Abstract
The prevalence of oropharyngeal squamous cell carcinoma (OPSCC) is increasing globally while the prevalence of other head and neck cancers is decreasing. The most likely reasons for this are a decreasing influence of smoking and the growing relevance of infections with the human papilloma virus (HPV) as a risk factor. A rise in the HPV‐attributable fraction (HPV‐AF) of OPSCC has been observed in many countries, yet a comprehensive review of prevalence rates and trends in Germany is lacking. To determine the current HPV‐AF of OPSCC in Germany and to assess whether it has changed during the last decades, we performed a systematic literature review. We screened Medline and EMBASE for studies that reported the tumor HPV status of newly diagnosed OPSCC patients treated at medical centers in Germany by testing for both HPV DNA and p16INK4a overexpression to confirm involvement of HPV in tumorigenesis. Out of 287 screened studies, 14 studies with data from a total of 1819 OPSCC patients treated between 1988 and 2015 were included in the data synthesis. The reported average HPV‐AF varied considerably between the studies, ranging from 11.5% (1988‐2008) to 55.0% (2004‐2009). Two of the included studies did not only provide the HPV‐AF for the entire observed calendar period but also for separate years, allowing to more accurately assess changes over time. These studies reported increases in the HPV‐AF from 21% in 2000 to 53% in 2015 and from 38% in 2004 to 71% in 2013, respectively.
Keywords: Germany, HNC, HPV, OPSCC, prevalence
1. INTRODUCTION
1.1. Background
Squamous cell carcinoma of the head and neck is the sixth most common cancer worldwide and constitutes about 90% of all head and neck cancers (HNC).1, 2, 3 In 2008, 15 583 new HNC cases and 6100 deaths were reported in Germany alone.2 Oropharyngeal squamous cell carcinoma (OPSCC) is a subtype of HNC that includes cancers originating from the oropharyngeal wall and oropharyngeal subsites (palatine tonsil, base of tongue). The main risk factors for OPSCC and other HNC that have been known for a long time are excessive alcohol and tobacco consumption.4, 5, 6 Over the last decades, changes in risk factors and incidence trends have been observed: while the number of tobacco users and the incidence of most types of HNC are declining, the incidence of OPSCC is stagnating or even increasing in many countries, especially in developed countries.7, 8, 9 For example, from 1983 to 2002, a recent global study found an increasing incidence in OPSCC in several developed countries including Denmark, the Netherlands, France, Poland, and Switzerland.10 The most likely explanation for this increase is its causal relation with the human papilloma virus (HPV), particularly with high‐risk types such as HPV16.10
High‐risk HPV is a mainly sexually transmitted oncovirus that has long been known to be responsible for nearly all cervical cancer cases worldwide.11 A link between HPV infection and OPSCC was first suspected when case‐control studies found a strong positive correlation between HPV16 seropositivity and OPSCC.12, 13, 14, 15 Further studies that investigated the presence of HPV DNA and mRNA including the expression of the viral oncogenes E6 and E7 in the tissue of HNC corroborated these results and confirmed a causal relation between HPV and OPSCC.9, 12, 16 Several lines of evidence strongly suggest that HPV‐related OPSCC is a distinct tumor entity that can be distinguished from HPV‐negative OPSCC by its etiology, molecular characteristics, and clinical presentation.17 It became evident that patients with HPV‐related OPSCC have a significantly better response to established treatment modalities and an improved survival compared to patients with HPV‐negative OPSCC.18 A difference can also be observed in the affected populations: while HPV‐negative OPSCC affects primarily elderly people and heavy tobacco users, mainly younger people develop HPV‐positive OPSCC regardless of tobacco consumption, with men being affected considerably more often than women.3, 9, 10
Both the HPV‐attributable fraction (HPV‐AF) of OPSCC and the total incidence of OPSCC have increased in many countries during the last decades.16, 19 For example, studies with patients from Australia,20 Sweden,21 and the United States16 all found a strong increase in the HPV‐AF of OPSCC, especially among men and patients below the age of 60. Globally, the HPV‐AF of OPSCC increased from 7.2% in 1990‐1994 to 32.7% in 2010‐2012.22 These results are in‐line with recent studies from Germany, which observed a strong increase in the HPV‐AF of OPSCC and a parallel decrease in tobacco‐related OPSCC over the last years.19, 23 It is expected that by 2020, the number of HPV‐positive OPSCCs will exceed the number of cervical cancers.16 Thus HPV‐associated OPSCC is increasingly becoming a core HPV disease, particularly in men.
1.2. Rationale
No in‐depth review of the literature on the HPV‐AF of OPSCC in Germany is currently available. While a recent publication assumed an HPV‐AF for OPSCC of only 16% for estimations of disease burden in Germany,24 this figure is derived from an international study that reported on the average HPV‐AF for Europe in general and not for Germany in particular.22 Even more importantly, the same study observed strong regional differences in the HPV‐AF ranging from about 7.6% in Southern Europe to 44.9% in Central Europe. Two recent publications based on data from two German medical centers reported an HPV‐AF of more than 50% that is much closer to the figure for Central Europe than to the European average.19, 23
1.3. Objectives
The aim of this systematic review was to determine the most recent HPV‐AF of OPSCC in Germany and to assess whether this fraction has changed during the last decades. For this purpose, the available literature was systematically screened for prospective and retrospective studies including patients with newly diagnosed OPSCC that were treated at a German medical center. The HPV status of the tumor had to be determined by HPV DNA PCR combined with p16INK4a immunohistochemistry on pretreatment tumor biopsies. The combined detection of HPV and p16INK4a has demonstrated good sensitivity and specificity in a recent meta‐analysis on the detection of HPV‐driven OPSCC.25
2. METHODS
We followed the PRISMA guidelines in our literature search and in the preparation of the manuscript.26
2.1. Study eligibility criteria
The PICOTS criteria (population, intervention, comparisons, outcome, time, study design) that were used for the selection of studies eligible for inclusion into the review are shown in Table 1.
Table 1.
PICOTS criteria for study inclusion
| Inclusion criteria | |
|---|---|
| Population | At least 10 patients with newly diagnosed OPSCC (including oropharyngeal sublocations such as tonsil) and treated at a German medical center |
| Intervention | HPV status determined by HPV DNA PCR combined with p16INK4a immunohistochemistry on pretreatment tumor biopsies |
| Comparisons | None |
| Outcome | HPV‐attributable fraction (prevalence of HPV DNA PCR and p16INK4a positive OPSCC) |
| Time | All eligible studies will be included regardless of publication time or study duration, if they were retrievable by the searched databases by January 10, 2018 |
| Study design | Prospective and retrospective studies including patients with newly diagnosed OPSCC without evident selection for eg stage or histology |
| Other | None |
2.2. Information sources and search
A literature search was conducted in Medline and EMBASE using the search string ([HPV AND oropharynx] AND Germany) OR ([HPV AND head and neck] AND Germany). No restrictions regarding language or publication dates were applied. The last search was run on January 10, 2018. Authors with more than one included study were contacted to confirm that study populations of multiple publications from the same medical center did not overlap, and authors were asked to provide additional data for individual years if these were not reported in the published study results.
2.3. Study selection and data extraction
The process of study selection including reasons for exclusion is illustrated in the PRISMA flow diagram (Figure 1). A data extraction sheet (Table 2) was developed, according to which extraction of the data was performed by one person and validated by a second person. Extracted data include the total number of analyzed tumors, the number of tumors that were positive for both HPV DNA and p16INK4a overexpression, and the following variables: study site, anatomic tumor location, case selection, calendar period of inclusion, and the type of HPV DNA PCR and p16INK4a test used. Tumor stage, age, gender, tobacco use, and alcohol consumption were additionally extracted if available.
Figure 1.
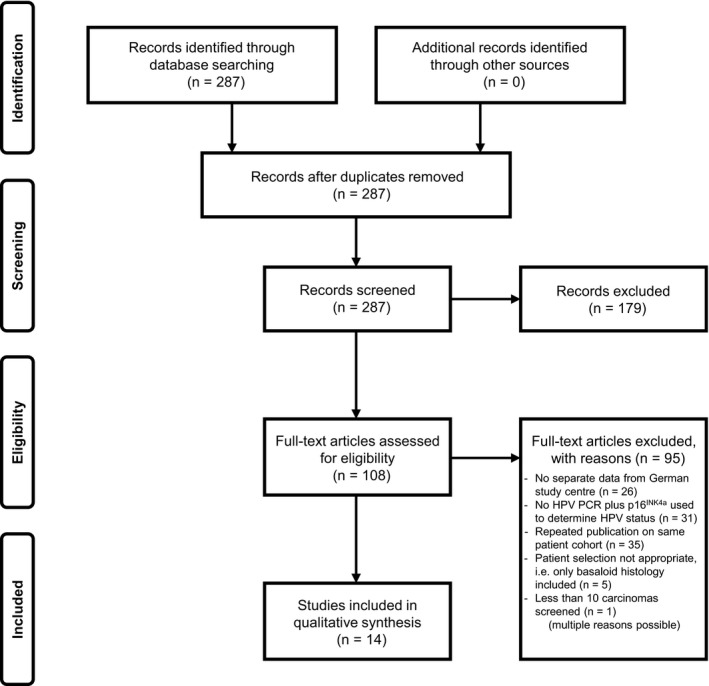
PRISMA flow diagram of the process of study selection
Table 2.
HPV prevalence and methodical details of the identified studies (FF=fresh frozen, FFPE=formalin‐fixed paraffin‐embedded)
|
Study (year of publication) N total cohort |
City | Calendar period | Case selection | OPSCC evaluable for HPV, n | OPSCC positive for HPV DNA/p16INK4a, n | OPSCC positive for HPV DNA/p16INK4a, % | HPV DNA detection method, (material, primers, read‐out) | p16INK4a detection method, (material, antibody clone, positive definition) |
|---|---|---|---|---|---|---|---|---|
|
Reimers (2007)43
N = 106 |
Cologne | 1997‐2002 | Consecutive patients with newly diagnosed and histologically confirmed OPSCC | 96 | 25 | 26.0 | FF, A5/A10 and A6/A8, electrophoresis | FFPE, 16P04, strong and diffuse cytoplasmic and nuclear staining |
|
Hoffmann (2010)44
N = 39 |
Kiel | 2004‐2007 | During surgery | 39 | 11 | 28.2 | FF, MY09/11 and GP5/GP6, multiplex genotyping (Luminex) and Southern blot | FFPE, E6H4, strong nuclear and cytoplasmic staining,> 25% |
|
Hoffmann (2012)28
N = 78 |
Kiel | 2004‐2009 | During surgery | 20 | 11 | 55.0 | FF, GP51/GP61 and MY09/11 plus a proprietary primer set, multiplex genotyping (Luminex) and PCR microarray | FFPE, E6H4, strong nuclear and cytoplasmic staining,> 25% |
|
Holzinger (2012)27
N = 196 |
Heidelberg | 1990‐2008 | Patients diagnosed with primary OPSCC and treated at the ENT department | 178 | 42 | 23.6 | FF, GP5+6+,multiplex genotyping (Luminex) | TMA, E6H4 and DSC‐106, strong nuclear and cytoplasmic staining in the proliferating tumor cells |
|
Maier (2013)45
N = 223 |
Ulm | 2001‐2012 | Patients treated at the ENT department | 102 | 30 | 29.4 | FFPE, MY09/11 plus a proprietary primer set, PCR microarray | FFPE, E6H4, strong nuclear and cytoplasmic staining |
|
Tahtali (2013)29
N = 104 |
Frankfurt | 1988‐2008 | Patients with primary tumors of the oropharynx treated at the ENT department | 104 | 12 | 11.5 | FF, MY09/11, electrophoresis | FF, E6H4, na |
|
Weiss (2013)46
N = 74 |
Münster | < 2013 | Patients diagnosed and treated at the ENT department | 59 | 28 | 47.5 | FF and FFPE, GP5+6+,na | FFPE, E6H4, strong and diffuse nuclear and cytoplasmic staining |
|
Lörincz (2014)47
N = 35 |
Hamburg | 2011‐2013 | Patients selected for TORS | 35 | 12 | 34.3 | na, na, na | na, na, na |
|
Meyer (2014)48
N = 106 |
Cologne | 2000‐2005 | Curative resection at ENT department | 93 | 25 | 26.9 | FF and FFPE, A5/A10 and A6/A8, electrophoresis | FFPE, 16P04, strong nuclear and cytoplasmic staining |
|
Quabius (2014)49
N = 36 |
Kiel | 2012 | During surgery at the ENT department | 19 | 5 | 26.3 | FF, MY09/11 and GP51/GP61, electrophoresis and Sanger sequencing | FFPE, E6H4, 30%, moderate 31‐75% and strong> 75% |
|
Tinhofer (2015)19
N = 227 |
Berlin | 2004‐2013 | Patients who had been treated at the radiation oncology department within the last 10 years | 227 | 91 | 40.1 | na, GP5+6+, multiplex genotyping (Luminex) | na, E6H4, strong nuclear staining in> 70% cells |
|
Hauck (2015)31
N = 424 |
Berlin | 1997‐2011 | Selected from pathology, according to availability of paraffin blocks | 122 | 42 | 34.4 | FFPE, GP5+6+, electrophoresis and Sanger sequencing | FFPE, E6H4, > 70% of neoplastic cells strong nuclear and/or cytoplasmic staining |
|
Hoffmann (2018)30
N = 126 |
Kiel | 2002‐2010 | During panendoscopy or surgery at the ENT | 126 | 48 | 38.1 | FFPE, MY09/11 and GP51/GP61, electrophoresis and Sanger sequencing | FFPE, E6H4, strong diffuse staining |
|
Würdemann (2017)23
N = 599 |
Giessen | 2000‐2015 | Patients diagnosed between 01/01/2000 and 07/15/2016 and sufficient tumor material available | 599 | 150 | 25.0 | FFPE, GP5+6+, multiplex genotyping (Luminex) | FFPE, E6H4, diffuse staining |
2.4. Risk of bias in individual and across studies
Only studies that tested tumors for both HPV DNA and for p16INK4a overexpression to indicate an actual involvement of HPV in tumorigenesis were included. Testing only for the presence of HPV DNA or overexpression of p16INK4a was considered insufficient. Additionally, we only included studies that reported results for the OPSCC cases separately from other HNC cases, that included patients at the time of diagnosis or treatment without evident selection for stage or histology, which provided separate data for a German medical center if an international cohort was analyzed and that tested tumors from at least 10 individual patients. To reduce bias resulting from studies with overlapping cohorts, we only included the most recent or most comprehensive publication from centers that repeatedly reported on the same patient population.
3. RESULTS
3.1. Characteristics of included studies
Out of 287 screened studies, 14 fulfilled the inclusion criteria and were included in the data synthesis. These 14 studies covered a total of 1819 individual OPSCC patients with no overlap between cohorts. A PRISMA flow diagram of the process of study selection is presented in Figure 1.
All included studies report the HPV‐AF of OPSCC in newly diagnosed patients that were treated at medical centers in Germany, using both HPV DNA PCR and p16INK4a immunohistochemistry to determine the HPV status of the tumor tissue. The studies cover varying time periods from 1988 to 2015 as shown in Table 2. The primary outcome obtained from all studies was the HPV‐AF of OPSCC. Secondary outcomes could be obtained from some of the included studies, however, often not separately for the OPSCC cases included in this review but for the entire studied HNC cohorts. These were tumor sub‐site (6/14 studies), tumor stage (12/14), age (all 14), gender, (all 14), tobacco use (8/14), and alcohol consumption (6/14). A summary of these results can be found in Table S1.
3.2. Reported HPV‐attributable fraction
The reported overall HPV‐AF varied considerably between the individual studies, ranging from 11.5%29 to 55.0%28 (Table 2). The HPV‐AF reported by the individual studies as a function of the median year of the observed time period is presented in Figure 2.
Figure 2.
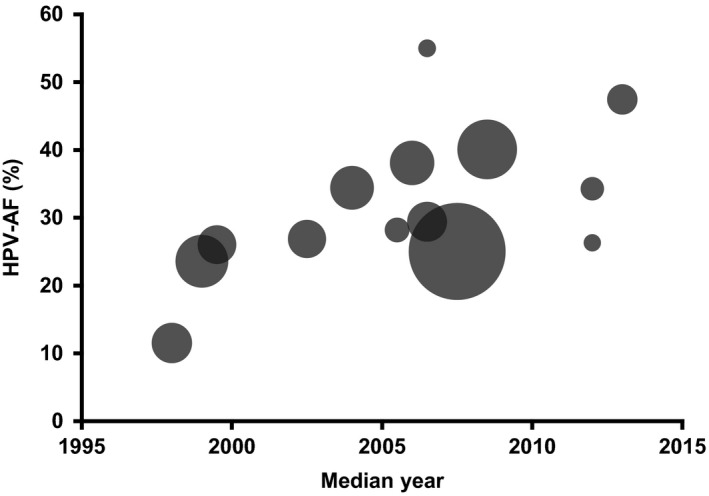
Bubble plot of the reported HPV‐AF of OPSCC as a function of the median year of the observed time period. Each bubble represents an individual study. Bubble area corresponds to the number of patients included in the study, ranging from 19 patients (smallest bubble) to 599 patients (largest bubble)
Two of the included studies did not only provide the HPV‐AF for the entire cohort of the observed period but also for separate years, allowing to more accurately assess the change in the HPV‐AF over time (Figure 3). One study (from Giessen) covering the years from 2000 to 2015 reported an HPV‐AF of 21% in 2000 and of 53% in 2015, with an average increase of 1.6% per year.23 The second study (from Berlin) covering the years from 2004 to 2013 obtained similar results, with an HPV‐AF of 38% in 2004 and 72% in 2013.19
Figure 3.
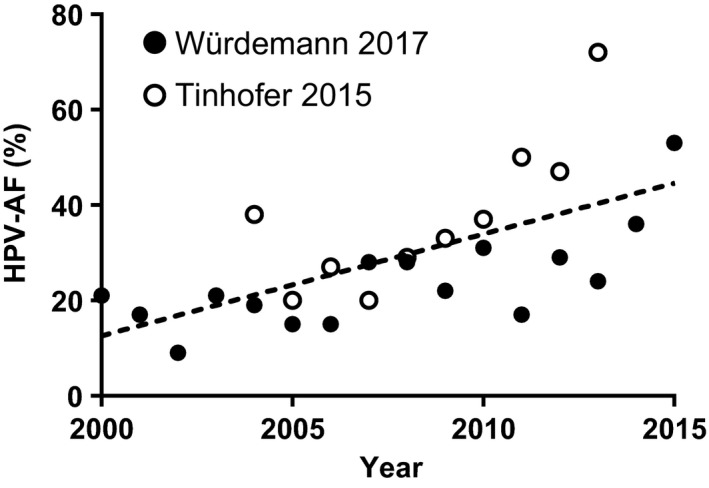
Changes in the HPV‐AF of OPSCC in two German cohorts observed between 2004‐2013 (empty circles)19 and 2000‐2015 (filled circles).23 The dashed trend line was derived by linear regression
3.3. Risk of bias within and across studies
To reduce the risk of bias in individual studies, we excluded studies that did not meet the inclusion requirements as shown in Figure 1 . To assess the risk of bias across the included studies, a funnel plot was used (Figure 4). The reported HPV‐AF was considered the primary outcome, and the number of included patients was used as a measure of study precision.
Figure 4.
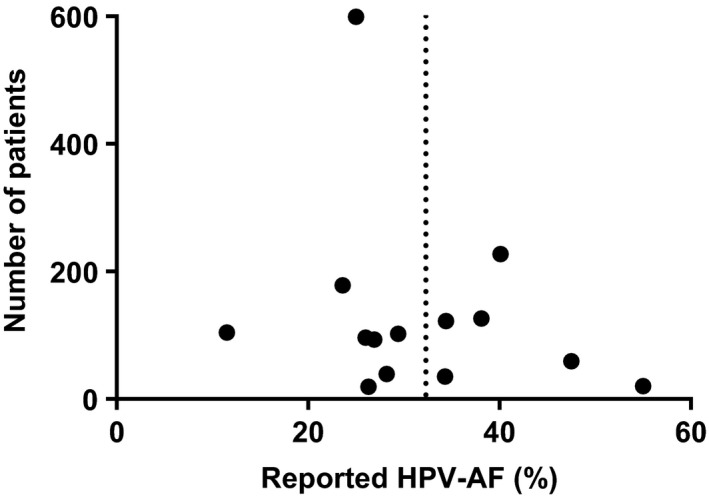
Funnel plot of study precision (number of included patients) vs primary outcome (HPV‐AF). The dotted line indicates the mean value
The distribution of the studies around the mean in the funnel plot showed no obvious asymmetry that might indicate a possible bias.
Since the reported HPV‐AF might artificially differ between studies that used HPV detection methods with different sensitivities, we tested for this potential bias by comparing the HPV‐AF reported by studies using different detection methods. To test for significance, we used either Student's t test (two parameters) or one‐way analysis of variance with Tukey's test (three or more parameters). Out of the 14 studies included into the data synthesis, 13 reported the DNA‐ and p16INK4a‐based detection method they used (Table 2). No significant difference was found between different materials, primers, or read‐outs in the case of DNA‐based methods or between different tissue fixation or antibodies in the case of p16INK4a‐based methods (Table S2). Taken together, no apparent bias based on differences in detection methods could be found.
4. DISCUSSION
4.1. Summary of evidence
This systematic literature review demonstrates that the published HPV‐AF of OPSCC in Germany varies strongly between different studies, ranging from 11.5%29 to 55.0%.28 The two included studies that provided the HPV‐AF for several successive years both observed a marked increase over time. For the most recent years they covered, 2013 and 2015, they reported a respective HPV‐AF of 72% and 53%.19, 23 It can thus be assumed that the HPV‐AF of OPSCC has increased strongly in Germany during the last decades and is currently above 50%.
4.2. Limitations
There are three major limitations that need to be considered when interpreting the data summarized in this review. The first limitation is related to the process of patient selection in the individual studies and insufficient definition and inconsistency in the published reports concerning anatomical tumor locations in the head and neck region. We therefore restricted the analysis to OPSCC in order to focus on the anatomic location most strongly associated with HPV. Studies were excluded if patient selection appeared not appropriate (ie if only basaloid histology was included). Still we were not able to separate the results for oropharyngeal sublocations, which might be important to account for the higher HPV‐AF known for tumors located at the palatine tonsils compared to base of tongue and other sites.
The second limitation of data on HPV‐AF is related to the method used to determine the HPV status, which is likely to contribute to heterogeneity between the individual studies. We only included studies that determined the HPV status by combining HPV PCR with p16INK4a immunohistochemistry to minimize this bias. Still, HPV PCR techniques are poorly harmonized and interpretation of p16INK4a immunohistochemistry can be heterogeneous. While testing for viral E6/E7 mRNA expression is a reliable alternative to testing for HPV association in OPSCC, it is less commonly used and still lacks standardization. It has been demonstrated that the combination of HPV DNA plus p16INK4a yields results comparable to E6/E7 mRNA testing.25
The third limitation is related to the varying calendar periods addressed in the studies. Since it is assumed that the HPV‐AF increased over the last decades and thus is influenced by the time period of diagnosis, data synthesis for several calendar periods would be desirable. However, the observation periods varied strongly between the individual studies, and only two studies report changes of prevalence over time. It was thus decided to restrict data synthesis and to report the prevalence of HPV in the individual studies without performing a meta‐analysis.
4.3. Comparison of the current HPV‐AF with previous studies
In a large international study from 2016, the HPV‐AF of OPSCC in Europe was reported to be 16% if determined by HPV DNA, p16INK4a and HPV mRNA, and 19.1% if determined by HPV DNA and p16INK4a as in the present review.22 This figure was presumed to be representative of the situation in Germany by some following studies.24 However, the vast majority of the samples on which the study is based were obtained before 2010, and several recent publications based on data from medical centers in Germany reported considerably higher figures for the HPV‐AF of OPSCC.19, 23, 30, 31 To address this discrepancy, the present study reviewed the available literature on the HPV‐AF of OPSCC in Germany.
The same study that calculated the average European HPV‐AF of OPSCC to be 19.1% also reported four distinct HPV‐AF values for different geographic regions of Europe as defined by the GLOBOCAN classification.32 These were 25% for Northern Europe (and up to about 50% with most test methods) and 45.7% for Central and Eastern Europe, yet only 18.1% for Western Europe and 7.7% for Southern Europe.22 However, a majority of 67.5% of the European cancer samples tested for HPV in this study was obtained from centers in Southern Europe, whereas the fractions of samples from Northern, Western, and Central/Eastern Europe were only 6.5%, 8.9%, and 17.1%, respectively. Since the total European figure was not corrected for these highly unequal regional contributions, it reflects mainly the situation in Southern Europe and not in Europe as a whole. A generalization of this figure to Europe in general or to individual European countries thus neglects the clear difference in the prevalence of HPV‐attributable OPSCC between northeastern and southwestern European countries that was reported by this study. If the four regions are weighed according to their respective population at the time of publication,33 the figure for the total European HPV‐AF of OPSCC rises to 27.8%, which is considerably higher and probably closer to the real percentage than the reported 19.1%. A recent study on the HPV‐AF of cancer cases in Germany draws on this figure, although the authors acknowledge that prevalence varies strongly between different countries and that a generalization of the European figure is thus likely an under‐ or overestimation.24 In particular, they point toward two recent studies which reported an HPV‐AF for the German OPSCC population that was much higher than the European estimate.19, 34 The results of the present systematic review support this notion since the most recent studies reviewed herein all report an HPV‐AF of OPSCC that is much higher than 19.1%.
4.4. Comparison of changes in the HPV‐AF with previous studies
Concerning a possible change in the HPV‐AF of OPSCC over time, our results indicate a strong increase during the last decades, particularly based on the results of two studies by Tinhofer et al and Würdemann et al.19, 23 In these two studies, HPV‐AF was determined individually for separate time periods (2004‐2006 to 2012‐2013) and years (2000 to 2015), respectively. Both studies concluded that there was a strong and highly significant increase in the HPV‐AF (by 115% and 152%) in the observed periods. Several studies show a similar trend for other countries such as the Netherlands,35 Denmark,36 Sweden,21 and the United States.9, 16 While we could not identify a systematic review on the HPV‐AF of OPSCC that focused on any individual European country, a recent systematic review and meta‐analysis of the literature on global trends in the prevalence of HPV in OPSCC reports a strong, significant increase from pre‐1995 to 2015 both globally and in Europe.37 That review included eight studies, which specifically reported on the situation in Germany and performed a meta‐analysis of all included studies with a median year of the sample collection period after 2000. In this meta‐analysis, the prevalence of HPV in OPSCC in Germany was calculated to be 46% (95% CI 36%‐56%). However, the presence of viral DNA in a tumor sample cannot be considered a precise surrogate for an actual etiological involvement of the virus in tumorigenesis. Since HPV DNA PCR alone was the only test for HPV performed in the studies included in that meta‐analysis, only the fraction of HPV‐positive tumors could be assessed, which is not necessarily the same as the HPV‐AF. In fact, more recent publications have shown that additional testing for either E6/E7 mRNA or p16INK4a expression is necessary to assume an actual involvement of HPV in tumorigenesis of OPSCC.12 Therefore, there was no overlap between the studies included in the review by Stein et al and the present review. It is accepted that testing for additional parameters provides a more robust picture of the actual HPV‐AF of OPSCC. A clear advantage of the present review is the requirement for p16INK4a expression testing in addition to HPV DNA among included studies. The combined analysis of p16INK4a plus HPV DNA has been demonstrated to accurately identify HPV E6/E7‐expressing OPSCC.25
While the reported increase in the HPV‐AF of OPSCC in Germany fits into the general observation of an increase in developed countries worldwide, the reasons for this increase remain unclear. Part of a possible explanation is the fact that the prevalence of tobacco use, which is a major risk factor for HPV‐negative OPSCC, has decreased markedly in developed countries since the 1980s (from 33.1% in 1980 to 23.5% in 2012), that is in parallel to the increase in HPV‐positive OPSCC.3, 38 This global trend is also reflected by the situation in Germany, where a decrease in the prevalence of tobacco use from 33.2% in 1980 to 25.0% in 201238 parallels the increase in the HPV‐AF of OPSCC observed in the present study. One might thus assume that a relative increase in the HPV‐AF can be attributed to decreasing tobacco use alone. However, since high‐risk HPV is mainly sexually transmitted, it is assumed that changes in sexual behavior in developed countries during the last decades also play a role in the increase in the HPV‐AF of OPSCC.9 An increasing number of lifetime sexual partners, increasing prevalence of oral sex, and younger age at first sexual contact are considered to be the major contributors to this trend.3, 12, 39 A recent systematic review of studies on sexual behavior as a risk factor for OPSCC found that a high number of sexual partners and performing oral sex were the two factors most strongly associated with a greater risk of OPSCC.40 In 2017, a survey of 2524 persons representative of the German population estimated the mean lifetime number of sexual partners of the opposite sex to be 10.23 for men and 5.46 for women. Regarding the prevalence of active oral sex, 51% of men and 45% of women interviewed reported to have engaged in this practice at least once in their life.41 Since this was the first ever assessment of sexual behavior in Germany based on a representative sample, no historical data are available for comparison. However, trends in behavior can be inferred from the data for different age groups obtained in this study. For example, about 75% of men and 68% of women in the age group of 24‐29 years reported to have performed oral sex on a partner at least once in their life, whereas these percentages were considerably lower in the age groups above 50 years, for example 43% and 36% in the age group of 60‐69 years and 24% and 17% in the age group of 80‐100 years, respectively. Since the reported prevalence of ever having engaged in vaginal intercourse was about equally high (88%‐98%) among all adult age groups, this data can be interpreted as evidence that the prevalence of oral sex has indeed increased in Germany since the middle of the 20th century. This assumption matches data from other countries such as the United States, where a similar trend has been reported based on historical data.37 In 2014, a study concluded that differences in oral sexual behavior alone may sufficiently explain epidemiological differences in oral infection with the OPSCC high‐risk strain HPV16 across gender, age, and race.42 This study reported that men had both a higher mean number of total sexual partners (18.4 vs 7.8) and of partners they performed oral sex on (9.9 vs 3.8) than women, which the authors conclude to be responsible for the higher prevalence of oral HPV infection among men (11.4% vs 3.3%). In general, the lifetime number of oral sex partners has been established as the factor most strongly associated with OPSCC.39
5. CONCLUSION
The majority of studies included in the present review report an HPV‐AF of OPSCC in Germany that is considerably higher than the previously assumed 16.0%. This is especially true for studies covering recent time periods, which report HPV‐AF values of up to 55%.28 Comparing the reported HPV‐AF with the observed time periods strongly indicates that the HPV‐AF of OPSCC in Germany has increased notably over the last decades. Since it is assumed that there is a time lag of about 20‐30 years between initial HPV infection and cancer development,37 a person that was exposed to HPV at age 25 would develop cancer at age 45‐55. Considering that the prevalence of performing oral sex has strongly increased in recent generations and is highest in individuals now aged 24‐29 years,41 it can be assumed that the incidence of HPV‐attributable OPSCC will continue to rise.
CONFLICT OF INTEREST
Miriam Reuschenbach is an employee of MSD Sharp & Dohme GmbH and MSD Sharp & Dohme GmbH Germany provided funding for this work. Jens Peter Klussmann received a research grant from MSD Sharp & Dohme GmbH Germany, and Claus Wittekindt and Steffen Wagner are participants of this granted research project. Ingeborg Tinhofer has nothing to disclose.
Supporting information
ACKNOWLEDGMENTS
The authors thank Markus Hoffmann for contributing valuable information on the patient cohorts covered by his publications. Writing assistance in the preparation of this manuscript was provided by Philip Böhler, KW medipoint.
Reuschenbach M, Tinhofer I, Wittekindt C, Wagner S, Klussmann JP. A systematic review of the HPV‐attributable fraction of oropharyngeal squamous cell carcinomas in Germany. Cancer Med. 2019;8:1908–1918. 10.1002/cam4.2039
REFERENCES
- 1. Gillison ML. Human papillomavirus‐associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744‐754. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893‐2917. [DOI] [PubMed] [Google Scholar]
- 3. Tribius S, Hoffmann M. Human papilloma virus infection in head and neck cancer. Dtsch Ärztebl Int. 2013;110:184‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maasland DH, van den Brandt PA, Kremer B, Goldbohm RA, Schouten LJ. Alcohol consumption, cigarette smoking and the risk of subtypes of head‐neck cancer: results from the Netherlands Cohort Study. BMC Cancer. 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewin F, Norell SE, Johansson H, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck. Cancer. 1998;82:1367‐1375. [DOI] [PubMed] [Google Scholar]
- 6. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282‐3287. [PubMed] [Google Scholar]
- 7. Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence. Cancer. 2007;110:1429‐1435. [DOI] [PubMed] [Google Scholar]
- 8. Conway DI, Stockton DL, Warnakulasuriya KA, Ogden G, Macpherson LM. Incidence of oral and oropharyngeal cancer in United Kingdom (1990–1999) – recent trends and regional variation. Oral Oncol. 2006;42:586‐592. [DOI] [PubMed] [Google Scholar]
- 9. Marur S, D'Souza G, Westra WH, Forastiere AA. HPV‐associated head and neck cancer: a virus‐related cancer epidemic. Lancet Oncol. 2010;11:781‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaturvedi AK, Anderson WF, Lortet‐Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550‐4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12‐19. [DOI] [PubMed] [Google Scholar]
- 12. Furniss CS, McClean MD, Smith JF, et al. Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120:2386‐2392. [DOI] [PubMed] [Google Scholar]
- 13. D'Souza G, Kreimer AR, Viscidi R, et al. Case‐control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944‐1956. [DOI] [PubMed] [Google Scholar]
- 14. Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772‐1783. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626‐1636. [DOI] [PubMed] [Google Scholar]
- 16. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vokes EE, Agrawal N, Seiwert TY. HPV‐associated head and neck cancer. J Natl Cancer Inst. 2015;107:djv344–djv. [DOI] [PubMed] [Google Scholar]
- 18. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tinhofer I, Johrens K, Keilholz U, et al. Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur J Cancer. 2015;51:514‐521. [DOI] [PubMed] [Google Scholar]
- 20. Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28:3269‐3272. [DOI] [PubMed] [Google Scholar]
- 21. Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620‐2623. [DOI] [PubMed] [Google Scholar]
- 22. Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:djv403. [DOI] [PubMed] [Google Scholar]
- 23. Würdemann N, Wagner S, Sharma SJ, et al. Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol. 2017;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buttmann‐Schweiger N, Delere Y, Klug SJ, Kraywinkel K. Cancer incidence in Germany attributable to human papillomavirus in 2013. BMC Cancer. 2017;17:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prigge ES, Arbyn M, von Knebel DM, Reuschenbach M. Diagnostic accuracy of p16(INK4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta‐analysis. Int J Cancer. 2017;140:1186‐1198. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holzinger D, Schmitt M, Dyckhoff G, Benner A, Pawlita M, Bosch FX. Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res. 2012;72:4993‐5003. [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann M, Tribius S, Quabius ES, et al. HPV DNA, E6*I‐mRNA expression and p16INK4A immunohistochemistry in head and neck cancer ‐ how valid is p16INK4A as surrogate marker? Cancer Lett. 2012;323:88‐96. [DOI] [PubMed] [Google Scholar]
- 29. Tahtali A, Hey C, Geissler C, et al. HPV status and overall survival of patients with oropharyngeal squamous cell carcinoma–a retrospective study of a German head and neck cancer center. Anticancer Res. 2013;33:3481‐3485. [PubMed] [Google Scholar]
- 30. Hoffmann M, Quabius ES, Tribius S, et al. Influence of HPV‐status on survival of patients with tonsillar carcinomas (TSCC) treated by CO2‐laser surgery plus risk adapted therapy ‐ A 10 year retrospective single centre study. Cancer Lett. 2018;413:59‐68. [DOI] [PubMed] [Google Scholar]
- 31. Hauck F, Oliveira‐Silva M, Dreyer JH, et al. Prevalence of HPV infection in head and neck carcinomas shows geographical variability: a comparative study from Brazil and Germany. Virchows Arch. 2015;466:685‐693. [DOI] [PubMed] [Google Scholar]
- 32. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 33. United Nations, World population prospects: the 2017 revision. ESA/P/WP/248., 2017.
- 34. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta‐analysis. Lancet Oncol. 2014;15:1319‐1331. [DOI] [PubMed] [Google Scholar]
- 35. Rietbergen MM, Leemans CR, Bloemena E, et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132:1565‐1571. [DOI] [PubMed] [Google Scholar]
- 36. Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733‐741. [DOI] [PubMed] [Google Scholar]
- 37. Stein AP, Saha S, Kraninger JL, et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21:138‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183‐192. [DOI] [PubMed] [Google Scholar]
- 39. Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus‐related oropharyngeal cancer. Ann Oncol. 2017;28:2386‐2398. [DOI] [PubMed] [Google Scholar]
- 40. Chancellor JA, Ioannides SJ, Elwood JM. Oral and oropharyngeal cancer and the role of sexual behaviour: a systematic review. Community Dent Oral Epidemiol. 2016;45:20‐34. [DOI] [PubMed] [Google Scholar]
- 41. Haversath J, Gärttner KM, Kliem S, et alSexual behavior in Germany: results of a representative survey. Dtsch Ärztebl Int. 2017;114:545‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D'Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS ONE. 2014;9:e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV‐DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731‐1738. [DOI] [PubMed] [Google Scholar]
- 44. Hoffmann M, Ihloff AS, Gorogh T, et al. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595‐1602. [DOI] [PubMed] [Google Scholar]
- 45. Maier M, Kraft K, Steinestel K, et al. [Human papillomavirus in squamous cell cancer of the head and neck. A study at the Ulm Military Hospital, Germany]. HNO. 2013;61:593‐601. [DOI] [PubMed] [Google Scholar]
- 46. Weiss D, Stockmann C, Schrodter K, Rudack C. Protein expression and promoter methylation of the candidate biomarker TCF21 in head and neck squamous cell carcinoma. Cell Oncol (Dordr). 2013;36:213‐224. [DOI] [PubMed] [Google Scholar]
- 47. Lörincz BB, Möckelmann N, Busch CJ, Knecht R. Functional outcomes, feasibility, and safety of resection of transoral robotic surgery: single‐institution series of 35 consecutive cases of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck. 2015;37:1618‐1624. [DOI] [PubMed] [Google Scholar]
- 48. Meyer MF, Seuthe IM, Drebber U, et al. Valosin‐containing protein (VCP/p97)‐expression correlates with prognosis of HPV‐ negative oropharyngeal squamous cell carcinoma (OSCC). PLoS ONE. 2014;9:e114170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quabius ES, Moller P, Haag J, et al. The role of the antileukoprotease SLPI in smoking‐induced human papillomavirus‐independent head and neck squamous cell carcinomas. Int J Cancer. 2014;134:1323‐1334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


