Abstract
The prevalence rate of obesity continues to rise in the U.S., but effective treatment options remain elusive resulting in increased emphasis on prevention. One such area of prevention research capitalizes on the relatively novel behavioral construct of food addiction, which has been implicated in obesity. Food addiction reflects an individual’s propensity for compulsive eating despite negative consequences, and shares not only symptoms with both eating and substance use disorders but also genetic and neural correlates within neural reward-circuitry modulated by dopamine. Here, we examined associations between food addiction scores, body mass index (BMI), reward-related ventral striatum activity, and a polygenic score approximating dopamine signaling in 115 non-Hispanic Caucasian young adult university students. As predicted, polygenic dopamine scores were related to ventral striatum activity, which in turn was associated with higher food addiction scores. In addition, food addiction was related to BMI. An exploratory post-hoc path analysis further indicated that polygenic scores were indirectly related to both food addiction and BMI, in part, through ventral striatum activity. Collectively, our results provide evidence supporting the utility of food addiction in weight gain prevention research by establishing links with known risk-related neural and genetic biomarkers.
Keywords: food addiction, obesity, dopamine, ventral striatum, BMI
As energy-dense foods have become widely available in the past decade, the prevalence of obesity has reached approximately 35% in U.S. adults (Flegal et al., 2012; Pi-Sunyer, 2003). Obesity is, in turn, increasingly recognized as a leading cause of major health complications, including type 2 diabetes, coronary heart disease, respiratory complications, osteoarthritis, and even certain forms of cancer (Kopelman, 2000). Often overlooked are the psychological processes associated with obesity, such as low self-esteem, chronic stress, anxiety, depressed affect, and diminished quality of life (Abilés et al., 2010; Faith, Matz, & Jorge, 2002; Kolotkin, Meter, & Williams, 2001). In fact, the prevalence of psychiatric illness, including eating disorders, attention deficit/hyperactivity disorder, and mood disorders, is significantly higher in overweight individuals (Agranat-Meged et al., 2005; McElroy et al., 2004; Yanovski, Nelson, Dubbert, & Spitzer, 1993). Despite growing evidence of the psychological and medical burden associated with obesity, few interventions using dietary restrictions and medications for weight loss have shown long-term effectiveness, partly due to weight regain and re-consumption of hyper-palatable foods following a period of highly restricted diet (Ayyad & Anderson, 2000; Mokdad, Marks, Stroup, & Gerberding, 2004). Given the current limitations of dietary interventions, prevention approaches that aim to identify individuals at risk of overeating prior to the onset of significant weight gain may hold more promise (Swinburn & Kumanyika, 2005).
Recent research suggests that food addiction, measured using a self-report questionnaire (Yale Food Addiction Scale; Gearhardt, Corbin, & Brownell, 2009), reflects a behavioral tendency to eat compulsively despite adverse consequences. Individuals with food addiction have higher body mass index (BMI) and body fat, greater number of unsuccessful attempts to control food intake, and increased emotional eating (Meule & Gearhardt, 2014; Pursey, Stanwell, Gearhardt, Collins, & Burrows, 2014). As a consequence of frequent hedonically-driven eating (Lutter & Nestler, 2009), food addiction is associated with a greater likelihood of obesity and diet-related disease (Flint et al., 2014). This is especially problematic because the very foods that promote obesity (e.g., high in fat and refined carbohydrates) have greater hedonic value, and therefore higher addictive potential (Cocores & Gold, 2009; Schulte, Avena, & Gearhardt, 2015; Nantha, 2014). It is important to note, however, that the concept of food addiction has been controversial, given the absence of validated diagnostic thresholds and biochemical evidence that foods have addictive properties (Finlayson, 2017). Additionally, food addiction has not been established as a clinically distinct or validated syndrome, although it is strongly correlated with binge eating disorder (BED; Davis et al., 2011) and other psychological traits underlying eating pathology, such as disinhibition and emotional eating (Mason et al., 2017; Price, Higgs, & Lee, 2015). What distinguishes food addiction from BED or other eating disorders are addiction-like symptoms that are directly derived from the DSM-IV (APA, 1994) criteria for substance dependence: the experience of withdrawal and tolerance as well as social and occupational impairment caused by distress related to food consumption or time spent acquiring, using, or recovering from excess food consumption (Corwin & Grigson, 2009; Ifland et al., 2009; Gearhardt et al., 2009).
Perhaps one of the most compelling findings supporting food addiction is that both drugs of abuse and hyper-palatable foods elicit overlapping responses in neural circuits supporting goal-directed behaviors (Blumenthal & Gold, 2010; Hone-Blanchet & Fecteau, 2014). For example, research using functional magnetic resonance imaging (fMRI) finds that substance dependence is associated with greater activation of the ventral striatum (VS), a brain region critical for goal-directed behaviors, motivation and action, when both anticipating (Beck et al., 2009; Becker, Kirsch, Gerchen, Kiefer, & Kirsch, 2017; David et al., 2005; Kühn & Gallinat, 2011; Li et al., 2012) and receiving a monetary reward (Breiter et al., 1997). Alternatively, other studies have shown that more heavily addicted individuals demonstrate blunted VS activity to drug-related cues (e.g., Bühler et al., 2010; Luijten et al. 2017; Vollstädt-Klein et al., 2011). Interestingly, obese individuals exhibit similarly increased VS activity when viewing pictures of foods, particularly those high in calories (Stoeckel et al., 2008). Furthermore, when individuals with higher food addiction scores view cues that predict consuming hyper-palatable food (i.e., chocolate milkshake), they exhibit relatively greater activation of the caudate, another reward processing region of the striatum (Gearhardt et al., 2011). Although the direction of dysfunction remains inconclusive, these fMRI findings suggest that aberrant VS activity may represent a shared mechanism through which drug and food addiction may emerge.
As a potential explanation to these patterns, Volkow et al. (2008) proposed that chronic drug users respond differently to non-drug and drug stimuli. One interpretation of this differential response is that individuals with drug addiction are less sensitive to natural rewards, and in order to compensate for this reduced sensitivity, turn to more powerful reinforcers, such as psychoactive drugs. For instance, when given monetary rewards, tobacco smokers exhibit reduced dopaminergic response in the VS than non-smokers (Martin-Sölch et al., 2001). While this finding was present in already addicted individuals, relatively diminished VS activity predicts future drug use in adolescents free of substance use (Büchel et al., 2017). Although neuroimaging studies of food addiction using general rewards are limited, Balodis et al. (2013) found that compared to lean healthy volunteers, those with BED show decreased activation of the VS and caudate when receiving non-food rewards, such as money.
One potential explanation for the above convergent patterns is that both food and drugs are primary reinforcers that engage mesolimbic dopaminergic signaling, which increases learned associations between these reinforcers and cues (Berridge & Robinson, 1998; Volkow, Wang, Fowler, & Telang, 2008). Although results are inconclusive (Karlsson et al., 2015), some positron emission tomography (PET) studies of addiction and obesity identify overlapping abnormalities characterized by reduced activity of dopamine D2 receptors in reward processing brain regions, including the VS, which are correlated with severity of substance dependence and BMI in addicted and morbidly obese participants, respectively (Volkow, Wang, Fowler, Tomasi, & Telang, 2011).
In the absence of direct measures of the dopaminergic system with PET, common functional polymorphisms in genes encoding components of dopamine signaling may be useful for modeling possible dopaminergic modulating of reward-related brain function in both drug and food addicted individuals. We have previously developed an additive polygenic score representing dopamine signaling, which predicts reward-related VS activity (Nikolova et al., 2011). Adapted versions of our original polygenic score have successfully predicted nicotine dependence and addiction-prone personality traits (e.g., impulsivity, neuroticism, and extraversion), as well as food addiction and cravings (David et al., 2013; Davis et al., 2013; Davis & Loxton, 2013). Additionally, individual functional variants used to create our polygenic scores, including the DRD2 −141C Ins/Del (rs1799732), DRD2/ANKK1 Taq1A (rs1800497), and the COMT Val158Met (rs4680), have been independently implicated in drug abuse (Doehring, Kirchhof, & Lotsch, 2009), obesity, and eating pathology (Nisoli et al., 2007; Stice, Spoor, Bohon, & Small, 2008; Stice, Yokum, Bohon, Marti, & Smolen, 2010, Yilmaz, Kaplan, Zai, Levitan, & Kennedy, 2011). However, evidence for shared genetic influences on substance and food addiction is inconclusive (Cornelis et al., 2016), which underscores the need to investigate the genetic underpinnings of shared neural processes (i.e., reward-related VS activity), rather than the shared genetics of these two forms of addiction directly.
In the current study, we attempt to bring these typically parallel lines of inquiry together by examining associations between food addiction scores, body mass, reward-related VS activity, and dopamine signaling polygenic scores using data from a sample of 115 young adult university students who successfully completed the Duke Neurogenetics Study. Based on the research summarized above, we hypothesized that (1) higher food addiction scores would be associated with higher BMI; (2) higher food addiction scores would be associated with lower VS activity to monetary rewards; (3) higher dopamine signaling polygenic scores would be associated with higher VS activity; (4) higher dopamine signaling polygenic scores would be associated with higher food addiction scores and BMI; and thus (5) dopamine signaling polygenic scores would be indirectly related to food addiction scores and BMI.
Method
Participants
Data were derived from an initial sample of 178 non-Hispanic Caucasian participants (91 women; mean age 19.95 ± 1.22 years) with overlapping behavioral measures and functional magnetic resonance imaging (fMRI) data collected through successful completion of the Duke Neurogenetics Study, which assessed a range of behavioral and biological traits among young adult, university students. Of these 178 participants, high quality fMRI data were available for 165 (see BOLD fMRI data pre-processing procedures below) and genotyping data for 115 (see imputation section below). The Duke Neurogenetics Study was approved by the Duke University School of Medicine Institutional Review Board, and all participants provided written informed consent prior to participation. All participants were in good general health and free of the following exclusion criteria: (1) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and (3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
As the Duke Neurogenetics Study seeks to examine the broad distribution of dimensional behavioral and biological variables, any past or current DSM-IV Axis I disorder or select Axis II disorders (antisocial personality disorder and borderline personality disorder) was not an exclusion to participation. However, no individuals, regardless of diagnosis, were taking any psychoactive medication during or at least 14 days prior to their participation. Categorical diagnosis was assessed with the electronic Mini International Neuropsychiatric Interview (Lecrubier et al., 1997) and Structured Clinical Interview for the DSM-IV subtests (First et al., 1995). Of the 165 participants included in our analyses, 40 individuals had at least one DSM-IV diagnosis, including 19 with alcohol use disorders, 8 with non-alcohol substance use disorders, 9 with major depressive disorders, 3 with bipolar disorders, 9 with panic disorder (no agoraphobia), 4 with panic disorder including agoraphobia, 2 with social anxiety disorder, 1 with generalized anxiety disorder, 1 with obsessive compulsive disorder, 1 with eating disorders, and 0 with posttraumatic stress disorder.
Self-reported Food Addiction
The Modified Yale Food Addiction Scale (mYFAS; Flint et al., 2014) is a 9-item, shortened version of the YFAS, which assesses food addiction based on the diagnostic criteria for substance dependence by assessing the frequency of food-seeking behaviors and the associated social and occupational impairments (Gearhardt et al., 2009). According to Flint et al. (2014), the prevalence rates and validity indices of mYFAS scores were comparable to those found using the full version (Gearhardt et al., 2009). Inasmuch as the majority of our sample did not meet the clinical criteria for food addiction, we adapted a new algorithm to score food addiction dimensionally to maximize variability in the measure and reduce skewness and kurtosis; that is, instead of assigning a score of 1 for each item for which the frequency of the FA symptom was “4 or more times per week” we assigned 0 = “never,” 1 = “once a month,” 2 = “2–4 times per month,” 3 = “2–3 times per week,” 4 = “4 or more.” For items regarding emotional distress caused by eating (e.g., “I kept consuming the same types or amounts of food despite significant emotional and/or physical problems related to my eating”) and tolerance (e.g., “Eating the same amount of food does not reduce negative emotions or increase pleasurable feelings the way it used to”), an additional point was assigned if the participant responded “yes,” which is consistent with the traditional scoring method. The sum of all scores from each question was employed, with a possible range from 0 to 22 (α = 0.82). This new scoring algorithm resulted in improved skewness of 0.460 (SE = 0.189) and kurtosis of −0.398 (SE = 0.376) compared to a symptom count scoring procedure (skewness = 2.402, SE = 0.189; kurtosis = 5.908, SE = 0.376; see Supplemental Figure 1 for histograms of these two scoring algorithms). The mYFAS scores resulting from this new scoring algorithm were strongly correlated with the total food addiction symptom count (r = .683) and were more strongly related to BMI (r = .257) than the total symptom count (r = .186).
Ventral Striatum Activity Paradigm
As described previously (Forbes et al. 2009), our blocked-design number-guessing paradigm consists of a pseudorandom presentation of three blocks of predominantly positive feedback (80% correct guess), three blocks of predominantly negative feedback (20% correct guess) and three control blocks. There are five trials, each with 3 seconds to guess, via button press, whether the value of a visually presented card is lower or higher than 5 (index and middle finger, respectively). The numerical value of the card is then presented for 500 milliseconds and followed by appropriate feedback (green upward-facing arrow for positive feedback; red downward-facing arrow for negative feedback) for an additional 500 milliseconds. A crosshair is then presented for 3 seconds, for a total trial length of 7 seconds. Each block comprises five trials, with three blocks each of predominantly positive feedback (80% correct) and three of predominantly negative feedback (20% correct) interleaved with three control blocks. During control blocks, participants are instructed to simply make button presses during the presentation of an “x” (3 seconds), which is followed by an asterisk (500 milliseconds) and a yellow circle (500 milliseconds). Each block is preceded by an instruction of “Guess Number” (positive or negative feedback blocks) or “Press Button” (control blocks) for 2 seconds resulting in a total block length of 38 seconds and a total task length of 342 seconds. Participants were unaware of the fixed outcome probabilities associated with each block and were led to believe that their performance would determine a net monetary gain at the end of the scanning session. Instead, all participants received $10. We included one incongruent trial within each task block (e.g., one of five trials during positive feedback blocks was incorrect resulting in negative feedback) to prevent participants from anticipating the feedback for each trial and to maintain participants’ engagement and motivation to perform well.
BOLD fMRI Data Acquisition
Each participant was scanned using one of two identical research-dedicated GE MR750 3T scanners equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1MHz at the Duke-UNC Brain Imaging and Analysis Center. A semi-automated high-order shimming program was used to ensure global field homogeneity. A series of 34 interleaved axial functional slices aligned with the anterior commissure-posterior commissure plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifacts (TR/TE/flip angle = 2000 ms/30 ms/60°; FOV = 240mm; 3.75×3.75×4mm voxels; interslice skip = 0). Four initial radiofrequency excitations were performed (and discarded) to achieve steady-state equilibrium. To allow for spatial registration of each participant’s data to a standard coordinate system, high-resolution three-dimensional structural images were acquired in 34 axial slices coplanar with the functional scans (TR/TE/flip angle = 7.7 s/3.0 ms/12°; voxel size = 0.9×0.9×4mm; FOV = 240mm, interslice skip = 0).
BOLD fMRI Data Pre-processing
Preprocessing was conducted using SPM8 (www.fil.ion.ucl.ac.uk/spm). Images for each participant were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model (final resolution of functional images = 2mm isotropic voxels), and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6-mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. The Artifact Recognition Toolbox (https://www.nitrc.org/projects/artifact_detect) was used to determine variability in single-subject whole-brain functional volumes. Individual whole-brain BOLD fMRI volumes meeting at least one of two criteria were included as nuisance regressors in determination of task-specific effects: (1) significant mean-volume signal intensity variation (i.e. within volume mean signal greater or less than 4 SD of mean signal of all volumes in time series) and (2) individual volumes where scan-to-scan movement exceeded 2mm translation or 2° rotation in any direction.
fMRI Quality Assurance Criteria
Quality control criteria for inclusion of a participant’s imaging data were: <5% volumes exceed artifact detection criteria for motion or signal intensity outliers and ≥90% coverage of signal within 5mm bilateral ventral striatum spheres centered at (±12, 10, −10). Additionally, data were only included in further analyses if the participant demonstrated sufficient engagement with the task, defined as responding to and receiving positive or negative feedback on at least 60% of trials within win and loss blocks, respectively. Imaging data for 13 out of the 178 non-Hispanic Caucasian DNS participants who completed the mYFAS were excluded based on the above criteria, resulting in a total of 165 participants with high quality imaging data available for subsequent analyses.
BOLD fMRI Data Analysis
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used to conduct fMRI data analyses. After preprocessing, linear contrasts using canonical hemodynamic response functions were used to estimate condition-specific (i.e., positive > negative feedback) BOLD responses for each individual and paradigm. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random-effects models to determine mean condition-specific neural reactivity using one-sample t-tests with a voxel-level statistical threshold of p < .05, familywise error (FWE) corrected for multiple comparisons across the entire search volume. Contrast estimates were then extracted from the peak activation voxel (MNI coordinates: x = ±12, y = 12, and z = −10) exhibiting a main effect of task using the above threshold within the left and right hemispheres of the anatomically defined VS ROI. The VS ROI was constructed using the Talairach Daemon option of the WFU PickAtlas Tool v2.4. BOLD parameter estimates exhibiting a main effect of contrast (e.g., positive > negative feedback) were extracted from the VS ROIs using the volume of interest (VOI) tool in SPM8 and used in our primary analyses described below.
Genotyping
DNA was isolated from saliva derived from Oragene DNA self-collection kits (DNA Genotek) customized for 23andMe (www.23andme.com). DNA extraction and genotyping were performed through 23andMe by the National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. One of two different Illumina arrays with custom content was used to provide genome-wide SNP data, the HumanOmniExpress or HumanOmniExpress-24 (Eriksson et al., 2010; Do et al., 2011; Tung et al., 2011; Hu et al., 2016).
Imputation
Genotype imputation was performed on all DNS participants with genome-wide chip data using the prephasing/imputation stepwise approach implemented in SHAPEIT/IMPUTE2 (Howie et al., 2011; Delaneau et al., 2012). Imputation was run separately for participants genotyped on the Illumina HumanOmniExpress and the Illumina HumanOmniExpress-24 arrays using biallelic SNPs only, the default value for effective size of the population (20,000), and chunk sizes of 3 Mb and 5 Mb for the respective arrays. Within each array batch, genotyped SNPs used for imputation were required to have missingness < 2%, Hardy-Weinberg equilibrium p > 10−6, and minor allele frequency >.01. The imputation reference set consisted of 2504 phased haplotypes from the full 1000 Genomes Project Phase 3 data set (May 2013, >70 million variants, release “v5a”). Imputed SNPs were retained if they had high imputation quality (Info >.9), low missingness (<5%), and minor allele frequency >.01. Of the 165 participants with high quality imaging data, imputed DRD2 −141C Ins/Del (rs1799732) data were excluded in 50 participants due to failing to meet these criteria.
Dopamine Polygenic Scores
Three of the five dopamine gene polymorphisms found by Nikolova et al. (2011) to predict ventral striatum activity were used to generate polygenic scores in the current analyses: DRD2 −141C Ins/Del (rs1799732), COMT Val158Met (rs4680), and DRD2 Taq1A (rs1800497). In our sample of 115 participants, there were 30 participants who were Del carriers and 85 Ins/Ins homozygotes for −141C Ins/Del; 26 participants homozygous for the Met allele, 37 for the Val allele, and 52 heterozygotes for Val158Met; 72 participants homozygous for the C allele, 3 for the T allele and 40 heterozygotes for Taq1A. All polymorphisms were in Hardy-Weinberg Equilibrium (genotype frequencies for each locus with associated X2 test p-values, are summarized in Supplemental Table 1).
According to Nikolova et al. (2011), genotypes that are associated with relatively higher dopamine signaling were assigned a score of 1; those with intermediate signaling were assigned a score of 0.5; those with lower signaling were assigned a score of 0 (Supplemental Table 2). For the DRD2 −141C Ins/Del polymorphism, Del carriers (Del+), who have been associated with increased dopamine signaling, were given a score of 1, and Ins/Ins homozygotes were given a score of 0. For the COMT Val158Met polymorphism, Met/Met homozygotes were given a score of 1 (high), Val/Met heterozygotes were given a score of 0.5 (intermediate), and Val/Val homozygotes were given a score of 0 (low). For the DRD2 Taq1A polymorphism, C/C homozygotes were given a score of 1 (high), C/T heterozygotes were given a score of 0.5 (intermediate), and T/T homozygotes were given a score of 0 (low). The polygenic score for each participant was the sum of the score at each locus, with a range from 0 to 3 (1.51 ± 0.68).
Statistical Analyses
Descriptive statistics were calculated for all measures and are displayed in Table 1. In our sample, the mean BMI was 23.44 (SD = ± 3.22). According to World Health Organization Criteria, 44 participants were classified as overweight/obese (BMI ≥ 25.00), 115 participants as normal (BMI between 18.5 and 25.00), and 6 participants as underweight (BMI <18.5). Table 1 also shows that there are no significant differences in any of the variables included in subsequent analyses among participants with and without polygenic scores. We also examined the bivariate associations among polygenic scores, right and left VS activity, mYFAS scores, BMI and all control variables (Table 2). Control variables included sex, age, and the top two ancestry principal components, which were computed using multidimensional scaling (MDS; i.e., no individuals were ±6 SDs from the mean on the top 10 components) in PLINK (Purcell et al., 2007).
Table 1.
Descriptive Statistics of Measures Included in Analyses and Test of Differences Between Participants With and Without Dopamine Signaling Polygenic Scores.
| PGS Present (N=115) | PGS Absent (N=50) | P-Value | Full Sample (N=165) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Min | Max | Mean | Std. Dev. | Min | Max | Mean | Std. Dev. | T-test | Min | Max | Mean | Std. Dev |
| Age | 18 | 22 | 19.86 | 1.18 | 18 | 22 | 20.16 | 1.30 | 0.15 | 18 | 22 | 19.95 | 1.22 |
| BMI | 17.48 | 43.38 | 23.55 | 3.44 | 18.73 | 29.82 | 23.18 | 2.67 | 0.50 | 17.48 | 43.38 | 23.44 | 3.22 |
| mYFAS | 0 | 16 | 5.71 | 3.74 | 0 | 13 | 5.92 | 3.57 | 0.74 | 0 | 16 | 5.78 | 3.68 |
| Right VS | −0.45 | 0.44 | 0.07 | 0.16 | −0.48 | 0.74 | 0.09 | 0.20 | 0.50 | −0.48 | 0.74 | 0.07 | 0.17 |
| Left VS | −0.24 | 1.12 | 0.09 | 0.17 | −0.18 | 0.43 | 0.08 | 0.13 | 0.55 | −0.24 | 1.12 | 0.09 | 0.16 |
| PGS | 0 | 3 | 1.51 | 0.68 | |||||||||
| % Sample | % Sample | X2 | % Sample | ||||||||||
| Sex | |||||||||||||
| Male | 43.5 | 48.0 | 0.59 | 44.8 | |||||||||
| Female | 56.5 | 52.0 | 55.2 | ||||||||||
Note. “PGS Present” refers to the 115 participants without missing PGS data. “PGS Absent” refers to the 50 participants with missing PGS data. “Full Sample” refers to the 165 participants with and without missing PGS data. The P-value column refers to independent samples t- and chi-square tests that compare differences between the PGS present (N=115) and PGS absent (N=50) samples on measures included in subsequent analyses. BMI = body mass index; mYFAS = Modified Yale Food Addiction Scale; VS = ventral striatum; PGS = polygenic scores.
Table 2.
Inter-Correlations Among All Measures.
| PGS | Right VS | Left VS | mYFAS | BMI | Sex | Age | C1 | C2 | |
|---|---|---|---|---|---|---|---|---|---|
| PGS | 1 | ||||||||
| Right VS | 0.230* | 1 | |||||||
| Left VS | −0.008 | −0.456*** | 1 | ||||||
| mYFAS | 0.083 | −0.204** | −0.020 | 1 | |||||
| BMI | −0.068 | −0.101 | 0.024 | 0.257** | 1 | ||||
| Sex | 0.160 | −0.019 | −0.026 | 0.420*** | −0.003 | 1 | |||
| Age | −0.003 | −0.055 | −0.001 | −0.071 | 0.155* | 0.004 | 1 | ||
| C1 | −0.162 | 0.030 | 0.067 | −0.166* | 0.018 | −0.040 | 0.027 | 1 | |
| C2 | 0.029 | −0.081 | −0.084 | 0.065 | 0.012 | −0.053 | −0.051 | −0.053 | 1 |
Note. Pearson’s r is reported in each cell. All correlations with PGS include N=115. All other correlations include N=165. PGS = polygenic scores; VS = ventral striatum; mYFAS = Modified Yale Food Addiction Scale; BMI = body mass index; C1 = MDS ancestry principal component 1; C2 = MDS ancestry principal component 2.
p < .05
p < .01
p < .001.
We conducted exploratory post-hoc path analyses with robust standard errors using the program Mplus (Muthén & Muthén, 2010). Based on both the results from the correlations and our hypotheses, we tested whether dopamine polygenic scores and BMI were indirectly related via the path through reward-related VS activity and mYFAS scores. Direct and indirect effects were computed using bias-corrected bootstrapping procedures with 5,000 samples (MacKinnon, Lockwood, & Williams, 2004). Standard methods for assessing goodness of fit were used, including the maximum likelihood goodness-of-fit chi-square test (p > .05), the comparative fit index (CFI > .95), the standardized root mean square residual (SRMR<0.05), and the root mean square error of approximation (RMSEA < .08) (Kline, 2011). We used full information maximum likelihood to estimate missing PGS scores for the 30% of the sample who did not have data available. This procedure is recommended when data are missing at random (Enders, 2010), a condition that was likely given that there were no demographic differences between participants with or without polygenic scores (see Table 1). We also conducted the path analyses excluding the 50 participants who did not have genetic data available to ensure that our results were not driven by any biases introduced by imputing missing scores.
Results
Simple Correlations
Consistent with our prior work (Nikolova et al., 2011), there was a significant positive correlation between dopamine signaling polygenic scores and reward-related activity in the right VS (see Table 2); however polygenic scores were not significantly associated with left VS activity, mYFAS scores, or BMI. There was a significant negative correlation between mYFAS scores and right but not left VS activity. Women had higher mYFAS scores, but mYFAS scores were positively correlated with BMI across men and women. BMI was not significantly associated with polygenic scores or VS activity in either hemisphere.
Exploratory Post-Hoc Indirect Path Model
Based on the results from the correlations described above, only right VS activity was included in the path model, given that polygenic scores, mYFAS scores, and BMI all were unrelated to left VS activity. The correlation between sex and mYFAS scores was added to the model, given the relatively strong bivariate relation between these variables (r = 0.43). Correlations also were added between polygenic scores and sex and ancestry MDS components.
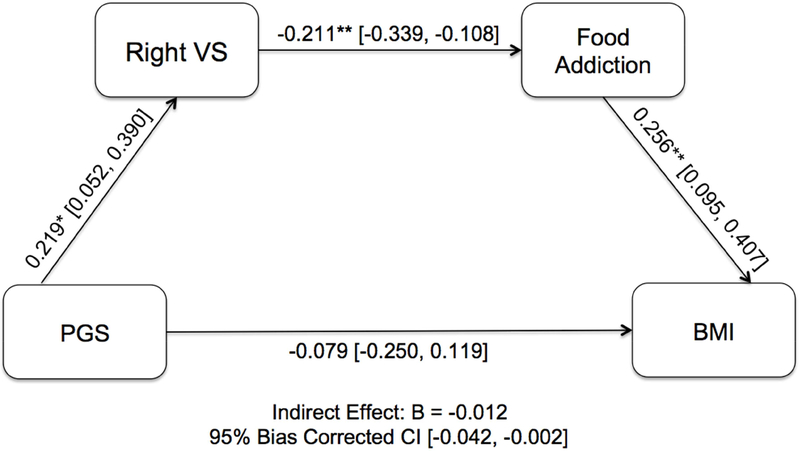
The model with a path from dopamine signaling polygenic scores to right reward-related VS activity to mYFAS scores to BMI demonstrated overall good fit (X2(11) = 11.352, p = 0.414; CFI = 0.993; TLI = 0.988; RMSEA = 0.014; SRMR = 0.042; see Table 3). Within the full model, the polygenic scores positively predicted right VS activity (βPGS→VS = 0.219, 95% CI [0.026, 0.406]), which, negatively predicted mYFAS scores (βVS→mYFAS = −0.211, 95% CI [−0.339, −0.108]), which, in turn, positively predicted BMI (βmYFAS→BMI = 0.256, 95% CI [0.095, 0.407]). The resulting indirect pathway from polygenic scores to BMI was significant (βPGS→BMI = −0.0012, 95% CI [−0.042, −0.002], Figure 1). Similarly, the indirect pathway from polygenic scores to mYFAS scores through right VS activity was significant as well (βPGS→mYFAS = −0.046, 95% CI [−0.123, −0.007]. The direct path between polygenic scores and BMI (βPGS→BMI = −0.079, 95% CI [−0.250, 0.119]) as well as polygenic scores and mYFAS scores were not significant (βPGS→mYFAS = 0.062, 95% CI [−0.129, 0.245]). When using listwise deletion (N = 115), the path model continued to demonstrate a good fit (X2(18) = 16.672, p = 0.546; CFI = 1.000; TLI = 1.068; RMSEA = 0.000; SRMR = 0.056). The indirect pathways from polygenic scores to right VS activity to mYFAS and polygenic scores to right VS activity to mYFAS to BMI also were significant in the smaller sample (see Supplemental Table 3 and Figure 3). The pathways from polygenic scores to mYFAS to BMI and polygenic scores to right VS activity to BMI were non-significant in the smaller sample.
Table 3.
Goodness of Fit Indices and Standardized Weights of Direct and Indirect Paths of the Model.
| Path | Estimate | 95% CI |
|---|---|---|
| Correlation | ||
| s PGSTM | ||
| Sex | 0.140 | (−0.047, 0.316) |
| C1 | −0.129 | (−0.295, 0.080) |
| C2 | 0.013 | (−0.180, 0.210) |
| Direct | ||
| PGS → | ||
| RVS | 0.219 | (0.026, 0.406) |
| mYFAS | 0.062 | (−0.129, 0.245) |
| BMI | −0.079 | (−0.250, 0.119) |
| RVS → | ||
| YFAS | −0.211 | (−0.339, −0.087) |
| BMI | −0.031 | (−0.166, 0.110) |
| mYFAS → | ||
| BMI | 0.256 | (0.095, 0.407) |
| Sex | 0.409 | (0.262, 0.531) |
| Indirect | ||
| PGS → mYFAS → BMI | 0.016 | (−0.025, 0.083) |
| PGS → RVS → BMI | −0.007 | (−0.054, 0.019) |
| PGS → RVS → mYFAS | −0.046 | (−0.123, −0.007) |
| PGS → RVS → mYFAS → BMI | −0.012 | (−0.042, −0.002) |
| X2/df | 11.352/11 | |
| CFI | 0.993 | |
| TLI | 0.988 | |
| RMSEA (90% CI) | 0.014 (0.00, 0.084) | |
| SRMR | 0.042 | |
Note. CI = confidence interval; PGS = polygenic scores; RVS = Right ventral striatum; mYFAS = Modified Yale Food Addiction Scale; BMI = body mass index; C1 = MDS ancestry principal component 1; C2 = MDS ancestry principal component 2; df = degrees of freedom; CFI = Comparative Fit Index; TLI = Tucker-Lewis Index; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual.
Figure 1.
Exploratory post-hoc path analysis model showing the association between dopamine signaling polygenic scores (PGS) and food addiction and body mass index (BMI) through right VS reward-related activity. Standardized regression coefficients are shown. The direct effect is reported along the lower path. * p < .05; ** p < .01. 95% bias corrected confidence intervals (CI) are shown in brackets. N=165.
Discussion
The overarching goal of this study was to examine a possible biological pathway through which prior risk-related genetic and neural phenotypes may be associated with an individual’s propensity for food addiction and, ultimately, likelihood for obesity. Consistent with the pattern of hypothesized simple inter-correlations between our individual variables of interest, a path model revealed that higher polygenic scores approximating dopamine signaling in vivo predicted higher food addiction symptoms and, ultimately, BMI via relatively blunted reward-related activity of the ventral striatum. Identifying these novel links with known risk-related neural and genetic biomarkers adds to existing findings supporting the utility of food addiction in obesity research.
Broadly, the results replicate our prior finding that the additive effect of individual alleles associated with relatively increased dopamine signaling map onto increased reward-related VS activity (Nikolova et al., 2011). However, in contrast to previous findings from fMRI studies of food addiction (Gearhardt et al, 2011; Stice et al., 2011), our analyses revealed that VS activity and food addiction were inversely correlated. This difference may be partly attributed to the different nature of the study samples. Prior work in food addiction, as well as in obesity, has focused on participants who were obese or diagnosed with binge eating disorder (Davis et al., 2011). However, relative to the national average BMI of 28.7 (Flegal et al., 2012), our sample consisted of lean healthy adults with an average BMI of 23.55 ± 3.44, and only six participants met diagnostic criteria for clinically significant food addiction.
Moreover, unlike previous studies in which the fMRI task specifically targeted response to food-related stimuli, such as images of food (Pelchat et al., 2004) and hyper-palatable food consumption (Gearhardt et al., 2011), our VS activity is associated with more general reward processing response to positive versus negative feedback within a monetary incentive paradigm. Notably, in a sample of lean participants, a similar polygenic score for dopamine signaling was negatively correlated to neural response to food reward (e.g., low dopamine signaling and greater response in reward-related regions), but positively to monetary reward (e.g., low dopamine signaling and blunted striatal response), emphasizing the critical role for the specific form of reward in neuroimaging studies of eating behaviors (Stice, Yokum, Burger, Epstein, & Smolen, 2012). To this end, conflicting patterns of results (e.g., hyper- and hypo-activation of the same regions) are commonly reported in substance addiction, and are often attributed to differences in fMRI tasks and clinical or demographic factors (Limbrick-Oldfield, van Holst, & Clark, 2013).
Because our sample consisted of largely lean healthy young adults, and most participants exhibited relatively low mYFAS scores, the majority of our sample did not meet the clinical threshold for food addiction. Although we were able to adapt the YFAS with a new dimensional scoring algorithm to capture a wider range of symptoms, given that only six participants met the diagnostic criteria for food addiction, our results should be interpreted with caution. Nevertheless, our results indicate that emerging symptoms of food addiction and risk for obesity may have identifiable biological correlates in lean individuals. Given that reward-seeking behaviors become compulsive only after a prolonged period of self-administration (Vanderschuren & Everitt, 2004), such risk-related biomarkers that predict subclinical symptoms of food addiction may inform increasing efforts for early identification and prevention approaches to weight gain and obesity.
Of course, our work is not without limitations that can be addressed in future research. First, our polygenic score assumes equal magnitude of effect for each locus on dopamine signaling, and models employing weighted effects may better capture the putative variability in dopamine signaling associated with the polygenic score. A reliable basis for such weighting, however, is not yet available. Second, reduced neural sensitivity to reward has been proposed as a consequence of overeating and weight gain (Stice, Yokum, Blum, & Bohon & 2010), and given the cross-sectional design of our study, it is difficult to conclude the extent to which the observed differences in VS activity are preexisting or acquired. Third, it is unclear whether the associations between dopamine signaling, food addiction, BMI, and VS activity are driven by either anticipating or receiving a reward given that our blocked-design does not allow for the separation of VS activity during those stages of reward processing. Future research should examine this question. Finally, relatively blunted reward responsiveness influences not only eating but multiple behavioral domains, such as affective state and the general ability to experience pleasure. Several studies suggest that hedonically-driven eating is a common method of self-medication in response to negative affect (Davis, Strachan, & Berkson, 2004; Macht, 2008). In fact, food addiction has been found to be closely associated with emotional eating, perhaps due to the “comforting” effect of palatable foods (Miller-Matero et al., 2014; Parylak, Koob, & Zorilla, 2001), and one study has reported that anxiety and depression were stronger predictors of food addiction than BMI (Miller-Matero et al., 2014). Therefore, it will be important to disentangle components of food addiction that are driven by addiction-like behavior and affective state for future research.
Supplementary Material
Acknowledgements
We thank the Duke Neurogenetics Study participants and the staff of the Laboratory of NeuroGenetics. The Duke Neurogenetics Study received support from Duke University as well as US-National Institutes of Health grants R01DA033369 and R01DA031579. ALR was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 1106401. ARH received further support from US-National Institutes of Health grant R01AG049789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adrienne L. Romer, Laboratory of NeuroGenetics, Department of Psychology & Neuroscience, Duke University, Durham, NC.
Min Su Kang, Laboratory of Affective and Translational Neuroscience, McLean Hospital, Belmont, MA.
Yuliya S. Nikolova, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
Ashley N. Gearhardt, Department of Psychology, University of Michigan, Ann Arbor, MI
Ahmad R. Hariri, Laboratory of NeuroGenetics, Department of Psychology & Neuroscience, Duke University, Durham, NC
References
- Abilés V, Rodríguez-Ruiz S, Abilés J, Mellado C, García A, de la Cruz AP, & Fernández-Santaella MC (2010). Psychological characteristics of morbidly obese candidates for bariatric surgery. Obesity Surgery, 20(2), 161–167. [DOI] [PubMed] [Google Scholar]
- Agranat-Meged AN, Deitcher C, Goldzweig G, Leibenson L, Stein M, & Galili-Weisstub E (2005). Childhood obesity and attention deficit/hyperactivity disorder: a newly described comorbidity in obese hospitalized children.International Journal of Eating Disorders, 37(4), 357–359. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (DSM). Washington, DC: American Psychiatric Association, 143–7. [Google Scholar]
- Ayyad C, & Andersen T (2000). Long term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999.Obesity Reviews, 1(2), 113–119. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, White MA, Stevens MC, Pearlson GD, … & Potenza MN (2013). Monetary reward processing in obese individuals with and without binge eating disorder. Biological Psychiatry, 73(9), 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, … & Wrase J (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–742. [DOI] [PubMed] [Google Scholar]
- Becker A, Kirsch M, Gerchen MF, Kiefer F, & Kirsch P (2017). Striatal activation and frontostriatal connectivity during non‐drug reward anticipation in alcohol dependence. Addiction Biology, 22(3), 833–843. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?. Brain Research Reviews, 28(3), 309–369. [DOI] [PubMed] [Google Scholar]
- Blumenthal DM, & Gold MS (2010). Neurobiology of food addiction. Current Opinion in Clinical Nutrition & Metabolic Care, 13(4), 359–365. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, … & Hyman SE (1997). Acute effects of cocaine on human brain activity and emotion. Neuron, 19(3), 591–611. [DOI] [PubMed] [Google Scholar]
- Büchel C, Peters J, Banaschewski T, Bokde AL, Bromberg U, Conrod PJ, … & Heinz A (2017). Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nature Communications, 8, 14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler M, Vollstädt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, … & Smolka MN (2010). Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry, 67(8), 745–752. [DOI] [PubMed] [Google Scholar]
- Cocores JA, & Gold MS (2009). The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Medical Hypotheses, 73(6), 892–899. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Flint A, Field AE, Kraft P, Han J, Rimm EB, & Dam R. M. van. (2016). A genome-wide investigation of food addiction. Obesity, 24(6), 1336–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, & Grigson PS (2009). Symposium overview—food addiction: fact or fiction?. The Journal of Nutrition, 139(3), 617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, & Walton RT (2005). Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry, 58(6), 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafò MR, … & Conti DV (2013). Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction, 108(12), 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, & Kennedy JL (2011). Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite, 57(3), 711–717. [DOI] [PubMed] [Google Scholar]
- Davis C, & Loxton NJ (2013). Addictive behaviors and addiction-prone personality traits: associations with a dopamine multilocus genetic profile. Addictive Behaviors, 38(7), 2306–2312. [DOI] [PubMed] [Google Scholar]
- Davis C, Loxton NJ, Levitan RD, Kaplan AS, Carter JC, & Kennedy JL (2013). ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiology & Behavior, 118, 63–69. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, & Berkson M (2004). Sensitivity to reward: implications for overeating and overweight. Appetite, 42(2), 131–138. [DOI] [PubMed] [Google Scholar]
- Delaneau O, Marchini J, & Zagury JF (2012). A linear complexity phasing method for thousands of genomes. Nature Methods, 9(2), 179. [DOI] [PubMed] [Google Scholar]
- Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, … & Wojcicki A. (2011). Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genetics, 7(6), e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehring A, Kirchhof A, & Lötsch J (2009). Genetic diagnostics of functional variants of the human dopamine D2 receptor gene. Psychiatric Genetics, 19(5), 259–268. [DOI] [PubMed] [Google Scholar]
- Enders CK Applied missing data analysis. New York: Guilford Press; 2010. [Google Scholar]
- Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S, … & Mountain J (2010). Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genetics, 6(6), e1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith MS, Matz PE, & Jorge MA (2002). Obesity–depression associations in the population. Journal of Psychosomatic Research, 53(4), 935–942. [DOI] [PubMed] [Google Scholar]
- Finlayson G (2017). Food addiction and obesity: unnecessary medicalization of hedonic overeating. Nature Reviews Endocrinology, 13(8), 493. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MWJB, & Williams JB (1995). Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, & Ogden CL (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA, 307(5), 491–497. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Gearhardt AN, Corbin WR, Brownell KD, Field AE, & Rimm EB (2014). Food-addiction scale measurement in 2 cohorts of middle-aged and older women. The American Journal of Clinical Nutrition, 99(3), 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR (2009). Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry, 14(1), 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, & Brownell KD (2009). Preliminary validation of the Yale food addiction scale. Appetite, 52(2), 430–436. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, & Brownell KD (2011). Neural correlates of food addiction. Archives of General Psychiatry, 68(8), 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone-Blanchet A, & Fecteau S (2014). Overlap of food addiction and substance use disorders definitions: Analysis of animal and human studies. Neuropharmacology, 85, 81–90. [DOI] [PubMed] [Google Scholar]
- Howie B, Marchini J, & Stephens M (2011). Genotype imputation with thousands of genomes. G3: Genes, Genomes, Genetics, 1(6), 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, & Hinds DA (2016). GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nature Communications, 7, 10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, … & Manso G (2009). Refined food addiction: a classic substance use disorder. Medical Hypotheses, 72(5), 518–526. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, … & Nummenmaa L (2015). Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. Journal of Neuroscience, 35(9), 3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB (2011). Principles and practice of structural equation modeling. New York: Guilford press. [Google Scholar]
- Kolotkin RL, Meter K, & Williams GR (2001). Quality of life and obesity. Obesity Reviews, 2(4), 219–229. [DOI] [PubMed] [Google Scholar]
- Kopelman PG (2000). Obesity as a medical problem. Nature, 404(6778), 635–643. [DOI] [PubMed] [Google Scholar]
- Kühn S, & Gallinat J (2011). Common biology of craving across legal and illegal drugs–a quantitative meta‐analysis of cue‐reactivity brain response. European Journal of Neuroscience, 33(7), 1318–1326. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, … & Dunbar GC (1997). The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry, 12(5), 224–231. [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, … & Zhao L (2012). Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Research, 1469, 63–72. [DOI] [PubMed] [Google Scholar]
- Limbrick-Oldfield EH, van Holst RJ, & Clark L (2013). Fronto-striatal dysregulation in drug addiction and pathological gambling: consistent inconsistencies?. NeuroImage: Clinical, 2, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MW, & Sescousse G (2017). Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry, 74(4), 387–398. [DOI] [PubMed] [Google Scholar]
- Lutter M, & Nestler EJ (2009). Homeostatic and hedonic signals interact in the regulation of food intake. The Journal of Nutrition, 139(3), 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht M (2008). How emotions affect eating: a five-way model. Appetite, 50 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavior Research 39, 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sölch C, Magyar S, Künig G, Missimer J, Schultz W, & Leenders K (2001). Changes in brain activation associated with reward processing in smokers and nonsmokers. Experimental Brain Research, 139(3), 278–286. [DOI] [PubMed] [Google Scholar]
- Mason AE, Vainik U, Acree M, Tomiyama AJ, Dagher A, Epel ES, & Hecht FM (2017). Improving assessment of the spectrum of reward-related eating: the RED-13. Frontiers in psychology, 8, 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, & Nemeroff CB (2004). Are mood disorders and obesity related? A review for the mental health professional. Journal of Clinical Psychiatry, 65(5), 634–651. [DOI] [PubMed] [Google Scholar]
- Meule A, & Gearhardt AN (2014). Five years of the Yale Food Addiction Scale: Taking stock and moving forward. Current Addiction Reports, 1(3), 193–205. [Google Scholar]
- Miller-Matero LR, Armstrong R, McCulloch K, Hyde-Nolan M, Eshelman A, & Genaw J (2014). To eat or not to eat; is that really the question? An evaluation of problematic eating behaviors and mental health among bariatric surgery candidates. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 19(3), 1–6. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. JAMA, 291(10), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO MPlus user’s guide (7th ed.). Muthén & Muthén: Los Angeles, CA, 1998–2013. [Google Scholar]
- Nantha YS (2014). Addiction to sugar and its link to health morbidity: A primer for newer primary care and public health initiatives in Malaysia. Journal of Primary Care & Community Health, 5(4), 263–270.. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, & Harari AR (2011). Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology, 36, 1940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Brunani A, Borgomainerio E, Tonello C, Dioni L, Briscini L, … & Carruba MO (2007). D2 dopamine receptor (DRD2) gene Taq1A polymorphism and the eatingrelated psychological traits in eating disorders (anorexia nervosa and bulimia) and obesity. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 12(2), 91–96. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, & Zorrilla EP (2011). The dark side of food addiction. Physiology & Behavior, 104(1), 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, & Ragland JD (2004). Images of desire: food-craving activation during fMRI. Neuroimage, 23(4), 1486–1493. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer X (2003). A clinical view of the obesity problem. Science, 299(5608), 859–860. [DOI] [PubMed] [Google Scholar]
- Price M, Higgs S, & Lee M (2015). Self-reported eating traits: underlying components of food responsivity and dietary restriction are positively related to BMI. Appetite, 95, 203–210. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, … & Sham PC (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics, 81(3), 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Gearhardt AN, Collins CE, & Burrows TL (2014). The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients, 6(10), 4552–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Avena NM, & Gearhardt AN (2015). Which foods may be addictive? The roles of processing, fat content, and glycemic load. PloS one, 10(2), e0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, & Small DM (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science, 322(5900), 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, & Bohon C (2010). Weight gain is associated with reduced striatal response to palatable food. Journal of Neuroscience, 30(39), 13105–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, & Smolen A (2010). Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage, 50(4), 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, & Small DM (2011). Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of Neuroscience, 31(12), 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, & Smolen A (2012). Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. The Journal of Neuroscience, 32(29), 10093–10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, & Cox JE (2008). Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage, 41(2), 636–647. [DOI] [PubMed] [Google Scholar]
- Swinburn B, Gill T, & Kumanyika S (2005). Obesity prevention: a proposed framework for translating evidence into action. Obesity Reviews, 6(1), 23–33. [DOI] [PubMed] [Google Scholar]
- Tung JY, Do CB, Hinds DA, Kiefer AK, Macpherson JM, Chowdry AB, … & Eriksson N (2011). Efficient replication of over 180 genetic associations with self-reported medical data. PloS One, 6(8), e23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, & Everitt BJ (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science, 305(5686), 1017–1019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, & Telang F (2008). Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B, 363, 3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, … & Pradhan K (2008). Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage, 42(4), 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, & Telang F (2011). Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences, 108(37), 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt‐Klein S, Kobiella A, Bühler M, Graf C, Fehr C, Mann K, & Smolka MN (2011). Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addiction Biology, 16(1), 166–175. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Nelson JE, Dubbert BK, & Spitzer RL (1993). Association of binge eating disorder and psychiatric comorbidity in obese subjects. The American Journal of Psychiatry, 150(10), 1472–1479. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Kaplan AS, Zai CC, Levitan RD, & Kennedy JL (2011). COMT Val158Met variant and functional haplotypes associated with childhood ADHD history in women with bulimia nervosa. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(4), 948–952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.