Abstract
Background: Diethylhexyl phthalate (DEHP) is widely used in industrial products, particularly as plasticizers and softeners. Because it is used extensively, DEHP has been detected in humans worldwide. Although epidemiological studies suggest that DEHP can disrupt the function of the hypothalamic–pituitary–thyroid (HPT) axis, evidence on the association between DEHP exposure and thyroid function remains inconclusive. Therefore, a comprehensive meta-analysis was performed to investigate the association between DEHP exposure and the HPT axis in humans.
Methods: A literature search of the MEDLINE, EMBASE, and Web of Science databases was conducted to search for studies in which the correlation coefficient values or regression coefficient values between three major DEHP metabolites (i.e., monoethylhexyl phthalate [MEHP], mono [2-ethyl-5-hydroxyhexyl] phthalate [MEHHP], and mono [2-ethyl-5-oxohexyl] phthalate) and thyrotropin, free thyroxine (T4), or total T4 were determined. The association between DEHPs and thyroid hormone levels were evaluated using Pearson's correlation coefficients.
Results: Thirteen eligible articles were included. Urinary MEHP and MEHHP concentration was negatively correlated with total T4. Pooled correlation coefficients between MEHP/MEHHP and total T4 were −0.02 [confidence interval (CI) −0.05 to 0.00] and −0.03 [CI −0.05 to −0.01], respectively. Urinary mono (2-ethyl-5-oxohexyl) phthalate concentration was positively correlated with thyrotropin, and the pooled correlation coefficient was 0.02 [CI 0.00–0.04].
Conclusions: The findings of this meta-analysis suggest a significant association between the exposure of DEHP metabolites and the function of the HPT axis.
Keywords: diethylhexyl phthalate, endocrine disruptors, thyroid hormones
Introduction
Thyroid dysfunction is among the most common diseases worldwide (1). Although the leading cause of thyroid dysfunction is iodine deficiency and autoimmune disease (2), many cases in which the cause is unclear have been reported. The recent massive increase in the use of chemicals worldwide has become a major health concern. Some of these chemicals can alter the function of the endocrine system, including that of the thyroid. These endocrine-disrupting chemicals (3,4), including phthalates, can interfere with the function of the hypothalamic–pituitary–thyroid (HPT) axis (4).
Phthalates are among the chemicals produced in high volume and are widely used as plasticizers and softeners in various commercial products, including food packaging, building materials, children's toys, medical devices, and cosmetics (5). Because phthalates are not chemically bound to the end products, they can be easily transferred to indoor dust, air, food, and water (6). Subsequently, humans can be exposed to phthalates through inhalation of contaminated air, ingestion of contaminated food or water, and dermal contact (5). Phthalates absorbed in the human body are rapidly metabolized to their metabolites and excreted in the urine or feces. Urinary concentration of phthalate metabolites is generally used as a biomarker for evaluating phthalate exposure in humans (7,8). Because of their extensive use, phthalates have been detected in humans worldwide (9,10).
Diethylhexyl phthalate (DEHP), one of the most commonly used phthalates, has been noted for its health effects (5). Recently, increasing evidence showed that DEHP can disrupt the function of the HPT axis (11). Animal experiments have shown that exposure to DEHP and its metabolites reduces the expression of the sodium–iodine symporter (NIS), decreases the level of transthyretin (one of the main thyroid hormone–binding proteins), and increases the levels of deiodinase 1 and UDP glucuronosyltransferase (UGT) in the liver, which metabolizes thyroid hormones (12–14). These observations suggest that DEHP can affect the thyroid hormone levels through effects on thyroid hormone synthesis, transport, and metabolism. In humans, Meeker et al. first reported that urinary concentration of monoethylhexyl phthalate (MEHP), one of the metabolites of DEHP, was negatively associated with free thyroxine (fT4) and total triiodothyronine levels in 408 men (15). Thereafter, several epidemiological studies in a diverse population supporting an association between DEHP exposure and thyroid hormone have been reported (16–22). However, the type of metabolites associated and the direction of association are different in each study, and some studies even reported absent associations. Therefore, a meta-analysis was conducted to determine the association of DEHP exposure with the function of HPT axis.
Methods
A meta-analysis was performed in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (23).
Search strategy
Two independent investigators (S.M. and M.J.K.) conducted a literature search of MEDLINE, EMBASE, and Web of Science in September 2017. The databases were searched with the following terms: “phthalate” or “diethylhexyl phthalate” or “Di (2-ethylhexyl) phthalate” or “Bis (2-ethylhexyl) phthalate” or “DEHP” and “thyroid.” Only articles published before September 1, 2017, in English were included.
Eligibility criteria
For studies to be included in this meta-analysis, the participants, interventions, comparators, outcomes, and study design framework was used (24). The participants of interest were the general population, including pregnant women and children. Neonates were excluded from this study because they might be exposed to DEHP through their mother rather than via direct exposure. Among different DEHP metabolites, urinary concentrations of MEHP, mono (2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) determined using liquid chromatography/tandem mass spectrometry were investigated to evaluate DEHP exposure. To evaluate thyroid function, blood concentrations of fT4, total T4 (TT4), and thyrotropin (TSH) were investigated. However, studies that presented thyroid status as categorized groups such as hyperthyroidism or hypothyroidism were excluded. Outcomes of interest were the association between urinary DEHP metabolite and thyroid hormone concentrations as continuous variables. Articles that reported Pearson's correlation coefficients, Spearman's correlation coefficients, or regression coefficients between fT4/TT4 and TSH and DEHP as continuous variables were included. Cross-sectional, case-control, and cohort studies were included.
Search and study selection
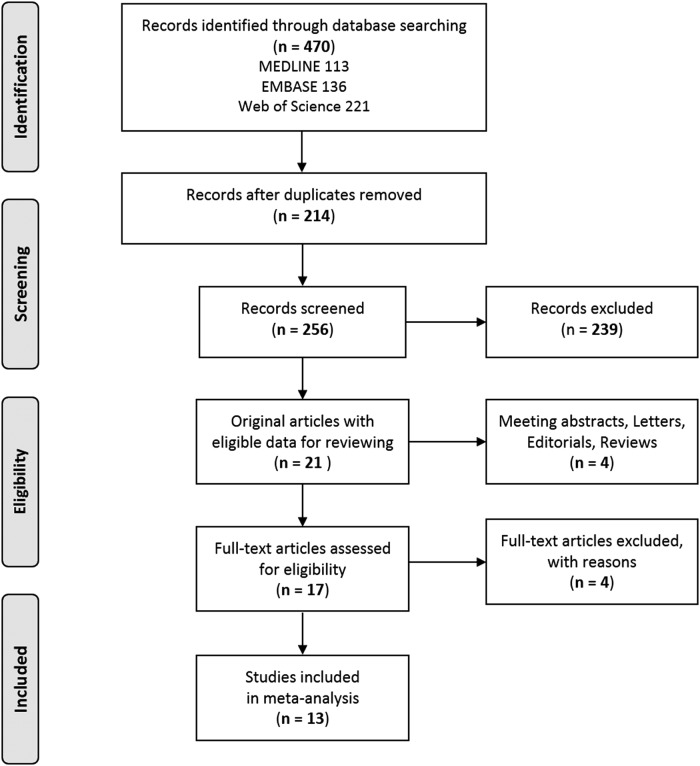
A literature search yielded 470 potentially relevant articles (Fig. 1). After excluding duplicate articles (n = 214), the titles and abstracts of 256 articles were further reviewed, and 239 articles were excluded based on the eligibility criteria. In addition, four articles published as meeting abstracts, letters, editorials, or reviews were excluded. Subsequently, the full texts of the 17 selected articles were reviewed by two independent investigators (S.M. and M.J.K.), and any disagreement was resolved by a third investigator (Y.J.P.). Four studies were excluded because they were in the same database (n = 1), analyzed with an interquartile range in DEHP concentration (n = 1), or had insufficient data for extraction (n = 2). Finally, 13 articles comprising five studies on children and adolescents (aged <18 years), four on pregnant women, two on adults (aged ≥18 years), and two analyzing a general population including children, adolescents, and adults were selected for the meta-analysis.
FIG. 1.
Representation of the search strategy.
Data extraction
The following variables were extracted by the two investigators independently based on the same rules: first author; publication year; country; number and age of subjects; the mean or median urinary concentration of MEHP, MEHHP, and MEOHP; TSH, fT4, and TT4; Pearson's correlation coefficient; Spearman's correlation coefficient; and regression coefficient.
Data analyses and statistical methods
The association between DEHPs and thyroid hormone levels was evaluated using Pearson's correlation coefficient. The z-values were calculated using Pearson's correlation coefficient after being transformed via Fisher's z-transformation (25). Pearson's correlation coefficient was known in only one of the 13 studies (26). The raw data were available in two studies (16,27), and a re-analysis was conducted to obtain Pearson's correlation coefficients. In the other studies, Pearson's correlation coefficient was calculated from existing Spearman's correlation coefficient or regression coefficient with the corresponding confidence interval (CI) using the following formulas:
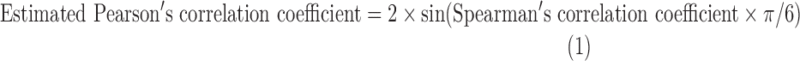 |
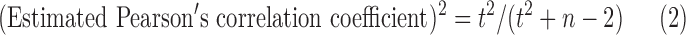 |
where t is the regression coefficient/the standard error of regression coefficient and the estimated Pearson's correlation coefficient × regression coefficient ≥0.
Meta-analysis of z-values was performed, and the pooled z-value was converted to a correlation coefficient again for ease of understanding according to the following formula: correlation coefficient = ([e2z − 1]/[e2z + 1]).
The Higgins' I2 statistic was used to test for heterogeneity. The random-effects model including a random intercept per study was used. Subgroup and sensitivity analyses were used to determine the cause of heterogeneity. The potential for publication bias was assessed using a funnel plot analysis and Egger's regression test. To examine the strength of the outcome, a sensitivity analysis was conducted to estimate the effects of the remaining studies without the effect of the larger one. All statistical analyses were calculated using the statistical program R v3.1.0 and the R package metafor (28).
Results
Characteristics of eligible studies
In total, 12,674 patients from 13 articles were included in this analysis. Sample sizes of these studies ranged from 76 to 6003 patients. The types of DEHP metabolites and thyroid hormones measured in the included studies are summarized in Table 1. The urinary concentration of DEHP metabolites were determined using liquid chromatography/tandem mass spectrometry. Among the several DEHP metabolites, a meta-analysis was performed for MEHP, MEHHP, and MEOHP.
Table 1.
Characteristics of Studies Included
| Urinary concentrations of DEHP metabolites (ng/mL), median (interquartile range) or geometric mean [CI] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Sampling year | Location | Population | n | Mean age (years) | Sex: n | MEHP | MEHHP | MEOHP | Thyroid hormone | Statistical analysis |
| Morgenstern et al. (2017) | 1998–2006 | United States | Children aged 3 years | 229 | 3.1 | M: 109; F: 120 | 3.2 [2.8–3.7] | 32.8 [27.9–38.5] | 19.2 [16.4–22.5] | fT4 TSH |
Multiple linear regression models |
| Tsai et al. (2016) | 2012–2013 | Taiwan | Children and adolescents aged <18 years | 240 | 4.8 (1.9–9.8) | 20.6 (10.3–35.9) | 14.7 (7.3–26.9) | fT4/TT4 TSH |
Multiple linear regression models | ||
| Wu et al. (2017) | 2013 | China | Children aged 5–7 years | 216 | 5–7 | M: 107; F: 109 | Urban: 6.1 (3.6–13.2); rural: 4 (3.2–6.3) | Urban: 15.4 (8.6–27.5); rural: 24.4 (10–94) | Urban: 6.1 (3.6–13.2); rural: 5.6 (4.1–12.3) | fT4/TT4 TSH |
Multiple linear regression models |
| Boas et al. (2010) | 2006–2007 | Denmark | Children aged 4–9 years | 845 | 7.0 | M: 503; F: 342 | M: 4.5 (2.5–7.7) F: 3.6 (1.8–7.2) |
M: 37 (19–64) F: 31 (14–55) |
M: 19 (9.6–32) F: 16 (7.8–29) |
fT4/TT4 TSH |
Multiple linear regression models |
| Weng et al. (2017) | 2013–2014 | Taiwan | Children aged 9–10 years (Taiwan birth Panel Study, TBPS) | 189 | 9–10 | M: 92; F: 97 | 9.4 (4.4–40) | 33.4 (17.2–70) | 21.9 (11.1–49.2) | fT4/TT4 TSH |
Multiple linear regression models |
| Huang et al. (2017) | 2013 | Taiwan | General population (Nutrition and Health Survey in Taiwan [NAHSIT]) | 79 | 12.6 | M: 47; F: 32 | 7.4 (2.4–12.6) | 25.5 (13.6–39.4) | 19.6 (9.3–32.3) | fT4/TT4 TSH |
Multiple linear regression models |
| Meeker et al. (2011) | 2007–2008 | United States | General population (National Health and Nutrition Examination Survey [NHANES]) | 329 | M: 185; F: 170 | 2.00 (LOD–4.50) | 20.33 (10.3–45.32) | 11.44 (5.79–24.74) | fT4/TT4 TSH |
Multiple linear regression models | |
| Yao et al. (2016) | N/A | China | Pregnant women (Ma'anshan birth Cohort [MABC]) | 2512 | 26.2 | All F | 2.50 (1.34–13.86) | 4.79 (3.01–20.19) | 6.61 (23.05) | fT4/TT4 TSH |
Spearman's correlations |
| Kuo et al. (2015) | 2009–2010 | Taiwan | Pregnant women | 148 | 29.3 | All F | 11.9 (8.2–19.3) | 20.5 (14.7–31.6) | 21.7 (14.8–33.8) | fT4/TT4 TT3 TSH |
Spearman's correlations |
| Huang et al. (2007) | 2005–2006 | Taiwan | Pregnant women | 76 | 33.6 | All F | 20.6 (13.1–38.6) | fT4/TT4 TSH |
Spearman's correlations | ||
| Huang et al. (2016) | 2013–2014 | Taiwan | Pregnant women | 97 | 35.1 | All F | 7.2 (ND–19.8) | 10.8 (2.2–17.7) | 9.5 (3.2–16.4) | fT4/TT4 TT3 TSH |
Pearson's correlations |
| Dirtu et al. (2013) | 2009–2012 | Belgium | Overweight and obese individuals and control (lean individuals) | 152 | Median 41 | M: 46; F: 106 | 3 [2–5] | fT4 TSH |
Multiple linear regression models | ||
| Huang et al. (2017) | 2013 | Taiwan | General population (NAHSIT) | 279 | 53.4 | M: 129; F: 150 | 6.7 (2.5–12.1) | 16.4 (9.8–30.1) | 10.2 (5.6–17) | fT4/TT4 TT3 TSH |
Multiple linear regression models |
| Meeker et al. (2011) | 2007–2008 | United States | General population (NHANES) | 1346 | 2.1 (0.8–5.4) | 20 (0.92–46) | 11.3 (5.2–25.6) | fT4/TT4 TSH |
Multiple linear regression models | ||
| Park et al. (2017) | 2012–2014 | Korea | General population (Korean National Environmental Health Survey) | 6003 | M: 2638; F: 3365 | 19.3 (10.7–21.1) | 13.2 (7.7–22.4) | TT4 TT3 TSH |
Multiple linear regression models | ||
DEHP, diethylhexyl phthalate; CI, confidence interval; MEHP, monoethylhexyl phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono (2-ethyl-5-oxohexyl) phthalate; fT4, free thyroxine; TSH, thyrotropin; TT4, total, thyroxine; LOD, lower limit of detection; TT3, total triiodothyronine; ND, not detected.
Correlation between MEHP exposure and thyroid function
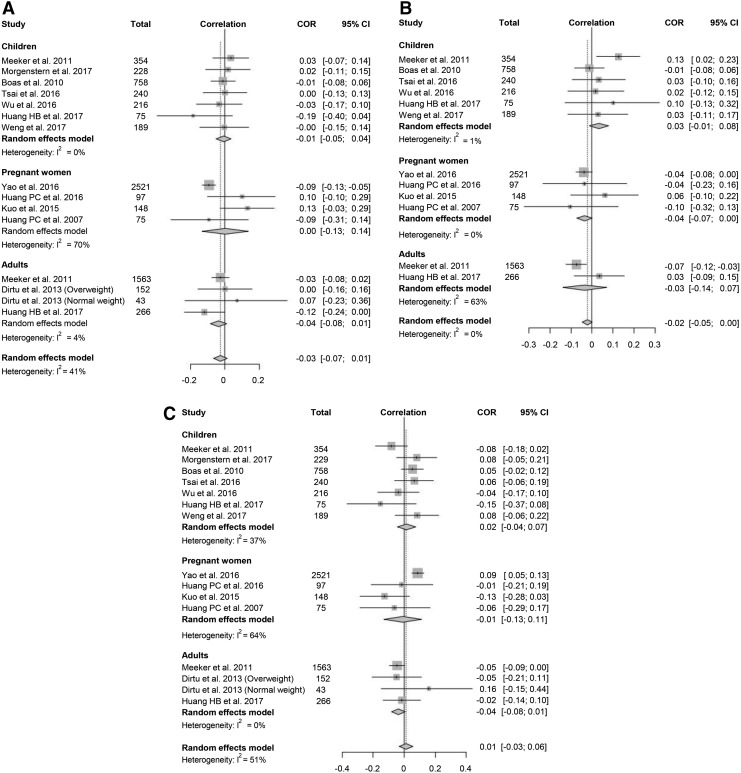
A total of 12 studies provided data suitable for a meta-analysis of the correlation between urine MEHP concentration and thyroid function. Data on fT4 and TSH were available in all 12 studies, while data on TT4 were available in 10/12 studies (Fig. 2). The analysis between MEHP and TT4 showed a negative correlation, and the pooled correlation coefficient was −0.02 [CI −0.05 to 0.00] without significant heterogeneity (I2 = 0%). The funnel plot for MEHP and TT4 was asymmetrical (Supplementary Fig. S1), and the p-value for Egger's test was 0.04. However, funnel plots for each subgroup did not show remarkable asymmetry (Supplementary Fig. S1). Subgroup analysis showed that MEHP was significantly associated with TT4 in pregnant women but not in adults and children. MEHP was not associated with fT4 and TSH (Fig. 2).
FIG. 2.
Forest plots of the correlation coefficient with corresponding confidence intervals (CIs) for the correlation between monoethylhexyl phthalate (MEHP) and thyroid hormone. (A) Correlation between MEHP and thyrotropin (TSH). (B) Correlation between MEHP and free thyroxine (fT4). (C) Correlation between MEHP and total thyroxine (TT4).
Correlation between MEHHP exposure and thyroid function
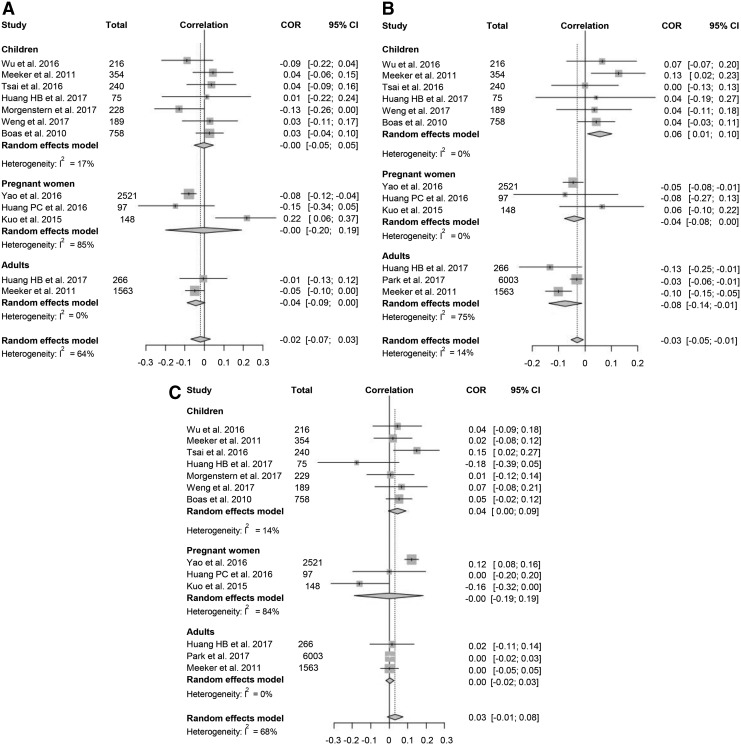
For a meta-analysis of the association between urine MEHHP concentration and thyroid function, 11 studies were included. Data for fT4 and TT4 were available in 10 studies, while data for TSH were available in all 11 studies. The analysis showed that the urinary MEHHP concentration was negatively associated with TT4 (pooled correlation coefficient −0.03 [CI −0.05 to −0.01], I2 = 14%; Fig. 3). Because the studies with fT4 were significantly heterogeneous (I2 = 64%), sensitivity analysis for fT4 was performed, and one outlier study was found (21). When this study was excluded, MEHHP was significantly associated with fT4 (pooled correlation coefficient −0.04 [CI −0.08 to 0.00], I2 = 39%). Subgroup analysis showed that MEHHP was negatively correlated with fT4/TT4 in adults, and the pooled correlation coefficients for fT4 and TT4 were −0.04 [CI −0.09 to 0.00] and −0.08 [CI −0.14 to −0.01], respectively. On the other hand, the MEHHP concentration in children was positively correlated with TT4 and TSH, and the pooled correlation coefficient for TT4 and TSH was 0.06 [CI 0.01–0.10] and 0.04 [CI 0.00–0.09], respectively. The funnel plot analysis and Egger's test revealed no significant publication bias (Supplementary Fig. S2).
FIG. 3.
Forest plots of the correlation coefficient with corresponding CIs for the correlation between mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and thyroid hormone. (A) Correlation between MEHHP and TSH. (B) Correlation between MEHHP and fT4. (C) Correlation between MEHHP and TT4.
Correlation between MEOHP exposure and thyroid function
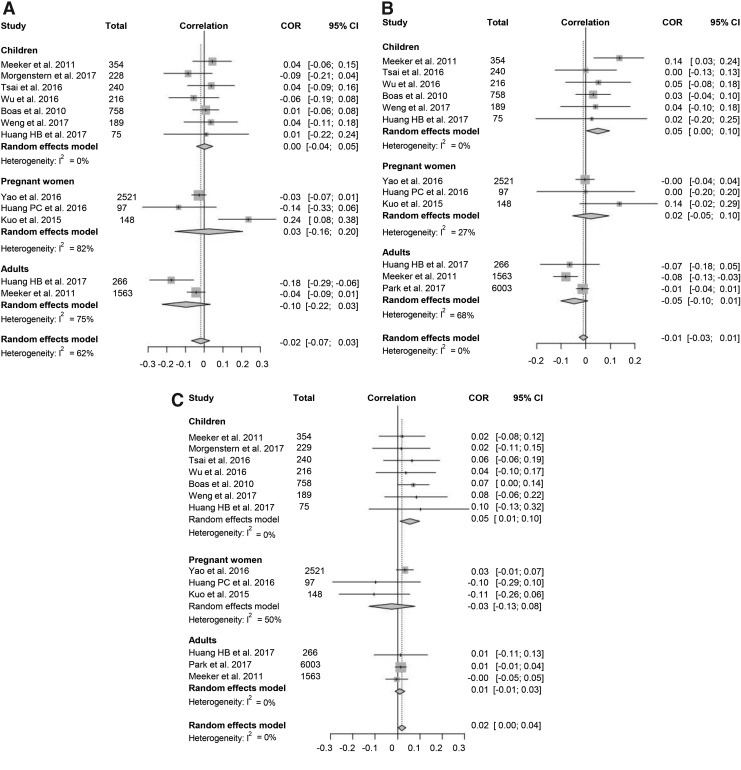
For a meta-analysis of the correlation between urine MEOHP and thyroid function, 11 studies met the eligibility criteria. Data for fT4 and TT4 were available in 10 studies, while data for TSH were available in all 11 studies (Fig. 4). A modest positive correlation between MEOHP and TSH was found (pooled correlation coefficient 0.02 [CI 0.00–0.04]), whereas no correlation between MEOHP and fT4/TT4 was noted. Because of significant heterogeneity of the analysis with fT4 (I2 = 53%), a sensitivity analysis was conducted where one outlier study was found (21). When this study was excluded, the heterogeneity disappeared, and the pooled correlation coefficient changed significantly (pooled correlation coefficient −0.03 [CI −0.05 to −0.01], I2 = 25%). In the subgroup analysis, MEOHP exposure in children was positively correlated with TT4 and TSH, and the pooled correlation coefficient for TT4 and TSH was 0.05 [CI 0.00–0.10] and 0.05 [0.01–0.10], respectively. The funnel plot was asymmetrical (Supplementary Fig. S3), but p-values for Egger's test were >0.05, suggesting no significant publication bias.
FIG. 4.
Forest plots of the correlation coefficient with corresponding CIs for the correlation between mono (2-ethyl-5-oxohexyl) phthalate (MEOHP) and thyroid hormone. (A) Correlation between MEOHP and TSH. (B) Correlation between MEOHP and fT4. (C) Correlation between MEOHP and TT4.
Discussion
The meta-analysis conducted in this study demonstrated that urinary MEHP and MEHHP concentrations were negatively correlated with serum TT4 concentration, and urinary MEOHP concentrations were positively correlated with serum TSH concentration. Furthermore, sensitivity analysis showed that urinary MEHHP and MEOHP concentrations were also negatively correlated with serum fT4 concentration. Interestingly, subgroup analysis showed a significant negative correlation between DEHP metabolites and TT4 in adults but a significant positive correlation between DEHP metabolites and TT4/TSH in children.
Previous experimental studies have suggested that DEHP can affect the function of the HPT axis. DEHP exposure in rat and zebrafish decreased fT4/TT4 concentration without any change in the serum TSH concentration (12,13). DEHP was associated with an antagonistic activity for thyroid hormone action in cell culture experiments (29,30). DEHP can disrupt the thyroid hormone system through various pathways, including thyroid hormone synthesis, transport, and metabolism. DEHP exposure induced histological changes of the thyroid gland in rats (12,31) and affected the expression of NIS in zebrafish (13), suggesting an effect of DEHP on thyroid hormone synthesis. Moreover, DEHP can interfere with thyroid hormone–binding proteins. DEHP exposure decreased transthyretin, a main thyroid hormone–binding protein in rats and zebrafish (12,13). However, the effects of DEHP on binding proteins in human is equivocal, and no association was found between DEHP exposure and thyroxine-binding globulin, the major thyroid hormone–binding protein in humans (19). Moreover, in this study, the association between DEHP exposure and fT4 was as relevant as that with TT4. Therefore, at least in humans, the influence of DEHP on binding proteins does not seem to be the main mechanism by which it affects thyroid hormone levels. Lastly, DEHP exposure can increase thyroid hormone metabolism. DEHP exposure affected deiodinase 1 activity and increased UGT in rat and zebrafish (12–14).
DEHP is rapidly metabolized when absorbed into the human body (32,33). Thus, DEHP metabolites, not the parent compound, may affect thyroid hormone levels. There is a wide variety of DEHP metabolites. However, only three of these were analyzed. Because each metabolite can have a different effect on thyroid hormone economy, this is a limitation of this study. Other studies measured various DEHP metabolites, calculated the sum of DEHP metabolites (ΣDEHP metabolites) (19,27), and reported that ΣDEHP metabolites were also negatively correlated with TT4. However, only three studies presented ΣDEHP metabolites, and each study used different types of DEHP metabolites when calculating ΣDEHP metabolites (19,27,34). Therefore, a meta-analysis was not conducted for that.
The effects of DEHP on the HPT axis in pregnant women can be different from those in the general population. Cross-sectional studies suggested an association between DEHP exposure and thyroid dysfunction in pregnant women, but this was not consistent (18,21,22,26). Therefore, a subgroup analysis was performed for pregnant women, and only the association between MEHP/MEHHP and TT4 was significant. In studies of pregnant women, the results can vary based on the timing of sample collection because thyroid function changes with gestational age. Therefore, to evaluate the effects of DEHP exposure, DEHP metabolites and thyroid hormone concentrations should be measured repeatedly at defined gestational ages. Longitudinal studies with repeated measurements revealed that MEHP was associated positively with TT4 and negatively with TSH in pregnant women (17), findings that contrast with the current results and suggesting that the effects of DEHP may vary depending on when the woman was exposed during pregnancy.
DEHP exposure of pregnant women can affect thyroid function or neurodevelopment of the baby. Prenatal DEHP exposure is negatively associated with a child's neurodevelopment (35,36). Because thyroid hormones play a pivotal role in neurodevelopment, DEHP-induced thyroid dysfunction is speculated to mediate the effect of DEHP on neurodevelopment. Some researchers investigated the association between maternal DEHP exposure and thyroid hormone levels in cord blood (18,21) or neonate (37), they but found no significant association between them. However, further research is necessary to verify these findings.
The results of studies that investigated the association between DEHP exposure and thyroid hormone levels in children and adolescents were inconsistent (16,19,38–42). In the subgroup analysis of children in this study, MEHHP and MEOHP were positively correlated with TSH and TT4. However, no association was observed between DEHP metabolites and fT4. These differences in the results between children and adults might be because children are less exposed to DEHP than adults. However, previous studies that included both children and adults reported that the urinary concentration of DEHP metabolites was inversely correlated with age (16,19,43). Hence, the difference in results between children and adults may come from the duration of DEHP exposure. Even if the urinary concentration of DEHP metabolites is similar in both adults and children, the duration of exposure to DEHP might be longer in adults than in children.
This meta-analysis has some limitations. First, DEHP metabolites and thyroid hormones were measured by different methods in each study. In addition, some studies corrected for urine dilution by using urine creatinine levels or specific gravity when analyzing Pearson's correlation or regression coefficients, while the others did not. Next, all included studies had a cross-sectional observational design. Because the half-life (<24 h) of DEHP is short (8) and exposure to DEHP can change over time, a single measurement of urinary DEHP metabolites cannot represent the total exposure throughout life. However, the urine concentration of phthalates with single urine spot can moderately represent long-term exposure (44,45). Even if the degree of DEHP exposure is constant, the duration of DEHP exposure may be different, an aspect that cannot be considered in the analysis. Finally, the analytical method can miss a nonlinear association between endocrine disrupting chemicals and thyroid function. Endocrine-disrupting chemicals may have non-monotonic or U-shape dose–response curves (4,46). Thus, a low or specific concentration may be more harmful than a higher concentration.
Urinary DEHP metabolites have been shown to have a strong correlation with other phthalates metabolites or bisphenol A (27). Furthermore, people are simultaneously exposed to various endocrine-disrupting chemicals. The mixture effects or endocrine-disrupting chemicals, including DEHP, on thyroid function can differ from the effects of DEHP alone (47). Further studies are needed in this area.
Conclusions
This meta-analysis shows that DEHP exposure can decrease TT4 and increase TSH. The results suggest that DEHP can affect thyroid function in children, adults, and pregnant women. Thus, exposure to DEHP should be avoided or reduced.
Acknowledgments
This research was supported by a grant (16182MFDS392) from Ministry of Food and Drug Safety in 2016. We are grateful for the statistical support from the Medical Research Collaborating Center, Seoul National University Hospital.
Author Disclosure Statement
All authors have nothing to disclose.
References
- 1. Garmendia Madariaga A, Santos Palacios S, Guillen-Grima F, Galofre JC. 2014. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab 99:923–931 [DOI] [PubMed] [Google Scholar]
- 2. Chaker L, Bianco AC, Jonklaas J, Peeters RP. 2017. Hypothyroidism. Lancet 390:1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization 2012. State of the Science of Endocrine Disrupting Chemicals—2012. Available at www.who.int/ceh/publications/endocrine/en (accessed January13, 2019)
- 4. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. 2015. EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36:E1–E150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agency for Toxic Substances & Disease Registry (ATSDR) 2002. Toxicological Profile for Di(2-Ethylheyl) Phthalate (DEHP). Department of Health and Human Servies, Atlanta, GA [Google Scholar]
- 6. Guo Y, Zhang Z, Liu L, Li Y, Ren N, Kannan K. 2012. Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J Agric Food Chem 60:6913–6919 [DOI] [PubMed] [Google Scholar]
- 7. Koch HM, Calafat AM. 2009. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 364:2063–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koch HM, Preuss R, Angerer J. 2006. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—an update and latest results. Int J Androl 29:155–165; discussion 181–185 [DOI] [PubMed] [Google Scholar]
- 9. Center for Disease Control and Prevention (CDC) 2018. Fourth National Report on Human Exposure to Environmental Chemicals. Available at www.cdc.gov/exposurereport/index.html (accessed January13, 2019)
- 10. Canada H. 2013. Second Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 2 (2009–2011). Available at www.healthyenvironmentforkids.ca/sites/healthyenvironmentforkids.ca/files/HumanBiomonitoringReport__EN.pdf (accessed January13, 2019)
- 11. Boas M, Feldt-Rasmussen U, Main KM. 2012. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355:240–248 [DOI] [PubMed] [Google Scholar]
- 12. Liu C, Zhao L, Wei L, Li L. 2015. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ Sci Pollut Res Int 22:12711–12719 [DOI] [PubMed] [Google Scholar]
- 13. Zhai W, Huang Z, Chen L, Feng C, Li B, Li T. 2014. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS One 9:e92465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong X, Dong J, Zhao Y, Guo J, Wang Z, Liu M, Zhang Y, Na X. 2017. Effects of long-term in vivo exposure to di-2-ethylhexylphthalate on thyroid hormones and the TSH/TSHR signaling pathways in Wistar rats. Int J Environ Res Public Health 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meeker JD, Calafat AM, Hauser R. 2007. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115:1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meeker JD, Ferguson KK. 2011. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect 119:1396–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. 2016. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environ Health Perspect 124:1808–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao HY, Han Y, Gao H, Huang K, Ge X, Xu YY, Xu YQ, Jin ZX, Sheng J, Yan SQ, Zhu P, Hao JH, Tao FB. 2016. Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 157:42–48 [DOI] [PubMed] [Google Scholar]
- 19. Huang HB, Pan WH, Chang JW, Chiang HC, Guo YL, Jaakkola JJ, Huang PC. 2017. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ Res 153:63–72 [DOI] [PubMed] [Google Scholar]
- 20. Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, Meeker JD. 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuo FC, Su SW, Wu CF, Huang MC, Shiea J, Chen BH, Chen YL, Wu MT. 2015. Relationship of urinary phthalate metabolites with serum thyroid hormones in pregnant women and their newborns: a prospective birth cohort in Taiwan. PLoS One 10:e0123884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. 2007. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod 22:2715–2722 [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269, W64 [DOI] [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–94 [DOI] [PubMed] [Google Scholar]
- 25. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to Meta-Analysis. John Wiley, Chichester, United Kingdom [Google Scholar]
- 26. Huang PC, Tsai CH, Liang WY, Li SS, Huang HB, Kuo PL. 2016. Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PLoS One 11:e0159398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park C, Choi W, Hwang M, Lee Y, Kim S, Yu S, Lee I, Paek D, Choi K. 2017. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population—Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci Total Environ 584–585:950–957 [DOI] [PubMed] [Google Scholar]
- 28. Viechtbauer W. 2010. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 36:1–48 [Google Scholar]
- 29. Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, Wang X. 2009. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett 191:9–14 [DOI] [PubMed] [Google Scholar]
- 30. Ghisari M, Bonefeld-Jorgensen EC. 2009. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189:67–77 [DOI] [PubMed] [Google Scholar]
- 31. Howarth JA, Price SC, Dobrota M, Kentish PA, Hinton RH. 2001. Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol Lett 121:35–43 [DOI] [PubMed] [Google Scholar]
- 32. Wittassek M, Angerer J. 2008. Phthalates: metabolism and exposure. Int J Androl 31:131–138 [DOI] [PubMed] [Google Scholar]
- 33. Koch HM, Bolt HM, Angerer J. 2004. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 78:123–130 [DOI] [PubMed] [Google Scholar]
- 34. Dirtu AC, Geens T, Dirinck E, Malarvannan G, Neels H, Van Gaal L, Jorens PG, Covaci A. 2013. Phthalate metabolites in obese individuals undergoing weight loss: urinary levels and estimation of the phthalates daily intake. Environ Int 59:344–353 [DOI] [PubMed] [Google Scholar]
- 35. Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, Hong YC, Chang N, Kim BN. 2011. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children's Environmental Health (MOCEH) study. Environ Health Perspect 119:1495–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, Meeker JD. 2013. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ 461–462:386–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minatoya M, Naka Jima S, Sasaki S, Araki A, Miyashita C, Ikeno T, Nakajima T, Goto Y, Kishi R. 2016. Effects of prenatal phthalate exposure on thyroid hormone levels, mental and psychomotor development of infants: The Hokkaido Study on Environment and Children's Health. Sci Total Environ 565:1037–1043 [DOI] [PubMed] [Google Scholar]
- 38. Morgenstern R, Whyatt RM, Insel BJ, Calafat AM, Liu X, Rauh VA, Herbstman J, Bradwin G, Factor-Litvak P. 2017. Phthalates and thyroid function in preschool age children: sex specific associations. Environ Int 106:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai HJ, Wu CF, Tsai YC, Huang PC, Chen ML, Wang SL, Chen BH, Chen CC, Wu WC, Hsu PS, Hsiung CA, Wu MT. 2016. Intake of phthalate-tainted foods and serum thyroid hormones in Taiwanese children and adolescents. Sci Rep 6:30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu W, Zhou F, Wang Y, Ning Y, Yang JY, Zhou YK. 2017. Exposure to phthalates in children aged 5–7 years: associations with thyroid function and insulin-like growth factors. Sci Total Environ 579:950–956 [DOI] [PubMed] [Google Scholar]
- 41. Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, Juul A, Main KM. 2010. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect 118:1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weng TI, Chen MH, Lien GW, Chen PS, Lin JC, Fang CC, Chen PC. 2017. Effects of gender on the association of urinary phthalate metabolites with thyroid hormones in children: a prospective cohort study in Taiwan. Int J Environ Res Public Health 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 112:1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 106:257–269 [DOI] [PubMed] [Google Scholar]
- 46. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33:378–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kortenkamp A. 2014. Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr Opin Pharmacol 19:105–111 [DOI] [PubMed] [Google Scholar]