Abstract
This study examined a range of anthropometric indices and their relationships with metabolic syndrome (MetS). Despite recommendations that central obesity assessment should be employed as a marker of metabolic health, there is no consensus regarding the protocol for measurement. The present study included 720 men aged 71 ± 8 years and 919 women aged 71 ± 7 years from a rural village. We examined the relationship between anthropometric indices {e.g., body mass index (BMI), waist-to-height ratio (WHtR), waist-to-hip ratio (WHpR)}, and MetS based on the modified criteria of the National Cholesterol Education Program’s Adult Treatment Panel (NCEP-ATP) III report in a cross-sectional (N = 1,639) and cohort (N = 377) data. A receiver operating curve (ROC) analysis was performed to determine the optimal cut-off value and best discriminatory value of each of these anthropometric indices to predict MetS. In the cross-sectional study, WHtR as well as BMI and WHpR showed significantly predictive abilities for MetS in both genders; and WHtR showed the strongest predictive ability for the presence of MetS. Also in the cohort study, WHtR as well as BMI and WHpR showed significantly predictive abilities for incident MetS in both genders, and in men WHtR showed the strongest predictive ability for incident MetS, but in women BMI showed the strongest predictive ability. In the cross-sectional study, the optimal WHtR cutoff values were 0.52 (sensitivity, 71.0%; specificity, 77.9%) for men and 0.53 (sensitivity, 79.8%; specificity, 75.7%) for women. In the cohort study, the optimal WHtR values were 0.50 (sensitivity, 60.7%; specificity, 73.2%) for men and 0.50 (sensitivity, 75.0%; specificity, 56.1%) for women. Increased WHtR was significantly and independently associated with prevalence of MetS in both genders. These results suggest that WHtR is a useful screening tool for determining metabolic risk in Japanese elderly community dwelling individuals.
Introduction
The underlying mechanism of metabolic syndrome (MetS), or a clustering of cardiovascular risk factors, such as hypertension, glucose intolerance, hypertriglyceridemia, and low high-density lipoprotein cholesterol (HDL-C) levels, is insulin resistance, which is also known as a pre-disease state that leads to an increased risk of cardiovascular disease (CVD) [1], [2], and type 2 diabetes [3], [4]. The incidence of MetS is increasing worldwide with the continuous increase in obesity prevalence [5]. Obesity is the most important underlying cause of insulin resistance, which has also been suggested as a pathogenetic mechanism of abdominal obesity because visceral fat plays an important role in lipid metabolism and insulin sensitivity [6], [7], [8], [9]. Various obesity-related anthropometric indices, such as body mass index (BMI), waist circumference (WC), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHpR), have been used to predict incident MetS in epidemiological studies [10], [11], [12], [13], [14]. BMI is a measurement of body fat by height and weight, while WC reflects abdominal obesity. WHtR and WHpR further reflect the fat distribution by WC, and all three indices are considered to be specific options to evaluate abdominal fat.
To address this hypothesis, we investigated the relationship between baseline visceral obesity indices and potential risk factors such as age, smoking status, drinking status, exercise habits, presence of CVD, low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), estimated glomerular filtration ratio (eGFR), and incident MetS using prospective cohort data from community-dwelling elderly individuals.
Materials & methods
Subjects
The subjects of this study population were recruited from the Nomura Health and Welfare Center in a rural town in Ehime prefecture of Japan through annual health checkup process closely related to the area (17). This study was started in 2014, and included 1639 community-dwelling participants aged 55–95 years. Follow-up assessment cycles are performed every three years.
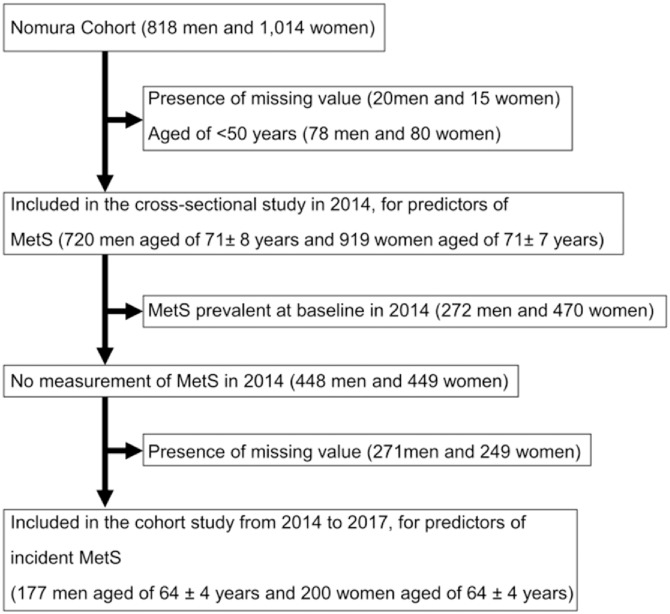
In the present study, we included data from the assessment cycles of 2014 and 2017. Blood samples were only obtained from respondents who participated in the medical interview at baseline. For the cross-sectional analyses, data of the 2014 cycle (n = 1639) were used as all five components of MetS were measured in this cycle. For the longitudinal analyses, a sub-cohort of the 2014 cycle was used including only participants in whom MetS was not prevalent at baseline in 2014 (n = 377). Fig 1 shows a flowchart of the inclusion of participants.
Fig 1. Flowchart.
For the cross-sectional analyses, data of the 2014 cycle (n = 1,639) that were used in this cycle were measured. For the longitudinal analyses, only participants in whom MetS was not prevalent at baseline in 2014 were included in the longitudinal analyses (n = 377).
This study complies with the Declaration of Helsinki, written informed consent was obtained from each subject, and the study was approved by the Ehime University Medical School Ethics Committee. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee in which the study was conducted. (IRB Approval number: 1402009).
Evaluation of confounding factors
Information on demographic characteristics and risk factors was collected using clinical files. Body mass index (BMI) was calculated by dividing weight (kilograms) by the square of height (meters). WHtR was calculated as WC (cm)/height (cm). WHpR was calculated as WC (cm)/hip circumference (cm). Other characteristics such as exercise, smoking habit, alcohol consumption, and medication, were investigated by individual interviews conducted using a structured questionnaire. Smoking habit was defined as the number of cigarette packs per day multiplied by the pack years (pack year), and participants were classified into never smokers, past smokers, light smokers (<30 pack year), and heavy smokers (≥30 pack year) [15]. Daily drinking status was measured using the Japanese alcoholic beverage unit equivalent to 22.9 g of ethanol, and the participants were classified into never drinkers, occasional drinkers (<1 unit/day), daily light drinkers (<2 unit /day), and daily heavy drinkers (≥2 unit/day) [16]. We measured blood pressure (BP) on the right upper arm of the subjects with an appropriate-sized cuff in the sedentary position using an automatic oscillometric blood pressure recorder after having rested for at least 5 min. For analysis, the mean of two consecutive measurements was used.
For all these individuals, triglycerides (TG), HDL-C, low-density lipoprotein cholesterol (LDL-C), hemoglobin A1c (HbA1c), serum uric acid (SUA), and creatinine (Cr) were measured during an overnight fast of over 11 hours. eGFR was calculated using CKD-EPI equations modified by the coefficient of Japan (eGFRCKDEPI): Male, Cr ≤0.9 mg/dl, 141 × (Cr/0.9) –0.411 × 0.993 age × 0.813; Cr >0.9 mg/dl, 141 × (Cr/0.9) –1.209 × 0.993 age × 0.813; Female, Cr ≤0.7 mg/dl, 144 × (Cr/0.7) –0.329 × 0.993 age × 0.813; Cr >0.7 mg/dl, 144 × (Cr/0.7) –1.209 × 0.993 age × 0.813 (18).
Criteria for clinical diagnosis of MetS
Based on the modified criteria of the National Cholesterol Education Program’s Adult Treatment Panel (NCEP-ATP) III report [17], MetS was defined as subjects having at least three of the following five conditions: 1) abdominal obesity of waist circumference ≧85 cm for men and ≧80 cm for women based on the adjusted waist circumference criteria in Japan [18]; 2) high BP with a systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure (DBP) ≥85 mmHg, and/or drug treatment for elevated blood pressure; 3) hypertriglyceridemia with a TG level ≥150 mg/dL; 4) low HDL cholesterolemia with a HDL-C <40 mg/dL for men and <50 mg/dL for women, and/or drug treatment for dyslipidemia; and 5) high fasting glucose with a HbA1c ≥5.6% (comparable with fasting plasma glucose (FPG) level ≥100 mg/dL [19] because FPG was not measured in this study) and/or drug treatment for elevated blood sugar.
Statistics
Unless otherwise specified, data are presented as the mean ± standard deviation (SD) and for parameters with non-normal distributions (i.e., TG, HbA1c) data are shown as median (interquartile range) values. For all analyses, parameters with non-normal distributions were used after log-transformation. Statistical analysis was performed using IBM SPSS Statistics Version 21 (Statistical Package for Social Science Japan, Inc., Tokyo, Japan). Subjects were divided into two groups based on gender and differences among the groups were analyzed by Student’s t-test for continuous variables or the χ2 -test for categorical variables. Multiple logistic linear regression analysis was used to evaluate the contribution of the baseline WHtR and confounding factors (i.e., gender, age, exercise habit, smoking habits, alcohol consumption, and prevalence of CVD, LDL-C, SUA, and eGFR) for prevalence of MetS in the cross-sectional study and incidence of MetS in the cohort study. In addition, areas under the receiver operating characteristic (ROC) curves were determined for each variable to identify the predictors of MetS. An ROC curve is a plot of sensitivity (true positive) versus 1–specificity (false positive) for each potential marker tested. Areas under the ROC curves are provided with standard errors. The area under the ROC curve is a summary of the overall diagnostic accuracy of the test. The best marker has an ROC curve shifted to the left with area under the curve close to unity. Predictive values were calculated as sensitivity/{sensitivity+(1 − specificity)} (positive predictive value) and specificity/{(1 − sensitivity)+specificity} (negative predictive value). To determine the optimal cutoffs for the MetS, the Youden index (sensitivity + specificity − 1) was calculated, and the corresponding value for the maximum of the Youden index was considered as the optimal cutoff point. A p-value <0.05 was considered significant.
Results
Baseline characteristics of study subjects categorized by gender
Baseline characteristics of the subjects categorized by gender are illustrated in Table 1. The study included 720 men aged 71 ± 8 (range, 55–95) years and 919 women aged 71 ± 7 (range, 55–90) years. BMI, WC, WHpR, smoking status, drinking status, prevalence of CVD, DBP, TG, HbA1c, presence of antidiabetic medication, and SUA were significantly higher in men, but WHtR, HDL-C, LDL-C, presence of antidyslipidemic medication, and eGFR were significantly lower. There were no differences in age, exercise habits, SBP, and presence of antihypertensive medication. In our study, prevalence of MetS was 37.8% in men and 51.1% in women, and mean (± SD) number of its component was 2.3 (± 1.1) in men and 2.6 (± 1.2) in women.
Table 1. Baseline characteristics of study subjects.
| Baseline Characteristics N = 1,639 | Men N = 720 | Women N = 919 | P-value* |
|---|---|---|---|
| Age (years) | 71 ± 8 | 71 ± 7 | 0.602 |
| Body mass index (kg/m2) | 23.1 ± 2.9 | 22.6 ± 3.2 | <0.001 |
| Waist circumference (cm) | 82.4 ± 8.1 | 80.5 ± 9.0 | <0.001 |
| Waist/height ratio | 0.51 ± 0.05 | 0.54 ± 0.06 | <0.001 |
| Waist/hip ratio | 0.90 ± 0.06 | 0.89 ± 0.06 | <0.001 |
| Smoking habit (never/past/light/heavy (%)) | 41.7/40.6/4.3/13.5 | 96.8/2.1/0.7/0.4 | <0.001 |
| Drinking Status (never/occasional/light/heavy (%)) | 24.7/22.1/16.8/36.4 | 71.6/22.1/4.6/1.7 | <0.001 |
| Exercise habits (%) | 36.9 | 38.6 | 0.505 |
| Cardiovascular disease (%) | 10.3 | 4.4 | <0.001 |
| Systolic blood pressure (mmHg) | 137 ± 17 | 137 ± 18 | 0.714 |
| Diastolic blood pressure (mmHg) | 80 ± 10 | 77 ± 10 | <0.001 |
| Antihypertensive medication (%) | 48.1 | 45.3 | 0.272 |
| Triglycerides (mg/dl) | 90 (68–131) | 87 (65–117) | <0.001 |
| HDL cholesterol (mg/dl) | 62 ± 16 | 68 ± 17 | <0.001 |
| LDL cholesterol (mg/dl) | 114 ± 28 | 124 ± 29 | <0.001 |
| Antidyslipidemic medication (%) | 14.0 | 30.0 | <0.001 |
| Hemoglobin A 1c (%) | 5.7 (5.4–6.0) | 5.7 (5.5–5.9) | 0.036 |
| Antidiabetic medication (%) | 13.6 | 5.5 | <0.001 |
| Serum uric acid (mg/dL) | 6.0 ± 1.3 | 4.7 ± 1.1 | <0.001 |
| Estimated GFR (ml/min/1.73 m2/year) | 69.4 ± 12.1 | 71.8 ± 10.8 | <0.001 |
| Number of metabolic syndrome component | 2.3 ± 1.1 | 2.6 ± 1.2 | <0.001 |
| Metabolic syndrome (%) | 37.8 | 51.1 | <0.001 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; GFR glomerular filtration ratio. Data presented are mean ± standard deviation. Data for triglycerides and HemoglobinA1c is skewed, and presented as median (interquartile range) values.
* P-value: Student’s t-test for the continuous variables or the χ2 -test for the categorical variables. Bold values indicate significance (p<0.05).
Results of the ROC curve analysis to identify optimal obesity indices to discriminate subjects with MetS in the cross-sectional and cohort studies
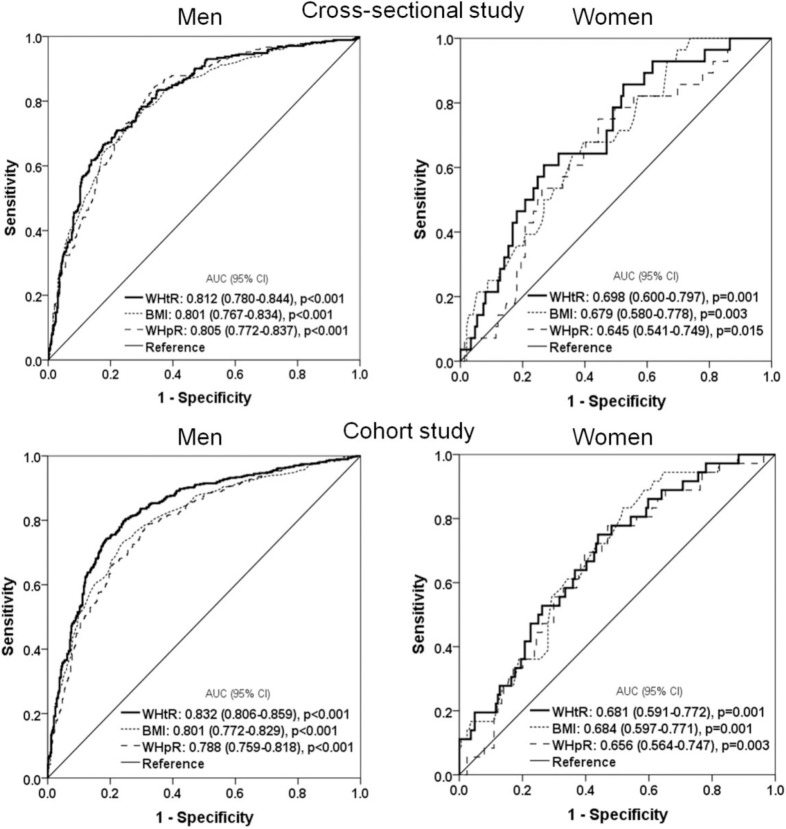
Fig 2 shows the AUC for WHtR, BMI, and WHpR for each MetS in both genders using ROC analyses. In the cross-sectional study, WHtR as well as BMI and WHpR showed significantly predictive ability for MetS in both genders, with WHtR showing the strongest predictive ability. Also in the cohort study, WHtR as well as BMI and WHpR showed a significantly high predictive ability for incident MetS in both genders. In men WHtR showed the strongest predictive ability for incident MetS, but in women BMI showed the strongest predictive ability.
Fig 2. Area under the receiver operating curve (AUC) values (95% CI) for selected obesity measurements to discriminate subjects with metabolic syndrome in the cross-sectional and cohort study.
Non-adjusted odds ratios and 95% CI for MetS and its components by quartile of WHtR in the cross-sectional and cohort study
The univariate effect of WHtR on MetS and its components is presented in Table 2. In the cross-sectional study, WHtR was significantly associated with presence of MetS and all of its components in both genders. In the cohort study, WHtR was significantly and independently associated with incident MetS and central obesity in both genders.
Table 2. Non-adjusted odds ratios and 95% CI for metabolic syndrome and its components of subjects according to baseline waist to height ratio in the cross-sectional and cohort studies.
| Cross-sectional study N = 1,639 | Men N = 720 | Women N = 919 | ||||
|---|---|---|---|---|---|---|
| Yes/No | Odds ratio (95% CI) | P-value* | Yes/No | Odds ratio (95% CI) | P-value | |
| Metabolic syndrome | 272/448 | 3.62 (2.95–4.43) | <0.001 | 470/449 | 3.46 (2.93–4.08) | <0.001 |
| Central obesity | 254/466 | 15.8 (10.6–23.3) | <0.001 | 471/448 | 19.9 (13.4–29.5) | <0.001 |
| Elevated blood pressure | 572/148 | 1.62 (1.34–1.97) | <0.001 | 716/203 | 1.66 (1.43–1.91) | <0.001 |
| Elevated triglycerides | 128/592 | 1.62 (1.35–1.95) | <0.001 | 112/807 | 1.87 (1.50–2.33) | <0.001 |
| Lowering HDL cholesterolemia | 139/581 | 1.62(1.35–1.95) | <0.001 | 349/570 | 1.54 (1.35–1.75) | <0.001 |
| Elevated hemoglobin A 1c | 527/193 | 1.24 (1.05–1.47) | 0.011 | 704/215 | 1.17 (1.02–1.34) | 0.026 |
| Cohort study N = 377 | Men N = 177 | Women N = 200 | ||||
| Metabolic syndrome | 28/149 | 1.99 (1.21–3.28) | 0.007 | 36/164 | 2.02 (1.34–3.06) | 0.001 |
| Central obesity | 41/136 | 5.38 (2.96–9.77) | <0.001 | 54/146 | 5.51 (3.29–9.23) | <0.001 |
| Elevated blood pressure | 125/52 | 1.18 (0.76–1.82) | 0.459 | 117/83 | 1.01 (0.74–1.39) | 0.956 |
| Elevated triglycerides | 21/156 | 1.03 (0.57–1.86) | 0.930 | 13/187 | 1.03 (0.55–1.93) | 0.933 |
| Lowering HDL cholesterolemia | 16/161 | 0.75 (0.36–1.57) | 0.450 | 31/169 | 1.50 (0.98–2.28) | 0.061 |
| Elevated hemoglobin A 1c | 107/70 | 0.89 (0.60–1.31) | 0.551 | 129/71 | 0.82 (0.59–1.13) | 0.219 |
CI, confidence interval.
*Bold values indicate significance (p<0.05).
Multivariate-adjusted odds ratios and 95% CI for MetS and its components by quartile of WHtR in the cross-sectional and cohort studies
To further investigate whether WHtR can explain MetS and its components independently of other confounding factors, a multiple logistic regression analysis using MetS and its components as dependent variables and various confounding factors (e.g., age, smoking status, drinking status, exercise habits, presence of CVD, LDL-C, SUA, and eGFR) as explanatory variables was performed with subjects categorized by gender (Table 3). In both the cross-sectional and cohort studies, increased WHtR showed an increasing trend with increased prevalence of MetS in both genders.
Table 3. Multivariate-adjusted odds ratios and 95% CI for metabolic syndrome and its components of subjects according to baseline waist to height ratio in the cross-sectional and cohort studies.
| Cross-sectional study N = 1,639 | Men N = 720 | Women N = 919 | ||||
|---|---|---|---|---|---|---|
| Yes/No | Odds ratio (95% CI) | P-value* | Yes/No | Odds ratio (95% CI) | P-value* | |
| Metabolic syndrome | 272/448 | 3.82 (3.08–4.72) | <0.001 | 470/449 | 3.33 (2.81–3.96) | <0.001 |
| Central obesity | 254/466 | 19.5 (12.6–30.1) | <0.001 | 471/448 | 31.0 (19.5–49.2) | <0.001 |
| Elevated blood pressure | 572/148 | 1.57 (1.28–1.93) | <0.001 | 716/203 | 1.43 (1.23–1.68) | <0.001 |
| Elevated triglycerides | 128/592 | 1.59 (1.30–1.95) | <0.001 | 112/807 | 1.75 (1.38–2.21) | <0.001 |
| Lowering HDL cholesterolemia | 139/581 | 1.70 (1.40–2.07) | <0.001 | 349/570 | 1.48 (1.28–1.70) | <0.001 |
| Elevated hemoglobin A1c | 527/193 | 1.24 (1.04–1.47) | 0.016 | 704/215 | 1.10 (0.95–1.28) | 0.200 |
| Cohort study N = 377 | Men N = 177 | Women N = 200 | ||||
| Metabolic syndrome | 28/149 | 1.94 (1.14–3.32) | 0.015 | 36/164 | 1.93 (1.23–3.03) | 0.004 |
| Central obesity | 41/136 | 5.75 (3.05–10.8) | <0.001 | 54/146 | 8.28 (4.28–16.0) | <0.001 |
| Elevated blood pressure | 125/52 | 1.07 (0.68–1.69) | 0.769 | 117/83 | 0.88 (0.62–1.24) | 0.467 |
| Elevated triglycerides | 21/156 | 1.07 (0.55–1.90) | 0.960 | 13/187 | 1.09 (0.55–2.19) | 0.803 |
| Lowering HDL cholesterolemia | 16/161 | 1.07 (0.48–2.37) | 0.869 | 31/169 | 1.27 (0.82–1.96) | 0.292 |
| Elevated hemoglobin A 1c | 107/70 | 0.90 (0.59–1.40) | 0.648 | 129/71 | 0.83 (0.58–1.17) | 0.280 |
*Multivariate-adjusted for age, smoking status, drinking status, exercise habits, presence of cardiovascular disease, low-density lipoprotein cholesterol, serum uric acid, and estimated GFR. Bold values indicate significance (p<0.05).
Best cutoff values of WHtR to predict MetS in the cross-sectional and cohort studies
In the cross-sectional study, the optimal WHtR cutoff values for predicting MetS according to WHtR were 0.52 (sensitivity, 71.0%; specificity, 77.9%) for men and 0.53 (sensitivity, 79.8%; specificity, 75.7%) for women (Table 4). In the cohort study, the optimal WHtR values were 0.50 (sensitivity, 60.7%; specificity, 73.2%) for men and 0.50 (sensitivity, 75.0%; specificity, 56.1%) for women.
Table 4. Best cutoff values of baseline waist to height ratio to predict metabolic syndrome in the cross-sectional and cohort studies.
| AUC (95% CI) | P-value | Cut off value | Sensitivity | specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Cross-sectional study N = 1639 | |||||||
| Men N = 720 | 0.812 (0.780–0.844) | <0.001 | 0.5185 | 71.0% | 77.9% | 76.3% | 72.9% |
| Women N = 919 | 0.832 (0.806–0.859) | <0.001 | 0.5349 | 79.8% | 75.7% | 76.7% | 78.9% |
| Cohort study N = 377 | |||||||
| Men N = 177 | 0.698 (0.600–0.797) | 0.001 | 0.4991 | 60.7% | 73.2% | 69.4% | 63.1% |
| Women N = 200 | 0.681 (0.591–0.772) | 0.001 | 0.4957 | 75.0% | 56.1% | 62.2% | 69.2% |
AUR, Area under the receiver operating curve; PPV: positive predictive value; NPV: negative predictive value. Bold values indicate significance (p<0.05).
Discussion
In this study where data from the Nomura study of 2014 and 2017 was used, the AUC analyses indicated that WHtR as well as BMI and WHpR had significant predictive ability for MetS in both genders, and that WHtR was significantly and independently associated with the prevalence of MetS in this cross-sectional study as well as the incidence of MetS in this cohort study. The usefulness of this cutoff value as a screening tool for the prediction of MetS was superior to those of BMI and WHpR, which are conventional obesity indices among both genders. This study showed that WHtR might be an appropriate definition from the point of view of knowing the presence and incidence of MetS. To the best of our knowledge, few epidemiologic studies have quantified the relevance between WHtR and incident MetS in Japanese elderly community-dwelling individuals.
This cross-sectional study showed that WHtR as well as BMI and WHpR was useful for predicting MetS, which is consist with Gu et al.’s research [20]. From the AUC analysis, BMI, WC and WHtR were predictive of high metabolic risks in men (0.698, 0.691, and 0.688, respectively), whereas female BMI and WC were similarly predictive of high metabolic risks (0.676 and 0.669) [20]. According to Liu et al. [21], ROC analyses of BMI, WC and WHtR values indicated that the presence of multiple metabolic risk factors can be equally predicted in Chinese adult population, and the AUC values of BMI, WC and WHtR did not differ in men (0.682, 0.661, and 0.651, respectively) and women (0.702, 0.671, and 0.674, respectively). The appropriate cut-off values for BMI, WC and WHtR were 22.9 and 23.3 kg/m2, 91.3 cm and 87.1 cm, and 0.51 and 0.53 in men and women, respectively. Zeng et al. [13] demonstrated that the optimal cut-off values to define overweight or obesity in Chinese adults were approximately 24·0 and 23·0 kg/m2 for BMI, 85·0 and 75·0 cm for WC, and 0·50 and 0·48 for WHtR for men and women, respectively. Ashwell et al. reported that in a systematic review and meta-analysis, WC improved identification of harmful cardiovascular risk outcomes by 3% compared with BMI, and WHtR improved discrimination by 4 to 5%. Moreover, WHtR was a stronger predictor than WC for hypertension, diabetes, CVD and all outcomes in both genders (p<0.005) [22]. The AUC analyses indicated that WHtR may be a more useful global clinical screening tool than WC and has a weighted mean boundary value of 0·5 [23]. In our prospective cohort study, WHtR was a convenient global clinical screening tool with a weighted mean boundary value of 0.50 in both genders.
The mechanisms that lead to increased incidence of MetS in individuals with increased WHtR remain to be clarified. BMI is strongly related to body fat but is not necessarily related to abdominal obesity. WC may accurately reflect the degree of visceral fat, but WC can overestimate or underestimate the risk of CVD as WC does not take into account differences in height [24], [25]. Hsieh et al. showed that people with a prominently large WC may have similar health risks of the above items irrespective of height, but short people have higher health risks than tall people in the moderately large WC population of Japanese men. [24]
Several limitations should be considered in this study. First, our cross-sectional study design does not eliminate the cause and effect on conventional obesity indices and MetS. Second, the measurement of WHtR is based on a single evaluation of the equation, which may introduce a misclassification bias. Third, we could not eliminate the influence that taking medications for hypertension, dyslipidemia, and hyperglycemia has on the present findings. Fourth, as the WC component is included in the MetS definition, the AUC estimate for the WHtR can be disturbed. Fifth, the longitudinal analyses were limited by a smaller sample size and discrepancies in the sequential measurements of the components of MetS in 2014 and 2017. The cohort was slightly younger and healthier compared to participants not included in the longitudinal analyses, this might have caused an underestimation of incident MetS after three years of follow-up. Therefore the demographics and referral source may limit generalizability of the study findings.
Conclusions
The present study showed that anthropometric indices such as WHtR, BMI, and WHpR are strongly associated with incident MetS among Japanese community-dwelling individuals. The underlying mechanism behind this relationship is unknown, but it seems to be independent of confounding factors such as age, exercise habits, smoking habits, drinking status, prevalence of CVD, LDL-C, SUA or eGFR. Thus, WHtR might be an important marker for the assessment of risk and become a therapeutic target for MetS. For healthy community residents, prospective population-based studies are necessary to investigate mechanisms such as effective lifestyle improvement and other interventions to control WHtR in adults.
Supporting information
(ODS)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported in part by a grant-in-aid from the Foundation for Development of Community (2018). No additional external funding was received for this study. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes care. 2005;28(2):385–90. Epub 2005/01/29. . [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288(21):2709–16. Epub 2002/12/04. . [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes care. 2003;26(11):3153–9. Epub 2003/10/28. . [DOI] [PubMed] [Google Scholar]
- 4.de Simone G, Devereux RB, Chinali M, Best LG, Lee ET, Galloway JM, et al. Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the Strong Heart Study. Diabetes care. 2007;30(7):1851–6. Epub 2007/04/19. 10.2337/dc06-2152 . [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. The New England journal of medicine. 2004;350(23):2362–74. Epub 2004/06/04. 10.1056/NEJMoa031049 . [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Tokunaga K, Shimomura I, Nishida M, Yoshida S, Kotani K, et al. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1994;107(2):239–46. Epub 1994/06/01. . [DOI] [PubMed] [Google Scholar]
- 7.Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K, et al. Insulin resistance and body fat distribution. Diabetes care. 1996;19(3):287–91. Epub 1996/03/01. . [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Nakamura T, Miyaoka K, Nishida M, Funahashi T, Yamashita S, et al. Visceral fat accumulation contributes to insulin resistance, small-sized low-density lipoprotein, and progression of coronary artery disease in middle-aged non-obese Japanese men. Japanese circulation journal. 2001;65(3):193–9. Epub 2001/03/27. . [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Hoshino K, Yokota K, Yokose T, Tajima N. Increased visceral fat and impaired glucose tolerance predict the increased risk of metabolic syndrome in Japanese middle-aged men. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2005;113(6):334–9. Epub 2005/06/25. 10.1055/s-2005-865680 . [DOI] [PubMed] [Google Scholar]
- 10.Lin WY, Lee LT, Chen CY, Lo H, Hsia HH, Liu IL, et al. Optimal cut-off values for obesity: using simple anthropometric indices to predict cardiovascular risk factors in Taiwan. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002;26(9):1232–8. Epub 2002/08/21. 10.1038/sj.ijo.0802040 . [DOI] [PubMed] [Google Scholar]
- 11.Al-Odat AZ, Ahmad MN, Haddad FH. References of anthropometric indices of central obesity and metabolic syndrome in Jordanian men and women. Diabetes & metabolic syndrome. 2012;6(1):15–21. Epub 2012/09/28. 10.1016/j.dsx.2012.05.012 . [DOI] [PubMed] [Google Scholar]
- 12.Brambilla P, Bedogni G, Heo M, Pietrobelli A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. International journal of obesity (2005). 2013;37(7):943–6. Epub 2013/03/13. 10.1038/ijo.2013.32 . [DOI] [PubMed] [Google Scholar]
- 13.Zeng Q, He Y, Dong S, Zhao X, Chen Z, Song Z, et al. Optimal cut-off values of BMI, waist circumference and waist:height ratio for defining obesity in Chinese adults. The British journal of nutrition. 2014;112(10):1735–44. Epub 2014/10/11. 10.1017/S0007114514002657 . [DOI] [PubMed] [Google Scholar]
- 14.Guan X, Sun G, Zheng L, Hu W, Li W, Sun Y. Associations between metabolic risk factors and body mass index, waist circumference, waist-to-height ratio and waist-to-hip ratio in a Chinese rural population. Journal of diabetes investigation. 2016;7(4):601–6. Epub 2016/05/18. 10.1111/jdi.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto R, Tabara Y, Kohara K, Miki T, Ohtsuka N, Kusunoki T, et al. Smoking status is associated with serum high molecular adiponectin levels in community-dwelling Japanese men. Journal of atherosclerosis and thrombosis. 2010;17(4):423–30. Epub 2010/01/13. . [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto R, Tabara Y, Kohara K, Miki T, Ohtsuka N, Kusunoki T, et al. Alcohol drinking status is associated with serum high molecular weight adiponectin in community-dwelling Japanese men. Journal of atherosclerosis and thrombosis. 2010;17(9):953–62. Epub 2010/09/02. . [DOI] [PubMed] [Google Scholar]
- 17.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2005;28(9):2289–304. Epub 2005/08/27. . [DOI] [PubMed] [Google Scholar]
- 18.Hu H, Kurotani K, Sasaki N, Murakami T, Shimizu C, Shimizu M, et al. Optimal waist circumference cut-off points and ability of different metabolic syndrome criteria for predicting diabetes in Japanese men and women: Japan Epidemiology Collaboration on Occupational Health Study. BMC public health. 2016;16:220 Epub 2016/03/05. 10.1186/s12889-016-2856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. The Journal of clinical endocrinology and metabolism. 2012;97(4):1067–72. Epub 2012/01/13. 10.1210/jc.2011-1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z, Li D, He H, Wang J, Hu X, Zhang P, et al. Body mass index, waist circumference, and waist-to-height ratio for prediction of multiple metabolic risk factors in Chinese elderly population. Scientific reports. 2018;8(1):385 Epub 2018/01/13. 10.1038/s41598-017-18854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Tong G, Tong W, Lu L, Qin X. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC public health. 2011;11:35 Epub 2011/01/14. 10.1186/1471-2458-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2012;13(3):275–86. Epub 2011/11/24. 10.1111/j.1467-789X.2011.00952.x . [DOI] [PubMed] [Google Scholar]
- 23.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutrition research reviews. 2010;23(2):247–69. Epub 2010/09/08. 10.1017/S0954422410000144 . [DOI] [PubMed] [Google Scholar]
- 24.Hsieh SD, Yoshinaga H, Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27(5):610–6. Epub 2003/04/22. 10.1038/sj.ijo.0802259 . [DOI] [PubMed] [Google Scholar]
- 25.Schneider HJ, Klotsche J, Silber S, Stalla GK, Wittchen HU. Measuring abdominal obesity: effects of height on distribution of cardiometabolic risk factors risk using waist circumference and waist-to-height ratio. Diabetes care. 2011;34(1):e7 Epub 2011/01/05. 10.2337/dc10-1794 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ODS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.