Abstract
Background
Palbociclib is a small‐molecule, cyclin‐dependent kinase 4 and 6 inhibitor, which prevents phosphorylation of the retinoblastoma (Rb) protein and inhibits cell‐cycle progression from G1 to S phase. We performed this meta‐analysis to estimate the safety and efficacy of palbociclib in cancer patients from clinical trials.
Methods
PubMed and EMBASE were searched for eligible studies. Adverse events (AE) of grade ≥3 and all‐grade (1‐5) were extracted to calculate event rates. Odds ratios (ORs) with 95% confidence interval (CI) were calculated to estimate the safety of palbociclib in endocrine treatment‐combined studies. A fixed effects model was used when homogeneity was low (I 2 ≤ 50%). A random effects model was adopted when there was a significant heterogeneity (I 2 > 50%). For efficacy endpoints, hazard ratio (HR) and 95% CI for progression‐free survival (PFS) or overall survival (OS) were extracted and analyzed.
Results
Nine clinical trials representing 1534 patients were identified. The most frequently observed all‐grade adverse events (AEs) in patients treated with palbociclib were neutropenia (event rate: 68.1%), leukopenia (51.7%), fatigue (35.9%), anemia (34.7%), and thrombocytopenia (30.9%). The most common grade 3 or more toxicities were neutropenia (51.6%), leukopenia (29.4%), and thrombocytopenia (7.5%). Hematologic adverse events had high occurrence in the palbociclib group. The pooled analysis of survival outcomes suggested that palbociclib produced clinical benefits in breast cancers and Rb‐positive tumors. More specifically, palbociclib was associated with significant improvement of PFS (HR: 0.518, 95% CI: 0.444‐0.604) in the treatment of ER‐positive and HER2‐negative breast cancer.
Conclusions
Hematologic adverse events were common in palbociclib‐treated cancer patients. Since palbociclib produced a higher PFS rate with a low serious complication rate, it can be a promising novel target therapy drug for treating ER‐positive and HER2‐negative breast cancer.
Keywords: CDK 4/6 inhibitor, efficacy, meta‐analysis, palbociclib, safety
1. INTRODUCTION
Cyclin‐dependent kinases 4 and 6 (CDK 4/6) are activated by D‐type cyclins. By phosphorylating the retinoblastoma (Rb) protein, they can promote cell‐cycle progression from G1 to S phase.1, 2, 3, 4 Abnormalities during the progression from G1 to S phase are closely related to many malignancies.5, 6 CDK 4/6 were considered as a potential therapeutic target in tumors with functional Rb protein. Palbociclib is a bioavailable, highly specific inhibitor of CDK 4/63 that prevents phosphorylation of the retinoblastoma (Rb) protein.7 Previous researches demonstrated that palbociclib had activity in reducing tumor growth in several Rb‐positive cell lines and xenograft models.2, 8 Furthermore, several clinical trials have suggested that palbociclib has antitumor activity in estrogen receptor (ER)‐positive breast cancer, genitourinary germ cell tumor, and some other retinoblastoma (Rb)‐positive tumors.9, 10, 11 From previous findings in clinical trials, we believed that CDK 4/6 inhibitor is potent in therapies for breast cancer and Rb‐positive tumor patients. At the same time, we should also pay more attention to the adverse effects caused by palbociclib or palbociclib‐based therapy. The adverse effects about palbociclib varied in different trials, which can be divided into treatment‐emergent adverse events (TEAE) and treatment‐related adverse events (TRAE). After reviewing these trials, we concluded that these therapies mainly manifested hematologic toxicity. To explore the potential clinical value of palbociclib, we conducted this study to find out the most meaningful adverse effects and efficacy outcomes of palbociclib and to direct further evaluation of the safety and efficacy of palbociclib.
2. METHODS
2.1. Search strategy
Clinical trials published in English considering this meta‐analysis were searched from PubMed database until 27 December 2017. The initial search keyword was “palbociclib” or “CDK 4/6 inhibitor” or “PD0332991.” The title and abstract of the identified studies were analyzed by two reviewers independently. Disagreements were resolved by consensus. In addition, references from selected publications were screened for potentially eligible studies. In addition, EMBASE was reviewed for following keywords including “palbociclib”, “CDK 4/6 inhibitor,” and “PD0332991” to avoid missing qualified studies (until 4 January 2018). Only the most complete and latest studies were included.
2.2. Study inclusion/exclusion criteria
Studies selected for final analysis should meet all of the following inclusion criteria: (a) prospective phase I, phase II, and III clinical trials used palbociclib in cancer patients; (b) data were available regarding the incidence of all‐grade adverse effects or grade ≥3 adverse effects or the survival outcome including overall survival (OS) or progression‐free survival (PFS); and (c) studies used palbociclib as a single‐agent or as combination therapy. The following exclusion criteria were applied: (a) repeated reports of same study group or repeated publications and (b) the study was not published in English.
2.3. Data extraction
Two independent investigators extracted the data needed from the selected studies, with disagreements resolved by consensus. Study characteristic information including the first author's name, publication year, sample size, study phase, treatment regime, tumor type, and other details of patients is listed in Table 1. Notably, studies used palbociclib combined with endocrine treatment were all randomized controlled trials, and studies adopted palbociclib as single agent were all non‐randomized controlled trials.
Table 1.
Baseline characteristics
| First author | Year | Phase | Histology | RB assessment (Biomarkers) | Treatment | Dose | Number | Age (median) | Gender M/F | Region |
|---|---|---|---|---|---|---|---|---|---|---|
| Cristofanilli M | 2016 | III | ER+, HER2‐, advanced BC | NR | Palbociclib‐Fulvestrant vs Placebo‐Fulvestrant | 125 mg | 521 (347/174) | 57 (57/56) | 0 + 521 | 17 countries |
| Finn RS | 2016 | II | ER+, HER2‐, advanced BC | NR | Palbociclib‐Letrozole vs Placebo‐Letrozole | 125 mg | 666 (444/222) | 62/61 | 0 + 666 | 17 countries |
| Finn RS | 2015 | II | ER+, HER2‐, advanced BC | NR | Palbociclib‐Letrozole vs Letrozole | 125 mg | 165 (84/81) | 63/64 | 0 + 165 | USA |
| Tamura K | 2016 | I | ER+, HER2‐, advanced BC | NR | Palbociclib | 125 mg | 12 | 55 (24‐76) | 0 + 12 | Japan |
| DeMichele A | 2014 | II | Metastatic or Advanced BC | IHC (Antibody of MS‐107‐P, clone 1F8) | Palbociclib | 125 mg | 37 | 59 (39‐88) | 0 + 37 | USA |
| Dickson MA | 2013 | II | Advanced or metastatic WDLS/DDLS | IHC (RB [4H1] mouse monoclonal antibody) | Palbociclib | 200 mg | 30 | 65 (37‐83) | 16 + 14 | USA |
| Flaherty KT | 2012 | I | Advanced solid tumors | IHC | Palbociclib | Dose finding | 41 | 54 (22‐77) | 20 + 21 | USA |
| Schwartz GK | 2011 | I | Rb‐positive advanced solid tumors or NHL | NR | Palbociclib | Dose finding 100/150/200/225 mg | 33 | 63 (35‐78) | 16 + 17 | USA |
| Vaughn DJ | 2015 | II | Metastatic GCTs | IHC (RB1 mouse monoclonal antibody) | Palbociclib | 125 mg | 29 | 31 (17‐56) | 26 + 4 | USA |
BC, breast cancer; GCTs, germ cell tumors; IHC, immunohistochemistry; NHL, non‐Hodgkin's lymphoma; NR, not report; WDLS/DDLS, well‐differentiated or dedifferentiated liposarcoma.
The clinical endpoints extracted from the trials were grade ≥3 and all‐grade (1‐5) adverse effects according to the National Cancer Institute (NCI) Common Toxicity Criteria version 3.0 or Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. For safety endpoints, the types of different adverse events and total patients were extracted to calculate adverse event ratio with 95% CI in trials which used palbociclib as a single agent and OR with 95% CI in endocrine treatment‐combined trials. For efficacy endpoints, HR and 95% CI for PFS or OS were extracted following Parmar's method.
2.4. Statistical analysis
Statistical analysis of pooled PFS, OS, or toxicities was performed using the software Review manager 5.3 (Copenhagen, Sweden) or Comprehensive Meta‐Analysis (CMA) program 2 (Biostat, Englewood, NJ). The Cochrane Q statistic (significant at P < 0.10) and the I 2 value (significant heterogeneity if >50%) were used to examine heterogeneity.12 The pooled toxicities were analyzed using a fixed or random effects model, depending on heterogeneity. A fixed effects model was used when homogeneity was low (P > 0.10, I 2 ≤ 50%). A random effects model was adopted when there was a significant heterogeneity (P < 0.10, I 2 > 50%). The survival outcome effects of palbociclib were estimated by using forest plots of HR
3. RESULTS
3.1. Search results and study characteristics
The initial search involved 434 potential studies, a total of 24 studies were identified as potentially relevant articles after title and abstract reviewing. The search of EMBASE publications did not supplement any additional results. After full‐text reviewing, fifteen studies were excluded due to lack of data on adverse effects or survival outcome. In total, 9 studies8, 9, 10, 11, 13, 14, 15, 16, 17 with 1534 patients which met our criteria were selected. The selection process is shown in Figure 1. Details of the nine eligible studies included in our final analysis are summarized in Table 1. These studies included three phase I, five phase II, and one phase III trials. Among these trials, six trials were designed of palbociclib as single agent, and three trials were designed of palbociclib plus other agent together as a therapy for patients. The eligible studies included four articles for ER‐positive and HER2‐negative breast cancer, one article for metastatic or advanced breast cancer, one article for metastatic germ cell tumor, one article for advanced or metastatic liposarcoma, and two articles for other Rb‐positive tumors.
Figure 1.
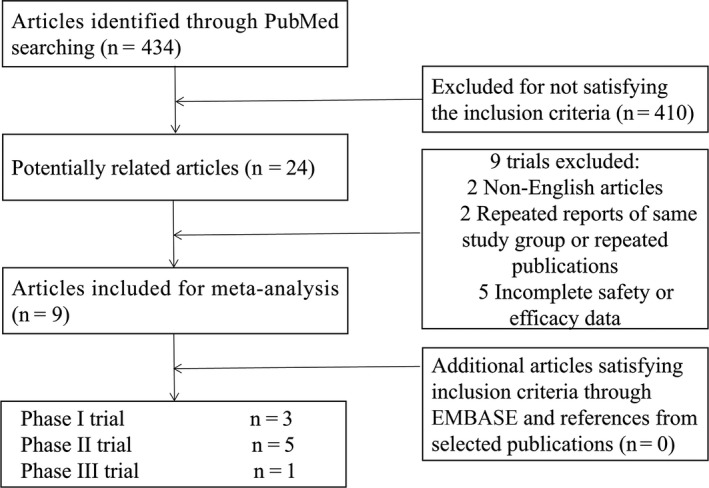
Flow diagram of the literature search and trial selection process
3.2. Adverse effect
Safety profiles were pooled together to analyze the risk factor for any side effects in the overall population with grade ≥3 or all‐grade adverse events (AEs). In all the single‐agent trials with all‐grade AEs analyzed with a fixed effects model, the highest risk was found for headache (event rate: 21.6%, 95% CI: 19.0%‐24.4%). In random model with all‐grade AEs of the single‐agent trials, neutropenia (68.1%, 95% CI: 52.4%‐80.5%), leukopenia (51.7%, 95% CI: 39.6%‐63.6%), fatigue (35.9%, 95% CI: 28.6%‐43.9%), anemia (34.7%, 95% CI: 24.8%‐46.1%), thrombocytopenia (30.9%, 95% CI: 20.9%‐43.0%), and nausea (30.1%, 95% CI 24.0%‐36.9%) were most common (Table 2A and Figure 2A).
Table 2.
(A) Top 10 all‐grade adverse events for single‐agent group (B) Top 10 grade ≥3 adverse events for single‐agent group
| Adverse events | Model | Event rate (%) | (95% CI) (%) | Z‐value | P‐value |
|---|---|---|---|---|---|
| (Lower limit‐Upper limit) | |||||
| (A) | |||||
| Headache | Fixed | 21.6 | 19.0‐24.4 | −15.622 | 0 |
| Constipation | Fixed | 18.5 | 16.1‐21.1 | −17.529 | 0 |
| Rash | Fixed | 16.5 | 14.1‐19.2 | −17.403 | 0 |
| Asthenia | Fixed | 16 | 13.2‐19.3 | −14.251 | 0 |
| Vomiting | Fixed | 15.5 | 13.3‐18.0 | −18.673 | 0 |
| Decreased appetite | Fixed | 15 | 12.8‐17.6 | −18.281 | 0 |
| Mucositis | Fixed | 13.9 | 11.8‐16.2 | −19.448 | 0 |
| Pain in extremity | Fixed | 13.8 | 11.6‐16.2 | −18.576 | 0 |
| Dyspnea | Fixed | 13.4 | 11.4‐15.8 | −19.345 | 0 |
| Dizziness | Fixed | 12.9 | 10.8‐15.3 | −18.83 | 0 |
| Neutropenia | Random | 68.1 | 52.4‐80.5 | 2.249 | 0.024 |
| Leukopenia | Random | 51.7 | 39.6‐63.6 | 0.267 | 0.789 |
| Fatigue | Random | 35.9 | 28.6‐43.9 | −3.379 | 0.001 |
| Anemia | Random | 34.7 | 24.8‐46.1 | −2.613 | 0.009 |
| Thrombocytopenia | Random | 30.9 | 20.9‐43.0 | −3.009 | 0.003 |
| Nausea | Random | 30.1 | 24.0‐36.9 | −5.341 | 0 |
| Diarrhea | Random | 25.1 | 19.6‐31.5 | −6.755 | 0 |
| Alopecia | Random | 20.1 | 11.7‐32.2 | −4.246 | 0 |
| Arthralgia | Random | 19.3 | 10.4‐33.1 | −3.861 | 0 |
| Lymphopenia | Random | 19 | 5.7‐47.7 | −2.091 | 0.036 |
| (B) | |||||
| Anemia | Fixed | 5.6 | 4.3‐7.3 | −19.9 | 0 |
| Fatigue | Fixed | 3 | 2.1‐4.4 | −17.563 | 0 |
| Asthenia | Fixed | 2.3 | 1.3‐4.0 | −12.872 | 0 |
| Diarrhea | Fixed | 1.7 | 0.9‐3.1 | −12.688 | 0 |
| Back pain | Fixed | 1.3 | 0.7‐2.3 | −14.36 | 0 |
| Decreased appetite | Fixed | 0.8 | 0.4‐1.7 | −12.642 | 0 |
| Rash | Fixed | 0.8 | 0.3‐1.7 | −11.83 | 0 |
| Arthralgia | Fixed | 0.6 | 0.3‐1.5 | −11.241 | 0 |
| Musculoskeletal pain | Fixed | 0.6 | 01‐2.3 | −7.224 | 0 |
| Vomiting | Fixed | 0.6 | 0.3‐1.6 | −10.649 | 0 |
| Neutropenia | Random | 51.6 | 42.7‐60.3 | 0.347 | 0.729 |
| Leukopenia | Random | 29.4 | 23.2‐36.6 | −5.292 | 0 |
| Thrombocytopenia | Random | 7.5 | 2.9‐18.1 | −4.914 | 0 |
| Dyspnea | Random | 1.6 | 0.6‐4.1 | −8.223 | 0 |
| Abdominal pain | Random | 1.3 | 0.4‐4.2 | −7.136 | 0 |
| Nausea | Random | 1.3 | 0.4‐4.6 | −6.557 | 0 |
| Bone pain | Random | 1.2 | 0.3‐4.7 | −6.134 | 0 |
| Constipation | Random | 0.7 | 0.1‐3.5 | −5.903 | 0 |
| Upper respiratory infection | Random | 0.7 | 0.1‐3.7 | −5.772 | 0 |
Figure 2.
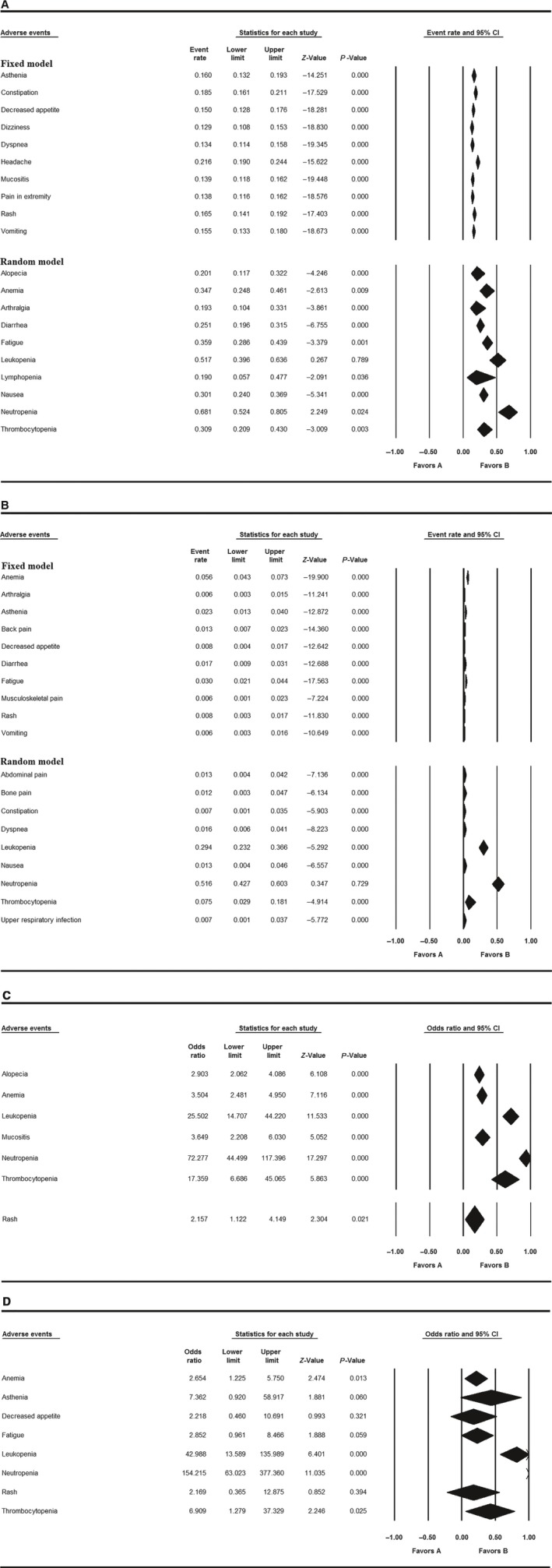
Adverse events and event rate/odds ratio with 95% CI. A, Top 10 all‐grade adverse events in single‐agent group; B, Top 10 grade ≥3 adverse events in single‐agent group; C, All‐grade adverse events with OR > 2 for endocrine treatment‐combined group; D, Grade ≥3 adverse events with OR > 2 for endocrine treatment‐combined group
Serious TEAEs (grade ≥ 3) were pooled to reveal the clinical risk of palbociclib. Neutropenia (51.6%, 95% CI: 42.7%‐60.3%), leukopenia (29.4%, 95% CI: 23.2%‐36.6%), and thrombocytopenia (7.5%, 95% CI: 2.9%‐18.1%) were most common in decreasing order of frequency (Table 2B and Figure 2B).
In the analysis of all‐grade AEs of the 3 endocrine treatment‐combined trials,9, 14, 15 the odds ratio (OR) of neutropenia was 72.277 (95% CI: 44.499‐117.396). The OR of leukopenia was 25.502 (95% CI: 14.707‐44.220). The OR of thrombocytopenia was 17.359 (95% CI: 6.686‐45.065). The OR of mucositis was 3.649 (95% CI: 2.208‐6.030). The OR of anemia was 3.504 (95% CI: 2.481‐4.950). The OR of alopecia was 2.903 (95% CI: 2.062‐4.086). In serious TEAEs (grade ≥ 3), the OR of neutropenia was 154.215 (95% CI: 63.023‐377.360). The OR of leukopenia was 42.988 (95% CI: 13.589‐135.989). The OR of asthenia was 7.362 (95% CI: 0.920‐58.917). The OR of thrombocytopenia was 6.909 (95% CI: 1.279‐37.329). The OR of fatigue was 2.852 (95% CI: 0.961‐8.466). The OR of anemia was 2.654 (95% CI: 1.225‐5.750; Table 3A,B and Figure 2C,D).
Table 3.
(A) All‐grade adverse events with OR > 2 for endocrine treatment‐combined group. (B) Grade ≥3 adverse events with OR > 2 for endocrine treatment‐combined group
| Adverse events | Model | Odds Ratio | 95% CI | Z‐value | P‐value |
|---|---|---|---|---|---|
| (Lower limit‐Upper limit) | |||||
| (A) | |||||
| Neutropenia | Fixed | 72.28 | 44.499‐117.396 | 17.297 | 0 |
| Leukopenia | Fixed | 25.5 | 14.707‐44.220 | 11.533 | 0 |
| Thrombocytopenia | Fixed | 17.36 | 6.686‐45.065 | 5.863 | 0 |
| Mucositis | Fixed | 3.649 | 2.208‐6.030 | 5.052 | 0 |
| Anemia | Fixed | 3.504 | 2.481‐4.950 | 7.116 | 0 |
| Alopecia | Fixed | 2.903 | 2.062‐4.086 | 6.108 | 0 |
| Rash | Random | 2.157 | 1.122‐4.149 | 2.304 | 0.021 |
| (B) | |||||
| Neutropenia | Fixed | 154.2 | 63.023‐377.360 | 11.035 | 0 |
| Leukopenia | Fixed | 42.99 | 13.589‐135.989 | 6.401 | 0 |
| Asthenia | Fixed | 7.362 | 0.920‐58.917 | 1.881 | 0.06 |
| Thrombocytopenia | Fixed | 6.909 | 1.279‐37.329 | 2.246 | 0.025 |
| Fatigue | Fixed | 2.852 | 0.961‐8.466 | 1.888 | 0.059 |
| Anemia | Fixed | 2.654 | 1.225‐5.750 | 2.474 | 0.013 |
| Decreased appetite | Fixed | 2.218 | 0.460‐10.691 | 0.993 | 0.321 |
| Rash | Fixed | 2.169 | 0.365‐12.875 | 0.852 | 0.394 |
Between the two endocrine treatment‐combined groups, the OR of hematologic adverse events of fulvestrant‐combined group was 15.131 (95% CI: 2.728‐83.919), and the OR of hematologic adverse events of letrozole‐combined group was 15.475 (95% CI: 5.312‐45.079), but the difference between the two groups was statistically insignificant (P = 0.983; Figure 3A).
Figure 3.
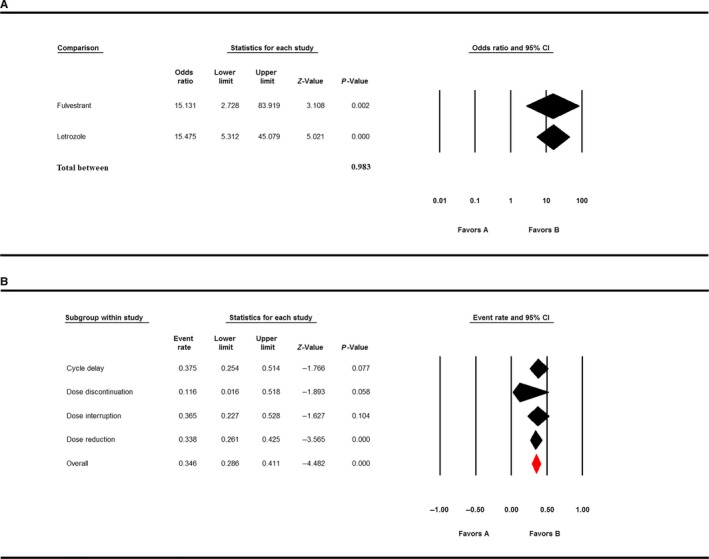
A, Comparison of adverse events in fulvestrant‐palbociclib group and letrozole‐palbociclib group. B, Unexpected treatment changes due to side effects
Serious adverse effects might affect treatment outcome; therefore, we analyzed the incidence of unexpected treatment changes due to side effects. Cycle delay was the most common events due to adverse effects (event rate: 37.5%, 95% CI: 25.4%‐51.4%). The event rate of dose interruption due to side effects was 36.5% (95% CI: 22.7%‐52.8%). The event rate of dose reduction due to adverse effects was 33.8% (95% CI: 26.1%‐42.5%). The event rate of dose discontinuation due to side effects was 11.6% (95% CI: 1.6%‐51.8%; Figure 3B).
Details including adverse events, study names, and statistical results are shown in Figures S1a, S1b, S2a, S2b, S3a, S3b, S4a, and S4b.
3.3. OS and PFS
The PFS analysis was based on three endocrine treatment‐combined trials and four single‐agent trials, including 1464 patients. In endocrine treatment‐combined studies, our analysis showed that the utility of palbociclib in treatment was beneficial in prolonging PFS (HR: 0.518, 95% CI: 0.444‐0.604). Subgroup analysis demonstrated that the utility of palbociclib combined with fulvestrant (HR: 0.460, 95% CI: 0.359‐0.589) was more beneficial than the utility of palbociclib combined with letrozole (HR: 0.559, 95% CI: 0.458‐0.681) in prolonging PFS; however, the difference was statistically insignificant (P = 0.229; Figure 4). Within the three endocrine treatment‐combined trials for breast cancer, Cristofanilli et al9 showed the median PFS was 9.5 months (95% CI: 9.2‐11.0) in the fulvestrant plus palbociclib group and 4.6 months (95% CI: 3.5‐5.6) in the fulvestrant plus placebo group (HR: 0.46, 95% CI: 0.36‐0.59, P < 0.0001). Finn et al15 reported the median PFS was 24.8 months (95% CI: 22.1—not estimable) in the palbociclib‐letrozole group, as compared with 14.5 months (95% CI: 12.9‐17.1) in the placebo‐letrozole group (HR: 0.58, 95% CI: 0.46‐0.72, P < 0.001). Finn et al14 manifested the median PFS was 10.2 months in the letrozole group (95% CI: 5.7‐12.6) and 20.2 months in the palbociclib‐letrozole group (95% CI: 13.8‐27.5; HR: 0.488, 95% CI: 0.319‐0.748; one‐sided P < 0.10).
Figure 4.
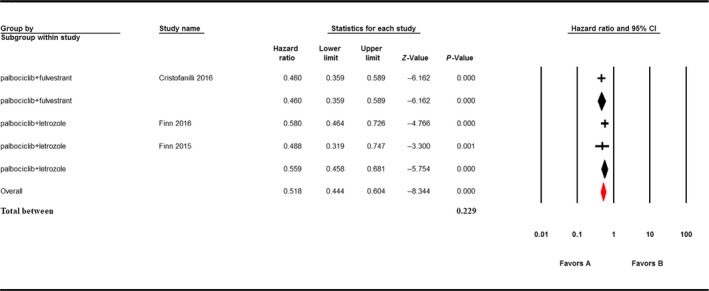
The HR and 95% CI for PFS in endocrine treatment‐combined group
In single‐agent studies, one phase II trial8 for well‐differentiated or dedifferentiated liposarcoma showed the median PFS was 4.5 months, which was 3.7 months in the phase II trial13 for Rb‐positive advanced breast cancer. Regarding the study11 for germ cell tumors, the median PFS for all evaluable patients who received treatment was 2.7 months. One phase I dose‐finding trial17 for solid tumor or NHL reported the duration of PFS ranged from 28 to 280 days. Only one study14 assessed OS. Median OS was 37.5 months in the palbociclib‐letrozole group (95% CI: 28.4—not estimable; 30 events) and 33.3 months in the letrozole group (95% CI: 26.4—not estimable; 31 events; HR: 0.813, 95% CI: 0.492‐1.345; two‐sided P = 0.317).
3.4. Quality assessment
Review Manager 5.3 (Copenhagen, Sweden) was used to measure quality assessment. QUADAS‐218 was used to estimate the quality of eligible studies. Overall, the quality of the studies was satisfactory. The results are shown in Figure 5A,B.
Figure 5.
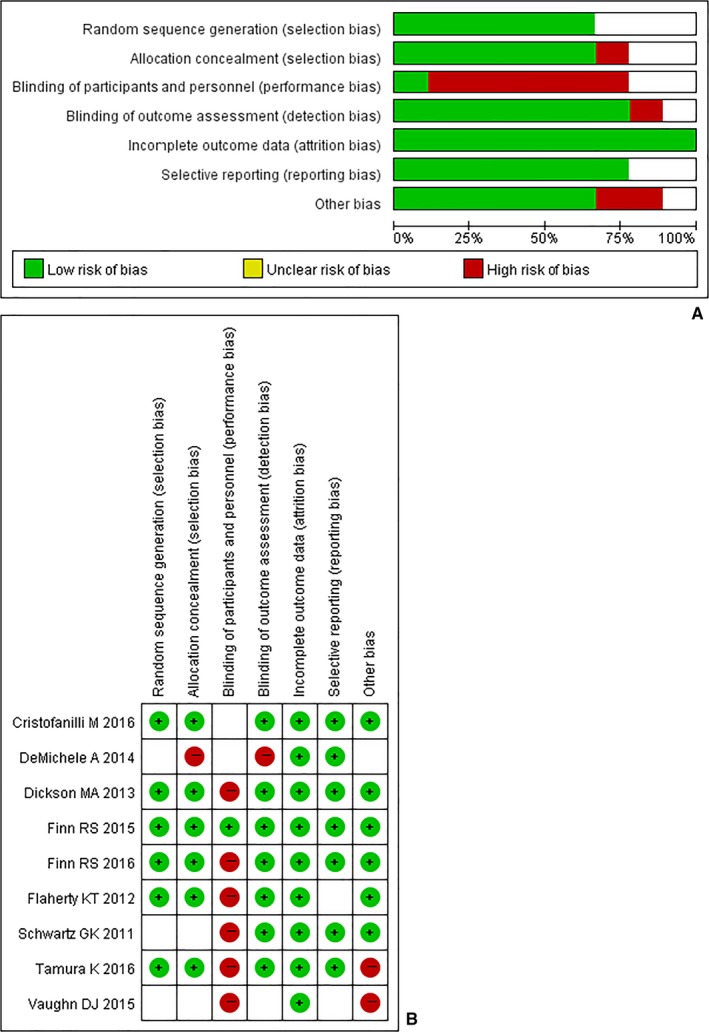
Quality assessment. A, Risk of bias graph; B, risk of bias summary
4. DISCUSSION
Our analysis pooled data from nine clinical trials involving 1534 patients. Based on safety and efficacy analysis, we provided evidence regarding the beneficial effect of palbociclib in ER‐positive and HER2‐negative breast cancer and the potential value of palbociclib in Rb‐positive germ cell tumors and Rb‐positive liposarcoma.
Serious adverse effect due to poor specificity usually restricts potential therapeutic value in oncology.19 Our results suggested there were more adverse effects in the group that used palbociclib‐containing regimen but all remained within expected parameters, at the same time, toxicities were relatively manageable. In the single‐agent trials, the most frequently observed all‐grade adverse events in patients treated with palbociclib were neutropenia, leukopenia, fatigue, anemia, thrombocytopenia, and nausea. The most common grade 3 or more toxicities were neutropenia, leukopenia, and thrombocytopenia. OR > 2 was considered as a criterion of strong clinical value.20 According to the OR value, within the three endocrine treatment‐combined trials, neutropenia had a higher occurrence in palbociclib regimen patients. The adverse events of palbociclib are CDK 4/6 inhibitor relevant, because palbociclib inhibits an upstream target of cell progression from G1 phase to S phase without specificity,21 which suggested that patients who were administered palbociclib should be strictly monitored and managed with preventive drugs or dose reduction.
Hematologic adverse events had high occurrence in the palbociclib group. The underlying mechanism of palbociclib‐related hematologic toxicities is possibly associated with myelosuppression, which may result from an effect of CDK 4/6 on‐target inhibition.14 On‐target refers to adverse effects caused by exaggerated and adverse pharmacologic effects of the target of interest.22 Currently, bone marrow hematopoietic stem and progenitor cells (HSPCs) have been found to require the activity of CDK 4/6 for proliferation.23 Due to poor sensitivity of this CDK 4/6 inhibitor, it resulted in the inhibition of HSPCs and caused hematologic adverse events. In addition, myelosuppression induced by CDK 4/6 inhibitor often causes dose reduction and therefore affects efficacy.19 Our results also demonstrated that the most common events due to side effects were cycle delay, dose interruption, and dose reduction, which might have effect on efficacy. To minimize myelosuppressive effects and related complications, current therapeutic approaches are depended on growth factors which are suboptimal19 and the US Food and Drug Administration has approved drugs for antitumor therapy‐induced myelosuppression such as filgrastim for neutropenia.24, 25, 26, 27, 28, 29, 30
In terms of efficacy, the addition of palbociclib in treatment regimen conferred a progression‐free benefit. Since CDK 4/6 can promote cell‐cycle entry from the G1 phase to the S phase by phosphorylating Rb protein, palbociclib inhibits CDK 4/CDK 6 therefore leading to tumor growth limitation.5, 31 In particular, the CDK 4/6 inhibitor palbociclib has shown high activity in ER‐positive and HER2‐negative advanced breast cancer, and it may result from the inhibition of CDK 4/6. Cyclin D1 is essential for breast cancer formation by coupling with CDK 4/6 to promote cell cycling. Binding to ER‐alpha subunit drives cyclin D1 transcription. By inhibiting CDK 4/6, palbociclib has a positive effect on the treatment of ER‐positive and HER2‐negative advanced breast cancer.32, 33 Our analysis showed that palbociclib is beneficial in prolonging PFS (HR: 0.518, 95% CI: 0.444‐0.604) in ER‐positive and HER2‐negative breast cancer patients. Three randomized controlled trials9, 14, 15 all demonstrated people could get the clinical benefit from palbociclib. One double‐blind, phase III trial included breast cancer patients that had relapsed or progressed during prior endocrine therapy. The result showed a prolonged median PFS from 4.6 months (placebo‐fulvestrant group) to 9.5 months (palbociclib 125 mg oral daily‐fulvestrant group; HR: 0.46 95% CI: 0.36‐0.59).9 In addition, in another phase II, multicenter open‐label randomized study,14 the result demonstrated that the median PFS was 20.2 months in the palbociclib‐letrozole group (125 mg daily) compared with 10.2 months in the letrozole group (HR: 0.488, 95% CI: 0.319‐0.748) and a phase III study9 showed that the median PFS increased from 14.5 months in the placebo‐letrozole group to 24.8 months in the palbociclib‐letrozole group (125 mg daily; HR: 0.58, 95% CI: 0.46‐0.72). The difference in median PFS within these RCTs might be related to different patient samples, pretreatment diseases, and treatment regimens. A phase II, single arm trial13 of palbociclib also demonstrated the single agent was well tolerated and active in patients with hormone receptor‐positive and Rb‐positive breast cancer. Finally, our estimation about OS did not manifest in statistical significance (P > 0.10).14 All above pooled results proved that palbociclib had high clinical value in the treatment for ER‐positive and HER2‐negative breast cancer, but the therapeutic value of palbociclib in other tumors is unclear.
Endocrine therapy has played the leading role in the treatment for ER‐positive breast cancer.34 Notably, our subgroup analysis revealed that there was no statistically significant difference in hematologic side effects and efficacy between fulvestrant‐palbociclib group and letrozole‐palbociclib group, which might be due to the small number of studies. Nowadays, more and more women have gained resistance to endocrine therapy, resulting a relapse of breast cancer.35 Results from our study supported the scientific evidence that palbociclib had high activity in ER‐positive and HER2‐negative breast cancer lines. In addition, the finding in one phase II14 and one phase III trial9 suggested that palbociclib is active in both patients who have acquired resistance to endocrine therapy and who have not received such therapy, when combined with endocrine therapy.
Remarkably, one phase I trial10 suggested the maximum tolerated dose and recommended phase II dose of palbociclib were 125 mg once daily, at which dose neutropenia was the sole significant toxicity. It should be pointed out that six9, 11, 13, 14, 15, 17 included trials adopted 125 mg palbociclib daily for patients. This dose of palbociclib might be associated with its relatively better survival outcome and lower toxicity.
Our analysis was limited by the small sample size and absence of blinding. Since palbociclib is a relatively new drug, trials about it are few, especially phase III trials. And only one study provided the OS data, so prolonged follow‐ups are essential. The previous study reported a strong association between PFS and QoL among cancer patients.36 Unfortunately, we did not analyze data concerning QoL because they were lacking or not homogeneous. More randomized controlled trials, OS data, and QoL included trials are needed to further validate our results.
In conclusion, our study showed that palbociclib has high activity in ER‐positive and HER2‐negative breast cancer and prolonged PFS in Rb‐positive tumors. In terms of adverse effect, hematologic adverse events were common, which suggested preventive measures should be adopted to reduce toxicity. More studies are needed to better understand the long‐term efficacy and toxicity of palbociclib.
CONFLICT OF INTEREST
None declared.
Supporting information
Guo L, Hu Y, Chen X, Li Q, Wei B, Ma X. Safety and efficacy profile of cyclin‐dependent kinases 4/6 inhibitor palbociclib in cancer therapy: A meta‐analysis of clinical trials. Cancer Med. 2019;8:1389–1400. 10.1002/cam4.1970
Linghong Guo, Yuanyuan Hu and Xuelei Ma contributed equally to this work
REFERENCES
- 1. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin‐dependent kinases in cancer therapy. Nat Rev Drug Discovery. 2015;14(2):130‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427‐1438. [PubMed] [Google Scholar]
- 3. Toogood PL, Harvey PJ, Repine JT, et al. Discovery of a potent and selective inhibitor of cyclin‐dependent kinase 4/6. J Med Chem. 2005;48(7):2388‐2406. [DOI] [PubMed] [Google Scholar]
- 4. Xu H, Yu S, Liu Q, et al. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol. 2017;10(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall M, Peters G. Genetic alterations of cyclins, cyclin‐dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67‐108. [DOI] [PubMed] [Google Scholar]
- 6. Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17(1):60‐65. [DOI] [PubMed] [Google Scholar]
- 7. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4‐amplified well‐differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31(16):2024‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425‐439. [DOI] [PubMed] [Google Scholar]
- 10. Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose‐escalation trial of the oral cyclin‐dependent kinase 4/6 inhibitor PD 0332991, administered using a 21‐day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18(2):568‐576. [DOI] [PubMed] [Google Scholar]
- 11. Vaughn DJ, Hwang WT, Lal P, Rosen MA, Gallagher M, O'Dwyer PJ. Phase 2 trial of the cyclin‐dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein‐expressing germ cell tumors. Cancer. 2015;121(9):1463‐1468. [DOI] [PubMed] [Google Scholar]
- 12. Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21(5):995‐1001. [DOI] [PubMed] [Google Scholar]
- 14. Finn RS, Crown JP, Lang I, et al. The cyclin‐dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first‐line treatment of oestrogen receptor‐positive, HER2‐negative, advanced breast cancer (PALOMA‐1/TRIO‐18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25‐35. [DOI] [PubMed] [Google Scholar]
- 15. Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925‐1936. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin‐dependent kinase inhibitor, administered in 3‐week cycles (Schedule 2/1). Br J Cancer. 2011;104(12):1862‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamura K, Mukai H, Naito Y, et al. Phase I study of palbociclib, a cyclin‐dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016;107(6):755‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐ 2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. [DOI] [PubMed] [Google Scholar]
- 19. Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC. Preclinical characterization of G1T28: a novel CDK4/6 inhibitor for reduction of chemotherapy‐induced myelosuppression. Mol Cancer Ther. 2016. [DOI] [PubMed] [Google Scholar]
- 20. Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6(4):375‐392. [DOI] [PubMed] [Google Scholar]
- 21. Berthet C, Kaldis P. Cell‐specific responses to loss of cyclin‐dependent kinases. Oncogene. 2007;26(31):4469‐4477. [DOI] [PubMed] [Google Scholar]
- 22. Rudmann DG. On‐target and off‐target‐based toxicologic effects. Toxicol Pathol. 2013;41(2):310‐314. [DOI] [PubMed] [Google Scholar]
- 23. Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120(7):2528‐2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double‐blind, placebo‐controlled trial. Lancet. 2003;362(9392):1255‐1260. [DOI] [PubMed] [Google Scholar]
- 25. Leyland‐Jones B, Investigators B, Study G . Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4(8):459‐460. [DOI] [PubMed] [Google Scholar]
- 26. Leyland‐Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first‐line chemotherapy: a survival study. J Clin Oncol. 2005;23(25):5960‐5972. [DOI] [PubMed] [Google Scholar]
- 27. Lyman GH. Balancing the benefits and costs of colony‐stimulating factors: a current perspective. Semin Oncol. 2003;30(4 Suppl 13):10‐17. [DOI] [PubMed] [Google Scholar]
- 28. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28(33):4996‐5010. [DOI] [PubMed] [Google Scholar]
- 29. Smith RE Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study. J Clin Oncol. 2008;26(7):1040‐1050. [DOI] [PubMed] [Google Scholar]
- 30. Wright JR, Ung YC, Julian JA, et al. Randomized, double‐blind, placebo‐controlled trial of erythropoietin in non‐small‐cell lung cancer with disease‐related anemia. J Clin Oncol. 2007;25(9):1027‐1032. [DOI] [PubMed] [Google Scholar]
- 31. Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin‐dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104(6):476‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cadoo KA, Gucalp A, Traina TA. Palbociclib: an evidence‐based review of its potential in the treatment of breast cancer. Breast cancer (Dove Medical Press). 2014;6:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen P, Lee NV, Hu W, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther. 2016;15(10):2273‐2281. [DOI] [PubMed] [Google Scholar]
- 34. Early Breast Cancer Trialists' Collaborative G . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687‐1717. [DOI] [PubMed] [Google Scholar]
- 35. Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy‐resistant breast cancer. Endocr Relat Cancer. 2011;18(3):333‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Booth CM, Eisenhauer EA. Progression‐free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030‐1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


