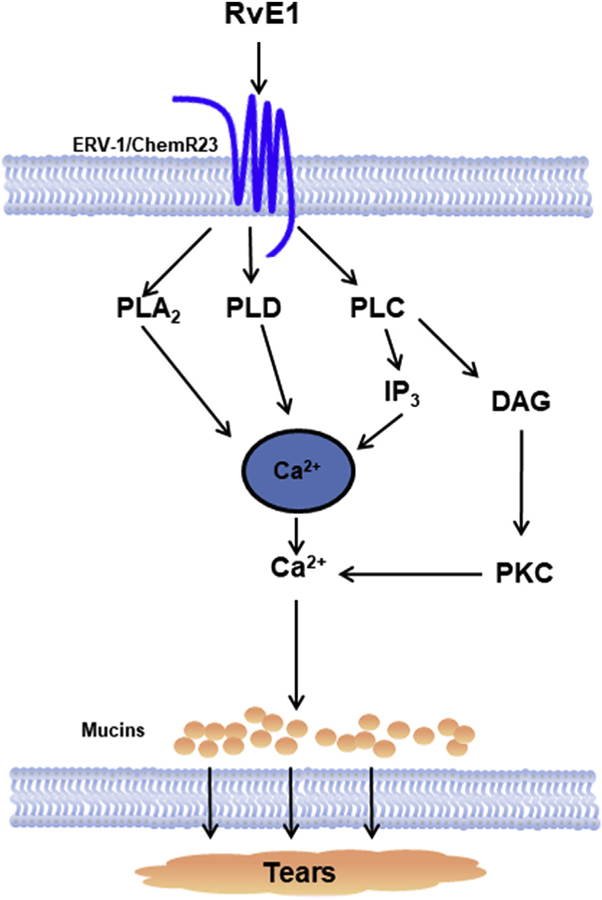
RvE1 binds to the ERV-1/ChemR23receptor and activates the signaling pathways of PLA2, PLD, and PLC. PLA2 and PLD activation increases [Ca2+]i. PLC increases IP3 and DAG. IP3 releases Ca2+ while DAG activates PKC. Activation of these pathways lead to mucin secretion. PLA2-phospholipase A2; PLD-phospholipase D; PLC-phospholipase C; IP3-inositol trisphosphate; DAG-diacylglycerol; PKC-protein kinase C.
In multiple types of chronic inflammatory diseases RvE1 is an active component of the resolution of inflammation (Hasturk et al., 2006; Aoki et al., 2010; Salic et al., 2016; Herrera et al., 2015; Kim et al., 2012; Haworth et al., 2008). Here, we presented supportive results that RvE1 may also regulate glycoconjugate secretion in conjunctival goblet cells in physiological conditions to maintain ocular surface health. Our results are consistent with earlier studies of the SPMs RvD1 and LXA4, which we showed also play a role in stimulating conjunctival goblet cell secretion under normal, physiological conditions. Similarly to RvE1, RvD1 and LXA4 also stimulate glycoconjugate mucin secretion thorough an increase in [Ca2+]i (Lippestad et al., 2017; Hodges et al., 2016b). RvE1 binds to the receptor ERV-1/ChemR23 (Arita et al., 2007), RvD1 activates DRV1/GPR32 (in humans) and ALX/FPR2 (Krishnamoorthy et al., 2010, 2012) and LXA4 stimulates ALX/FPR2 (Chiang et al., 2006). Although RvE1, RvD1, and LXA4 activate different receptors, they act in a surprisingly similar manner. All the SPMs studied activated PLC, PLD and PLA2 pathways when interacting with goblet cells from the conjunctiva. The only significant difference we found was that RvD1 and LXA4 also induced [Ca2+]i through Ca2+/ CaMK. Our results indicate that SPMs have a common regulating function on goblet cell glycoconjugate mucin secretion, which is key in maintaining a healthy ocular surface. A physiological role for the SPMs is strengthened by LXA4 and RvD1 being found in emotional tears from human (English et al., 2017). Although RvE1 was not identified in tears this may reflect the nutritional status of EPA of the individuals since 18-HEPE, the RvE1 precursor, was present in tears from males. Hence, RvE1 could still be effective in maintaining ocular surface health if added topically to the tear film.
None of the inhibitors gave a complete blockage of RvE1-stimulated [Ca2+]i. RvE1 works through several different signaling pathways. Here, we studied three possible pathways that RvE1 could activate and found that all three were used by RvE1. Although one pathway may be inhibited, [Ca2+]i can still be increased by RvE1 through other pathways, and stimulate to glycoconjugate secretion. This redundancy signifies the importance of RvE1 as a regulator of glycoconjugate mucin secretion.
When activation of the PLC pathway was studied using the PLC inhibitor U73122 and its negative control U73343, the negative control inhibited RvE1-induced [Ca2+]i increase more than the inhibitor. A similar problem occurred when U73343 also inhibited RvD1-induced [Ca2+]i increase (Lippestad et al., 2017). In contrast, U73122 inhibited the [Ca2+]i increase induced by the cholinergic agonist carbachol, but the negative control U73343 did not (Lippestad et al., 2017). There are several possibilities for the difference between RvE1, RvD1, and carbachol. First, even though all three agonists each bind to G protein coupled receptors, the receptors are different and thus the coupling to PLC could differ. Perhaps different Gα proteins are used. Cholinergic agonists activate three of the different muscarinic receptors, M1AChR, M2AChR, and M3AChR (Rios et al., 1999, 2000; Kanno et al., 2003; Hodges et al., 2012b). These receptors usually act through Gαq (Zenko and Hislop, 2017). In contrast, RvE1 can use Gαi (Jo et al., 2016). Second, RvE1 and RvD1 are lipids, whereas carbachol is a carbamate ester. The lipids could bind to their receptors with different affinities and time-dependencies than carbachol thus altering the activation of PLC. Regardless of the difference in action of the three compounds, it was not possible to conclude if RvE1 activated PLC using only a PLC inhibitor. We therefore studied compounds distal in the PLC pathway. Activation of PLC produces IP3 and DAG. IP3 then binds to an IP3 receptor on the ER, which leads to rise in [Ca2+]i by depleting Ca2+ stored in the ER. DAG activates PKC. In the present study we found that an IP3-receptor inhibitor blocked the RvE1-stimulated increase in [Ca2+]i. In addition, when the ER store of Ca2+ was emptied using thapsigargin, we found a complete blockage of the RvE1-stimulated [Ca2+]i increase. Furthermore, we found that an inhibitor of PKC also blocked the RvE1-stimulated [Ca2+]i increase. These results support the conclusion that RvE1 increases [Ca2+]i via activating the PLC pathway.
The goal of studying RvE1 in goblet cells is to determine if it may be used to preserve ocular surface homeostasis and as a treatment of ocular inflammatory diseases. We found that RvE1 increased glycoconjugate secretion after 2 h, not at 4 h. This suggests that RvE1 has a short, but potent, action on regulating goblet cell secretion. Similar results were obtained for RvD1 and AT-RvD1, where glycoconjugate secretion was measured every hour for 0–4 h. Peak secretion was observed for both RvD1 and AT-RvD1 after 1 h (Li et al., 2013). This time dependency is similar to that of EGF but shorter than the effect of histamine that was still effective after 4 h in rat conjunctival goblet cells (Hayashi et al., 2012; Hodges et al., 2012a). Thus, RvE1 could function to provide short-term stimulation of high molecular weight glycoconjugates including MUC5AC secretion without overproducing mucin that can be harmful to the ocular surface.
The data presented in this manuscript along with studies by Lippested et al. using RvD1 (Lippestad et al., 2017) and Hodges et al. (2017) using LXA4, support the hypothesis that these SPMs play a dual role in the conjunctival goblet cells. The first role is to maintain homeostasis in normal, non-inflamed conjunctiva. In support of this, we demonstrated that resolvins RvE1, RvD1, and LXA4 alone stimulate [Ca2+]i and secretion. In these experiments, the resolvins were added (with no other additions) and [Ca2+]i or secretion were measured. The second role is the resolution of inflammation and as such they inhibit histamine- and leukotriene-stimulated increase in [Ca2+]i and secretion. This was demonstrated by incubation of goblet cells for 30 min with resolvins prior to addition of either histamine or leukotrienes (Dartt et al., 2011; Li et al., 2013).
In summary, RvE1 stimulates conjunctival goblet cells to secrete high molecular weight glycoconjugates including MUC5AC secretion by increasing [Ca2+]i. which in turns activates the PLC, PLD and PLA2 signaling pathways. Previous results have shown that RvE1, like RvD1 and LXA4, counter-regulate inflammatory mediator induced glycoconjugate secretion (Dartt et al., 2011; Hodges et al., 2016a). RvE1, RvD1 and LXA4 appear to act in similar ways to regulate glycoconjugate mucin secretion during physiological conditions using different GPCR to evoke intracellular signals. We conclude that RvE1, as well as other SPMs, help to maintain stable, normal glycoconjugate mucin production in the ocular surface. Thus, RvE1 may be useful both to maintain ocular surface health and as a treatment of ocular surface inflammatory diseases.
Acknowledgments
The authors thank Drs. Dayu Li and Marie Shatos for their helpful assistance and advice.
Funding
This work was supported by The Norwegian Research Council to M.L., National Institute of Health Grant EY019470 to D.A.D, and R01GM038765 to C.N.S.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.exer.2018.04.015.
References
- Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, et al. , 2010. September 10 Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem. Biophys. Res. Commun 400 (1), 128–133. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN, 2007. March 15 Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol 178 (6), 3912–3917. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, 2009. June Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 1793 (6), 933–940. [DOI] [PubMed] [Google Scholar]
- Burke JE, Dennis EA, 2009. April Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res 50, S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. , 2006. September The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev 58 (3), 463–487. [DOI] [PubMed] [Google Scholar]
- Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S, 2013. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediat. Inflamm 2013, 636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA, Masli S, 2014. October Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol 14 (5), 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA, Rios JD, Kanno H, Rawe IM, Zieske JD, Ralda N, et al. , 2000. December Regulation of conjunctival goblet cell secretion by Ca(2+)and protein kinase C. Exp. Eye Res 71 (6), 619–628. [DOI] [PubMed] [Google Scholar]
- Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN, 2011. April 1 Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J. Immunol 186 (7), 4455–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Schwartz CE, Gjorstrup P, Pflugfelder SC, 2012. November Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea 31 (11), 1299–1303. [DOI] [PubMed] [Google Scholar]
- English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN, 2017. February Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot. Essent. Fatty Acids 117, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayton JL, 2009. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol 3, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes PJ, 2014. October Trends in prevalence and treatment of ocular allergy. Curr. Opin. Allergy Clin. Immunol 14 (5), 451–456. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Gipson IK, 2010. June Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res 90 (6), 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. , 2006. February RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. Faseb. J 20 (2), 401–403. [DOI] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD, 2008. August Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol 9 (8), 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, Dartt DA, 2012. May 17 Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest. Ophthalmol. Vis. Sci 53 (6), 2993–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera BS, Hasturk H, Kantarci A, Freire MO, Nguyen O, Kansal S, et al. , 2015. February Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect. Immun 83 (2), 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA, 2012. Octobera. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp. Eye Res 103, 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA, 2012. Octoberb. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp. Eye Res 103, 99–113 [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Serhan CN, Dartt DA, 2016. November 8a. Lipoxin A4 counter-regulates histamine-stimulated glycoconjugate secretion in conjunctival goblet cells. Sci. Rep 6, 36124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, et al. , 2016. April 13b. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol 10, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, et al. , 2017. January Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol 10 (1), 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, et al. , 2009. October Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest. Ophthalmol. Vis. Sci 50 (10), 4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YY, Lee JY, Park CK, 2016. Resolvin E1 inhibits substance P-Induced potentiation of TRPV1 in primary sensory neurons. Mediat. Inflamm 2016, 5259321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Jumblatt JE, 1999. January MUC5AC mucin is a component of the human precorneal tear film. Invest. Ophthalmol. Vis. Sci 40 (1), 43–49. [PubMed] [Google Scholar]
- Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, et al. , 2003. April Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am. J. Physiol. Cell Physiol 284 (4), C988–C998. [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim GD, Jin YH, Park YS, Park CS, 2012. December Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharm 14 (4), 384–391. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. , 2010. January 26 Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. U. S. A 107 (4), 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN, 2012. May Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol 180 (5), 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, et al. , 2013. March 14 Allergic conjunctivitis: a comprehensive review of the literature. Ital. J. Pediatr 39, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Sun Y, Gjorstrup P, Pearlman E, 2015. April Inhibition of corneal inflammation by the resolvin E1. Invest. Ophthalmol. Vis. Sci 56 (4), 2728–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE, 2010. October Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Therapeut 26 (5), 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Carozza RB, Shatos MA, Hodges RR, Dartt DA, 2012. October 5 Effect of histamine on Ca(2+)-dependent signaling pathways in rat conjunctival goblet cells. Invest. Ophthalmol. Vis. Sci 53 (11), 6928–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, et al. , 2013. November Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol 6 (6), 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA, 2017. September 1 Resolvin D1 increases mucin secretion in cultured rat conjunctival goblet cells via multiple signaling pathways. Invest. Ophthalmol. Vis. Sci 58 (11), 4530–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Broad LM, Bird GS, Putney JW Jr., 2001. February 23 Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J. Biol. Chem 276 (8), 5613–5621. [DOI] [PubMed] [Google Scholar]
- Mantelli F, Argueso P, 2008. October Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol 8 (5), 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilligan VE, Gregory-Ksander MS, Li D, Moore JE, Hodges RR, Gilmore MS, et al. , 2013. Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS One 8 (9), e74010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA, 2005. October Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr 82 (4), 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN, 2010. January 29 Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem 285 (5), 3451–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT, 2011. February 1 Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J. Immunol 186 (3), 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA, 1999. May Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Investig. Ophthalmol. Vis. Sci 40 (6), 1102–1111 [Research Support, U.S. Gov’t, P.H.S.]. [PubMed] [Google Scholar]
- Rios JD, Forde K, Diebold Y, Lightman J, Zieske JD, Dartt DA, 2000. July Development of conjunctival goblet cells and their neuroreceptor subtype expression. Investig. Ophthalmol. Vis. Sci 41 (8), 2127–2137 [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. [PubMed] [Google Scholar]
- Salic K, Morrison MC, Verschuren L, Wielinga PY, Wu L, Kleemann R, et al. , 2016. July Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 250, 158–165. [DOI] [PubMed] [Google Scholar]
- Serhan CN, 2014. June 5 Pro-resolving lipid mediators are leads for resolution physiology. Nature 510 (7503), 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, 2013. August Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol 13 (4), 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Arita M, Hong S, Gotlinger K, 2004. November Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 39 (11), 1125–1132. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J, Levy BD, 2014. October 30 Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol 7 (2), a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA, 2001. June Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest. Ophthalmol. Vis. Sci 42 (7), 1455–1464. [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Agrawal V, Wilson SE, 2014. Resolvin E1 analog RX-10045 0.1% reduces corneal stromal haze in rabbits when applied topically after PRK. Mol. Vis 20, 1710–1716. [PMC free article] [PubMed] [Google Scholar]
- Uchino M, Schaumberg DA, 2013. June Dry eye disease: impact on quality of life and vision. Curr. Ophthalmol. Rep 1 (2), 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau S, Maire MA, Pasquis B, Gregoire S, Acar N, Bron AM, et al. , 2009. August Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes Arch. Clin. Exp. Ophthalmol 247 (8), 1039–1050. [DOI] [PubMed] [Google Scholar]
- Zenko D, Hislop JN, 2017. November 11 Regulation and trafficking of muscarinic acetylcholine receptors. Neuropharmacology [Review] [DOI] [PubMed]