Abstract
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are dietary factors involved in the prevention of cardiovascular, inflammatory, and neoplastic diseases. A multidisciplinary approach – based on recent findings in nutritional science, lipid biochemistry, biotechnology, and biology of inflammation and cancer – has been recently employed to develop ω-3 PUFA-containing nanoformulations with an aim to protect these fatty acids from degradation, increase their bioavailability and delivery to target tissues, and, thus, enhance their bioactivity. In some cases, these nanoformulations were designed to administer ω-3 PUFAs in combination with other nutraceuticals or conventional/innovative drugs. The aim of this strategy was to increase the activities of the compounds contained in the nanoformulation and to reduce the adverse effects often induced by drugs. We herein analyze the results of papers evaluating the potential use of ω-3 PUFA-containing nanomaterials in fighting cardiovascular diseases and cancer. Future directions in this field of research are also provided.
Keywords: innovative biotechnologies, cardiovascular diseases, nanoparticles, ω-3 PUFAs, cancer
Introduction
There is a large body of evidence supporting a beneficial role of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) against several pathologies, including cardiovascular diseases (CVDs) and cancer.1–3 The results have been obtained by using either the essential fatty acid α-linolenic acid (ALA, 18:3 ω-3), mostly found in vegetables and nuts or, and particularly, its metabolic products, the long-chain (LC)-ω-3 eicosapentaenoic acid (EPA, 20:5 ω-3) and docosahexaenoic acid (DHA, 22:6 ω-3). However, in mammal cells these two highly bioactive compounds are produced endogenously from ALA at very low levels; therefore, it is necessary to increase their main dietary sources (fish and seafood) to reach sufficient amounts in tissues. However, this requires a frequent intake of fish and seafood that appears unsustainable, particularly in the future.4 Moreover, wild fish is often contaminated with heavy metals or pesticides,5 whereas farmed fish contains much lower levels of LC-ω-3 PUFAs and high levels of antibiotics.6 In order to overcome these problems, alternative LC-ω-3 PUFA sources are now being explored, such as microalgae grown in controlled environments,7 or genetically modified plants and marine protists.8–11 An alternative approach may be the new nanotechnology-based strategies that are being developed to effectively deliver purified ω-3 PUFAs to the target tissues. These strategies are aimed to overcome the scarce solubility of these fatty acids, protect them from degradation, make them specifically active to target the site of injury, and/or distribute them in strict combination with other bioactive compounds/drugs. The ultimate goal is to enhance their bioavailability, thus reducing the level of intake of these fatty acids or of other co-transported drugs.12–14 For the first time, we have comprehensively and critically analyzed in the present review all the reports concerning the nanotechnological ω-3 PUFA-containing formulations hitherto developed, limiting our investigation to the in vitro and in vivo preclinical studies concerning the use of these nanoformulations in cellular and animal models of CVDs and cancers.
Literature search
A systematic literature search of the PubMed database was conducted from July 2017 to July 2018 to identify published peer-reviewed original articles regarding in vitro studies, in vivo animal studies, and human studies on the delivery of ω-3 PUFAs, alone, or in combination with other bioactive compounds, through nanoformulations. The key words used for the search of titles and abstracts were: “omega-3” or “n-3 PUFA” or “docosahexaenoic acid” or “eicosapen-taenoic acid” or “α-linolenic acid” or “fish oil;” and “animal studies,” or “in vitro studies,” or “in vivo studies” or “human studies;” and “nanoparticles” or “nanoformulations” and “encapsulation” and “delivery” and “nanomedicine” and “cancer” or “tumor” and “cardiovascular diseases” or “heart” and “inflammation.” We identified full-text articles written in English. The papers were chosen without restriction of time. We analyzed only the studies evaluating the biological effects of ω-3 PUFA nanoformulations and, in particular, in the cardiovascular and cancer conditions.
ω-3 PUFA-containing nanoformulations for the prevention of CVD and therapy
The prevention of CVDs is considered the main setting for ω-3 PUFA clinical application, and the major processes involved in the pathogenesis of most CVDs, including inflammation, oxidative stress, and abnormal cell proliferation,15,16 also represent the main targets of these fatty acids. However, the research investigating potential innovative nanomedicine strategies in CVDs is still very scarce, and currently restricted only to occlusive vasculopathies and atherosclerosis17,18 (Table 1; Figure 1). In the first report on this topic, Deshpande et al17 investigated nanotechnology-based approaches to deliver ω-3 PUFAs in combination with other nutraceuticals/drugs to vascular walls in order to prevent occlusive vasculopathies following vascular injuries. The study was based on the recent observations demonstrating that ω-3 PUFAs and some of their bioactive metabolic derivatives (ie, the specialized proresolving mediators, including resolvins, protectins, and maresins) are crucial endogenous signals to maintain vascular homeostasis, either by exerting anti-inflammatory properties,19 or by modulating the resolving phase of vascular injury and accelerating repair.20
Table 1.
Application of ω-3 PUFA nanoformulations in cardiovascular disease settings
| Nanoparticle (NP) type | Chemical form of ω-3 PUFA used | NP size (nm) | EE (%) | Cargo molecule | Zeta potential (mV) | Function of the NP | Experimental model | Mechanisms involved in the NP effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ALA-rich* flaxseed oil-based oil-in-water nanoemulsion | ALA-rich (56% by weight) flaxseed oil | 138–144 | 92–98 | CER and/or 17-βE | 32.30±1.81 (CER) 33.80±2.45 (17-βE) | To improve 17-βE and/or CER internalization in vascular cells | Human EC and VSMC in vitro | Increased CER and 17-βE uptake by cultured cells; increased EC and decreased VSMC proliferation (p38 MAPK inhibition) | 17 |
| ALA-rich* flaxseed oil-based oil-in-water nanoemulsion | ALA-rich (56% by weight) flaxseed oil | 176 | 94.6 | CREKA and 17-βE | 33.80±2.45 (17-βE); NR (CREKA) | To improve 17-βE internalization in vascular cells | Human EC in vitro; IV administration to apoE−/− high-fat diet mice | Increased NO production by EC in vitro; decreased atherosclerotic plaque size in mice; improved plasma lipid profile in mice; reduced ICAM-1, VCAM-1, IL-6, TNF in mice atherosclerotic plaque | 18 |
Note:
56% by weight.
Abbreviations: ALA, α-linolenic acid; CER, ceramide; CREKA, cysteine–arginine–glutamic acid–lysine–alanine peptide; 17-βE, 17β-estradiol; EC, endothelial cells; EE, encapsulation efficiency; IV, intravenous; MAPK, mitogen-activated protein kinase; NO, nitric oxide; NR, not reported; PUFA, polyunsaturated fatty acid; VSMC, vascular smooth muscle cells; TNF, tumor necrosis factor.
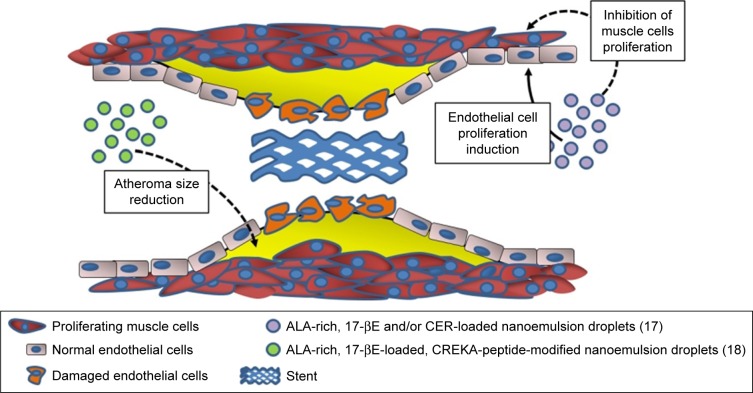
Figure 1.
Potential use of ALA-containing nanoemulsions against the development of restenosis and atheroma.
Note: The biological activity of nanoemulsions were evaluated in vitro or in vivo.
Abbreviations: ALA, α-linolenic acid; 17-βE, 17β-estradiol; CER, C6-ceramide; CREKA, cysteine–arginine–glutamic acid–lysine–alanine.
In particular, ω-3 PUFAs and their derivatives have been considered potential anti-restenosis agents, and Deshpande et al17 included an ALA-rich oil (flaxseed oil) in an oil-in-water nanoemulsion system they engineered for 17β-estradiol (17-βE) and C6-ceramide (CER) (Table 1; Figure 1). This combination appeared remarkably encouraging, since ALA has been largely reported to improve CV health, and both 17-βE and CER were previously demonstrated to be cardio-protective and vasoactive.21,22 However, it has been reported that their effect in vivo may be markedly reduced by a scarce release due to their high hydrophobicity and capability to bind with circulating proteins.23,24 In addition to the observation that the two cell systems related to restenosis (human aortic endothelial cells [ECs] and vascular smooth muscle cells [VSMCs]) showed a high in vitro uptake of 17-βE and CER, the most remarkable finding was a greater biological activity of the nanoemulsion compared to each single component administered alone (ie, increased or decreased platelet-derived growth factor-induced proliferation in EC and VSMC, respectively).17 The authors explained the results on the basis of the more efficient delivery of 17-βE and CER to vascular cells when they were encapsulated in ALA-based nanodroplets. For the muscular cells, they associated the findings with the increased inhibitory effect exerted by encapsulated 17-βE on the p38 MAP-kinase activity, known to be involved in VSMC proliferation. In a more recent work by the group,18 the same flaxseed oil-based nanoemulsion encapsulating 17-βE was used with the clot-binding peptide cysteine–arginine–glutamic acid–lysine–alanine, for its ability to selectively bind to the atherosclerotic plaque (Table 1; Figure 1). This strategy was aimed to specifically target atherosclerotic lesions and, thus, better counteract their development and progression. The nanoemulsion was administered either to EC cultured in vitro or, intravenously, to a mouse model of atherosclerosis (apoE−/− mice fed a high-fat diet). The nanoemulsion components 17-βE and ALA were considered responsible for the increased production of nitric oxide by the EC cultured in vitro. They also minimally reduced the levels of plasma cholesterol and decreased the atherosclerotic plaque size, as well as the expression of atherosclerosis-related markers (intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), IL-6, tumor necrosis factor [TNF], etc.) within the aorta of the nanoemulsion-treated apoE−/− mice. The apoE−/− mice showed significantly increased levels of both triglycerides and cholesterol after a 10-week high-fat diet administration. Interestingly, the treatment with 17-βE and ALA containing nanoemulsion was able to slightly decrease, yet significantly, the level of plasma cholesterol which, conversely, remained unchanged when 17-βE was administered alone as a solution. The authors suggested that the marginal change in cholesterol induced by 17-βE and ALA nanoemulsion could partially contribute to the protective anti-atherosclerotic effects of the nanoparticles. However, they were induced to hypothesize that it was not responsible for the lesion-size reduction triggered by these 17-βE/ALA nanoformulations, since previous observations in the same mouse model of atherosclerosis had shown that the levels of circulating cholesterol were not related to the size and area of the atherosclerotic lesion.25 Probably, the endothelial modifications induced by the nanoemulsion could be responsible for the size reductions of the atherosclerotic lesions.
It is worth pointing out that a recent meta-analysis did not detect any result of increased dietary intake of ALA in the blood concentrations of the same inflammation markers (soluble ICAM and VCAM, IL-6, and TNF).26 This suggests that a more efficient delivery of ALA – such as the one demonstrated in the apoE−/− mouse model – could allow this fatty acid to reach vascular walls at adequate amounts to induce CV health effects, particularly if the delivery occurs in combination with other cardioprotective compounds (such as 17-βE).
ω-3 PUFA-containing nanoformulations for cancer prevention and therapy
Developed ω-3 PUFA-containing nanoformulations for liver cancer prevention and therapy
Most of the studies on new nanotechnology-based strategies for a more efficient delivery of ω-3 PUFAs to cancer cells in vitro and in vivo have been conducted on liver cells. A relevant approach was used by the Corbin group.12,27–29 They created low-density lipoprotein (LDL)-based nanoparticles with DHA as bioactive cargo (Table 2; Figure 2A), and tested them on a normal mouse liver cell line, as well as on mice,12,28 rats,29 or human liver cancer cell lines.27 Interestingly, this DHA nanoformulation was successfully employed by the same group of authors also for the delivery of DHA to the brain.30 In this case, they used the systemic administration of DHA-LDL nanoparticles combined with pulsed focused ultrasound exposures. In the LDL nanoparticles, DHA was placed inside the hydrophobic core, where its stability and biological activity can be preserved. The strategy of using LDLs as ω-3 PUFA transporters to liver cells has several advantages, since LDLs have a great capacity of carrying active substances, and they are naturally present in circulation, so that they are not recognized and internalized by mononuclear circulating phagocytes.31 Moreover, LDLs carry and supply cells with lipids also in physiological conditions via LDL receptor-mediated endocytosis and seem particularly suitable to deliver bioactive lipid factors to cancer cells, which show higher avidity for lipids than normal cells, due to their sustained membrane turnover.32–34 Indeed, one particularly interesting feature of these engineered nanoparticles was their ability to be selectively cytotoxic to hepatocarcinoma cells, over the nonmalignant counterparts.12,28 This specificity for cancer cells is highly advantageous for antineoplastic agents, and, presumably, it was related to the fact that high concentrations of the cell growth inhibitor DHA were achieved only inside the cancer cells. Particularly, the selective growth-inhibitory effect of the LDL-DHA nanoparticles in liver cancer cells was explained on the basis of the specific and powerful anti-inflammatory and pro-resolving properties of the ω-3 PUFAs and their bioactive derivatives. Moreover, it was also related to increased levels of reactive oxygen species (ROS) and iron found in cancer cells, as well as to the reported reduced glutathione (GSH) cellular depletion.35,36 This pro-oxidative condition of cancer cells may easily induce peroxidation of a highly unsaturated compound, such as DHA.37 In turn, the peroxidation of intracellular membranes is triggered, and that may lead to lysosomal, mitochondrial, and nuclear disruption, and, lastly, cell death.28 Of great significance, a single intra-arterial injection of LDL-DHA in rats bearing transplanted orthotopic hepatocarcinoma markedly reduced tumor growth by altering redox balance and inducing tumor cell death.29 The most recent application of LDL-DHA particles by these authors convinced them to formulate the stimulating hypothesis that DHA enclosed in these particles could induce a recently described regulated type of necrotic cell death called ferroptosis in liver cancer cells.27 This non-apoptotic iron-dependent form of cell death38 is characterized by the simultaneous intracellular occurrence of increased lipid peroxidation, high levels of intracellular ROS originating from iron metabolism, and glutathione depletion. Moreover, it is critically and negatively regulated by the lipid antioxidant enzyme glutathione peroxidase-4 (GPX-4).27 In line with this, LDL-DHA also substantially reduced the expression and activity of GPX-4 in liver cancer cells, along with causing all the pro-oxidative changes typical of ferroptosis.27 Since it is usually reported that DHA – given as a free fatty acid – mainly causes apoptotic death in cancer cells,39 the finding that LDL-DHA, on the other hand, triggers ferroptosis would imply that, depending on the delivery system used, ω-3 PUFAs may induce different death pathway inside the cells (ie, apoptosis or ferroptosis). The authors associated this effect to the fact that – while free DHA enters the cells by diffusion or facilitated transport40 – LDL-DHA nanoparticles are endocytosed in large amounts. This different entrance pathway into the cells could enable DHA to easily reach more internal subcellular structures and, thus, exert considerable effects on them, thus contributing to the ferroptotic pathway of cytotoxicity.27
Table 2.
Application of ω-3 PUFA nanoformulations in liver cancer
| Nanoparticle (NP) type | Chemical form of ω-3 PUFA used | NP size (nm) | EE (%) | Cargo molecule | Zeta potential (−mV) | Function of the NP | Experimental model | Mechanisms involved in the NP effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| LDL-based nanoparticles | Unesterified DHA incorporated in LDL | 18.3 | 13 | DHA | 22 | To enhance physical, oxidative stability, and delivery of DHA to target cells | Normal TIB-73 and neoplastic TIB-75 murine hepatocytes in vitro | Enhanced oxidative and physical stability of LDL-based nanoparticles over free DHA; selective cytotoxicity toward cancer cells | 12 |
| LDL-based nanoparticles | Unesterified DHA incorporated in LDL | 18.3 | 13 | DHA | Not reported | To enhance physical, oxidative stability, and delivery of DHA to target cells | Rat and human HCC cell lines in vitro; mice transplanted with human HCC cells | Enhanced tumor cell death through ferroptosis; reduced tumor volumes in transplanted mice | 27 |
| LDL-based nanoparticles | Unesterified DHA incorporated in LDL | 18.3 | 13 | DHA | 24±2.6 | To enhance physical, oxidative stability, and delivery of DHA to target cells | Normal TIB-73 and neoplastic TIB-75 murine hepatocytes in vitro | Selective cytotoxicity toward cancer cells through an impairment of lysosomal, mitochondrial, and nuclear function | 28 |
| LDL-based nanoparticles | Unesterified DHA incorporated in LDL | 18.3 | 13 | DHA | 25 | To enhance physical, oxidative stability, and delivery of DHA to target cells | H4IIE rat hepatoma cells IV injected in rats | Reduced tumor volumes; tumor-specific necrosis induced by selective redox balance alteration within cancer cells | 29 |
| Tuftsin-tagged liposomes | ALA esterified to 2,6-di-isopropylphenol (propofol) | 100–130 | 74 | 2,6-propofol ALA conjugate | 43.23±1.4 | To enhance the delivery and anticancer activity of ALA in target cells | Mice subject to DEN-induced hepatocarcinoma | Increased mice survival; reduced expression of COX-2 and Bcl-2; increased expression of Bax in hepatocarcinomas | 41 |
Abbreviations: ALA, α-linolenic acid; DEN, diethylnitrosamine; DHA, docosahexaenoic acid; EE, encapsulation efficiency; HCC, hepatocellular carcinoma; IV, intravenous; LDL, low-density lipoproteins; PUFA, polyunsaturated fatty acid.
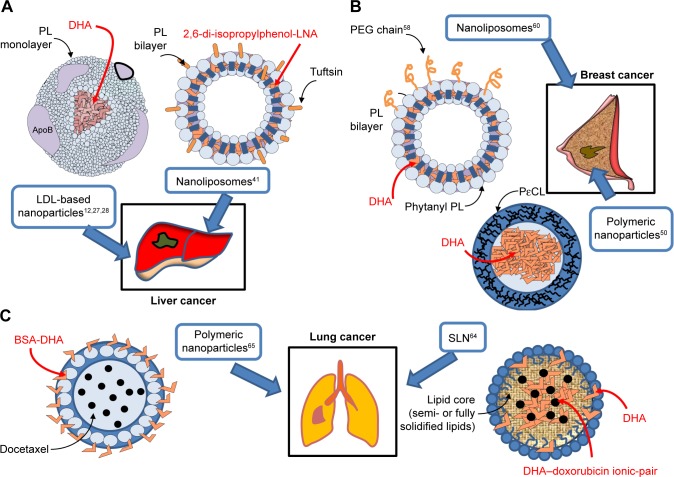
Figure 2.
Omega-3 PUFA-containing nanomaterials developed and evaluated in vitro or/and in vivo for their potential use against liver (A), breast (B) and lung (C) cancer.
Abbreviations: DHA, docosahexaenoic acid; LDL, low-density lipoprotein; LNA, linolenic acid; PEG, polyethylene glycol; PL, phospholipids; PεCL, poly-ε-caprolactone; PUFA, polyunsaturated fatty acid; SLN, solid lipid nanoparticle.
Another interesting approach for the nanodelivery of ω-3 PUFAs was recently used to deliver ALA to diethylnitrosamine-induced hepatocarcinomas in mice.41 In this case, ALA was conjugated with 2,6-di-isopropylphenol, an anesthetic agent being structurally similar to vitamin E. The chemical structure of this conjugate was reported in Khan et al.42 This compound, conjugated with DHA, was previously shown to possess antioxidant properties and the ability to induce apoptosis and decrease the metastatic potential of human cancer cells.43 For an efficient delivery, the 2,6-di-isopropylphenol-linolenic acid conjugate was enclosed in nanoliposomes tagged with the tetrapeptide tuftsin (Thr-Lys-Pro-Arg),44 a fraction of the immunoglobulin G molecule (Table 2; Figure 2A). The use of tuftsin-tagged liposomes was previously and successfully used to deliver antibiotics in the area of infections,45 due to the immunomodulatory properties of tuftsin.44 It is worth highlighting that tuftsin was also previously administered in combination with antineoplastic drugs and shown to enhance their antitumor activity.46,47 The treatment with tuftsin-tagged liposomes containing the ω-3 PUFA conjugate41 increased the survival of the hepatocarcinoma-bearing mice, and this effect was partly related to the markedly reduced expression of COX-2 and Bcl-2, as well as the upregulation of Bax observed in hepatocellular carcinomas. In our opinion, the co-presence of tuftsin and ω-3 PUFAs inside the same nanoliposomes is significant, since ω-3 PUFAs also show both immunomodulatory and antineoplastic activities at the same time, and their possible applications in the field of cancer immunotherapy have been recently suggested.48,49 Moreover, due to the safe and non-toxic nature of both ω-3 PUFAs and tuftsin and their common immunomodulating properties, the application of this liposomal delivery could be potentially useful, not only for the treatment of hepatocar-cinoma, but also for its prevention in populations at high risk (such as hepatitis C virus (HCV)-positive patients, alcoholics, or patients with inborn errors of metabolism, including type I glycogenosis or type I tyrosinemia).
Developed ω-3 PUFA-containing nanoformulations for breast cancer prevention and therapy
Roy et al50 developed polymeric nanoparticles (150–200 nm) encapsulating DHA (Table 3; Figure 2B) with the aim of using them in breast cancer. The polymeric wall of the nano-capsules (NCs) consisted of poly-ε-caprolactone, a polyester able to protect the small liquid droplets of DHA included in the internal part (core) from chemical and enzymatic degradation, protecting DHA against the adverse conditions present in the gastrointestinal tract. This nanoencapsulation approach was previously used for the delivery of different drugs achieving positive results, such as enhanced drug efficacy and decreased drug toxicity.51 Roy et al50 observed a clear cytotoxic effect (measured by the MTT assay) when they administered 50–100 µM DHA in the form of free fatty acid for 4 days to MDA-MB-231 cultured in vitro, whereas lower concentrations showed no effect. This finding was in line with the results of several papers52–56 analyzed by us in a recent review,57 showing that treatments of the triple-negative MDA-MB-231 or MDA-MB-453 breast cancer cells with EPA or/and DHA in the 30–100 µM range for 1–5 days were necessary to inhibit cell growth and proliferation, and to enhance apoptosis or breast cancer cell sensitivity to docetaxel (DTX). Unexpectedly, however, Roy et al50 found that, when lower concentrations of DHA (1–10 µM) were enclosed in NCs, they induced the proliferation of MDA-MB-231 cancer cells. On the other hand, higher concentrations (30–50 µM) of encapsulated DHA reduced breast cancer cell proliferation, similarly to what was obtained with free DHA. The simultaneous presence of vitamin E inside the NCs eliminated the growth inhibiting effect of DHA. Moreover, encapsulated DHA became more toxic than free DHA if H2O2 was added inside the NCs. Based on these results, the authors hypothesized that DHA should be oxidized and, thus, release secondary toxic products of lipid peroxidation which may be responsible for its antiproliferative effects. However, the authors suggested that H2O2 could have also acted by disrupting the nanoparticle wall, releasing DHA outside the nanoparticles and, therefore, increasing its biological activity. Otherwise, due to its hydrophobic nature, this fatty acid tends to remain bound to the oily core of these nanoparticles and lose its bioactivity. Overall, however, the delivery of DHA to scarcely differentiated breast cancer cells through this kind of polymeric nanoparticles does not appear promising for breast cancer therapy, as the antineoplastic activity of this fatty acid appears to decrease when it is encapsulated at high concentrations. Moreover, when it is encapsulated at lower concentrations, it seems to even promote breast cancer cell growth.50
Table 3.
Application of ω-3 PUFA nanoformulations in breast cancer
| Nanoparticle (NP) type | Chemical form of ω-3 PUFA used | NP size (nm) | EE (%) | Cargo molecule | Zeta potential (−mV) | Function of the NPs | Experimental model | Mechanisms involved in the NP effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Polymeric PεCL nanocapsules | DHA-free fatty acid | 183 | NR | DHA | 29.7±0.7 | To protect DHA from oxidation; to provide a delivery system to be used orally (through a higher resistance to adverse environmental conditions of GI tract) | Human breast epithelial MCF-10A and tumor MDA-MB-231 cancer cells in vitro | Inhibition of cell proliferation (MTT assay) (only in the presence of H2O2 within the nanoparticle) | 50 |
| PEGylated liposomes | DHA-free fatty acid | 99 | 81.4 | DHA | 15.7±2.5 | To enhance cell permeability and retention and facilitate local delivery of DHA | Murine 4T1 breast cancer cells in vitro; murine RAW264.7 and human THP-1 monocytes in vitro | Inhibition of cancer cell proliferation (BrdU assay); decreased inflammation (reduced MCP-1 and TNF-α production by monocytes)* | 58 |
| Phytanyl lipid-based liposomes | DHA-sodium salt | 130–160 (depending on pH values) | 60–80 | DHA | 1.6 | To enhance DHA chemical and oxidative stability | Human MDA-MB-231 and MCF-7 breast cancer cells in vitro | Reduction of cell viability (MTT assay); increased apoptosis; reduced p-Akt expression# | 60 |
Notes:
Compared to unloaded nanoparticles or free ω-3 PUFA.
Compared to free DHA.
Abbreviations: DHA, docosahexaenoic acid; EE, encapsulation efficiency; GI, gastrointestinal; NR, not reported; PEGylated, polyethyleneglycolylated; PεCL, poly-ε-caprolactone; PUFA, polyunsaturated fatty acid; TNF, tumor necrosis factor.
On the other hand, Alaarg et al58 enclosed DHA into liposomes by using 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, cholesterol, and distearoyl phospha-tidylethanolamine (PE); and polyethylene glycol (PEG) and these liposomes were used for the delivery of DHA to murine breast cancer cells in vitro (Table 3; Figure 2B). PEGylated liposomes were chosen for their acknowledged ability to stay in the circulation for a longer period (about 1 day half-life) compared to non-PEGylated particles that are cleared from the circulation in ≤1 hour.59 This implies that a bioactive compound (in this case, DHA) enclosed in these nanoparticles can have more chances to be taken up by cells and tissues. DHA-loaded liposomes were able to inhibit the proliferation of murine 4T1 breast cancer cells, and other kinds of epithelial cells. However, it is worth underlining that, in this study, the effects induced by the DHA-loaded liposomes were compared only to those induced by the unloaded liposomes. For a clearer understanding, however, it would have been essential also to evaluate the effects of DHA administered in its free fatty acid form.
Skibinski et al60 evaluated the improvement in the bioactivity of DHA included in a different kind of lipo-somes administered to MCF-7 and MDA-MB-231 breast cancer cells in vitro. Their DHA-loaded liposomes contained 1,2-dihexadecyl-sn-glycero-3-phosphocholine and 1,2-diphytanyl-sn-glycero-3-phosphoethanolamine (Table 3; Figure 2B), phytanyl lipids similar to those present in Archaea prokaryotes and known to be highly resistant to pH and temperature changes. The authors observed that DHA loaded into the liposomes reduced more the viability of breast cancer cells and induced apoptosis more efficiently in both the cell lines. Inside the liposomes, only DHA was responsible for these effects, since the empty phytanyl lipid liposome did not significantly alter cell viability and apoptosis. Moreover, the authors found that DHA enclosed in the liposomes could more effectively decrease the expression of the activated form of Akt (p-AkT). They hypothesized that this effect could explain the increased antineoplastic activity showed by DHA loaded in the liposomes. This hypothesis is particularly interesting, since p-Akt represents a biomarker associated with poor prognosis in breast cancer, and used to evaluate the activity of the tyrosine kinase receptor signaling, often deregulated in breast cancer carcinogenesis.57,61
A possible future ω-3 PUFA-containing nano-based approach for breast cancer may be suggested on the basis of the above analyzed remarkable results, obtained by both Alaarg et al58 and Skibinski et al60 with DHA-loaded nanoliposomes. Since ω-3 PUFAs are known to potentially act as an adjuvant therapy to strengthen the activity of conventional chemotherapeutics or targeted drugs in the therapy of breast cancer,57,62 it would be remarkable to develop nanoliposomal structures that are able to deliver simultaneously both ω-3 PUFAs and drugs with previously acknowledged activity in breast cancer. This liposomal combined approach could also be experimented in the clinical setting, since some liposomes are known to be safe and have already been clinically approved. Moreover, liposomes are able to encapsulate both hydrophobic and hydrophilic compounds and show a high capacity, making them particularly suitable to transport combinations of antineoplastic agents with different activities (conventional, targeted, or immunoactive agents),63 including also safe nutritional factors with multiple anticancer functions, such as ω-3 PUFAs.
Developed ω-3 PUFA-containing nanoformulations for lung cancer prevention and therapy
To date, only two studies64,65 have evaluated the anticancer properties of ω-3 PUFA-containing nanoformulations on lung cancer cells (Table 4; Figure 2C), and their main aim was to improve the delivery and antitumor efficacy of doxorubicin (DOX) and DTX, two chemotherapeutic agents widely used in the therapy of solid tumors. A variety of nanotechnological approaches have been investigated so far to improve the limited uptake and distribution of DOX in solid tumors.66,67 Indeed, DOX is a weakly basic compound that becomes protonated when exposed to the slightly acidic pH of the microenvironment surrounding cancer cells, making its cellular uptake difficult.66 Liposomes, solid lipid nanoparticles (SLNs), and polymeric micelles are among the different types of DOX nanocarriers designed to overcome this problem. A PEGylated liposomal formulation containing DOX (Doxil) has also been approved for the treatment of some cancers (ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma).68 Mussi et al64 designed DOX-loaded SLN for lung cancer, where the oily phase included DHA and surfactants, such as Compritol, Tween 80, and triethanolamine (Table 4; Figure 2C). The SLN formulation allows a site-specific release of DOX in a slightly acidic environment, such as tumor tissues.66,69 Moreover, the SLN solid matrix allows a more controlled release, and the lipophilicity of these nanoparticles improves the interaction with the cellular membranes. Furthermore, the SLNs obtained by encapsulating 0.4% DHA (w/v) were of smaller size (94±1 nm), which is advantageous, being the nanoparticles in the range of 100–200 nm taken up and retained at a higher concentration inside cancer cells compared to particles with a higher size.70–72 The simultaneous encapsulation of DOX with a lipophilic anion, such as DHA in the SLN, was aimed to overcome the low level of incorporation in lipidic nanostructures that DOX usually exhibits, due to its cationic amphiphilic nature. Moreover, the SLN exhibited negative charges due to the presence of DHA, and, inside the SLN, DOX formed an ion-pairing with DHA that could explain the high DOX encapsulation effi-ciency (almost 100%), as well as the enhanced cytotoxicity shown by the DHA-DOX-loaded SLN against A549 lung adenocarcinoma cells, as compared to free DOX and DHA administered in combination.
Table 4.
Application of ω-3 PUFA nanoformulations in lung and prostate cancer
| Nanoparticle (NP) type | Chemical form of ω-3 PUFA used | NP size (nm) | EE (%) | Cargo molecule | Zeta potential (−mV) | Function of the NP | Experimental model | Mechanisms involved in the NP effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| SLN | DHA-free fatty acid | 94 (0.4% DHA) | 99 (0.4% DHA) | DOX ± DHA | 34±0.2 (0.4% DHA) | To reduce SLN size; to induce negative charges in SLN; to enhance DOX EE, release in medium and solubility in lipids | Human A549 lung adenoCa cells in vitro | Enhanced DOX cytotoxicity§ (TB exclusion assay) | 64 |
| DHA-BSA polymeric nanoparticles | DHA-free fatty acid | 146.9±22.9 | 98.6±9.3 | DTX | 31.5±1.5 | To specifically target cancer cells | Murine LLC cells and human A549 adenoCa in vitro; murine LCC cells transplanted in C57BL/6 mice | ¥In vitro: enhanced cancer cell targeting; enhanced cell uptake efficiency; enhanced DTX antiproliferative effect (MTT assay); decreased cancer cell migration. @In vivo: decreased lung cancer metastases to bone; increased mice survival | 65 |
| Oil-in-water nanoemulsion | DHA-SBT-1214 conjugate | 228±7 | 97 | DHA-SBT-1214 | 24.9±4.3 | To enhance permeability and retention of SBT-1214 in cancer cells | Human CSC-enriched PPT2 prostate cancer cells in vitro and transplanted in NOD/SCID mice | Enhanced drug cytotoxicity in vitro*; enhanced tumor growth suppression in vivo* | 87 |
| Oil-in-water nanoemulsion | DHA-SBT-1214 conjugate | 213.2 | 97 | DHA-SBT-1214 | 28.9±4.3 | To improve the targeted delivery of SBT-1214 to cancer cells | Human CSC-enriched PPT2 prostate cancer cells transplanted in NOD/SCID mice | Slower in vitro drug release; improved pharmacokinetic properties in plasma and tissues; enhanced stability# | 88 |
Notes:
Compared to free DHA + DOX and to empty SLN.
Compared to free DTX or to DTX-BSA nanoparticles.
Compared to DTX-BSA nanoparticles.
Compared to both Abraxane® and placebo nanoemulsion formulation.
Compared to aqueous solution.
Abbreviations: EE, encapsulation efficiency; LLC, Lewis lung carcinoma; NOD, nonobese diabetic; SCID, severe combined immunodeficiency; SLN, solid lipid nanoparticles; adenoCa, adenocarcinoma; CSC, cancer stem cells; DHA, docosahexaenoic acid; DOX, doxorubicin; DTX, docetaxel; PUFA, polyunsaturated fatty acid.
The second study was performed by Zu et al,65 who evaluated the anticancer activity of ω-3 PUFA-containing polymeric nanoparticles on lung cancer cells. The nanoparticle surface contained DHA covalently bound to BSA, a natural biodegradable polymer often used to prepare polymeric nanoformulations73 (Table 4; Figure 2C). Also in this case, the size of the newly developed nanoparticle (141.7±7.9 nm) was within the range of sizes allowing a more efficient uptake by cancer cells.70–72 The DHA-BSA nanoparticles were designed to enclose DTX, an approved semisynthetic antineoplastic drug belonging to the taxane family and, at present, largely used for the treatment of a variety of solid cancers, including lung and breast cancer.74,75 Its inclusion in the nanoparticle was aimed to overcome the scarce aqueous solubility of the drug. Numerous formulations of taxanes have been developed over the last decades in order to increase the aqueous solubility of the hydrophobic taxanes, such as cyclodextrin, liposomes, and albumin-bound nanoparticles (Abraxane) formulation.73,76 In the nanoformulation analyzed,65 the addition of DHA to the albumin on the surface of the DTX-BSA nanoparticle was aimed to specifically target cancer cells, since it was previously observed that DHA preferentially accumulates in tumor cells and is considered a target ligand for cancer cells.77–79 This tumor targeting ability of DHA has been typically associated with the increased fluidity shown by cancer cell membranes which contain high levels of this fatty acid.77–79 In turn, the enhanced fluidity was related to their higher content in phospholipids, and, particularly, in PE, which was found to have a specific affinity for DHA.77 Above all, targeting lung cancer cells with these nanoparticles seems mainly appropriate since PE has been found to be overexpressed not only in the cancer cells used in this study (murine Lewis Lung Carcinoma [LLC] cells)77 but also, more in general, in the human non-small-cell lung cancers.80 Furthermore, the addition of an antineoplastic natural agent, such as DHA, to polymeric DTX-BSA nanoparticle65 was also related to the results of several human studies demonstrating that the therapeutic efficacy of this taxane can be increased after its conjugation to DHA, which is also able to decrease the side effects usually induced by taxane therapy, and improve the taxane delivery to the tumor tissues.81–86 Only the choice of BSA to prepare these polymeric nanoformulations could be controversial, since BSA was reported to antagonize the effect of DHA.13 The new DTX-DHA-BSA nanoparticles were administered to lung cancer cells cultured in vitro and to mice injected with syngeneic LLC cells in the tibia of the right limb, which mimicked lung cancer metastasis in the bone.65 The finding demonstrated that the simultaneous presence of DHA in the nanoparticles enhanced the anticancer activity of taxanes both in vitro and in vivo, and also increased the mean survival time of the mouse.
Developed ω-3 PUFA-containing nanoformulations for prostate cancer prevention and therapy
Similarly to the results of the work by Zu et al,65 where nanoformulations containing DHA bound to a taxane were tested in lung cancer, two studies performed on prostate cancer used an oil-in-water nanoemulsion formulation,87,88 where the starting oil phase consisted of fish oil with a novel DHA-taxoid conjugate (DHA-SBT-1214) (Table 4; Figure 3A). The oil phase was mixed with the water phase formed by egg phosphatidylcholine (Lipoid E80®), polysorbate 80 (Tween80®), and 1,2-distearoyl-sn-glycero-3-phosphoeth-anolamine-N-(amino[polyethylene glycol]-2000) (DSPE-PEG2000). Both the empty nanoemulsion (not containing drugs) and the nanoemulsion containing DHA-SBT-1214 showed spherical shape and a very similar particle size (225±7 and 228±7 nm, respectively). The surface charge of the oil droplets in the two formulations did not change even using the maximum encapsulation of DHA-SBT-1214 within the oil droplets. The two studies are remarkable, since they benefit from a new-generation taxoid (SBT-1214), bound to DHA, and avoid the use of albumin, which can reduce the anticancer effect of DHA.13 In particular, the use of SBT-1214 was aimed to overcome the mechanism through which placlitaxel89 or DTX90 was observed to induce drug resistance in prostate cancer (by mechanisms related to the induced expression of either P-glycoprotein or transforming growth factor-β superfamily components, respectively). On the other hand, the rationale of SBT-1214 binding to DHA was related to the above-mentioned ω-3 PUFA ability to exert cancer-specific toxicity, to the synergistic antineoplastic effects that these fatty acids exert when bound to anticancer drugs, and to their ability to protect non-transformed prostate cells from cytotoxicity and reduce the secondary systemic harmful effects of conventional anticancer drugs, including taxanes. Moreover, the use of the non-ionic emulsifying agent Tween 80 in the preparation of this nanoemulsion is relevant, as it allows to obtain optimal oil droplet size and charge by inhibiting aggregation through competitive absorption to interfaces. This surfactant was also used for the preparation of other nanoemulsions containing ω-3 PUFAs and herein analyzed.64,91,92 The newly designed nanoemulsion of DHA-SBT-1214 (NE-DHA-SBT-1214) was tested for its antineoplastic activity against human prostatic cancer PPT2 cells cultured in vitro, or transplanted subcutaneously in NOD/SCID mice.87,88 These prostate cancer cells have the features of stem-like cells and are considered a good model for testing new drugs for prostate cancer. This is a highly recurrent tumor, which has been related to the presence of high levels of cancer stem cells with the potential of self-renewing the tumor.93–95 The NE-DHA-SBT-1214 exerted higher cytotoxicity toward the PPT2 cells cultured in vitro, compared with DHA-SBT-1214 in solution. In addition, intravenous administrations of this nanoemulsion carrier system containing DHA-SBT-1214 in fish oil droplets to the transplanted mice markedly suppressed prostate cancer cell growth in vivo compared to a placebo nanoemulsion formulation previously approved for the treatment of metastastic human breast cancer (Abraxane™, ie, paclitaxel albumin-bound particles). Moreover, the prostate cancer cells that still remained viable after the in vivo treatment with NE-DHA-SBT-1214 had reduced neoplastic potential, which was shown by their decreased ability to give rise to colonies and spheres when analyzed ex vivo for clonogenic and sphere-forming capacities. These results suggest that the use of NE-DHA-SBT-1214 for the treatment of prostate cancer could be a promising strategy.
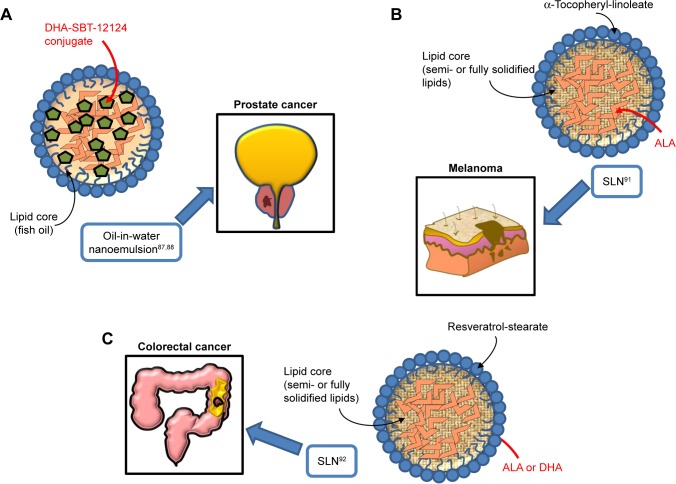
Figure 3.
Omega-3 PUFA-containing nanomaterials developed and evaluated in vitro or/and in vivo for their potential use against prostate (A), melanoma (B), and colorectal cancer (C).
Abbreviations: PUFA, polyunsaturated fatty acid; DHA, docosahexaenoic acid; SLN, solid lipid nanoparticle; ALA, α-linolenic acid.
Developed ω-3 PUFA-containing nanoformulations for melanoma and colorectal cancer (CRC) prevention and therapy
We have studied the antineoplastic potential of ω-3 PUFAs in CRC and melanoma for many years96–101 and investigated the possible mechanisms underlying the beneficial action of these fatty acids. We have recently developed new SLNs for the delivery of ω-3 PUFAs to C32 melanoma cells91 and HT-29 and HCT116 CRC cells,92 to reduce possible ω-3 PUFA oxidative degradation of these compounds, and, thus, prolong their shelf-life, enhance their bioavailability, and improve their antineoplastic activity (Table 5; Figure 3B and C). In particular, the matrix of the SLN tested in melanoma cells contained ALA esterified to α-tocopherol and encapsulated ALA itself. ALA carried in these nanoformulations was always more cytotoxic than ALA or α-tocopherol administered alone to melanoma cells.91 On the other hand, the lipid matrix of the SLN formulation tested in CRC cells contained stearic acid esterified to the natural dietary antioxidant resveratrol (RV). Furthermore, these RV-based SLN (RV-SLN) carried either ALA or DHA inside.92 Compared with the other SLN tested on melanoma cells, the substitution of ALA in the matrix with stearic acid was aimed to further increase the hydrophobicity of the encapsulated ω-3 PUFAs, thus potentially improving the efficiency of their delivery. Even though stearic acid is a saturated fatty acid, it has been shown that it may lower serum LDL cholesterol levels,102 and also protect from the development of prostate cancer.103,104 In particular, this protective action appears remarkable, since it has been recently hypothesized that the incidence of prostate cancer may be associated with a high intake of ω-3 PUFAs. Thus, the combination stearic acid/ω-3 PUFAs inside the SLN could neutralize possible prostate-specific adverse effects of these fatty acids.105 Moreover, the encapsulation of both ALA and DHA makes them more efficient in inhibiting CRC cell growth, by enhancing their inhibitory effect on cell proliferation rather than further increasing their already high ability to induce apoptosis.92 These increased antineoplastic effects were strictly related to the enhanced cellular incorporation observed when DHA and ALA were enclosed in the RV-SLN to CRC cells. It was remarkable to find that, only after the encapsulation, ALA induced an evident cellular increase in both EPA and DHA and that encapsulated DHA was more efficiently retroconverted to EPA. This implies that the encapsulation of either DHA or ALA into RV-based SLN could also markedly increase the CRC content of EPA, which, according to several recent reports, seems to protect against CRC risk better than all the other main ω-3 PUFAs.106–108 The more efficient conversion of SLN-encapsulated ALA is particularly interesting when considering that ALA is present at high levels in a variety of seeds and vegetables, and its increased intake could be achieved more easily and in a more sustainable way compared to an increased consumption of EPA itself.
Table 5.
Application of ω-3 PUFA nanoformulations in melanoma and colon cancer
| Nanoparticle (NP) type | Chemical form of ω-3 PUFA used | NP size (nm) | EE (%) | Cargo molecule | Zeta potential (−mV) | Function of the NP | Experimental model | Mechanisms involved in the NP effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| α-Tocopheryl linoleate-based SLN | ALA-free fatty acid | 181 | 77 | ALA | ND | To improve the delivery and stability of ALA | Human C32 melanoma cells in vitro | Enhanced antioxidant efficiency*; enhanced cytotoxicity# | 91 |
| Resveratrol-based SLN | ALA- or DHA free fatty acids | 842 (DHA-containing SLN); 1000 (ALA-containing SLN) | 77 (ALA); 100 (DHA) | DHA or ALA | ND | To protect ω-3 PUFA from degradation and to increase their incorporation in cancer cells | Human HT29 and HCT116 colon cancer cells in vitro | Increased ω-3 PUFA incorporation in tumor cells; increased cancer cell growth inhibition; reduced cancer cell proliferation§ | 92 |
Notes:
Compared to the empty SLN.
Compared to free ALA.
Compared to free DHA and ALA.
Abbreviations: ALA, α-linolenic acid; DHA, docosahexaenoic acid; EE, encapsulation efficiency; ND, not determined; SLN, solid lipid nanoparticles; PUFA, polyunsaturated fatty acid.
In conclusion, it is important to underline that the possibility to further enhance the protective activity of these dietary compounds against CRC is appealing, since the possible prevention and treatment of CRC with PUFA has involved many researchers in the past two decades.96,109–112 In particular, possible successful future investigations in the field of CRC nanomedicine-based therapy could be related to the powerful modulator effect recently demonstrated for ω-3 PUFAs on the intestinal microbiota.107,113 Indeed, since the microbiome may deeply affect the individual response to cancer immunotherapy, it would be worth investigating the approach of combined nanodelivery of immunomodulating innovative drugs, already used for CRC114,115 with ω-3 PUFAs against CRC in vivo. Moreover, co-delivering powerful anti-inflammatory agents, such as ω-3 PUFAs, and these drugs in nanoparticles could also have the potential to overcome the severe colon immune-related adverse inflammatory events induced by these innovative treatments in CRC patients.116
Mixed and contrasting outcomes of recent human studies and the well-known benefits of ω-3 PUFAs in CVD and cancer
We cannot avoid focusing hereinafter on the topic outlined by the following question: are the contrasting outcomes of recent large human trials weakening the accepted dogma of ω-3 PUFA healthy effects? In our opinion, this topic deserves particular attention, particularly by the researchers who have been studying for decades the beneficial effects of ω-3 PUFAs in the prevention of CVD and cancer.1,39,57,62,96,98,117 Even though this issue cannot be tackled exhaustively in just one paragraph, it is essential to outline some considerations.
As far as the ω-3 PUFA cardioprotective role is concerned, the beneficial outcomes of early human studies (epidemiological studies, prospective cohort studies, and randomized controlled trials) appear in contrast with the mixed or less positive results of some recent prospective trials, and meta-analysis of clinical trials.117 Indeed, they provided scarce or no evidence that ω-3 PUFA may reduce CV events or mortality (for a review see Bowen et al118). It has been hypothesized117 that these discrepancies may be attributed to the difficulty of ω-3 PUFA to induce healthy effects in a completely changed scenario. In fact, in recent years, the enrolled patients have often been simultaneously subject to the administration of statins and/or other pharmaco-logic agents widely used to prevent CVD. Moreover, higher levels of ω-3 PUFA containing foods have been generally added to the diet following the numerous recommendations formulated over the last two decades. The results of the recently published VITAL study119 also obtained mixed and less promising results. It had the novelty of being a large, 5-year randomized, double-blind, placebo-controlled trial of a daily fish oil supplementation (1 g, Omacor® fish oil) for primary prevention in healthy subjects at baseline. On the contrary, the earlier trials mostly evaluated whether a supplementation with fish oil or purified ω-3 PUFAs could prevent CV adverse events in patients with a history of CVD or at high risk for CVD. Interestingly, the primary outcomes of the VITAL study indicated only a small but non-significant decrease in the rate of a major adverse CV event (ie, myocardial infarction [MI] or stroke) in the ω-3 PUFA group with respect to the placebo group. However, a secondary analysis in those patients that were specifically affected by stroke or MI revealed that the intervention was able to significantly reduce the risk of MI (by 28%) and the death rate of MI (by 50%). Interestingly, a much higher reduction in MI risk (by 77%) was observed in the African subpopulation, thus enforcing the notion that the ω-3 PUFA beneficial effects may be modified depending on the individual genetic background.120 Remarkably, the need for angioplasty was also significantly reduced (by 22%) by the fish oil supplementation. The accuracy of this trial was also demonstrated by the evaluation of the basal plasma ω-3 index (EPA + DHA as a percentage of total fatty acids) of the subjects, which was evaluated also after 1-year treatment to show its increase (by about 50%). The outcomes of another recently published randomized double-blind, placebo-controlled trial (REDUCE-IT) reported more positive results. However, it evaluated the effects of simultaneous treatment with a very high dose (4 g/day) of purified EPA and statin therapy for almost 5 years in patients with CVD or at high risk for CVD and high fasting triglyceride levels.121 The study showed that the EPA treatment reduced the levels of serum triglycerides, CV events, and CV death. In particular, the rates of emergency or urgent revascularizations or MI were reduced by the EPA supplementation, even though the high dose used slightly increased the rate of bleeding events (see next paragraph), as well as the onset of atrial arrhythmias. It can be underlined that many differences do exist between these two most recent and largest trials, which can however help us to better understand the actual potential of ω-3 PUFAs in preventing CVD. For instance, the use of fish oil at a quite low dose may lack in showing the induction of health CV effects. On the contrary, EPA administered alone at a very high dose, even though in the presece of a statin therapy, may elicit beneficial effects, increasing, however, also the risk of some detrimental properties. Overall, the debate on the ω-3 PUFA-induced CV benefits is ongoing, also considering the manifold data from in vitro studies and in vivo animal studies supporting a variety of different biological and molecular mechanisms whereby ω-3 PUFA may confer coronary protection and reduce the risk of CVD.122 Therefore, further large interventional trials comparing different doses and types of fatty acid (EPA and DHA) are needed for a better knowledge of this topic. Moreover, it would be more advantageous that the subjects/patients would be stratified according to the ω-3 index expressed as the content of EPA and DHA in red cell membranes, which is considered a more reliable and stable indicator of the real ω-3 PUFA intake than their plasma level.123,124 Moreover, it would be important to evaluate the ω-3 index several times along the long-term studies.
With regard to cancer, plenty of evidence obtained from in vitro and in vivo preclinical studies has clearly indicated the anticancer activity of omega-3 PUFA.120 On the contrary, as we have widely discussed in a recent critical review of the literature,120 the results of the human studies are more controversial. In particular, most of the recent results confirmed the beneficial effects of the preclinical studies. However, there are studies where no effect was observed, or the antineoplastic effects were confined only to specific subpopulations (obese subjects, with specific dietary habits or with a particular fatty acid pattern, or having a specific T-cell infiltration in the tumor microenvironment). Only a few reports found positive associations between the development/progression of cancer (mainly for prostate cancer), and the high level of ω-3 PUFA introduced with the diet, present in blood, or accumulated in tissues. In our opinion, to clarify the issue, it would be essential to know the actual levels of ω-3 PUFA intake and the efficiency of their incorporation in the tissues of each subject along the study period. To this end, we recommended repeated evaluations of biomarkers reflecting the intake of ω-3 PUFAs, and in particular, the ω-3 index expressed as the content of EPA and DHA in red cell membranes.120 Moreover, we suggested, whenever possible, not to overlook some characteristics of the subjects being studied, such as the race/genetic background, and the metabolic/nutritional status.
Interestingly, the outcomes of the more recent interventional VITAL study119 indicated that the daily supplementation with 0.8 g/day ω-3 EPA + DHA was unable to lower the incidence of different kinds of invasive cancer of any type, as well as that of colorectal, breast, and prostate cancer, or the incidence of death from cancer. These findings were consistent with the results of two recent meta-analyses of ω-3 PUFA trials of CVD that had shown either no association between the supplementations with ω-3 PUFA and the risk of cancer in general125 or a slightly increased but not significant risk.126 Again, however, attention should be focused on the doses supplemented and the consequent ω-3 PUFA tissue enrichment, that is, in our experience, the most related parameter to the antineoplastic activity of these fatty acids.124 It is possible that the daily dose of about 1.0 g (generally recommended for the secondary prevention of CVD) may not be sufficient to induce any anticancer effect. In a recently published review,124 we critically analyzed the doses of ω-3 PUFA that had been able to induce anticancer effects both in the animal studies and in the few available interventional human studies. We found that supplementation to humans with safe doses of EPA + DHA of ~2.0 g/day produced a plasma and tissue enrichment in these fatty acids of about 2–5 folds, which was associated with significant anticancer effects.127–129 Higher doses were not able to further increase the amount of EPA and DHA incorporated in tissues. In mice or rats the dietary doses of EPA + DHA that have clear antineoplastic effects were variable in relation to their body mass and surface. However, these doses always produced an increase of ~2–5 folds of these fatty acids in the plasma, as well as in the normal and neoplastic tissues. Altogether, these observations prompted us to suggest that a daily dose of about 2 g could be the most appropriate for future human studies evaluating the anticancer effects of ω-3 PUFAs.
Possible adverse effects of ω-3 PUFAs: are they real obstacles to their use?
Increased bleeding risk
It is not possible to completely overlook here the controversies regarding the possible adverse effects of high doses of ω-3 PUFAs. In particular, high intakes of ω-3 PUFAs have been suggested to exert adverse effects on bleeding risk, since, in an earlier study, the diet with high levels of ω-3 PUFAs of the Greenland Inuit population was associated with an increased bleeding time.130 The finding that these fatty acids were able to displace the ω-6 PUFA arachidonic acid from cell membranes and decrease its metabolic conversion to the powerful pro-thrombotic platelet-aggregating factor thromboxane A2, further substantiated this hypothesis.96 Moreover, increased intakes of ω-3 PUFAs were also reported to be associated with reduced blood levels of several clotting factors such as factor V, prothrombin, and von Willebrand factor.131 However, a recent review of the literature132 examining this issue supported the safe consumption of EPA and DHA even in highly vulnerable and sensitive populations, such as patients having gastrointestinal cancer or hospitalized in Intensive Care Units and consuming fish oil–enriched medical nutrition. No increased risk of bleeding-related event was identified in the analyzed reports, even if the patients were simultaneously treated with platelet aggregation inhibitors. On the contrary, the results concurred to support the safety of either very high daily doses of EPA + DHA given for a short period (up to 10 g/day) or much lower doses consumed for 1 year. Results obtained more recently by the large VITAL study having a 5-year median follow-up confirmed these findings.119 In this study, healthy subjects supplemented with 1 g/day fish oil (providing 460 mg EPA + 380 mg DHA) did not show increased major bleeding episodes as compared to the subjects of the placebo group. The other recent large REDUCE-IT study121 was performed on patients already receiving statin therapy and showing high levels of serum triglycerides, patients with a previously diagnosed CVD or at high risk of developing CVD. They were supplemented for almost 5 years with a high daily dose (4 g) of purified EPA. This study reported a modestly but significantly higher overall rate of adverse events, but not fatal bleeding events in the EPA group (2.7% vs 2.1% in the placebo group). However, it was observed that there were no significant differences between the placebo end EPA groups in severe bleeding events, such as hemorrhagic stroke, serious central nervous system bleeding and gastrointestinal bleeding. Moreover, CV patients are often subject to chronic antithrombotic therapies, and this factor was not considered in the study. In any case, the inclusion of ω-3 PUFA in nanoformulation and the consequent increased efficiency of their delivery and absorbance at specific sites may prevent the induction of adverse bleeding effects at the circulatory level.
Immunosuppression
Concern has also been expressed/raised over the possibility that ω-3 PUFAs could have the potential to suppress any possible basal immunoreactivity against cancer, and decrease the impact of cancer immunotherapy.133 This alarming possibility is related to the widely acknowledged anti-inflammatory and immunosuppressive activities of ω-3 PUFAs, and it is clear that their inclusion in nanoformulations to increase their bioactivity could also intensify these effects. However, in the last decades, a great deal of information has been gathered supporting the anti-cancer activities of ω-3 PUFAs, which oppose the preconceived alarm of a possible ω-3 PUFA cancer-promoting activity.120 Moreover, it should be underlined that, whereas acute inflammation is a response to antagonize infections, repair, and resolve tissue damage, chronic inflammation has been involved in the promotion of cancer, by inducing immunosuppression in tumor microenvironment.134,135 Per se, it is considered a hallmark of cancer. To this respect, ω-3 PUFAs reveal their multifaceted nature, on one hand, by inhibiting persistent inflammation, thus preventing the promotion of cancer, and by also exerting pro-inflammatory actions to inhibit the immunosuppression existing in the tumor environment.136 In fact, recent findings have shown that one of the mechanisms through which ω-3 PUFAs may inhibit tumor development in vivo is the induction of a local antitumor immune response.137,138 In particular, Liang et al137 demonstrated a decreased expression of markers of tumor-associated macrophages in the tumor microenvironment of immunocompetent mice bearing prostate cancer and fed a fish oil–enriched diet. They also observed a decreased expression of a chemokine (CCL-2) that specifically recruits monocytes and macrophages to inflammatory sites.137 It was also hypothesized that more than the dietary intake of ω-3 PUFAs by themselves, an increased dietary ω-3 PUFA/ω-6 PUFA ratio could maximally and beneficially influence the immune characteristics of the tumor microenvironment and reduce the risk of developing some forms of cancer.139 This was demonstrated by Gevariya et al138 that used mice transplanted with syngeneic prostate transgenic cell line and supplemented with a high ω-3 PUFA/ω-6 PUFA ratio diet (3.3). They found increased levels of cytokines associated with Th1 and Th2 immune response in the tumors growing in these mice compared to those of mice fed a diet with a much lower ω-3 PUFA/ω-6 PUFA ratio (0.002). These findings are in line with recent in vitro studies showing that ω-3 PUFAs are able to stimulate the innate and the adaptive immune response, both in the humoral and in the cellular branch.140–142
Finally, considering the immunosuppressive activity of ω-3 PUFAs, it could be argued that they may have the potential to inhibit the immune response to infections. Again, this detrimental effect could be augmented by their inclusion in nanoformulations aimed to increase their efficacy. On the contrary, however, according to recent relevant results comprehensively analyzed by Costantini et al,143 dietary ω-3 PUFA can positively modulate the immune response to microbe infections. In particular, evidence has been provided that the strict relationship between ω-3 PUFAs, gut microbiota, and immune system may influence the activity of the host immune cells.143 In particular, plenty of animal studies and some evidence in humans have demonstrated that dietary supplementations with ω-3 PUFAs are able to normalize the gut microbial imbalance.143 This appears interesting, since the intestinal microbiota has been reported to exert a crucial role in the development of the systemic and gut immune response.144,145
Conclusion
To date, only a limited number of recent studies performed in vitro or in animals have directly investigated the biological effects of newly developed ω-3 PUFA-containing nanomaterials in CVDs and cancer. To date, none of these nanoformulations have been tested in the clinical setting. The studies have almost univocally demonstrated that a more efficient delivery of ω-3 PUFAs through these newly developed nanosystems may markedly enhance the therapeutic potential of nutraceuticals and other co-delivered drugs toward inflammatory lesions and cancer. Different strategies have been used, and these fatty acids have generally been included in the newly developed systems because of their ability to induce healthy effects. However, in some cases, they were also used just as a component of the lipidic phase to obtain nanoparticles carrying different bioactive products. Moreover, their position in the external part of some nanoparticles was also thought to specifically target cancer cells, which are avid of lipids necessary for their high-level metabolism and high proliferative turnover rate. Thus, through this expedient, such ω-3 PUFA-coated nanoparticles could be preferentially taken up by cancer cells.
Notably, the inclusion of these fatty acids – showing both anti-inflammatory and anti-neoplastic activities – in nanoformulations could be significant in making them more bioavailable and to better deliver them to cancer tissues. Therefore, ω-3 PUFAs could better inhibit cancer cell growth, but simultaneously decrease the adverse inflammatory effects induced by the currently used conventional and innovative antineoplastic agents and, also, reduce the cachexia associated with the advanced cancer stages.
It is worth observing that ALA becomes more bioactive when enclosed in some of the nanoformulations analyzed in this review. This happened since ALA in this form was more efficiently delivered to the target cells and converted into its more bioactive products (EPA and DHA). This implies that these ALA-containing nanoformulations could allow to easily enrich our tissues with EPA and DHA, which, unlike ALA, largely found in vegetables and nuts, are mainly obtained from fish that indeed are not considered a sustainable food source for the entire world population.
Altogether, the reports analyzed univocally suggest that multiple potential advantages could be obtained with the administration of nanoformulations containing ω-3 PUFAs for the prevention and cure of both CVDs and cancer. In particular, the inclusion in nanoparticles has been often designed to offer protection to these quite unstable compounds from oxidative insults and to increase and enhance their delivery to the sites of the pathological lesions, resulting in an increased efficacy. Moreover, the safety of ω-3 PUFAs at the doses generally used in human studies,124 their inexpensiveness, and the results so far obtained made them optimal candidates to be enclosed in nanoparticles in combination with other pharmacological agents already used in the clinical setting. The potential of these therapeutic combinations is to deliver all the agents enclosed successfully, increase their activity, and make the therapy more effective.
Acknowledgments
This work was funded in part by grants Linea D1 2016, Linea D.3.2 2013, and D.3.2 2015 to GC from Università Cattolica del S Cuore, Rome, Italy, within its program of promotion and diffusion of scientific research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013;2013:743171. doi: 10.1155/2013/743171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marventano S, Kolacz P, Castellano S, et al. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? Int J Food Sci Nutr. 2015;66(6):611–622. doi: 10.3109/09637486.2015.1077790. [DOI] [PubMed] [Google Scholar]
- 3.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitessa SM, Abeywardena M, Wijesundera C, Nichols PD. DHA-containing oilseed: a timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients. 2014;6(5):2035–2058. doi: 10.3390/nu6052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushkin-Bedient S, Carpenter DO. Benefits versus risks associated with consumption of fish and other seafood. Rev Environ Health. 2010;25(3):161–191. doi: 10.1515/reveh.2010.25.3.161. [DOI] [PubMed] [Google Scholar]
- 6.Nichols PD, McManus A, Krail K, Sinclair AJ, Miller M. Recent advances in omega-3: health benefits, sources, products and bioavailability. Nutrients. 2014;6(9):3727–3733. doi: 10.3390/nu6093727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells ML, Potin P, Craigie JS, et al. Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol. 2017;29(2):949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MR, Nichols PD, Carter CG. n-3 Oil sources for use in aquaculture–alternatives to the unsustainable harvest of wild fish. Nutr Res Rev. 2008;21(2):85–96. doi: 10.1017/S0954422408102414. [DOI] [PubMed] [Google Scholar]
- 9.Lenihan-Geels G, Bishop KS, Ferguson LR. Alternative sources of omega-3 fats: can we find a sustainable substitute for fish? Nutrients. 2013;5(4):1301–1315. doi: 10.3390/nu5041301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins DA, Custódio L, Barreira L, et al. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs. 2013;11(7):2259–2281. doi: 10.3390/md11072259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague M, Betancor MB, Tocher DR. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol Lett. 2017;39(11):1599–1609. doi: 10.1007/s10529-017-2402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds L, Mulik RS, Wen X, Dilip A, Corbin IR. Low-density lipoprotein-mediated delivery of docosahexaenoic acid selectively kills murine liver cancer cells. Nanomedicine (Lond) 2014;9(14):2123–2141. doi: 10.2217/nnm.13.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanno S, Kurauchi K, Tomizawa A, Yomogida S, Ishikawa M. Albumin modulates docosahexaenoic acid-induced cytotoxicity in human hepa-tocellular carcinoma cell lines. Toxicol Lett. 2011;200(3):154–161. doi: 10.1016/j.toxlet.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Namani T, Ishikawa T, Morigaki K, Walde P. Vesicles from docosahexaenoic acid. Colloids Surf B Biointerfaces. 2007;54(1):118–123. doi: 10.1016/j.colsurfb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56(7):1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 16.Innes JK, Calder PC. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. 2018;19(2):E532. doi: 10.3390/ijms19020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshpande D, Janero DR, Amiji M. Engineering of an ω-3 polyunsaturated fatty acid-containing nanoemulsion system for combination C6-ceramide and 17β-estradiol delivery and bioactivity in human vascular endothelial and smooth muscle cells. Nanomedicine. 2013;9(7):885–894. doi: 10.1016/j.nano.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande D, Kethireddy S, Janero DR, Amiji MM. Therapeutic effi-cacy of an ω-3-fatty acid-containing 17-β estradiol nano-delivery system against experimental atherosclerosis. PLoS One. 2016;11(2):e0147337. doi: 10.1371/journal.pone.0147337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansbury BE, Spite M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ Res. 2016;119(1):113–130. doi: 10.1161/CIRCRESAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cong B, Xu Y, Sheng H, et al. Cardioprotection of 17β-estradiol against hypoxia/reoxygenation in cardiomyocytes is partly through up-regulation of CRH receptor type 2. Mol Cell Endocrinol. 2014;382(1):17–25. doi: 10.1016/j.mce.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14:55. doi: 10.1186/s12944-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowey K, Tanguay JF, Tabrizian M. 2-Dioleoyl-sn-glycero-3-phosphocholine-based nanoliposomes as an effective delivery platform for 17β-estradiol. Eur J Pharm Biopharm. 2014;86(3):369–375. doi: 10.1016/j.ejpb.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 25.Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su H, Liu R, Chang M, Huang J, Jin Q, Wang X. Effect of dietary alpha-linolenic acid on blood inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. 2018;57(3):877–891. doi: 10.1007/s00394-017-1386-2. [DOI] [PubMed] [Google Scholar]
- 27.Ou W, Mulik RS, Anwar A, McDonald JG, He X, Corbin IR. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597–607. doi: 10.1016/j.freeradbiomed.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss LR, Mulik RS, Van Treuren T, Kim SY, Corbin IR. Investigation into the distinct subcellular effects of docosahexaenoic acid loaded low-density lipoprotein nanoparticles in normal and malignant murine liver cells. Biochim Biophys Acta. 2016;1860(11 Pt A):2363–2376. doi: 10.1016/j.bbagen.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen X, Reynolds L, Mulik RS, et al. Hepatic arterial infusion of low-density lipoprotein docosahexaenoic acid nanoparticles selectively disrupts redox balance in hepatoma cells and reduces growth of ortho-topic liver tumors in rats. Gastroenterology. 2016;150(2):488–498. doi: 10.1053/j.gastro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulik RS, Bing C, Ladouceur-Wodzak M, Munaweera I, Chopra R, Corbin IR. Localized delivery of low-density lipoprotein docosahexaenoic acid nanoparticles to the rat brain using focused ultrasound. Biomaterials. 2016;83:257–268. doi: 10.1016/j.biomaterials.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotto AM, Jr, Pownall HJ, Havel RJ. Introduction to the plasma lipoproteins. Methods Enzymol. 1986;128:3–41. doi: 10.1016/0076-6879(86)28061-1. [DOI] [PubMed] [Google Scholar]
- 32.Favre G. Targeting of tumor cells by low density lipoproteins: principle and use of ellipticin derivatives. C R Seances Soc Biol Fil. 1992;186(1–2):73–87. [PubMed] [Google Scholar]
- 33.Gal D, Ohashi M, MacDonald PC, Buchsbaum HJ, Simpson ER. Low-density lipoprotein as a potential vehicle for chemotherapeutic agents and radionucleotides in the management of gynecologic neoplasms. Am J Obstet Gynecol. 1981;139(8):877–885. doi: 10.1016/0002-9378(81)90952-2. [DOI] [PubMed] [Google Scholar]
- 34.Ho YK, Smith RG, Brown MS, Goldstein JL. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood. 1978;52(6):1099–1114. [PubMed] [Google Scholar]
- 35.Casaril M, Corso F, Bassi A, et al. Decreased activity of scavenger enzymes in human hepatocellular carcinoma, but not in liver metastases. Int J Clin Lab Res. 1994;24(2):94–97. doi: 10.1007/BF02593907. [DOI] [PubMed] [Google Scholar]
- 36.Yang LY, Chen WL, Lin JW, et al. Differential expression of antioxidant enzymes in various hepatocellular carcinoma cell lines. J Cell Biochem. 2005;96:622–631. doi: 10.1002/jcb.20541. [DOI] [PubMed] [Google Scholar]
- 37.Merendino N, Loppi B, D’Aquino M, et al. Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation. Nutr Cancer. 2005;52:225–233. doi: 10.1207/s15327914nc5202_12. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand RL. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med Hypotheses. 2017;101:69–74. doi: 10.1016/j.mehy.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Serini S, Piccioni E, Merendino N, Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis. 2009;14(2):135–152. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- 40.Schwenk RW, Holloway GP, Luiken JJFP, Bonen A, Glatz JFC. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4–6):149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Khan AA, Alanazi AM, Jabeen M, Hassan I, Bhat MA. Targeted nano-delivery of novel omega-3 conjugate against hepatocellular carcinoma: regulating COX-2/bcl-2 expression in an animal model. Biomed Pharmacother. 2016;81:394–401. doi: 10.1016/j.biopha.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Khan AA, Alam M, Tufail S, Mustafa J, Owais M. Synthesis and characterization of novel PUFA esters exhibiting potential anticancer activities: an in vitro study. Eur J Med Chem. 2011;46(10):4878–4886. doi: 10.1016/j.ejmech.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 43.Altenburg JD, Harvey KA, McCray S, Xu Z, Siddiqui RA. A novel 2,6-diisopropylphenyl-docosahexaenoamide conjugate induces apoptosis in T cell acute lymphoblastic leukemia cell lines. Biochem Biophys Res Commun. 2011;411(2):427–432. doi: 10.1016/j.bbrc.2011.06.172. [DOI] [PubMed] [Google Scholar]
- 44.Siemion IZ, Kluczyk A. Tuftsin: on the 30-year anniversary of Victor Najjar’s discovery. Peptides. 1999;20(5):645–674. doi: 10.1016/s0196-9781(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 45.Khan MA, Aljarbou A, Khan A, Owais M. Immune stimulating and therapeutic potential of tuftsin-incorporated nystatin liposomes against Cryptococcus neoformans in leukopenic BALB/C mice. FEMS Immunol Med Microbiol. 2012;66(1):88–97. doi: 10.1111/j.1574-695X.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- 46.Khan A, Khan AA, Dwivedi V, Ahmad MG, Hakeem S, Owais M. Tuftsin augments antitumor efficacy of liposomized etoposide against fibrosarcoma in Swiss albino mice. Mol Med. 2007;13(5–6):266–276. doi: 10.2119/2007-00018.Khan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai KB, Láng O, Orbán E, et al. Design, synthesis, and in vitro activity of novel drug delivery systems containing tuftsin derivatives and methotrexate. Bioconjug Chem. 2008;19(11):2260–2269. doi: 10.1021/bc800115w. [DOI] [PubMed] [Google Scholar]
- 48.Halder RC, Almasi A, Sagong B, Leung J, Jewett A, Fiala M. Curcuminoids and ω-3 fatty acids with anti-oxidants potentiate cytotoxicity of natural killer cells against pancreatic ductal adenocarcinoma cells and inhibit interferon γ production. Front Physiol. 2015;6:129. doi: 10.3389/fphys.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song M, Nishihara R, Cao Y, et al. Marine ω-3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor-infiltrating T cells. JAMA Oncol. 2016;2(9):1197–1206. doi: 10.1001/jamaoncol.2016.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy J, Oliveira LT, Oger C, et al. Polymeric nanocapsules prevent oxidation of core-loaded molecules: evidence based on the effects of docosahexaenoic acid and neuroprostane on breast cancer cells proliferation. J Exp Clin Cancer Res. 2015;34:155. doi: 10.1186/s13046-015-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leite EA, Grabe-Guimarães A, Guimarães HN, Machado-Coelho GLL, Barratt G, Mosqueira VCF. Cardiotoxicity reduction induced by halofantrine entrapped in nanocapsule devices. Life Sci. 2007;80(14):1327–1334. doi: 10.1016/j.lfs.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92(2):187–195. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 53.Rogers KR, Kikawa KD, Mouradian M, et al. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31(9):1523–1530. doi: 10.1093/carcin/bgq111. [DOI] [PubMed] [Google Scholar]
- 54.Chauvin L, Goupille C, Blanc C, et al. Long chain n-3 polyunsaturated fatty acids increase the efficacy of docetaxel in mammary cancer cells by downregulating Akt and PKCε/δ-induced ERK pathways. Biochim Biophys Acta. 2016;1861(4):380–390. doi: 10.1016/j.bbalip.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Sato SB, Park J, Kawamoto J, Sato S, Kurihara T. Inhibition of constitutive Akt (PKB) phosphorylation by docosahexaenoic acid in the human breast cancer cell line MDA-MB-453. Biochim Biophys Acta. 2013;1831(2):306–313. doi: 10.1016/j.bbalip.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Lee EJ, Yun UJ, Koo KH, et al. Down-regulation of lipid raft-associated oncoproteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim Biophys Acta. 2014;1841(1):190–203. doi: 10.1016/j.bbalip.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Serini S, Calviello G. Modulation of Ras/ERK and phosphoinositide signaling by Long-Chain n-3 PUFA in breast cancer and their potential complementary role in combination with targeted drugs. Nutrients. 2017;9(3):E185. doi: 10.3390/nu9030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alaarg A, Jordan NY, Verhoef JJ, Metselaar JM, Storm G, Kok RJ. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: an in vitro assessment. Int J Nanomedicine. 2016;11:5027–5040. doi: 10.2147/IJN.S115995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of PEGylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 60.Skibinski CG, Das A, Chen KM, et al. A novel biologically active acid stable liposomal formulation of docosahexaenoic acid in human breast cancer cell lines. Chem Biol Interact. 2016;252:1–8. doi: 10.1016/j.cbi.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 61.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1(9):707–717. [PubMed] [Google Scholar]
- 62.Serini S, Ottes Vasconcelos R, Fasano E, Calviello G. How plausible is the use of dietary n-3 PUFA in the adjuvant therapy of cancer? Nutr Res Rev. 2016;29(1):102–125. doi: 10.1017/S0954422416000044. [DOI] [PubMed] [Google Scholar]
- 63.Vaidya T, Straubinger RM, Ait-Oudhia S. Development and evaluation of tri-functional immunoliposomes for the treatment of HER2 positive breast cancer. Pharm Res. 2018;35(5):95. doi: 10.1007/s11095-018-2365-x. [DOI] [PubMed] [Google Scholar]
- 64.Mussi SV, Silva RC, Oliveira MC, Lucci CM, Azevedo RB, Ferreira LA. New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur J Pharm Sci. 2013;48(1–2):282–290. doi: 10.1016/j.ejps.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 65.Zu Y, Hu Y, Yu X, Jiang S. Docetaxel-loaded bovine serum albumin nanoparticles conjugated docosahexaenoic acid for inhibiting lung cancer metastasis to bone. Anticancer Agents Med Chem. 2017;17(4):542–551. doi: 10.2174/1871520616666160817143656. [DOI] [PubMed] [Google Scholar]
- 66.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 67.Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11(24 Pt 1):8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 68.Johnson-Arbor K, Dubey R. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2017. 2018 pp. [Google Scholar]
- 69.Subedi RK, Kang KW, Choi HK. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009;37(3–4):508–513. doi: 10.1016/j.ejps.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64(1):29–39. doi: 10.1016/j.addr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8(1):34–50. doi: 10.1016/j.apsb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alken S, Kelly CM. Benefit risk assessment and update on the use of docetaxel in the management of breast cancer. Cancer Manag Res. 2013;5:357–365. doi: 10.2147/CMAR.S49321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He X, Wang J, Li Y. Efficacy and safety of docetaxel for advanced non-small-cell lung cancer: a meta-analysis of Phase III randomized controlled trials. Onco Targets Ther. 2015;8:2023–2031. doi: 10.2147/OTT.S85648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17(5):735–749. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 77.Li S, Qin J, Tian C, et al. The targeting mechanism of DHA ligand and its conjugate with Gemcitabine for the enhanced tumor therapy. Oncotarget. 2014;5(11):3622–3635. doi: 10.18632/oncotarget.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Ye E, Wang J, et al. Variations of cellular membrane phospholipids with genesis of gastric cancer. Mod Oncol. 2010;18:08550857. [Google Scholar]
- 79.Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 80.Marien E, Meister M, Muley T, et al. Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int J Cancer. 2015;137(7):1539–1548. doi: 10.1002/ijc.29517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolff AC, Donehower RC, Carducci MK, et al. Phase I study of docosahexaenoic acid-paclitaxel: a taxane-fatty acid conjugate with a unique pharmacology and toxicity profile. Clin Cancer Res. 2003;9(10 Pt 1):3589–3597. [PubMed] [Google Scholar]
- 82.Fracasso PM, Picus J, Wildi JD, et al. Phase 1 and pharmacokinetic study of weekly docosahexaenoic acid paclitaxel, Taxoprexin, in resistant solid tumor malignancies. Cancer Chemother Pharmacol. 2009;63(3):451–458. doi: 10.1007/s00280-008-0756-0. [DOI] [PubMed] [Google Scholar]
- 83.Payne M, Ellis P, Dunlop D, et al. DHA-paclitaxel (Taxoprexin) as first-line treatment in patients with stage IIIB or IV non-small cell lung cancer: report of a phase II openlabel multicenter trial. J Thorac Oncol. 2006;1(9):984–990. [PubMed] [Google Scholar]
- 84.Homsi J, Bedikian AY, Kim KB, et al. Phase 2 open-label study of weekly docosahexaenoic acid-paclitaxel in cutaneous and mucosal metastatic melanoma patients. Melanoma Res. 2009;19(4):238–242. doi: 10.1097/CMR.0b013e32832a1e2f. [DOI] [PubMed] [Google Scholar]
- 85.Bedikian AY, De Conti RC, Conry R, et al. Phase 3 study of docosahexaenoic acid-paclitaxel versus dacarbazine in patients with metastatic malignant melanoma. Ann Oncol. 2011;22(4):787–793. doi: 10.1093/annonc/mdq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Homsi J, Bedikian AY, Papadopoulos NE, et al. Phase 2 open-label study of weekly docosahexaenoic acid paclitaxel in patients with metastatic uveal melanoma. Melanoma Res. 2010;20(6):507–510. doi: 10.1097/CMR.0b013e3283403ce9. [DOI] [PubMed] [Google Scholar]
- 87.Ahmad G, El Sadda R, Botchkina G, Ojima I, Egan J, Amiji M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017;406:71–80. doi: 10.1016/j.canlet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmad G, Gattacecca F, El Sadda R, et al. Biodistribution and phar-macokinetic evaluations of a novel taxoid DHA-SBT-1214 in an oil-in-water nanoemulsion formulation in naïve and tumor-bearing mice. Pharm Res. 2018;35(4):91. doi: 10.1007/s11095-018-2349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vredenburg MR, Ojima I, Veith J, et al. Effects of orally active taxanes on P-glycoprotein modulation and colon and breast carcinoma drug resistance. J Natl Cancer Inst. 2001;93(16):1234–1245. doi: 10.1093/jnci/93.16.1234. [DOI] [PubMed] [Google Scholar]
- 90.Marín-Aguilera M, Codony-Servat J, Kalko SG, et al. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol Cancer Ther. 2012;11(2):329–339. doi: 10.1158/1535-7163.MCT-11-0289. [DOI] [PubMed] [Google Scholar]
- 91.Cassano R, Mellace S, Marrelli M, Conforti F, Trombino S. α-Tocopheryl linolenate solid lipid nanoparticles for the encapsulation, protection, and release of the omega-3 polyunsaturated fatty acid: in vitro anti-melanoma activity evaluation. Colloids Surf B Biointerfaces. 2017;151:128–133. doi: 10.1016/j.colsurfb.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 92.Serini S, Cassano R, Corsetto PA, Rizzo AM, Calviello G, Trombino S. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int J Mol Sci. 2018;19(2):E586. doi: 10.3390/ijms19020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 94.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea – a paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 95.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124(6):1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Calviello G, Serini S, Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Curr Med Chem. 2007;14(29):3059–3069. doi: 10.2174/092986707782793934. [DOI] [PubMed] [Google Scholar]
- 97.Fasano E, Serini S, Piccioni E, et al. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta. 2012;1822(11):1762–1772. doi: 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Serini S, Ottes Vasconcelos R, Fasano E, Calviello G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin Ther Targets. 2016;20(7):843–858. doi: 10.1517/14728222.2016.1139085. [DOI] [PubMed] [Google Scholar]
- 99.Serini S, Fasano E, Piccioni E, et al. DHA induces apoptosis and differentiation in human melanoma cells in vitro: involvement of HuR-mediated COX-2 mRNA stabilization and β-catenin nuclear translocation. Carcinogenesis. 2012;33(1):164–173. doi: 10.1093/carcin/bgr240. [DOI] [PubMed] [Google Scholar]
- 100.Serini S, Fasano E, Celleno L, Cittadini A, Calviello G. Potential of long-chain n-3 polyunsaturated fatty acids in melanoma prevention. Nutr Rev. 2014;72(4):255–266. doi: 10.1111/nure.12093. [DOI] [PubMed] [Google Scholar]
- 101.Serini S, Zinzi A, Ottes Vasconcelos R, et al. Role of β-catenin signaling in the anti-invasive effect of the omega-3 fatty acid DHA in human melanoma cells. J Dermatol Sci. 2016;84(2):149–159. doi: 10.1016/j.jdermsci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 102.Mensink RP. Effects of stearic acid on plasma lipid and lipoproteins in humans. Lipids. 2005;40(12):1201–1205. doi: 10.1007/s11745-005-1486-x. [DOI] [PubMed] [Google Scholar]
- 103.Crowe FL, Allen NE, Appleby PN, et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European prospective investigation into cancer and nutrition. Am J Clin Nutr. 2008;88(5):1353–1363. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- 104.Liu YZ, Wu K, Huang J, et al. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int J Oncol. 2014;45(1):104–112. doi: 10.3892/ijo.2014.2392. [DOI] [PubMed] [Google Scholar]
- 105.Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105(15):1132–1141. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hull MA, Sandell AC, Montgomery AA, et al. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized controlled trial. Trials. 2013;14:237. doi: 10.1186/1745-6215-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piazzi G, D’Argenio G, Prossomariti A, et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int J Cancer. 2014;135(9):2004–2013. doi: 10.1002/ijc.28853. [DOI] [PubMed] [Google Scholar]
- 108.Nakanishi M, Hanley MP, Zha R, et al. A novel bioactive derivative of eicosapentaenoic acid (EPA) suppresses intestinal tumor development in ApcΔ14/+ mice. Carcinogenesis. 2018;39(3):429–438. doi: 10.1093/carcin/bgx136. [DOI] [PubMed] [Google Scholar]
- 109.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83(3):217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 110.Roynette CE, Calder PC, Dupertuis YM, Pichard C. n-3 polyunsaturated fatty acids and colon cancer prevention. Clin Nutr. 2004;23(2):139–151. doi: 10.1016/j.clnu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Dupertuis YM, Meguid MM, Pichard C. Colon cancer therapy: new perspectives of nutritional manipulations using polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2007;10(4):427–432. doi: 10.1097/MCO.0b013e3281e2c9d4. [DOI] [PubMed] [Google Scholar]
- 112.Lee JY, Sim TB, Lee JE, Na HK. Chemopreventive and chemotherapeutic effects of fish oil derived omega-3 polyunsaturated fatty acids on colon carcinogenesis. Clin Nutr Res. 2017;6(3):147–160. doi: 10.7762/cnr.2017.6.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Noriega BS, Sanchez-Gonzalez MA, Salyakina D, Coffman J. Understanding the impact of omega-3 rich diet on the gut microbiota. Case Rep Med. 2016;2016:3089303. doi: 10.1155/2016/3898307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nappi A, Berretta M, Romano C, et al. Metastatic colorectal cancer: role of target therapies and future perspectives. Curr Cancer Drug Targets. 2018;18(5):421–429. doi: 10.2174/156800961766617020095143. [DOI] [PubMed] [Google Scholar]
- 115.Nikpoor AR, Tavakkol-Afshari J, Sadri K, Jalali SA, Jaafari MR. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: in vitro and in vivo studies. Nanomedicine. 2017;13(8):2671–2682. doi: 10.1016/j.nano.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 116.Coutzac C, Adam J, Soularue E, et al. Colon immune-related adverse events: anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entities. J Crohns Colitis. 2017;11(10):1238–1246. doi: 10.1093/ecco-jcc/jjx081. [DOI] [PubMed] [Google Scholar]
- 117.Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med. 2017;6(1):25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bowen KJ, Harris WS, Kris-Etherton PM. Omega-3 fatty acids and cardiovascular disease: are there benefits? Curr Treat Options Cardiovasc Med. 2016;18(11):69. doi: 10.1007/s11936-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Serini S, Calviello G. Long-chain omega-3 fatty acids and cancer: any cause for concern? Curr Opin Clin Nutr Metab Care. 2018;21(2):83–89. doi: 10.1097/MCO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 121.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 122.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 123.Harris WS, von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39(1):212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 124.Fasano E, Serini S, Cittadini A, Calviello G. Long-chain n-3 PUFA against breast and prostate cancer: which are the appropriate doses for intervention studies in animals and humans? Crit Rev Food Sci Nutr. 2017;57(11):2245–2262. doi: 10.1080/10408398.2013.850060. [DOI] [PubMed] [Google Scholar]
- 125.Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang YF, Gao HF, Hou AJ, Zhou YH. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: a meta-analysis of randomized controlled trials. BMC Public Health. 2014;14:204. doi: 10.1186/1471-2458-14-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anti M, Armelao F, Marra G, et al. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology. 1994;107(6):1709–1718. doi: 10.1016/0016-5085(94)90811-7. [DOI] [PubMed] [Google Scholar]
- 128.Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101(12):1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59(7):918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 130.Dyerberg J, Bang HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979;2:433–435. doi: 10.1016/s0140-6736(79)91490-9. [DOI] [PubMed] [Google Scholar]
- 131.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin Nutr. 2009;28:378–386. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Jeansen S, Witkamp RF, Garthoff JA, van Helvoort A, Calder PC. Fish oil LC-PUFAs do not affect blood coagulation parameters and bleeding manifestations: analysis of 8 clinical studies with selected patient groups on omega-3-enriched medical nutrition. Clin Nutr. 2018;37:948–957. doi: 10.1016/j.clnu.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 133.D’Eliseo D, Velotti F. Omega-3 fatty acids and cancer cell cytotoxicity: implications for multi-targeted cancer therapy. J Clin Med. 2016;5(2):E15. doi: 10.3390/jcm5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 135.Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 136.Ilag LL. Are long-chain polyunsaturated fatty acids the link between the immune system and the microbiome towards modulating cancer? Medicines (Basel) 2018;5(3):E102. doi: 10.3390/medicines5030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liang P, Henning SM, Schokrpur S, et al. Effect of dietary omega-3 fatty acids on tumor-associated macrophages and Prostate Cancer Progression. Prostate. 2016;76(14):1293–1302. doi: 10.1002/pros.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gevariya N, Besançon M, Robitaille K, et al. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate. 2019;79(1):9–20. doi: 10.1002/pros.23706. [DOI] [PubMed] [Google Scholar]
- 139.Khadge S, Sharp JG, McGuire TR, et al. Immune regulation and anti-cancer activity by lipid inflammatory mediators. Int Immunopharmacol. 2018;65:580–592. doi: 10.1016/j.intimp.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guesdon W, Kosaraju R, Brophy P, et al. Effects of fish oils on ex vivo B-cell responses of obese subjects upon BCR/TLR stimulation: A pilot study. J Nutr Biochem. 2018;53:72–80. doi: 10.1016/j.jnutbio.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hou TY, McMurray DN, Chapkin RS. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol. 2016;785:2–9. doi: 10.1016/j.ejphar.2015.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wachtel N, Rohwer N, Pietzner A, Loew A, Weylandt KH. Omega-3 fatty acid supplementation-a possible dietary adjunct to enhance immune therapy in cancer? J Cell Biotech. 2018;1(4[1–2]):83–88. [Google Scholar]
- 143.Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18(12):E2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 145.Sanz Y, De Palma G. Gut microbiota and probiotics in modulation of epithelium and gut-associated lymphoid tissue function. Int Rev Immunol. 2009;28:397–413. doi: 10.3109/08830180903215613. [DOI] [PubMed] [Google Scholar]