Abstract
Mesenchymal stem cells (MSCs) are multipotent stromal cells present in various adult tissues. Several studies suggest that MSCs secrete exosomes that perform as mediators in the tumor niche and play several roles in tumorigenesis, angiogenesis, and metastasis. In contrast, there are other studies supporting the tumor-suppressing effects of MSC-derived exosomes. Therefore, the exact association of MSC exosomes and tumor cells remains open to debate. This review aimed to demonstrate the present knowledge of MSC-derived exosomes in cancer research and to illustrate current approaches to make use of modified exosomes as a platform in therapeutic strategies in cancer.
Keywords: mesenchymal stem cells, exosome, drug delivery, exosome engineering, cancer therapy
Introduction
In recent years, exosomes have been explored as key performers in intercellular communication. Exosomes are nano-sized vesicles containing biological signaling molecules that mediate cell–cell signaling. Mesenchymal stem cells (MSCs) are believed to have antitumor effects and are preferred for their properties, such as immune-modulating capacity and ability to accumulate at the tumor site. Recent data indicate that MSCs mediate their therapeutic functions in a paracrine rather than a cellular manner. Growing evidence suggests that MSC-derived exosomes could transfer proteins and RNAs to recipient cells and exert several effects on the growth of various tumor cells. Furthermore, MSCs are the only human cell type known to have a scalable capacity for the mass production of exosomes for drug delivery. There are controversial reports on whether MSC-derived exosomes suppress or promote tumor growth. Apart from regulating the tumor cell fate, MSC-derived exosomes are capable of being applied for delivery of anticancer therapeutics. Cell-derived exosomes have numerous benefits as therapeuticagents compared to cells or synthetic nanoparticles including the potential to be engineered, exceptional biocompatibility/stability features, and a superior capacity for loading with various cargoes. They can be modified with certain ligands or proteins on their surface to deliver the therapeutic payload into target cells and tissues. In this study, current findings regarding the role of MSC-derived exosomes in cancer therapy are reviewed. In addition, current approaches to make use of engineered exosomes as a platform in therapeutic strategies in cancer will be discussed.
Extracellular vesicles
Extracellular vesicles (EVs) represent a group of cell-derived structures that were first described in the 1970s. EVs are composed of lipid bilayer membranes and have been demonstrated to associate with other cells in the body as a novel mechanism of intercellular communication, and are currently recognized as sources of circulating biomarkers of disease.1–3 These vesicles fluctuate from 30 to 1,000 nm in size and are dissimilar with respect to protein, nucleic acid, and lipid composition.4,5 EVs are commonly classified into three subtypes; exosomes, microvesicles (MVs), and apoptotic bodies.6,7 Nanovesicles ranging from 8 to 12 nm have also been recently described.8
Exosome structure, content, and biogenesis
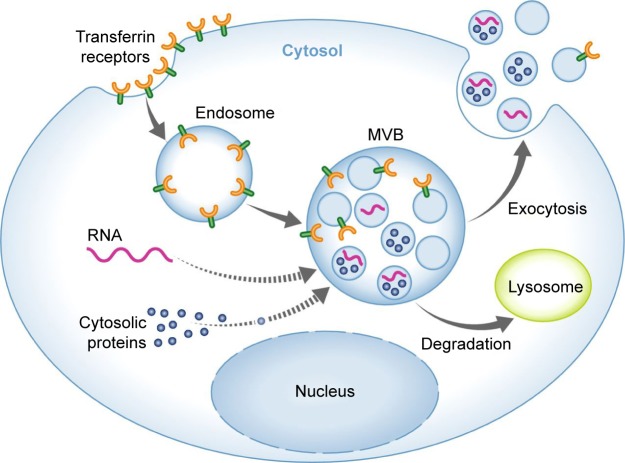
Exosomes were first defined as EVs arising from maturing reticulocytes and appreciated as a means to remove obsolete material. A role of exosomes in intercellular communication was suggested by Raposo and Stoorvogel,9 indicating that B cell-derived exosomes can deliver functional antigen-presenting complexes. Exosomes consist of proteins, mRNA, and noncoding miRNA surrounded by a lipid bilayer membrane.10–13 Further RNA forms including transfer RNA, ribosomal RNA, nucleolar RNA, and long noncoding RNA (lncRNA) have also been identified in exosomes.14 Topical studies have demonstrated that fragments of DNA may exist in exosomes as well.15–17 The exosome biogenesis process consists of four phases: initiation, endocytosis, multivesicular body (MVB) development, and release.18 Initially, endocytic vesicles are produced from the plasma membrane, forming an early endosome that subsequently develops into late endosomes. The late endosomes undergo inward budding leading to formation and accumulation of intraluminal vesicles inside the lumen, termed MVBs. The MVBs will merge with lysosome for degradation; otherwise they will merge with the plasma membrane, releasing intraluminal vesicles into extracellular spaces, which are now called exosomes (Figure 1).19–21 Secreted exosomes can subsequently be directed to other cells via a rather poorly determined mechanism, probably through proteins located at the surface such as tetraspanins.7 The exosomes are taken up by the target tissues through membrane fusion, endocytosis, or receptor–ligand interaction, which later can be transferred to lysosomes in order to be degraded or can release its cargo within the receiver cells.
Figure 1.
Exosome biogenesis and secretion.
Note: Reprinted from Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2015;16(1):24–43, with permission from John Wiley and Sons.21
Abbreviation: MVB, multivesicular body.
The protein content of exosomes has been well identified using different proteomic approaches.22 Mass spectrometry indicated the presence of over 4,000 various proteins within exosomes while there appears to be a unique preserved protein collection in exosomes derived from certain kinds of cells.23 Alix, heat shock proteins, and TSG101 proteins have been recognized in most of the exosomes.24 Exosomes have tetraspanins, such as CD9, CD81, and CD63, which are involved in cellular interactions through binding with various proteins, such as integrins and major histocompatibility complex (MHC) molecules.25 Rab, annexins, myosin, actin, tubulin, ribosomal proteins, and signal transduction molecules are demonstrated to be enriched in exosomes.9,24 The exosomal components differ based on various physiological/pathological conditions and certain kinds of cells. Furthermore, the exosomal content can be dissimilar from the cells of origin owing to the discriminating sorting of the cargo into exosomes.
Exosome isolation methods
Several accepted techniques, such as differential ultracentrifugation, density gradients, precipitation, filtration, and size exclusion chromatography, have been applied for exosome separation.19 Traditional ultracentrifugation has become a reliable technique among researchers in exosome research. It consists of a series of centrifugation cycles of varying centrifugal force and duration to isolate exosomes according to their density and size differences. Recently, a number of commercial kits have been introduced to isolate exosomes for different purposes.9 These kits are superior to ultracentrifugation due to being less time consuming, less technique sensitive, and more compatible with limited volumes of samples. The relative success of these different methods needs to be considered in terms of recovery and specificity.
Roles of MSC-derived EVs/exosomes in cancer
The microenvironment of a tumor is composed of different cell types, such as fibroblasts, immune cells, and endothelial cells. The crosstalk of this microenvironment with the tumor seems to be vital for growth and progression of tumor cells.26,27 Previous studies have revealed that MSCs produce exosomes, which could perform as paracrine mediators by transferring signaling molecules, which regulate tumor cell proliferation, angiogenesis, and metastasis via controlling a number of cellular pathways.28,29
Tumor growth
The impact of exosomes on tumor progression has been extensively reported in the past decade. MSC-derived exosomes affect tumor development in both supporting and suppressing ways. Among the proposed mechanisms, the miRNA content of exosomes has been widely investigated. As indicated by Vallabhaneni et al,30 stressed MSC-derived EVs including exosomes promoted breast cancer cell proliferation and metastasis via transferring tumor-supportive miRNAs and proteins (Table 1). It was demonstrated that a variety of tumor-promoting miRNAs, such as miR-21 and miR-34a, have been enriched in MSC-derived EVs. This tumor-supportive function may rely on epigenetic changes induced under stress conditions. Along with miRNAs, several possible mechanisms have been proposed to be associated in the protumor or antitumor features of MSC-derived exosomes. According to Qi et al,31 MSC-derived exosomes from bone marrow (BM MSCs) have stimulated the Hedgehog signaling pathway in osteosarcoma and gastric cancer cell lines, and thus promoted tumor growth. It was also demonstrated that exosomes derived from MSCs hold matrix metalloproteinase-2 that might result in tumor microenvironment reorganization and growth.32 While MSC-derived exosomes largely mediate tumor-promoting effects in the tumor microenvironment, there is also evidence for antitumor activity of EVs derived from MSCs. Wu et al33 reported that EVs from human umbilical cord Wharton’s jelly MSCs reversed the development of bladder carcinoma cells possibly by down-regulating phosphorylation of Akt protein kinase and up-regulating cleaved caspase-3 (Table 2). Similarly, adipose MSC-derived exosomes were demonstrated to inhibit prostate cancer via delivery of miR-145 by reducing the activity of Bcl-xL and promoting apoptosis through the caspase-3/7 pathway.34 In the same way, EVs obtained from normal human BM MSCs were found to inhibit proliferation and to promote apoptosis in liver carcinoma, Kaposi’s sarcoma, and ovarian tumor cell lines.35 The inconsistency between the results pointed to a need for additional research in developing a standardized condition for MSC culture as the MSC culture condition may affect the overall features of the secreted vesicles. In addition, the source of MSCs where exosomes were obtained was also demonstrated to be important for the final tumor-suppressive or tumor-promoting effects. Roccaro et al36 noticed that EVs derived from BM MSCs of patients with multiple myeloma could support multiple myeloma tumor/cell progression while EVs isolated from normal individuals suppressed the development of multiple myeloma tumor/cells perhaps by transferring a lower content of miR-15a. Besides higher miRNA content, other factors such as superior amounts of cytokines and adhesion molecules in patient-derived exosomes might also be involved in the tumor-promoting effects.
Table 1.
Natural or modified MSC EVs/exosomes that promote tumors or extend their metastatic effect
| Source of exosome | Study mode | Therapeutic agent | Target cancer/cells | Outcome | Reference |
|---|---|---|---|---|---|
| Stressed EVs derived from BM MSCs | In vitro and in vivo | No | Breast cancer cell | Supported breast cancer cell proliferation and metastasis possibly by transferring miR-21 and miR-34a | 30 |
| Human BM MSC-derived exosomes | In vitro | No | Osteosarcoma and gastric cancer cell lines | Supported cancer cell growth by promoting the Hedgehog signaling pathway | 31 |
| Human umbilical cord MSC-derived exosomes | In vitro | No | Ovary and breast cancer cells | Supported cancer cell reorganization and growth by transferring MMP-2 enzyme | 32 |
| Multiple myeloma BM MSC-derived exosomes | In vitro and in vivo | No | Multiple myeloma | Supported tumor progression possibly by transferring lower amount of exosomal tumor inhibitor miR-15a | 36 |
| Exosomes derived from BM MSCs | In vitro and in vivo | No | Gastric cancer | Stimulated tumor angiogenesis by inducing VEGF expression via activating ERK1/2 and p38 mitogen-activated protein kinase pathways | 37 |
| Placental exosomes derived from MSCs | In vitro | No | Placental microvascular endothelial cells | Promoted migration and angiogenesis | 38 |
| Adipose MSC-derived exosomes | In vitro and in vivo | No | Human microvascular endothelial cells | Promoted angiogenesis | 39 |
| Exosomes derived from gastric cancer tissue-derived MSCs | In vitro and in vivo | No | Gastric cancer | Facilitated growth and migration of tumor cells | 44 |
| Exosomes derived from MSCs | In vitro | No | Breast cancer | Induced Wnt signaling activation, hence facilitating migration and growth of the tumor cell line | 45 |
Abbreviations: BM, bone marrow; EV, extracellular vesicle; miR, microRNA; MMP-2, matrix metalloproteinase-2; MSC, mesenchymal stem cell; VEGF, vascular endothelial growth factor.
Table 2.
Natural or modified MSC EVs/exosomes that have an inhibitory effect on tumors
| Source of exosome | Study mode | Therapeutic agent | Target cancer/cells | Outcome | Reference |
|---|---|---|---|---|---|
| Human umbilical cord Wharton’s jelly MSC-derived EVs | In vitro and in vivo | No | Bladder carcinoma | Inhibited tumor growth by down-regulating phosphorylation of Akt protein kinase and up-regulating cleaved caspase-3 | 33 |
| Adipose MSC-derived exosomes | In vitro and in vivo | No | Prostate cancer | Inhibited tumor growth possibly by transferring miR-145 to reduce the activity of Bcl-xL and promote apoptosis through the caspase-3/7 pathway | 34 |
| Human BM MSC-derived EVs | In vitro and in vivo | No | Hepatoma/Kaposi’s sarcoma/ovarian tumor cell lines | Inhibited tumor growth | 35 |
| Exosomes derived from normal BM MSCs | In vitro and in vivo | No | Multiple myeloma | Inhibited tumor promotion | 36 |
| Mouse exosomes derived from BM MSCs | In vitro and in vivo | No | Mouse breast cancer cell line (4T1) | Suppressed angiogenesis via delivery of miR-16 and down-regulation of VEGF in the tumor | 41 |
| Exosomes derived from MSCs | In vivo | No | N/A | Cancer cells targeting capability using the exosome-mediated transfer of proteasomes | 48 |
| Exosomes derived from MSCs | In vitro | Paclitaxel | Pancreatic adenocarcinoma | Mediated powerful antitumorigenic outcomes | 53 |
| Exosomes derived from MSCs | In vitro | Anti-miR-9 | Glioblastoma multiforme | Reversed the expression of multidrug transporters and reversed the chemoresistance | 54 |
| Exosomes derived from MSCs | In vitro and in vivo | miR-146b | Glioma | Decreased glioma xenograft progress in a rat brain tumor model and declined in vitro progression and metastasis | 55 |
| Exosomes derived from MSCs | In vitro | miR-143 | Osteosarcoma cells | Decreased the migration of osteosarcoma cells | 56 |
| Exosomes derived from adipose MSCs | In vitro and in vivo | miR-122 | Hepatocellular carcinoma | Increased tumor cell sensitivity to chemotherapeutic agents and tumor growth | 57 |
| Exosomes derived from adipose MSCs | In vitro and in vivo | miR-379 | Breast cancer | Reduced tumor activity over the 6 weeks of monitoring | 58 |
| MSC-derived EVs | In vitro | TRAIL | Various cancer cell lines | Increased cell death in a range of cancer cell lines | 59 |
| Exosomes derived from MSCs | In vitro | PLK-1 siRNA | Bladder cancer | Successfully applied as a delivery vector to transfer PLK-1 siRNA to cancer cells of the bladder | 50 |
| Exosomes derived from MSCs | In vitro and in vivo | miR-124/miR-145 mimics | Glioma | Reduced the tumor cells migration and the stem cell properties of glioma cells | 60 |
| Human foreskin fibroblast-like/MSCs | In vivo | siRNA for oncogenic Kras | Mouse model of pancreatic cancer | Repressed cancer in multiple models of pancreatic cancer in the mouse and improved overall survival | 61 |
Abbreviations: BM, bone marrow; EV, extracellular vesicle; miR, microRNA; MSC, mesenchymal stem cell; N/A, not available; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
Angiogenesis
Angiogenesis is an essential physiological multistep procedure involved in tumorigenesis. Exosomes hold several angiogenic factors that control tumor angiogenesis. Exosomes derived from MSCs were shown to stimulate angiogenesis through elevating vascular endothelial growth factor (VEGF) production in tumor cells and via stimulating ERK1/2 and p38 mitogen-activated protein kinase pathways.37 MSCs secrete exosomes that transport mRNAs and miRNAs to target cells and induce endothelial cell proliferation, thus leading to angiogenic effects, superior blood flow restoration, and capillary network formation. Placental MSC-derived exosomes have been shown to facilitate placental microvascular endothelial cell migration and vascularization.38 It was shown that adipose MSC-derived exosomes and MVs are internalized by human microvascular endothelial cells and lead to angiogenesis.39 According to this study, platelet-derived growth factor enhances angiogenesis by causing adipose MSCs to secrete exosomes and MVs that are rich in proangiogenic factors. In a similar study, injection of MSC-derived exosomes into stroke rats was shown to lessen severe symptoms by stimulating angiogenesis, neurite remodeling, and neurogenesis.40 Lee et al,41 however, showed that exosomes derived from MSCs blocked vessel formation by delivery of miR-16 via downregulating VEGF within the tumor microenvironment. Similarly, Huang et al42 revealed that the angiogenic function of MSCs was mostly mediated through MVs while exosomes and paracrine factors were shown to prevent HIMF and Smad2, which lead to anti-vascular remodeling. Although several reports have shown that MSCs play a key role in angiogenesis, the role of MSC exosomes in angiogenesis is still controversial. It seems that some miRNAs found in MSC exosomes are supposed to be specifically involved in tumor angiogenesis. According to a bioinformatic analysis conducted by Ferguson et al,43 various genes targeted by the miRNA content of exosomes derived from MSCs were associated with angiogenesis and vessel formation, and thus the angiopoietin network might be a key target of these exosomes in angiogenesis induction. Other factors such as amounts of oncogenic proteins, cytokines, and adhesion molecules are thought to be involved in the exosome-mediated angiogenesis.
Metastasis/invasion
The role of exosomes derived from MSCs has also been examined in metastasis and the premetastatic niche. Exosomes derived from MSCs were reported to transfer miR-221 to HGC27 cells, thus enabling the growth and migration of tumor cells.44 The results of this study showed that while MSC-derived exosomes from both normal and cancer tissue shared similarities in promoting tumor proliferation and migration, MSCs obtained from cancer tissue secreted exosomes enriched in tumor-supportive miR-221 and thus favored cancer promotion and migration. Similarly, exosomes isolated from MSCs induced Wnt signaling activation, hence facilitating the growth and migration of the breast tumor cells.45 It has been shown that MSCs induced the invasiveness of breast cancer cells partly through MSC-derived MVs.46 Furthermore, BM-derived MSCs were revealed to release exosomes that encompass miRNAs, stimulating dormancy in invasive breast cancer cells.47 Exosomes are likewise made up of multi functional proteins. Lai et al48 noticed that abundant amounts of all seven α chains and seven β chains of the 20S proteasome and the three β subunits of the immunoproteasome were present in MSC-derived exosomes, showing that exosomes were capable of targeting tumor cells via proteasome transfer.
In conclusion, even though exosomes hold a hopeful future as drug delivery vehicles, their application has been restricted by the lack of standardized approaches in the case of production, isolation, and purification. The protumor or antitumor features of stem cell exosomes may possibly rely on the settings utilized to cultivate the stem cells and promote vesicle formation and on the tumor model to be utilized, as both the tumor microenvironment and the systemic environment of the host can differ seriously from tumor suppression to tumor promotion. Therefore, the main outcomes in some studies are not adequately supported by the experiments performed and detailed data need to be given to allow replication. It is crucial to demonstrate all detailed procedures for reported parameters, such as culture media composition and harvesting condition as well as any supplements, such as antibiotics and growth factors, as these can influence EV production and the relevant content. Furthermore, the method of isolation needs to be stated as exosomes and other EVs mediate quite different or overlapping functions. The controversial effects of MSC-derived exosomes might be partially associated with the complexity of mechanisms applied by stem cells to sense and home to tumors that might overlap with strategies that direct them to sites of injury and inflammation. Besides, miRNAs are crucial players in the ultimate biological function of exosomes as these miRNAs are formed as a central fraction of the exosomal content.
Modification of MSC-derived exosomes for cancer drug delivery
In the previous section, we have highlighted the effect of natural (nonmodified) MSC-derived exosomes on cancer biology. In this section, we will summarize the application of modified MSC-derived exosomes as a novel therapeutic strategy for drug delivery in cancer. As demonstrated by Smyth et al,49 internalization of exosomes within tumor cells is ten times greater than liposomes of comparable size, representing a superior specificity of exosomes for cancer targeting. In addition, cancer cells were demonstrated to internalize a greater percentage of exosomes when compared to normal cells.50 In conventional therapies, the lack of selectivity to the diseased site is considered the major drawback. Many stem cells types, however, show intrinsic tropism toward tumors, making them attractive candidates for the targeted delivery of anticancer drugs. Additionally, by engineering/modifying these cells to express anticancer agents, they can effectively target tumor sites.51 This novel exosome-based therapy might be an interesting alternative due to their benefits over the corresponding stem cells. They are smaller, less complex, and less immunogenic than their parent cells since they have a lower content of membrane-bound proteins.52 Furthermore, production and storage of exosomes are easier than for their parental cells. Pascucci et al53 demonstrated that MSCs treated with paclitaxel-mediated powerful anti-tumorigenic outcomes due to their potential to get the drug and subsequently packed it in EVs. Besides approaches utilized for loading drugs into exosomes, various loading methods can also be applied to encapsulate miRNA inside exosomes. According to Munoz et al,54 anti-miR-9-loaded MSC-derived exosomes reversed the expression of multi-drug transporters in drug-resistant glioblastoma multiforme cells and reversed the chemoresistance. Alternative research showed that intratumoral injection of miR-146b-expressing MSC-derived exosomes resulted in considerable reduction in glioma xenograft development in a rat brain tumor model and decreased the growth, migration, and invasion of cells.55 According to these findings, miRNAs could be packaged into MSC-derived exosomes and later suppressed glioma tumor cells, suggesting that engineered MSCs to secrete exosomes enriched with miRNAs could be an effective strategy for malignant glioma treatment. In a similar study, the obtained results indicated that the transfer of miR-143 by means of MSC exosomes decreased the in vitro migration of osteosarcoma cells.56 Likewise, miR-122-transfected adipose MSCs can generate miR-122 encapsulated exosomes to deliver miR-122 into hepatocellular tumor cells, which elevated tumor cell sensitivity to chemotherapeutic agents via gene expression alternations and tumor proliferation in vitro and in vivo.57 Transfected MSCs to release exosomes encapsulated with miR-379 have been administered for breast cancer therapy in vivo.58 According to the results of this study, the modified exosomes were delivered to the tumor site and have therapeutic effects. Application of EVs for anticancer protein delivery by genetic manipulation of parental MSCs has also been investigated. Yuan et al59 examined EV-mediated TRAIL delivery in various cancer cells. The modified MSC EVs expressing TRAIL promoted apoptotic death in 11 cancer cell lines in a dose-dependent manner. A similar result was observed in MSC-derived exosome-mediated small interfering RNA (siRNA) gene delivery.50 Selective gene silencing of PLK-1 was achieved using MSC-derived exosomes as a vehicle to transfer PLK-1 siRNA to tumor cells of the bladder. The approach led to effective repression of PLK-1 mRNA and protein. Lee et al60 noticed that exosomes derived from MSCs delivered particular miRNA mimics (miR-124 and miR-145) and reduced glioma cell migration and the stem cell properties of cancer cells. siRNA loading of exosomes was also investigated by an electro-poration approach. According to the study conducted by Kamerkar et al,61 electroporated MSC-derived exosomes with siRNA against oncogenic Kras suppressed cancer in mouse pancreatic tumor models and remarkably improved overall survival. Bliss et al62 demonstrated that breast cancer cells prime MSCs to secrete exosomes with a particular profile of miRNAs, which account for the cellular quiescence effect and drug resistance in some types of cancer cells. According to this finding, a beneficial approach to target dormant breast cancer cells was introduced by systemic application of MSCs encapsulated with antagomiR-222/223 resulting in chemosensitization and an increased survival rate.
In summary, these findings demonstrate the possible effectiveness of exosomes in drug and/or nucleic acid delivery. Several features including the selection of cells from which exosomes are obtained, yield of exosomes, cargo encapsulation process, choice of targeting biomolecules on the surface, biodistribution, and immune response are important elements that need to be considered for exosome-mediated drug transfer in upcoming clinical settings. Encapsulation of small-molecule drugs may be effective, although further investigation is essential for genetic materials encapsulation. The quantity and nature of RNA in exosomes may differ based on the type and origin of the parental cells; however, certain RNAs are enriched.63,64 This raises concepts that specific mechanisms may occur for RNA sorting into EVs; hence, discovering these mechanisms might be used to deliver selective therapeutic RNA cargoes. In addition, a full characterization and comparison of exosome contents of various sources will be essential to demonstrate whether stem cell-derived exosomes differ from others. Application of recent technologies such as a highly versatile single-cell assay offers a suitable and low-cost approach allowing quantitative and time-lapsed examination of single-cell features including secreted exosomes.65
Strategies to modify exosomes for drug delivery
In this section, we highlight the exciting potential for exosomes as therapeutic vehicles for drug delivery and to develop strategies to engineer/modify the exosome surface and content.
Cargo loading
With regard to the intrinsic capability of exosomes to transfer genetic materials, it is possible to engineer vesicles for gene therapy through exosomal-mediated delivery of some important regulating miRNAs, various noncoding RNAs, or tumor suppressors.66,67 One of the most widely used methods is to bring about transgene expression in the parental cell, which is inspired by the regular action of the exosome for intercellular transfer. Using this manipulation strategy, mRNA or noncoding RNAs could be packed into exosomes using a donor cell transfection method.55,64,68 Another approach for manufacturing exosome-based formulation is genetic fusions of polypeptides to the enriched protein in exosomes to guarantee the ideal localization of the expressed product. For example, application of poly A binding protein that attaches mature mRNAs to selectively recruit mRNAs into exosomes. Otherwise, a zip-like, approximately 25-nucleotide fragment present in the 3′-UTR of particular mRNAs can be fused into the 3′-UTR of the mRNA of interest and be recruited by Z-DNA binding protein 1 (ZBP1).64 Similarly, to selectively enrich exosomes with miRNAs, Ago protein or catalytically dead Dicer/Drosha can be attached to EV-specific proteins such as tetraspanin CD63 to cargo miRNAs or pre-miRNAs and pri-miRNAs, respectively.69 Likewise, polycomb repressive complex 2 (gene symbols EZH1 and EZH2) identifies specific lncRNAs selectively, and thus could be applied to enrich exosomal lncRNAs.70 For drug incorporation into exosomes, one method is to introduce exogenous materials to the parental cells. The encapsulation efficiency normally depends on the quantity of material to be transported to the cell. Hence, in order to maximize cell loading, high concentrations and prolonged incubation periods are required. The main restriction of this method is that it depends on phagocytosis as an uptake mechanism. Therefore, it is challenging to obtain equivalent encapsulation. Another strategy for drug loading is the passive approach, in which the drug is incorporated into exosomes through post-isolation methods. This approach is not restricted to biological cargo and can include small-molecule drugs. The simplest passive approach of cargo loading is obtained by incubation of cargo with exosomes. This approach efficiently works with some hydrophobic molecules that affect lipid rearrangement and modify the lipid fluidity. For those large and charged molecules that cannot diffuse across the membrane, loading can be obtained via electroporation. Loading therapeutic RNAs into exosomes has several challenges as the process can be inefficient and generates RNA precipitates.71 Regarding the exosomal-mediated protein delivery, incorporation of catalase into exosomes was practical by several encapsulation procedures, such as incubation at room temperature, freeze/thaw cycles, and sonication.72 Recently, an exosome-based delivery system called EXPLORs has been developed for protein loading via optically reversible protein–protein interactions.73 Taken together, these studies indicate the potential application of exosome-mediated nucleic acid, protein, and small-molecule drug delivery. For cargo loading, each method has its own pros and cons, and varies based on the therapeutic payload, site of the disease, and proper settings for a certain type of exosome-cargo vehicle.
Exosome display technology
Despite the feasibility of exosomes as natural carriers for various types of RNAs, proteins, and artificial drugs,74 systemically administered exosomes might be gathered in some other tissues. Exosome display technology is a procedure allowing re-engineering of the exosomal protein composition to modify exosomes with novel desired features. Using this technology, several forms of ligands such as a multi-meric antigen, which does not typically exist on exosomes, can be produced at the surface of exosomes in a natural conformation.75 A popular application of this technology is to engineer exosomes with targeting ligands by transfection of the parental cells in order to obtain production of targeting moieties attached to exosome native membrane proteins (Figure 2). Lysosomal-associated membrane protein 2 (Lamp2b) is a well-known exosome membrane protein that has been widely investigated for exosome targeting.76–78 Alvarez-Erviti et al79 used the rabies virus glycoprotein (RVG) peptide to target exosomes to the mouse brain by manipulation the parental dendritic cells to express Lamp2b, fused to the neuron-specific peptide derived from RVG. Despite the effectiveness of the method, there are serious concerns about the longstanding stability of Lamp-2b hybrids;77 hence, more stable substitutes to Lamp-2b such as glycosylphosphatidylinositol (GPI) have been introduced. As demonstrated by this group, EGF-expressing tumor cells were targeted by EVs displaying anti-EGF receptor nanobodies fused to GPI. Likewise, others have established a human embryonic kidney cell line that stably expressed EGF binding peptide fused with the transmembrane receptor of platelet-derived growth factor for targeted tumor therapy.80 Another exosome membrane protein candidate is the C1C2 domain of lactadherin. Lactadherin has been indicated to localize to the lipid membrane of exosome through binding of its C1C2 domain.81 Inspired from display technology, a group of researchers used the exosomal surface structure in order to discover potential sites on the tetraspanin CD63 for integration of fluorescent fusion proteins on both sides of the exosomal membrane.82 Zhao et al83 used the cell’s own machinery to engineer a chimeric multidomain transmem-brane targeting protein, which contained the intracellular and transmembrane domains of the transferrin receptor capable of targeting EVs to specific populations of cells.
Figure 2.
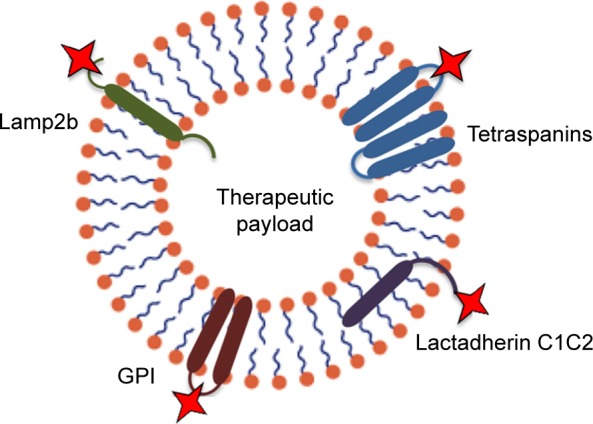
Strategies for targeting extracellular vesicles to particular target cells can be achieved by genetic modification of exosomes to express targeting moieties fused with exosome native membrane proteins, such as lysosomal-associated membrane protein 2 (Lamp2b), tetraspanins, glycosylphosphatidylinositol (GPI), and lactadherin C1C2.
Taken together, this technology demonstrates an approach to display targeting of oligonucleotides and proteins on the surface of EVs. However, such strategies might be vulnerable since they require modifications of producer cells that are often time-consuming and challenging, particularly in the case of primary cells. Besides, a number of targeting moieties protein that attaches inappropriate expression and degradation that restricts their functional demonstration on EVs.
Hybrid membrane engineering
For further applications of exosomes in drug transfer, it may be essential to alter and tune the exosome interface to improve the surface features to reduce exosome immunogenicity, increase stability, and improve its half-life in blood. Recently, a new approach was proposed to provide stealth in addition to tumor cell-targeting features to pre-isolated exosomes. This group developed a technique for tailoring exosomes with targeting moieties attached to polyethylene glycol.4 While exosomes without modification were quickly cleared from the bloodstream within a few minutes after systematic injection in mice, the engineered exosomes were detectable in plasma for prolonged circulation times. This approach enables a dual strategy to design targeted liposomes, where multiple ligands can be inserted into a variety of preformed liposomes containing a number of drugs, allowing the treatment to be personalized based on the needs of individuals. In an alternative approach to optimize the performance of exosomal carriers, a novel membrane-engineering strategy was introduced to modify the exosome interface using direct membrane fusion between synthetic liposomes and exosomes in pre-isolated vesicles (Figure 3).84 Furthermore, Goh et al85 introduced a hybrid system called EXOPLEXs, obtained by fusion of EVs and liposomes as a novel drug delivery vehicle. Interestingly, similar loading efficiencies were obtained when doxorubicin was encapsulated into liposomes and EXOPLEXs, which recommends that the fusion procedure does not adversely affect the encapsulation capacity of EXOPLEXs. In conclusion, it is assumed that these characteristics would be favorable for the exosome-based drug transfer, as exogenously administered exosomes have been indicated to be cleared quickly from the bloodstream by the reticuloendothelial system, therefore preventing their accumulation in the target tissue.
Figure 3.
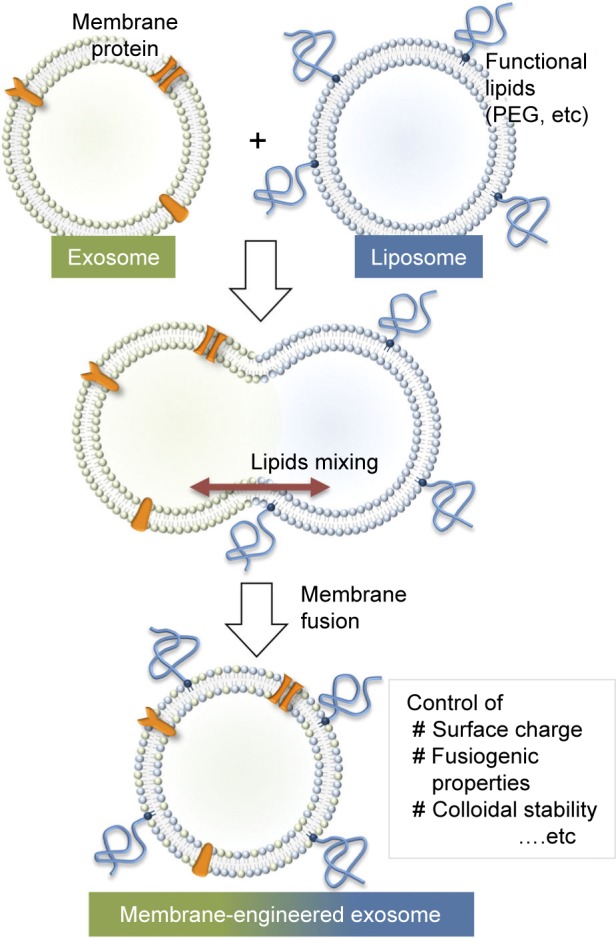
Schematic of the procedure used to engineer the exosome–liposome hybrids. Reprinted from Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933.84
Surface functionalization
Surface modification is another strategy to improve the targeting capability of exosomes.4,5 Click chemistry is a method that enables the bioconjugation of functional ligands to the surfaces of exosomes by copper-catalyzed azidealkyne cycloaddition.86 Furthermore, it is rapid and highly efficient in comparison with traditional cross-linking chemistries. Tian et al87 recently proposed conjugation of the cyclo (Arg-Gly-Asp-d-Tyr-Lys) peptide, which displays high affinity toward integrin αvβ3 on the MSC-derived exosome surface. Another strategy for modification of exosomes is the application of the ApoA-I mimetic peptide (L-4F), which enables the interaction of EVs and a therapeutic/targeted peptide by attachment to the phospholipid vesicles. Ye et al88 have shown that methotrexate-loaded EVs functionalized with a synthetic multifunctional peptide facilitated the membrane receptor-mediated internalization procedure both in vitro and in vivo in a glioma model. In summary, surface functionalization of exosomes using click chemistry could improve the targeting capability of exosomes. This approach seems to be superior compared to exosome display technology as it does not have the challenges of modifying producer cells.
Large-scale production and storage of exosomes for clinical use
One of the key issues in developing exosome-mediated drug delivery systems is to scale up the exosome production.89 Large-scale production of therapeutic exosomes necessitates exosomal isolation and purification under controlled conditions. In order to manufacture therapeutic exosomes, the exosome yield per cell is important and has an impact on ultimate production cost and clinical applications. MSCs are superior in large-scale production of exosomes and can be applied on a clinical scale.90 The MSC source is one of the key factors that should be considered in exosome-based drug delivery systems. The ideal source would be a high-exosome-yielding cell with a high expansion capacity. Another important consideration is the age of the donor tissues, as the exosome production might be inversely associated with the age of the donor tissue. The prolonged donor cell cultivation may result in significantly increased production. According to the literature, prolonged culture and maintaining cells at low pH improved exosomal release.91,92 In order to enhance vesicle release from cells, other strategies have also been proposed. Raising intracellular calcium, serum starvation, or endotoxin can increase vesicle production. Establishment of immortalized cells from MSCs is another strategy to scale up EV production.93 Overexpression of oncogene c-myc, for instance, was reported to increase EV production in MSCs.94 Extrusion or filtration of cells is another approach which might be easily scaled up to obtain high yields of exosomes. Isolation of exosome-like nanoparticles following the breakdown of parental cells encapsulated with antitumor drugs led to a 100-fold higher yield of the drug carriers.95 Lastly, manufacturing bioreactors similar to those for tissue engineering might be employed for large-scale production of exosomes.96 Particularly, MSCs may be cultured from different origins, propagated, and later loaded with the desired therapeutic biomolecules. The choice of the loading method and the therapeutic cargo are other features that need to be considered for successful exosome-based drug delivery. Strategies to preserve exosomes are challenges that need to be further addressed. Previous reports have demonstrated that the size of exosome drops by 60% after 2 days of storage at 37°C. Nevertheless, the initial size was maintained when exosomes are stored at −80°C for 2 days.97 It is possible to preserve exosomes at −80°C for extended periods. Based on the promising preclinical studies, EV-based therapies are making their way into the clinic. Our knowledge of exosome-mediated therapies is rapidly expanding although exosomes have already been approved for application in several clinical trials.
Conclusion and future perspectives
In recent years, the rapid development of the MSC exosome field has attracted the attention of many researchers. MSC exosomes hold remarkable potential in therapeutic applications considering their relative ease of isolation and manipulation of both contents and surface. The lipid and protein composition of exosomes, which enhance exosomal stability and slow clearance in circulation are other features that make these vesicles as perfect carriers. Owing to their small size, lack of toxicity, and target specificity as well as being tolerated by the body, these vehicles might be regarded as the next-generation drug delivery system. Furthermore, exosomes could be generated on a large scale, are easier to handle, are less expensive, and do not raise potential ethical and legal concerns compared to MSCs. These characteristics demonstrate potentials of exosomes for upcoming therapeutic applications. Research in exosome biology has been in the early stage of development and much effort needs to be made in order to guarantee the safe and effective application of them for therapeutic use. In addition, component characterization, immune reactions, and loading of exosomes without modifying the natural characteristics of the donor cell require to be obviously understood. Translating therapy from the lab into the clinics requires scale up of exosome isolation. Therefore, adequate standards for exosome manipulation, isolation, and characterization need to be established to bring this exciting development a step closer to clinical reality.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boriachek K, Islam MN, Möller A, et al. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. 2018;14(6):1702153. doi: 10.1002/smll.201702153. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Li P, Shangguan L, et al. A novel bacterial cellulose membrane immobilized with human umbilical cord mesenchymal stem cells-derived exosome prevents epidural fibrosis. Int J Nanomedicine. 2018;13:5257–5273. doi: 10.2147/IJN.S167880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kooijmans SA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: new nanotools for cancer treatment. Pharmacol Res. 2016;111:487–500. doi: 10.1016/j.phrs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatischeff I. Cell-derived extracellular vesicles open new perspectives for cancer research. Cancer Res Front. 2015;1:208–224. [Google Scholar]
- 7.Neviani P, Fabbri M. Exosomic microRNAs in the tumor microenvironment. Front Med. 2015;2:47–53. doi: 10.3389/fmed.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H-G, Cao P, Teng Y, et al. Isolation, identification, and characterization of novel nanovesicles. Oncotarget. 2016;7(27):41346–41362. doi: 10.18632/oncotarget.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin G. Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ. 2015;22(1):34–45. doi: 10.1038/cdd.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B-R, Kim J-H, Choi E-S, Cho J-H, Kim E. Effect of young exosomes injected in aged mice. Int J Nanomedicine. 2018;13:5335–5345. doi: 10.2147/IJN.S170680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Hao C, Yao S, et al. Exosomal miRNA profiling to identify nanoparticle phagocytic mechanisms. Small. 2018;14(15):1704008. doi: 10.1002/smll.201704008. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14(1):319–333. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahlert C, Melo SA, Protopopov A, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi A, Okada R, Nagao K, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287–15301. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8(1):83–96. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 20.Alenquer M, Amorim M. Exosome biogenesis, regulation, and function in viral infection. Viruses. 2015;7(9):5066–5083. doi: 10.3390/v7092862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2015;16(1):24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welton JL, Khanna S, Giles PJ, et al. Proteomic analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9(6):1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tickner JA, Urquhart AJ, Stephenson S-A, Richard DJ, OByrne KJ. Functions and therapeutic roles of exosomes in cancer. Front Oncol. 2014;4:127–135. doi: 10.3389/fonc.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomes-structure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol. 2015;81(1):2–10. doi: 10.1111/sji.12247. [DOI] [PubMed] [Google Scholar]
- 26.Corcoran C, Rani S, OBrien K, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7(12):e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y, Lai X, Yu S, et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147(2):423–431. doi: 10.1007/s10549-014-3037-0. [DOI] [PubMed] [Google Scholar]
- 28.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 29.Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhaneni KC, Penfornis P, Dhule S, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–4967. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi J, Zhou Y, Jiao Z, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem. 2017;42(6):2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Bucan V, Baehre H, Von Der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol. 2015;47(1):244–252. doi: 10.3892/ijo.2015.3001. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Ju G-Q, Du T, Zhu Y-J, Liu G-H. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One. 2013;8(4):e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahara K, Inamoto T, Ibuki N, et al. 245 microRNA-145 mediates the inhibitory effect of adipose-derived stem cells on androgen-independent prostate cancer. Eur Urol Suppl. 2016;15(3):e245. doi: 10.1016/S1569-9056(16)60247-6. [DOI] [PubMed] [Google Scholar]
- 35.Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2012;22(5):758–771. doi: 10.1089/scd.2012.0304. [DOI] [PubMed] [Google Scholar]
- 36.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8(7):e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12(1):26–38. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J-K, Park S-R, Jung B-K, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8(12):e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int J Biol Sci. 2015;11(2):238–245. doi: 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson SW, Wang J, Lee CJ, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8(1):1419–1431. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Zhao C, Shi H, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110(5):1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20. doi: 10.1007/s11010-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 46.Maffey A, Storini C, Diceglie C, et al. Mesenchymal stem cells from tumor microenvironment favour breast cancer stem cell proliferation, cancerogenic and metastatic potential, via ionotropic purinergic signalling. Sci Rep. 2017;7(1):13162–13171. doi: 10.1038/s41598-017-13460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ono M, Kosaka N, Tominaga N, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 48.Lai RC, Tan SS, Teh BJ, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012:971907–971921. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smyth TJ, Redzic JS, Graner MW, Anchordoquy TJ. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim Biophys Acta Biomembr. 2014;1838(11):2954–2965. doi: 10.1016/j.bbamem.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology. 2016;91(241):241, e1–241.e7. doi: 10.1016/j.urology.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer. 2014;14(10):683–691. doi: 10.1038/nrc3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascucci L, Cocce V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 54.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimbo K, Miyaki S, Ishitobi H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381–387. doi: 10.1016/j.bbrc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8(1):122–133. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Brien K, Khan S, Gilligan K, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 59.Yuan Z, Kolluri KK, Gowers KH, Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. 2017;6(1):1265291. doi: 10.1080/20013078.2017.1265291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HK, Finniss S, Cazacu S, et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4(2):346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bliss SA, Sinha G, Sandiford OA, et al. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016;76(19):5832–5844. doi: 10.1158/0008-5472.CAN-16-1092. [DOI] [PubMed] [Google Scholar]
- 63.Koppers-Lalic D, Hogenboom MM, Middeldorp JM, Pegtel DM. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv Drug Deliv Rev. 2013;65(3):348–356. doi: 10.1016/j.addr.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolukbasi MF, Mizrak A, Ozdener GB, et al. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 2012;1:e1–e10. doi: 10.1038/mtna.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiu YJ, Cai W, Shih YRV, Lian I, Lo YH. A single-cell assay for time lapse studies of exosome secretion and cell behaviors. Small. 2016;12(27):3658–3666. doi: 10.1002/smll.201600725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sempere LF, Keto J, Fabbri M. Exosomal microRNAs in breast cancer towards diagnostic and therapeutic applications. Cancers (Basel) 2017;9(7):71. doi: 10.3390/cancers9070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanez-Mo M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maguire CA, Balaj L, Sivaraman S, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2012;20(5):960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riazifar M, Pone EJ, Lotvall J, Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–154. doi: 10.1146/annurev-pharmtox-061616-030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marcus ME, Leonard JN, editors. Engineering exosomes as therapeutic delivery vehicles. Mol Ther. 2014;22:S96. [Google Scholar]
- 72.Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2016;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yim N, Ryu S-W, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nature Rev Endocrinol. 2016;12(9):504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 75.Lu M, Xing H, Xun Z, et al. Exosome-based small RNA delivery: progress and prospects. Asian J Pharm Sci. 2017;13(1):1–11. doi: 10.1016/j.ajps.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 77.Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem. 2015;290(13):8166–8172. doi: 10.1074/jbc.M114.621383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naseri Z, Oskuee RK, Jaafari MR, Moghadam MF. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomedicine. 2018;13:7727–7747. doi: 10.2147/IJN.S182384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 80.Ohno S-I, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delcayre A, Estelles A, Sperinde J, et al. Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis. 2005;35(2):158–168. doi: 10.1016/j.bcmd.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B. Development of exosome surface display technology in living human cells. Biochem Biophys Res Commun. 2016;472(1):53–59. doi: 10.1016/j.bbrc.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 83.Zhao C, Busch DJ, Vershel CP, Stachowiak JC. Multifunctional transmembrane protein ligands for cell-specific targeting of plasma membrane-derived vesicles. Small. 2016;12(28):3837–3848. doi: 10.1002/smll.201600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goh WJ, Zou S, Lee CK, et al. EXOPLEXs: chimeric drug delivery platform from the fusion of cell-derived nanovesicles and liposomes. Biomacromolecules. 2017;19(1):22–30. doi: 10.1021/acs.biomac.7b01176. [DOI] [PubMed] [Google Scholar]
- 86.Hein CD, Liu X-M, Wang D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm Res. 2008;25(10):2216–2230. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian T, Zhang H-X, He C-P, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Ye Z, Zhang T, He W, et al. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl Mater Interfaces. 2018;10(15):12341–12350. doi: 10.1021/acsami.7b18135. [DOI] [PubMed] [Google Scholar]
- 89.Nordin JZ, Lee Y, Vader P, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11(4):879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Yeo RWY, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Ban J-J, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461(1):76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 92.Chen L, Charrier A, Zhou Y, et al. Epigenetic regulation of connective tissue growth factor by microRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nano-carriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen TS, Arslan F, Yin Y, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9(1):47–57. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 96.Portner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. Bioreactor design for tissue engineering. J Biosci Bioeng. 2005;100(3):235–245. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- 97.Shao Y, Shen Y, Chen T, Xu F, Chen X, Zheng S. The functions and clinical applications of tumor-derived exosomes. Oncotarget. 2016;7(37):60736. doi: 10.18632/oncotarget.v7i37. [DOI] [PMC free article] [PubMed] [Google Scholar]