Abstract
BACKGROUND
People with the human immunodeficiency virus (PWHIV) have improved survival because of the advent of antiretroviral therapy. Consequently, PWHIV experience higher rates of non-acquired immunodeficiency syndrome-defining malignancies (NADMs). Previous studies have demonstrated worsened cancer-specific survival in PWHIV, partly because of advanced cancer stage at diagnosis. The objective of the current systematic review was to evaluate screening disparities for NADMs among PWHIV.
METHODS
The PubMed, Cochrane, EMBASE, and ClinicalTrials.gov databases were searched from January 1, 1996 through April 10, 2018 to identify studies related to screening disparities for NADMs among PWHIV. Eligibility criteria included any study performed in a high-income country that compared screening for NADMs by HIV status. After title/abstract screening and full-text review, articles that met eligibility criteria were analyzed.
RESULTS
Of 613 unique articles identified through the search, 9 studies were analyzed. Three studies addressed breast cancer screening, 4 addressed colorectal cancer screening, and 2 addressed prostate cancer screening. Five of the reviewed studies demonstrated that PWHIV were less likely to receive indicated cancer screenings compared with the general population, whereas 3 indicated that screening proportions were higher among PWHIV, and 1 demonstrated that screening proportions were comparable. In most of the studies, PWHIV who had regular access to health care were more likely to undergo cancer screening.
CONCLUSIONS
The available evidence does not uniformly confirm that PWHIV are less likely to receive cancer screening. Social determinants of health (insurance status, access to health care, education, income level) were associated with the receipt of appropriate cancer screening, suggesting that these barriers need to be addressed to improve cancer screening in PWHIV.
Keywords: acquired immunodeficiency syndrome, early detection of cancer, health care disparities, human immunodeficiency virus (HIV), social determinants of health
INTRODUCTION
The advent of antiretroviral therapy (ART) has led to decreased morbidity and mortality in people with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) (PWHIV). From 1999 to 2011, the rates of all-cause death in this population decreased from 17.5% to 9.5%.1 Because PWHIV are living longer with better controlled HIV, they are experiencing higher incidence of chronic and age-related diseases like cancer. The burden of cancer in PWHIV has increased over time,2,3 and cancer has now become a leading non-AIDS cause of death in the HIV population.1
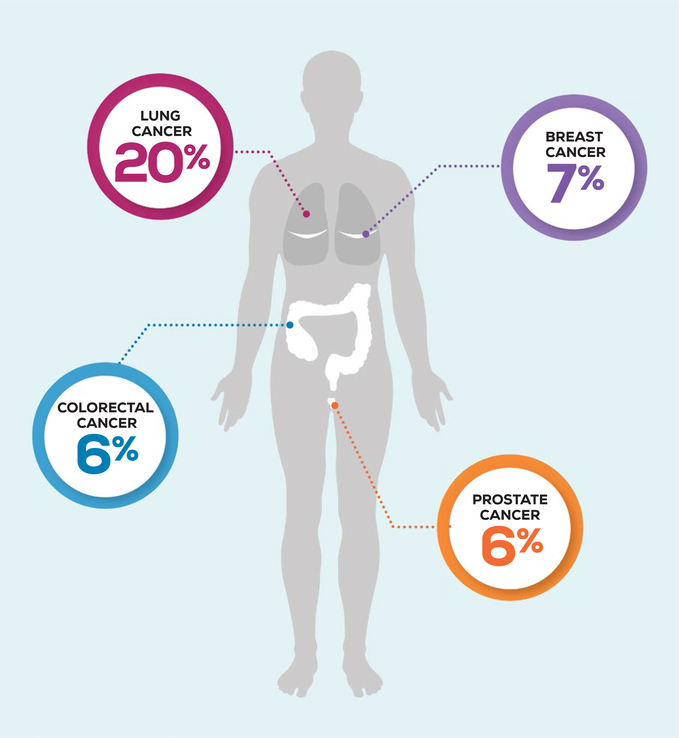
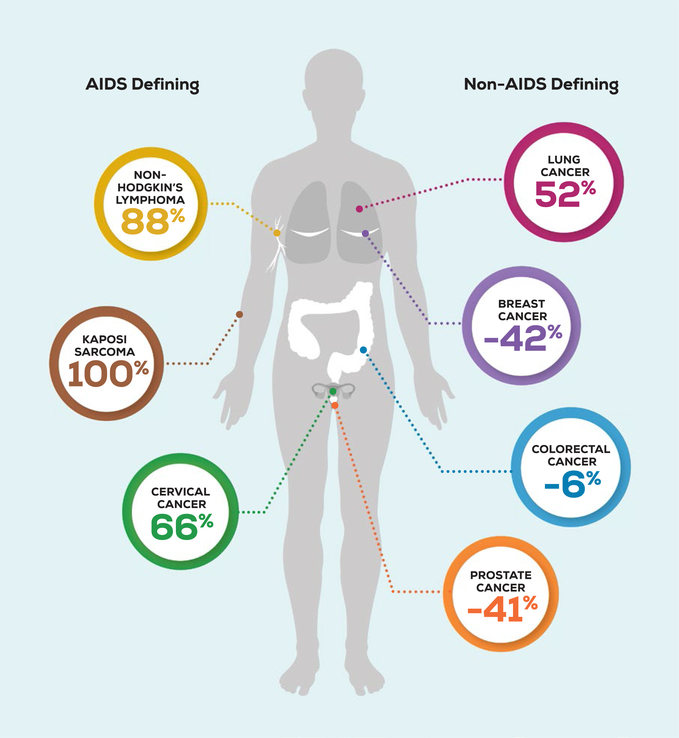
Cancer epidemiology among PWHIV has shifted since the introduction of ART.4 Over a 10-year time period in the United States, rates of AIDS-defining malignancies, including Kaposi sarcoma, non-Hodgkin lymphoma, and cervical cancer, decreased, whereas rates of many non-AIDS–defining malignancies (NADMs) substantially increased.2 Lung cancer is the most common NADM in PWHIV, accounting for 20% of the cancer burden.2 Of the other NADMs with recommended screening guidelines, breast cancer constitutes 7%, prostate cancer constitutes 6%, and colorectal cancer constitutes 6% of cancers in PWHIV (Fig. 1).2 Compared with the general population, PWHIV have a decreased risk for some of these screen-detectable cancers (Fig. 2). This may be underdiagnosis from inadequate screening; however, little is known about the utilization of cancer screening among PWHIV.
Figure 1.
The prevalence of screen detectable non-acquired immunodeficiency syndrome (AIDS)-defining malignancies is illustrated among people living with human immunodeficiency virus in the United States.
Figure 2.
The excess risk of cancers among people living with human immunodeficiency virus in the United States is illustrated. The decreased risks of breast, prostate, and colorectal cancer may be reflective of underdiagnosis caused by a lack of appropriate screening. AIDS indicates acquired immunodeficiency syndrome.
Unfortunately, PWHIV have worse cancer survival,5 in part because of their later stage at presentation compared with their uninfected counterparts.6 Although differences in survival persist even when factoring in cancer stage at diagnosis, detecting disease at a later stage leads to more complex treatment approaches and worse survival. Thus, the utilization of age-appropriate cancer screening may improve outcomes for PWHIV. In the current systematic review, we summarized the available literature on disparities in cancer screening for NADM among PWHIV to highlight opportunities to improve cancer diagnosis, treatment, and outcomes for this special population.
MATERIALS AND METHODS
A comprehensive literature search was performed to identify any published article that compared cancer screening for NADMs in PWHIV with an uninfected population. We searched the PubMed, Cochrane, and EMBASE databases using the following key terms: cancer/ neoplasm/malignancy, screening/early detection of cancer, HIV/AIDS, and health care disparities/ barriers. We searched for unpublished studies using the US National Library of Medicine of the National Institutes of Health ClinicalTrials.gov registry. The search was limited to adults and included English-language studies done in high-income countries that were published after January 1, 1996. This date was chosen because ART became widely available in 1996. We defined high-income countries using the United Nation’s classification list.7 We included all study types given the paucity of data on cancer screening in this population. We excluded pediatric studies and studies that focused on AIDS-defining malignancies. For a more detailed overview of our eligibility criteria and search strategy, see Supporting Tables 1 and 2.
All titles and abstracts were reviewed independently by K.L.C. and K.C.W. to determine eligibility using our predetermined inclusion criteria. Articles that met possible inclusion through initial screening were subjected to dual, independent, full-text review by K.L.C. and K.C.W. to assess for eligibility. Discrepancies were further reviewed for consensus. We evaluated the results of all studies that met our inclusion criteria. Specifically, we extracted descriptive data on the study design, size, population, intervention, comparator group, and outcome. We also extracted data on the reported proportion of HIV and non-HIV participants who underwent cancer screening in each study. Studies were pooled for comparison according to cancer type.
We evaluated the quality and risk of bias of the included publications using the Newcastle Ottawa Scale (NOS) for evaluating nonrandomized studies.8 The studies were rated as good quality if they met 8 or 9 of the 9 NOS criteria, fair quality if they met 6 or 7 of the 9 NOS criteria, or poor quality if they met less than 6 NOS criteria. For more information regarding quality designations for the studies, see Supporting Table 3.
RESULTS
General Description of Studies
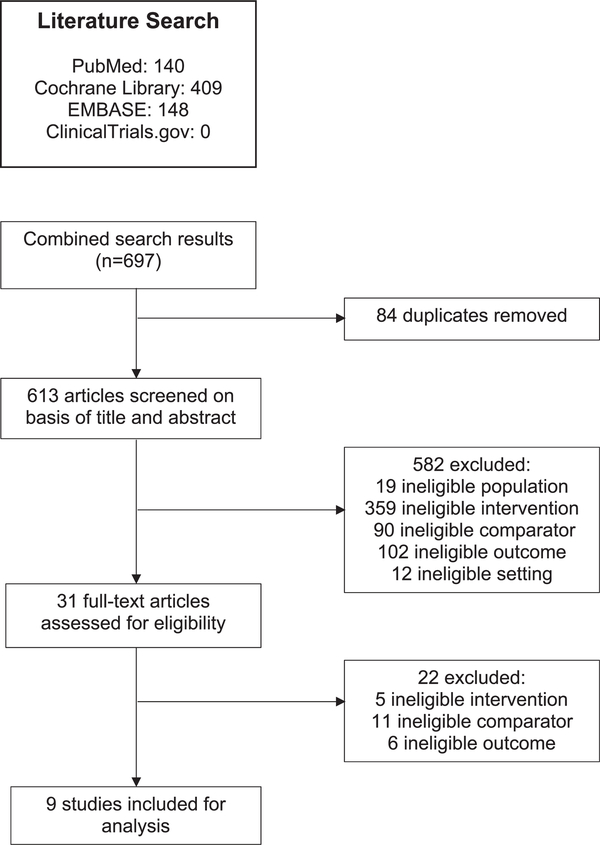
In total, 613 unique articles were identified through the search (Fig. 3). Of these, 31 were potentially relevant and subjected to full-text review. Nine studies met full eligibility criteria for the current systematic review. Figure 3 illustrates the study selection process and includes exclusion reasoning at the full-text review stage. Of the 9 included studies, 3 addressed breast cancer screening, 4 addressed colorectal cancer screening, and 2 addressed prostate cancer screening. Although many studies have analyzed screening for anal cancer, those studies did not calculate screening rates or did not include uninfected population comparators and thus did not meet our inclusion criteria. No studies that addressed lung cancer or other non-AIDS–defining malignancies met our inclusion criteria. Characteristics of the included studies are described below, grouped by cancer type.
Figure 3.
This is a flow diagram of study selection for the current review.
Breast Cancer
Three studies assessed breast cancer screening among HIV-infected and uninfected patients (Table 1).9–11 Two studies were cross-sectional, and 1 used a prospective cohort design. All 3 studies were scored as good quality. One study defined breast cancer screening as biennial mammography starting at age 40 years.9 For the remaining 2 studies, breast cancer screening was defined as biennial mammography starting at age 50 years.10,11 All 3 studies designated women who had a screening mammogram in the 2-year timeframe surrounding the study period as being up-to-date with breast cancer screening. The studies were done in 3 different countries: the United States, France, and Canada. The French study population was limited to individuals living in metropolitan areas, whereas the US and Canadian study populations were more geographically diverse. The Canadian study included women at “average” risk of developing breast cancer, and the US and French studies included women with any baseline cancer risk, including high-risk women.
TABLE 1.
Characteristics of the Included Studies Targeting Breast Cancer
| Screening Proportion: No./Total No. (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Study Type | Size | Population | Intervention | Comparator | Primary Outcomes | HIV Population | HIV Non-Population | Quality |
| Preston-Martin 20029 | Cohort study | N = 2626 Participants (2059 HIV, 569 non-HIV) | HIV-positive women aged 40–73 y in the United States with any baseline cancer risk | Breast cancer screening using biennial mammography starting at age 40 y | HIV-negative women in the United States | Breast cancer screening proportions | 1380/2059 (67) | 353/569 (62) | Good |
| Tron 201710 | Cross-sectional study | N = 886 participants (225 HIV 661 non-HIV) | HIV-positive women aged 50–75 y in metropolitan France with any baseline cancer risk | Breast cancer screening using biennial mammography, ages 50–75 y | HIV-negative women in metropolitan France | Breast cancer screening proportions | 182/225 (81) | 588/661 (89) | Good |
| Kendall 201711 | Cross-sectional study | N = 1,447,015 participants (623 HIV, 1,446,392 non-HIV) | HIV-positive women aged 50–74 y in Ontario, Canada, with “average” breast cancer risk | Breast cancer screening using biennial mammography, ages 50–74 y | HIV-negative women in Ontario, Canada | Breast cancer screening proportions | 312/623 (50) | 911,227/1,446,392 (63) | Good |
Abbreviations: HIV, human immunodeficiency virus.
Across all 3 studies, the results were inconsistent; 2 of the studies indicated that HIV-infected women were screened less often, and 1 indicated that these women were screened more often than uninfected women. In 2002, Preston-Martin and colleagues analyzed breast cancer screening among women aged 40 to 73 years who lived in the United States.9 Their study included 2059 HIV-positive women and 569 HIV-negative women and the results indicated that a higher proportion of HIV-positive women received breast cancer screening (67% vs 62%, respectively). A second study enrolled women aged 50 to 75 years who lived in metropolitan France (225 HIV-infected women and 661 women from the general population).10 That study revealed that a lower proportion of HIV-infected women received breast cancer screening compared with women in the general population (81% vs 89%, respectively). The third study analyzed breast cancer screening among women aged 50 to 74 years in Ontario, Canada.11 Among 623 HIV-positive women and 1,446,392 HIV-negative women, fewer HIV-positive women had breast cancer screening than HIV-negative women (50% vs 63%, respectively; P < .05).
All 3 studies identified factors beyond HIV status that were associated with breast cancer screening. Women with insurance, medical comorbidities, recent physician visits, receipt of other preventative care, and dental care were more likely to have up-to-date breast cancer screening.9–11 These studies also identified factors specific to women with HIV that were associated with decreased breast cancer screening, including low/intermediate education level, irregular gynecologic care, a CD4 count <500 cells/mm3, and having a nonfemale primary care provider.10,11
Colorectal Cancer
Four studies assessed colorectal cancer screening among HIV-infected and uninfected patients (Table 2).12–15 Two of those publications were cohort studies, and 2 were casecontrol studies. Three studies were done in the United States, and 1 was done in Canada. Two studies were performed at Veterans Affairs (VA) medical centers, 1 was done at an outpatient primary care clinic, and 1 was done using a general medical record database review. All 4 studies were evaluated as fair quality. To fulfill up-to-date screening criteria, colorectal cancer screening was defined as a fecal occult blood test in the past year, flexible sigmoidoscopy in the past 5 years, an air-contrast barium enema in the past 5 years, or colonoscopy in the past 10 years. There were 2 exceptions to this screening definition. Guest et al did not include air-contrast barium enema as a colorectal cancer screening test,14 and Antoniou et al required that all endoscopic tests had to be done within the past 5 years.15 Two of the studies included patients who had an “average” or “high” risk of developing colorectal cancer, 1 study included only average-risk patients, and the final study included individuals with any baseline cancer risk.
TABLE 2.
Characteristics of the Included Studies Targeting Colorectal Cancer
| Screening Proportion: No./Total No. (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Study Type | Size | Population | Intervention | Comparator | Primary Outcomes | HIV Population | HIV Non-Population | Quality |
| Reinhold 200512 | Case-control study | N = 604 participants (302 HIV,302 non-HIV) | HIV-positive patients aged >50 y at a VA clinic in the United States with any baseline cancer risk | Colorectal cancer screening using FOBT in the past y, flexible sigmoidoscopy in the past 5 y, ACBE in the past 5 y, or colonoscopy in the past 10 y | HIV-negative matched controls at the same VA outpatient clinic | Colorectal cancer screening proportions | 148/302 (49) | 199/302 (66) | Fair |
| Iqbal 201013 | Cohort study | N = 205 participants (114 HIV, 91 non-HIV) | HIV-positive individuals aged >50 y in the United States with “average” or “high” colorectal cancer risk | Colorectal cancer screening using FOBT in the past y, flexible sigmoidoscopy in the past 5 y, ACBE in the past 5 y, or colonoscopy in the past 10 y | HIV-negative individuals | Colorectal cancer screening proportions | 47/114 (41) | 61/91 (67) | Fair |
| Guest 201414 | Case-control study | N = 1884 participants (942 HIV, 942 non-HIV) | HIV-positive patients aged >45 y at a VA clinic in the United States with “average” colorectal cancer risk | Colorectal cancer screening using FOBT in the past y, flexible sigmoidoscopy in the past 5 y, or colonoscopy in the past 10 y | HIV-negative matched controls at the same VA clinic | Colorectal cancer screening proportions | 480/942 (51) | 452/942 (48) | Fair |
| Antoniou 201515 | Cohort study | N = 743,801 participants (1432 HIV, 742,369 non-HIV) | HIV-positive men aged 50 to 65 y in Ontario, Canada, with “average” or “high” colorectal cancer risk | Colorectal cancer screening using FOBT in the past y, flexible sigmoidoscopy in the past 5 y, ACBE in the past 5 y, or colonoscopy in the past 5 y | HIV-negative men in Ontario, Canada | Colorectal cancer screening proportions | 702/1432 (49) | 304,371/742,369 (41) | Fair |
Abbreviations: ACBE, air-contrast barium enema; FOBT, fecal occult blood test; HIV, human immunodeficiency virus; VA, US Department of Veterans Affairs.
The results were inconsistent across the 4 studies. Two studies indicated that the HIV-infected population was screened less often, 1 reported similar screening proportions, and another indicated that HIV-infected individuals were screened more often than the general population. The first study evaluated the proportion of HIV-positive individuals and matched controls from an outpatient VA clinic who were aged ≥50 years.12 That study included 302 HIV-positive patients and 302 matched HIV-negative controls and demonstrated that there was a lower proportion of HIV-positive patients who were up to date with colorectal cancer screening according to the recommended screening intervals in place at the time of the study (49% vs 66%, respectively; P < .05; note that screening guidelines have changed since that study). Subsequently, Iqbal et al compared screening among 114 HIV-infected and 91 non-HIV– infected individuals aged ≥50 years.13 Their study indicated that a lower proportion of HIV-infected individuals received colorectal cancer screening compared with non-HIV– infected individuals (41% vs 67%, respectively; P < .05). More recently, Guest et al included 942 individuals with HIV infection and 942 matched controls without HIV, all aged ≥45 years, and observed that the proportion of patients with HIV infection who received colorectal screening was similar to that among the controls without HIV (51% vs 48%, respectively).14 The final study analyzed screening proportions between 1432 HIV-positive men and 742,369 men without HIV aged 50 to 65 years who received care in Ontario, Canada.15 That study indicated that HIV-positive men had a higher proportion of colorectal cancer screening compared with their uninfected counterparts (49% vs 41%, respectively).
These studies also discovered additional factors that contributed to colorectal cancer screening. HIV-infected individuals with a detectable viral load, younger age, no family history of cancer, no comorbidities, and <10 physician visits over 2 years were less likely to be screened for colorectal cancer.12,13
Prostate Cancer
Two studies reported on prostate cancer screening among HIV-infected and uninfected patients as secondary outcomes (Table 3).16,17 Because the primary objective of those studies was not to assess screening itself but, instead, to analyze the relatedness of low prostate cancer incidence to low cancer screening rates among PWHIV, they did not fulfill as many of the NOS quality criteria used in the current review. One study was evaluated as poor quality,16 and the other was evaluated as fair quality.17 Both were cohort studies set in the United States and included individuals with any baseline cancer risk. One study16 used any prostate-specific antigen (PSA) test in the patient’s record during the study period as a measure of prostate cancer screening, and the other study17 used the first PSA test done in the study period that was not followed by a prostate biopsy as the screening measure. The study populations differed vastly between the 2 studies; 1 used a low-income population, and the other used a population that had health insurance and better health care access. Neither study explored other factors beyond HIV status that were associated with prostate cancer screening.
TABLE 3.
Characteristics of the Included Studies Targeting Prostate Cancer
| Screening Proportion: No./Total No. (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Study Type | Size | Population | Intervention | Comparator | Primary Outcomes | HIV Population | HIV Non-Population | Quality |
| Shiels 201016 | Cohort study | N = 50,036 participants (721 HIV, 49,315 non-HIV) | HIV-positive, low-income men aged >40 y in Baltimore, MD, with any baseline cancer risk | Prostate cancer screening using at least 1 PSA test | External HIV-negative general population | Prostate cancer incidence and screening proportions | 135/721 (19) | 28,109/49,315 (57) | Poor |
| Marcus 201417 | Cohort study | N = 200,223 participants (17,424 HIV, 182,799 non-HIV) | HIV-positive men aged ≤55 y enrolled in Kaiser Permanente in the United States with any baseline cancer risk | Prostate cancer screening using at least 1 PSA test | HIV-negative men enrolled in Kaiser Permanente | Prostate cancer incidence and screening proportions | 15,856/17,424 (91) | 157,207/182,799 (86) | Fair |
Abbreviations: HIV, human immunodeficiency virus; PSA, prostate-specific antigen.
The 2 studies produced different results; 1 study indicated that HIV-infected men had higher screening proportions, whereas the other indicated that these men were screened less often than men in the general population. The first study compared longitudinal data from a cohort of 721 low-income, HIV-infected, African American men with data from 49,315 men in the general population who participated in the 2001 Behavioral Risk Factor Surveillance System survey conducted by the US Centers for Disease Control and Prevention.16,18 The study indicated that, among men aged ≥40 years, a lower proportion of HIV-infected men received screening compared with men in the general population (19% vs 57%, respectively). More recently, Marcus et al analyzed prostate cancer screening in 17,424 HIV-positive and 182,799 HIV-negative men who were enrolled in Kaiser Permanente.17 Their study indicated that a higher proportion of HIV-positive men received prostate cancer screening by age 55 years compared with HIV-negative men (91% vs 86%, respectively; P < .05).
DISCUSSION
Non-AIDS–Defining Cancer Screening Disparities
To our knowledge, the current systematic review is the first to compile and compare studies that assessed screening for NADMs among HIV-infected and uninfected cohorts. Across the 9 included studies, there was no consistent difference in cancer screening between PWHIV and uninfected individuals. Instead, screening differed according to cancer type, education level, insurance status, income level, the presence of comorbidities, and the number of visits to health care providers. This suggests that HIV status alone may not negatively influence cancer screening. However, several other factors, such as low income and education, as well as underinsurance, which are more prevalent in the US HIV-positive population, could explain the underutilization of cancer screening.
In this review, we also identified gaps in the available literature. Although many studies have analyzed screening for AIDS-defining malignancies (eg, cervical cancer) and historically common NADMs (eg, anal cancer), data were scarce on screening for other NADMs.19–23 One significant gap in the literature was the absence of studies focused on lung cancer screening in the HIV/ AIDS population. This is concerning, because lung cancer is among the most common cancers in PWHIV, who are more likely to smoke, less likely to receive lung cancer treatment, and have higher lung cancer-specific mortality compared with the general population.24,25 Although non-AIDS–defining malignancies have increased in prevalence among PWHIV over the past 20 years, research on NADM screening in this population is limited. Future studies should investigate whether the current cancer-screening recommendations aimed at the general population are adequate for the HIV-infected population and whether HIV specialists, who often serve as primary care providers for PWHIV, are offering cancer screening at appropriate intervals.
Breast Cancer Screening in PWHIV
Disparate results for breast cancer screening between PWHIV and the general population may reflect issues related to setting and population. The studies were performed in different countries (France, the United States, Canada), and each had differing access to care and established screening guidelines. In the French study, much higher percentages of both HIV-infected and uninfected women underwent breast cancer screening compared with women in the United States and Canada. In France, breast cancer screening is fully paid for by public health insurance, and reminders are sent to women aged 50 to 74 years. This system has been associated with high rates of cancer screening and may account in part for the differing degrees of breast cancer screening between France, the United States, and Canada.26 In addition, different age ranges and baseline risk profiles were used in the studies. The studies that used a wider age range and included individuals at a higher risk for breast cancer may have had increased screening proportions.
All 3 studies identified characteristics that were associated with higher screening rates for women with HIV, including the presence of health insurance, more annual primary care visits, and a woman primary care physician. Although PWHIV are more likely to be uninsured or underinsured,27,28 they may have a higher number of annual visits as part of routine HIV care, which may lead to improved cancer screening. In addition, Tron and colleagues and Kendall et al identified specific barriers for screening among women living with HIV, including low educational background and low income level.10,11 These challenges are identical to those identified in the review by Lambert et al, who studied barriers to cervical cancer screening among women with HIV.22 Thus, social determinants of health play a major role in the receipt of cancer screening among HIV-infected women and may contribute to disparities in cancer care.
Colorectal Cancer Screening in PWHIV/AIDS
Although all 4 studies that analyzed colorectal cancer screening were designated as fair quality, the results from those studies should be reviewed with care. The studies by Reinhold et al and Guest and colleagues were both performed at VA medical centers.12,14 The results from those studies may overestimate screening rates because of universal health coverage and high compliance with colorectal cancer screening in VA clinics.29 In addition, Antoniou et al could not distinguish between coding for diagnostic and screening endoscopic tests; therefore, the results from their study likely overestimate colorectal cancer screening rates. Although all 4 studies that assessed colorectal cancer screening used similar age ranges for their populations, they used different baseline risk profiles. The studies that analyzed populations with a high risk of developing cancer likely observed greater screening proportions than studies that did not include high-risk patients. These differences in patient populations and issues with data quality may account for the differing conclusions in these 4 studies.
Two of the studies, which were conducted in populations from a local VA clinic and a community hospital, indicated that HIV-infected patients are less likely to be screened for colorectal cancer.12,13 There are several potential explanations for this observed lower proportion of colorectal cancer screening among PWHIV. First, PWHIV have reported anxiety associated with colorectal cancer screening as well as lack of time and low priority because of competing health concerns.30 In addition, Iqbal et al reported that PWHIV who had comorbidities and more annual physician visits were more likely to be up to date with colorectal cancer screening.13 Like similar findings for breast cancer screening, a lack of contact with the health care system is a significant barrier to screening for colorectal cancer. Finally, of the 2 studies at VA medical centers, 1 study population received primary care solely through their infectious disease physicians,12 and the other population received primary care through both infectious disease physicians and primary care providers.14 The former study reported a significantly lower proportion of colorectal cancer screening among the HIV-infected population compared with the uninfected population, whereas the latter study reported comparable screening proportions in the HIV-infected and uninfected populations. These results suggest that the delivery of primary care services by an infectious disease specialist may lead to reduced screening rates. This hypothesis has not yet been studied in the published literature; however, literature from other fields suggests that HIV specialists may focus on HIV control to the detriment of other routine health screening services, such as blood pressure control or lipid monitoring.31–33
Prostate Cancer Screening in PWHIV/AIDS
The 2 studies that analyzed prostate cancer screening in HIV-infected patients reported discrepant results, likely because of differences in study settings, populations, and cancer screening criteria. The main purpose of the study by Shiels et al was to ascertain whether the low incidence of prostate cancer among PWHIV was because of differential screening. Although the methodologic rigor was high, for the purpose of the current review, it was designated as poor quality, because there was no direct comparison with an uninfected group; instead, external data were used as the comparator for screening in the general population.16 In addition, that study was conducted in a small cohort of low-income men in 1 US city, making the results less generalizable. In contrast, Marcus et al used HIV-infected and non-HIV–infected individuals from the same study population that had full access to primary care services through Kaiser Permanente.17 The discrepant results are likely related to the markedly different access to care between the 2 study populations, again indicating that health systems factors (such as insurance status, income level, and access to care) may influence cancer screening more than HIV status alone. Finally, 1 limitation in both studies was the inability to distinguish between a screening PSA test and a diagnostic PSA test, thus the results of both studies likely overestimate prostate cancer screening rates.
Impact of Cancer Screening Disparities
In our review, we observed that, although HIV does not appear to drive disparities in cancer screening for NADMs, many factors that disproportionately affect the US HIV-infected population also affect the likelihood of receiving cancer screening. First, low income level and lower educational attainment are associated with decreased cancer screening. Several studies have indicated that there is a higher prevalence of HIV in areas with low socioeconomic status and higher HIV/AIDS-related mortality in these areas.34–36 Consequently, low socioeconomic status exacerbates cancer screening inequalities among PWHIV and could be 1 source of worsened cancer outcomes in this population. In addition, the findings from our review demonstrate that increased contact with the health care system increases the likelihood of receiving cancer screening. This has both a positive and a negative effect on PWHIV. Individuals with HIV who are able to attend regular appointments with their infectious disease physician or primary care provider may be more likely to remain up to date with their cancer screening, in some instances even more so than the general population because of this increased health care contact. However, for PWHIV with low socioeconomic status, the lack of health care accessibility worsens their likelihood of receiving cancer screening. Consequently, improvements in access to primary care for PWHIV are needed to increase the use of high- quality preventive services.
One potential explanation for decreased cancer screening among PWHIV is poor patient compliance despite physician recommendations for screening. Past studies have indicated that patients with HIV are more likely to have adherence challenges because of difficulty remembering appointments, lack of time, low prioritization of non-HIV/AIDS–related disease, and enhanced anxiety toward medical procedures.30,37 In addition, a qualitative study that analyzed cervical cancer screening barriers demonstrated that HIV-infected women had lower awareness, limited transportation, and concern over the Papanicolaou smear procedure that prevented them from obtaining recommended cervical cancer screening.38 Thus, patient challenges and fears regarding cancer screening should be addressed by physicians and further investigated in future studies.
Another important consideration for cancer screening in PWHIV is the younger age at which this population develops cancer compared with uninfected individuals.39,40 Patients with HIV/AIDS who are at risk for developing cancer may not be eligible for screening because they do not meet age thresholds devised for the general population. Physicians may want to consider early screening for PWHIV, although limited data are available weighing the benefits and harms of screening in this younger population of PWHIV.
The low prevalence of cancer screening observed among PWHIV raises concerns about the utility of available guidelines and the transitioning physician roles for patients with HIV/AIDS. The HIV Medicine Association has published guidelines for primary care management of PWHIV.41 However, these guidelines provide HIV-specific, evidence-based recommendations only for cervical and anal cancer screening. Although the guidelines incorporate breast and colorectal cancer screening extrapolated from screening recommendations for the general population, there is no HIV-specific evidence available for these recommendations and no specific screening guidelines for lung or prostate cancer in PWHIV. Without clear cancer screening guidelines for PWHIV, physicians and patients may question the utility and efficacy of screening in this specific patient population. In addition, because infectious disease specialists are increasingly assuming the role of the primary care providers for PWHIV, there is added concern over the appropriate administration of preventative services, including cancer screening.31 Additional research should focus on optimal screening strategies for PWHIV as well as forming recommendations for the utilization of cancer screening services in this population.
The current review has several limitations. First, our search strategy may have missed relevant articles; however, the systematic approach and review by 2 independent reviewers minimized this risk. Second, direct comparison of data was difficult because of the heterogeneity of study designs, settings, and populations used across the relevant publications. In addition, studies from low-income and middle-income countries, where the burden of HIV is higher than in high-income countries, were excluded. This was done to maximize homogeneity within study groups and to enhance applicability to the HIV population in the United States. Finally, it is possible that screening utilization was not completely captured in the studies analyzed in this review. However, the general population screening rates were comparable to those reported previously in the literature, suggesting that the reported screening rates were appropriately captured.42–44
CONCLUSION
This review has synthesized findings about non-AIDS– defining malignancy screening trends among PWHIV to investigate disparities and enhance cancer care in this vulnerable population. Although we identified discrepancies on the impact of HIV in the published literature, we consistently observed that several sociodemographic factors disproportionally affecting the US HIV-infected population do affect cancer screening. These factors include insurance-status, income-level, education-level, as well as access to and amount of contact with the health care system. In addition, patient preferences and physician factors may contribute to underutilization of cancer screening services. PWHIV/AIDS are living longer because of improved antiretroviral medications, and they are experiencing a rapidly increasing burden of cancer. PWHIV and cancer have worse cancer outcomes compared with the general population, in part because of their advanced stage at diagnosis. Improvements in cancer screening are urgently needed to provide this population with high-quality cancer care, improve health outcomes, and enhance the quality of life for PWHIV.
Supplementary Material
FUNDING SUPPORT
John A. Bartlett is supported by grants P30AI064518, D43TW009595, and D32TW010138 from the US National Institutes of Health. Gita Suneja is supported by grants K08CA228631 and P30AI064518 from the US National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. [DOI] [PubMed] [Google Scholar]
- 2.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaberg EC, Wiley D, Martinez-Maza O, et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010;116:5507–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgi A, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. [DOI] [PubMed] [Google Scholar]
- 5.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer- specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiels MS, Copeland G, Goodman MT, et al. Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer. 2015;121:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drysdale C, ed. World Economic Situation and Prospects 2018. New York: United Nations Department of Economic and Social Affairs; 2018. [Google Scholar]
- 8.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses Ottawa, Ontario, Canada: Ottawa Hospital Research Institute; 2000. [Google Scholar]
- 9.Preston-Martin S, Kirstein LM, Pogoda JM, et al. Use of mammographic screening by HIV-infected women in the Women’s Interagency HIV Study (WIHS). Prev Med. 2002;34:386–392. [DOI] [PubMed] [Google Scholar]
- 10.Tron L, Lert F, Spire B, Dray-Spira R. Levels and determinants of breast and cervical cancer screening uptake in HIV-infected women compared with the general population in France. HIV Med. 2017;18:181–195. [DOI] [PubMed] [Google Scholar]
- 11.Kendall CE, Walmsley S, Lau C, et al. A cross-sectional population-based study of breast cancer screening among women with HIV in Ontario, Canada. CMAJ Open. 2017;5:E673–E681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinhold JP, Moon M, Tenner CT, Poles MA, Bini EJ. Colorectal cancer screening in HIV-infected patients 50 years of age and older: missed opportunities for prevention. Am J Gastroenterol. 2005;100:1805–1812. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal S, Browne-McDonald V, Cerulli MA. Recent trends for colorectal cancer screening in HIV-infected patients. Dig Dis Sci. 2010;55:761–766. [DOI] [PubMed] [Google Scholar]
- 14.Guest JL, Rentsch CT, Rimland D. Comparison of colorectal cancer screening and diagnoses in HIV-positive and HIV-negative veterans. AIDS Care. 2014;26:1490–1493. [DOI] [PubMed] [Google Scholar]
- 15.Antoniou T, Jembere N, Saskin R, Kopp A, Glazier RH. A population-based study of the extent of colorectal cancer screening in men with HIV [serial online]. BMC Health Serv Res. 2015;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiels MS, Goedert JJ, Moore RD, Platz EA, Engels EA. Reduced risk of prostate cancer in US men with AIDS. Cancer Epidemiol Biomarkers Prev. 2010;19:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus JL, Chao CR, Leyden WA, et al. Prostate cancer incidence and prostate-specific antigen testing among HIV-positive and HIV-negative men. J Acquir Immune Defic Syndr. 2014;66:495–502. [DOI] [PubMed] [Google Scholar]
- 18.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289:1414–1420. [DOI] [PubMed] [Google Scholar]
- 19.Ogunwale AN, Coleman MA, Sangi-Haghpeykar H, et al. Assessment of factors impacting cervical cancer screening among low-income women living with HIV-AIDS. AIDS Care. 2016;28:491–494. [DOI] [PubMed] [Google Scholar]
- 20.Williams M, Moneyham L, Kempf MC, Chamot E, Scarinci I. Structural and sociocultural factors associated with cervical cancer screening among HIV-infected African American women in Alabama. AIDS Patient Care STDS. 2015;29:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nankya E, Wood C, Ainsworth J, Schwenk A, Waters A, Johnston V. P186. Assessing the uptake of cervical screening amongst HIV-positive women attending an HIV clinic in the UK [abstract]. HIV Med. 2015;16(suppl 2):71. [Google Scholar]
- 22.Lambert CC, Chandler R, McMillan S, Kromrey J, Johnson-Mallard V, Kurtyka D. Pap test adherence, cervical cancer perceptions, and HPV knowledge among HIV-infected women in a community health setting. J Assoc Nurses AIDS Care. 2015;26:271–280. [DOI] [PubMed] [Google Scholar]
- 23.Burchell AN, Andany N, Antoniou T, et al. O039. Pap cytology testing among HIV-positive and HIV-negative women in Ontario, 2008–2013: suboptimal for cervical cancer prevention [abstract]. Can J Infect Dis Med Microbiol. 2015;26(suppl B):19B. [Google Scholar]
- 24.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS. 2013;27:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. [DOI] [PubMed] [Google Scholar]
- 26.Pivot X, Rixe O, Morere JF, et al. Breast cancer screening in France: results of the EDIFICE survey. Int J Med Sci. 2008;5:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman DP, Leibowitz AA, Joyce GF, et al. Insurance status of HIV-infected adults in the post-HAART era: evidence from the United States. Appl Health Econ Health Policy. 2003;2:85–90. [PubMed] [Google Scholar]
- 28.Bhattacharya J, Goldman D, Sood N. The link between public and private insurance and HIV-related mortality. J Health Econ. 2003;22:1105–1122. [DOI] [PubMed] [Google Scholar]
- 29.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348:2218–2227. [DOI] [PubMed] [Google Scholar]
- 30.Campbell J, Young B. Use of screening colonoscopy in ambulatory HIV-infected patients. J Int Assoc Physicians AIDS Care (Chic). 2008;7:286–288. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmi S, Beekmann SE, Polgreen PM, Rodriguez A, Alcaide ML. HIV primary care by the infectious disease physician in the United States—extending the continuum of care. AIDS Care. 2018;30:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fultz SL, Goulet JL, Weissman S, et al. Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clin Infect Dis. 2005;41:738–743. [DOI] [PubMed] [Google Scholar]
- 33.Duffus WA, Barragan M, Metsch L, et al. Effect of physician specialty on counseling practices and medical referral patterns among physicians caring for disadvantaged human immunodeficiency virus-infected populations. Clin Infect Dis. 2003;36: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 34.Denning P, DiNenno E. Communities in Crisis: Is There a Generalized HIV Epidemic in Impoverished Urban Areas of the United States? Atlanta, GA: National Center for HIV Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 35.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the US HIV epidemic. Am Psychol. 2013;68:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis [serial online]. PLoS Med. 2016;13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher FE, Buchberg M, Schover LR, et al. Perceptions of barriers and facilitators to cervical cancer screening among low-income, HIV-infected women from an integrated HIV clinic. AIDS Care. 2014;26:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goedert JJ, Schairer C, McNeel TS, et al. Risk of breast, ovary, and uterine corpus cancers among 85,268 women with AIDS. Br J Cancer. 2006;95:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Office of Analysis and Epidemiology, National Center for Health Statistics, Centers for Disease Control and Prevention. Table 83. Use of mammography among women 40 years of age and over, by selected characteristics: United States, selected years 1987–2008. Atlanta, GA: Centers for Disease Control and Prevention; 2010. https://www.cdc.gov/nchs/data/hus/2013/083.pdf. Accessed July 1, 2018.
- 43.American Cancer Society. Colorectal Cancer: Facts & Figures 2017–2019. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 44.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.