Abstract
Three QS-21-based vaccine adjuvant candidates with a terminal-functionalized side chain incorporated in the west wing trisaccharide have been synthesized. The terminal polar functional group serves to increase the solubility of these analogues in water. Two of the synthetic analogues have been shown to have adjuvant activity comparable to that of GPI-0100. The stand-alone adjuvant activity of the new synthetic analogues again confirmed that it is a feasible way to develop new saponin-based vaccine adjuvants through derivatizing at the west wing branched trisaccharide domain. Inclusion of an additional polar functional group such as a carboxyl group (as in 3x) or a monosaccharide (as in 4x and 5x) is sufficient to increase the water solubility of the corresponding synthetic analogues to a level comparable to that of GPI-0100 and suitable for immunological studies and clinical application. The structure of the incorporated side chain has a significant impact on the adjuvant activity in terms of the magnitude and nature of the host’s responses.
Graphical Abstract
INTRODUCTION
Recent efforts in developing new vaccines to combat cancer and infectious diseases have relied heavily on subunit antigen constructs. However, the refined and homogeneous antigens are often less immunogenic, which necessitates the use of immune adjuvants to enhance the ability of vaccines to elicit strong and durable immune responses to specific antigens.1–6 Despite the obvious benefits, the choice of adjuvant for human vaccines is severely limited. Alum (aluminum salts) was the sole adjuvant used in licensed human vaccines for over 80 years until the 1990s. But it is only efficient in eliciting a high antibody response with a Th2 profile instead of eliciting a protective Th1 response. A Th1 response is necessary for vaccines against cancers and intracellular pathogens such as HIV, TB, and malaria.2,7–9 Since the 1990s, only a few other adjuvants have been approved for use in defined human vaccines, including oil in water emulsions (MF59 and AS03) used in influenza vaccines and a combination adjuvant (i.e., AS04, composed of monophosphoryl lipid A (MPL) adsorbed to alum) used in HBV and HPV vaccines in Europe and the United States. Despite the progress, developing subunit vaccines is still bottlenecked by the lack of safe and effective adjuvants. Because of their urgent need, the discovery and development of novel adjuvants has emerged as a critical frontline effort in vaccine research.
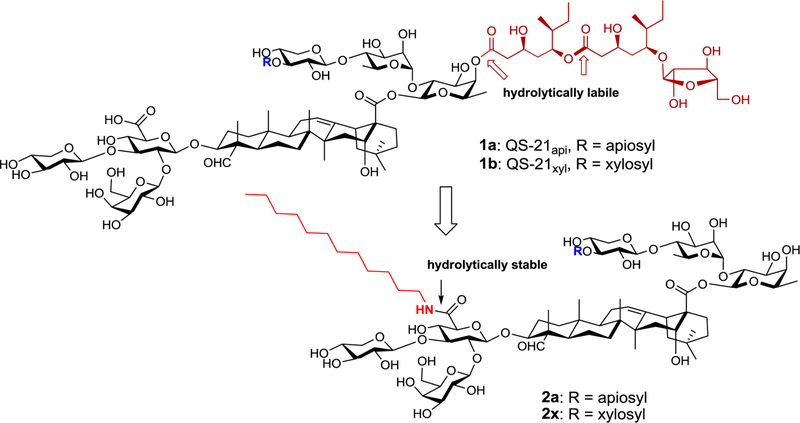
Having promising lead compounds is the critical first step toward successful development of synthetic vaccine adjuvants. Naturally occurring QS saponins can be promising leads in this regard. Among various vaccine adjuvants studied, QS-21 (1, Figure 1), a saponin adjuvant obtained from the bark of Quillaja saponaria (QS) Molina, stimulates mixed Th1 and Th2 responses. It significantly outperformed other classes of adjuvants (including glucan formulations, peptidoglycans, amphiphilic block copolymers, bacterial nucleosides, and bacterial lipopolysaccharide)10,11 and has been evaluated in over 100 clinical trials of vaccines against cancer and infectious diseases.10 Although it is the immunostimulant of choice in many clinical trials of vaccines, QS-21 has its own drawbacks. One of them is its chemical instability in stock solution, originated from the hydrolytically unstable ester moieties (i.e., the one that connects the side chain to the east wing oligosaccharide domain and the internal one in the side chain). SAR studies confirmed that removal of the east wing chain leads to QS-21’s loss of the capability of boosting a lymphoproliferative response along with CTL production.12–16 These inherent drawbacks prevent QS-21 from wider use. Despite recent progress in circumventing these limitations of QS-21,17,18 (1) QS-based synthetic analogues appropriate for clinical use are still not available, (2) the molecular mechanisms by which QS saponins work are not understood, and (3) synthesis of the unnatural QS-21 analogues remains a challenging and time-consuming task.
Figure 1.
Natural QS-21 (1) and synthesized analogues (2).
To address these challenges, we have recently looked into a different type of synthetic QS-21 analogue. Different from the natural QS-21, which has a hydrolytically labile acyl side chain incorporated to the east wing tetrasaccharide domain, the new analogues feature a plain aliphatic chain incorporated to the branched west wing trisaccharide domain through a hydrolytically stable amide bond. The design is inspired by the early work of developing the semisynthetic saponin analogue GPI-0100.12–15,19 Marciani and co-workers prepared GPI-0100 from Quil A, a complex mixture of QS tree bark extracts (containing QS-21). They completely removed the east wing acyl side chain and replaced it with a plain dodecylamine chain on the other side of the saponins through the amide formation reaction. The obtained complex mixture retains adjuvanticity similar to that of Quil A, stimulating humoral and T-cell immunity along with antigen-specific CTL production. Although the immune stimulatory activity of GPI-0100 is lower than that of the natural saponins, toxicology studies indicated that GPI-0100 is 20 times less lethal in mice than QS-21. In mice, the ratio of acute toxic dose to effective dose is about 40–50, which allows the GPI-0100 dose to be significantly increased to achieve the desired immune response without early onset of toxicity. However, GPI-0100 is highly heterogeneous, with high variability in content and composition, which affects its efficacy and formulation and prevents its use in a clinical setting. Nevertheless, the early work of Marciani et al. on GPI-010012–15,19 and the structure–function studies of Soltysik et al.20 provide us a valuable clue on the initial molecular design of QS analogues (as shown in Figure 1). We have recently synthesized structurally defined 2a and 2x,21 the QS-21-derived components in GPI-0100.12,16 The stand-alone adjuvant activity of 2x confirmed that our new design is a feasible path to chemically stable QS-based saponin adjuvants. However, we noticed that 2x has lower adjuvant activity and water solubility than GPI-0100. Herein, we report our efforts aimed at increasing the water solubility and improving the adjuvant activity of the synthetic analogues.
RESULTS AND DISCUSSION
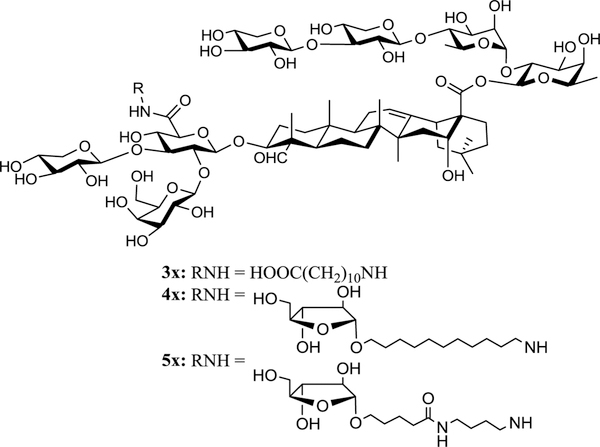
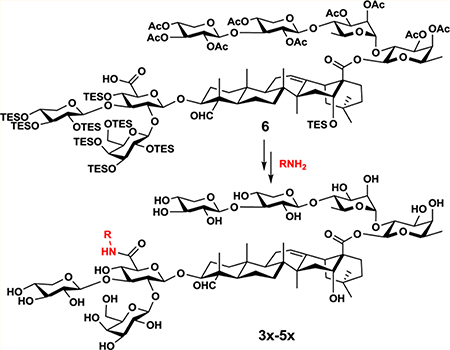
We infer that inclusion of a polar functional group such as a carboxyl group (Figure 2, as in 3x) or one extra sugar unit (Figure 2, as in 4x and 5x) will increase the water solubility. In 3x, the small polar terminal carboxyl group is expected to retain the adjuvant activity of GPI-0100 due to minimal structural variation. In 4x and 5x, we included a terminal L-arabinose unit to mimic the terminal structure of the natural acyl side chain in QS-21 (Figure 1). The analogue 5x differs from 4x in having an internal polar functional group, mimicking the internal ester moiety in the acyl side chain of the natural QS-21.
Figure 2.
Design of new QS-21 analogues.
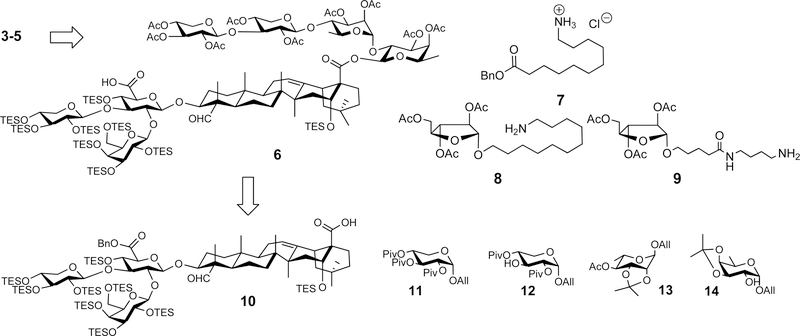
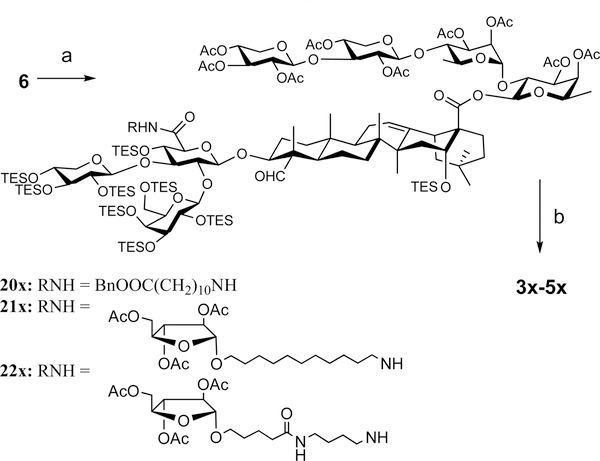
Retrosynthesis of the targets 3x–5x leads to the common intermediate 6 and the different side chains 7–9 (Scheme 1). The intermediate 6 was also used in the synthesis of 2x.21 It can be obtained from the conjugate 10 (prepared in three steps from Quil A)22,23 and a tetrasaccharide synthesized from the allyl glycoside building blocks 11–14 by using the two-stage activation of allyl glycosyl donor method recently developed in our laboratory.21,24–26
Scheme 1.
Retrosynthesis of the Analogues 3–5
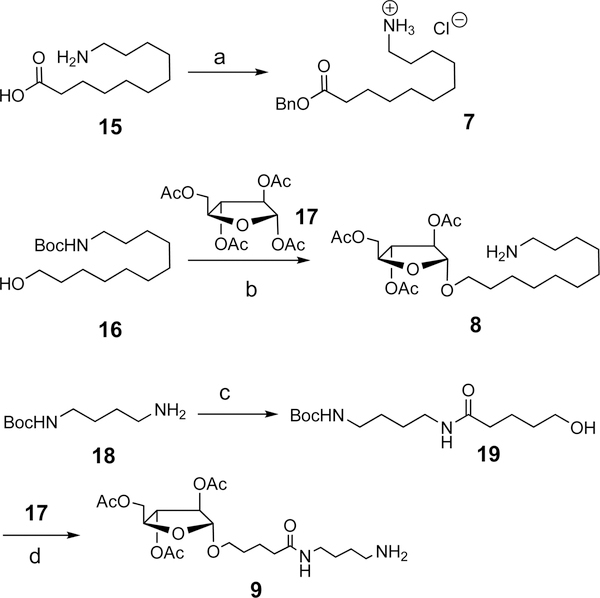
The side-chain 11-aminoundecanoic acid benzyl ester hydrochloride (7) for the synthesis of 3x can be synthesized from the commercially available 11-aminoundecanoic acid (15) in a quantitative yield (Scheme 2).27 From the compound 1628and peracetyl L-arabinoside (17),29 acid-promoted glycosylation reaction led to the protected side chain 8 with an unoptimized yield of 51% for the synthesis of the analogue 4x. For the synthesis of the side chain to be incorporated into 5x, we started with the commercially available N-Boc-1,4-butanediamine (18). Thus, the reaction of 18 with δ-lactone resulted in the formation of 19 in a 94% yield.30 In the presence of 2 equiv of TESOTf, the glycosyl acceptor 19 and the donor 17 provided the protected side chain 9 in a 79% yield.29
Scheme 2. Synthesis of the Side Chainsa.
aReagents and conditions: (a) SOCl2, BnOH, > 99%; (b) TESOTf (2 equiv), 0 to rt, 51%; (c) δ-lactone, THF, 75 °C, 94%; (d) TESOTf (2 equiv), 0 °C to rt, 79%.
With the three side chains in hand, we attempted the divergent synthesis of the fully protected adjuvant analogues from the common intermediate 6 (Scheme 3). Thus, coupling of 6 with the side chains 7–9 by using a standard coupling reagent such as HATU31 provided the conjugates 20x, 21x, and 22x in 94%, 82%, and 67% yield, respectively. The subsequent deprotection consists of two steps, i.e., removal of the silyl protecting groups under acidic conditions (TFA/H2O (4:1 v/v) at 0 °C) followed by removal of the acetyl groups under basic conditions (K2CO3 in MeOH). The ester moiety between the east wing linear tetrasaccharide and the quillaic acid remained intact under the reaction conditions.32,33 After reversed-phase HPLC separation and lyophilization, the final products 3x–5x were obtained as white powder in 74%, 70%, and 50% yield, respectively. Inclusion of a polar functional group such as a carboxyl group (as in 3x) or one extra sugar unit (as in 4x and 5x) proved to be sufficient to increase their water-solubility to a level comparable to that of GPI-0100.
Scheme 3. Synthesis of the Adjuvants 3x–5xa.
aReagents and conditions: (a) side chain 7, 8, or 9, HATU, DIPEA, CHCl3, 23 °C, 94% for 20x, 82% for 21x, 67% for 22x; (b) TFA/H2O (4:1), DCM, 0 °C; K2CO3, MeOH, 23 °C, 74% for 3x, 70% for 4x, 50% for 5x.
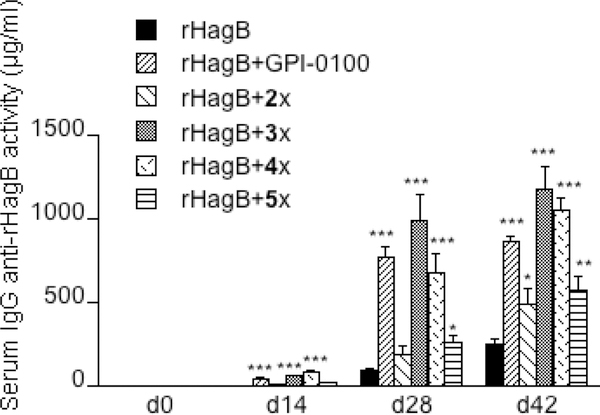
For immunological study, we first evaluated the effectiveness of 3x–5x in augmenting immune responses to rHagB. The antigen rHagB is a recombinant, nonfimbrial adhesion hemagglutinin B from Porphyromonas gingivalis, a periodontal pathogen.34–40 We used GPI-0100 and 2x as the positive controls. BALB/c mice (female, 8–10 weeks of age, six per group) were injected with rHagB (20 μg) alone or rHagB (20 μg) with different adjuvants (i.e., GPI-0100, 2x and 3x–5x) via a subcutaneous route (s.c.). Mice were immunized on days 0, 14, and 28. Prior to each immunization and at 42 days post the last immunization, mice were weighed and then serum was collected from each mouse and analyzed for anti-rHagB activity using an enzyme-linked immunosorbent assay (ELISA). GPI-0100 augments significantly higher (P < 0.001) IgG anti-rHagB antibody responses than that seen with rHagB alone at days 14, 28, and 42, and 2x showed potentiation at day 42 (P < 0.05) (Figure 3).21 The adjuvants 3x and 4x were also effective in enhancing the serum IgG anti-rHagB response after the initial immunization (P < 0.001) and subsequent immunizations, compared to that seen with antigen alone. Similarly, the adjuvant 5x enhanced the anti-rHagB IgG responses at days 28 (P < 0.05) and 42 (P < 0.01). However, mice immunized with rHagB+3x or +4x had higher serum IgG anti-rHagB antibody responses compared to mice immunized with rHagB+2x or +5x at days 28 (P < 0.001) and 42 (P < 0.01). Interestingly, although the differences were not significant, the anti-rHagB responses potentiated by 3x at days 28 and 42 were higher than that seen with GPI-0100. No difference was seen in the mean body weights of mice between groups (see Figure S1), indicating that the adjuvants lacked toxicity.
Figure 3.
Serum IgG anti-rHagB in mice immunized (s.c.) with antigen ± adjuvant on days 0, 14, and 28. Values are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with mice immunized with rHagB alone.
We then assessed the subclass of the IgG antibody responses (Table 1). Immunization of mice with rHagB+GPI-0100 or with rHagB+2x, +3x, or +4x resulted in significantly higher (P < 0.05 or P < 0.001) serum IgG1 anti-rHagB antibody levels by day 42 than seen in mice immunized with rHagB alone. A higher IgG1 response was also seen in mice receiving rHagB+5x than in mice immunized with antigen alone. Only a slight IgG2a response was induced in the mice receiving rHagB+5x or rHagB alone. Although the IgG2a response induced by rHagB+2x was higher than that seen with rHagB alone, GPI-0100, 3x, and 4x potentiated significantly higher (P < 0.001) serum IgG2a anti-rHagB responses. The IgG2a/IgG1 ratio of the anti-rHagB responses indicated that rHagB and rHagB+2x preferentially induced IgG1 antibody responses and that rHagB+5x further potentiated the response toward the IgG1 direction. However, both IgG1 and IgG2a responses to rHagB were potentiated by GPI-0100, 3x, and 4x. These findings suggest that following s.c. immunization, rHagB selectively induces a Th2-like response, whereas the adjuvants 3x and 4x, like GPI-0100, potentiate a mixed Th1- and Th2-like response to rHagB and that 5x mainly enhanced a Th2-like response. Taken together, our results demonstrated that QS-21 derivatives 3x and 4x are comparable to GPI-0100 for potentiating systemic responses to rHagB.
Table 1.
Serum IgG Subclass Anti-rHagB Activity at Day 42a
| entry | adjuvant | IgG1 (μg/mL) | IgG2a (μg/mL) | IgG2a/IgG1 |
|---|---|---|---|---|
| 1 | none | 264.6 ± 30.3 | 9.7 ± 2.8 | 0.036 ± 0.007 |
| 2 | GPI-0100 | 756.4 ± 39.33d | 327.2 ± 70.2c | 0.5 ± 0.1c |
| 3 | 2x | 546.2 ± 41.7b | 24.2 ± 6.3 | 0.04 ± 0.01 |
| 4 | 3x | 950.5 ± 90.5d | 380.0 ± 72.9d | 0.40 ± 0.06b |
| 5 | 4x | 813.3 ± 88.8d | 495.9 ± 65.2d | 0.7 ± 0.1d |
| 6 | 5x | 460.7 ± 28.0 | 4.5 ± 0.8 | 0.010 ± 0.002 |
Values are the mean ± SEM.
P < 0.05.
P < 0.01.
P < 0.001.
In summary, three synthetic QS-21-based immune adjuvants have been derived. These new analogues are equipped with a terminal-functionalized side chain that connects to the west wing branched trisaccharide domain through a hydrolytically stable amide bond with the glucuronic acid moiety. The stand-alone adjuvant activity of the new synthetic analogues again confirmed that our molecular design is a feasible approach to new saponin adjuvants with different adjuvant properties. Addition of one polar functional group such as a carboxyl group (as in 3x) or an extra sugar unit (as in 4x) is sufficient to increase the water solubility of the corresponding synthetic analogues to a level comparable to that of GPI-0100 and suitable for immunological studies and potential clinical application. Thus, the structure of the incorporated side chain has a significant impact on adjuvant activity in terms of the magnitude and nature of the responses.
EXPERIMENTAL SECTION
General Methods
Organic solutions were concentrated by rotary evaporation at ca. 12 Torr. Flash column chromatography was performed employing 230–400 mesh silica gel. Thin-layer chromatography was performed using glass plates precoated to a depth of 0.25 mm with 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). IR data are presented as frequency of absorption (cm−1). 1H NMR or 13C NMR spectra were recorded on 300, 400, and 700 MHz NMR spectrometers; chemical shifts are expressed in parts per million (δ scale) downfield from tetramethylsilane. Data are presented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and/or multiple resonances), coupling constants in hertz (Hz), integration. HRMS was conducted with either an ESI or MALDI ionization method and with a TOF mass analyzer.
Materials
Tetrahydrofuran (THF), toluene, dichloromethane (DCM), and acetonitrile (MeCN) were distilled from appropriate drying reagents under a nitrogen atmosphere at 760 Torr. Other chemicals and solvents (such as methanol (MeOH), ethyl acetate (EtOAc), and petroleum ether (PE)) were obtained from commercial vendors and used without further purification.
Synthesis of side chain 8: A solution of peracetyl L-arabinoside (83 mg, 0.26 mmol) and N-Boc-11-amino-1-undecanol (57 mg, 0.20 mmol) in 3.0 mL of DCM at 0 °C was treated with TESOTf (91 μL, 0.40 mmol). The reaction was stirred for 2 h at 0 °C and quenched with triethylamine. The reaction solution was concentrated for column purification on silica gel (eluted with DCM/MeOH 10:1) to afford 8 (70 mg, 79%) as a colorless oil: Rf = 0.5 (DCM/MeOH, 10:1); 1H NMR (400 MHz, CDCl3) δ 5.09 (s, 1 H), 5.03 (s, 1 H), 4.98 (d, J = 3.3 Hz, 1 H), 4.45 (m, 1 H), 4.26−4.23 (m, 2 H), 3.71 (m, 1 h), 3.46 (m, 1 H), 2.95 (s, 2 H), 2.11 (s, 9 H), 1.69−1.58 (m, 4 H), 1.35−1.28 (m, 14 H); 13C NMR (101 MHz, CDCl3) δ 171.2, 170.7, 170.2, 105.9, 81.7, 80.6, 68.0, 63.8, 40.5, 29.9, 29.8, 29.74, 29.69, 29.3, 27.9, 26.7, 26.4, 21.2; IR (neat) 2927, 28.56, 1744, 1679; HRMS (ESI-TOF) m/e [M + H]+ calcd for C22H40NO8 446.2754, found 446.2751.
Synthesis of side chain 9: N-Boc-1,4-butanediamine (4.50g, 24 mmol) and δ-lactone (2.87g, 28.7 mmol) were refluxed in THF for 2 days. The reaction solution was concentrated, and the residue was taken in EtOAc and washed with water. The organic layer was dried over anhydrous Na2SO4, concentrated, and recrystallized in EtOAc/DCM/PE to provide the intermediate 19 (6.5 g, 94%). A solution of peracetyl L-arabinoside (83 mg, 0.26 mmol) and 19 (58 mg, 0.20 mmol) in 3.0 mL of DCM at 0 °C was treated with TESOTf (120 μL, 0.52 mmol). The reaction was stirred for 1.5 h at 0 °C and then 12 h at room temperature before quenched with triethylamine. The reaction solution was concentrated for column purification on silica gel (eluted with DCM/MeOH 9:1) to afford 9 (77 mg, 84%) as a colorless oil: Rf = 0.6 (DCM/MeOH, 9:1); 1H NMR (400 MHz, CDCl3) δ 6.80 (t, J = 5.5 Hz, 1 h), 5.01 (s, 1 H), 5.00 (s, 2 H), 4.43 (m, 1 H), 4.43−4.20 (m, 2 H), 3.73 (m, 1 H), 3,44 (m, 1 H), 3.25 (m, 2 H), 3.01 (s, 2 H), 2.24 (m, 2 H), 2.14 (s, 3 H), 2.12 (s, 3 H), 2.10 (s, 3 H), 1.73−1.60 (m, 8 H); 13C NMR (101 MHz, CDCl3) δ 175.0, 171.2, 170.7, 170.6, 106.0, 82.0, 80.4, 67.5, 63.6, 39.9, 38.9, 36.2, 31.3, 28.9, 26.6, 24.8, 22.9, 21.2, 21.0; IR (neat) 2938, 1733, 1673, 1638, 1547; HRMS (ESI-TOF) m/e [M + H]+ calcd for C20H35N2O9 447.2343, found 447.2339.
Synthesis of 20x
The QA-trisaccharide conjugate 6 (30 mg, 0.01 mmol)21 and the side chain 7 (11 mg, 0.03 mmol) in 1.0 mL of chloroform were treated with HATU (14 mg, 0.04 mmol) and N,N-diisopropylethylamine (11 μL, 0.06 mmol) at room temperature overnight. The reaction mixture was then concentrated and purified directly with column chromatography on silica gel (eluted with PE/EtOAc gradient) to afford the amide 20x (31 mg, 94%) as a white amorphous solid: Rf = 0.6 (PE/EtOAc, 1:1); [α]D23 = −28.8 (c = 1.53, CHCl3); 1H NMR (400 MHz, CDCl3) (characteristic protons) δ 9.68 (s, 1 H), 7.37−7.33 (m, 5 H), 6.11 (t, J = 5.5 Hz, 1 H), 5.44 (d, J = 7.8 Hz, 1 H), 5.28 (m, 1 H), 5.20 (d, J = 3.2 Hz, 1 H), 5.10−4.82 (m, 10 H), 4.79 (dd, J = 7.8, 5.8 Hz, 1 H), 4.58 (d, J = 6.0 Hz, 1 H), 4.55 (d, J = 7.3 Hz, 1 H), 4.52 (d, J = 7.0 Hz, 1 H), 4.48 (s, 1 H), 4.41 (d, J = 7.3 Hz, 1 h), 4.26 (d, J = 7.2 Hz, 1 H), 4.10 (dd, J = 12.0, 4.6 Hz, 1 H), 4.05 (dd, J = 11.9, 4.9 Hz, 1 H), 3.94 (s, 1 H), 3.90 (t, J = 8.1, 1 h), 3.84−3.65 (m, 7 H), 3.65−3.53 (m, 4 H), 3.50 (m, 1 H), 3.40−3.15 (m, 7 H), 3.12 (t, J = 10.0 Hz, 1 H), 2.83 (d, J =11.6 Hz, 1 H), 2.35 (t, J = 7.6 Hz, 2 h), 2.25−2.20 (m, 1 H), 2.15 (s, 3 H), 2.10 (s, 3 H), 2.09−2.00 (m, 20 H), 1.20 (d, J = 6.2 Hz, 3 H), 1.07 (d, J = 6.2 Hz, 3 H), 1.02−0.81 (m, 91 H), 0.79 (s, 3 H), 0.78−0.55 (m, 50 H); 13C NMR (176 MHz, CDCl3) δ 212.2, 175.6, 173.7, 170.4, 170.2, 170.1, 169.9, 169.7, 169.4, 169.2, 169.1, 168.2, 143.4, 136.1, 128.5, 128.4, 128.3, 128.2, 121.6, 108.3, 107.3, 105.8, 104.7, 102.9, 102.4, 101.4, 101.3, 100.7, 100.6, 98.4, 93.9, 79.6, 78.8, 78.7, 77.5, 76.3, 76.2, 76.1, 75.8, 75.7, 75.4, 75.0, 72.6, 72.47, 72.45, 72.0, 71.6, 71.4, 71.36, 70.3, 70.27, 70.0, 69.8, 69.5, 69.2, 68.7, 68.2, 66.1, 65.4, 62.4, 61.5, 60.4, 57.7, 56.0, 53.9, 49.5, 49.3, 46.8, 46.1, 41.7, 40.9, 39.9, 39.3, 38.1, 36.1, 35.1, 34.5, 34.3, 32.8, 32.5, 31.9, 30.7, 30.5, 29.7, 29.6, 29.4, 29.3, 29.2, 29.1, 27.0, 26.4, 25.8, 24.9, 24.3, 23.4, 22.7, 21.0, 20.9, 20.8, 20.7, 20.6, 20.57, 20.53, 20.3, 17.7, 17.5, 15.92, 15.90, 14.1, 12.1, 7.6, 7.5, 7.3, 7.2, 7.1, 7.05, 6.9, 6.8, 5.9, 5.6, 5.4, 5.3, 5.26, 5.23, 5.22, 5.0, 4.4; IR (neat) 2952, 2875, 1750; MS (MALDI) m/e [M + Na]+ (rel intens) calcd for C159H279 NNaO46Si9 3214.7378 (100.0), 3215.7411 (85.4), 3213.7344 (58.1), 3216.7445 (48.4), 3215.7374 (45.7), 3216.7407 (39.0), 3216.7346 (30.1), 3214.7340 (26.6), 3217.7380 (25.7), 3217.7441 (22.1), 3217.7479 (20.4), 3215.7313 (17.5), 3218.7413 (14.6), 3217.7342 (12.2), 3218.7376 (10.5), found 3213.420, 3214.436, 3215.444, 3216.446, 3217.446, 3218.446.
Synthesis of 21x
The QA-trisaccharide conjugate 6 (30 mg, 0.01 mmol) and the side chain 8 (13 mg, 0.03 mmol) in 1.0 mL of chloroform were treated with HATU (11 mg, 0.03 mmol) and N,N-diisopropylethylamine (11 μL, 0.06 mmol) at room temperature overnight. The reaction mixture was then concentrated and purified directly with column chromatography on silica gel (eluted with PE/EtOAc gradient) to afford the amide 21x (27 mg, 82%) as a white amorphous solid: Rf = 0.4 (PE/EtOAc, 1:1); [α]d23 = −36.0 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) (characteristic protons) δ 9.68 (s, 1 H), 6.12 (t, J = 5.5 Hz, 1 H), 5.44 (d, J = 7.8 Hz, 1 H), 5.27 (s, 1 H), 5.2 (d, J = 4.5 Hz, 1 h), 5.1−4.95 (m, 6 H), 4.94−4.83 (m, 4 H), 4.79 (dd, J = 7.7, 6.0 Hz, 1 H), 4.58 (d, J = 5.9 Hz, 1 H), 4.56 (d, J = 7.3 Hz, 1 H), 4.51 (d, J = 7.3 Hz, 1 H), 4.48 (s, 1 H), 4.44 (t, J = 5.8 Hz, 1 H), 4.41 (d, J = 5.7 Hz, 1 H), 4.29−4.21 (m, 3 H), 4.11 (dd, J = 12.2,4.3 Hz, 1 H), 4.05 (dd, J = 11.8, 5.2 Hz, 1 H), 3.93 (d, J = 1.7Hz, 1 h), 3.90 (t, J = 8.0 Hz, 1 H), 3.85−3.20 (m, 25 H), 3.11 (t, J = 11.2 Hz, 1 H), 2.82 (d, J =15.5 Hz, 1 H), 2.24 (t, J =15.8 Hz, 1 h), 2.16 (s, 3 H); 13C NMR (176 MHz, CDCl3) δ 212.2, 175.6, 170.7, 170.4, 170.3, 170.1, 169.9, 169.72, 169.70, 169.4, 169.2, 169.1, 168.2, 143.4, 121.6, 105.5, 102.8, 101.4, 101.3, 100.7, 100.6, 98.4, 93.9, 86.0, 81.3, 80.1, 79.6, 78.75, 78.73, 77.5, 76.3, 76.2, 76.1, 75.8, 75.7, 75.4, 75.0, 72.6, 72.5, 72.4, 72.0, 71.6, 71.39, 71.36, 70.30, 70.27, 69.95, 69.8, 69.5, 69.3, 68.7, 68.2, 67.7, 65.4, 63.4, 62.4, 61.5, 60.4, 58.2, 53.9, 49.5, 49.3, 46.8, 46.1, 41.7, 40.9, 39.9, 39.3, 38.1, 36.1, 35.1, 34.5, 32.8, 32.5, 31.9, 30.6, 30.5, 29.71, 29.66, 29.62, 29.60, 29.58, 29.40, 29.37, 29.32, 27.0, 26.4, 26.0, 25.4, 24.3, 23.4, 22.7, 21.0, 20.9, 20.84, 20.82, 20.78, 20.71, 20.60, 20.57, 20.53, 20.3, 17.7, 17.5, 15.92, 15.90, 14.1, 12.1, 8.0, 7.6, 7.5, 7.4, 7.3, 7.15, 7.12, 7.09, 7.05, 7.0, 6.9, 6.8, 5.9, 5.6, 5.4, 5.3, 5.26, 5.23, 5.22, 4.9, 4.4; IR (neat) 2953, 2876, 1751; MS (MALDI) m/e [M + Na]+ (rel intens) calcd for C163H289 NNaO82Si9 3368.7855 (100.0), 3369.7889 (87.6), 3367.7822 (56.7), 3370.7922 (50.8), 3369.7851 (45.7), 3370.7885 (40.0), 3370.7824 (30.1), 3371.7857 (26.4), 3368.7817 (25.9), 3371.7918 (23.2), 3371.7956 (21.4), 3369.7790 (17.1), 3372.7891 (15.3), 3371.7819 (12.2), 3370.7898 (10.7), 3372.7853 (10.6), found 3367.388, 3368.398, 3369.404, 3370.405, 3371.406.
Synthesis of 22x
The QA-trisaccharide conjugate 6 (61 mg, 0.02 mmol) and the side chain 9 (27 mg, 0.06 mmol) in 1.5 mL of chloroform were treated with HATU (24 mg, 0.06 mmol) and N,N-diisopropylethylamine (10 μL, 0.05 mmol) at room temperature overnight. The reaction mixture was then concentrated and purified directly with column chromatography on silica gel (eluted with PE/EtOAc gradient) to afford the amide 22x (45 mg, 67%) as a white amorphous solid: Rf = 0.2 (PE/EtOAc, 1:2); [α]d23 = −39.0 (c = 0.39, CHCl3); 1H NMR (400 MHz, CDCl3) (characteristic protons) δ 9.66 (s, 1 h), 6.27 (t, J = 5.8 Hz, 1 H), 5.91 (t, J = 5.5 Hz, 1 H), 5.45 (d, J = 7.8 Hz, 1 H), 5.28 (s, 1 H), 5.20 (d, J = 3.3 Hz, 1 h), 5.12−5.09 (m, 2 H), 5.07−5.00 (m, 3 H), 4.90 (m, 1 H), 4.88−4.83 (m, 3 H), 4.78 (t, J = 6.8 Hz, 1 H), 4.59 (d, J = 6.0 Hz, 1 H), 4.56 (d, J = 7.2 Hz, 1 H), 4.51 (d, J = 7.4 Hz, 1 H), 4.48 (s, 1 H), 4.43−4.39 (m, 2 H), 4.31 (d, J = 7.5 Hz, 1 H), 4.26−4.21 (m, 2 H), 4.11 (dd, J = 12.2, 4.5 Hz, 1 H), 4.04 (dd, J =11.8, 5.0 Hz, 1 H), 3.94 (s, 1 H), 3.90 (t, J = 7.9 Hz, 1 H), 3.82−3.71 (m, 9 H), 3.67−3.55 (m, 5 H), 3.51−3.43 (m, 2 H), 3.41−3.18 (m, 12 H), 3.12 (t, J = 10.7 Hz, 1 H), 2.84 (dd, J = 13.9, 3.4 Hz, 1 H), 2.24 (t, J = 13.8 Hz, 1 H), 2.20 (t, J = 7.6 Hz, 1 H), 2.16 (s, 3 H), 2.12−2.09 (m, 13 H), 2.08−2.00 (m, 23 H), 1.36 (s, 3 H), 1.30−1.25 (m, 8 H), 1.19 (d, J = 6.2 Hz, 3 H), 1.07 (d, J = 6.4 Hz, 3 H), 1.00−0.92 (m, 100 H), 0.88 (s, 3 H), 0.79 (s, 3 h), 0.77−0.57 (m, 61 H); 13C NMR (176 MHz, CDCl3) δ 211.5, 175.5, 172.8, 170.6, 170.3, 170.2, 170.0, 169.9, 169.8, 169.6, 169.3, 169.2, 169.0, 168.6, 143.4, 127.8, 121.6, 114.0, 105.7, 102.4, 101.5, 101.4, 100.7, 100.6, 98.4, 93.9, 85.3, 81.5, 80.1, 79.8, 78.8, 78.6, 76.3, 76.2, 75.8, 75.7, 75.5, 72.6, 72.4, 72.0, 71.7, 71.5, 71.4, 70.4, 70.3, 69.9, 69.6, 68.7, 68.2, 67.4, 65.4, 63.3, 62.4, 61.6, 60.5, 56.0, 54.0, 49.4, 49.3, 46.8, 46.3, 41.7, 40.9, 39.9, 39.1, 38.8, 38.1, 36.6, 36.2, 36.1, 35.1, 34.5, 32.8, 32.5, 31.9, 31.0, 30.6, 30.5, 29.7, 29.6, 29.4, 28.8, 28.5, 27.2, 26.6, 26.4, 25.2, 24.7, 24.4, 23.4, 23.3, 22.7, 22.5, 20.91, 20.86, 20.78, 20.76, 20.73, 20.68, 20.66, 20.58, 20.54, 20.5, 20.3, 17.7, 17.5, 15.91, 15.90, 14.09, 11.9, 7.9, 7.5, 7.42, 7.35, 7.22, 7.13, 7.12, 7.09, 6.99, 6.91, 6.82, 6.78, 5.8, 5.7, 5.5, 5.4, 5.31, 5.27, 5.24, 5.08, 5.0, 4.5; IR (neat) 2954, 2914, 2877, 1749, 1372, 1224, 1080; MS (MALDI) m/e [M + Na]+ (rel intens) calcd for C161H284 N2NaO53Si9 3369.7444 (100.0), 3370.7478 (86.5), 3368.7410 (57.4), 3371.7511 (49.5), 3370.7440 (45.7), 3371.7473 (39.5), 3371.7412 (30.1), 3369.7406 (26.2), 3372.7446 (26.1), 3372.7507 (22.6), 3372.7545 (20.6), 3370.7379 (17.3), 3373.7479 (14.9), 3372.7408 (12.2), 3371.7486 (10.9), 3373.7442 (10.5), found 3368.824, 3369.708, 3370.736, 3371.762, 3372.767, 3373.731.
Synthesis of 3x
To the fully protected conjugate 20x (30 mg, 9.4 μmol) in 2.0 mL of THF was added Pd/C (8 mg, 10%). After being shaken under hydrogen (55 psi) for 23 h, the reaction mixture was filtered through a Celite 545 plug and concentrated. The residue in 0.1 mL of DCM was treated with 0.6 mL of TFA/H2O (4:1) at 0 °C for 40 min, and the liquid was then removed under vacuum at 0 °C. The residue was dissolved in 1.2 mL of MeOH with five drops of chloroform and treated with K2CO3 (20 mg) overnight. The reaction solution was centrifuged, and half of the solution was concentrated and purified with RP-HPLC (MeCN/H2O gradient). The product fraction was concentrated on a rotary evaporator at room temperature to remove MeCN, and the remaining water was then removed on a lyophilizer to afford 3x (4.6 mg, 74%) as a white powder: 1H NMR (400 MHz, CD3OD) (characteristic protons) δ 9.35 (s, 1 H), 5.33 (d, J = 1.5 Hz, 1 H), 5.21 (s, 1 H), 5.19 (d, J = 8.3 Hz, 1 H), 4.70 (d, J = 7.6 Hz, 1 H), 4.52 (s, 1 H), 4.47 (d, J = 7.8 Hz, 1 H), 4.44−4.40 (m, 2 H), 4.38 (s, 1 H), 4.33 (d, J = 7.2 Hz, 1 H), 3.85−3.25 (m, 32 H), 3.20−3.05 (m, 7 H), 2.85 (dd, J = 13.9, 3.2 Hz, 1 H), 2.20 (t, J = 13.6 Hz, 1 H), 2.12 (t, J = 7.5 Hz, 1 H), 1.90−1.77 (m, 5 H), 1.72−1.58 (m, 4 H), 1.54−1.47 (m, 3 H), 1.45−1.40 (m, 5 H), 1.29 (s, 3 H), 1.27−1.20 (m 20 H), 1.11 (d, J = 6.4 Hz, 3 h), 1.07 (s, 3 H), 0.90 (s, 3 H), 0.84 (s, 3 H), 0.78 (s, 3 H), 0.65 (s, 3 h); 13C NMR (176 MHz, CD3OD) δ 212.2, 178.0, 171.6, 145.7, 124.0, 107.9, 106.5, 105.8, 105.4, 104.6, 102.1, 95.9, 88.2, 87.8, 87.2, 86.1, 79.1, 78.8, 78.6, 77.85, 77.76, 77.3, 76.3, 76.2, 76.1, 75.9, 75.3, 75.1, 74.5, 74.4, 73.5, 73.2, 72.7, 72.2, 71.8, 71.7, 71.5, 70.3, 69.4, 68.1, 68.0, 67.8, 62.8, 57.1, 50.9, 48.8, 43.7, 43.1, 41.9, 41.0, 40.2, 38.0, 37.5, 34.4, 34.2, 32.2, 31.7, 31.52, 31.47, 31.45, 31.36, 31.1, 28.7, 28.0, 27.8, 26.8, 25.6, 25.3, 22.3, 19.2, 18.5, 17.4, 17.3, 11.9; HRMS (eSI-TOF) m/e [M - H]− calcd for C80H128NO37 1694.8165, found 1694.8182.
Synthesis of 4x
To the fully protected conjugate 21x (15 mg, 4.5 μmol) in 0.1 mL of DCM cooled in an ice-water bath was added 0.5 mL of TFA/H2O (4:1). After being stirred at 0 °C for 40 min, the liquid was removed under vacuum at 0 °C. The residue was dissolved in 1.0 mL of MeOH with five drops of chloroform and treated with K2CO3 (10 mg) overnight. The reaction solution was centrifuged, and the solution was concentrated and purified with RP-HPLC (MeCN/H2O gradient). The product fraction was concentrated on a rotary evaporator at room temperature to remove MeCN, and the remaining water was then removed on a lyophilizer to afford 4x (5.6 mg, 70%) as a white powder: 1H NMR (400 MHz, CD3Od) (characteristic protons) δ 9.51 (s, 1 H), 5.47 (s, 1 H), 5.33 (s, 1 H), 5.32 (d, J = 8.2 Hz, 1 H), 4.83 (d, J = 7.6 Hz, 1 H), 4.60 (d, J = 7.7 Hz, 1 h), 4.554.50 (m, 3 H), 4.46 (d, J = 6.6 Hz, 1 H), 4.00−3.64 (m, 30 H), 3.623.37 (m, 19 H), 2.98 (d, J = 13.8 Hz, 1 H), 2.34 (t, J = 13.6 Hz, 1 H), 2.00−1.91 (m, 7 H), 1.85−1.70 (m, 5 H), 1.68−1.60 (m, 4 H), 1.581.45 (m, 7 H), 1.42−1.31 (m, 32 h), 1.24 (d, J =6.3 Hz, 3 H), 1.21 (s, 3 H), 1.03 (s, 3 H), 0.98 (s, 6 H), 0.91 (s, 3 H), 0.78 (s, 3 H); 13C NMR (176 MHz, CD3Od) δ 209.9, 175.8, 169.3, 143.5, 121.7, 108.0, 105.6, 104.2, 103.6, 103.1, 102.3, 99.8, 93.6, 85.9, 84.9, 83.8, 83.7, 82.2, 77.3, 76.8, 76.6, 76.4, 75.6, 75.5, 75.0, 73.9, 73.8, 73.6, 72.8, 72.2, 71.2, 71.0, 70.4, 69.6, 69.5, 68.0, 67.5, 67.2, 65.8, 65.6, 61.6, 60.6, 56.3, 56.2, 56.1, 55.9, 55.8, 54.8, 41.4, 40.8, 39.6, 38.7, 37.9, 35.7, 35.2, 32.0, 30.8, 30.0, 29.5, 29.4, 29.3, 29.2, 29.1, 28.9, 26.5, 25.9, 25.8, 24.6, 23.3, 23.1, 20.7, 20.1, 16.9, 16.2, 16.1, 16.0, 15.9, 15.8, 15.7, 15.1, 15.0, 9.6; HRMS (ESI-TOF) m/e [M - H]− calcd for C85H138NO40 1812.8795, found 1812.8800.
Synthesis of 5x
To the fully protected conjugate 22x (11 mg, 3.3 μmol) in 0.1 mL of DCM cooled in an ice-water bath was added 0.5 mL of TFA/H2O (4:1). After stirred at 0 °C for 40 min, the liquid was removed under vacuum at 0 °C. The residue was dissolved in 1.0 mL of MeOH with five drops of chloroform and treated with K2CO3 (10 mg) overnight. The reaction solution was centrifuged, and the solution was concentrated and purified with RP-HPLC (MeCN/H2O gradient). The product fraction was concentrated on a rotary evaporator at room temperature to remove MeCN, and the remaining water was then removed on a lyophilizer to afford 3x (3.0 mg, 50%) as a white powder. 1H NMR (400 MHz, CD3Od) (characteristic protons) δ 9.51 (s, 1 H), 5.49 (s, 1 H), 5.36 (s, broad, 1 H), 5.34 (d, J = 8.3 Hz, 1 H), 4.00−3.70 (m, 26 H), 3.70−3.50 (m, 13 H), 3.00 (d, J = 13.8 Hz, 1 H), 2.40−2.23 (m, 3 h), 1.80−0.70 (m, 47 h), 0.99 (s, 3 H), 0.93 (s, 3 H); 13C NMR (176 MHz, CD3OD) δ 210.0, 175.8, 174.7, 169.4, 168.9, 143.4, 121.7, 108.1, 105.6, 104.2, 103.6, 103.1, 102.3, 99.9, 93.6, 85.9, 85.6, 85.0, 83.9, 83.8, 82.2, 77.3, 76.8, 76.5, 76.3, 76.0, 75.5, 75.0, 74.0, 73.9, 73.8, 73.6, 73.0, 72.8, 72.24, 72.15, 71.2, 71.0, 70.4, 70.0, 69.55, 69.47, 69.2, 68.0, 67.1, 67.0, 65.9, 65.8, 65.5, 61.7, 60.5, 56.32, 56.20, 56.07, 55.97, 55.8, 54.9, 48.1, 46.63, 46.56, 41.4, 40.8, 39.6, 38.6, 38.4, 37.9, 35.7, 35.4, 35.2, 32.1, 32.0, 30.9, 30.0, 28.6, 26.2, 25.7, 24.5, 23.3, 23.1, 22.6, 20.1, 16.9, 16.2, 16.1, 16.0, 15.9, 15.8, 15.7, 15.6, 15.1, 15.0, 9.6; HRMS (ESI-TOF) m/e [M + H]+ calcd for C83H135N2O41 1815.8540, found 1815.8558.
Immunological Evaluation of QS-21-Based Immune Adjuvants
Mice and Immunization
BALB/c mice were used in this study and were purchased from Frederick Cancer Research (Fredrick, MD). Mice were maintained within an environmentally controlled, pathogen-free animal facility at the University of Alabama at Birmingham. To assess the adjuvant activity of the QS-21-based immune adjuvant, groups of mice (8–10 weeks of age; six female mice per group) were immunized by a subcutaneous (s.c.) route with rHagB (20 μg) along or with GPI-0100 (100 μg) or with a synthetic adjuvant (100 μg) on days 0, 14, and 28. We used the s.c. route of immunization since we21,36,39,40 and others12–16,20 have used this route in previous studies to assess vaccines and since this route is used to vaccinate humans. Mice were weighed, and blood samples were collected prior to and at various time points following the initial immunization. Blood samples were obtained via the retro-orbital plexus by using heparinized capillary pipettes. The blood samples were centrifuged, and the serum was collected and stored at −20 °C until it was assayed. All studies were done in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All protocols involving animal research were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (Protocol No. IACUC-20222 under Institutional Animal Assurance No. A-3255–01).
ELISA
The levels of specific serum IgG and IgG subclasses against rHagB in each group were determined by ELISA using Maxisorp microtiter plates (NUNC International, Roskilde, Denmark) coated with rHagB (1 μg/mL) or with optimal amounts of goat antimouse IgG, IgG1, or IgG2a (Southern Biotechnology Associates, Inc., Birmingham, AL) in borate buffer saline (BBS; 100 mM NaCl, 50 mM boric acid, 1.2 mM Na2B4O7, pH 8.2) at 4 °C overnight. Plates were washed, and then BBS containing 1% bovine serum albumin (BSA) and 0.02% sodium azide was added to wells for 2 h at room temperature. Serial 2-fold dilutions of serum samples were added in duplicate to the plates. In addition, serial dilutions of a mouse immunoglobulin reference serum (MP Biomedicals, Solon, OH) were added to two rows of wells in each plate that had been coated with the appropriate antimouse IgG or IgG subclass reagent, in order to generate standard curves. After incubation (overnight at 4 °C) and washing of the plates, horseradish peroxidase-conjugated goat antimouse IgG or IgG subclass antibody (Southern Biotechnology Associates, Inc.) was added to the appropriate wells. After 4 h of incubation at room temperature, plates were washed and developed by o-phenylenediamine substrate with hydrogen peroxide. Color development was recorded at 490 nm. The concentrations of antibodies were determined by interpolation on standard curves generated by using the mouse immunoglobulin reference serum and constructed by a computer program based on four-parameter logistic algorithms (Softmax/Molecular Devices Corp., Menlo Park, CA).
Statistical Analysis
Statistical significance in antibody responses and body weights between groups was evaluated by ANOVA and the Tukey multiple-comparisons test using the InStat program (Graph Pad Software, San Diego, CA). Differences were considered significant at a P value < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the NIH (AI099407) and the UAB CAS Interdisciplinary Innovation Team Award. We also thank Dr. Michael J. Jablonsky for assistance with NMR spectroscopy.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.6b00922.
1H and 13C NMR spectra of the new compounds (PDF)
REFERENCES
- (1).Brunner R; Jensen-Jarolim E; Pali-Scholl I Immunol. Lett 2010, 128, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kensil CR; Mo AX; Truneh A Front. Biosci., Landmark Ed 2004, 9, 2972. [DOI] [PubMed] [Google Scholar]
- (3).Leroux-Roels G Vaccine 2010, 28, C25. [DOI] [PubMed] [Google Scholar]
- (4).Sharp FA; Lavelle EC In Development of Therapeutic Agents Handbook, 1 ed.; Gad SC, Ed.; John Wiley & Sons: Hoboken, 2012; p 533. [Google Scholar]
- (5).Wang W World J. Vaccines 2011, 1, 33. [Google Scholar]
- (6).Weeratna RD; McCluskie MJ In Emerging Trends in Antibacterial Discovery: Answering the Call to Arms; Miller AA, Miller PF, Eds.; Caister Academic Press: Great Britain, 2011; p 303. [Google Scholar]
- (7).Klebanoff CA; Acquavella N; Yu Z; Restifo NP Immunol. Rev 2011, 239, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Plotkin SA Nat. Med 2005, 10, S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Rappuoli R; Aderem A Nature 2011, 473, 463. [DOI] [PubMed] [Google Scholar]
- (10).Ragupathi G; Gardner JR; Livingston PO; Gin DY Expert Rev. Vaccines 2011, 10, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kensil CR; Liu G; Anderson C; Storey J In Vaccine Adjuvants: Immunological and Clinical Principles; Hackett CJ, Harn DAJ, Eds.; Humana Press: Totowa, NJ, 2005; p 221. [Google Scholar]
- (12).Marciani D; Press JB; Reynolds RC; Pathak AK; Pathak V; Gundy LE; Farmer JT; Koratich MS; May RD Vaccine 2000, 18, 3141. [DOI] [PubMed] [Google Scholar]
- (13).Marciani DJ; Pathak AK; Reynolds RC; Seitz L; May RD Int. Immunopharmacol 2001, 1, 813. [DOI] [PubMed] [Google Scholar]
- (14).Marciani DJ; Ptak RG; Voss TG; Reynolds RC; Pathak AK; Chamblin TL; Scholl DR; May RD Int. Immunopharmacol 2002, 2, 1703. [DOI] [PubMed] [Google Scholar]
- (15).Marciani DJ; Reynolds RC; Pathak AK; Finley-Woodman K; May RD Vaccine 2003, 21, 3961. [DOI] [PubMed] [Google Scholar]
- (16).Liu G; Anderson C; Scaltreto H; Barbon J; Kensil CR Vaccine 2002, 20, 2808. [DOI] [PubMed] [Google Scholar]
- (17).Adams MM; Damani P; Perl NR; Won A; Hong F; Livingston PO; Ragupathi G; Gin DY J. Am. Chem. Soc 2010, 132, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chea EK; Fernandez-Tejada A; Damani P; Adams MM; Gardner JR; Livingston PO; Ragupathi G; Gin DY J. Am. Chem. Soc 2012, 134, 13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Marciani DJ; Pathak AK; Reynolds RC. Vaccine 2002, 20, 3237. [DOI] [PubMed] [Google Scholar]
- (20).Soltysik S; Wu J-Y; Recchia J; Wheeler DA; Newman MJ; Coughlin RT; Kensil CR Vaccine 1995, 13, 1403. [DOI] [PubMed] [Google Scholar]
- (21).Wang P; Dai Q; Thogaripally P; Zhang P; Michalek SM J. Org. Chem 2013, 78, 11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Deng K; Adams MM; Damani P; Livingston PO; Ragupathi G; Gin DY Angew. Chem., Int. Ed 2008, 47, 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Higuchi R; Tokimitsu Y; Fujioka T; Komori T; Kawasaki T; Oakenful DG Phytochemistry 1986, 26, 229. [Google Scholar]
- (24).Wang P; Haldar P; Wang Y; Hu HJ Org. Chem 2007, 72, 5870. [DOI] [PubMed] [Google Scholar]
- (25).Wang Y; Zhang X; Wang P Org. Biomol. Chem 2010, 8, 4322. [DOI] [PubMed] [Google Scholar]
- (26).Yang H; Wang PJ Org. Chem 2013, 78, 1858. [DOI] [PubMed] [Google Scholar]
- (27).Solleder SC; Zengel D; Wetzel KS; Meier MA R. Angew. Chem, Int. Ed 2016, 55, 1204. [DOI] [PubMed] [Google Scholar]
- (28).Perez EM; Dryden DTF; Leigh DA; Teobaldi G; Zerbetto F J. Am. Chem. Soc 2004, 126, 12210. [DOI] [PubMed] [Google Scholar]
- (29).D’Souza FW; Cheshev PE; Ayers JD; Lowary TL J. Org. Chem 1998, 63, 9037. [Google Scholar]
- (30).MaGee DI; Beck EJ Can. J. Chem 2000, 78, 1060. [Google Scholar]
- (31).Carpino L J. Am. Chem. Soc 1993, 115, 4397. [Google Scholar]
- (32).Wang P; Kim Y-J; Navarro-Villalobos M; Rohde BD; Gin DY J. Am. Chem. Soc 2005, 127, 3256. [DOI] [PubMed] [Google Scholar]
- (33).Pillion DJ; Amsden JA; Kensil CR; Recchia J J. Pharm. Sci 1996, 85, 518. [DOI] [PubMed] [Google Scholar]
- (34).Gaddis DE; Maynard CL; Weaver CT; Michalek SM; Katz J J. Leukocyte Biol 2013, 93, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Gaddis DE; Michalek SM; Katz J Mol. Immunol 2009, 46, 2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Gaddis DE; Michalek SM; Katz J J. Immunol 2011, 186, 5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yang Q-B; Martin M; Michalek SM; Katz J Infect. Immun 2002, 70, 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zhang P; Martin M; Michalek SM; Katz J Infect. Immun 2005, 73, 3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhang P; Martin M; Yang Q-B; Michalek SM; Katz J Infect. Immun 2004, 72, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhang P; Yang Q-B; Marciani DJ; Martin M; Clements JD; Michalek SM; Katz J Vaccine 2003, 21, 4459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.