Abstract
Background
Treatment with epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) leads to initial response in most patients with EGFR‐mutated non‐small cell lung cancer (NSCLC). In contrast, little is known of the subpopulation of patients with NSCLC with EGFR mutations who exhibit clinical outcomes that require treatment with immune checkpoint inhibitors (ICIs). Therefore, to identify eligible cases to treat with ICIs, we retrospectively analyzed the correlation between clinical features and the efficacy of ICIs in patients with EGFR mutations.
Patients and Methods
We retrospectively analyzed patients with advanced NSCLC harboring EGFR mutations who were treated with ICIs after developing resistance to EGFR‐TKIs between February 2016 and April 2018 at 6 institutions in Japan. The association between clinical outcomes and the efficacy of ICIs was investigated.
Results
We enrolled 27 patients who harbored EGFR‐activating mutations. The objective response and disease control rates were higher in patients with uncommon EGFR mutations than in those with common EGFR mutations (71% vs 35.7% and 57% vs 7%, P = 0.14 and P < 0.01, respectively). Patients with uncommon EGFR mutations or without T790M mutations exhibited a significantly longer median progression‐free survival than those with common EGFR mutations or with T790M mutations (P = 0.003 and P = 0.03, respectively).
Conclusion
Patients with uncommon EGFR mutations and without T790M mutations are associated with the best outcomes for treatment with immunotherapy among those with EGFR‐mutated NSCLC, based on retrospective analysis. Further research is needed to validate the clinical biomarkers involved in ICI responders with EGFR mutations.
Keywords: biomarker, EGFR mutation, immunology, non‐small cell lung cancer
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths worldwide.1 Recently, some types of molecular‐targeted therapy and angiogenesis inhibitors have been successfully introduced as lung cancer treatments. Epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) have been shown to be effective in the treatment of non‐small cell lung cancer (NSCLC) in patients with mutant EGFR.2, 3, 4, 5 Although EGFR‐TKIs may lead to initial clinical benefits in most patients with EGFR‐mutated NSCLC, these patients develop acquired resistance to various EGFR‐TKIs. Therefore, novel therapeutic strategies after resistance to EGFR inhibitors are still needed to improve the prognosis of patients with EGFR‐driven lung cancers.
Recently, programmed cell death protein 1 (PD‐1)/programmed death ligand 1 (PD‐L1) checkpoint inhibitors are promising alternative treatments for NSCLC. Of them, nivolumab, pembrolizumab, and atezolizumab have been approved in the United States, Japan, and other countries for the treatment of patients with metastatic NSCLC based on some phase III trials that showed the superior outcomes of PD‐1/PD‐L1 checkpoint inhibitors compared with standard systemic chemotherapy in patients with NSCLC.6, 7, 8, 9, 10 Several mechanisms for poor responses to immune checkpoint inhibitors (ICIs) have been reported, such as a lower tumor mutation burden and an uninflamed and immunosuppressive tumor microenvironment.11 A recent retrospective study showed that subgroups with oncogenic driver mutations, including EGFR and anaplastic lymphoma kinase (ALK), tend to show a reduced response to PD‐1/PD‐L1 inhibitors regarding objective response rates and progression‐free survival (PFS) when compared with wild‐type EGFR and ALK‐negative/unknown subgroups among patients with NSCLC.12 To date, little is known about the subpopulation of patients with NSCLC with EGFR mutations who exhibit clinical outcomes upon receiving ICI treatments. Therefore, to identify eligible patients to treat with ICIs, we retrospectively analyzed the correlations between clinical features and the efficacy of ICIs in patients with EGFR mutations.
2. MATERIALS AND METHODS
2.1. Patients
We enrolled 27 patients with EGFR‐activating mutations who were diagnosed with NSCLC, and treated with EGFR‐TKIs and ICIs at 6 different institutions in Japan (University Hospital Kyoto Prefectural University of Medicine, Japanese Red Cross Kyoto Daiichi Hospital, Japanese Red Cross Kyoto Daini Hospital, Uji‐Tokushukai Medical Center, Fukuchiyama City Hospital, and Niigata University Medical and Dental Hospital) between February 2016 and May 2018, regardless of receiving any previous cytotoxic chemotherapy‐containing treatment. We obtained the patients’ clinical data from retrospective medical records, as follows: age, sex, histological subtype, the levels of PD‐L1 expression in tumors, EGFR mutation status at baseline, with or without the emergence of EGFR‐T790M mutation, disease stage, Eastern Cooperative Oncology Group PS, smoking status, the progression‐free survival (PFS), the time to treatment failure (TTF), response rate, disease control rate of patients on initial EGFR‐TKI, and ICI treatments based on the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST). This study protocol was approved by the ethics committees of each hospital. The tumor–node–metastasis (TNM) stage was classified using version 7 of the TNM stage classification system.
2.2. Tumor genomic analysis
EGFR mutations were detected using one of the following methods: the peptide nucleic acid–locked nucleic acid clamp (LSI Medience, Tokyo, Japan), Cycleave PCR (Takara bio, Kusatsu, Japan), or Cobas EGFR mutation test (Roche Molecular Systems, Pleasanton, CA), with sequencing of exons 18‐21 being performed at commercial clinical laboratories (SRL, Inc and BML, Inc, Tokyo, Japan).
2.3. Tumor PD‐L1 analysis
PD‐L1 expression was analyzed at SRL, Inc with the PD‐L1 IHC 22C3 pharmDx assay or 28‐8 pharmDx assay (Agilent Technologies, Santa Clara, CA). The PD‐L1 tumor proportion score (TPS) was calculated as the percentage of at least 100 viable tumor cells for complete or partial membrane staining. The pathologists of the commercial vendor provided the TPS interpretation.
2.4. Immunotherapy
The anti‐PD‐1 antibodies administered were nivolumab and pembrolizumab. Nivolumab and pembrolizumab were intravenously administered at doses of 3 mg/kg every 2 weeks and 1200 mg every 3 weeks, respectively. In general, these treatments continued until disease progression, intolerable toxicity, or patient refusal was encountered.
2.5. Statistical analysis
Cox proportional hazards models were used, considering age, sex, PS, smoking history, histological type, best response to initial EGFR‐TKIs, metastatic lesions, staging, regimen of ICIs, status of EGFR mutation, EGFR‐T790M mutation, levels of PD‐L1 expression in tumors, levels of serum albumin, and neutrophil/lymphocyte ratios (NLRs). To analyze the TTF and PFS, times to events were estimated using the Kaplan–Meier method and compared using the log‐rank test. The TTF and PFS were censored at the date of the last visit for patients who remained alive without any documented disease progression. The tumor response was evaluated in accordance with the RECIST, version 1.1. All statistical analyses were performed using Prism (version 7.02; GraphPad Software Inc, CA). All p values less than 0.05 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics
A total of 27 patients with NSCLC who received ICIs, as well as EGFR‐TKIs, which were treated more than one compound, between February 2016 and April 2018 at 6 institutions in Japan were included. Of them, 8 (30%) patients were male and 20 (74%) were never‐smokers, and the median age of all patients was 67 years (range, 37‐82 years). The histological subtypes were adenocarcinoma in 26 patients (96%) and large cell neuroendocrine carcinoma in 1 patient (4%). Twenty‐three patients (85%) had a performance status of 0 or 1. The sites of metastatic disease were the bone, brain, and liver in 12 (44%), 11 (41%), and 4 (15%) patients, respectively. Eighteen patients (67%) had stage IV disease and 9 patients (33%) exhibited recurrence. Twenty‐one (78%) and 6 (22%) patients were administered nivolumab and pembrolizumab, respectively. EGFR mutations at baseline were detected as follows: 8 patients harbored a deletion in exon 19, 12 patients harbored an L858R missense mutation in exon 21, 4 patients harbored a G719X mutation in exon 18, and 3 patients harbored an insertion mutation in exon 20. EGFR‐T790M mutations after developing resistance to initial EGFR‐TKI treatment were detected in 8 patients, and were not detected in 19 patients (Table 1).
Table 1.
Patients’ characteristics
| Patient characteristics | No. of patients (N = 27) |
|---|---|
| N (%) | |
| Age | |
| Median (Range) | 67.0 (37.0‐82.0) |
| Sex | |
| Male | 8 (29.6) |
| Female | 19 (70.4) |
| PS | |
| 0/1 | 23 (85.2) |
| 2 | 4 (14.8) |
| Histology | |
| Adenocarcinoma | 26 (96.3) |
| LCNEC | 1 (3.7) |
| Smoking status | |
| Never‐smoker | 20 (74.1) |
| Smoker | 7 (25.9) |
| Best response to EGFR‐TKIs | |
| CR/PR | 14 (51.9) |
| SD | 8 (29.6) |
| PD | 1 (3.7) |
| NE | 4 (14.8) |
| Sites of metastatic disease | |
| Bone | 12 (44.4) |
| Brain | 11 (40.7) |
| Liver | 4 (14.8) |
| Stage | |
| IV | 18 (66.7) |
| Postoperative recurrence | 9 (33.3) |
| ICI treatment | |
| Nivolumab | 21 (77.8) |
| Pembrolizumab | 6 (22.2) |
| EGFR mutations | |
| EGFR Ex19del | 8 (29.6) |
| EGFR L858R | 12 (44.4) |
| EGFR G719X | 4 (14.8) |
| EGFR exon20 ins | 3 (11.1) |
| Best overall response for ICIs | |
| CR/PR | 6 (22.2) |
| SD | 5 (18.5) |
| PD | 13 (48.1) |
| NE | 3 (11.1) |
| PD‐L1 TPS | |
| TPS ≧50% | 6 (22.2) |
| TPS 1~49% | 5 (18.5) |
| TPS <1% | 6 (22.2) |
| NE | 10 (37.0) |
3.2. Association between clinical features and outcomes for patients with EGFR‐mutated NSCLC who were treated with ICIs
We showed that a small proportion of patients with lung adenocarcinoma with EGFR mutations exhibited favorable clinical benefits when treated with ICIs, nivolumab and pembrolizumab. Of 27 patients with NSCLC with EGFR mutations, no patients achieved complete response (CR; 0%), 6 achieved partial response (PR; 22.2%), 5 achieved stable disease (18.5%), 13 achieved progressive disease (48.1%), and 3 were unevaluable (11.1%) when treated with ICIs, which was indicated in a response rate of 22% and disease control rate of 41% (Figure 1A). The median PFS was 57.5 days (8‐612 days) and median TTF was 76.5 days (8‐612 days) (Figure 2A,B).
Figure 1.
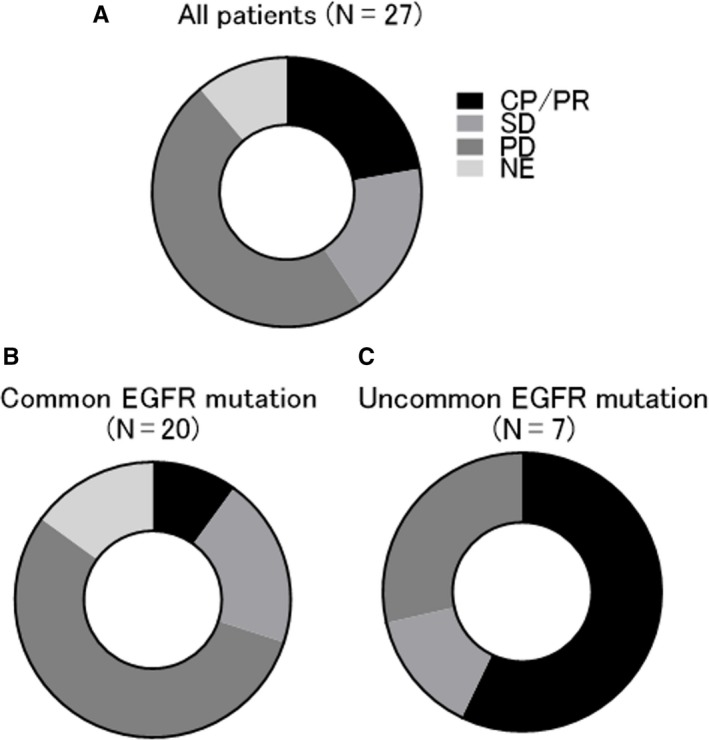
Frequency of best overall response to immune checkpoint inhibitors (ICIs) after acquired resistance to EGFR‐TKI treatment in patients with EGFR‐mutated NSCLC. Frequency of best overall response to ICIs for all patients (N = 27) (A), patients with common EGFR mutations (N = 20) (B), and patients with uncommon EGFR mutations (N = 7) (C) are shown in the pie chart. ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non‐small cell lung cancer
Figure 2.
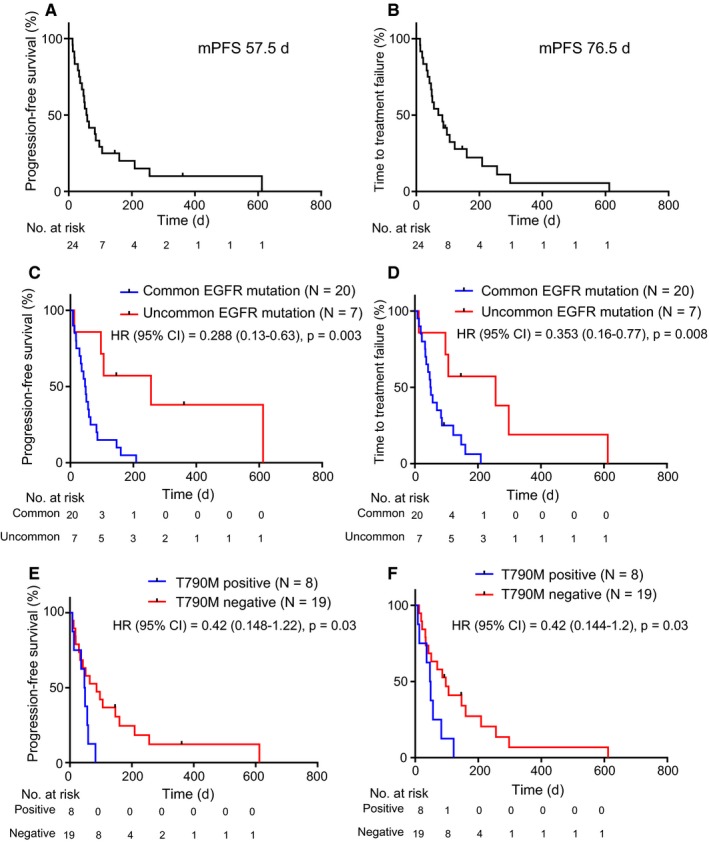
Kaplan‐Meier curves for PFS and TTF in patients with EGFR‐mutated NSCLC treated with immune checkpoint inhibitors after acquired resistance to EGFR‐TKI treatment. (A, B) PFS (A) and TTF (B) curves for all patients (N = 27), and (C, D) PFS (C) and TTF (D) curves for patients with common (N = 20) and uncommon (N = 7) EGFR mutations. (E, F) PFS (E) and TTF (F) curves for patients with T790M‐positive (N = 7) and T790M‐negative (N = 17) EGFR mutations. Column signs denote censoring. PFS, progression‐free survival; TTF, time to treatment failure; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non‐small cell lung cancer
To assess whether the clinical features might be a determinant of ICI efficacy in patients with EGFR mutations, we next investigated the association between some clinical parameters and the responders to ICI treatment, which were defined as the CR and PR cases based on the evaluation of RECIST criteria. Of various clinical parameters, only patients with uncommon EGFR mutations, including G719X in exon 18 and insertion in exon 20, significantly correlated with responding to ICIs, compared to those with common EGFR mutations (hazard ratio of 0.047 with 95% confidence interval of 0.004‐0.557, P = 0.015) (Figure 1B,C, Table 2). Although the PD‐L1 TPS in tumors, smoking status, and location of metastatic lesions were known to be predictive factors for therapeutic effects of ICI treatment in NSCLC with wild‐type EGFR, there were no significant differences in this study. We next evaluated the correlation between the disease control rate following ICI administration and patient factors, including EGFR mutation status and the detection of T790M mutations, that were demonstrated to be relatively promising predictors in the analysis of ICI responders, as shown in Table 2. Although these factors tend to be adequate candidates for prediction, there was no significant correlation with the disease control rate following ICI administration (Supplementary Table S1). We next evaluated the correlation between patient profiles and clinical outcomes of ICI treatment, such as PFS and TTF. Of them, patients with uncommon EGFR mutations had significantly better PFS and TTF compared with those in patients with common EGFR mutations (256 days vs 50 days, 256 days vs 48 days; hazard ratios of 0.288 and 0.353 with 95% confidence intervals of 0.13‐0.63 and 0.16‐0.77; P = 0.003 and 0.008; respectively) (Figure 2C, 2D). In addition, patients with T790M mutations when treated with ICIs had significantly better PFS and TTF compared with those in patients without T790M mutations (86 days vs 48 days, 97 days vs 48 days; hazard ratios of 0.42 and 0.42 with 95% confidence intervals of 0.148‐1.22 and 0.144‐1.2; P = 0.03 and 0.03; respectively) (Figure 2E,F). Finally, we validated two promising predictive factors: uncommon EGFR mutations and the absence of T790M mutations. T790M mutations were not detected in our analysis in all 7 patients with uncommon EGFR mutations. In the cases of common EGFR mutations, T790M mutations were detected in 8 patients and but not in 12 patients. These 3 groups (uncommon EGFR mutation plus T790M mutation‐positive, common EGFR mutation plus T790M mutation‐positive, and common EGFR mutation plus T790M mutation‐negative) were statistically different according to PFS and TTF following ICI treatment (P = 0.006 and P = 0.012, respectively) (Figure 3). These results showed that uncommon EGFR mutations in the absence of T790M mutations in patients might be a most potent predictor to detect the responders of ICIs among patients with EGFR‐mutated NSCLC.
Table 2.
Predictive factors according to the response to immune checkpoint inhibitors based on single‐variable analysis
| Characteristic | Patients (N = 24) | ICI Response | Odds ratio | p value | |
|---|---|---|---|---|---|
| Responder (N = 6) | non‐Responder (N = 18) | (95% CI) | |||
| Age (year) | |||||
| <70 | 17 | 4 | 13 | 0.769 | >0.999 |
| ≧70 | 7 | 2 | 5 | (0.11~5.10) | |
| Gender | |||||
| Male | 8 | 2 | 6 | 1 | >0.999 |
| Female | 16 | 4 | 12 | (0.16~6.50) | |
| PS | |||||
| 0/1 | 22 | 6 | 16 | ||
| 2 | 2 | 0 | 2 | ||
| Smoking status | |||||
| Never‐smoker | 17 | 4 | 13 | 0.769 | >0.999 |
| Smoker | 7 | 2 | 5 | (0.11~5.10) | |
| Best response to EGFR‐TKIs | |||||
| CR/PR | 11 | 2 | 9 | 0.5 | 0.649 |
| SD+PD+NE | 13 | 4 | 9 | (0.08~3.04) | |
| Sites of CNS metastasis | |||||
| Present | 9 | 3 | 6 | 2 | 0.635 |
| Absent | 15 | 3 | 12 | (0.37~10.42) | |
| Sites of Bone metastasis | |||||
| Present | 11 | 3 | 8 | 1.25 | >0.999 |
| Absent | 13 | 3 | 10 | (0.24~6.49) | |
| Sites of Liver metastasis | |||||
| Present | 2 | 1 | 1 | 3.4 | 0.446 |
| Absent | 22 | 5 | 17 | (0.15~67.66) | |
| Stage | |||||
| IV | 16 | 3 | 13 | 0.385 | 0.362 |
| Postoperative recurrence | 8 | 3 | 5 | (0.073~2.18) | |
| ICI treatment | |||||
| Nivolumab | 18 | 5 | 13 | 1.92 | >0.999 |
| Pembrolizumab | 6 | 1 | 5 | (0.21~26.71) | |
| EGFR mutation | |||||
| Common mutation | 17 | 1 | 16 | 0.047 | 0.015 |
| Uncommon mutation | 7 | 4 | 3 | (0.004~0.557) | |
| EGFR‐T790M mutation | |||||
| Present | 7 | 0 | 7 | 0.121 | |
| Absent | 17 | 6 | 9 | ||
| PD‐L1 TPS | |||||
| TPS ≧50% | 6 | 2 | 4 | 1.75 | 0.618 |
| TPS <50%+NE | 18 | 4 | 14 | (0.25~14.64) | |
| Alb | |||||
| <3.5 mg/dl | 8 | 2 | 6 | 1 | >0.999 |
| ≧3.5 mg/dl | 16 | 4 | 12 | (0.16~6.53) | |
| NLR | |||||
| <4.0 | 17 | 6 | 11 | 0.13 | |
| ≧4.0 | 7 | 0 | 7 | ||
CI, confidence interval.
Figure 3.
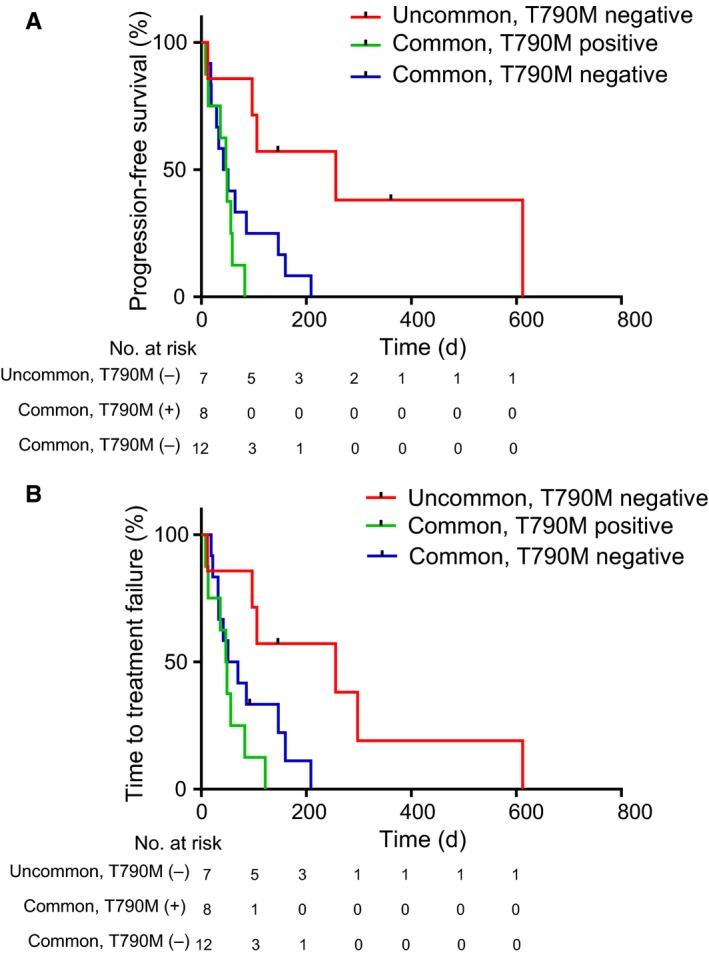
Kaplan–Meier curves for progression‐free survival (PFS) and time to treatment failure (TTF) following treatment with immune checkpoint inhibitors according to EGFR mutation status and/or acquired T790M mutations in patients with EGFR‐mutated NSCLC. (A, B) PFS (A) and TTF (B) curves for uncommon EGFR mutation plus T790M‐positive (red line), common EGFR mutation plus T790M ‐positive (green line), and common EGFR mutation plus T790M‐negative (blue line) (N = 27) NSCLC. Column signs denote censoring. PFS, progression‐free survival; TTF, time to treatment failure; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer
4. DISCUSSION
Many promising drugs have been developed for NSCLC, such as molecular‐targeted therapies for EGFR and immunotherapy. To date, the effectiveness of ICIs has been reported to be associated with various biomarkers, such as PD‐L1 overexpression in tumors, tumor mutation burden, and smoking exposure in patients with NSCLC.13, 14 Of them, the frequency of a PD‐L1 TPS of ≥50% is lower in patients with EGFR‐mutated or ALK‐rearranged NSCLC compared with that in those negative for these genetic changes.8, 15, 16 In addition, the efficacy of PD‐1/PD‐L1 inhibitors is less related to PD‐L1 expression in EGFR‐mutated lung cancer.12 A meta‐analysis of 3 randomized trials demonstrated that ICIs are less sensitive than docetaxel in subsets of EGFR‐mutated advanced NSCLC.17 Thus, patients with EGFR‐mutated NSCLC generally are unlikely to be ideal candidates to receive ICI treatment. Meanwhile, preclinical studies have shown that activation of EGFR signaling pathways involved in the production of PD‐L1 expression in NSCLC cells and anti‐PD‐1 antibodies are effective in mouse models with EGFR mutant‐driven tumors.18, 19, 20 In fact, 2 patients with EGFR mutations survived for more than 5 years in a phase 1 study of nivolumab treatment. Interestingly, uncommon EGFR mutations, exon20 insertion and G719A, but not common mutations, were detected in these patients.21 More recently, a phase III study demonstrated that patients with EGFR mutations who were pretreated with EGFR‐TKIs showed superior PFS upon receiving combination therapy of anti‐PD‐L1 antibody atezolizumab plus platinum‐based chemotherapy, compared with that upon receiving chemotherapy alone.22 However, the subpopulation of patients with EGFR‐mutated tumors who are ideal candidates to receive immunotherapy is still unclear. Therefore, in this retrospective study, we focused on screening for efficacy cases with ICIs among patients with EGFR‐mutated NSCLC.
Liver metastases are known as a poor prognostic factor in patients with lung cancer, regardless of various histologic types,23, 24 and the presence of liver metastases in patients with NSCLC is associated with shorter PFS and tends to reduce effectivity to PD‐1 inhibition compared with those in patients without liver metastases.25 General conditions, including malnutrition, are important factors to consider for successful administration of systemic treatments. In our study, neither metastatic lesions, serum levels of albumin, or PS had an impact on the sensitivity to PD‐1 inhibitors in EGFR‐mutated NSCLC. In addition, the serum NLR was not correlated with the outcomes of ICI treatment in EGFR‐mutated NSCLC, although the serum NLR was reported to be a potent biomarker according to the benefit of ICI treatments in patients with advanced‐stage cancer.26
A previous study reported that a higher Brinkman index, defined as the number of cigarettes smoked per day times the smoking years, greater than 600 may be one of the predictive factors for the efficacy of nivolumab in patients with NSCLC with EGFR mutations [10]. Our results indicated that patients with uncommon EGFR mutations, G719X and exon 20 insertions, had significantly better response, PFS, and TTF than those in patients with common EGFR mutations, such as exon 19 deletions and L858R in exon 21, consistent with findings reported previously.27 These findings suggested that patients with uncommon EGFR mutations might associate with more specific characteristics of smokers than patients with common EGFR mutations, although our study could not indicate the significant relationship between smoking history and the efficacy of ICIs.28 As it was reported as a controversial finding, smoking history might be an inadequate and confounding factor in the outcomes of ICIs in EGFR‐mutated NSCLC. A previous report showed the negative correlation between the detection of T790M mutations and outcomes of ICI treatment,29 which was consistent with our observations that the detection of T790M mutations inversely predicted the PFS and TTF following treatment with ICIs. The EGFR‐T790M, the gatekeeper mutation, is the most common mechanism of acquired resistance and is detectable in approximately 50% of patients who develop acquired resistance to first‐ and second‐generation EGFR‐TKIs.30, 31 The tumors with EGFR‐T790M mutations showed low mutation burden in next‐generation sequencing analysis, suggested that these tumors were likely to exhibit reduced responses to ICIs. However, our findings showed that all patients without EGFR‐T790M mutations include those with uncommon EGFR mutations that are most promising predictors in analyzing ICI responders. In addition, patients with common EGFR mutations poorly responded to immunotherapy, independent of EGFR‐T790M mutations. Therefore, further investigations are needed to determine the important roles of EGFR‐T790M mutations on the detection of responders to immunotherapy. Importantly, this is the first report to show that uncommon EGFR mutations without T790M mutations when initiating treatment with ICIs is a promising predictive factor to identify responders of ICIs among patients with EGFR‐mutated NSCLC.
This study has several limitations; first, it included a small sample size and was a retrospective study. However, previous retrospective observations regarding these associations have been based on similar sample sizes as that included in this study.27 Second, this study only recruited a Japanese cohort. Third, we had various biases on patient conditions when ICI treatment was initiated, such as the number of pretreatment regimens and PS of patients. Fourth, we could not validate the cases harboring uncommon EGFR mutations with T790M mutations. Further studies are warranted to develop useful biomarkers of PD‐1/PD‐L1 inhibitors in EGFR‐mutated NSCLC.
In summary, NSCLC with uncommon EGFR mutations and without T790M mutations was found to be positively associated with clinical benefits of ICI treatment and to be an independent positive prognostic factor in patients with NSCLC with EGFR mutations. Further studies are warranted to identify responders to ICIs among patients with EGFR mutation‐positive NSCLC.
CONFLICT OF INTEREST
All authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGMENTS
This study was supported by a research grant for developing innovative cancer chemotherapy from the Kobayashi Foundation for Cancer Research (to T. Yamada), a Grant for Lung Cancer Research, founded by Japan Lung Cancer Society (to T. Yamada).
Yamada T, Hirai S, Katayama Y, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR‐mutated non‐small cell lung cancer. Cancer Med. 2019;8:1521–1529. 10.1002/cam4.2037
REFERENCES
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283‐298. [DOI] [PubMed] [Google Scholar]
- 2. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380‐2388. [DOI] [PubMed] [Google Scholar]
- 3. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735‐742. [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327‐3334. [DOI] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540‐1550. [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 10. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet (London, England). 2017;389(10066):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soo RA, Lim SM, Syn NL, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non‐small cell lung cancer: current controversies and future directions. Lung Cancer (Amsterdam, Netherlands). 2018;115:12‐20. [DOI] [PubMed] [Google Scholar]
- 12. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585‐4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grizzi G, Caccese M, Gkountakos A, et al. Putative predictors of efficacy for immune checkpoint inhibitors in non‐small‐cell lung cancer: facing the complexity of the immune system. Expert Rev Mol Diagn. 2017;17(12):1055‐1069. [DOI] [PubMed] [Google Scholar]
- 14. Shukuya T, Carbone DP. Predictive markers for the efficacy of anti‐PD‐1/PD‐L1 antibodies in lung cancer. J Thorac Oncol. 2016;11(7):976‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoneshima Y, Ijichi K, Anai S, et al. PD‐L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer (Amsterdam, Netherlands). 2018;118:36‐40. [DOI] [PubMed] [Google Scholar]
- 16. Rangachari D, VanderLaan PA, Shea M, et al. Correlation between classic driver oncogene mutations in EGFR, ALK, or ROS1 and 22C3‐PD‐L1 >/=50% expression in lung adenocarcinoma. J Thorac Oncol. 2017;12(5):878‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung cancer‐a meta‐analysis. J Thorac Oncol. 2017;12(2):403‐407. [DOI] [PubMed] [Google Scholar]
- 18. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov. 2013;3(12):1355‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen N, Fang W, Zhan J, et al. Upregulation of PD‐L1 by EGFR activation mediates the immune escape in EGFR‐Driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910‐923. [DOI] [PubMed] [Google Scholar]
- 20. Azuma K, Ota K, Kawahara A, et al. Association of PD‐L1 overexpression with activating EGFR mutations in surgically resected nonsmall‐cell lung cancer. Ann Oncol. 2014;25(10):1935‐1940. [DOI] [PubMed] [Google Scholar]
- 21. Gettinger S, Horn L, Jackman D, et al. Five‐year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: results from the CA209‐003 study. J Clin Oncol. 2018;36(17):1675‐1684. [DOI] [PubMed] [Google Scholar]
- 22. Reck M, Socinski MA, Cappuzzo F, et al. LBA1_PRPrimary PFS and safety analyses of a randomized phase III study of carboplatin + paclitaxel +/− bevacizumab, with or without atezolizumab in 1L non‐squamous metastatic nsclc (IMPOWER150). Ann Oncol. 2017;28(suppl_11):mdx760.002‐mdx760.002. [Google Scholar]
- 23. Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non‐small‐cell lung cancer. Mol Clin Oncol. 2015;3(1):217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer (Amsterdam, Netherlands). 2014;86(1):78‐84. [DOI] [PubMed] [Google Scholar]
- 25. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang T, Qiao M, Zhao C, et al. Pretreatment neutrophil‐to‐lymphocyte ratio is associated with outcome of advanced‐stage cancer patients treated with immunotherapy: a meta‐analysis. Cancer Immunol Res. 2018;67(5):713‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida H, Kim YH, Ozasa H, et al. Nivolumab in non‐small cell lung cancer with EGFR mutation. Ann Oncol. 2018;29(5):777‐778. [DOI] [PubMed] [Google Scholar]
- 28. Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non‐small cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2017;114:96‐102. [DOI] [PubMed] [Google Scholar]
- 29. Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation‐positive non‐small‐cell lung cancer based on T790M status after disease progression during EGFR‐TKI treatment. Ann Oncol. 2017;28(7):1532‐1539. [DOI] [PubMed] [Google Scholar]
- 30. Sequist LV, Waltman BA, Dias‐Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu SG, Liu YN, Tsai MF, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor‐afatinib in lung adenocarcinoma patients. Oncotarget. 2016;7(11):12404‐12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


