Abstract
RATIONALE AND OBJECTIVES:
Additive manufacturing may be used as a form of personalized medicine in interventional radiology by allowing for the creation of customized bioactive constructs such as catheters that can act as a form of localized drug delivery. The purpose of the present in vitro study was to use 3D printing to construct bioactive laden bioabsorbable catheters impregnated with antibiotics and chemotherapeutics.
MATERIALS AND METHODS:
Polylactic acid bioplastic pellets were coated with the powdered bioactive compounds gentamicin sulfate (GS) or methotrexate (MTX) in order to incorporate these drugs into the 3D printed constructs. The pellets were then extruded into drug-impregnated filament for fused deposition modeling 3D printing. Computer-aided design files were generated in the shapes of 14-F catheters. Scanning electron microscope imaging was used to visualize the presence of the additive powders on the surface of the printed constructs. Elution profiles were run on the antibiotic laden catheter and MTX-laden catheters. Antibiotic laden catheters were tested on bacterial broth and plate cultures.
RESULTS:
Both GS and MTX catheter constructs had sustained drug release up to the 5-day limit of testing. The 3D printed GS-enhanced catheters inhibited all bacterial growth in broth cultures and had an average zone of inhibition of 858 ± 118 mm2 on bacterial plates while control catheters had no effect.
CONCLUSION:
The 3D printing manufacturing method to create instruments in percutaneous procedures is feasible. Further in vivo studies will substantiate these findings.
Keywords: 3D printing, Three-dimensional printing, Personalized medicine, Additive manufacturing, Interventional radiology, catheter
INTRODUCTION
Bioimaged three-dimensional printing (B3DP) is an emerging modality in radiology that transcribes patient imaging data to replicate a patient’s anatomy to deliver personalized medicine. 3D printing can construct custom anatomical models, custom prostheses, and procedural instruments tailored to patient-specific anatomy. Additive manufacturing has the potential to serve as a form of personalized medicine in interventional radiology. One example of this could be through the creation of bioactive constructs such as antibiotic-laden catheters customized to patient anatomy and clinical presentation. One challenge to widespread implementation of B3DP in medicine is reimbursement and regulatory challenges (1, 2) along with the time to design and print catheter.
Few reports detail how 3D printing was implemented in creating custom surgical instruments (1, 3). With appropriate development and practice setting, there is potential in interventional radiology – to manufacture “just in time” delivery of patient-specific procedural instruments. Conceivably, 3D printed catheters could be designed using pre-procedural CT, MR, or conventional angiograms to account for bend, branching, and length. This would allow for customizability with shape, bend, and thickness. The instruments could be printed in a sterile field or sterilized using chemical techniques, including glutaraldehyde, autoclaving, and ultraviolet light (3). 3D printing offers the unique possibility of incorporating drug within the structure of an instrument or construct itself (4, 5). The authors hypothesized that fused deposition modeling 3D printing technology could be used to incorporate antibiotics and chemotherapeutics within the structure of the 3D printed catheters with pharmaceutical activity still intact following the manufacturing process. The purpose of this study was to create drug-eluting procedural instruments and profile their drug release in an in vitro model.
MATERIALS AND METHODS
Institutional Review Board-approval was not required for this in vitro study. The work was conducted by generating computer-aided three-dimensional renderings of constructs, fabricating bioactive filaments, printing constructs with bioactive filaments and finally conducting testing of the printed bioactive constructs. Additive manufacturing methods were done in accordance with previously published methods of incorporating drugs within a 3D printed construct’s structure (4).
A computer modeling software program was used to generate three-dimensional models of a 14-F catheter tip (SolidWorks; Dassault Systems SolidWorks Corporation Waltham, MA). The models were saved in the stereolithographic (STL) format. A solid model of the distal section of a 5 cm catheter tip model with a small orifice at its tip (modeled for guidewire passage) was created as the drug-eluting section of the full-length model and to focus on a small area in testing drug profiles. “Catheters” and “catheter tips” are used interchangeably in this study.
Bioresorbable bioplastic pellets consisting of polylactic acid (PLA) pellets (NatureWorks, LLC; Minnetonka, MN) was used as the base polymer. The PLA pellets were coated in gentamicin sulfate (GS; Sigma Aldrich; St. Louis, MO) at 1 wt% or coated in methotrexate (MTX) at 2.5 wt% and 5 wt% (Sigma Aldrich; St. Louis, MO) by placing pellets in a sterile plastic tube, adding GS or MTX powder, then placing them into a vortex mixer until coated. The GS and MTX-embedded pellets were then extruded into 1.75 mm diameter filaments with a modified filament extruder and a proprietary thermoplastic extrusion protocol (ExtrusionBot, LLC; Phoenix, AZ).
The catheters were fabricated using the GS and MTX-embedded filaments. Control catheters without drug-embedded filaments were also fabricated. The filaments were loaded in a 3D printer (MakerBot 3D printer; Brooklyn, NY) with a 300 μm layer height, at 220 °C GS, 170 °C for MTX, and with an in-fill rate set at 100%.
Drug elution profiles of drug-loaded catheters were tested using a NANODROP 2000 UV-Visible Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, Massachusetts,). Simulated body fluid was used to collect the samples from the constructs at 1 minute, 2 minutes, 5 minutes, 10 minutes, 20 minutes, 40 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, and then daily for a total of 5 days. For MTX, direct detection at 300 nm was performed. Since GS could not be directly detected, indirect determination was performed using OPTA reagent. Equal volumes of collected sample, isopropyl alcohol and OPTA reagent were added, and this mixture was analyzed by absorbance using a spectrophotometer with a set wavelength at 330 nm. The amount of drug eluted in the collected samples were calculated using the mean absorbance in triplicate from each catheter. Scanning electron microscope (SEM) imaging was used to visualize the presence of the additive powders on the surface of the printed constructs.
Printed constructs laden with gentamicin were tested with E. coli bacteria in either liquid nutrient broth or on agar plates. Mueller Hinton broth or Mueller Hinton agar plate cultures were inoculated according to ISO standards (Fischer Scientific; Hampton, NH). Sets of three PLA control catheters and three gentamicin antibiotic enhanced catheters were tested with individual bacterial agar plate or broth cultures. Cultures were incubated for 24hrs at 37 °C then analyzed for bacterial growth. Bacterial zones of inhibition were measured using calipers on agar plate cultures. Data are summarized with descriptive statistics.
RESULTS
14-F catheter tips 5 cm in length were modeled and successfully printed to specification using both GS and MTX-laden filaments (Figure 1). Catheters printed with GS had a mean weight of 1052 ± 60 mg and catheters printed with MTX had a mean weight of 1140 ± 61 mg. The calculated amount of GS in the catheter is 10.5 ± 0.6 mg and for the MTX catheters containing 2.5 % is 28.5 ± 1.5 mg and for catheters with 5% is 57.1 ± 3 mg. The actual amount would have required complete destruction/degradation of the PLA, which was not performed (kept for further testing). SEM suggested the bioactive additive particles (GS or MTX) on the surface of the catheter filaments. SEM illustrated the layer-by-layer construction of the catheters (Figure 2).
Figure 1.

Photograph of the methotrexate-laden 3D printed catheter.
Figure 2.

Scanning electron microscope images of gentamicin-laden 3D printed catheters (A, B) and methotrexate(MTX)-laden 3D printed catheters (C-D) and filaments (E-F). A, B). A and B). Multiple amorphous defects seen at 35x magnification suggest gentamicin incorporation into the catheter structure (by lack of a normal uniform filament structure) (A, arrows). This is confirmed at 20,000x magnification, which highlights the amorphous configuration of gentamicin (B, circles). C-D). Multiple coarse outpouchings are seen on the surface of the 3D printed catheter surface at 35x magnification, suggesting MTX incorporation on the catheter’s surface (arrows). Clusters of the crystalline structure MTX is demonstrated at the higher 10,000x magnification view (circles). E, F). MTX-laden individual filaments with the crystalline structure demonstrated at 1,000x (E, circle) and 20,000x (F) magnification views.
Elution profiles of GS-laden catheters and MTX-laden catheter tips are shown in Figure 3 and Figure 4. The pattern showed an initial burst release during the first few hours followed by a steady release. Continuing after 5 days, all catheters were still releasing GS and MTX within the working concentrations. The 3D printed GS enhanced catheters inhibited all bacterial growth in broth cultures and had an average zone of inhibition of 858 ± 118 mm2 on bacterial plates while control catheters had no effect (Figure 5).
Figure 3.
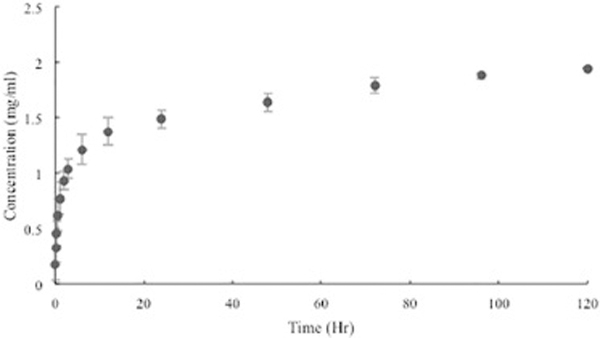
Cumulative concentration of GS eluted from 3D printed PLA catheters (mean ± SD, n = 3).
Figure 4.
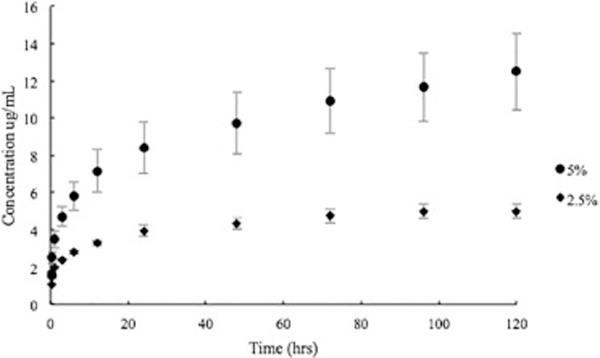
Cumulative concentration of MTX released from 2.5wt% and 5wt% 3D printed PLA catheters (mean ± SD, n = 3).
Figure 5.
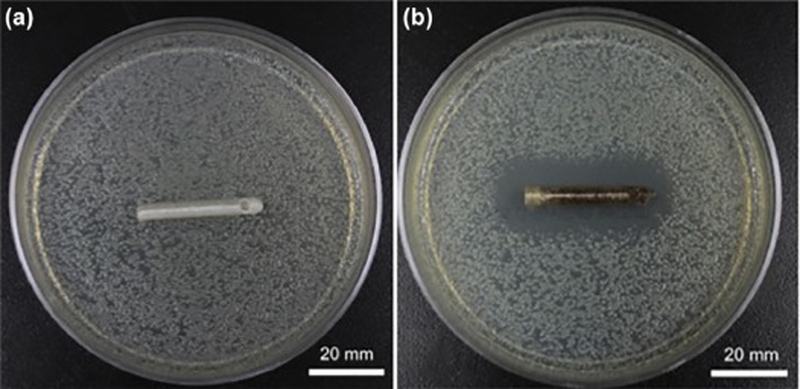
Antimicrobial properties of PLA GS-laden catheters control (A) and 1wt% gentamicin (B) PLA catheters. The GS-laden catheter retards bacterial growth (B).
DISCUSSION
This study demonstrates the feasibility of B3DP constructs of instruments used in interventional radiology. In the in vitro model, GM and MTX-eluting catheters showed a sustained drug release profile. The method of incorporating drugs into the structure of the construct itself offers a unique drug delivery system compared to conventional coatings. In biodegradable plastics, once the initial diffusion of drugs on the surface occurs new layers are ‘introduced’ which have drugs that have not reached the surface medium (1, 4, 5). During the 3D printing process of the 3D printed drug-laden catheters in the present study, the bioactive agents are dispersed into the matrix of PLA polymer and, the layer-by-layer construction of catheters increases their surface area of drug-impregnated filaments. Exploiting such drug-incorporation capabilities integrated with B3DP can create on-demand instruments for use in interventional radiology. Specialized biomaterials such as drug-coated beads or drug eluting stents have historically been used to achieve these effects (6, 7). One challenge in the use of biomaterials for drug delivery is the need to create absorbable materials that overcome the permanent placement of a material in the body. The 3D printing method B3DP as described in the present study may optimize therapeutic drug delivery in implantable constructs.
Bioprinting includes B3DP, such as the current study, and tissue or tissue constructs. Antibiotic and chemotherapeutic-laden 3D printed constructs have been described by Weisman et al. (4). In that study, several constructs were 3D printed and demonstrated similar drug release profiles compared to the present study. Ballard et al. (5) impregnated surgical meshes with gentamicin, which demonstrated inhibition of bacterial growth in an in vitro model. Tappa et al. (8) reported 3D printing of hormone-laden 3D printed intrauterine devices with estrogen and progesterone. Of note, all these studies have been performed in vitro and not in animals or humans.
There are limitations to our study including the in vitro design and design of the catheters. These 3D printed catheters in the present study were proof-of-concept and the process of transitioning this to a functional catheter for use in patients would need further development. The larger sizes were fabricated for visibility and analogous to 14-F drainage catheters in clinical practice, rather than small diameter endovascular catheters. It is unknown if such custom drug-eluting constructs would offer advantages compared to commercially available constructs. Printing drug-laden constructs in a sterile manner would require a more rigorous set-up than used in the present study and it is unknown what effect chemical sterilization techniques would have on the drug elution profiles and bioactivity. Future studies are needed with different materials commonly used in catheters – silastic, silicone, polyurethane. The drug-elution profiles, absorbability, and architecture will inevitably vary with different materials used in different constructs. There are unique possibilities to explore with the drug-eluting properties of biodegradable constructs. With the fused deposition modeling 3D printing used in the constructs in our study, the elution profiles in situ may be dynamic as the constructs dissolve. The advantage of layering individual filaments in manufacturing catheters, stents, and other implants, is that, as superficial layers dissolve, new drug-laden filaments are exposed. Animal studies are needed to substantiate efficacy. Material and property testing of the drug-containing 3D printed catheters in the present study were not performed. An analysis of this, especially compared to commercial catheters, should be performed in future follow-up studies.
Although there is great potential for 3D printing to personalize patients’ treatments in interventional radiology, there are challenges with implementing personalized 3D printing in human studies and clinical practice. An immediate advantage and implication of using additive manufacturing in interventional radiology research is the development of customized equipment and instruments. Regulatory and reimbursement issues remain hurdles to widespread implementation in clinical practice and these indications will require further demonstration of the safety and value of additive manufacturing techniques. The present study was therefore conducted with the objective of combining the customized nature of additive manufacturing with the proper biomaterials for localized drug delivery. Thus, we have demonstrated proof-of-concept of a new manufacturing method to create bioactive 3D printed constructs, in-vitro studies that have shown the eluted drugs are bioactive. Further in-vivo studies are warranted.
Acknowledgments
Conference Presentation
This study was presented as on oral presentation at the 2015 SIR Annual Scientific Meeting as: Weisman JA, Jammalamadaka U, Tappa K, Nicholson JC, Ballard DH, Wilson CG, D’Agostino HB, Mills DK. (2015). 3D printing antibiotic and chemotherapeutic eluting catheters and constructs. Oral Presentation Presented at: 2015 Society of Interventional Radiology Annual Scientific Meeting; Atlanta, GA.
Footnotes
Disclosures
JAW and DKM are co-inventors on a patent application which related to the methods in this paper: “Methods and Devices For Three-Dimensional Printing Or Additive Manufacturing Of Bioactive Medical Devices”, Application number US14822275. All other authors claim no conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Ballard DH, Trace AP, Ali S, Hodgdon T, Zygmont ME, DeBenedectis CM, et al. Clinical applications of 3d printing: primer for radiologists. Acad Radiol 2018; 25:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgdon T, Danrad R, Patel MJ, Smith SE, Richardson ML, Ballard DH, et al. Logistics of three-dimensional printing: primer for radiologists. Acad Radiol 2018; 25:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rankin TM, Giovinco NA, Cucher DJ, Watts G, Hurwitz B, Armstrong DG. Three-dimensional printing surgical instruments: are we there yet? J Surg Res 2014; 189:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisman JA, Nicholson JC, Tappa K, Jammalamadaka U, Wilson CG, Mills DK. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int J Nanomedicine 2015;10:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard DH, Weisman JA, Jammalamadaka U, Tappa K, Alexander JS, Griffen FD. Three-dimensional printing of bioactive hernia meshes: In vitro proof of principle. Surgery 2017;161:1479–1481. [DOI] [PubMed] [Google Scholar]
- 6.Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park C-H, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012; 57:1244–1250. [DOI] [PubMed] [Google Scholar]
- 7.Lemos PA, Serruys PW, Sousa JE. Drug-eluting stents: cost versus clinical benefit. Circulation 2003; 107:3003–3007. [DOI] [PubMed] [Google Scholar]
- 8.Tappa K, Jammalamadaka U, Ballard DH, Bruno T, Israel MR, Vemula H, et al. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS One 2017;12:e0182929. [DOI] [PMC free article] [PubMed] [Google Scholar]


