Abstract
Permanent hearing loss affects more than 5% of the world’s population, yet there are no nondevice therapies that can protect or restore hearing. Delivery of therapeutics to the cochlea and vestibular system of the inner ear is complicated by their inaccessible location. Drug delivery to the inner ear via the vasculature is an attractive noninvasive strategy, yet the blood-labyrinth barrier at the luminal surface of inner ear capillaries restricts entry of most blood-borne compounds into inner ear tissues. Here, we compare the blood-labyrinth barrier to the bloodbrain barrier, discuss invasive intratympanic and intracochlear drug delivery methods, and evaluate noninvasive strategies for drug delivery to the inner ear.
HEARING LOSS AND COCHLEAR CELL DEATH
More than 5% of the world’s population suffers from disabling hearing loss according to a 2015 report by the World Health Organization (https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss)). Hearing loss is classified as sensorineural in ~90% of cases, most involving irreversible loss of auditory neurons or cochlear sensory cells. External causes of acquired hearing loss include noise trauma (1), ototoxic treatments such as head and neck radiation therapy (2), platinum-based chemotherapy (3), and aminoglycoside antibiotic treatment (4). Furthermore, more than 500 genetic mutations have been identified that account for more than 50% of cases of congenital deafness (5). Unfortunately, there are no drug therapies that can protect or restore hearing. Currently, the principal U.S. Food and Drug Administration–approved treatment for profound hearing loss is the cochlear implant, which requires invasive surgery to insert the frequency-specific electrode array into the cochlea. Preclinical research has predominantly focused on the regeneration or repair of auditory sensory cells or neurons, as well as the delivery of candidate otoprotective agents to prevent hearing loss. These approaches include the delivery of cellbased or macromolecular therapeutics to the inner ear, such as stem cells (6) or growth factors (7). Such approaches also include gene therapy to introduce a viral vector carrying a therapeutic gene into the inner ear, as exemplified by a recent clinical trial for delivery of the master hair cell gene Hath1 (the human equivalent of the Atoh1 gene in mouse) to the inner ear of patients (https://clinicaltrials.gov/ct2/show/NCT02132130). However, the physical inaccessibility of the inner ear and the blood-labyrinth barrier (BLB) create challenges for effective delivery of these therapeutics. The BLB, the barrier between the vasculature and fluids of the inner ear, restricts entry of most blood-borne compounds into inner ear tissues.
The bony labyrinth of the inner ear contains the cochlea that detects sound, the primary focus of this Review, and the vestibular system that senses motion and gravity (Fig. 1). The mechanosensory hair cells of the cochlea mechanoelectrically transduce sound stimuli into neuronal action potentials with high fidelity and have been extensively reviewed elsewhere (8–10). The organ of Corti containing these mechanosensory hair cells and their support cells spirals from the base to the apex of the cochlea. All mammals have a limited number of sensory hair cells that cannot be regenerated when damaged, resulting in permanent hearing loss. Nonmammalian vertebrates, such as birds, fish, and amphibians, can regenerate their sensory hair cells, providing insight into the genes and signaling pathways activated by damage or injury that can promote cellular regeneration (11). Exogenous activation of the hair cell master gene, Atoh1, in a damaged mammalian auditory system can drive the conversion of support cells into hair cells, facilitating partial functional recovery of hearing in guinea pigs (12) and mice (13). Hair cells are innervated by primary auditory neurons (also called spiral ganglion neurons). Primary auditory neurons (90 to 95%) are type I neurons that innervate inner hair cells and carry action potentials to the brainstem. The remaining 5 to 10% type II neurons extend unmyelinated dendrites to innervate outer hair cells (14). Type II neurons may regulate the ability of outer hair cells to amplify the incoming acoustic signal and are postulated to play nociceptive roles in noise-induced damage (15, 16). There is increasing evidence for hearing loss after degeneration of primary auditory neurons, as well as degeneration that occurs as a secondary effect of hair cell loss (17). Indeed, degeneration of primary auditory neurons without hair cell damage has been observed in the aged human cochlea (18) and also after exposure to aminoglycoside antibiotics (4) or noise (19). Thus, sensorineural hearing loss can be caused by dysfunction or death of primary auditory neurons and sensory hair cells.
Fig. 1. Structure and blood supply of the cochlea.
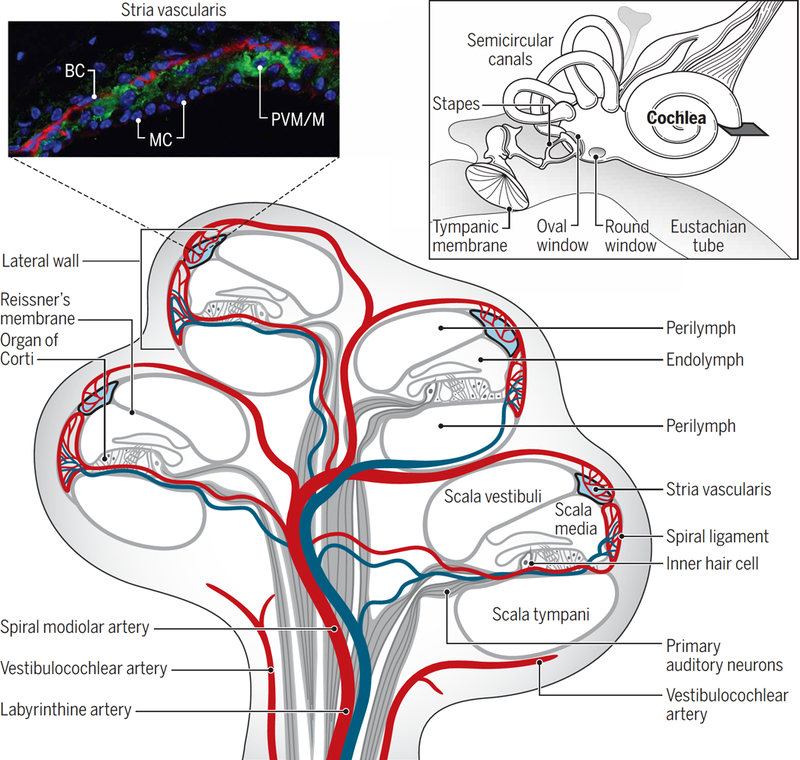
Inset: (top left) Fluorescence micrograph of the mouse stria vascularis showing three cell layers comprising basal cells (BC), marginal cells (MC), and an intermediate cell layer of perivascular-resident macrophage-like melanocytes (PVM/Ms). Basal cells are immunostained for actin (red), and PVM/ Ms are immunostained for the K+ channel Kir4.1 (green); nuclei are stained with DAPI (4′,6-diamidino-2-phenylindole) counterstain (blue). Inset: (top right) Structure of the middle ear containing the tympanic membrane, oval window, round window, and auditory ossicles. Also shown are the cochlea and semicircular canals (vestibular system) of the inner ear. Main figure: The blood supply to the cochlea (shown in cross section) comes from the common cochlear artery, which divides into the spiral modiolar artery and the vestibulocochlear artery. The spiral modiolar artery supplies the apical turns of the cochlea, and the cochlear branch of the vestibulocochlear artery supplies the basal turns of the cochlea. The spiral modiolar artery supplies the organ of Corti and primary auditory neurons of the modiolus and forms the capillaries of the spiral ligament and stria vascularis in the cochlear lateral wall. The blue vessels represent the venous return route of deoxygenated blood. Image credit: Sophie Nyberg, University of Toronto.
THE STRIA VASCULARIS AND HEARING LOSS
Mechanotransduction of auditory signals by cochlear sensory hair cells relies on the precise electrochemical composition of the cochlear fluids. The stria vascularis is a highly vascularized epithelial-mesodermal tissue in the lateral wall of the cochlea (Fig. 1). It is essential for generating and maintaining the unique ionic composition of the endolymph in the scala media. The stria vascularis is composed of three cell layers that form a distinct compartment, delineated by tight junctions at its medial and lateral borders (Fig. 2). On the medial surface, adjacent marginal cells are coupled together by apical tight junctions to form a continuous secretory barrier epithelium. On the lateral side, tight junctions between adjacent basal endothelial cells separate the stria vascularis from the fibrocytes populating the spiral ligament in the cochlear lateral wall. A perivascular intrastrial space, located between the marginal and basal cell layers, contains a dense network of strial capillaries together with pericytes and perivascular-resident macrophage-like melanocytes (PVM/Ms) also called intermediate cells (20). These intermediate cells wrap around capillaries to form a third cell layer sandwiched between the marginal cell and basal cell epithelia. K+ ions are actively transported from blood or perilymph by Na+- and K+-dependent ATPases (Na+/K+ ATPases), Na+/K+/Cl− cotransporters, and potassium rectifying channels (Kir4.1) across the stria vascularis. They are secreted into endolymph in the scala media to generate and maintain the endocochlear potential of +80 to 100 mV required for neurotransmission of sounds by the cochlea (21, 22). Malfunction of the stria vascularis can alter the electrochemical composition of the endolymph, resulting in loss of the endocochlear potential, elevated auditory thresholds, and hearing loss. This commonly occurs after mutations in the KCNJ10 gene that encodes the Kir4.1 channels required for generation of the endocochlear potential (23). In the cochlea, the scala vestibuli and scala tympani are filled with perilymph that is similar in ionic composition to cerebrospinal fluid (CSF), although the perilymph of the two scalae differs slightly in ion and protein concentrations. CSF-perilymph exchange occurs via pressure-driven oscillations (24). The scala media endolymph bathing the upper surface of the organ of Corti is primarily regulated by the stria vascularis. The ionic composition of extracellular endolymph is uniquely characterized by a high concentration of K+ ions and a low concentration of Na+ ions, more closely resembling the average intracellular environment in mammalian cells than an extracellular fluid. The composition of perilymph and endolymph is further regulated by ion transport across Reissner’s membrane, which separates the two fluids (Fig. 1) (25). A third distinct fluid, intrastrial fluid between strial cells, has a very low K+ ion concentration and a low Na+ ion concentration generating an electrical potential of +115 to 120 mV, making the intrastrial fluid unique compared to other fluids in the nervous system (Table 1) (23, 26).
Fig. 2. Comparison of the BBB and BLB.
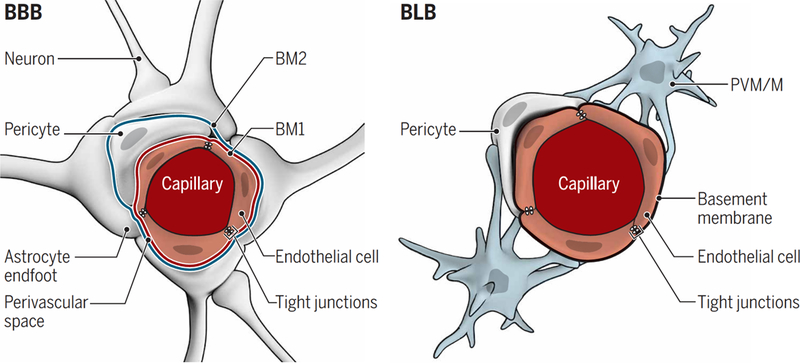
Shown are cross sections of the capillaries and cell types forming the BBB and BLB. (Left) The BBB endothelium is surrounded by a basement membrane that splits to accommodate pericytes so that the basement membrane (BM1) is shared between endothelial cells and pericytes. A second basement membrane (BM2) is formed by the endfeet of astrocytes, generating a perivascular space between the two basement membranes. Within the CNS, most of the outer basement membrane encapsulating BBB capillaries is covered by astrocytic endfeet. (Right) The BLB endothelium is similarly surrounded by a basement membrane with pericytes wrapping around the basement membrane enclosing the capillaries, but there is no outer basement membrane as in the BBB. PVM/Ms are wrapped around the basement membrane surrounding the BLB endothelium. In both the BBB and BLB, tight junctions are present between endothelial cells and are composed of similar proteins.
Table 1.
Differences in fluid composition between compartments of the CNS and peripheral nervous system.
| Component | Blood (127) | Brain interstitial fluid (127, 128) | CSF (129, 130) | Scala vestibule perilymph (129) | Scala tympani perilymph (129) | Intrastrial fluid (129) | Scala media endolymph (129) |
|---|---|---|---|---|---|---|---|
| [Na+] (mM) | 145 | 154 | 152–156 | 141 | 148 | 85 | 1.3 |
| [K+] (mM) | 4.6 | 2.9 | 3 | 6.0 | 4.2 | 1–2 | 157 |
| pH | 7.4 | 7.3 | 7.33 | 7.3 | 7.3 | Unknown | 7.4 |
| Protein (mg/dl) | 4238 | Unknown | 24 | 242 | 178 | Unknown | 38 |
| Free [Ca2+] (mM) | 2.4 | 1.2 | Unknown | 0.6 | 1.3 | 0.8 | 0.023 |
| Potential (mV) | 0 | 0 | 0 | <0.3 | 0 | +115–120 | +85 |
In the central nervous system (CNS), ion homeostasis is maintained through the combined activity of the bloodbrain barrier (BBB) at the brain capillary interface and the blood-CSF barrier at the choroid plexus in the brain’s ventricles. Homeostasis of endolymph and the intrastrial fluid between cells within the stria vascularis makes the stria vascularis physiologically more complex than its CNS counterparts. Strial capillaries are nonfenestrated with tight junctions between adjacent endothelial cells, forming a barrier that separates intrastrial fluids from blood. The barrier properties are maintained through the expression of a combination of intercellular tight junction proteins. Basal cells of the stria vascularis primarily express claudin-11, which is essential for strial integrity because mice lacking claudin-11 are deaf (27). Marginal cells express claudin-1, claudin-3, and claudin-4 and form a barrier that separates the intrastrial fluid from the endolymph (28). PVM/Ms surrounding strial capillaries in the intrastrial space help to maintain compartmentalization of the stria, as loss of these cells causes barrier disruption and deafness in mice (29). Noise damage causes an increase in permeability of the BLB due to down-regulation of occludin and claudin-5 in the basal and marginal cells of the stria (1). Loss of BLB integrity and strial degeneration is observed in a mouse model of Pendred syndrome, where the loss of the protein pendrin results in hyperpigmentation of the stria vascularis, disorganization of the marginal cell layer, and an inability to generate an endocochlear potential (30). Abnormalities in the stria vascularis of adult mice lacking pendrin are associated with recruitment of CD68+ CD83+ macrophages (31). Last, the gap junction protein connexin 30 is required for strial integrity, and loss of connexin 30 causes congenital deafness in mice. Strial capillaries in mice lacking connexin 30 display multiple endothelial cell layers and enlarged paracellular spaces between endothelial cells, resulting in loss of the endocochlear potential and leakage of serum proteins into the stria vascularis (32).
Decreased vascularization in the stria vascularis is a primary consequence of aging. A corrosion cast study using resin to capture the blood vessel structure of the stria vascularis in murine presbycusis (age-related hearing loss) found marked narrowing and degeneration of strial capillaries in the basal turn of the cochlea, whereas strial capillaries in apical turns of the cochlea were less affected (33). Aged mice show lower endocochlear potentials that correlate with loss of marginal cells and decreased melanin in the stria vascularis (34). The effects of age-related metabolic changes in the inner ear vasculature upon hearing loss require further characterization. There is evidence for age-dependent degeneration of the stria vascularis in humans that precedes shifts in auditory thresholds (35, 36). Thus, there is a window of therapeutic opportunity to prevent degeneration of the inner ear vasculature before the onset of hearing loss. Exploration of therapies that prevent or reduce hearing loss resulting from strial degeneration could include gene therapy to up-regulate expression of tight junction proteins to repair a leaky stria vascularis, delivery of angiogenesis-inducing growth factors, or antioxidant treatments to ameliorate the effects of inflammation on barrier leakiness.
THE BLOOD-LABYRINTH BARRIER
The concept of a distinct barrier in the inner ear originated from the observed difference in chemical composition of blood and inner ear fluids. Two key publications from the 1980s, nearly a century after the discovery of the BBB, reported a functional barrier referred to as the blood-perilymph barrier or the BLB. This barrier is characterized by nonfenestrated capillaries with tight junctions and a decreasing rate of entry into perilymph from blood by compounds of increasing molecular weight (Fig. 2) (37, 38). The precise anatomical sites of the BLB remain poorly characterized, and there are several barriers separating the inner ear fluid compartments from capillaries of the vasculature. In the cochlea, the BLB is composed of vascular endothelial cells coupled together by tight junctions. The analogous blood-endolymph barrier is more complex, with tight junction-coupled strial endothelial cells separating the capillary lumen from the intrastrial interstitial fluid. Tight junction-coupled epithelial marginal cells together with endothelial basal cells form the intrastrial compartment, separating intrastrial fluid from endolymph. Recent publications refer to this as the blood-strial barrier or intrastrial fluid–blood barrier (32, 39), highlighting the role of the stria vascularis in compartmentalizing and maintaining the distinct fluidic compositions within the cochlea. Here, we use the more general term BLB, as this term covers all of the primary anatomical barrier structures separating cochlear (and vestibular) fluids and tissues from blood.
Where does the BLB begin? Although the cochlea is part of the peripheral nervous system, the blood vessels supplying the cochlea originate from the CNS (Fig. 1). This poses additional difficulties in defining the fluid interface barriers of the inner ear. It is clear that strial capillary beds have many barrier properties akin to those of the BBB and that all vascular barriers of the inner ear contribute to form a heterogeneous BLB. More proximal blood vessels, such as capillaries supplying the primary auditory neurons, could arguably be part of the BBB because the blood supply comes from branches of vessels supplying the CNS. The barrier properties of nonstrial vessels throughout the inner ear should also be investigated and may form an even tighter barrier than do strial vessels (40, 41). In addition, nerve fibers adjacent to the spiral modiolar artery (Fig. 1) regulate cochlear blood flow by providing vasoactive peptides and noradrenaline to the vasculature and may form a blood-nerve barrier (42). Overall, the permeability and barrier properties of the BLB are likely to be heterogeneous across the inner ear and require detailed characterization to elucidate the functional range of BLB properties.
The BLB at the stria vascularis is fundamentally similar to the BBB that separates brain interstitial fluid from blood. Table 2 compares the properties of the strial BLB and the BBB. The BLB is less permeable than the blood-CSF barrier located at the choroid plexus and arachnoid membrane. This was shown by intravenous injection of the radioactive tracer molecule trimethylphenylammonium, which resulted in 14.3% of the dye concentration being measured in CSF after 90 min, compared to 6.3 and 3.7% in the perilymph of the scala tympani and scala vestibuli, respectively (43). By contrast, the aminoglycoside antibiotic gentamicin can readily cross the BLB but apparently not the BBB (44). This suggests differences in BBB and BLB tightness or differential barrier transendothelial cell transport mechanisms (40) that await specific characterization in the BLB. Structurally, the strial BLB is similar to the BBB but simpler (Fig. 2 shows a structural comparison of the two barriers in cross section). The overall permeability of the BBB is modulated locally by CNS-resident pericytes, the end feet of astrocytic glia, and neurons, which all function together as a local neurovascular unit (45). The endothelium of strial capillaries appears to have similar interactions among closely associated cells, functioning as a cochlear-vascular unit (39). Pericytes are embedded within the basement membrane of strial capillaries, although more sparsely than pericytes in capillaries of the adjacent spiral ligament (46). Intermediate cells or PVM/Ms are in close contact with strial vessels through numerous cytoplasmic processes (47), which are similar to astrocytic end feet covering brain endothelial cells. PVM/Ms are essential to the integrity of the blood-strial barrier because their ablation causes leaky vessels and increased hearing thresholds due to down-regulation of zonula occludens-1 and occludin in tight junctions, as well as down-regulation of vascular endothelial cadherin (VE-cadherin) in adherens junctions (48). Overall, more data comparing the BBB and BLB are needed to fully elucidate the properties of the BLB.
Table 2.
Physiological characteristics of the BBB and BLB.
| BBB | BLB | |
|---|---|---|
| Principal cell type | Capillary endothelial cells | Capillary endothelial cells |
| Support cells | Pericytes, smooth muscle cells, astrocytes, and neurons | Pericytes, PVM/Ms in stria vascularis; pericytes and fibrocytes in cochlear lateral wall |
| Tight junction composition | Claudin-5, occludin, zonula occludens 1, and VE-cadherin (80) | Claudin-5, claudin-11, occludin, zonula occludens 1, and VE-cadherin in basal cells; claudin-1, claudin-3, and claudin-4 in marginal cells (1, 28, 131, 132) |
| Time of development (rat) | Embryonic day 1 to postnatal day 0 (133) | Established by postnatal day 14 (134) |
| Fluids separated by the BBB or BLB | Blood/brain interstitial fluid | Blood/perilymph, blood/endolymph, and endolymph/ intrastrial fluid |
| Permeability modulators | Osmotic agents, inflammation, and trauma (134–137) | Osmotic agents (including diuretics), inflammation, and trauma (noise) |
| Receptors mediating endocytosis or transcytosis* | Transferrin receptor, LRP1, LRP2, insulin receptor, and diphtheria toxin receptor (87, 138, 139) | LRP2, transferrin receptor, and insulin receptor (92, 140, 141) |
Note that although receptors known to mediate transcytosis elsewhere are expressed at the BLB, literature on receptor-mediated transcytosis across the BLB is sparse.
COCHLEAR BLOOD FLOW AND BLB PERMEABILITY
The stria vascularis and adjacent spiral ligament have complementary roles in regulating the cochlear microenvironment. The spiral ligament is rich in fibrocytes derived from mesenchymal stem cells. Cochlear macrophages in the spiral ligament and PVM/Ms in the stria vascularis comprise the immune presence in the cochlea and are able to modulate barrier permeability. Fibrocytes produce inflammatory cytokines, such as tumor necrosis factor–α, interleukin-1β, and interleukin-6 in response to noise damage (49). Cytokine production, in conjunction with reduced connexin 26 expression, may contribute to recruitment and infiltration of blood-borne immune cells into the cochlea and increased barrier permeability during inflammation (50). Cochlear infiltration by CD45+ Iba1+ CX3R1+ macrophages occurs after noise exposure (51), and these cells may have a role in clearing dead or dying cells by phagocytosis. Noise-induced activation of the cochlear immune response is coupled with vasodilation of spiral ligament capillaries and increased cochlear blood flow, regulated by fibrocyte vascular–induced calcium signaling in a cyclooxygenase-1–dependent mechanism (52).
Pericytes in the spiral ligament are associated with endothelial cells at a ratio of 1:1 to 1:2 compared to a lower ratio of 1:5 in brain endothelium (46, 53). Spiral ligament pericytes are positive for the contractile proteins α-smooth muscle actin, tropomyosin, and nonmuscle myosin II, which are absent from pericytes in the stria vascularis (54, 55). Calcium imaging revealed that spiral ligament fibrocytes are functionally coupled to adjacent vascular cells, and calcium wave propagation through the lateral wall is followed by capillary vasodilation (56). Thus, the two microvasculature systems of the stria vascularis and spiral ligament influence blood flow and, indirectly, BLB permeability through the release of inflammatory cytokines. However, further studies are warranted to understand the molecular mechanisms underlying vasodilation and vasoconstriction of cochlear blood vessels.
CURRENT APPROACHES FOR DRUG DELIVERY TO THE INNER EAR
Systemic delivery of therapeutics to any location faces numerous challenges, in particular, avoiding adverse effects as a consequence of off-target binding (such as ototoxicity from antibiotic treatment). The therapeutic must have affinity for the target, bind to the target, and induce a biological effect. Because of the ubiquitous expression of most cell surface receptors in the body, systemic administration of drugs tends to cause adverse effects as the drug perfuses, binds, and elicits responses in different tissues. The BLB presents additional challenges for delivery of therapeutics to the inner ear. However, noninvasive yet targeted drug delivery to the inner ear through the vasculature is a challenge that is becoming more feasible to overcome with the advance of synthetic and biological drug delivery vehicles. Nanoscale engineering of drug delivery vehicles opens up many avenues of “smart” targeting, due to the ability to incorporate properties such as receptor targeting moieties, controlled release mechanisms, and characteristics to avoid premature elimination from the bloodstream, all of which may help to enhance therapeutic effects and reduce off-target effects associated with systemic delivery (57).
The cochlea and vestibular system are encased in the bony otic capsule of the petrous bone, one of the densest bones in the body, making surgery and imaging difficult. The tympanic membrane separates the external auditory canal from the middle ear, and the round window and oval window separate the middle ear from the inner ear perilymph (Fig. 3). Local delivery of therapeutics to the inner ear is inherently invasive, requiring needles or scalpels to gain access (Fig. 3). Advances in local delivery to the inner ear have been discussed in several publications (58–60). Table 3 summarizes current delivery methods, such as intratympanic, intracochlear, and systemic delivery, and provides examples of approaches for local delivery, including gene therapy, cell-based therapies, and nanoparticles.
Fig. 3. Invasive and noninvasive delivery of drugs to the cochlea.
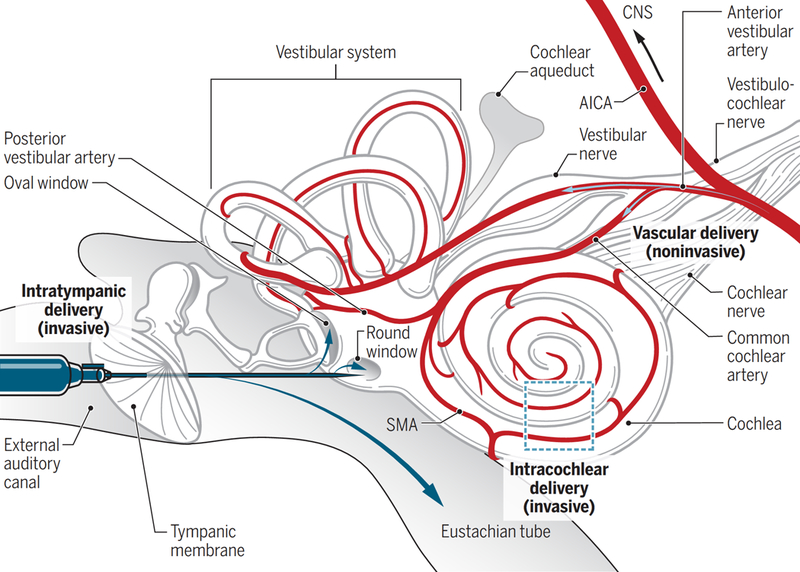
Intratympanic delivery involves using a syringe needle to penetrate the tympanic membrane and to inject the therapeutic into the middle ear. Blue arrows indicate the potential fates of water-soluble therapeutics after injection into the middle ear, including uptake by the oval window or round window epithelial membranes or clearance by the Eustachian tube. Intracochlear delivery can be performed after a cochleostomy through the temporal bone of the skull and can be used to deliver drugs via injections or catheters directly to the cochlea. This is usually only performed in conjunction with surgery to implant a cochlear device. Water-soluble therapeutics delivered through the vasculature enter via the anterior cerebellar inferior artery branching into the cochlear and vestibular blood vessels. Only arteries and arterioles are shown for the blood supply. SMA, spiral modiolar artery; AICA, anterior inferior cerebellar artery.
Table 3.
Comparison of methods for drug delivery to the inner ear.
| Delivery method | Advantages | Limitations | Approaches tested/type of therapeutic |
|---|---|---|---|
| Intratympanic | Can combine injections with catheters for delivery over weeks (142) Avoids problems with systemic targeting and premature systemic clearance |
Invasive Round window membrane is a major epithelial barrier to delivery Clearance by Eustachian tube; half-life of corticosteroids in middle ear ~27 min (124, 143) Toxicity from high-dose single injection (144) |
Clinical: Antibiotics, corticosteroids for Meniere’s disease, and sudden sensorineural hearing loss (145, 146) Preclinical: Poloxamer hydrogels with dexamethasone for extended delivery; hydrogels loaded with neurotrophin-3 growth factor for cochlear synapse regeneration (139, 147) |
| Intracochlear | Avoids problems with systemic targeting and premature systemic clearance Useful technique for preclinical studies |
Highly invasive, requires surgical access to cochlea Risk of trauma and postoperative complications such as protein fouling and inflammation |
Clinical: Implantation of medical devices i.e., cochlear implant Preclinical: Implantation for intracochlear delivery of infusions, complex macromolecular therapeutics e.g., neurotrophin genes for protection of primary auditory neurons and sensory hair cells (61–63; 148–150) |
| Semicircular canal injection | Gives direct access to perilymph Avoids problems with systemic targeting and premature systemic clearance |
Invasive Not feasible for use in humans due to anatomical location |
Preclinical: Gene therapy using adenoviral vector delivery of the therapeutic gene in animal models (151) |
| Systemic | Non-invasive intravenous delivery is easy to use clinically. BLB transporters can be targeted for delivery that does not disrupt the BLB. Smart drug delivery vehicles can carry macromolecular therapeutics. |
BLB is a major hurdle. Off-target effects and lower concentrations compared to local delivery (98) Protein fouling and elimination by the immune system |
Preclinical: Delivery of diphtheria toxin to the cochlea to kill macrophages (48) |
Adeno-associated virus (AAV) vectors are frequently used for therapeutic gene delivery in preclinical animal models. AAV vectors have been used in animal models to deliver neurotrophin genes to the cochlea to protect primary auditory neurons (61, 62) or sensory hair cells (63, 64). Several AAV vector serotypes have enabled targeting of a therapeutic gene, such as the hair cell gene Atoh1, to the inner ear (12, 65). Delivery of the gene encoding vesicular glutamate transporter 3 using AAV1 led to functional restoration of hearing in mice lacking this protein (66). The virulence of certain AAV strains has been problematic for clinical translation in the past due to adverse effects observed in preclinical and clinical trials (67). Newer generations of AAV vectors are being developed to improve targeting and safety, such as the synthetic vector Anc80L65, which was demonstrated to target sensory hair cells in the cochlea safely and efficiently (68). Cell transplantation is a promising option for nonviral delivery of therapeutic genes. Stem cell transplants have been successful in restoring hearing in preclinical models (69, 70). Auditory stem cell–like cells have been isolated from human fetal (71) and adult (72) inner ear tissues. Otic progenitor cells derived from human embryonic stem cells transplanted into ouabain-treated gerbils with primary auditory neuron damage resulted in partial recovery of auditory evoked response thresholds (6). Hair cells can also be generated in vitro from induced pluripotent stem cells, and the yield can be increased by using a three-dimensional (3D) matrix cell culture system (73). An alternative to embryonic stem cells is genetically engineered NIH3T3 fibroblasts, which, when transplanted into the mouse inner ear produced brain-derived neurotrophic factor (BDNF) for up to 4 weeks after transfection (7). In conjunction with cochlear implant stimulation, primary auditory neurons survived for more than 6 months in the deafened guinea pig ear after delivery of Schwann cells overexpressing BDNF encapsulated in alginate microspheres (74). Low transplant efficacy and long-term risks of postimplantation tumorigenesis in the case of stem cells (75) are issues that need to be addressed before cell-based therapies can safely undergo clinical trials.
Nanoparticles with targeting peptides are able to selectively target specific cell types within the inner ear. Polymersomes are synthetic nanoscale cargo-carrying vesicles formed from the self-assembly of polymers in water and can be engineered to have a range of properties including biocompatibility, immune evasion via addition of poly(ethylene glycol) (PEG) chains, and smart release mechanisms (76). Polymersomes decorated with a human nerve growth factor–derived peptide targeting moiety selectively bind to primary auditory neurons in organotypic explants (77). Polymersomes equipped with a prestin-binding peptide have targeted the outer hair cells of rat cochlear explants (78). PEG-based polymersomes with a Tet1 targeting mechanism (a trisialoganglioside of clostridial toxin that binds to neuronal receptors) targeted the cochlear nerve after surgically accessing the cochlea through cochleostomy, but not after transtympanic injections (79). The inability of Tet1 polymersomes to reach the target after transtympanic injections could be due to the limited permeability of the round window membrane, creating a need for a targeting mechanism across the BLB. Some targeting peptides may not be able to achieve nanoparticle delivery across the BLB but may be of utility for local delivery.
MECHANISMS OF ENTRY ACROSS THE BLB
Mechanisms enabling trafficking of therapeutics across the BLB are conceptually similar to those for the BBB (80) but require specialized adaptations specific to the cochlea and the vestibular system. Therapeutics could cross both the BBB (80) and the BLB (40) by diffusion through membranes if they are sufficiently lipophilic. The proportion of drugs with these physicochemical characteristics is estimated to be <1%. For a small-molecule drug to cross the BBB, it must generally have fewer than eight hydrogen bonds and a molecular weight of <400 Da (81). Requirements for drug entry across the BLB are far less well characterized. The BLB and BBB can be opened by osmotic disruption using glycerin or mannitol (82); this has enabled delivery of antioxidants to the cochlea as otoprotectants during cisplatin chemotherapy in guinea pig models (83) and in human patients (84). Cyclodextrin, a common drug carrier, solubilizes the plasma membrane by releasing glycosylphosphatidylinositol-anchored proteins and sphingolipid domains (85). Cyclodextrin entry into the inner ear environment may be possible by perforating the BLB. When used in this manner, high doses of cyclodextrin are toxic to outer hair cells, causing moderate to severe hearing loss (86).
Cellular processes could also transport therapeutics across the BLB, as occurs in the BBB (80). These include specific and nonspecific endocytosis, as well as ion channels, ion exchangers, and transporters if the therapeutic is a bona fide substrate. Endocytosis includes the transcellular trafficking of cargo across the BBB endothelial cell barrier in either direction, which has been shown for cargo such as transferrin (87) and amyloid-β (88). Aminoglycosides are ototoxic antibiotics that readily cross the strial and perilymphatic BLB through as yet unidentified mechanisms, which are presumed to be intracellular transport processes due to saturable uptake kinetics (89). In other cells, aminoglycosides use several classes of nonselective cation channels such as transient receptor potential channels to permeate across endothelial cells (90). The expression or activity of nonselective cation channels can be up-regulated, potentially contributing to the increase in drug uptake by vascular endothelial cells during inflammation (40). Transporters that are up-regulated during inflammation could potentially be harnessed to increase drug delivery to the inner ear. A caveat is that, whereas such channels are present the cargo is protected from premature degradation and can retain its biological activity (95). Immunoglobulin G antibody was delivered to the mouse CNS as a model macromolecular cargo encapsulated in PEG-based polymersomes decorated with the peptide Angiopep-2, which enabled targeting of the transcytosis receptor LRP1 at the BBB (93). A similar targeting mechanism would be desirable for the BLB. However, little is known about the entry of systemically administered nanoparticles into the inner ear. Future research should explore targeting specific receptors at the BLB to enable transcytosis of ototherapeutics. Mapping trafficking pathways across the BLB for essential nutrients, macromolecules, and ototoxic compounds will provide essential information to enable noninvasive vascular drug delivery to the inner ear. at the BLB, they are ubiquitously expressed, thus rendering targeting and control of uptake kinetics problematic. Within the category of mechanisms of drug entry using cellular processes, receptor-mediated transcytosis is of particular interest for the targeting of macromolecular therapeutics across the BLB without disrupting endothelial tight junctions (81). It is not known whether bulk (fluid-phase or adsorptive-mediated transcytosis or receptor-mediated transcytosis) occurs across the BLB. There are few known putative receptors mediating transcytosis at the BLB, but the answer may lie in mapping whether the corresponding receptors at the BBB are expressed at the BLB. For instance, low-density lipoprotein receptor– related protein 1 (LRP1) is one receptor that mediates transcytosis at the BBB (88). LRP2 or megalin is structurally similar to LRP1 (91) and is strongly expressed at the apical surface of stria vascularis marginal cells (92). At the BBB, exploiting receptors mediating transcytosis has enabled successful trafficking of macromolecular cargo across the BBB without disrupting barrier integrity (93). This strategy is particularly useful in combination with hollow nanoscale vectors such as liposomes or polymersomes, which enable more efficient delivery to the target (94) partly because
PHARMACOKINETICS AND DRUG DELIVERY TO THE INNER EAR
Noninvasive targeting of therapeutics to the inner ear must consider several different pharmacokinetic variables. The therapeutic or its carrier must be water soluble for distribution in the blood. Absorption depends on lipophilicity and solubility (96). Greater lipophilicity not only generally correlates with a greater ability to cross cell membranes but also results in more rapid removal from the blood. Besides the route of administration, variables such as drug dose, singular or repeated injections, and properties of the vehicle (ionic composition, osmolarity, and pH) are factors that determine the distribution and clearance (rate of removal) of the drug from blood (97).
The bioavailability of the drug delivered will vary depending on the route of administration, with local delivery currently achieving better results compared to conventional systemic administration. Intratympanic injections of dexamethasone achieve higher concentrations in perilymph than does intravenous administration (98). Gentamicin in a fibrin hydrogel applied to the round window of the guinea pig ear resulted in a peak concentration of 14% of the drug dose in the vestibular perilymph after 10 hours, followed by clearance of the drug over several days (99). By contrast, systemically administered gentamicin in the chicken resulted in a perilymph peak concentration of 0.00025% of the drug dose after 4 hours (100). The low concentrations reached after intravenous injection highlight the need for an active targeting strategy for systemically administered therapeutics. The spiral organization of the cochlea establishes an additional challenge for drug delivery because equal distribution of therapeutics from the base to the apex of the cochlea cannot be assumed. Computer modeling of drug pharmacokinetics with single injection strategies shows drug clearance into the middle ear and steep concentration gradients of the drug in the inner ear, resulting in rapid clearance of the drug from the basal turns of the cochlea (101). This was demonstrated experimentally in the guinea pig cochlea where a basal-to-apical concentration gradient of gentamicin was observed after both local and systemic administration (102). Similarly, application of dexamethasone to the guinea pig ear round window resulted in a basal-to-apical gradient in the perilymph of the scala tympani (103). The concentration gradient along the cochlea from base to apex depends on the molecular weight of the drug, favoring a more even distribution for smaller molecules due to their higher diffusion coefficients. Drug removal from the inner ear can occur through a number of routes, for example, by diffusion into adjacent fluid compartments (e.g., into CSF via the cochlear aqueduct), through metabolism by inner ear cells, or by export across the BLB into the vascular or lymphatic system. The strial BLB has an array of enzymes and transporters that can export or metabolize drugs including P-glycoprotein, Na+/K+ ATPase α1, glutathione S-transferase, prosaposin, leukotriene A4 hydrolase, and glutamate oxaloacetate transaminase, indicative of a high metabolic demand and robust transport activity (104, 105).
Delivery of complex macromolecular therapeutics, such as viral vectors and their therapeutic genes, small interfering RNAs, antibodies, or cargo-carrying nanoparticles, pose additional challenges. Avoidance of elimination by the immune system or metabolism in the periphery is particularly important factors; for instance, BDNF has a serum half-life of 10 min due to rapid clearance in the periphery (106), limiting its long-term therapeutic value without multiple injections or controlled release approaches. Furthermore, large nanoparticles are cleared more rapidly from the circulation than are their smaller-diameter counterparts (107). Protein fouling, that is, adsorption of serum proteins onto the surface of a therapeutic or drug delivery vehicle, reduces bioavailability because the gradual adsorption of proteins causes recognition and engulfment by phagocytes. Detection and elimination of a therapeutic by the immune system or opsonization of the therapeutic can be reduced by incorporating antiprotein “fouling” properties. The conjugation of PEG to small-molecule drugs, therapeutic antibodies, or nanoscale drug delivery vehicles is the most commonly used method for immune system evasion and for prolonging circulation time (108). Lack of toxicity and biodegradability of the drug delivery vehicle are essential for controlled release approaches.
ALTERED BLB PERMEABILITY IN DISEASE AND IMPACT ON DRUG DELIVERY
Several external factors can modulate permeability of the BLB including diuretics (109), inflammation (110, 111), and acoustic trauma (1, 112). Different disease states can alter BLB physiology and increase its permeability to specific compounds; for example, inflammation caused by either local (acoustic trauma) or systemic immunogenic stimuli alters uptake of aminoglycoside antibiotics (40, 113). Therapeutics can also cross the BLB through leaky blood vessels where the tight junctions between adjacent endothelial cells have broken down; this occurs with autoimmune disease that affects the auditory system and severe inflammation that affects the BBB (114, 115).
Inflammation generally dilates blood vessels (vasodilation) to facilitate paracellular flux into the interstitial extracellular space. In the tight junction-coupled BLB and BBB, vasodilation typically occurs without increased paracellular flux but does result in increased cochlear uptake of aminoglycosides into the stria vascularis or of fluorophores into the perilymph (40, 111). A mutant mouse that is hyporesponsive to immunogenic stimuli and shows reduced vasodilation in response to inflammation, exhibits reduced uptake of aminoglycosides by the cochlea (40). Pharmacological induction of vasodilation with serotonin or Ginkgo biloba enhances cochlear uptake of aminoglycosides in rodents (116, 117). Although vasodilation could have confounding effects on drug uptake by the cochlea, it is plausible to consider that inflammation-induced or pharmacologically induced vasodilation could provide a mechanism to enhance the cochlear uptake of pharmaceuticals administered systemically.
Acoustic trauma may affect BLB permeability and subsequent uptake of drugs indirectly by inducing inflammation. Loud sounds affect almost all cochlear cell types and induce cochlear inflammation and drug-induced cochleotoxicity (113, 118). Intriguingly, the synergistic ototoxicity of loud sound exposure and aminoglycosides is not confined to simultaneous exposure. Exposure to loud sound days or weeks before aminoglycoside treatment can also potentiate drug-induced hearing loss (119), suggesting that cochlear inflammation can persist for days after induction. Furthermore, although limited data exist, hypoxia also appears to induce inflammatory signaling cascades in the modiolus and cochlear lateral wall and, especially, in the spiral ligament populated by fibrocytes (120, 121). Little information exists on the effects of hypertension on BLB permeability. Loop diuretics, a class of antihypertensive drugs that promote diuresis and reduce blood volume, appear to increase BLB permeability. Loop diuretics readily increase cochlear uptake of aminoglycosides (109) but do not appear to increase the paracellular flux of horseradish peroxidase into the cochlea (122), which is suggestive of transcellular flux of aminoglycosides across the BLB. Overall, data suggest an increased permeability of the BLB in inflammation, hypertension, hypoxia, and trauma, which could be exploited for enhanced systemic delivery of therapeutics under these conditions.
CHALLENGES AND FUTURE DIRECTIONS
There is a profound lack of pharmacotherapies available for treating hearing loss. The design of drug delivery systems to target the cochlea for protecting, restoring, or regenerating hearing faces many obstacles, regardless of the route of administration. Delivery through a systemic vascular route requires a better understanding of the functional properties of the BLB and the mechanisms that enable trafficking of molecules across this barrier. Questions such as where the BLB begins and ends must be answered to achieve more effective drug targeting. We need to better understand which cochlear microcirculatory systems most efficiently deliver systemically administered drugs to the cochlea and how these drugs are eliminated from the cochlea. Characterization of the routes of transport across the capillary endothelium is a key to designing receptor-mediated targeting strategies similar to those exploited for drug delivery at the BBB. A comparison of permeability and active transport mechanisms between the BBB and endothelia associated with the inner ear would be useful in determining the nature of the BLB. There are currently technical limitations in distinguishing strial vascularis capillaries from spiral ligament capillaries in vivo, although a thin window technique for observing blood vessels has been developed (123). Indeed, there are numerous experimental limitations that must be addressed to facilitate successful translational research on drug delivery to the inner ear.
The size and location of the cochlea complicate assessment of drug delivery and clearance. Because of the extremely low volumes of cochlear fluids, it is difficult to measure drug concentrations in these fluids without creating artifacts or contamination by other fluids such as perilymph or CSF upon extraction (124). Methods are also lacking for imaging the cochlea in situ due to its anatomical location, encased by the otic capsule. However, advances in optical clearing techniques that preserve tissue integrity and the use of fluorophores have enabled imaging of intact tissues ex vivo at single-cell resolution (125). A recently developed in vivo technique uses optical coherence tomography to image blood flow, with a whole cochlea 3D acquisition time of a few seconds (126). Last, the translation of preclinical research on ototherapeutics into human patients has been slow. This may be due to continued use of delivery methods and techniques in preclinical models that are impractical for clinical use (e.g., semicircular canal injections) or the use of approaches such as stem cell transplantation that do not readily translate between species particularly with respect to the inner ear.
Vascular delivery should be further explored for the delivery of complex therapeutics such as genes and growth factors across the BLB. The ability to manipulate materials at the nanoscale level to yield smart drug delivery vehicles creates opportunities to solve issues in targeting, toxicity, and premature elimination that are major concerns for systemic delivery. BLB delivery faces major challenges but is feasible. A mass spectrometry shotgun proteomics approach used on isolated strial capillaries identified more than 600 strial capillary proteins (105), many of which are involved in metabolism and transport. The robust transport activity at the BLB indicates that targeting active transport mechanisms at the strial BLB is a feasible strategy to import certain drugs and chemicals to the inner ear. Such a strategy would greatly increase the options available for delivering therapeutics to the cochlea noninvasively rather than surgically to protect, restore, or regenerate hearing in patients. Drug delivery to the inner ear should also be investigated in different disease states, for example, systemic inflammation may enhance cochlear uptake of some therapeutics. BLB permeability modulators such as acoustic trauma, inflammation, and diuretics may create a window of opportunity for enhancing delivery of otoprotective therapeutics to the inner ear. Continued elucidation of the molecular underpinnings of the BLB and advancement in strategies for drug delivery across fluid interface barriers will provide the required platform for translating preclinical research into therapeutics to treat patients with hearing loss.
Acknowledgments:
We thank X. Wang, K. Nishimura, and T. Le for valuable comments.
Funding: This work was supported by the Koerner Foundation (A.D.) and the Sunnybrook Hearing Regeneration Initiative (A.D. and S.N.), as well as NIDCD R01 grant nos. DC004555 and DC12588 (P.S.S.), NIH/NIDCD R21 grant no. DC016157 (X.S.), and NIH/NIDCD R01 DC015781 (X.S.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Wu Y-X, Zhu G-X, Liu X-Q, Sun F, Zhou K, Wang S, Wang C-M, Jia J-W, Song J-T, Lu L-J, Noise alters guinea pig’s blood-labyrinth barrier ultrastructure and permeability along with a decrease of cochlear Claudin-5 and Occludin. BMC Neurosci 15, 136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhandare N, Antonelli PJ, Morris CG, Malayapa RS, Mendenhall WM, Ototoxicity after radiotherapy for head and neck tumors. Int. J. Radiat. Oncol. Biol. Phys 67, 469–479 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Schell MJ, McHaney VA, Green AA, Kun LE, Hayes FA, Horowitz M, Meyer WH, Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J. Clin. Oncol 7, 754–760 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Sone M, Schachern PA, Paparella MM, Loss of spiral ganglion cells as primary manifestation of aminoglycoside ototoxicity. Hear. Res 115, 217–223 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Müller U, Barr-Gillespie PG, New treatment options for hearing loss. Nat. Rev. Drug Discov 14, 346–365 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN, Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490, 278–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okano T, Nakagawa T, Kita T, Endo T, Ito J, Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol. Ther 14, 866–871 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Schwander M, Kachar B, Müller U, The cell biology of hearing. J. Cell Biol 190, 9–20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fettiplace R, Hackney CM, The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci 7, 19–29 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Hudspeth AJ, Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci 15, 600–614 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Rubel EW, Furrer SA, Stone JS, A brief history of hair cell regeneration research and speculations on the future. Hear. Res 297, 42–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y, Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med 11, 271–276 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV, Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537–541 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Primary Auditory Neurons of the Mammalian Cochlea, Dabdoub A, Fritzsch B, Popper AN, Fay RR, Eds. (Springer-Verlag New York, 2016), vol. 52. [Google Scholar]
- 15.Froud KE, Wong AC, Cederholm JM, Klugmann M, Sandow SL, Julien JP, Ryan AF, Housley GD, Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat. Commun 6, 7115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores EN, Duggan A, Madathany T, Hogan AK, Márquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, García-Añoveros J, A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr. Biol 25, 606–612 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberman MC, Kujawa SG, Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res 349, 138–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN, Age-related primary cochlear neuronal degeneration in human temporal bones. J. Assoc. Res. Otolaryngol 12, 711–717 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kujawa SG, Liberman MC, Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci 29, 14077–14085 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neng L, Zhang F, Kachelmeier A, Shi X, Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J. Assoc. Res. Otolaryngol 14, 175–185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salt AN, Melichar I, Thalmann R, Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97, 984–991 (1987). [PubMed] [Google Scholar]
- 22.Wangemann P, Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol 576, 11–21 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Zhao H-B, The role of an inwardly rectifying K+ channel (Kir4.1) in the inner ear and hearing loss. Neuroscience 265, 137–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salt AN, Gill RM, Hartsock JJ, Perilymph kinetics of FITC-dextran reveals homeostasis dominated by the cochlear aqueduct and cerebrospinal fluid. J. Assoc. Res. Otolaryngol 16, 357–371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi T, Hamrick PE, Walsh PJ, Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol 86, 22–34 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y, The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Natl. Acad. Sci. U.S.A 105, 1751–1756 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitajiri S.-i., Miyamoto T, Mineharu A, Sonoda N, Furuse K, Hata M, Sasaki H, Mori Y, Kubota T, Ito J, Furuse M, Tsukita S, Compartmentalization established by claudin-11based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J. Cell Sci 117, 5087–5096 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Florian P, Amasheh S, Lessidrensky M, Todt I, Bloedow A, Ernst A, Fromm M, Gitter AH, Claudins in the tight junctions of stria vascularis marginal cells. Biochem. Biophys. Res. Commun 304, 5–10 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Steel KP, Barkway C, Another role for melanocytes: Their importance for normal stria vascularis development in the mammalian inner ear. Development 107, 453–463 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC, Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2, 30 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jabba SV, Oelke A, Singh R, Maganti RJ, Fleming S, Wall SM, Everett LA, Green ED, Wangemann P, Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC Med 4, 37 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin J-P, Janel N, Meda P, Petit C, Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc. Natl. Acad. Sci. U.S.A 104, 6229–6234 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carraro M, Harrison RV, Degeneration of stria vascularis in age-related hearing loss; a corrosion cast study in a mouse model. Acta Otolaryngol 136, 385–390 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Ohlemiller KK, Rice ME, Lett JM, Gagnon PM, Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hear. Res 249, 1–14 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Gratton MA, Schmiedt RA, Schulte BA, Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear. Res 102, 181–190 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Nomoto Y, Nakagawa T, Kuwahata N, Ogawa H, Suzuki Y, Ito J, Omori K, Age-dependent degeneration of the stria vascularis in human cochleae. Laryngoscope 116, 1846–1850 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Jahnke K, The blood-perilymph barrier. Arch. Otorhinolaryngol 228, 29–34 (1980). [DOI] [PubMed] [Google Scholar]
- 38.Juhn SK, Rybak LP, Prado S, Nature of blood-labyrinth barrier in experimental conditions. Ann. Otol. Rhinol. Laryngol 90, 135–141 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Shi X, Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res 338, 52–63 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koo J-W, Quintanilla-Dieck L, Jiang M, Liu J, Urdang ZD, Allensworth JJ, Cross CP, Li H, Steyger PS, Endotoxemia-mediated inflammation potentiates aminoglycosideinduced ototoxicity. Sci. Trans. Med 7, 298ra118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Steyger PS, Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J. Assoc. Res. Otolaryngol 10, 205–219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlisle L, Aberdeen J, Forge A, Burnstock G, Neural basis for regulation of cochlear blood flow: Peptidergic and adrenergic innervation of the spiral modiolar artery of the guinea pig. Hear. Res 43, 107–113 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Inamura N, Salt AN, Permeability changes of the blood-labyrinth barrier measured in vivo during experimental treatments. Hear. Res 61, 12–18 (1992). [DOI] [PubMed] [Google Scholar]
- 44.Neuwelt EA, Baker DE, Pagel MA, Blank NK, Cerebrovascular permeability and delivery of gentamicin to normal brain and experimental brain abscess in rats. J. Neurosurg 61, 430–439 (1984). [DOI] [PubMed] [Google Scholar]
- 45.Hawkins BT, Davis TP, The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev 57, 173–185 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Axelsson A, The vascular anatomy of the cochlea in the guinea pig and in man. Acta Otolaryngol 1968 (suppl. 243), 1–134 (1968). [PubMed] [Google Scholar]
- 47.Shi X, Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res 342, 21–30 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X, Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. U.S.A 109, 10388–10393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H, Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res 83, 575–583 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Ichimiya I, Yoshida K, Hirano T, Suzuki M, Mogi G, Significance of spiral ligament fibrocytes with cochlear inflammation. Int. J. Pediatr. Otorhinolaryngol 56, 45–51 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Hirose K, Discolo CM, Keasler JR, Ransohoff R, Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol 489, 180–194 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Dai M, Shi X, Fibro-vascular coupling in the control of cochlear blood flow. PLOS ONE 6, e20652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank RN, Dutta S, Mancini MA, Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest. Ophthalmol. Vis. Sci 28, 1086–1091 (1987). [PubMed] [Google Scholar]
- 54.Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL, The cochlear pericytes. Microcirculation 15, 515–529 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly JJ, Forge A, Jagger DJ, Contractility in type III cochlear fibrocytes is dependent on non-muscle myosin II and intercellular gap junctional coupling. J. Assoc. Res. Otolaryngol 13, 473–484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai M, Nuttall A, Yang Y, Shi X, Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear. Res 254, 100–107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mura S, Nicolas J, Couvreur P, Stimuli-responsive nanocarriers for drug delivery. Nat. Mater 12, 991–1003 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT, Inner ear drug delivery for auditory applications. Adv. Drug Deliv. Rev 60, 1583–1599 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Kechai N, Agnely F, Mamelle E, Nguyen Y, Ferrary E, Bochot A, Recent advances in local drug delivery to the inner ear. Int. J. Pharm 494, 83–101 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Rivera T, Sanz L, Camarero G, Varela-Nieto I, Drug delivery to the inner ear: Strategies and their therapeutic implications for sensorineural hearing loss. Curr. Drug Deliv 9, 231–242 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Nakaizumi T, Kawamoto K, Minoda R, Raphael Y, Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol. Neurootol 9, 135–143 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Staecker H, Gabaizadeh R, Federoff H, Van De Water TR, Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol. Head Neck Surg 119, 7–13 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Stone IM, Lurie DI, Kelley MW, Poulsen DJ, Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol. Ther 11, 843–848 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Yagi M, Magal E, Sheng Z, Ang KA, Raphael Y, Hair cell protection from aminoglycoside ototoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor. Hum. Gene Ther 10, 813–823 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Yang S-M, Chen W, Guo W-W, Jia S, Sun J-H, Liu H-Z, Young W-Y, He DZZ, Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PLOS ONE 7, e46355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, Lustig LR, Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75, 283–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas CE, Ehrhardt A, Kay MA, Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet 4, 346–358 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Landegger LD, Pan B, Askew C, Wassmer SJ, Gluck SD, Galvin A, Taylor R, Forge A, Stankovic KM, Holt JR, Vandenberghe LH, A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol 35, 280–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS, Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of Corti. J. Neurobiol 66, 1489–1500 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi F, Corrales CE, Liberman MC, Edge ASB, BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci 26, 3016–3023 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Chen W, Johnson SL, Marcotti W, Andrews PW, Moore HD, Rivolta MN, Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells 27, 1196–1204 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Hu Z, Luo X, Zhang L, Lu F, Dong F, Monsell E, Jiang H, Generation of human inner ear prosensory-like cells via epithelial-to-mesenchymal transition. Regen. Med 7, 663–673 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E, Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillespie LN, Zanin MP, Shepherd RK, Cell-based neurotrophin treatment supports long-term auditory neuron survival in the deaf guinea pig. J. Control. Release 198, 26–34 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Nishimura K, Nakagawa T, Sakamoto T, Ito J, Fates of murine pluripotent stem cell-derived neural progenitors following transplantation into mouse cochleae. Cell Transplant 21, 763–771 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Messager L, Gaitzsch J, Chierico L, Battaglia G, Novel aspects of encapsulation and delivery using polymersomes. Curr. Opin. Pharmacol 18, 104–111 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Roy S, Johnston AH, Newman TA, Glueckert R, Dudas J, Bitsche M, Corbacella E, Rieger G, Martini A, Schrott-Fischer A, Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: Potential tool for drug delivery. Int. J. Pharm 390, 214–224 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Surovtseva EV, Johnston AH, Zhang W, Zhang Y, Kim A, Murakoshi M, Wada H, Newman TA, Zou J, Pyykkö I, Prestin binding peptides as ligands for targeted polymersome mediated drug delivery to outer hair cells in the inner ear. Int. J. Pharm 424, 121–127 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Zhang W, Johnston AH, Newman TA, Pyykkö I, Zou J, Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. Int. J. Nanomedicine 7, 1015–1022 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbott NJ, Rönnbäck L, Hansson E, Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci 7, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Pardridge WM, The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2, 3–14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Juhn SK, Prado S, Pearce J, Osmolality changes in perilymph after systemic administration of glycerin. Arch. Otolaryngol 102, 683–685 (1976). [DOI] [PubMed] [Google Scholar]
- 83.Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, Egorin MJ, Neuwelt EA, Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin. Cancer Res 6, 309–315 (2000). [PubMed] [Google Scholar]
- 84.Doolittle ND, Muldoon LL, Brummett RE, Tyson RM, Lacy C, Bubalo JS, Kraemer DF, Heinrich MC, Henry JA, Neuwelt EA, Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin. Cancer Res 7, 493–500 (2001). [PubMed] [Google Scholar]
- 85.Ilangumaran S, Hoessli DC, Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J 335 (Pt. 2), 433–440 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crumling MA, King KA, Duncan RK, Cyclodextrins and iatrogenic hearing loss: New drugs with significant risk. Front. Cell. Neurosci 11, 355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE, Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res 18, 299–304 (1987). [DOI] [PubMed] [Google Scholar]
- 88.Pflanzner T, Janko MC, André-Dohmen B, Reuss S, Weggen S, Roebroek AJM, Kuhlmann CRW, Pietrzik CU, LRP1 mediates bidirectional transcytosis of amyloid-β across the blood-brain barrier. Neurobiol. Aging 32, 2323.e1–e11 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Wang Q, Kachelmeier A, Steyger PS, Competitive antagonism of fluorescent gentamicin uptake in the cochlea. Hear. Res 268, 250–259 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marcotti W, van Netten SM, Kros CJ, The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechanoelectrical transducer channels. J. Physiol 567, 505–521 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK, LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev 88, 887–918 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tauris J, Christensen EI, Nykjaer A, Jacobsen C, Petersen CM, Ovesen T, Cubilin and megalin co-localize in the neonatal inner ear. Audiol. Neurootol 14, 267–278 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Tian X, Nyberg S, Sharp PS, Madsen J, Daneshpour N, Armes SP, Berwick J, Azzouz M, Shaw P, Abbott NJ, Battaglia G, LRP-1-mediated intracellular antibody delivery to the central nervous system. Sci. Rep 5, 11990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joseph A, Contini C, Cecchin D, Nyberg S, Ruiz-Perez L, Gaitzsch J, Fullstone G, Tian X, Azizi J, Preston J, Volpe G, Battaglia G, Chemotactic synthetic vesicles: Design and applications in blood-brain barrier crossing. Sci. Adv 3, e1700362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Messager L, Burns JR, Kim J, Cecchin D, Hindley J, Pyne ALB, Gaitzsch J, Battaglia G, Howorka S, Biomimetic hybrid nanocontainers with selective permeability. Angew. Chem. Int. Ed. Engl 55, 11106–11109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mannhold R, The impact of lipophilicity in drug research: A case report on β-blockers. Mini Rev. Med. Chem 5, 197–205 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Salt AN, Pharmacokinetics of drug entry into cochlear fluids. Volta Rev 105, 277–298 (2005). [PMC free article] [PubMed] [Google Scholar]
- 98.Chandrasekhar SS, Intratympanic dexamethasone for sudden sensorineural hearing loss: Clinical and laboratory evaluation. Otol. Neurotol 22, 18–23 (2001). [DOI] [PubMed] [Google Scholar]
- 99.Balough BJ, Hoffer ME, Wester D, O’Leary MJ, Brooker CR, Goto M, Kinetics of gentamicin uptake in the inner ear of Chinchilla langier after middle-ear administration in a sustained-release vehicle. Otolaryngol. Head Neck Surg 119, 427–431 (1998). [DOI] [PubMed] [Google Scholar]
- 100.Bunting EC, Park DL, Durham D, Girod DA, Gentamicin pharmacokinetics in the chicken inner ear. J. Assoc. Res. Otolaryngol 5, 144–152 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Plontke SK, Salt AN, Simulation of application strategies for local drug delivery to the inner ear. ORL J. Otorhinolaryngol Relat. Spec 68, 386–392 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Imamura S.-i., Adams JC, Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J. Assoc. Res. Otolaryngol 4, 176–195 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN, Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol 29, 401–406 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saito T, Zhang ZJ, Tsuzuki H, Ohtsubo T, Yamada T, Yamamoto T, Saito H, Expression of P-glycoprotein in inner ear capillary endothelial cells of the guinea pig with special reference to blood-inner ear barrier. Brain Res 767, 388–392 (1997). [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X, Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLOS ONE 6, e16547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sakane T, Pardridge WM, Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res 14, 1085–1091 (1997). [DOI] [PubMed] [Google Scholar]
- 107.Alexis F, Pridgen E, Molnar LK, Farokhzad OC, Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm 5, 505–515 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harris JM, Chess RB, Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov 2, 214–221 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Taylor RR, Nevill G, Forge A, Rapid hair cell loss: A mouse model for cochlear lesions. J. Assoc. Res. Otolaryngol 9, 44–64 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kastenbauer S, Klein M, Koedel U, Pfister HW, Reactive nitrogen species contribute to blood-labyrinth barrier disruption in suppurative labyrinthitis complicating experimental pneumococcal meningitis in the rat. Brain Res 904, 208–217 (2001). [DOI] [PubMed] [Google Scholar]
- 111.Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN, Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J. Assoc. Res. Otolaryngol 15, 707–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki M, Yamasoba T, Ishibashi T, Miller JM, Kaga K, Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear. Res 164, 12–18 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Li H, Wang Q, Steyger PS, Acoustic trauma increases cochlear and hair cell uptake of gentamicin. PLOS ONE 6, e19130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin DW, Trune DR, Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol. Head Neck Surg 117, 530–534 (1997). [DOI] [PubMed] [Google Scholar]
- 115.Banks WA, Erickson MA, The blood–brain barrier and immune function and dysfunction. Neurobiol. Dis 37, 26–32 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Koo J-W, Wang Q, Steyger PS, Infection-mediated vasoactive peptides modulate cochlear uptake of fluorescent gentamicin. Audiol. Neurootol 16, 347–358 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miman MC, Ozturan O, Iraz M, Erdem T, Olmez E, Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear. Res 169, 121–129 (2002). [DOI] [PubMed] [Google Scholar]
- 118.Vethanayagam RR, Yang W, Dong Y, Hu BH, Toll-like receptor 4 modulates the cochlear immune response to acoustic injury. Cell Death Dis 7, e2245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ryan AF, Bone RC, Potentiation of kanamycin ototoxicity by a history of noise exposure. Otolaryngology 86, ORL-125–ORL-128 (1978). [DOI] [PubMed] [Google Scholar]
- 120.Gross J, Olze H, Mazurek B, Differential expression of transcription factors and inflammation-, ROS-, and cell death-related genes in organotypic cultures in the modiolus, the organ of Corti and the stria vascularis of newborn rats. Cell. Mol. Neurobiol 34, 523–538 (2014). [DOI] [PubMed] [Google Scholar]
- 121.Khan M, Szczepek AJ, Haupt H, Olze H, Mazurek B, Expression of the proinflammatory cytokines in cochlear explant cultures: Influence of normoxia and hypoxia. Neurosci. Lett 479, 249–252 (2010). [DOI] [PubMed] [Google Scholar]
- 122.Duvall AJ III, Robinson KS, Cochlear vessel permeability to horseradish peroxidase after diuretic administration in the chinchilla. Acta Otolaryngol 108, 397–403 (1989). 123. [DOI] [PubMed] [Google Scholar]
- 123.Shi X, Zhang F, Urdang Z, Dai M, Neng L, Zhang J, Chen S, Ramamoorthy S, Nuttall AL, Thin and open vessel windows for intra-vital fluorescence imaging of murine cochlear blood flow. Hear. Res 313, 38–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salt AN, Plontke SKR, Local inner-ear drug delivery and pharmacokinetics. Drug Discov. Today 10, 1299–1306 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Susaki EA, Ueda HR, Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: Toward organism-level systems biology in mammals. Cell Chem. Biol 23, 137–157 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Subhash HM, Davila V, Sun H, Nguyen-Huynh AT, Shi X, Nuttall AL, Wang RK, Volumetric in vivo imaging of microvascular perfusion within the intact cochlea in mice using ultra-high sensitive optical microangiography. IEEE Trans. Med. Imaging 30, 224–230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janigro D, Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia 53, 26–34 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hansen AJ, Effect of anoxia on ion distribution in the brain. Physiol. Rev 65, 101–148 (1985). [DOI] [PubMed] [Google Scholar]
- 129.Wangemann P, Schacht J, in The Cochlea, Dallos P, Popper AN, Fay RR, Eds. (Springer New York, 1996), pp. 130–185. [Google Scholar]
- 130.Hladky SB, Barrand MA, Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 13, 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trowe M-O, Maier H, Petry M, Schweizer M, Schuster-Gossler K, Kispert A, Impaired stria vascularis integrity upon loss of E-cadherin in basal cells. Dev. Biol 359, 95–107 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B, Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J. Neurosci 24, 7051–7062 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, Structure and function of the blood–brain barrier. Neurobiol. Dis 37, 13–25 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Suzuki M, Kaga K, Development of blood-labyrinth barrier in the semicircular canal ampulla of the rat. Hear. Res 129, 27–34 (1999). [DOI] [PubMed] [Google Scholar]
- 135.Rapoport SI, Osmotic opening of the blood-brain barrier: Principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol 20, 217–230 (2000). [DOI] [PubMed] [Google Scholar]
- 136.Abbott NJ, Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol 20, 131–147 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Chodobski A, Zink BJ, Szmydynger-Chodobska J, Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res 2, 492–516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pardridge WM, Drug and gene targeting to the brain with molecular trojan horses. Nat. Rev. Drug Discov 1, 131–139 (2002). [DOI] [PubMed] [Google Scholar]
- 139.Salt AN, Hartsock J, Plontke S, LeBel C, Piu F, Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol. Neurootol 16, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mazurek B, Amarjargal N, Haupt H, Fuchs J, Olze H, Machulik A, Gross J, Expression of genes implicated in oxidative stress in the cochlea of newborn rats. Hear. Res 277, 54–60 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Murillo-Cuesta S, Camarero G, González-Rodríguez A, Rodríguez-de la Rosa L, Burks DJ, Avendaño C, Valverde AM, Varela-Nieto I, Insulin receptor substrate 2 (IRS2)-deficient mice show sensorineural hearing loss that is delayed by concomitant protein tyrosine phosphatase 1B (PTP1B) loss of function. Mol. Med 18, 260–269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hill SL III, Digges EN, Silverstein H, Long-term follow-up after gentamicin application via the Silverstein MicroWick in the treatment of Ménière’s disease. Ear Nose Throat J 85, 494, 496, 498 (2006). [PubMed] [Google Scholar]
- 143.Plontke SK, Mikulec AA, Salt AN, Rapid clearance of methylprednisolone after intratympanic application in humans. Comment on: Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007;28:1124–30. Otol. Neurotol. 29, 732–733 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Okuda T, Sugahara K, Shimogori H, Yamashita H, Inner ear changes with intracochlear gentamicin administration in Guinea pigs. Laryngoscope 114, 694–697 (2004). [DOI] [PubMed] [Google Scholar]
- 145.Barrs DM, Keyser JS, Stallworth C, McElveen JT Jr., Intratympanic steroid injections for intractable Ménière’s disease. Laryngoscope 111, 2100–2104 (2001). [DOI] [PubMed] [Google Scholar]
- 146.Plontke SK, Löwenheim H, Mertens J, Engel C, Meisner C, Weidner A, Zimmermann R, Preyer S, Koitschev A, Zenner H-P, Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope 119, 359–369 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Suzuki J, Corfas G, Liberman MC, Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci. Rep 6, 24907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bas E, Goncalves S, Adams M, Dinh CT, Bas JM, Van De Water TR, Eshraghi AA, Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front. Cell. Neurosci 9, 303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Havenith S, Lammers MJ, Tange RA, Trabalzini F, della Volpe A, van der Heijden GJMG, Grolman W, Hearing preservation surgery: Cochleostomy or round window approach? A systematic review. Otol. Neurotol 34, 667–674 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Shepherd RK, Xu J, A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear. Res 172, 92–98 (2002). [DOI] [PubMed] [Google Scholar]
- 151.Husseman J, Raphael Y, Gene therapy in the inner ear using adenovirus vectors. Adv. Otorhinolaryngol 66, 37–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


