Abstract
Aims
This real‐world study is conducted to evaluate the efficacy and safety of recombinant human endostatin (rh‐endostatin) combined with chemotherapy as first‐line treatment for non‐driver genes mutation non‐small cell lung cancer (NSCLC) patients, and establish evidence‐based optimal regimen for rh‐endostatin.
Patients and Methods
Using propensity score matching (cut‐off: 0.01), 88 patients were eligible for our study, 34 of which received platinum‐based chemotherapy alone (chemotherapy group), 54 patients received platinum‐based chemotherapy plus rh‐endostatin (rh‐endostatin group). Among those 54 patients in the rh‐endostatin group, 27 patients received rh‐endostatin administered at 7.5 mg/m2 from day 1 to day 14 (rh‐endostatin 14d group), and the other 27 patients were administered at 15 mg/m2 from day 1 to day 7 (rh‐endostatin 7d group). The primary endpoint was progression‐free survival (PFS) and secondary endpoints were overall survival (OS), overall response rate (ORR), disease control rate (DCR), and safety.
Results
There were no differences in clinic characteristics among 3 groups. Compared with chemotherapy group, rh‐endostatin group improved PFS and OS significantly. The median PFS was 6 months vs 4.5 months (P = 0.047), and median OS was 20 months vs 10 months (P < 0.001). The ORR was 33.3% vs 20.6% (P = 0.197) and DCR was 83.3% vs 64.7% (P = 0.046) in the rh‐endostatin group and chemotherapy group, respectively. The comparisons between the rh‐endostatin 7d and 14d groups revealed a significant improvement in PFS for the rh‐endostatin 7d group (P = 0.044), but no significant differences in OS (P = 0.111), ORR (P = 0.074), or DCR (P = 0.234). The incidences of grade 3 and 4 adverse events were similar among 3 groups.
Conclusion
Chemotherapy combined with rh‐endostatin was more effective than chemotherapy alone for non‐driver gene mutation NSCLC patients. The administration of rh‐endostatin for 7 days at 15 mg/m2 was non‐inferior to 14 days at 7.5 mg/m2 in prolonging patients’ PFS. Further evaluation should be conducted before its application in clinical work.
Keywords: chemotherapy, different administration, non‐small cell lung cancer, real‐world study, recombinant human‐endostatin
1. INTRODUCTION
Non‐small cell lung cancer (NSCLC) is the most common type of epithelial lung accounting for nearly 85% of all lung cancer patients. Its cancer incidence and mortality are the highest worldwide and the 5‐year overall survival (OS) of patients with advanced NSCLC is <5%.1 Currently, the development of platinum‐based chemotherapy mostly are limited to better drug tolerance and less toxic side effects, but the progress in efficacy is inadequate. In past decades, great clinical improvements have been achieved in NSCLC with the participation of target therapy like EGFR tyrosine kinase inhibitors.2, 3 However, patients with non‐driver genes have shown far less clinical response to those target therapy.
Recombinant human endostatin (rh‐endostatin) inhibits the proliferation of vascular endothelial cells through multiple targets, thereby suppressing angiogenesis and tumor growth.4 It has been reported that the combination of rh‐endostatin with cisplatin/vinorelbine significantly increased time to progression and overall response rate (ORR) in NSCLC patients.5 On the basis of this study, the China Food and Drug Administration (CFDA) approved rh‐endostatin as the first‐line treatment for advanced NSCLC patients in 2005. After that the efficacy of rh‐endostatin has been proved in several researches.6, 7, 8 Rh‐endostatin in those studies was administered intravenously at 7.5 mg/m2 daily from day 1 to 14 every 3 weeks, which has been widely used in clinical practice. However, the administration for 14 days could result in several problems, including increasing hospital stay and cost, leading to a lower compliance of patients and reduced treatment response. Some researchers later adjusted the dose of rh‐endostatin to 15 mg/m2 from day 1 to 7 every 3 weeks to solve the problem.
We conducted this project to investigate the routine practice different administration rh‐endostatin combined with chemotherapy as first‐line treatment in advanced non‐driver gene mutation NSCLC patients. Also we investigated effect of different administration modes on patient outcome.
2. PATIENTS AND METHODS
2.1. Patients
Between April 2014 and April 2017, 136 advanced NSCLC patients who received first‐line chemotherapy combined with rh‐endostatin at Hunan Cancer Hospital were enrolled in this study. All patients were ≥18 years old and histologically diagnosed with inoperable stage III or IV NSCLC, with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0‐3. Patients with hepatic or renal dysfunction and cardiac disease were excluded. We used propensity score matching (PSM) to normalize the baseline characteristics among the 3 groups. The characteristics of the patients including sex, age, ECOG PS, smoking history, histological grade, pathology, and metastasis were listed in Table 1. All the patients signed written informed consent. The study was approved by Hunan Cancer Hospital Ethic Committee. The study was also conducted in accordance with the Declaration of Helsinki.
Table 1.
Patients’ characteristics
| Variables |
Chemotherapy alone group n (%) |
Rh‐endostatin 14d group n (%) |
Rh‐endostatin 7d group n (%) |
P |
|---|---|---|---|---|
| Total | 34 | 27 | 27 | |
| Age, years | ||||
| Median | 57 | 59 | 59 | |
| Range | 41‐68 | 39‐78 | 44‐69 | |
| ≥65 | 9 (26.5) | 4 (14.8) | 8 (29.6) | 0.399 |
| <65 | 25 (73.5) | 23 (85.2) | 19 (70.4) | |
| Sex | ||||
| Male | 27 (79.4) | 18 (66.7) | 24 (88.9) | 0.137 |
| Female | 7 (20.6) | 9 (33.3) | 3 (11.1) | |
| ECOG PS | ||||
| 0, 1 | 33 (97.0) | 27 (100.0) | 27 (100.0) | 0.448 |
| 2, 3 | 1 (3.0) | 0 (0) | 0 (0) | |
| Smoke | ||||
| Yes | 24 (70.6) | 17 (63.0) | 22 (81.5) | 0.316 |
| No | 10 (29.4) | 10 (37.0) | 5 (18.5) | |
| Grade | ||||
| III | 15 (44.1) | 10 (37.0) | 14 (51.9) | 0.548 |
| Ⅳ | 19 (55.9) | 17 (63.0) | 13 (48.1) | |
| Pathology | ||||
| AD | 8 (23.5) | 9 (33.3) | 3 (11.1) | 0.215 |
| SCC | 24 (70.6) | 17 (63.0) | 24 (88.9) | |
| Other | 2 (5.9) | 1 (3.7) | 0 (0) | |
| Metastasis | ||||
| Lung | 3 (8.8) | 4 (14.8) | 4 (14.8) | 0.264 |
| Liver | 7 (20.6) | 2 (7.4) | 1 (3.7) | |
| Bone | 8 (23.5) | 6 (22.2) | 6 (22.2) | |
| Brain | 4 (11.8) | 5 (18.5) | 1 (3.7) | |
| Other | 5 (14.7) | 11 (40.7) | 8 (29.6) | |
| None | 16 (47.1) | 9 (26.5) | 14 (51.9) | |
AD, adenocarcinoma; ECOG, eastern cooperative oncology group; PS, performance status; SCC, squamous cell carcinoma.
2.2. Treatment
After selection through PSM, 34 patients only received platinum‐based chemotherapy every 21 days was set as chemotherapy alone group. Fifty‐four patients received platinum‐based chemotherapy plus rh‐endostatin was set as rh‐endostatin group. Among those 54 patients, 27 patients (rh‐endostatin 14d group) received platinum‐based chemotherapy plus rh‐endostatin administered daily at 7.5 mg/d, from day 1 to 14 every 21 days (d1‐14, q21d), and 27 patients (rh‐endostatin 7d group) received chemotherapy plus rh‐endostatin administered daily at 15 mg/d, from day 1 to 7 every 21 days (d1‐7, q21d). All chemotherapy regimens in this study included TP: paclitaxel (175 mg/m2, d1) + cisplatin (100 mg/m2, d1), TC: paclitaxel + carboplatin (area under the curve, 6), GP: gemcitabine (1250 mg/m2, d1, d8) + cisplatin, GC: gemcitabine + carboplatin, PP: pemetrexed (500 mg/m2, d1) + cisplatin, and PC: pemetrexed + carboplatin. Treatment will continue until progressive disease (PD), consent withdrawal or intolerable toxicity.
2.3. Assessment
Patients were evaluated for response by computed tomography every 2 treatment cycles. Treatment response was evaluated as complete response (CR), partial response (PR), stable disease (SD), PD, or not evaluable according to the Response Evaluation Criteria in Solid Tumor criteria 1.1.9 The ORR was defined as the sum of CR and PR. The disease control rate (DCR) was defined as the sum of CR, PR, and SD. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Informed consent was obtained from all patients. The primary endpoint was progression‐free survival (PFS). Secondary endpoints were OS, ORR, and DCR.
2.4. Statistics analysis
The proportions in 3 groups and treatment responses were compared by the χ2 test. Survival distributions were estimated with the Kaplan‐Meier method and compared using the log‐rank test. All statistical analyses were performed using the SPSS 22.0 software for Windows (SPSS Corp., Armonk, NY, USA); P < 0.05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Patient characteristics
The characteristics of the patients, including age, sex, performance, smoking status, grade, pathology, and metastasis, which are similar among the 3 groups, are summarized in Table 1. A total of 21 patients (23.9%) were 65 years old or older, 19 patients (21.6%) were female, and 25 patients (28.4%) had never smoked. Only 1 patient (1.1%) had a poor PS (2). According to the TNM classification for NSCLC patients (seventh edition), 39 patients (44.3%) were at stage III, and 49 patients (55.7%) were at stage IV. Twenty (22.7%) patients were diagnosed with adenocarcinoma, and 65 (73.9%) patients with squamous cell carcinoma.
3.2. Treatment response
The treatment responses are listed in Table 2. The ORR and DCR in the chemotherapy group were 20.6% and 64.7% vs 33.3% and 83.3% in the rh‐endostatin group, respectively. The difference of ORR was no statistical significance (P = 0.197), but the DCR was significantly improved in the rh‐endostatin group (P = 0.046). The ORR and DCR in the rh‐endostatin 14d group were 44.4% and 88.9%, vs 22.2% and 77.8% in the rh‐endostatin 7d group, respectively. No significant differences were observed in the comparison between the 2 groups (ORR: P = 0.074, DCR: P = 0.234). No patient achieved CR in the whole population.
Table 2.
Summary of treatment efficacy
| Response |
Chemotherapy group n (%) |
Rh‐endostatin group n (%) |
P |
Rh‐endostatin group n (%) |
||
|---|---|---|---|---|---|---|
|
Rh‐endostatin 14d group n (%) |
Rh‐endostatin 7d group n (%) |
P | ||||
| CR | 0 (0) | 0 (0) | — | 0 (0) | 0 (0) | — |
| PR | 7 (20.6) | 18 (33.3) | 0.1970 | 12 (44.4) | 6 (22.2) | 0.07 |
| ORR | 7 (20.6) | 18 (33.3) | 0.1970 | 12 (44.4) | 6 (22.2) | 0.07 |
| SD | 15 (44.1) | 27 (50.0) | 0.5910 | 12 (44.4) | 15 (55.6) | 0.29 |
| DCR | 22 (64.7) | 45 (83.3) | 0.0460 | 24 (88.9) | 21 (77.8) | 0.23 |
| PD | 6 (17.6) | 2 (3.7) | 0.0250 | 1 (3.7) | 1 (3.7) | 0.76 |
| NE | 6 (17.6) | 7 (13.0) | — | 2 (7.4) | 5 (18.5) | — |
CR, complete response; DCR, disease control rate; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
3.3. Survival
The median PFS in the rh‐endostatin group was 6.0 months vs 4.5 months in the chemotherapy group (P = 0.047, Figure 1A) and the median OS in the rh‐endostatin group was 20.0 months vs 10.0 months in the chemotherapy group (P < 0.001; Figure 1B). Both PFS and OS were longer in rh‐endostatin group and of statistical significance. Also the 1 year survival rate in the chemotherapy alone and rh‐endostatin group was 71.47%, higher than chemotherapy group of 41.18% (and 71.47% respectively, P < 0.01). In addition, there was a significant advantage in the PFS of the rh‐endostatin 7d group compared with that of the rh‐endostatin 14d group (6.5 months vs 6.0 months; P = 0.044, Figure 2A). Although a noticeable improvement was detected in the OS of the rh‐endostatin 14d group compared to 7d group, there was no significant difference (22 months vs 14 months; P = 0.111; Figure 2B).
Figure 1.
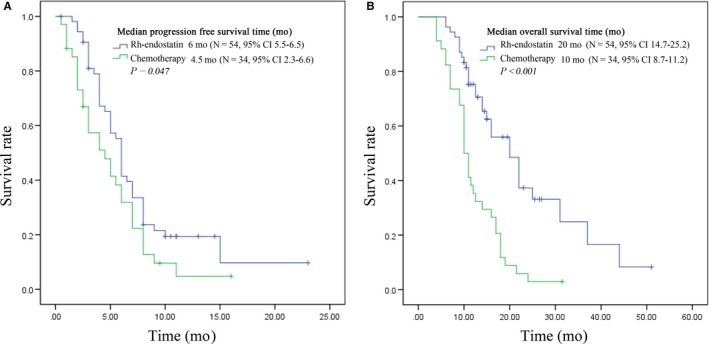
Plot of Kaplan‐Meier estimates for progression‐free survival time (A) and overall survival time (B) for the recombinant human endostatin (Rh‐endostatin) group compared with chemotherapy group
Figure 2.
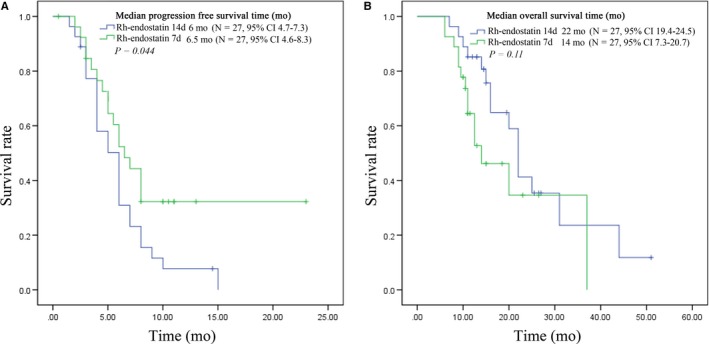
Plot of Kaplan‐Meier estimates for progression‐free survival time (A) and overall survival time (B) for the endostar 7d group compared with recombinant human endostatin (Rh‐endostatin) 14d group
3.4. Toxicity
Among the 88 patients enrolled, 16 (18.2%) had severe adverse events (grade 3‐4). In addition, the incidence of severe myelosuppression in the chemotherapy group, the rh‐endostatin 14d group, and the rh‐endostatin 7d groups was 11.6%, 22.2%, and 3.7%, respectively. Common severe adverse reactions included 3 cases of leukopenia, 3 cases of neutropenia, 4 cases of thrombocytopenia, and 3 cases of vomiting. The incidence of each severe adverse reaction was lower than 10% in all groups. Other rare adverse reactions included mild liver damage in 2 cases, mild constipation in 2 cases, and fatigue in 1 case. Details of these adverse reactions are given in Table 3. In the chemotherapy group, there were a total of 16 cases (47.1%) of adverse events and 7 cases (20.6%) of serious adverse events. Anemia was the most common adverse reaction, including 3 cases (8.8%) of mild to moderate anemia and 1 case (2.9%) of severe anemia. In the rh‐endostatin 14d group, there were a total of 41 cases of adverse events, most of which were mild‐to‐moderate (Grades 1‐2) adverse events, and 15 patients had mild nausea with an incidence rate of 55.6%. These were the most common adverse reactions. Mild vomiting occurred in 6 patients with an incidence rate of 22.2%. Serious adverse events occurred in 6 patients (22.2%), including 2 patients with leukopenia (7.4%), 2 patients with granulocytopenia (7.4%), and 2 patients with thrombocytopenia (7.4%). In the rh‐endostatin 7d group, a total of 16 cases (59.3%) of adverse reactions occurred, and 3 cases (11.1%) of serious adverse events, including 1 case of grade 4 thrombocytopenia (3.7%), 1 case of nausea (3.7%), and 1 case of vomiting (3.7%). Compared with the rh‐endostatin 14d group, the incidence of gastrointestinal adverse reactions and severe myelosuppression was lower in the rh‐endostatin 7d group.
Table 3.
Treatment related adverse events
| Adverse events |
Chemotherapy group n (%) |
Rh‐endostatin 14d group n (%) |
Rh‐endostatin 7d group n (%) |
|||
|---|---|---|---|---|---|---|
| Grade 1‐2 | Grade 3‐4 | Grade 1‐2 | Grade 3‐4 | Grade 1‐2 | Grade 3‐4 | |
| Leukopenia | 5 (14.7) | 6 (17.6) | 8 (29.6) | 7 (25.9) | 6 (22.2) | 7 (25.9) |
| Neutropenia | 5 (14.7) | 5 (14.7) | 7 (25.9) | 5 (18.5) | 5 (18.5) | 4 (14.8) |
| Anemia | 3 (8.8) | 1 (2.9) | 1 (3.7) | 1 (3.7) | 1 (3.7) | 2 (7.4) |
| Thrombocytopenia | 2 (5.9) | 1 (2.9) | 3 (11.1) | 2 (7.4) | 0 (0) | 1 (3.7) |
| Nausea | 1 (2.9) | 1 (2.9) | 15 (55.6) | 2 (7.4) | 3 (11.1) | 1 (3.7) |
| Fatigue | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.7) | 0 (0) |
| Vomiting | 1 (2.9) | 2 (5.9) | 6 (22.2) | 0 (0) | 0 (0) | 1 (3.7) |
| Liver dysfunction | 1 (2.9) | 0 (0) | 2 (7.4) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 0 (0) | 0 (0) | 2 (7.4) | 0 (0) | 0 (0) | 0 (0) |
| Hemoptysis | 0 (0) | 0 (0) | 2 (7.4) | 0 (0) | 2 (7.4) | 1(3.7) |
| Cardiotoxicity | 0 (0) | 0 (0) | 3 (11.1) | 0 (0) | 2 (7.4) | 0 (0) |
| Hypertension | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Proteinuria | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
4. DISCUSSION
Endostatin was a 20 kDa c‐terminal fragment of collagen XVIII,10 originally isolated by O’ Reilly from supernatant of a murine hemangio‐endothelioma.11 In preclinical studies, endostatin was demonstrated to play key role in anti‐angiogenesis by normalizing tumor microvessels and inhibiting the activities of vascular endothelial cells via multiple targets.4, 12 It showed a strong suppression on various murine tumors growth in vivo, such as ovarian cancer,13 renal cell cancer,14 JC breast carcinoma,15 Lewis lung carcinoma, T241 fibrosarcoma, and B16F10 melanoma.10
Rh‐endostatin (rh‐endostatin) is a new and modified recombined human endostatin developed by Chinese scientist, which had been demonstrated to offer clinical benefits for patients with advanced NSCLC in several clinical trials.6, 16, 17, 18 Based on the encouraging outcomes in a large randomized, double‐blind phase III clinical trial,19 rh‐endostatin was approved by CFDA in combination with vinorelbine/cisplatin for patients with advanced NSCLC in 2005. In recent years, Chinese scholars have begun to investigate rh‐endostatin in greater extent for the further improvement in efficacy. He et al20 have reported that the expression of regulator of G–protein signaling 5 (RGS5) and carbonic anhydrase 9 (CA) were reduced during the vascular normalization window, which suggested that RGS5 and CA may be the key to define the optimal administration time of rh‐endostatin. Li et al21 used gold nanoparticles (AuNPs) as a drug delivery system, which enhanced the concentration of rh‐endostatin in tumors.
In this study, we screened 136 patients with stage III/IV NSCLC, after PSM (cut‐off = 0.01) the selected 88 patients were administered 2 different doses of rh‐endostatin combined with platinum‐based chemotherapy or platinum‐based chemotherapy only, and their efficacy and safety were compared. The results revealed that the median PFS in the rh‐endostatin group and in the chemotherapy group was 6 months and 4.5 months, respectively (P = 0.047), reaching the primary endpoint. The median OS in the rh‐endostatin group was significantly longer (20 months vs 10 months, P < 0.001), and the DCR was significantly increased (83.3% vs 64.7%, P = 0.046). Our study firstly revealed rh‐endostatin plus chemotherapy to be better efficient compared with chemotherapy only group in real‐world practice, and provided evidence for the combined treatment. Rh‐endostatin plus chemotherapy should be recommended as first line standard treatment strategy for non‐driver gene mutation NSCLC patients. In our study, the platinum‐based chemotherapy regimen for each patient was different, including pemetrexed, gemcitabine, and paclitaxel combined with cisplatin or carboplatin. However, as shown in clinical trials of ECOG 1594 and JMDB, the efficacy and safety of all the 4 regimens were similar.22, 23 So, we think out data prove evidence to show rh‐endostatin combined chemotherapy improved the efficacy.
In the ECOG 4599 study,24 the median PFS in patients receiving paclitaxel/carboplatin combined with bevacizumab (15 mg/kg) was prolonged by nearly 2 months compared with those receiving chemotherapy only (6.2 months vs 4.5 months), the OS for the first time exceeded 1 year (12.3 months vs 10.3 months), and the ORR was significantly increased (35% vs 15%). While, in BEYOND study,25 the median OS in the experimental group was 24.3 months, which was 6.6 months longer than that of the control group (24.3 months vs 17.7 months, P = 0.015), the median PFS was prolonged by 2.7 months (9.2 months vs 6.5 months, P < 0.001),and the ORR was also significantly increased (54% vs 26%). In our study, the median OS was 22 months and 14 months in the rh‐endostatin 14d group and the rh‐endostatin 7d group, respectively, which was both significantly, improved compared with results of the ECOG 4599 study (12.3 months). The median PFS in the rh‐endostatin 14d group (6 months) and the rh‐endostatin 7d group (6.5 months) were similar with the ECOG 4599 study (6.2 months), but less than BEYOND. In addition, the ORR in the rh‐endostatin 14d group of this study was higher than that in the ECOG 4599 study (44.4% vs 35.0%) but lower than BEYOND. The ORR in the rh‐endostatin 7d group (22.2%) was not as high as that in the ECOG 4599 study or BEYOND study (54.0%). This discrepancy may be due to the enrolled patients in the BEYOND study25 had higher proportion of women and non‐smokers who tended to which carried driver gene mutations, while in our study, all patients were negative for driver gene mutations. In safety analysis, severe adverse reactions in the rh‐endostatin group included leukopenia in 14 cases (25.9%), granulocytopenia in 9 cases (16.7%), anemia in 3 cases (5.6%), thrombocytopenia in 3 cases (5.6%), and nausea in 3 cases (5.6%). Additionally, their incidence (leukopenia: 25.9%; neutropenia: 18.5%) was slightly higher than those in the chemotherapy group. The incidence of severe vomiting was 3.7%, lower than that in the chemotherapy group (5.9%). Grade 4 adverse reactions occurred in only 1 patient of rh‐endostatin 7d group who developed thrombocytopenia after treatment, and platelet count gradually returned to normal after treatment of megakaryocyte (recombinant interleukin 11). Other adverse events associated with rh‐endostatin included 1 case of hemoptysis (1.9%) and 3 cases of cardiac toxicity (5.6%), no hypertension and proteinuria events were observed in our study. This was significantly lower compared with ECOG4599 and BEYOND.24 Our results partially showed that rh‐endostatin was safer. Our results partially showed that rh‐endostatin was safer compared with bevacizumab for side effect management.
We also compared the efficacy, survival time, and safety of the 2 different administration regimens of rh‐endostatin. The median PFS in the rh‐endostatin 14d group and the rh‐endostatin 7d group was similar (6 months vs 6.5 months) but statistically significant (P = 0.044). Although the OS was significantly longer in the rh‐endostatin 14d group than that in the rh‐endostatin 7d group, the difference was not of statistical significance (P = 0.111). In addition, although the ORR and DCR in the rh‐endostatin 14d group showed more advantage, there was no significant difference compared with the rh‐endostatin 7d group (ORR: P = 0.074, DCR: P = 0.234). Regarding the safety, the incidence of severe myelosuppression was similar in the rh‐endostatin 14d group and the rh‐endostatin 7d group, which was 55.6% and 51.9%, respectively. Likewise, the incidence of severe nausea and vomiting was also roughly equivalent in rh‐endostatin 14d group (7.4% and 0%) and rh‐endostatin 7d group (both 3.7%). However, among the grade 1‐2 adverse events, nausea, and vomiting events were more common in the rh‐endostatin 14d group. In the rh‐endostatin 14d group, mild nausea occurred in more than half (55.6%) of the patients, mild vomiting occurred in 6 patients (22.2%), while in the rh‐endostatin 7d group, mild nausea occurred in only 3 cases (11.1%) and no grade 1 or 2 vomiting occurred. Generally, there was no significant difference between 7‐day administration and 14‐day administration of rh‐endostatin; 7‐day and 14‐day for both efficacy and safety. Treatment of rh‐endostatin 7d will also be a reliable administration method for patients, and with less hospital stay time. Large sample size random trials should be conducted for further evaluation.
Certainly, there are limitations in this study, including the small sample size, and difficulties of some patients in the follow‐up period that led to truncated data. In conclusion, rh‐endostatin plus platinum‐based chemotherapy significantly improved the PFS, OS, and DCR in advanced non‐driver gene mutation NSCLC patients with well tolerance. The 7‐day administration regimen was not inferior to the 14‐day administration regimen of rh‐endostatin in efficacy and safety. The 7‐day administration regimen of rh‐endostatin needs further studies before its adoption in clinical work.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
Conception and design: Yongchang Zhang, Nong Yang; Analysis and interpretation of data: Zhongtai Wang; Acquisition of data: Chunhua Zhou, Xiaoyan Long; Writing, review, and/or revision of the manuscript: Zhongtai Wang and Hui Zhang.
Wang Z, Zhang H, Zhou C, et al. Real‐world outcomes of various regimens of recombinant human endostatin combined with chemotherapy in non‐driver gene mutation advanced non‐small cell lung cancer. Cancer Med. 2019;8:1434–1441. 10.1002/cam4.2014
Zhongtai Wang, Hui Zhang, and Chunhua Zhou contributed equally.
Contributor Information
Nong Yang, Email: yangnong0217@163.com.
Yongchang Zhang, Email: zhangyongchang@csu.edu.cn.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2. Gridelli C, Ciardiello F, Gallo C, et al. First‐line erlotinib followed by second‐line cisplatin‐gemcitabine chemotherapy in advanced non‐small‐cell lung cancer: the TORCH randomized trial. J Clin Oncol. 2012;30:3002‐3011. 10.1200/JCO.2011.41.2056 [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13:239‐246. 10.1016/s1470-2045(11)70393-x [DOI] [PubMed] [Google Scholar]
- 4. Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF‐induced tyrosine phosphorylation of KDR/Flk‐1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79‐84. 10.1016/j.bbrc.2007.06.155 [DOI] [PubMed] [Google Scholar]
- 5. Wang J, Sun Y, Liu Y, et al. Results of randomized, multicenter, double‐blind phase III trial of rh‐endostatin (YH‐16) in treatment of advanced non‐small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2005;8:283‐290. 10.3779/j.issn.1009-3419.2005.04.07 [DOI] [PubMed] [Google Scholar]
- 6. Zhao X, Mei K, Cai X, et al. A randomized phase II study of recombinant human endostatin plus gemcitabine/cisplatin compared with gemcitabine/cisplatin alone as first‐line therapy in advanced non‐small‐cell lung cancer. Invest New Drugs. 2012;30:1144‐1149. 10.1007/s10637-011-9631-7 [DOI] [PubMed] [Google Scholar]
- 7. Rong B, Yang S, Li W, Zhang W, Ming Z. Systematic review and meta‐analysis of endostar (rh‐endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non‐small cell lung cancer. World J Surg Oncol. 2012;10:170 10.1186/1477-7819-10-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Sun Y, Qin S; Endostar Phase IV Study Group . Results of phase IV clinical trial of endostar with chemotherapy for treatment of (NSCLC). J Clin Oncol. 2010;28(15 suppl):a7598. [Google Scholar]
- 9. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205‐216. 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 10. Oh SP, Kamagata Y, Muragaki Y, et al. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly‐Xaa‐Yaa repeats identify a distinct family of collagenous proteins. Proc Natl Acad Sci USA. 1994;91:4229‐4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277‐285. [DOI] [PubMed] [Google Scholar]
- 12. Boehle AS, Kurdow R, Schulze M, et al. Human endostatin inhibits growth of human non‐small‐cell lung cancer in a murine xenotransplant model. Int J Cancer. 2001;94:420‐428. [DOI] [PubMed] [Google Scholar]
- 13. Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190‐2196. [PubMed] [Google Scholar]
- 14. Blezinger P, Wang J, Gondo M, et al. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene. Nat Biotechnol. 1999;17:343‐348. 10.1038/7895 [DOI] [PubMed] [Google Scholar]
- 15. Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus‐mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci USA. 2000;97:4802‐4807. 10.1073/pnas.090065597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang L, Wang JW, Tang ZM. A phase I clinical trial for recombinant human endostatin. Chin J New Drugs. 2004;13:548‐553. [Google Scholar]
- 17. Sun X‐J, Deng Q‐H, Yu X‐M, et al. A phase II study of endostatin in combination with paclitaxel, carboplatin, and radiotherapy in patients with unresectable locally advanced non‐small cell lung cancer. BMC Cancer. 2016;16:266 10.1186/s12885-016-2234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bao Y, Peng F, Zhou Q‐C, et al. Phase II trial of recombinant human endostatin in combination with concurrent chemoradiotherapy in patients with stage III non‐small‐cell lung cancer. Radiother Oncol. 2015;114:161‐166. 10.1016/j.radonc.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 19. Sun Y, Wang JW, Liu YY, et al. Long‐term results of a randomized, double‐blind, and placebo‐controlled phase III trial: endostar (rh‐endostatin) versus placebo in combination with vinorelbine and cisplatin in advanced non‐small cell lung cancer. Thorac Cancer. 2013;4:440‐448. 10.1111/1759-7714.12050 [DOI] [PubMed] [Google Scholar]
- 20. He L, Zhao C, Li Y, et al. Antiangiogenic effects of recombinant human endostatin in lung cancers. Mol Med Rep. 2018;17:79‐86. 10.3892/mmr.2017.7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li W, Zhao X, Du B, et al. Gold nanoparticle‐mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy. Sci Rep. 2016;6:30619 10.1038/srep30619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med. 2002;346:92‐98. 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 23. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol. 2008;26:3543‐3551. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 24. Sandler A, Gray R, Perry MC, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med. 2006;355:2542‐2550. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 25. Zhou C, Wu Y‐L, Chen G, et al. BEYOND: a randomized, double‐blind, placebo‐controlled, multicenter, phase III study of first‐line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2015;33:2197‐2204. 10.1200/JCO.2014.59.4424 [DOI] [PubMed] [Google Scholar]


