Abstract
Disease is a leading cause of diminished welfare and productivity in pig systems, but its spread among pigs within commercial herds can be limited through early detection. Identifying specific behavioral changes at the onset of disease can have a substantial diagnostic value by improving treatment success through timely intervention. Our study aimed to identify key behaviors that visibly change at the group level when only a few individuals are acutely sick. First, we quantified the behavioral changes seen during an acute health challenge in groups of pigs, using total pen vaccination as an artificial sickness model. Then we investigated the minimum proportion of sick pigs needed to detect group level behavioral changes using three treatments: a control (Con; 0% pigs), low (±20% pigs), or a high (±50% pigs) number of pigs vaccinated in the pens. Total pen vaccination in Trial 1 produced group level behavioral changes, including reduced feeding (P < 0.001), non-nutritive visits to the feeder (P < 0.01), drinking (P < 0.001), standing (P < 0.001), and interaction with pen enrichment (P < 0.001), accompanied by increased lying rates (P < 0.01) and elevated body temperatures (P < 0.001), confirming that vaccination is an appropriate model to study effects of acute sickness. In Trial 2, group level declines in interaction with the enrichment device (P < 0.001) and standing rates (P = 0.064), along with an increase in pen lying rates (P < 0.001), were apparent in the Low treatment when compared to the Con rates, which suggests these key behaviors could serve an important diagnostic value for early disease detection in groups. These changes lasted for up to 3 h post vaccination. In contrast, feeding rates (treatment × time of day: P < 0.01) only showed a decrease from the Con in the High treatment after vaccination, with pen drinking showing a similar trend (treatment: P = 0.07), suggesting that these behaviors would be more appropriate for confirming the spread of disease within a herd. Identifying key behaviors that alert to the presence of disease is critical to further refine automated early warning systems using pen level sensors for commercial pig operations.
Keywords: disease detection, early warning system, pig health, subclinical disease, sickness behavior, swine
INTRODUCTION
Subclinical and clinical disease is mainly responsible for reduced productivity on commercial pig farms and has significant welfare implications (Pritchard et al., 2005). For livestock species, the behavioral indicators of sickness are often initially subtle, perhaps as a hard wired strategy to hide signs of vulnerability, which makes detection more difficult (Millman, 2007). These subtle changes (Matthews et al., 2016; Fernández-Carrión et al., 2017) may be the most important in terms of early detection of health and welfare compromises. To facilitate more sustainable pig production, early detection of disease is essential to prevent major losses (Fernández-Carrión et al., 2017; Maselyne et al., 2018). Once one animal in a group becomes infected, the remaining individuals in the group are highly susceptible to acquiring the disease. Close spatial proximity to the sick animal heightens pathogen spread in group-housed animals through direct skin-to-skin contact, respiration, and environmental contamination with infected pig waste (Ribbens et al., 2004). Therefore, it is critical to identify, treat, and separate sick individuals before the appearance of clinical symptoms to prevent spread of the infectious disease to the remaining herd.
Detection of disease is traditionally done by direct observation of the animals by staff during routine checks. However, on a commercial scale, direct observation at the individual level is impractical and observations are intermittent, meaning only substantial changes in behavior (e.g., when the animal is unable to stand) are possible to detect (Millman, 2007; Weary et al., 2009). Moving forward, it is critical to identify key changes in behavior that occur at the onset of disease and that can be detected at the group level. Considerable advancements in automated detection of behavior for longitudinal on-farm health and welfare monitoring are being developed, focusing on a group level approach. These early warning systems use video cameras to monitor for changes in group behavior, such as overall movement (Fernández-Carrión et al., 2017; Martínez-Avilés et al., 2017; Süli et al., 2017) or specific behavioral patterns (e.g., feeding and standing; Matthews et al., 2017). In this study, we aimed to determine key behaviors that can be identified at the group level, which offer a diagnostic value for early detection of acute sickness in growing pigs. Our first objective was to evaluate an artificial model of behavioral disturbance in groups of pigs, akin to what would be seen during an acute health challenge. Key behavioral changes that can be used to detect this change at the pen level could then be identified. Our second objective was to quantify the minimum proportion of individuals required to detect these behavioral changes at the group level. We expect that key behaviors (e.g., standing, feeding, drinking rates, and interaction with environmental enrichment) will exhibit group level changes when only a few individuals are ill, suggesting monitoring efforts should focus on these behaviors for early disease detection. In this paper, we quantified group level behavior through direct observation of pigs in a commercial setting, but our aspiration is that these changes will be monitored automatically in the future through advances in technology. Freedom from disease is a critical priority for maintaining a high level of health and welfare on modern commercial pig farms (Farm Animal Welfare Council, 1993), so determining the quantitative behavioral changes from illness will help to refine automated systems to improve early disease detection and allow infected pens to be treated promptly.
MATERIALS AND METHODS
All procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986, Euenrichmentan Directive EU 2010/63, and with the approval of the Newcastle University Animal Welfare and Ethical Review Body.
Animals
Seventy-six pigs (Landrace/Large White × synthetic sire line, Hermitage Seaborough Ltd., North Tawton, UK) approximately 9–10 wk of age from the resident herd at Cockle Park Farm (Newcastle University, UK) were used. The study consisted of two separate trials, with 35 pigs in two pens used in Trial 1 (17 to 18 pigs/pen, start weight 19.6 ± 5.6 kg) and 61 pigs in three pens used in Trial 2 (20 to 21 pigs/pen, start weight 29.7 ± 10.1 kg). Before allocation to treatments, pigs were managed according to routine farm husbandry. In both trials, pigs were weighed before allocation into groups using a randomized block design, so that the mean weight and SD were as similar as possible at the start of each trial. For both trials, each group was allocated at random to a single fully slatted pen (4 m × 2.4 m), where they remained for the duration of the study. A 7 d acclimation period was given to the animals before commencing the study. Food and water were provided ad libitum for the duration of the study via four feeding troughs and four drinking nipples per pen. A hanging chain, covered in plastic pipe, was provided in each pen as enrichment akin to standard commercial conditions. The ambient temperature ranged from 17.0 to 24.3 °C (mean: 20.1 °C) with a mean relative humidity of 50% (range: 43% to 61%). All pigs were individually identifiable by a numbered ear tag.
Experimental Design
In the first trial, the behavior of all pigs within the pens was temporarily disrupted via the deep intramuscular injection of 2 mL Porcilis Glasser vaccine behind one ear (Intervet UK Ltd., Milton Keyes, UK). This was carried out across 2 d with each pig acting as their own control, receiving the vaccine on 1 d and a control dose of saline on the other day. On d 1 at 8:50, one person entered the pig room and removed all pigs from pen 1 (n = 17). Each individual pig was then vaccinated by the same trained member of staff and immediately returned to the home pen (total Vacc treatment). At 9:00, all pigs were removed from pen 2 (n = 18) and administered an equivalent volume of saline and immediately returned to their home pen (Con treatment). This process was reversed on d 2 so all animals received one vaccine dose and all animals acted as their own controls. On the first vaccination day, the rectal temperatures of 10 randomly selected pigs in each of the two pens were measured at 13:00 (Maximum thermometer, TFA Dostmann GmbH & Co. KG, Wertheim-Reicholzheim, Germany) to confirm the vaccinated pigs were experiencing a febrile response from the challenge. Only a subset of pigs had their temperatures recorded to limit the amount of disruption within the pen.
In the second trial, the behavior of a subset of the pigs in a given pen was temporarily disrupted using an identical injection of the Porcilis Glasser vaccine as in Trial 1. Each pen had three test days: control (Con), low subset (Low), and high subset (High) vaccination, with a Con day immediately before both the Low and High treatment days. The treatment order was the same for all pens (i.e., Con, Low, Con, and High). For the Con treatment, no pigs in the pen received an injection of the vaccine or saline. For the Low vaccination treatment, a mean of 20% of pigs in each pen was vaccinated. For the High treatment, a mean of 50% of pigs within a given pen were vaccinated. As in Trial 1, vaccinations were administered by the same staff member at approx. 10:00 on each vaccination day. After vaccination, the pigs were left undisturbed for 3 h and then were again checked to ensure there were no adverse reactions. No animal received more than 1 vaccination.
All pigs recovered from the vaccine as expected, with no adverse events observed. In Trial 2, one pig was removed, before vaccination, due to lameness and was treated accordingly by the named veterinary surgeon. After completion of the study, all the other pigs were weighed, checked by the veterinarian, and released back into the commercial stock.
Equipment Setup
For both trials, two cameras (Microsoft Kinect for Xbox One, Microsoft, Redmond, Washington) were used to capture the entire floor area of each pen. The cameras were housed in ingress-protected enclosures and attached to the ceiling of each pen at a perpendicular angle to the floor. Videos for behavioral annotation were produced from the camera color stream and were encoded at 30 frames/s and split and stored as five (Trial 1) or 10 min video files (Trial 2). Data capture was synchronized across all cameras by time with Network Time Protocol.
Behavioral Observations
Manual behavioral observations were carried out by four trained, treatment blinded observers retrospectively using ELAN software (version 4.9.2, Max Planck Institute for Psycholinguistics, Nijmegen, The Netherlands) based on the ethogram in Table 1. For Trial 1, behavioral observations were completed for one control day and one vaccination day per pen. For Trial 2, behavioral observations were completed for the two vaccination days (one Low and one High day) and two control days per pen. The frequency and duration of time spent performing each behavior were recorded at the pen level, either continuously from 8:00 to 13:00 (Trial 1) or for 10 min periods every 20 min from 9:00 to 14:00 (Trial 2) for all study days. The behaviors selected for observation were those that could be scored at the group level and have been shown to be affected by an acute health disease challenge (Krsnik et al., 1999; Escobar et al., 2007; Ahmed et al., 2015).
Table 1.
The six pig behaviours manually annotated for both trials using Elan version 5.2 software
| Behaviour | Description |
|---|---|
| Standing | Pig only has feet (and possibly snout) in contact with pen floor |
| Lying | Trunk of the pig is in contact with the floor |
| Feeding | Pig has head inside a food trough |
| Drinking | The pig’s snout is in contact with a nipple drinker |
| Non-nutritive visit (NNV) | Pig enters the black mat of the feeding area with two or more feet (one must be a front foot), then leaves the area without putting head in food trough |
| Enrichment interaction | Pig uses its head to bite, nose, or knock the plastic pipe and chain suspended from the ceiling |
Statistical Analysis
To ensure consistent behavioral scoring, the four observers underwent thorough training and rescored three of the same 90 s video files before completing the video analysis. Kendall’s coefficient of concordance demonstrated high inter-observer reliability in the recording of both the durations (video file 1: W = 0.97, χ2 = 19.43, P = 0.002; video file 2: W = 0.95, χ2 = 19.00, P = 0.002; video file 3: W = 1.00, χ2 = 20.00, P = 0.001) and frequencies (video file 1: W = 0.97, χ2 = 19.32, P = 0.002; video file 2: W = 0.92, χ2 = 18.30, P = 0.003; video file 3: W = 1.00, χ2 = 20.00, P = 0.001) for all six behaviors in both trials.
For Trials 1 and 2, 210 and 166 observation files were included in the final statistical analyses. Observations at 9:00 and 10:00 in the second trial were removed from the analysis as ≥50% files were missing or incomplete due to disturbance from daily husbandry activities or the inability to synchronize the timing of the two videos covering each pen. Before analysis, the observation files for both trials were rounded down to the near whole min to account for several video files being <5 (Trial 01) or <10 min in length (Trial 2). For both trials, the observational data for each behavior were expressed as a pen level rate (s/min) for each time point, which were calculated as the duration of time pigs spent engaged in the specific behavior (s) multiplied by the frequency of pigs performing that behavior at that particular time point over the rounded observation file length (min). Consequently, the pen level durations spent performing each behavior (s) could exceed real-time values to account for >1 pig engaged in a behavior at a particular time (e.g., three pigs standing for 20 s would be a 60 s standing duration at the group level). To evaluate the impact of time of day on the different behavioral rates, the observations in each trial were grouped together into five time periods and presented as the number of hours (h) pre- or postvaccination [Trial 1: −2 h (8:00 to 8:55), −1 h (9:00 to 9:55), 0 h (10:00 to 10:55), +1 h (11:00 to 11:55), and +2 h (12:00 to 12:55); Trial 2: −1 h (9:20 to 9:50), 0 h (10:20 to 11:10), +1 h (11:20 to 12:10), +2 h (12:20 to 13:10), and +3 h (13:20 to 14:10)]. For Trial 2, the behavioral rates for each pen were averaged over the two control days.
The rectal temperature measurements from Trial 1 were analyzed in SPSS (IBM SPSS Statistics Version 24, Armonk, NY) using an independent t-test with treatment type as the grouping factor. For both trials, the pen level rates for each of the six untransformed behaviors were evaluated separately using a generalized linear mixed model procedure (Proc Glimmix) in SAS 9.4 (SAS Institute Inc., Cary, NC). The fixed effects in each model were treatment type (Trial 1: Con or Total Vacc; Trial 2: Con, Low, or High vaccination treatment), time of day of the observation, and the interaction between treatment type × time of day. The experimental unit for all the analyses was pen and the random effects accounted for repeated measures of each pen over the different trial days and multiple observations within each trial day. The repeated covariance type (i.e., variance components, banded main diagonal, or heterogeneous compound symmetry) for each behavioral model was chosen separately for each behavior based on the smallest Akaike’s information criterion value. The denominator degrees of freedom in each model were calculated using a Satterthwaite approximation. When a fixed independent variable showed a significant effect, pairwise comparisons for that fixed effect were completed post hoc with Tukey Honestly Significant Difference confidence interval adjustments. The results from both trials are reported as least square means ± SEM. A significant effect was detected when P < 0.05, whereas a trend was considered a P-value between 0.05 and 0.10.
RESULTS
Trial 1 – Total Pen Vaccination
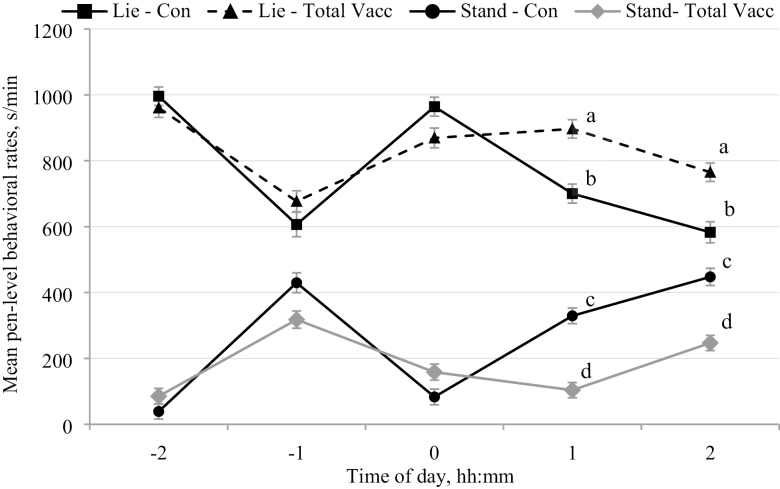
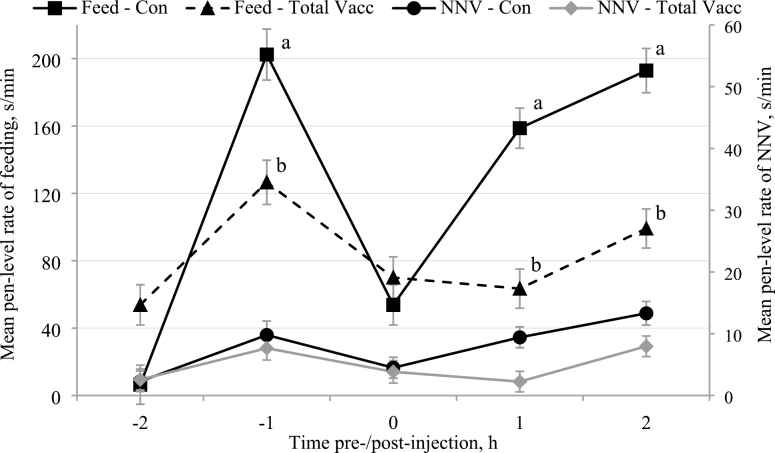
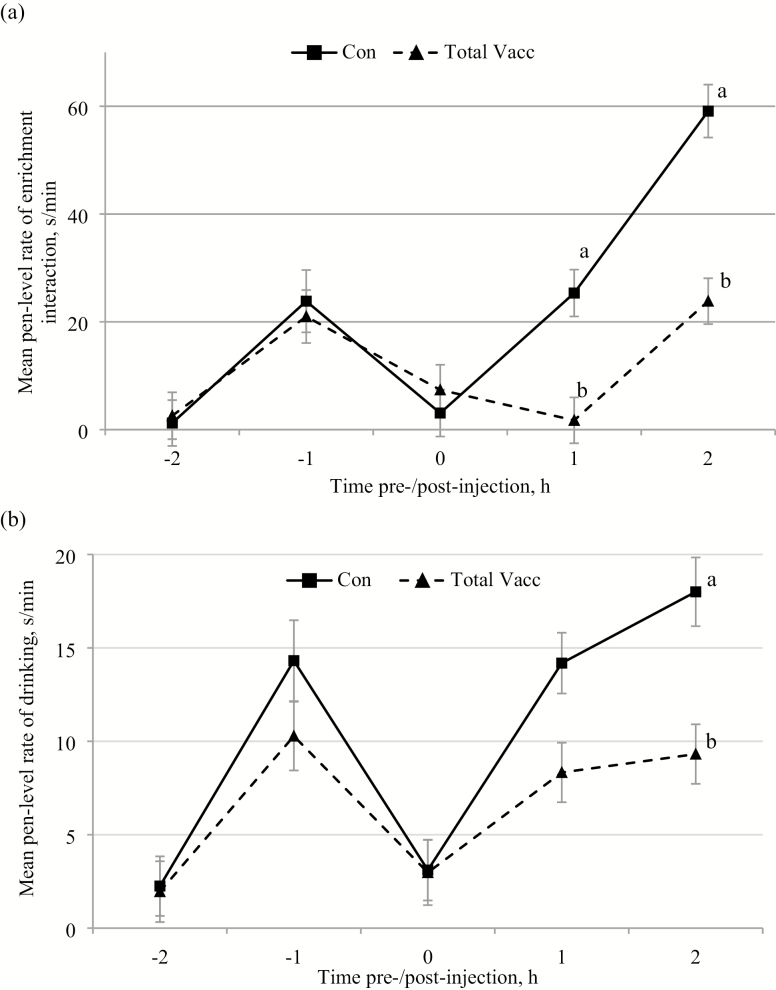
The rectal temperatures of the vaccinated pigs (total Vacc) were significantly greater (41.1 ± 0.2 °C; mean ± SEM) than the saline-injected control pigs (Con; 39.9 ± 0.1 °C; P < 0.001). Total pen vaccination also had a significant effect on all of the measured behaviors (Table 2) resulting in greater pen level lying rates (Figure 1), but lower rates of standing (Figure 1), feeding (Figure 2), non-nutritive visits (NNV; Figure 2), enrichment interaction (Figure 3a), and drinking (Figure 3b). Time of day had a significant effect on all of the behaviors with the greatest rates of lying, but the lowest rates of standing (Figure 1), feeding, NNV (Fig. 2), drinking, and enrichment interaction (Figure 3), at the start of recording (−2 h) and during vaccination (0 h) than any other time period (Table 3).
Table 2.
The mean pen level behavioral rates (±SEM) for the control days (Con) and total pen vaccination days (total Vacc) during Trial 1 taken from the full Glimmix models
| Behaviour | Treatment | |
|---|---|---|
| Con | Total Vacc | |
| Lying | 770.03 ± 13.93a | 833.75 ± 13.16b |
| Standing | 265.80 ± 11.41a | 182.51 ± 10.79b |
| Feeding | 122.84 ± 5.74a | 82.60 ± 5.43b |
| Non-nutritive visits (NNV) | 7.86 ± 0.83a | 4.86 ± 0.79b |
| Drinking | 10.37 ± 0.80a | 6.57 ± 0.75b |
| Enrichment interaction | 22.52 ± 2.13a | 11.30 ± 2.01b |
a,bWithin a row, least square means with different superscripts differ by P < 0.05.
Figure 1.
The mean (±SEM) pen level hourly rates of lying (Lie) and standing (Stand) before and after injections (time of day of injections: 0 h) during the saline control days (Con) and the days of total vaccination of all pigs within the pens (total Vacc). Effect of treatment – lying: P < 0.002, standing: P < 0.001; time of day – lying: P < 0.001, standing: P < 0.001; and treatment × time of day – lying: P < 0.001; standing: P < 0.001. Differences of P < 0.05 in the least square mean rates of that specific behavior between the Con and total Vacc treatments at the same time points are marked with different superscripts.
Figure 2.
The mean (±SEM) pen level hourly rates of feeding (feed) and non-nutritive visits (NNV) before and after injections (time of day of injections: 0 h) during the saline control days (Con) and the days of total vaccination of all pigs within the pens (total Vacc). Effect of treatment – feeding: P < 0.001, NNV: P < 0.010; time of day – feeding: P < 0.001, NNV: P < 0.001; and treatment × time of day – feeding: P < 0.001; NNV: P = 0.150. Differences of P < 0.05 in the least square mean feeding rates between the Con and total Vacc treatments at the same time points are marked with different superscripts.
Figure 3.
The mean (±SEM) pen level hourly rates of (a) enrichment interaction and (b) drinking before and after injections (time of day of injections: 0 h) during the saline control days (Con) and the days of total vaccination of all pigs within the pens (total Vacc). Effect of treatment – enrichment: P < 0.001, drinking: P < 0.001; time of day – enrichment: P < 0.001, drinking: P < 0.001; and treatment × time of day – enrichment: P < 0.001; drinking: P = 0.052. Differences of P < 0.05 in the least square mean rates of that specific behavior between the Con and total Vacc treatments at the same time points are marked with different superscripts.
Table 3.
The mean pen level behavioural rates (±SEM) for each hourly time period before or after injections (time of day of injections: 0 h) during Trial 1 from the full Glimmix models
| Behaviour | Time pre-/post-injection, h | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |
| Lying | 978.21 ± 20.09a | 641.85 ± 24.53b | 916.76 ± 20.81a | 798.60 ± 20.09c | 674.01 ± 21.27b |
| Standing | 62.18 ± 16.57a | 373.83 ± 19.96b | 120.87 ± 17.04a | 216.60 ± 16.57c | 347.30 ± 17.44b |
| Feeding | 30.11 ± 8.34a | 164.48 ± 10.03b | 61.92 ± 8.57a | 111.08 ± 8.34c | 146.00 ± 8.77b |
| Non-nutritive visits (NNV) | 2.44 ± 1.19a | 8.73 ± 1.48bc | 4.17 ± 1.25ab | 5.83 ± 1.19ab | 10.64 ± 1.27c |
| Drinking | 2.10 ± 1.14a | 12.30 ± 1.42b | 3.04 ± 1.19a | 11.26 ± 1.14b | 13.66 ± 1.22b |
| Enrichment interaction | 1.90 ± 3.04a | 22.41 ± 3.80b | 5.22 ± 3.19a | 13.54 ± 3.04ab | 41.47 ± 3.25c |
a–cWithin a row, least square means with different combinations of superscripts differ by P < 0.05.
There were significant interactions between treatment × time of day for all measured behaviors except NNV. Group level rates of standing (Figure 1), feeding (Figure 2), and enrichment interaction (Figure 3a) decreased significantly +1 h and +2 h postvaccination for total Vacc compared with those time points for Con. In addition, the average time spent feeding was also greater −1 h prevaccination for total Vacc than at that same time during the Con (P = 0.008; Figure 2). Mean pen lying rates were greater +1 h (P < 0.001) and +2 h after vaccination (P < 0.002) for total Vacc than at those time points during the Con treatment (Figure 1). However, drinking rates were only significantly lower +2 h postvaccination for total Vacc when compared with the same time of day for the Con treatment (P = 0.016; Figure 3b).
Trial 2: Proportional Pen Vaccination
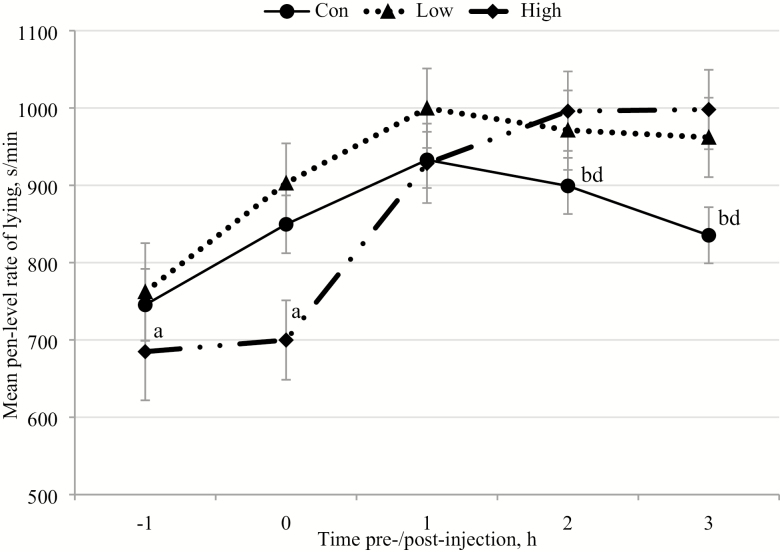
Vaccination treatment affected or tended to affect the rates of pen level enrichment interaction (P = 0.001), drinking (P = 0.077), lying (Figure 4), and standing (P = 0.087) in Trial 2 (Table 4). Specifically, the group level rates of enrichment interaction, standing, and lying differed between the Con and Low treatments with lower average rates of enrichment interaction (P < 0.001) and standing (P = 0.036), but greater lying rates during the Low treatment (P = 0.030; Table 4). The pen level rates of enrichment interaction (P = 0.007) and drinking (P = 0.028) were lower for the High than Con treatments, but none of the other behavioral rates varied between these two treatments (Table 4). There were no differences between the Low and High treatments in the rates of any of the measured behaviors. Additionally, the rates of feeding and NNV showed no variation among any of the treatment groups in this trial (Table 4).
Figure 4.
The mean (±SEM) hourly pen level rates of lying before and after injections (time of day of injections: 0 h) on the days of the control (Con), low subset vaccination (Low), and high subset vaccination treatments (High). Effect of treatment: P = 0.07, time of day: P < 0.0001, and treatment × time of day: P = 0.05. Differences of P < 0.05 in the least square mean lying rates between time points in High treatment are marked with different superscripts.
Table 4.
The mean pen level behavioural rates (±SEM) on the days of the control (Con), low subset vaccination (Low), and high subset vaccination treatments (High) during Trial 2 taken from the full Glimmix models
| Behavior | Treatment | ||
|---|---|---|---|
| Con | Low | High | |
| Lying | 852.46 ± 17.33a | 919.60 ± 24.10b | 861.38 ± 24.10ab |
| Standing | 327.35 ± 13.98a | 276.91 ± 19.42b | 293.79 ± 19.42ab |
| Feeding | 153.74 ± 7.12 | 149.72 ± 9.90 | 156.31 ± 9.90 |
| Non-nutritive visits (NNV) | 13.31 ± 1.52 | 11.71 ± 1.87 | 15.90 ± 2.42 |
| Drinking | 17.69 ± 1.38a | 16.42 ± 1.40ab | 13.53 ± 1.27b |
| Enrichment interaction | 26.53 ± 3.35a | 10.92 ± 2.35b | 14.77 ± 2.63b |
a,bWithin a row, least square means with different superscripts differ by P < 0.05.
Time of the day had a significant effect with feeding (Figure 5), NNV (P < 0.001), and standing (P < 0.001) being significantly greater at the start of the recording (−1 h pre-injection) than during any other time point (Table 5). The enrichment interaction rates for these pens were only significantly greater at the start of the day (−1 h) than +1 h postinjection (P < 0.002; Table 5). Mean lying rates were also significantly greater in the hours post-injection. The mean drinking rates for these pens showed no variation with time of day (P = 0.077; Table 5).
Figure 5.
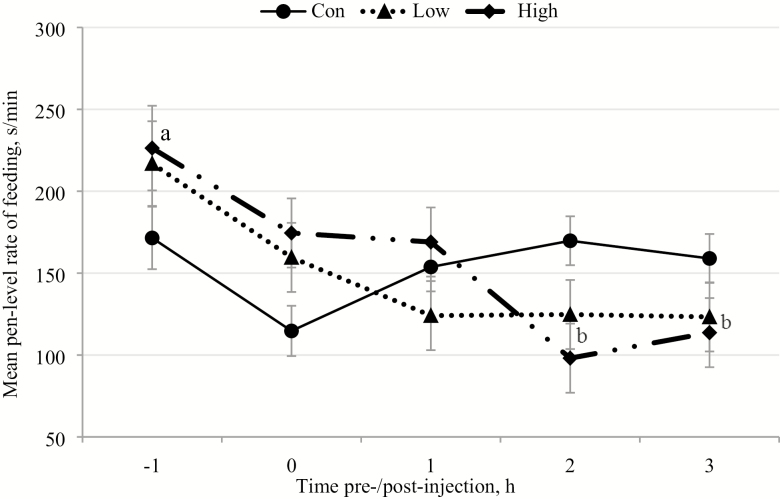
The mean (± SEM) hourly pen level rates of feeding before and after injections (time of day of injections: 0 h) on the days of the control (Con), low subset vaccination (Low), and high subset vaccination treatments (High). Effect of treatment: P = 0.89, time of day: P = 0.0004, and treatment × time of day: P = 0.0037. Differences of P < 0.05 in the least square mean feeding rates between time points in High treatment are marked with different superscripts.
Table 5.
The mean pen level behavioural rates (±SEM) for each hourly time period before or after injections (time of day of injections: 0 h) during Trial 2 taken from the full Glimmix models
| Behaviour | Time pre-/post-injection, h | ||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | |
| Lying | 730.40 ± 33.46a | 817.40 ± 27.23a | 953.65 ± 27.07b | 955.46 ± 27.07b | 931.76 ± 27.07b |
| Standing | 429.79 ± 26.97a | 328.16 ± 21.95b | 241.68 ± 21.82c | 238.20 ± 21.82c | 258.92 ± 21.82b |
| Feeding | 204.90 ± 13.74a | 149.60 ± 11.18b | 148.95 ± 11.12b | 130.86 ± 11.12b | 131.97 ± 11.12b |
| Non-nutritive visits (NNV) | 27.78 ± 2.97a | 14.84 ± 2.43b | 6.69 ± 2.42b | 8.87 ± 2.42b | 10.04 ± 2.42b |
| Drinking | 17.03 ± 2.05 | 15.98 ± 1.66 | 13.62 ± 1.65 | 16.56 ± 1.65 | 16.19 ± 1.65 |
| Enrichment interaction | 25.17 ± 4.31a | 20.27 ± 3.48ab | 8.92 ± 3.42b | 15.30 ± 3.42ab | 17.34 ± 3.42ab |
a,bleast square means with different superscripts differ by P < 0.05.
There were significant interactions of treatment × time of day for the pen level rates of lying (Figure 4) and feeding (Figure 5) in the High treatment, which were not seen for any of the other behavioral rates. Furthermore, for any of the behaviors, there were no interactions between treatment × time of day within the Con or Low treatments, or between the three treatment groups. During the High treatment, the mean lying rates were significantly lower −1 h before injection than +2 h (P < 0.001) and +3 h post-injection (P < 0.001). Similarly, the pen level lying rates for these pens were also lower at the time of injection (0 h) than +2 h (P < 0.001) and +3 h after injection (P < 0.001; Figure 4). The pen level feeding rates were also significantly greater −1 h pre-injection than both +2 h (P < 0.001) and +3 h post-injection (P < 0.001) for the High treatment (Figure 5).
DISCUSSION
The objectives of this research were first to create an acute sickness challenge in growing pigs through total pen vaccination, and then identify which key behaviors would show immediate changes that could be detected at the group level when only a subset of animals were ill. Trial 1 demonstrated that pen level changes in all the included behavioral parameters (i.e., decreased standing, enrichment interaction, drinking, feeding, and NNV, but increased rates of lying) can be detected when the entire group of pigs is ill, whereas the results from Trial 2 support our hypothesis that group level changes in key behaviors (i.e., decreased standing and enrichment interaction with increased time spent lying) are apparent when only a few individuals are acutely sick in a pen.
In Trial 1, an acute health challenge was created in these pigs through controlled exposure to an inactivated pathogen via vaccination. After vaccination, we expected to see changes in key behaviors at the pen level if all pigs were experiencing acute sickness, which is a nonspecific immune response (Hart, 1988; Weary et al., 2009; Szyszka et al., 2012). Exposure of pigs to infectious agents has been shown to cause short-term behavioral and physiological changes, characteristic of acute sickness, including anorexia, adipsia, reduced activity, increased lying, decreased social interaction, and elevated body temperatures (Krsnik et al., 1999; Escobar et al., 2007; Ahmed et al., 2015). Furthermore, intramuscular vaccinations in pigs are known to produce behavioral changes akin to illness for up to 6 h after injection (Fangman et al., 2011; Cook et al., 2015, 2018). In this trial, the vaccination procedure mimicked infection by producing significant behavioral and physiological changes (i.e., elevated body temperatures) that were observable at the group level. Our findings suggest that vaccination of pigs acts as a successful challenge model by producing the expected group level behavioral changes during the onset of disease.
It is well established that pigs decrease their overall activity when ill, which was observed during total pen vaccination in Trial 1. Reduced activity serves to conserve energy by engaging an immune response and protects sick animals by limiting their exposure to predators (Hart, 1988). Pigs are known to spend a large proportion of their daily time budgets lying when healthy (Costa et al., 2009; Maselyne et al., 2014), but the additional postural shift from standing to lying during illness allows for greater conservation of the heat and energy needed to fight infection (Hart, 1988; Escobar et al., 2007; Reiner et al., 2009). The feeding and drinking activity of group-housed pigs has also been well demonstrated to decrease during an acute health challenge (Krsnik et al., 1999; Escobar et al., 2007; Reiner et al., 2009; Brown-Brandl et al., 2013). The severity of the decline in feeding and drinking is closely linked (Ahmed et al., 2015), and the extent of the feeding reduction can vary based on several factors, including pathogen type (Kyriazakis and Houdijk, 2007; González et al., 2008; Rostagno et al., 2011).
The significant decline in NNVs and enrichment interaction during total pen vaccination also likely reflects an adaption to preserve energy by reducing low-resilience behaviors not critical for short-term survival (Littin et al., 2008; Weary et al., 2009; Deen, 2010). Animals are thought to perform NNVs to learn where food is available in their environment, so when an animal’s health is compromised they will shift their resources from engaging in these exploratory behaviors (Kyriazakis et al., 1998; Svensson and Jensen, 2007; Weary et al., 2009). Healthy indoor-housed pigs have been shown to spend up 10% of their active time interacting with a suspended enrichment or chain enrichment (Van de Weerd et al., 2003; Trickett et al., 2009). Additionally, Docking et al., (2008) showed pigs exhibit a degree of synchronisation in enrichment-directed behavior with group members, which could be partially attributed to social facilitation. So groups of pigs might also reduce their enrichment interaction during a disease outbreak due to reduced interest and/or less interest in the enrichment device amongst pen mates.
In Trial 2, when only a proportion of pigs in a pen were experiencing acute illness, the onset and degree of the pen level behavioral changes differed from the sickness-induced changes seen during the total pen vaccination. When only some pigs in a pen are acutely sick from vaccination, the changes in key behaviors might still be apparent in overall pen behavior, but this depends on the proportion of pigs affected. The behaviors that serve a diagnostic value for early disease detection are those that exhibit group level changes when only a small proportion of the pigs are affected (i.e., the Low treatment) and before clinical signs are apparent (Fernández-Carrión et al., 2017; Martínez-Avilés et al., 2017; Süli et al., 2017). However, specific behavioral changes that are only apparent during the High treatment, when a larger percentage of the pigs in a pen are sick, would be less important for the initial timely diagnosis of illness. In Trial 2, the decrease in pen level standing, while lying rates increased, was only apparent when comparing the Con and Low vaccination treatments (Fernández-Carrión et al., 2017; Martínez-Avilés et al., 2017; Süli et al., 2017). The pen level shift from standing to inactive lying when only a few of pigs were ill has been previously validated as a sensitive behavioral indicator for early disease detection in pigs (Fernández-Carrión et al., 2017; Martínez-Avilés et al., 2017; Süli et al., 2017).
Once subclinical behavioral changes are apparent during illness, behavioral rates are expected to exhibit either a completely linear relationship as more animals are infected or to initially change linearly then remain constant once a certain threshold of animals are sick within the group (Szyszka et al., 2013). In this trial, the overall rates of enrichment interaction at the group level decreased significantly from the Con to both the Low and High treatments (Littin et al., 2008; Deen, 2010), which suggests the group level decline in the enrichment rate levels off once only a percentage of pigs are infected (Szyszka et al., 2013). The decrease in enrichment interaction in the Low treatment demonstrates the value of monitoring for reductions in exploratory behaviors for accelerated disease diagnosis. Littin et al. (2008) showed mice with transgenic Huntington disease had a steep decline in enrichment use before the appearance of clinical symptoms. Low-resilience behaviors, such as enrichment use, play and grooming, would be expected to decrease before behaviors critical to survival (i.e., feeding and drinking) as an effort to conserve energy resources (Littin et al., 2008; Weary et al., 2009; Mandel et al., 2017). The results of the proportional vaccination trial suggest that close monitoring for group variation in lying, standing, and enrichment interaction is particularly valuable for identifying the onset of disease outbreaks in pigs.
In contrast, the drinking rates in Trial 2 only showed a tendency to decline between the Con and High treatments, suggesting that reduced drinking from illness only becomes apparent at the pen level when more than 40% of the pens were acutely sick (Szyszka et al., 2013). Given the close temporal relationship of feed and water consumption in pigs, we would expect the decrease in drinking rates to occur concomitantly with the decline in feeding in this trial (Krsnik et al., 1999; Reiner et al., 2009; Ahmed et al., 2015), which would explain why both of these behavioral decreases were first detectable in the same treatment group (i.e., High). Furthermore, Martínez-Avilés et al. (2017) proposed reduced movement during the onset of illness would lead to less feeding and drinking behavior, which suggest changes in feeding and drinking should be less of a focus for early disease detection in pigs. Although the cumulative rates of feeding and NNV did not vary between the treatments, the feeding rates declined in the hours immediately after vaccination in the High treatment. Martínez-Avilés et al. (2017) found the decrease in feed intake occurs more slowly than the rise in body temperature in African swine fever-infected pigs, which is known for having a rapid course of infection. Similarly, the drinking rates for these pens were also lower in the High than Con vaccination treatment. While these specific behavioral changes are less relevant for early disease detection, this group level decrease in feeding and drinking rates could be useful when confirming a disease has already spread to large proportion of pigs.
Across both trials, the behavioral rates showed a time of day effect, which likely reflects the normal activity pattern of pigs consisting of several active bouts, but with a general decline in mid-day activity due to vaccination (Costa et al., 2009; Maselyne et al., 2014). However, the active behaviors (i.e., feeding, NNV, standing, and drinking) in Trial 1 were lowest at the start of observations −2 h pre-injection, but activity peaked at the initial observation time (i.e., −1 h pre-injection) in the second study. This discrepancy between trials could be explained by the later time of the start of behavioral recordings in Trial 2, which overlaps with the first daily peak in activity (Costa et al., 2009; Maselyne et al., 2014).
This research has identified key behaviors for early disease detection in pigs at the group level, but should still be considered preliminary due to the small samples sizes of both trials. Future research should employ more levels of proportional vaccination and greater treatment replication (as in Cook et al., 2015) to more clearly pinpoint the onset of key behavioral changes from acute sickness. Using the data from Trial 2, the authors suggest future studies should employ a minimum sample size of six groups (80% power, α = 0.05) to more thoroughly investigate the relationship between the proportion of ill animals and group level behavioral changes. Precisely identifying the onset of key behavioral changes is vital to improving the accuracy of early warning systems for disease outbreaks in commercial pig operations. However, the degree of these key behavioral changes will quantifiably vary between groups of pigs, so monitoring efforts for disease detection should instead focus on variation from normal behavioral patterns of each group. Future efforts to refine these automated systems should also widen the repertoire of monitored behaviors. For instance, monitoring variation in more specific social interactions (e.g., social organization) during illness could allow for better understanding of how social dynamics change as disease spreads within a group (Reiner et al., 2009; Matthews et al., 2016).
In this paper, we quantified group behavioral changes from an acute health challenge manually, but recent advances in sensor technology should enable these changes to be detected automatically. Early warning systems have been developed to gather information from group level or individual sensors, but determining what type of sensors to use is dependent on a number of factors, including herd size, cost, the type of information sought, and the time input for stockpersons to implement and manage the sensors (Matthews et al., 2016; Süli et al., 2017). Group level sensors for early disease detection are currently the most immediate, cost-effective solution for large scale commercial pig farms, but these systems still need to establish the minimum thresholds for group level behavioral changes to ensure timely detection of health and welfare challenges. In Trial 1, total pen vaccination produced short-term quantitative behavioral and physiological changes at the group level akin to an acute disease challenge. When only a small number of pigs within a pen were given an acute health challenge in Trial 2, the group level rates of enrichment interaction and standing decreased, while the lying rate increased, which suggests group sensors should focus on variation in these specific behaviors for enhancing early disease detection. In contrast, the rates of feeding and drinking only declined at the group level in the High treatment, which suggests that these behaviors would be better suited for confirming disease spread within a herd.
Conflict of interest statement. None declared.
Footnotes
This study was supported by the Biotechnology and Biological Sciences Research Council, UK, through an Agri-tech Research grant (BB/M011364/1) in conjunction with Zoetis Inc., Harbro Nutrition Ltd., Innovent UK Ltd., and RAFT solutions Ltd.
LITERATURE CITED
- Ahmed S. T., H. S. Mun H. Yoe, and Yang C. J.. 2015. Monitoring of behavior using a video-recording system for recognition of salmonella infection in experimentally infected growing pigs. Animal. 9:115–121. doi:10.1017/S1751731114002213 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Rohrer G. A., and Eigenberg R. A.. 2013. Analysis of feeding behavior of group housed growing-finishing pigs. Comput. Electron. Agric. 96:246–252. doi:10.1016/j.compag.2013.06.002 [Google Scholar]
- Cook N. J., C. J. Bench T. Liu B. Chabot, and Schaefer A. L.. 2018. The automated analysis of clustering behaviour of piglets from thermal images in response to immune challenge by vaccination. Animal. 12:122–133. doi:10.1017/S1751731117001239 [DOI] [PubMed] [Google Scholar]
- Cook N. J., B. Chabot T. Lui C. J. Bench, and Schaefer A. L.. 2015. Infrared thermography detects febrile and behavioural responses to vaccination of weaned piglets. Animal. 9:339–346. doi:10.1017/S1751731114002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., Borgonovo F., Leroy T., Berckmans D., Guarino M.. 2009. Dust concentration variation in relation to animal activity in a pig barn. Biosyst. Eng. 104:118–124. doi:10.1016/j.biosystemseng.2009.05.009 [Google Scholar]
- Deen J. 2010. Pigs: behavior and welfare assessment. In: Breed M. D., Moore J., editors, Encyclopedia of animal behavior. Cambridge, MA:Academic Press; p. 731–739. [Google Scholar]
- Docking C. M., Van de Weerd H. A., Day J. E. L., and Edwards S. A.. 2008. The influence of age on the use of potential enrichment objects and synchronisation of behavior in pigs. Appl. Anim. Behav. Sci. 110:244–257. doi:10.1016/j.applanim.2007.05.004 [Google Scholar]
- Escobar J., Van Alstine W. G., Baker D. H., and Johnson R. W.. 2007. Behavior of pigs with viral and bacterial pneumonia. Appl. Anim. Behav. Sci. 105:42–50. doi:10.1016/j.applanim.2006.06.005 [Google Scholar]
- Fangman T. J., Johnson A. K., Okones J., and Elder R. A.. 2011. Willingness-to-approach behavior of weaned pigs after injection with Mycoplasma hyopneumoniae vaccines. J. Swine Health Prod. 19:19–25. doi:10.1080/10888700902720292 [Google Scholar]
- Farm Animal Welfare Council 1993. Second report on priorities for research and development in farm animal welfare. Surbiton, Surrey, UK:Ministry of Agriculture Fisheries and Food. [Google Scholar]
- Fernández-Carrión E., Martínez-Avilés M., Ivorra B., Martínez-López B., Ramos Á. M., and Sánchez-Vizcaíno J. M.. 2017. Motion-based video monitoring for early detection of livestock diseases: the case of African swine fever. PLoS ONE 12:e0183793. doi:10.1371/journal.pone.0183793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González L. A., B. J. Tolkamp M. P. Coffey A. Ferret, and Kyriazakis I.. 2008. Changes in feeding behavior as possible indicators for the automatic monitoring of health disorders in dairy cows. J. Dairy Sci. 91:1017–1028. doi:10.3168/jds.2007-0530 [DOI] [PubMed] [Google Scholar]
- Hart B. L. 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12:123–137. doi:10.1016/S0149-7634(88)80004–6 [DOI] [PubMed] [Google Scholar]
- Krsnik B., R. Yammine Z. Pavicić T. Balenović B. Njari I. Vrbanac, and Valpotić I.. 1999. Experimental model of enterotoxigenic Escherichia coli infection in pigs: potential for an early recognition of colibacillosis by monitoring of behavior. Comp. Immunol. Microbiol. Infect. Dis. 22:261–273. doi:10.1016/S0147-9571(99)00016-8 [DOI] [PubMed] [Google Scholar]
- Kyriazakis I., and Houdijk J.. 2007. Food intake and performance of pigs during health, disease and recovery. Proceedings of the 62nd Easter School in the Agricultural and Food Sciences Sutton Bonington, UK; p. 493–513. [Google Scholar]
- Kyriazakis I., I, Tolkamp B. J., and Hutchings M. R.. 1998. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim. Behav. 56:265–274. doi:10.1006/anbe.1998.0761 [DOI] [PubMed] [Google Scholar]
- Littin K., A. Acevedo W. Browne J. Edgar M. Mendl D. Owen C. Sherwin H. Würbel, and Nicol C.. 2008. Towards humane end points: behavioural changes precede clinical signs of disease in a Huntington’s disease model. Proc. Biol. Sci. 275:1865–1874. doi:10.1098/rspb.2008.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel R., C. J. Nicol H. R. Whay, and Klement E.. 2017. Short communication: detection and monitoring of metritis in dairy cows using an automated grooming device. J. Dairy Sci. 100:5724–5728. doi:10.3168/jds.2016-12201 [DOI] [PubMed] [Google Scholar]
- Martínez-Avilés M., Fernández-Carrión E., López García-Baones J. M., and Sánchez-Vizcaíno J. M.. 2017. Early detection of infection in pigs through an online monitoring system. Transbound. Emerg. Dis. 64:64–373. doi:10.1111/tbed.12372 [DOI] [PubMed] [Google Scholar]
- Maselyne J., Saeys W., De Ketelaere B., Briene P., Millet S., Tuyettens F., and Van Nuffel A.. 2014. How do fattening pigs spend their day? Proceedings of 6th International Conference on the Assessment of Animal Welfare at Farm and Group Level (WAFL) Clemont-Ferrand, France; p. 157. [Google Scholar]
- Maselyne J., Van Nuffel A., Briene P., Vangeyte J., De Ketelaere B., Millet S., Van den Hof J., Maes D., and Saeys W.. 2018. Online warning systems for individual fattening pigs based on their feeding pattern. Biosystm. Eng. 173: 143–156. doi:10.1016/j.biosystemseng.2017.08.006 [Google Scholar]
- Matthews S. G., A. L. Miller J. Clapp T. Plötz, and Kyriazakis I.. 2016. Early detection of health and welfare compromises through automated detection of behavioural changes in pigs. Vet. J. 217:43–51. doi:10.1016/j.tvjl.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S. G., A. L. Miller T. PlÖtz, and Kyriazakis I.. 2017. Automated tracking to measure behavioural changes in pigs for health and welfare monitoring. Sci. Rep. 7:17582. doi:10.1038/s41598-017-17451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman S. T. 2007. Sickness behavior and its relevance to animal welfare assessment at the group level. Anim. Welf. 16:123–125. [Google Scholar]
- Pritchard G., Dennis I., and Waddilove J.. 2005. Biosecurity: reducing disease risks to pig breeding herds. In Pract. 27:230–237. doi:10.1136/inpract.27.5.230 [Google Scholar]
- Reiner G., Hübner K., and Hepp S.. 2009. Suffering in diseased pigs as expressed by behavioral, clinical and clinical-chemical traits, in a well defined parasite model. Appl. Anim. Behav. Sci. 118:222–231. doi:10.1016/j.applanim.2009.02.010 [Google Scholar]
- Ribbens S., J. Dewulf F. Koenen H. Laevens, and de Kruif A.. 2004. Transmission of classical swine fever. A review. Vet. Q. 26:146–155. doi:10.1080/01652176.2004.9695177 [DOI] [PubMed] [Google Scholar]
- Rostagno M. H., S. D. Eicher, and Lay D. C. Jr. 2011. Immunological, physiological, and behavioral effects of salmonella enterica carriage and shedding in experimentally infected finishing pigs. Foodborne Pathog. Dis. 8:623–630. doi:10.1089/fpd.2010.0735 [DOI] [PubMed] [Google Scholar]
- Süli T., M. Halas Z. Benyeda R. Boda S. Belák M. Martínez-Avilés E. Fernández-Carrión, and Sánchez-Vizcaíno J. M.. 2017. Body temperature and motion: evaluation of an online monitoring system in pigs challenged with porcine reproductive & respiratory syndrome virus. Res. Vet. Sci. 114:482–488. doi:10.1016/j.rvsc.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Svensson C., and Jensen M. B.. 2007. Short communication: identification of diseased calves by use of data from automatic milk feeders. J. Dairy Sci. 90:994–997. doi:10.3168/jds.S0022-0302(07)71584-9 [DOI] [PubMed] [Google Scholar]
- Szyszka O., and Kyriazakis I.. 2013. What is the relationship between level of infection and ‘sickness behavior’ in cattle? Appl. Anim. Behav. Sci. 147:1–10. doi:10.1016/j.applanim.2013.05.007 [Google Scholar]
- Szyszka O., B. J. Tolkamp S. A. Edwards, and Kyriazakis I.. 2012. The effects of acute versus chronic health challenges on the behavior of beef cattle. J. Anim. Sci. 90:4308–4318. doi:10.2527/jas.2011-4765 [DOI] [PubMed] [Google Scholar]
- Trickett S. L., Guy J. H., and Edwards S. A.. 2009. The role of novelty in environmental enrichment for the weaned pig. Appl. Anim. Behav. Sci. 116:45–51. doi:10.1016/j.applanim.2008.07.007 [Google Scholar]
- Van de Weerd H. A., Docking C. M., Day J. E. L., Avery P. J., and Edwards S. A.. 2003. A systematic approach towards developing environmental enrichment for pigs. Appl. Anim. Behav. Sci. 84:101–118. doi:10.1016/S0168-1591(03)00150-3 [Google Scholar]
- Weary D. M., J. M. Huzzey, and von Keyserlingk M. A.. 2009. Board-invited review: using behavior to predict and identify ill health in animals. J. Anim. Sci. 87:770–777. doi:10.2527/jas.2008-1297 [DOI] [PubMed] [Google Scholar]