Abstract
Recycling of samarium–cobalt (SmCo) magnets is essential due to the limited resources of the mentioned metals and their high economic importance. The ionic liquid (IL) trihexyltetradecylphosphonium trichloride, [P666,14][Cl3], which can safely store chlorine gas in the form of the trichloride anion, was used as an oxidizing solvent for the recovery of metals from spent SmCo magnets. The dissolution was studied considering various mixtures of the ILs [P666,14][Cl3] and [P666,14]Cl, solid-to-liquid ratios and different temperatures. The results showed that the maximum capacity of [P666,14][Cl3] for SmCo magnets was 71 ± 1 mg/g of [P666,14][Cl3], in the presence of an extra source of coordinating chloride ions. The maximum loading of the IL could be reached within 3 h at 50 °C. Four stripping steps effectively removed all metals from the loaded IL, where sodium chloride solution (3 mol L–1), twice water and ammonia solution (3 mol L–1) were used consecutively as the stripping solvents. The regenerated IL showed a similar dissolution performance as fresh IL. Oxidative dissolution of metals in trichloride ILs is easily transferable to the recycling of valuable metals from other end-of-life products such as neodymium–iron–boron magnets and nickel metal hydride batteries.
Keywords: Oxidative dissolution, Polyhalides, Solvometallurgy, Rare earths, Recycling
Short abstract
Metals in SmCo magnet powders can be recovered by dissolving in a recyclable trichloride ionic liquid and separated by stripping with aqueous solutions.
Introduction
Samarium–cobalt (SmCo) magnets have either SmCo5 (1:5 series) or Sm2Co17 (2:17 series) as the main phase, but besides samarium and cobalt the 2:17 series also contain minor elements such as iron, copper and zirconium.1,2 SmCo magnets are used mainly for high-temperature applications due to their high coercivity (resistance to demagnetization), good corrosion resistance and excellent thermal stability.3,4 The current share of SmCo magnets in the permanent magnet market is less than 2%, because they have been largely replaced by neodymium–iron–boron (NdFeB) magnets after 1985. However, the global SmCo magnets market is growing thanks to new applications in consumer electronics, automotive and medical technology, aerospace and military equipment.
Recycling of SmCo magnets can lower the supply risk of samarium and cobalt, close the materials loop (circular economy), and reduce the environmental issues associated with primary mining and ore processing.3 The easiest way to recycle SmCo permanent magnets is direct recycling in which the magnet is converted to an alloy powder that can be processed to new magnets.5 This is only possible if a waste stream with a fairly constant chemical composition is available and if the magnets are not broken and oxidized at the surface. A more general approach is indirect recycling in which the metals are separated and recovered by hydrometallurgical methods. Oxidative dissolution is needed to chemically recycle metals in their elemental state. This is often achieved by dissolving the metals in solutions of strong mineral acids such as hydrochloric acid, sulfuric acid or nitric acid.6−8 The disadvantages of this hydrometallurgical method are the generation of hydrogen gas and the consumption of large amounts of acid. Other types of oxidizing agents for metal dissolution are solutions of chlorine, bromine or iodine in an organic solvent. For example, a solution of chlorine in N,N-dimethylformamide has been used for chlorination of metallic rhenium and zirconium.9,10 However, both halogens and organic solvents are volatile and hazardous. Moreover, halogens can attack the organic solvent to form undesirable decomposition products.11
Ionic liquids (ILs) consisting entirely of ions are generally considered as environmentally friendly solvents due to their negligible vapor pressure. They are being applied in the fields of synthesis, separations, catalysis and electrochemistry.12−16 ILs have also shown potential for safely storing halogens by forming trihalide or polyhalide anions, such as [Cl3]−, [Br3]−, [I3]− or [ClBr2]−.17−20 Recently, trichloride ILs have been synthesized and used for dissolution of various metals and alloys at mild conditions.17 Using trichloride ILs for oxidative dissolution of metals is greener than using directly chlorine gas in conventional processes, because the oxidizing agent can be effectively consumed by quantitatively controlling the ratio of trichloride anion to metal ion. In conventional processes, large excesses of chlorine was usually purged to the reactor to efficiently chlorinate metals.9
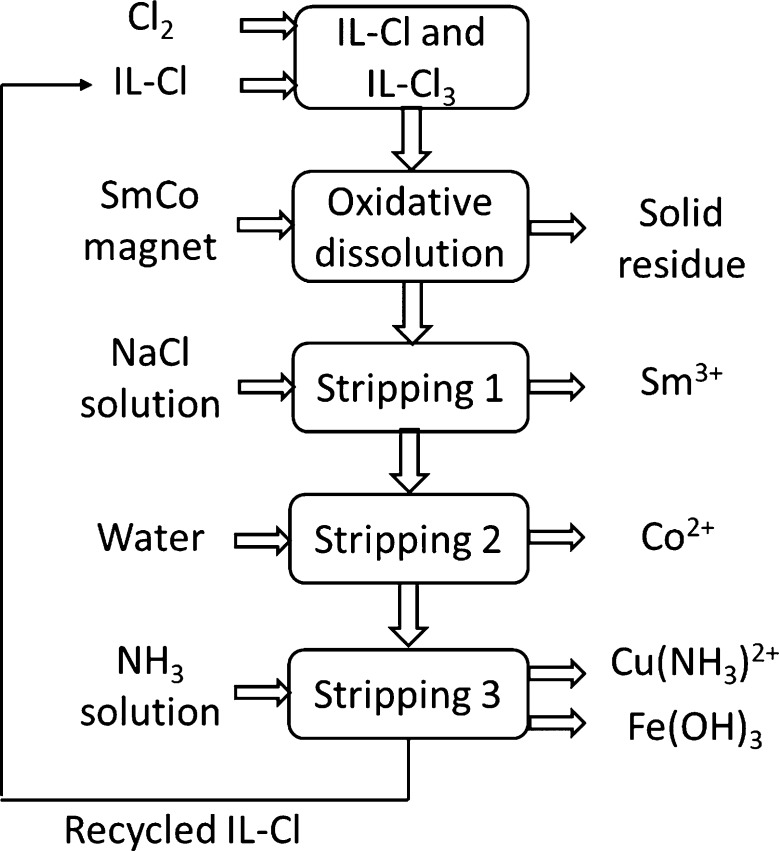
In this paper, trihexyl(tetradecyl)phosphonium trichloride, [P666,14][Cl3], was synthesized and employed as a reactive solvent for the recycling of SmCo magnets. This IL was selected on the basis of the hydrophobicity of [P666,14]Cl, which made it possible to use aqueous solutions for poststripping processes. The dissolution efficiency has been studied by varying the volume fraction of [P666,14][Cl3] in [P666,14]Cl (the latter as an additional source of coordinating chloride ions), the solid-to-liquid ratio and the temperature. Stripping of metals from the loaded IL was studied with different aqueous solutions, followed by a reusability study of the ILs. Finally, a conceptual process flow sheet was proposed, in which iron, copper, cobalt and samarium were separated in different streams.
Experimental Section
Materials and Instrumentation
The details for materials and instrumentation are presented in the Supporting Information (SI).
Milling of SmCo Magnet and Sm Pieces
SmCo magnet samples were first crushed by a hydraulic press to reduce them to a particle size ≤5 mm, to be suitable for the next milling step. A planetary ball mill (Fritsch, model Pulverisette 7) was used to further reduce the magnets into a powder using the following milling conditions: stainless steel pots and balls (diameter 10 and 1 mm), ball-to-powder ratio 3/1, 600 rpm, 3 cycles of milling (5 min) and pause (5 min). The obtained powder was shaken on an automatic stack of sieves for 15 min and the powders with size <45 μm were used in this work. The collected magnet powder was stored under inert argon atmosphere to prevent further oxidation by air. The crystalline structure of the milled, unsieved SmCo powder was determined by XRD analysis.
Samarium powder was obtained from the samarium pieces by ball-milling with the same procedures as mentioned above, except that the material of the pot and balls was zirconium oxide. Two fractions of powders were obtained by sieving: ≥125 μm and <125 μm. The latter one was further used in this work.
Synthesis and Characterization of Trichloride IL
[P666,14][Cl3] was prepared according to the same procedure described in the previous work.17 A detailed description of the synthesis method, setup and safety considerations can be found there. Briefly, chlorine gas (5.56 g, 78 mmol) was purged into dry [P666,14]Cl (40.66 g, 78 mmol) at room temperature in a flask protected from light. The chlorine gas was controlled by a chlorine flow meter made with chlorine compatible materials.
Dissolution Experiments
Dissolution was conducted in two series of tests with 4 mL glass vials protected from light. First, IL mixture (1 mL) with various volume fractions of [P666,14][Cl3] in [P666,14][Cl] (from 0 vol % to 100 vol %) were mixed with 21 ± 1 mg of SmCo magnet powder at 200 rpm for 24 h at room temperature (23 °C). Each leachate was then separated from the solid residue by centrifugation. Second, 1 mL of IL mixture [P666,14]2[Cl3]Cl (50 vol % [P666,14][Cl3] in [P666,14][Cl]) were mixed with different amounts of SmCo powder. The procedures for mixing and phase separation were the same as the methods mentioned above. The metal concentrations in the IL phases were measured by ICP-OES analysis. To measure metal contents in IL samples by ICP-OES analysis, a certain amount of IL samples was digested with strong acids into aqueous solutions with a microwave digestion instrument (see Instrumentation section in SI). This microwave digestion procedure is only for analytical purpose and has no influence on the leaching experiments.
The magnet dissolution rate was studied at 23 and 50 °C; 40 ± 1 mg of SmCo powder was mixed with 1 mL [P666,14]2[Cl3]Cl at 200 rpm. After a certain time, 10 μL of the IL leachate was taken out and treated though microwave digestion prior to ICP-OES analysis. The dissolution efficiency was calculated using eq 1:
| 1 |
where mL is the mass of the dissolved metal (mg) and m0 is the mass of metal in the initial sample (mg). The reported data were the average values from duplicate experiments with error bars indicated in the corresponding figures or in the caption of the figures.
Stripping Experiments
A synthetic solution was prepared by adding 121 mg of CuCl2, 780 mg of FeCl3, 1134 mg of CoCl2 and 680 mg of SmCl3·6H2O to 27.6 g of [P666,14][Cl]. After mixing at 50 °C overnight, some light-yellow crystals were found at the bottom, which are very likely to be SmCl3·6H2O, due to the poor solubility of this samarium salt in the IL. Thus, the supernatant was used as the first synthetic solution (IL-Feed-1). A second synthetic solution (IL-Feed-2) that contained copper(II) and iron(III) salts only, was prepared by adding 120 mg of CuCl2 and 780 mg of FeCl3 to 27.6 g of [P666,14][Cl]. After mixing at 50 °C overnight, no precipitate was observed. The accurate metal contents were determined by ICP-OES.
IL-Feed-1 was stripped consecutively with aqueous solutions with varying sodium chloride concentrations (0–5 mol L–1) and twice with pure water. Two liquid phases with a volume ratio of 1:1 were mixed at 200 rpm and 23 °C for 1 h and then separated by centrifugation at 5000 rpm for 5 min. Metal concentrations in the aqueous phases were measured by ICP-OES analysis.
IL-Feed-2 was stripped with various aqueous ammonia solutions (0.1–4 mol L–1) with a volume ratio of 1:1. Two liquid phases were mixed at 200 rpm and 23 °C for 1 h. After centrifuging at 5000 rpm for 30 min the mixture was separated to 2 or 3 phases: a top IL phase, a bottom aqueous phase and, if observed, a solid precipitate at the interface. Metal concentrations in both the aqueous and IL phases were determined by TXRF analysis. The stripping efficiency was calculated using eq 2:
| 2 |
where mS is the mass of the metal (mg) in the stripping solutions and m0 is the mass of metal in the initial IL feed solutions (mg). The reported data were the average values from duplicate experiments with error bars indicated in the corresponding figures or in the caption of the figures.
IL Recycling
The IL [P666,14]Cl was used in five cycles to study its reusability. Each cycle included dissolution, stripping and IL regeneration. In the first cycle, 314 mg of SmCo magnet were mixed with 8 mL of fresh [P666,14]2[Cl3]Cl at 200 rpm and at 50 °C for 3 h. The loaded IL was separated with the solid residue by centrifuging at 4000 rpm for 5 min. Four stripping steps were performed to remove metals from the loaded IL with 3 mol L–1 NaCl solution, twice water and 3 mol L–1 ammonia solution. The volume ratio of two phases was 1:1 in each step. [P666,14]Cl was recovered after removing the remaining ammonia and water at 50 °C under vacuum with a Buchi rotary evaporator. By purging desired amount of chlorine to the recovered [P666,14]Cl, [P666,14]2[Cl3]Cl was regenerated and was used in the next cycle. Identical procedures were performed for the other four cycles. Metal concentrations in aqueous solutions, in IL leachate and in recovered IL phase were determined by ICP-OES analysis. The recovered [P666,14]Cl after cycle 2 and 5 was analyzed with NMR (1H, 13C and 31P).
Results and discussion
Trichloride IL
The starting material [P666,14]Cl and the prepared [P666,14][Cl3] were characterized by NMR and Raman spectroscopy (Figures S1–S4). The overlapping NMR spectra of [P666,14][Cl3] and [P666,14]Cl indicate that the cationic structure of [P666,14][Cl3] was not destroyed by the addition of chlorine. The band at 270 cm–1 in the Raman spectrum of [P666,14][Cl3] proves the presence of the trichloride anion.17,21 The peak of free chlorine gas was not observed in Raman spectra, suggesting that all the chlorine has reacted with chloride to form trichloride anion.
Characterization of SmCo Powder
The SmCo powder was composed of 42.3 wt % Co, 22.8 wt % Sm, 22.4 wt % Fe, 4.3 wt % Cu, 2.5 wt % Zr and 5.7 wt % other elements, obtained by ICP-OES analysis after dissolving the powder into aqueous solution by microwave digestion. A total of 65 wt % of Sm and Co make recycling of these powders economically feasible. The crystalline structure of the milled magnet powder was characterized by X-ray powder diffraction. There are three main phases in the powders: Sm2Co17, SmCo5 and Sm13.7Co68.2Fe18.1 (Figure S5). Copper might be freely spread in the matrix.
Dissolution of Sm Powders
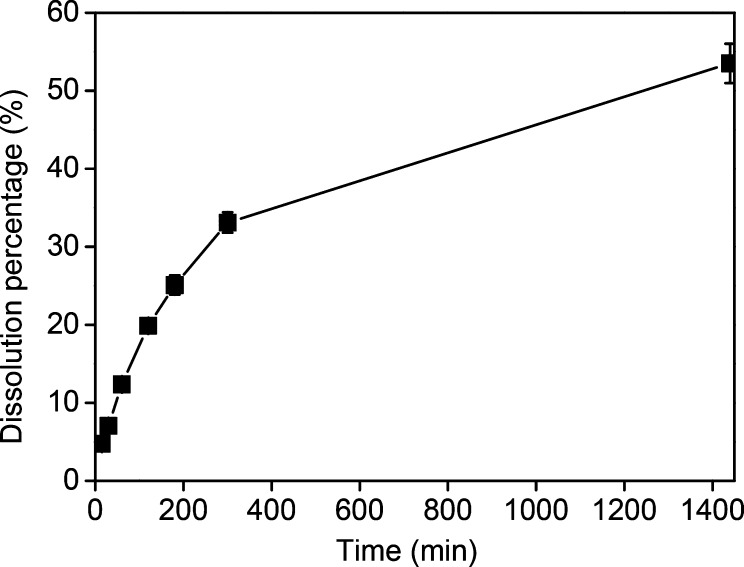
Samarium powders (<125 μm) obtained by ball-milling of metallic samarium pieces were studied for oxidative dissolution in [P666,14][Cl3] with a molar solid-to-liquid ratio of 1:12. As expected, the powders gradually dissolved in [P666,14][Cl3] and the dissolution efficiencies in Figure 1 were calculated according to eq 1. Up to 53% of samarium was dissolved after 24 h, which is 10.7 mg/g IL. As reported previously, pieces of samarium metal were unexpectedly difficult to dissolve in trichloride ILs, most likely because of the presence of a protective layer on the surface which prevents the contact of the oxidizing trichloride anion with the metal.17 Samarium powder can be dissolved in [P666,14][Cl3] but samarium pieces cannot. This is because the large surface area of the powder was composed of metal form rather than metal oxide.
Figure 1.
Dissolution percentages of samarium powder (< 125 μm) in pure [P666,14][Cl3] with a molar solid-to-liquid ratio of 1:12 as a function of time at room temperature.
Dissolution of SmCo Magnets
We first studied the dissolution efficiency of SmCo magnets in IL mixtures with various volume fractions of [P666,14][Cl3] in [P666,14]Cl at room temperature with a solid-to-liquid ratio of 20 g/L for 24 h. It is seen from Figure 2 that an increasing trend of dissolution efficiency for all metals was observed with increasing volume fraction of [P666,14][Cl3]. When the fraction of [P666,14][Cl3] was more than 40 vol %, no solid residues were observed in the vial. The full dissolution was further confirmed by ICP-OES analysis of the IL solutions. Three solutions with vol % of [P666,14][Cl3] = 80, 90 or 100% were not analyzed by ICP-OES and the dissolution efficiency was determined to be 100% according to visual observations. Raman spectra of the loaded ILs (Figure S7) confirm that when volume fraction of [P666,14][Cl3] was lower than 40%, no trichloride anion remained in the ILs, indicating that all trichloride anions were effectively consumed and reduced to chloride ions, i.e., the maximum capacity of [P666,14][Cl3] for the dissolution of SmCo magnets was reached. On the basis of the dissolution efficiency, the calculated maximum capacity was 72 ± 4 mg of SmCo magnet per gram of [P666,14][Cl3]. As shown in Figure S7, when [P666,14][Cl3] was above 40%, trichloride anions were detected, suggesting that more solids could be dissolved in the IL. Moreover, the different metals in the SmCo solid phase had the same dissolution efficiency although their reactivities toward [P666,14][Cl3] were different when pure metal was used.17 This is because these metals are alloyed in SmCo magnets in the form of three crystal phases as presented in Figure S5.
Figure 2.
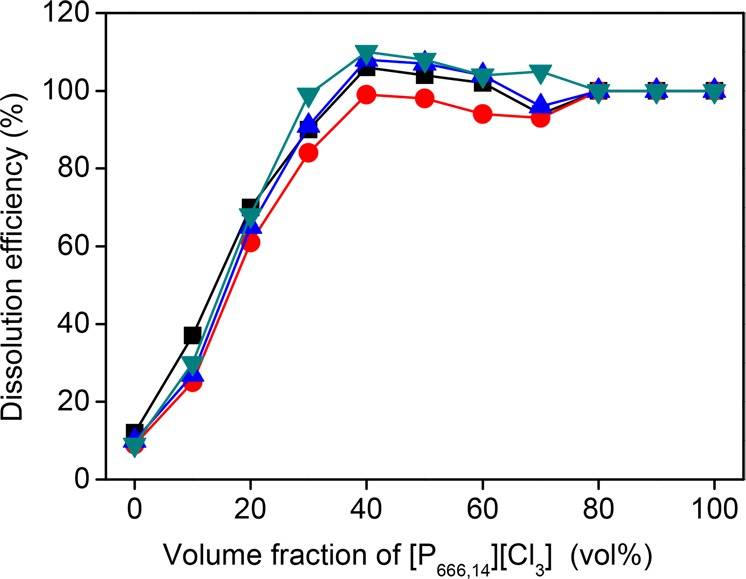
Effect of [P666,14][Cl3] concentrations in [P666,14][Cl] on the dissolution efficiency of metals (±10%) with a solid-to-liquid ratio of 20 g/L at room temperature after 24 h. Fe (■), Sm (●), Co (▲), Cu (▼).
Theoretically, two processes are involved in oxidative dissolution, i.e., oxidation and dissolution of the oxidation products. Oxidation converts the metals to the corresponding metal chlorides and simultaneously each trichloride anion is reduced to three chloride ions. In other words, after oxidation dissolution of metals, trichloride ILs are transferred to chloride ILs. The generated metal chlorides are subsequently dissolved in the IL by complexation. It has been shown by extended X-ray absorption fine structure (EXAFS) and UV–vis absorption spectroscopy that chloride salts of Fe(III), Cu(II) and Co(II) form the anionic complexes [FeCl4]−, [CuCl4]2– and [CoCl4]2–, respectively, when being dissolved in [P666,14]Cl.17,22,23 The samarium species in water-saturated [P666,14]Cl are the same as in aqueous solutions, namely [Sm(H2O)9]3+, because of the high hydration energy of trivalent lanthanide ions. But the species in dry [P666,14]Cl are different from those in aqueous solution, which is confirmed by the UV–vis absorption spectra in Figure S6. Lanthanide hexachloro complexes can be prepared in aprotic solvents or in chloride ILs.24,25 Thus, the dissolution mechanism of iron, cobalt, copper and samarium in [P666,14][Cl3] or mixture of [P666,14][Cl3] and [P666,14][Cl] is a combination of oxidation reaction and complexation according to eqs 3–6, respectively:
| 3 |
| 4 |
| 5 |
| 6 |
It can be derived from these equations that the dissolution of cobalt, copper and samarium requires not only the oxidizing agent [P666,14][Cl3], but also additional coordinating chloride ions, which can be provided by [P666,14]Cl. In other words, addition [P666,14]Cl or other complexing agent is required for oxidation dissolution of metals in [P666,14][Cl3]. When little or no [P666,14][Cl] was present in the initial mixture, [P666,14][Cl3] served both as oxidizing and complexing agent.
The dissolution of SmCo magnets was studied by varying the solid-to-liquid ratio to obtain the maximum capacity of the IL solvent [P666,14][Cl3] in [P666,14]Cl (50 vol %) (further mentioned as [P666,14]2[Cl3]Cl). As shown in Figure S8, all solids were dissolved when 20 mg of magnet was added. When increasing the amount of magnet, the dissolution efficiency decreased. The dissolved amount remained constant at 35–36 mg when 40, 60 or 80 mg of magnets was added. This indicates that the maximum dissolution capacity of the liquid was reached, which was calculated to be 71 ± 1 mg of SmCo magnet per gram of [P666,14][Cl3] in the presence of [P666,14]Cl. This value is in agreement with the results from Figure 2, suggesting that when a sufficient amount of solids were added, [P666,14][Cl3] could be effectively consumed to form [P666,14]Cl. When too large excess of solids was added, the mixture became extremely viscous after dissolution, which could give problems for the separation of the solid residues. Thus, the obtained maximum capacity of [P666,14][Cl3] is an important factor for a large-scale design where Cl2 in the trichloride anion could be fully consumed and solids (nearly) fully leached. This may lower the reagent waste and make the postseparation processes easier.
The dissolution rate of the SmCo magnet in IL mixture [P666,14]2[Cl3]Cl was studied at 23 and 50 °C. Dissolution efficiency of Sm as representative is presented in Figure 3, and the efficiencies for other metals are shown in Figure S9. It is evident that the oxidative dissolution of SmCo powders proceeded faster at higher temperature, especially at the beginning of the dissolution process. After 15 min, more than 60 wt % of the solids was dissolved at 50 °C, whereas only about 20 wt % dissolved at 23 °C. As the dissolution process proceeded, the dissolution rate decreased. Two factors simultaneously contribute to this. Chemically, the amount of the oxidizing trichloride anions was reduced, so that the contact of the reactants was limited. Physically, the viscosity of the liquid phase increased dramatically, due to the increasing amount of metal chloro-complexes. Although the formation of [FeCl4]− could lower the viscosity of ILs,26 the presence of the divalent cations Cu2+ and Co2+ in the form of the divalent complex anions [CuCl4]2– and [CoCl4]2–, increases the viscosity strongly.23 It is recommended to perform the dissolution at slightly higher temperature to reduce the process time. At 50 °C, the optimal time to reach the maximum solubility is 3 h.
Figure 3.
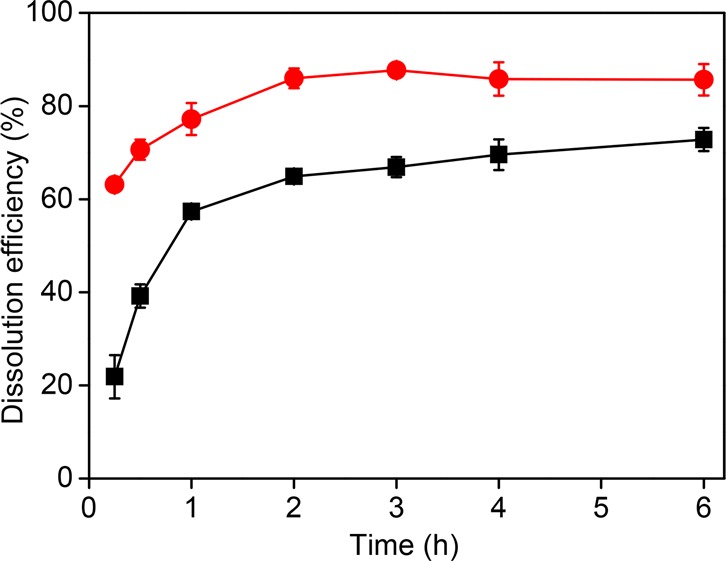
Temperature effect on the dissolution rate of Sm in SmCo magnet with IL mixture (50 vol % [P666,14][Cl3] in [P666,14][Cl]) with a solid-to-liquid ratio of 40 g/L at 23 °C (■) and 50 °C (●).
Stripping of Metals from Synthetic IL Leachates
A synthetic IL leachate labeled as IL-Feed-1 was employed for the study of the metal stripping, which was prepared by dissolving the chloride salts of iron(III), copper(II), cobalt(II) and samarium(III) in [P666,14]Cl. The metal concentrations in the synthetic IL solution were 9.0, 1.8, 12.3 and 6.2 mg g–1 for iron, copper, cobalt and samarium, respectively, mimicking those in the IL leachate when maximum amount (35 mg) of SmCo magnet was dissolved in 1 mL of [P666,14]2[Cl3]Cl. In all the stripping experiments, the volume ratio of the two phases was 1:1.
Aqueous solutions with different concentrations of NaCl were studied as stripping agent for samarium, because samarium has a higher affinity for water than for [P666,14]Cl, whereas other metals have the opposite trend of affinity.22,27 NaCl served as chloride source for the salting-out effect. It is shown in Figure 4 that when pure water was used, iron, cobalt and samarium could be stripped. As the chloride content increased, the stripping efficiency for cobalt and iron decreased, up to almost zero at a NaCl content of ≥3 mol L–1. In the entire range of tested NaCl concentrations, more than 90% of samarium could be stripped to the aqueous phase, while no copper was stripped at all. Thus, aqueous solutions of NaCl with a concentration ≥3 mol L–1 could selectively strip samarium while leaving iron, cobalt and copper in the IL phase.
Figure 4.
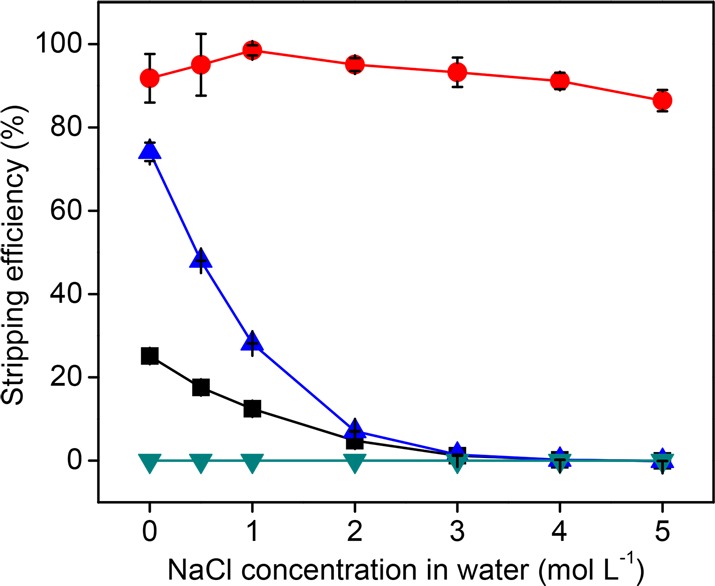
Effect of chloride concentrations in water on the stripping efficiency of metals from synthetic solution IL-Feed-1 with a liquid–liquid phase ratio of 1:1 at room temperature for 1 h. Fe (■), Sm (●), Co (▲), Cu (▼).
Pure water was used to remove cobalt from the samarium-depleted IL leachate. A two-stage cross-current stripping process was carried out to achieve complete cobalt removal. The orange bars in Figure 5 indicate the stripping efficiency of metals in the second step where pure water was used. After one contact with water, about 90% of cobalt was stripped from the samarium-depleted IL leachate. However, selective removal of cobalt was only achieved when 3 mol L–1 NaCl solution was used in the first stripping step. After second contact with water (purple bars in Figure 5), cobalt in the IL phase was completely removed, with costripping of copper and iron, but the majority in the aqueous phase was still composed of cobalt. The impurities of iron and copper in the obtained aqueous solution might be removed by extraction with [P666,14]Cl, due to its high selectivity for iron and copper over cobalt. This extraction process is not included in the present work. After the above-described stripping steps, only iron and copper remained in the IL phase.
Figure 5.
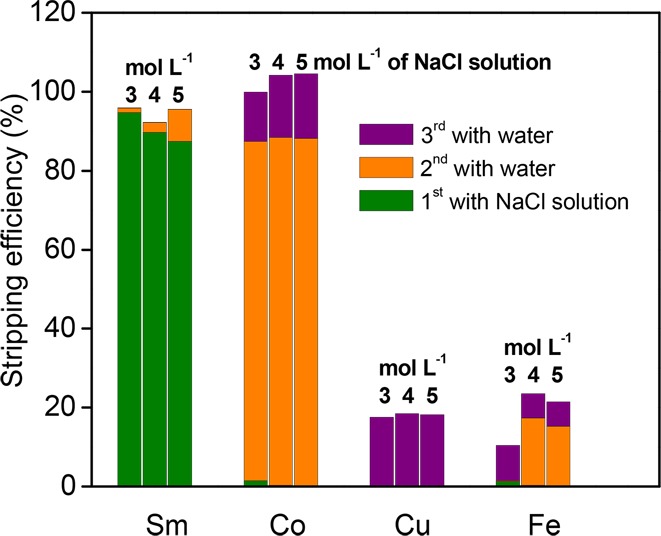
Stripping efficiency of metals (±3%) from synthetic solution IL-Feed-1 with a NaCl solution, followed by two stripping steps with pure water with a liquid–liquid phase ratio of 1:1 at room temperature for 1 h.
A synthetic solution, labeled as IL-Feed-2 and containing similar concentrations of iron and copper as IL-Feed-1, was used to study the fourth stripping step with aqueous solutions of varying NH3 concentration (0–4 mol L–1). When pure water or 0.1 mol L–1 NH3 solution was used, no color change was visually observed (Figure S10). A precipitate was formed when using 0.5 mol L–1 NH3 solution. With increasing NH3 concentration, more precipitate was formed and the color of the aqueous solution changed to blue, due to the formation of [Cu(NH3)4]2+ complex (eq 7).28
| 7 |
The concentration of metals in both phases were analyzed with TXRF analysis. It can be seen in Figure 6 that a certain amount of iron was stripped to the aqueous phase when stripping with pure water or 0.1 mol L–1 NH3 solution. The amount of iron in both phases decreased with increasing NH3 concentration, indicating that iron precipitated. When 1 mol L–1 NH3 solution was used, iron completely precipitated as a solid and iron was detected in neither the aqueous nor the IL phase. No change was observed by incrementally increasing the NH3 concentration up to 4 mol L–1. Copper remained in the IL phase and was not stripped at all, when a low concentration of NH3 (≤0.5 mol L–1) was used. When increasing the NH3 concentration to 2 mol L–1, copper was stripped to aqueous phase in the form of [Cu(NH3)4]2+. By further increasing NH3 concentration, the copper content in the IL phase dropped below 100 ppm and remained constant in the aqueous phase.
Figure 6.
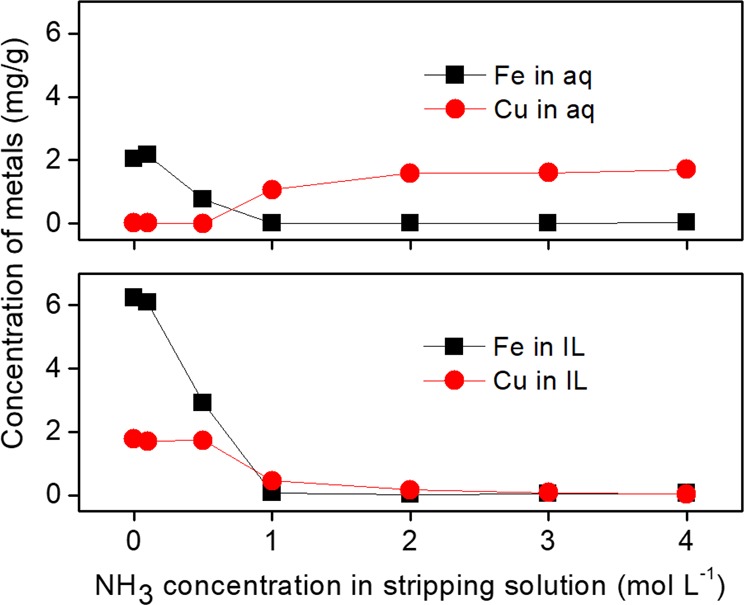
Concentration of metals (±0.05 mg/g) in the aqueous and IL phases after stripping with aqueous NH3 solutions from synthetic solution IL-Feed-2 with a liquid–liquid phase ratio of 1:1 at room temperature for 1 h.
It can be concluded from the above stripping study that four stripping steps are required to selectively remove all metals from the IL leachate to obtain single-element containing streams for further metal recovery, and to allow the recycling of IL. These stripping processes were adopted in the following section for the recycling of ILs for dissolution of real SmCo magnet.
Reusability of IL for Dissolution of SmCo Magnet
The IL mixture [P666,14]2[Cl3]Cl was used to dissolve real SmCo magnet powders and the reusability of IL [P666,14]Cl was studied in five cycles. Each cycle included dissolution of the SmCo magnet, four stripping steps, recovery of [P666,14]Cl by removing ammonia and water, and regeneration of [P666,14]2[Cl3]Cl by purging the desired amount of chlorine gas through [P666,14]Cl. All the trichloride anions [Cl3]− were converted to [Cl]− after leaching, because no trichloride peak was observed in the Raman spectra of IL leachate after leaching and IL phase after four stripping steps (see Figure S11). The metal contents in the IL leachate after cycles 1 to 5 and in the recovered IL [P666,14]Cl in the cycles 2 and 5 were analyzed with ICP-OES. The results in Table 1 show that metal contents in IL leachate in the first cycle (labeled as IL leachate 1) were in agreement with those in leachate 2–5, suggesting that the regenerated IL had similar dissolution performance as the fresh IL. Metal concentrations in both the IL and the aqueous phase after each stripping step in the first cycle are reported in Figure S12. The results of metal stripping from the real IL leachate were in agreement with those from synthetic solutions. Trace amounts of metals were observed in the recovered [P666,14]Cl in the second and fifth cycle (labeled as recovered IL 2 and 5). The similar metal concentrations in recovered IL 2 and 5 suggests that these metals did not accumulate in IL during the different cycles. NMR spectra of the starting material [P666,14]Cl and the recovered IL 2 and 5 were compared (Figures S13–S15). The similarity of the 1H, 13C and 31P NMR spectra suggests that the cation of the IL is stable and can be recycled, even after dissolution at elevated temperatures (50 °C) and stripping with alkaline NH3 solutions.
Table 1. Metal Concentrations in the IL Leachate in Each Cycle and the Recovered IL [P666,14]Cl in Cycles 2 and 5.
| Fe (g kg–1) | Sm (g kg–1) | Co (g kg–1) | Cu (g kg–1) | |
|---|---|---|---|---|
| IL leachate 1 | 8.16 | 8.34 | 15.60 | 1.74 |
| IL leachate 2 | 8.03 | 7.98 | 15.23 | 1.65 |
| IL leachate 3 | 7.92 | 7.76 | 15.25 | 1.77 |
| IL leachate 4 | 8.02 | 7.92 | 15.42 | 1.78 |
| IL leachate 5 | 7.95 | 7.93 | 15.29 | 1.77 |
| Recovered IL 2 | 0.15 | 0.03 | 0.08 | 0.05 |
| Recovered IL 5 | 0.10 | 0.02 | 0.06 | 0.04 |
Conceptual Flow Sheet
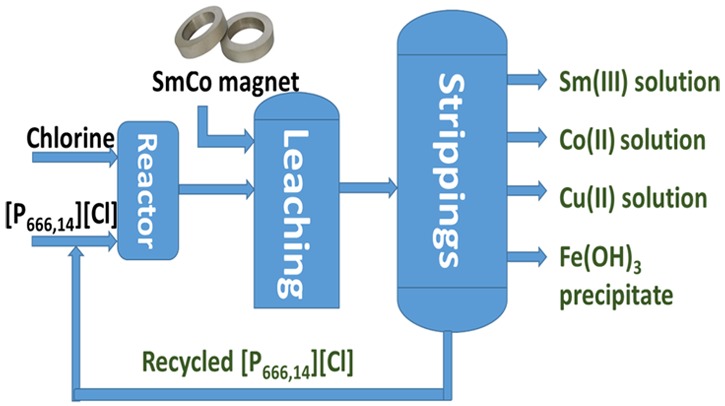
Finally, a conceptual flow sheet was proposed for the recovery of metals from SmCo magnet using a trichloride IL in which single-element aqueous streams are obtained and the IL is recycled (Figure 7). In our lab-scale experiments, stripping with water was performed in two steps, but in a large-scale process, this should be optimized by adjusting the conditions, such as phase volume ratio, temperature or process mode (counter-current instead of cross-current), etc. In our conceptual flow-sheet, the two stripping steps with water were merged into one step. The proposed process combines dissolution (leaching) and solvent extraction in one step, is thus a form of process intensification compared with the conventional processes, which require dissolution, solvent extraction and stripping. The application of trichloride ILs for dissolving metals could be used as well for other valuable waste streams such as neodymium–iron–boron magnets and nickel–metal hydride batteries, where dissolution of metals and separation of rare-earth elements from transition metals are required.
Figure 7.
Conceptual process for recovery of metals from SmCo magnets using a trichloride IL.
Conclusion
Metals in spent SmCo magnets have been recovered by a combined dissolution and stripping process. By tuning the fraction of [P666,14][Cl3] in [P666,14]Cl and the solid-to-liquid ratio, an optimized process was designed where the oxidizing agent was effectively consumed and the amount of solid residues minimized. Moreover, an elevated temperature for dissolution largely enhanced the dissolution rate of the magnet. Several stripping steps with NaCl, water and ammonia solutions were applied to subsequently recover the metals, respectively samarium, cobalt, copper and iron in different streams. Meanwhile, the IL can be regenerated and reused for next cycles. The designed conceptual process consists only of a dissolution step and a sequence of stripping steps, and is therefore a form of process intensification in comparison with the traditional approach, where also solvent extraction is required. The process developed in this work is easily transferrable to the recycling of valuable metals from other end-of-life products.
Acknowledgments
The research leading to these results received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for critical metals (SOLCRIMET) and from the European Community’s Horizon 2020 Programme ([H2020/2014-2019]) under Grant Agreement 674973 (MSCA-ETN DEMETER). The authors also thank Magneti Ljubljana for providing the magnets, Tony Debecker and Kevin Wierinckx for the pretreatment of the magnets and Tony Debecker, Paul Wijnants and Dirk Henot for their assistance in building the setup for working with chlorine gas.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.8b05604.
Materials and instrumentations, NMR and Raman spectra of ILs, X-ray diffractogram of milled SmCo magnet, UV–vis absorption spectra of samarium species, dissolution efficiencies of SmCo magnet with various solid-to-liquid ratio and temperatures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Strnat K. J.; Strnat R. M. W. Rare-earth cobalt permanent-magnets. J. Magn. Magn. Mater. 1991, 100 (1–3), 38–56. 10.1016/0304-8853(91)90811-N. [DOI] [Google Scholar]
- Buschow K. H. J. Intermetallic compounds of rare-earth and 3d transition-metals. Rep. Prog. Phys. 1977, 40 (10), 1179–1256. 10.1088/0034-4885/40/10/002. [DOI] [Google Scholar]
- Binnemans K.; Jones P. T.; Blanpain B.; Van Gerven T.; Yang Y.; Walton A.; Buchert M. Recycling of rare earths: a critical review. J. Cleaner Prod. 2013, 51, 1–22. 10.1016/j.jclepro.2012.12.037. [DOI] [Google Scholar]
- Binnemans K.; Jones P. T. Rare Earths and the Balance Problem. J. Sustain. Metall. 2015, 1 (1), 29–38. 10.1007/s40831-014-0005-1. [DOI] [Google Scholar]
- Eldosouky A.; Škulj I. Recycling of SmCo5 magnets by HD process. J. Magn. Magn. Mater. 2018, 454, 249–253. 10.1016/j.jmmm.2018.01.064. [DOI] [Google Scholar]
- Sinha M. K.; Pramanik S.; Kumari A.; Sahu S. K.; Prasad L. B.; Jha M. K.; Yoo K.; Pandey B. D. Recovery of value added products of Sm and Co from waste SmCo magnet by hydrometallurgical route. Sep. Purif. Technol. 2017, 179, 1–12. 10.1016/j.seppur.2017.01.056. [DOI] [Google Scholar]
- Xu T.; Zhang X.; Lin Z.; Lü B.; Ma C.; Gao X. Recovery of rare earth and cobalt from Co-based magnetic scraps. J. Rare Earths 2010, 28, 485–488. 10.1016/S1002-0721(10)60355-9. [DOI] [Google Scholar]
- Zhou K.; Wang A.; Zhang D.; Zhang X.; Yang T. Sulfuric acid leaching of Sm Co alloy waste and separation of samarium from cobalt. Hydrometallurgy 2017, 174, 66–70. 10.1016/j.hydromet.2017.09.014. [DOI] [Google Scholar]
- Trifonova E. N.; Drobot D. V.; Krenev V. A. Chlorination of Metallic Rhenium by Chlorine Gas in Dimethylformamide. Inorg. Mater. 2003, 39 (5), 529–531. 10.1023/A:1023637014713. [DOI] [Google Scholar]
- Chekmarev A. M.; Buchikhin E. P.; Sidorov D. S.; Koshcheev A. M. Zirconium dissolution by low-temperature chlorination in dimethylformamide. Theor. Found. Chem. Eng. 2007, 41 (5), 752–754. 10.1134/S0040579507050521. [DOI] [Google Scholar]
- Brunzie G. F.; Johnson T. R.; Steunenberg R. K. Selective Dissolution of Uranium from Uranium-Uranium Oxide Mixtures by Bromine-Ethyl Acetate. Anal. Chem. 1961, 33 (8), 1005–1006. 10.1021/ac60176a043. [DOI] [Google Scholar]
- Dai C. N.; Zhang J.; Huang C. P.; Lei Z. G. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117 (10), 6929–6983. 10.1021/acs.chemrev.7b00030. [DOI] [PubMed] [Google Scholar]
- Hallett J. P.; Welton T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111 (5), 3508–3576. 10.1021/cr1003248. [DOI] [PubMed] [Google Scholar]
- Li X.; Kersten S. R. A.; Schuur B. Extraction of Guaiacol from Model Pyrolytic Sugar Stream with Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55 (16), 4703–4710. 10.1021/acs.iecr.6b00100. [DOI] [Google Scholar]
- Dewilde S.; Vander Hoogerstraete T.; Dehaen W.; Binnemans K. Synthesis of Poly-p-phenylene Terephthalamide (PPTA) in Ionic Liquids. ACS Sustainable Chem. Eng. 2018, 6 (1), 1362–1369. 10.1021/acssuschemeng.7b03727. [DOI] [Google Scholar]
- Gai H. J.; Qiao L.; Zhong C. Y.; Zhang X. W.; Xiao M.; Song H. B. Designing Ionic Liquids with Dual Lewis Basic Sites to Efficiently Separate Phenolic Compounds from Low-Temperature Coal Tar. ACS Sustainable Chem. Eng. 2018, 6 (8), 10841–10850. 10.1021/acssuschemeng.8b02119. [DOI] [Google Scholar]
- Li X.; Van den Bossche A.; Vander Hoogerstraete T.; Binnemans K. Ionic liquids with trichloride anions for oxidative dissolution of metals and alloys. Chem. Commun. 2018, 54 (5), 475–478. 10.1039/C7CC08645H. [DOI] [PubMed] [Google Scholar]
- Bortolini O.; Bottai M.; Chiappe C.; Conte V.; Pieraccini D. Trihalide-based ionic liquids. Reagent-solvents for stereoselective iodination of alkenes and alkynes. Green Chem. 2002, 4 (6), 621–627. 10.1039/b209436n. [DOI] [Google Scholar]
- Chiappe C.; Leandri E.; Pieraccini D. Highly efficient bromination of aromatic compounds using 3-methylimidazolium tribromide as reagent/solvent. Chem. Commun. 2004, (22), 2536–2537. 10.1039/b410796a. [DOI] [PubMed] [Google Scholar]
- Van den Bossche A.; De Witte E.; Dehaen W.; Binnemans K. Trihalide ionic liquids as non-volatile oxidizing solvents for metals. Green Chem. 2018, 20 (14), 3327–3338. 10.1039/C8GC01061G. [DOI] [Google Scholar]
- Brückner R.; Haller H.; Ellwanger M.; Riedel S. Polychloride Monoanions from [Cl3]– to [Cl9]–: A Raman Spectroscopic and Quantum Chemical Investigation. Chem. - Eur. J. 2012, 18 (18), 5741–5747. 10.1002/chem.201103659. [DOI] [PubMed] [Google Scholar]
- Li Z.; Li X.; Raiguel S.; Binnemans K. Separation of transition metals from rare earths by non-aqueous solvent extraction from ethylene glycol solutions using Aliquat 336. Sep. Purif. Technol. 2018, 201, 318–326. 10.1016/j.seppur.2018.03.022. [DOI] [Google Scholar]
- Wellens S.; Thijs B.; Binnemans K. An environmentally friendlier approach to hydrometallurgy: highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem. 2012, 14 (6), 1657–1665. 10.1039/c2gc35246j. [DOI] [Google Scholar]
- Löble M. W.; Keith J. M.; Altman A. B.; Stieber S. C. E.; Batista E. R.; Boland K. S.; Conradson S. D.; Clark D. L.; Lezama Pacheco J.; Kozimor S. A.; Martin R. L.; Minasian S. G.; Olson A. C.; Scott B. L.; Shuh D. K.; Tyliszczak T.; Wilkerson M. P.; Zehnder R. A. Covalency in Lanthanides. An X-ray Absorption Spectroscopy and Density Functional Theory Study of LnCl6x– (x = 3, 2). J. Am. Chem. Soc. 2015, 137 (7), 2506–2523. 10.1021/ja510067v. [DOI] [PubMed] [Google Scholar]
- Binnemans K. Lanthanides and Actinides in Ionic Liquids. Chem. Rev. 2007, 107 (6), 2592–2614. 10.1021/cr050979c. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhou Q.; Lu X.; Zhang S. Densities and viscosities of binary mixtures of magnetic ionic liquids 1-alkyl-3-methylimidazolium tetrachloroferrate with ethyl acetate at temperatures (293.15 to 323.15)K. J. Mol. Liq. 2017, 243, 285–292. 10.1016/j.molliq.2017.08.014. [DOI] [Google Scholar]
- Vander Hoogerstraete T.; Wellens S.; Verachtert K.; Binnemans K. Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: separations relevant to rare-earth magnet recycling. Green Chem. 2013, 15 (4), 919–927. 10.1039/c3gc40198g. [DOI] [Google Scholar]
- Hu H.; Liu C.; Han X.; Liang Q.; Chen Q. Solvent extraction of copper and ammonia from ammoniacal solutions using sterically hindered β-diketone. Trans. Nonferrous Met. Soc. China 2010, 20 (10), 2026–2031. 10.1016/S1003-6326(09)60412-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.