Abstract
The objective of this study was to determine the effects of different forms of hydrolyzable tannin [HT; source (chestnut, CN; tannic acid, TA); subunit (gallic acid, GA)] on apparent total-tract digestibility, methane (CH4) production, and nitrogen (N) utilization in beef cattle fed an alfalfa silage-based diet. Eight ruminally cannulated heifers with an initial BW of 480 ± 29.2 kg (mean ± SD) were used in a double 4 × 4 Latin square experiment. The experiment consisted of four 28-d periods (14-d adaptation, 14-d measurements) and a 7-d washout between periods. The animals received a basal diet with 19.8% CP (DM basis) content containing 75% alfalfa silage, 20% barley silage, and 5% supplement (DM basis) with or without different forms of HT. The dietary treatments were as follows: control (no HT), GA (1.5% of diet DM), TA (1.5% of diet DM), and CN (2% of diet DM). Animals were fed 95% of their ad libitum intake during the measurement phase. Total fecal excretion was collected for 4 d, CH4 was measured for 72 h using respiration chambers, and ruminal fermentation variables and plasma urea N (PUN) concentration were measured on 2 nonconsecutive days before and after feeding. The restricted DM (DMI; 10.79 ± 1.076 kg/d) and nutrient intakes did not differ (P ≥ 0.22) among treatments. Furthermore, apparent DM digestibility (60.3 ± 0.86%) was not affected (P = 0.20) by treatment, but CP digestibility decreased for TA and CN compared with control and GA treatments (63.1 vs. 69.0%; P < 0.001). Total VFA concentration tended (P = 0.089) to increase for GA compared with control and TA (134 vs. 125 and 126 mM) and intermediate for CN (129 mM). The PUN concentration was lower for all HT treatments compared with control (196 vs. 213 mg/L; P = 0.02). Both TA and CN increased the proportion of N excreted in feces and decreased the proportion in urine compared with control and GA (43.9% vs. 37.8% and 56.1% vs. 62.2%; respectively; P < 0.001). However, the proportion of urea N in urinary N decreased for all HT treatments compared with control (47.2% vs. 51.2%; P = 0.02). Also, GA tended to decrease CH4/DMI (20.4 vs. 22.3 g/kg DMI; P = 0.07) and decreased the proportion of GE intake emitted as CH4 (5.16 vs. 5.71%; P = 0.04) compared with control. Thus, among the different forms of HT applied to a high-protein alfalfa silage-based diet, both TA and CN had no effect on CH4 production, but decreased CP digestibility and shifted N excretion from urine to feces, whereas GA (i.e., HT subunit) decreased CH4 production and decreased the proportion of urea N in urinary N in beef cattle without affecting CP digestibility. Thus, feeding the HT subunit, GA, has the potential to decrease environment impact of ruminants (lower CH4 and ammonia emissions), without decreasing animal performance.
Keywords: chestnut, digestibility, enteric methane, gallic acid, nitrogen utilization, tannic acid
INTRODUCTION
There is growing concern that livestock production contributes significantly to anthropogenic greenhouse gas (GHG) emissions, mainly due to enteric methane (CH4) and nitrous oxide (N2O) from manure. Globally, enteric fermentation from ruminants contributes about 39% of the livestock sector GHG emissions (Gerber et al., 2013). In addition to contributing to GHG emissions, ruminant animals utilize dietary nitrogen (N) with low efficiency with only 10% to 40% of consumed N retained in products (Calsamiglia et al., 2010). The high loss of N in feces and urine leads to an increase in ammonia-N (NH3-N) and N2O emissions from manure (Dijkstra et al., 2013). Eliminating livestock production and adoption of a vegan diet is often promoted as a means of reducing environmental impact (Veeramani et al., 2017). However, a recent modeling study of U.S. agriculture without animal production showed that adoption of a plant-derived diet decreased CH4 emission, but the food supply did not support the population’s nutritional requirements (White and Hall, 2017).
The negative environmental impacts of ruminant livestock production have stimulated interest in finding suitable mitigation strategies. One such possible mitigation approach is incorporation of tannins in the diets consumed by ruminants. Hydrolyzable tannins (HT) and condensed tannins (CT) are secondary compounds in plants with the ability to form complexes with protein and carbohydrate fractions through hydrogen bonds. Tannins have been shown to improve N utilization and decrease CH4 production from ruminants (Patra and Saxena, 2011). The inhibitory effects of tannins on CH4 production have been suggested to result from direct effects on methanogens, indirect effects on protozoal-associated CH4 production, and reduction of fiber digestion (Patra and Saxena, 2011). However, most of the research on feeding tannins to ruminants has focused on CT rather than HT because it is assumed that HT would have negative effects on OM digestibility and animal performance (Beauchemin et al., 2008).
Unlike CT, HT are complexes of low molecular weight (500 to 3,000 Da) formed from a monosaccharide (glucose or glucitol) at the central core that is partially or totally esterified with gallic acid (GA) or ellagic acid (Patra and Saxena, 2011). Thus, HT is either gallotannin (i.e., glucose core surrounded by several GA units, with more GA attached through depside bonding of additional galloyl residues) or ellagitannin (sugar core often a glucose unit surrounded by hexahydroxydiphenic acid formed from oxidative coupling of galloyl groups; Hagerman, 2011). However, the effects of tannins on microbes and CH4 emissions may depend on the unit structure (McAllister et al., 2005; Tavendale et al., 2005). An in vitro study showed that HT did not decrease OM degradability and was more effective in reducing enteric CH4 emission than CT (Jayanegara et al., 2010). Tannic acid (TA), a HT with about 10 molecules of GA, decreased CH4 production of beef cattle by 11.1%, 14.7%, and 33.6%, respectively, when applied at 0.65%, 1.3%, and 2.6% of dietary DM to a 50:50 forage:concentrate diet (Yang et al., 2017).
Hydrolyzable tannins can bind to microbes thereby affecting their function and to proteins decreasing their degradation in the rumen and consequently altering N excretion. For example, feeding GA to beef cattle altered the pattern of N excretion by increasing the ratio of fecal N:urinary N and decreasing the ratio of urinary urea N:urinary N (Wei et al., 2016). Also, HT extract from chestnut (CN) applied at 1% to 3% dietary DM in sheep (Liu et al., 2011) or combined with quebracho (CT extract) at 1.5% dietary DM in beef cattle (Aboagye et al., 2018) lowered ruminal NH3 concentration and CH4 production. Thus, differences in unit structure and molecular weight of HT may affect rumen microbes differently and consequently CH4 production and N excretion differently. However, little is known about the effect of the different sources (TA or CN) and components of HT (GA) on diet digestibility, CH4 production, and N excretion.
Alfalfa, which does not contain tannin, is widely used as a source of forage for ruminants, particularly grazing cattle and dairy cows (Berard et al., 2011). Alfalfa has high crude protein (CP) and soluble protein contents such that its dietary inclusion can elevate ruminal NH3-N concentration and excretion of excess N into the environment. Feeding a source of HT to ruminants fed alfalfa-based diets may decrease methanogenesis and N excretion without negatively affecting feed digestibility. Thus, we hypothesized that feeding HT or a component of HT to cattle fed a high-protein diet based on alfalfa silage would decrease both urinary N excretion and enteric CH4 production and that the response to HT would depend on the source of HT or its subunit. Therefore, the objective of this study was to determine the effects of different forms of HT on CH4 production, N utilization, diet digestibility, protozoal populations, ruminal fermentation, and blood metabolite profile in beef cattle fed a high-protein diet mainly containing alfalfa silage.
MATERIALS AND METHODS
The Animal Care Committee of the Lethbridge Research and Development Centre (LeRDC) reviewed the experimental protocol (ACC 1633), and throughout the experiment, the animals were cared for according to the guidelines of the Canadian Council on Animal Care (2009).
Alfalfa Silage Preparation, Animals, Diets, and Experimental Design
Alfalfa (Medicago sativa L.) was from a stand grown in Lethbridge County, AB, Canada, and harvested at 9% bloom as second cut on 4 August 2016. The fresh forage was wilted to 34% DM, and the windrows were raked (FELLA, TS880; Jackson, MN) and chopped to a 10-mm theoretical length (John Deere 6810; John Deere, Moline, IL) into a truck. An inoculant (11 GFT; Pioneer Hi-Bred Ltd., Chathan, ON, Canada) was applied at the manufacturer’s recommended rate of 1 g/T fresh forage during chopping. Loaded trucks delivered the chopped forages to LeRDC (15 km from the harvested site) where they were packed in a horizontal plastic silo bag (Hyplast; RKW Klerks Inc., Hoogstraten, Belgium) with a silage bagger (Ag-bagger 7000; Ag-Bag, St. Nazianz, WI). After 60 d of ensiling, the bag was opened and the alfalfa silage was used to formulate a basal diet consisting of 75% alfalfa silage, 20% barley silage (from a bunker silo at LeRDC), and 5% supplement containing minerals and vitamins to meet or exceed the nutrients requirement of beef cattle gaining 1 kg/d (National Academies of Sciences, Engineering, and Medicine, 2016; Table 1). The diet was formulated to reflect a high-protein forage diet such as lush pasture for grazing animals.
Table 1.
Feed ingredients and chemical composition of the dietary treatments
| Treatment1 | ||||
|---|---|---|---|---|
| Item | Control | GA | TA | CN |
| Ingredients, % DM | ||||
| Alfalfa silage2 | 75.0 | 75.0 | 75.0 | 75.0 |
| Barley silage3 | 20.0 | 18.5 | 18.5 | 18.0 |
| Supplement4 | 5.0 | 5.0 | 5.0 | 5.0 |
| Barley ground | 4.9 | 4.9 | 4.9 | 4.9 |
| Salt (sodium chloride) | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin and mineral premix5 | 0.05 | 0.05 | 0.05 | 0.05 |
| GA6 | — | 1.5 | — | — |
| TA6 | — | — | 1.5 | — |
| CN6 | — | — | — | 2.0 |
| Chemical composition7, % of DM | ||||
| DM (as is) | 34.4 ± 0.13 | 34.4 ± 0.15 | 34.3 ± 0.19 | 34.4 ± 0.29 |
| OM | 85.1 ± 1.14 | 85.1 ± 0.92 | 85.2 ± 1.08 | 84.8 ± 1.04 |
| CP | 19.8 ± 0.79 | 19.7 ± 1.00 | 20.2 ± 0.30 | 19.3 ± 0.90 |
| NDF | 42.2 ± 0.94 | 41.7 ± 0.70 | 42.1 ± 0.57 | 41.3 ± 0.81 |
| ADF | 33.1 ± 0.52 | 32.4 ± 1.03 | 32.2 ± 0.91 | 32.6 ± 1.45 |
| Starch | 5.9 ± 1.41 | 5.4 ± 1.66 | 4.3 ± 1.13 | 5.9 ± 2.25 |
| GE, Mcal/kg DM | 5.2 ± 0.07 | 5.2 ± 0.12 | 5.2 ± 0.16 | 5.3 ± 0.18 |
1GA = gallic acid; TA = tannic acid; CN = chestnut.
2Contained 31.4 ± 0.19% DM, 80.6 ± 2.35% OM, 21.6 ± 0.93% CP (soluble CP, 25.2% CP), 43.7 ± 1.35% NDF, and 35.0 ± 1.24% ADF on a DM basis using pooled samples from each period during digestibility measurement (mean ± SD; n = 8).
3Contained 37.0 ± 0.40% DM, 91.7 ± 1.00% OM, 13.2 ± 0.61% CP, 46.4 ± 1.45% NDF, 25.5 ± 1.07% ADF, and 17.8 ± 5.57% starch on a DM basis using pooled samples from each period during digestibility measurement (mean ± SD; n = 8).
4Contained 90.1 ± 0.51% DM, 96.2 ± 0.83% OM, 10.8 ± 0.23% CP, 19.7 ± 3.39% NDF, 6.13 ± 1.19% ADF, and 59.9 ± 4.44% starch on a DM basis using samples from each period during digestibility measurement (mean ± SD; n = 8); provided as mash.
5Contained 35.01% CaCO3, 10.37% CuSO4, 28.23% ZnSO4, 0.15% ethylenediamine dihydroiodide (80% concentration), 5.01% Se (10,000 mg Se/kg), 0.1% CoSO4, 14.54% MnSO4, 1.71% vitamin A (500,000,000 IU/kg), 0.17% vitamin D (500,000,000 IU/kg), and 4.7% vitamin E (500,000 IU/kg).
6Gallic acid (99% GA; Rhus chinensis; J & K Scientific Ltd., Beijing, China); tannic acid (95% TA; J & K Scientific Ltd.); chestnut (74% tannin; Castanea sativa; Tanin Sevnica, Sevnica, Slovenia); all in powdered forms; CN was applied such that 2% CN and 1.5% TA added to the diet both supplied 1.43% HT.
7Determined using samples pooled by diet for each period during digestibility measurement (mean ± SD; n = 8).
Eight ruminally cannulated beef heifers with an initial BW of 480 ± 29.2 kg (mean ± SD) were used in a double 4 × 4 Latin square experiment. Before the start of the experiment, the animals were adapted to the basal (control) diet for 14 d. The experiment consisted of four 28-d periods (14-d adaptation, 14-d measurement) and a 7-d washout between periods during which all animals were fed the basal diet. The heifers were housed in individual tie-stalls fitted with rubber mats and bedded with wood shavings, and they were permitted access to a group exercise pen for 1 h daily (except during measurements). The BW of heifers was measured at the beginning of the experiment and before and after digestibility and CH4 measurements. The heifers were assigned to 2 groups (4 animals per group) based on their initial BW, and the 4 periods were staggered by 1 wk between groups 1 and 2 to facilitate measurements.
Each heifer received a unique sequence of 4 dietary treatments over time. The dietary treatments were as follows: control (basal diet, no tannin), GA (1.5% of diet DM; 99% GA; extracted from Rhus chinensis Mill.; J & K Scientific Ltd., Beijing, China), TA (1.5% of diet DM; 95% TA; J & K Scientific Ltd., Beijing, China), and CN (2% of diet DM; 74% HT; extracted from Castanea sativa; Tanin Sevnica, Sevnica, Slovenia). The GA, TA, and CN were substituted for barley silage in the diet. They were mixed into the diet in powdered form using a feed mixer (Data Ranger; American Calan Inc., Northwood, NH), and the diets were fed as total mixed rations (TMR). Chestnut was applied on a HT equivalent basis such that the 2% CN and 1.5% TA added to the diet both supplied 1.43% HT. The level of 1.5% GA or 1.5% TA was chosen based on 2 dose studies in beef cattle, where a maximum concentration of GA (2.1%; Wei et al., 2016) or TA (2.6%; Yang et al., 2017) added to the dietary DM did not cause toxicity in the beef cattle.
At the start of each period, the heifers were gradually adapted to the experimental diets. Animals fed GA or TA received a diet containing 0.75% GA or 0.75% TA, respectively, for 5 d of the adaptation phase before stepping up to 1.5% for the remainder of the period. Animals fed CN received a diet with 0.75% CN for 5 d, 1.5% CN for the next 5 d, and 2% CN for the rest of the period. The heifers were fed once daily at 1030 h and had free access to water. Animals were fed for ad libitum intake during the adaptation phase and fed 95% of their ad libitum intake during the measurement phase to minimize feed sorting.
Feed Sampling
Diets offered and orts (when available) were weighed daily for individual animals. During the digestibility and CH4 measurements, DMI was calculated using the DM contents of the dietary treatments and ort samples (if any). Orts were sampled, composited for each animal to provide representative samples corresponding to the digestibility and CH4 measurements. Samples were stored at −20 °C until analyzed for DM and chemical composition including OM, NDF, ADF, CP, starch, and GE. Sampling of the dietary treatments and feed ingredients (alfalfa silage, barley silage and supplement) was performed weekly to monitor DM content and where DM content of the silages varied by more than 3%, an adjustment in diet composition was made. A subsample of the ingredients was composited by period and stored at −20 °C until analyzed for chemical composition. Daily dried samples of dietary treatments were pooled by period and stored for chemical analysis to provide representative samples corresponding to the digestibility and CH4 measurements.
Rumen Fermentation and Plasma Urea Nitrogen Measurements
Ruminal contents (1 L of fluid and solids) were collected from multiple sites in the rumen on days 15 and 25 of each period before feeding (0 h) and at 3-h intervals after feeding for 12 h (i.e., 3, 6, 9, and 12 h). The ruminal contents were sieved through a polyester screen (355 µm pore size; B & S H Thompson, Ville Mont-Royal, QC, Canada) and retained for analysis of VFA, NH3-N concentrations, and protozoa enumeration. For VFA determination, 5 mL of the filtered ruminal fluid was added to 1 mL of 25% meta-phosphoric acid (wt/vol) and for NH3-N determination another 5 mL was added to 1 mL of 1% sulfuric acid (vol/vol). The collected samples were immediately frozen with liquid N and stored at −80 °C until analyzed. For protozoa enumeration, 5 mL of the filtrate from the 0-, 6-, and 12-h samples was mixed with 5 mL of methyl green-formalin-saline solution and stored in the dark at room temperature until analyzed.
On days 15 and 25, as rumen contents were sampled, dissolved hydrogen (dH2) concentration was measured (0, 3, 6, 9, and 12 h) using a polarized hydrogen gas (H2) sensor (1,000 mV; H2-500; Unisense, Aarhus, Denmark) connected to a glass flow-through cell (2 mm internal diameter, 6 mm external diameter) as described by Guyader et al. (2017). Briefly, the H2 sensor was connected to a microsensor multimeter (Unisense), which was controlled by the Unisense logger computer software (SensorTrace Suite; Version 2.5.0) recording dH2 concentration every second. The sensor was standardized each day before the first measurement using a 2-point calibration curve (0 and 592.7 µM), which was created using water without and with H2 gas bubbling [80% H2 and 20% carbon dioxide (CO2) gas mixture]. For standardization, the flow cell was connected through a closed system using H2-impermeable chemical tubing (Masterflex Tygon; Cole-Parmer Instrument Co., Vermon Hills, IL), beginning and ending in an Erlenmeyer flask filled with water and kept in a 39 °C water bath. The water in the flask circulated in the system via a peristaltic pump (model 1001, Medical Technology Products, Inc., Huntington Station, NY). At each time point of sampling, a 15-cm-long polyvinyl chloride pipe (18 mm internal diameter, 20 mm external diameter) with closed ends and a 2.5-cm cut on the side (covered with a mesh) was connected at one end to H2-impermeable chemical tubing (Masterflex Tygon; Cole-Parmer Instrument Co., Vermon Hills, IL) and inserted into the rumen. A 40-mL syringe was used to sample the ruminal fluid (40 mL) and inject it directly into the flow cell of the H2 sensor for dH2 concentration.
Starting on day 18, pH data loggers (LeRDC pH data logger system, Dascor, Escondido, CA; Penner et al., 2006) were placed in the ventral sac of the rumen. The ruminal pH was recorded every min continuously for 6 d, coinciding with digestibility measurements. The loggers were standardized in buffers pH 4 and 7 at the start and end of each measurement.
Blood samples were collected from heifers on days 15 and 25 at 0 and 6 h after feeding. Blood samples were taken from the jugular vein into sterile evacuated tubes containing an anticoagulant (10 mL, lithium heparin, Vacutainer, Becton Dickinson, Oakville, ON). The blood was centrifuged at 3,000 × g and 4 °C for 20 min to obtain plasma and stored at −20 °C until analysis of plasma urea N (PUN) concentration.
Digestibility and Nitrogen Excretion
On day 18, heifers were housed in metabolism stalls (without bedding) for 4 d (days 18 to 21) for apparent total-tract digestibility and N excretion determination. The animals were fitted with urinary indwelling balloon catheters (Bardex Lubricath Foley catheter, balloon size: 75 cm3, catheter diameter: 8.7 mm; Bard Canada Inc., Oakville, ON, Canada) to enable separate collection of urine and feces. Urine was collected into a container containing 4 N H2SO4 to ensure the pH < 2 to prevent microbial activity and volatilization of NH3. Total feces and urine were collected and weighed daily. A subsample (1 kg) of the daily fecal output was dried at 55 °C in an oven for 72 h to determine the DM content. A composite sample of dried feces for each animal within period was obtained by pooling daily samples based on DM contents, and these were stored at an ambient temperature until ground and analyzed for OM, NDF, ADF, CP, and GE. Total urine output from each animal was measured daily, and samples of diluted (15 to 60 mL of deionized water) urine were stored frozen (−20 °C). The diluted urine was composited (10 mL) by animal within period based on daily output until analyzed for total N, urea, allantoin, and uric acid.
Methane Emission Measurement
Near the end of each period (days 26 to 28), heifers were moved to 4 environmentally controlled chambers to obtain 72 h of continuous CH4 measurement. Prior to starting the experiment, the animals had been adapted to the chambers to minimize stress. The dimensions of the chambers were 4.4 m wide × 3.7 m deep × 3.9 m tall (C1330, Conviron Inc., Winnipeg, MB), and the methodology used for CH4 measurement was previously described by Beauchemin and McGinn (2006). Briefly, CH4 concentrations in the intake and exhaust ducts were measured in succession (3 or 4 min from the intake or from the exhaust ducts per chamber) using a CH4 analyzer (model Ultramat 5E; Siemens Inc., Karlsruhe, Germany). For each chamber, intake and exhaust airflow was monitored (FE-1500-FX-12; Paragon Controls Inc., Santa Rosa, CA), and airflow CH4 concentration was sampled every 30 min (i.e., 27 min plus 3 min of zero reference gas measurement using pure N gas). The difference between the incoming and outgoing mass and air flow of CH4 was used to calculate the amount generated in each chamber.
Before and after the experiment, the chambers were calibrated by releasing a known quantity of CH4 into each chamber, and the recovered amount (i.e., to adjust each chamber to 100% recovery) were then used to correct chamber CH4 emission data from the experiment. The chambers were opened daily for feeding and cleaning, and corresponding CH4 fluxes were removed from the analysis. As the time required for gas concentration to reach steady state was 5 min, the interruptions from daily feeding and cleaning had limited impact on emissions.
Laboratory Analyses
Samples of TMR, ingredients, orts, and feces were dried in a forced-air oven at 55 °C for 72 h to determine DM content. The dried samples were ground through a 1-mm screen (Wiley mill; A.H. Thomas, Philadelphia, PA), and duplicate samples were used to determine analytical DM (method 930.15; AOAC, 2005), which was used to correct the chemical analysis to a DM basis. Ash (OM = 100 − ash; method 942.05; AOAC, 2005), NDF (with heat-stable amylase and sodium sulfite; Ankom A200, Ankom Technology, Fairport, NY), and ADF (Ankom Technology) were analyzed. Gross energy content was determined using a bomb calorimeter (model E2k; CAL2k, Johannesburg, South Africa). The 1-mm-size samples were further ground using a ball grinder (Mixer Mill MM 2000; Retsch, Haan, Germany) and analyzed for total N (CP = N × 6.25) concentration using flash combustion and thermal conductivity detection (Carlo Erba Instruments, Milan, Italy). Total urinary N was analyzed the same way using freeze-dried urine samples. The ball ground samples of the ingredients were analyzed for starch content (Koenig et al., 2013).
Urea-N concentrations in the blood and urine were determined using micro-segmented flow analysis (model Astoria2; Astoria Pacific Inc., Clackamas, OR). Uric acid N was determined using uric acid standard (5 mg/dL), reagent, and control set obtained from the manufacturer (Pointe Scientific Inc., Canton, MI), and the absorbance of the mixture (duplicate of 200 µL) was read at a wavelength of 520 nm (Thermo Scientific Appliskan; Thermo Fisher Scientific, Vantaa, Finland). Allantoin N was determined as described by Chen and Gomes (1992). Microbial N flow was estimated from uric acid and allantoin (Chen and Gomes, 1992).
Concentrations of VFA in ruminal fluid were analyzed using gas chromatography (model 5890; Hewlett Packard, Wilmington, DE) with crotonic acid as an internal standard. Ruminal NH3-N concentration was determined by the salicylate–nitroprusside–hypochlorite method using segmented flow analyzer (Rhine et al., 1998). Ruminal protozoa were enumerated under a light microscope using a counting chamber (Neubauer Improved Bright-Line counting cell, 0.1 mm depth; Hausser Scientific, Horsham, PA) as described by Veira et al. (1983).
Statistical Analyses
All data were analyzed using a mixed procedure of SAS (SAS Inst., Inc., Cary, NC). Animal was the experimental unit for all variables. The 6 d of continuous ruminal pH data were summarized by day for mean, minimum, maximum, and range values. Data for ruminal fermentation variables, protozoa, and blood samples were averaged across days for each time point and animal within period before analysis (i.e., for VFA and dH2 at 0, 3, 6, 9, and 12 h; protozoa at 0, 6, and 12 h; NH3-N and PUN at 0 and 6 h). Data for apparent digestibility and N excretion were averaged for each animal for each period before analysis. Daily CH4 production was determined for each animal within period and CH4 yield was calculated as CH4 production expressed relative to DMI, GE, and DE intakes using daily intakes when animals were in the chambers. Therefore, the fixed effects in the model included treatment, interval (time point or day), and treatment × interval interaction with time point interval as a repeated measure to analyze ruminal fermentation variables, protozoa and blood sample data, and day interval as repeated measure for CH4 production data, respectively. However, the fixed effect in the model used to analyze data for apparent digestibility and N excretion was treatment. All data were analyzed using the random effects of group, animal nested within group, and period nested within group.
Normality of distribution and homogeneity of variance were determined using the univariate procedure of SAS. For normality of the protozoa data, a log10 transformation was applied and an inverse log10 of the least square means were reported. For all data, covariance structure (autoregressive) that yielded the smallest Akaike and Bayesian information criteria value was used, and means were separated at P < 0.05, whereas tendencies were indicated at 0.05 ≤ P < 0.10. Least square differences were used to determine significant differences among means.
RESULTS
Nutrient Intakes and Apparent Nutrient Digestibilities
The restricted DMI during the apparent digestibility and N excretion measurements averaged 10.79 ± 1.076 kg/d and did not differ (P = 0.23) among treatments (Table 2). Organic matter, CP, NDF, ADF, and GE contents were comparable across dietary treatments. Therefore, nutrient intakes were not affected by treatment (mean ± SEM; 9.16 ± 0.878, 2.13 ± 0.219, 4.53 ± 0.430, 3.53 ± 0.378 kg/d and 56.72 ± 5.646 Mcal/d, respectively; P ≥ 0.22). Similarly, apparent DM digestibility was not affected by treatment (60.3 ± 0.86%; P = 0.20), and apparent OM, NDF, ADF, and GE digestibilities also did not differ among treatments (64.0 ± 0.92%, 45.7 ± 1.48%, 39.0 ± 3.20%, 59.6 ± 1.46%, respectively; P ≥ 0.13). However, apparent CP digestibility decreased for TA and CN treatments compared with the control and GA treatments (63.1% vs. 69.0%; P < 0.001), with no difference (P = 0.26) between control and GA.
Table 2.
Effects of different forms of hydrolyzable tannin on nutrient intake and apparent digestibility of heifers (n = 8) fed an alfalfa silage-based high forage diet
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item2 | Control | GA | TA | CN | SEM | P |
| Intake | ||||||
| DM, kg/d | 10.55 | 10.58 | 11.19 | 10.82 | 1.076 | 0.23 |
| OM, kg/d | 8.95 | 8.98 | 9.54 | 9.18 | 0.878 | 0.22 |
| CP, kg/d | 2.09 | 2.10 | 2.20 | 2.12 | 0.219 | 0.55 |
| NDF, kg/d | 4.46 | 4.44 | 4.73 | 4.48 | 0.430 | 0.63 |
| ADF, kg/d | 3.50 | 3.44 | 3.62 | 3.55 | 0.378 | 0.63 |
| GE, Mcal/d | 54.98 | 55.67 | 58.83 | 57.39 | 5.646 | 0.23 |
| Digestibility, % | ||||||
| DM | 61.6 | 60.1 | 58.9 | 60.0 | 0.86 | 0.20 |
| OM | 65.2 | 64.7 | 62.7 | 63.5 | 0.92 | 0.13 |
| CP | 69.6a | 68.3a | 62.2b | 64.0b | 0.87 | <0.001 |
| NDF | 48.3 | 45.8 | 44.2 | 44.3 | 1.48 | 0.20 |
| ADF | 40.3 | 38.1 | 37.5 | 40.1 | 3.20 | 0.77 |
| GE | 60.6 | 60.2 | 57.1 | 60.4 | 1.46 | 0.24 |
a–bWithin a row, means without a common superscript letter differ at P < 0.05.
1GA = gallic acid; TA = tannic acid; CN = chestnut.
2Nutrient intakes and apparent total-tract digestibility at 95% ad libitum intake.
Ruminal Fermentation and Plasma Urea Nitrogen Measurements
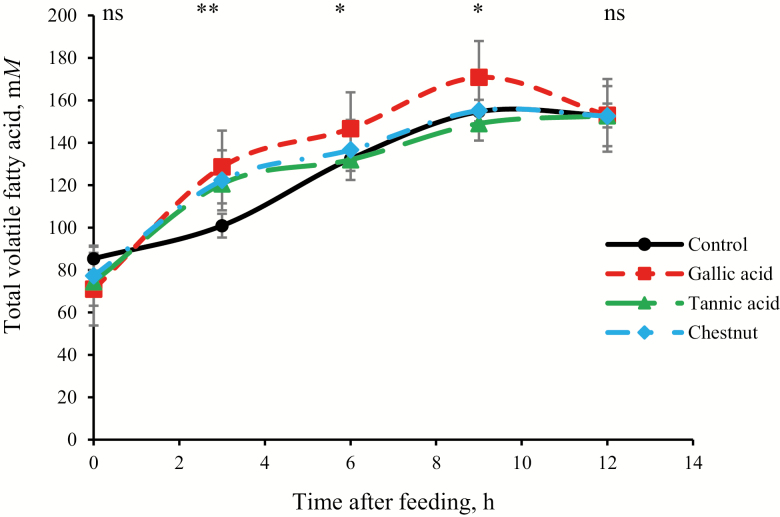
Ruminal pH in terms of minimum, mean, maximum, and range values did not differ among treatments (6.21 ± 0.069, 6.66 ± 0.042, 7.07 ± 0.057, 0.87 ± 0.078, respectively; P ≥ 0.13; Table 3). Also, ruminal pH variables did not differ among days, and there was no treatment × day interaction (P ≥ 0.22). Total VFA differed among treatments at specific time points (P = 0.048), with an increase for the different forms of HT compared with control at 3 h after feeding (124 vs. 101 ± 5.98 mM; P < 0.01; Fig. 1). The increase in total VFA remained at 9 h after feeding for GA compared with the control, TA, and CN (171 vs. 153 mM; P ≤ 0.04). However, at 12 h after feeding, total VFA did not differ among treatments (153 ± 5.98 mM; P ≥ 0.96). Consequently, over all time points, total VFA tended (P = 0.089) to increase for GA compared with the control and TA (134 vs. 125 and 126 mM) with CN not different from the other treatments (129 mM; Table 3). The proportion of isobutyrate tended (P = 0.06) to decrease for tannin treatments compared with control (1.73 vs. 1.84 mol/100 mol), but treatment or treatment × time interaction effects were not observed for the molar proportions of acetate, propionate, butyrate, valerate, and isovalerate and acetate:propionate ratio (P ≥ 0.13). However, sampling time affected (P ≤ 0.01) the molar proportion of VFA, whereby acetate proportion and acetate:propionate ratio decreased after feeding, whereas the proportions of propionate, butyrate, valerate, isobutyrate, and isovalerate increased after feeding.
Table 3.
Effects of different forms of hydrolyzable tannin on ruminal fermentation, protozoa enumeration, and plasms urea-N concentration of heifers (n = 8) fed an alfalfa silage-based high forage diet
| Treatment1 | P 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | Control | GA | TA | CN | SEM | Trt | I | Trt × I |
| Ruminal pH3 | ||||||||
| Minimum | 6.26 | 6.21 | 6.16 | 6.19 | 0.069 | 0.23 | 0.55 | 0.42 |
| Mean | 6.74 | 6.66 | 6.60 | 6.64 | 0.042 | 0.13 | 0.22 | 0.70 |
| Maximum | 7.14 | 7.08 | 7.01 | 7.05 | 0.057 | 0.19 | 0.65 | 0.99 |
| Range | 0.88 | 0.87 | 0.85 | 0.86 | 0.078 | 0.96 | 0.39 | 0.70 |
| Total VFA4, mM | 125y | 134x | 126y | 129xy | 3.25 | 0.089 | <0.001 | 0.048 |
| VFA4, mol/100 mol | ||||||||
| Acetate | 69.0 | 69.7 | 69.8 | 69.1 | 0.47 | 0.13 | <0.001 | 0.21 |
| Propionate | 14.5 | 14.1 | 14.1 | 14.5 | 0.46 | 0.39 | 0.01 | 0.84 |
| Butyrate | 9.75 | 9.68 | 9.62 | 9.75 | 0.68 | 0.96 | <0.001 | 0.99 |
| Valerate | 1.83 | 1.76 | 1.78 | 1.84 | 0.068 | 0.58 | <0.001 | 0.29 |
| Isobutyrate | 1.84x | 1.73y | 1.72y | 1.74y | 0.044 | 0.06 | <0.001 | 0.13 |
| Isovalerate | 2.41 | 2.29 | 2.23 | 2.28 | 0.084 | 0.23 | <0.001 | 0.31 |
| Acetate:propionate ratio | 4.82 | 5.03 | 4.98 | 4.83 | 0.15 | 0.13 | <0.001 | 0.32 |
| dH24, µM | 34.4 | 31.0 | 31.4 | 34.1 | 4.24 | 0.67 | <0.001 | 0.65 |
| Protozoa5, cells × 105/mL | 5.23 | 4.08 | 4.68 | 4.51 | 0.07 | 0.59 | 0.45 | 0.91 |
| NH3-N6, mM | 14.4x | 13.0xy | 11.8y | 12.7xy | 0.80 | 0.05 | <0.001 | 0.51 |
| PUN6, mg/L | 213a | 195b | 195b | 198b | 14.58 | 0.02 | <0.001 | 0.76 |
a–b, x–yWithin a row, means without a common superscript letter differ or tend to differ at P < 0.05 or 0.05 ≤ P < 0.10, respectively.
1GA = gallic acid; TA = tannic acid; CN = chestnut.
2Trt = treatment; I = interval (day or time); Trt × I = treatment × interval interaction.
3Determined for 6 d during digestibility measurements; range = maximum ruminal pH − minimum ruminal pH.
4Total VFA = total volatile fatty acids; dH2 = dissolved hydrogen; determined at 0, 3, 6, 9, and 12 h using average values of days 15 and 25 samples.
5Data were log10 transformed before statistical analysis and inverse log10 least squares mean reported herein for 0, 6, and 12 using average values of days 15 and 25 of each period.
6NH3-N = ammonia-nitrogen; PUN = plasma urea nitrogen; determined at 0 and 6 h using average values of days 15 and 25 samples of each period.
Figure 1.
Mean daily pattern of total volatile fatty concentration (mM) averaged over 2 d (days 15 and 25) for heifers (n = 8) fed an alfalfa silage-based high forage diet. Error bars indicate the SEM. Treatment significance for each time point is indicated by ns = not significant, *P < 0.05, and **P < 0.01.
The dH2 concentration was not affected by treatment or treatment × time (32.7 ± 4.24 µM; P ≥ 0.65). Also, total protozoa numbers in rumen contents were not affected by treatment (mean; 4.63 × 105/mL; P = 0.59). The ruminal NH3-N concentration tended to decrease in animals fed TA compared with control (11.8 vs. 14.4 mM; P = 0.05) with GA and CN not different from the other treatments (13.0 and 12.7 mM, respectively). However, PUN was reduced for GA, TA, and CN treatments compared with the control (196 vs. 213 mg/L; P = 0.02).
Nitrogen Retention, Excretion, and Urinary N Fraction
Nitrogen intake and estimated microbial N flow were not affected by treatment (341 ± 35.4 and 91 ± 15.3 g/d, respectively; P ≥ 0.19; Table 4). Fecal output of animals fed TA and CN increased compared with control animals (4.49 vs. 4.07 kg DM/d; P = 0.02), but fecal output of GA animals (4.20 kg DM/d) only differed from that of TA animals. In contrast, total urinary output was not affected by treatment (22.2 ± 1.23 L/d; P = 0.15). Consequently, urinary N excretion was not affected by treatment (171 ± 25.5 g/d; P = 0.24), but fecal N excretion was greatest for animals fed TA, followed by those fed CN, compared with animals fed control and GA diets (134, 124, vs. 104 g/d, respectively; P < 0.001). Thus, total N excreted tended (P = 0.07) to increase for TA fed animals compared with control animals (300 vs. 270 g/d). However, the proportion of total N excreted increased in feces and decreased in urine for TA and CN compared with control and GA (43.9% vs. 37.8% and 56.1% vs. 62.2%; respectively; P < 0.001). Similarly, the proportion of N excreted as a proportion of N consumed increased in feces for TA and CN compared with the control and GA (37.3% vs. 31.0%; P < 0.001). However, the proportion of N excreted as a proportion of N consumed increased in urine for animals fed GA compared with animals fed control, TA, and CN diets (53.5% vs. 48.2%; P = 0.02). Urea N excreted in urine was not different among treatments (80.8 ± 9.23 g/d; P = 0.15); however, the proportion of urea N in urinary N was reduced for all tannin treatments compared with the control (47.2% vs. 51.2%; P = 0.02). Allantoin N excreted in urine or its proportion in urinary N was not affected by treatment (21.7 ± 2.97 g/d or 12.9 ± 0.60%, respectively; P ≥ 0.13). The amount of uric acid N excreted in urine and the proportion of uric acid N in urinary N were reduced for GA compared with control, TA, and CN (1.57 vs. 2.28 g/d and 0.91% vs. 1.32%, respectively; P < 0.001). The N retained did not differ among treatments irrespective of how it was expressed (54.2 g/d, 16.3% of N intake and 24.5% N digested; P ≥ 0.21).
Table 4.
Effects of different forms of hydrolyzable tannin on N intake, excretion, and retention, and urinary N fractions of heifers (n = 8) fed an alfalfa silage-based high forage diet
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item2 | Control | GA | TA | CN | SEM | P |
| N intake, g/d | 335 | 336 | 352 | 340 | 35.4 | 0.53 |
| Microbial N flow3, g/d | 93 | 91 | 96 | 83 | 15.3 | 0.19 |
| Output | ||||||
| Feces, kg DM/d | 4.07c | 4.20bc | 4.59a | 4.39ab | 0.42 | 0.02 |
| Urine, L/d | 23.1 | 22.2 | 22.0 | 21.3 | 1.23 | 0.15 |
| N excretion, g/d | ||||||
| Feces | 102c | 106c | 134a | 124b | 13.1 | <0.001 |
| Urine | 168 | 181 | 166 | 167 | 25.5 | 0.24 |
| Total | 270y | 287xy | 300x | 291xy | 38.3 | 0.07 |
| N excretion, % of total N excretion | ||||||
| Feces | 38.3b | 37.3b | 44.7a | 43.1a | 1.05 | <0.001 |
| Urine | 61.7a | 62.7a | 55.3b | 56.9b | 1.05 | <0.001 |
| N excretion, % of N intake | ||||||
| Feces | 30.3b | 31.7b | 37.8a | 36.8a | 0.09 | <0.001 |
| Urine | 49.3b | 53.5a | 46.8b | 48.6b | 0.03 | 0.02 |
| Urinary N fraction, g/d | ||||||
| Urea-N | 82.8 | 85.2 | 78.1 | 77.1 | 9.23 | 0.15 |
| Allantoin N | 21.8 | 22.2 | 22.5 | 20.3 | 2.97 | 0.20 |
| Uric acid N | 2.28a | 1.57b | 2.21a | 2.02a | 0.21 | <0.001 |
| Urinary N fraction, % of urine N | ||||||
| Urea-N | 51.2a | 47.8b | 47.4b | 46.5b | 2.03 | 0.02 |
| Allantoin N | 13.1 | 12.3 | 13.6 | 12.4 | 0.60 | 0.13 |
| Uric acid N | 1.32a | 0.91b | 1.36a | 1.26a | 0.12 | <0.001 |
| N retention | ||||||
| g/d | 65.0 | 50.1 | 52.7 | 48.8 | 7.59 | 0.30 |
| % of N intake | 20.4 | 15.0 | 15.4 | 14.5 | 0.03 | 0.21 |
| N retention, % N digested | 29.0 | 21.9 | 24.6 | 22.6 | 4.27 | 0.28 |
a–c, x–yWithin a row, means without a common superscript letter differ or tend to differ at P < 0.05 or 0.05 ≤ P < 0.10, respectively.
1GA = gallic acid; TA = tannic acid; CN = chestnut.
2Nitrogen intakes and excretion were sampled over 4 d and averaged before analysis.
3Estimated based on Allantoin N and uric acid N (Chen and Gomes, 1992).
Methane Emission Measurement
During CH4 measurement, the DMI averaged 10.06 ± 1.109 kg/d and was not affected by treatment (P = 0.95; Table 5). The daily CH4 produced did not differ among treatments (213 ± 21.3 g/d; P = 0.31). However, adding GA to the diet tended to decrease CH4/DMI (P = 0.07), and it decreased the proportion of GE intake emitted as CH4 (P = 0.04) by 9% compared with the control (20.4 vs. 22.3 g/kg DMI; 5.16% vs. 5.71%). Also, the proportion of DE intake emitted as CH4 decreased by 9% for animals fed GA diet compared with the control and TA (8.57% vs. 9.42%; P = 0.02), whereas it was intermediate for CN (8.83%).
Table 5.
Effects of different forms of hydrolyzable tannin on methane (CH4) emission of beef steers (n = 8) fed an alfalfa silage-based high forage diet
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item2 | Control | GA | TA | CN | SEM | P |
| DMI, kg/d | 9.92 | 10.10 | 10.15 | 10.07 | 1.11 | 0.95 |
| Methane3 | ||||||
| g/d | 220 | 204 | 216 | 213 | 21.3 | 0.31 |
| g/kg of DMI | 22.3x | 20.4y | 21.2xy | 21.2xy | 0.87 | 0.07 |
| % of GE intake | 5.71a | 5.16b | 5.39ab | 5.34ab | 0.21 | 0.04 |
| % of DE intake | 9.42a | 8.57b | 9.42a | 8.83ab | 0.35 | 0.02 |
a–b, x–yWithin a row, means without a common superscript letter differ or tend to differ at P < 0.05 or 0.05 ≤ P < 0.10, respectively.
1GA = gallic acid; TA = tannic acid; CN = chestnut.
2Treatment means reported herein because the effects of day and treatment × day interaction did not differ (P > 0.10).
3DMI = DM intake; GE = gross energy; DE = digestible energy at 95% ad libitum intake.
DISCUSSION
Nutrient Intakes and Apparent Nutrient Digestibilities
Alfalfa is a forage crop cultivated for silage production and used as pasture by beef and dairy producers due to its high digestibility and high CP content (Berard et al., 2011). However, the use of high-protein alfalfa silage to improve animal performance has the potential to negatively affect the environment because high intake of soluble protein can lead to an increase in N excreted in feces and urine (Dijkstra et al., 2013). Thus, this study examined the effects of supplementing different forms of HT as a means of improving N utilization and reducing CH4 emission of beef cattle fed a high-protein forage-based diet. Hydrolyzable tannins have the ability to bind to proteins, and so, they may decrease protein degradation in the rumen (Getachew et al., 2008). Therefore, the alfalfa used for this study was at 9% bloom (21.6% CP) and used to formulate a diet high in CP (19.8%) to evaluate the hypothesis that adding HT to a high-protein diet would decrease enteric CH4 and urinary N excretion and that the response to HT would depend on the form of HT.
The effect of added HT was examined using different sources of HT (TA; 1.5% DM and CN; 2% DM) and a component of HT from TA (GA; 1.5% DM). The GA was extracted from the R. chinensis plant and is a subunit of gallotannin (i.e., a glucose core surrounded by several GA units, with more GA attached through depside bonding of additional galloyl residues; Djakpo and Yao, 2010). Tannic acids from commercial sources are usually gallotannins (Hagerman, 2011), and the GA and TA were obtained from the same commercial source. The HT extracted from CN is usually a mix of gallotannins and ellagitannins (a sugar core often a glucose unit surrounded by hexahydroxydiphenic acid formed from oxidative coupling of galloyl groups; Hagerman, 2011; Chiarini et al., 2013). Thus, accounting for the HT concentrations of the TA and CN in the diets, both supplied 1.43% HT.
Adding tannins to ruminant diets may have a negative effect on palatability and digestibility and consequently decrease DMI as reviewed by Waghorn (2008). In the present study, limit-feeding (95% ad libitum) of the animals may have obscured any palatability issues associated with tannin-feeding. The lack of effect of HT treatments on apparent total-tract DM, OM, NDF, ADF, and GE digestibilities indicates no negative effects of tannin treatments on energy availability of the diets. On the contrary, the observed 9% decrease in apparent CP digestibility for TA and CN compared with GA and control indicates that only the more complex form of HT decreased CP digestibility. This effect of HT is consistent with Frutos et al. (2004) who reported that CN did not affect in situ DM degradability of soybean meal, but it decreased CP degradability by 12%. Also, similar to our findings for total-tract digestibility, supplementing alfalfa hay with TA reduced in vitro CP degradability compared with a control, but CP degradability was not affected by GA addition (Getachew et al., 2008). The capacity of different types of HT to form complexes with proteins is generally greater for those with greater molecular weight (Kawamoto et al., 1995). Thus, the lack of effect of GA on apparent CP digestibility can be attributed to its rapid and complete hydrolyzation in the rumen compared with HT (Murdiati et al., 1992). Thus, the complex forms of HT (TA and CN) were more effective in decreasing CP digestibility than the subunit of HT.
Ruminal Fermentation and PUN
The lack of effect of the HT treatments on ruminal pH agrees with the results of other studies with HT fed to cattle (Krueger et al., 2010; Aboagye et al., 2018) or sheep (Liu et al., 2011). The tendency for GA to increase VFA concentration in the present study is similar to the study by Getachew et al. (2008) who compared GA and TA in vitro and found that GA incubated with alfalfa hay produced more total VFA compared with a control, whereas there was no effect for TA. As GA is easily hydrolyzed in the rumen to pyrogallol, resorcinol, and phloroglucinol, it may have been used as an energy source by the microbes (Murdiati et al., 1992; McSweeney et al., 2001), which would account for the increase in VFA concentration. The lack of effect of the HT treatments on the main VFA proportions (acetate and propionate) is consistent with the lack of effect of treatments on apparent DM and fiber digestibility.
Usually, greater ruminal NH3-N concentration is associated with greater PUN concentration because of excess dietary protein intake relative to dietary energy. However, in the present study, there was inconsistency between ruminal NH3-N and PUN concentrations. Although all HT treatments decreased PUN concentration, only TA lowered NH3-N concentration, indicating that the forms of HT functioned differently. Tannins complex with dietary protein and prevent ruminal degradation of protein, thereby, decreasing ruminal NH3-N concentration. This effect may increase amino acid absorption from undegraded feed protein or cause an offset by decreasing microbial protein synthesis rendering no net effect of metabolizable protein flow to the small intestine. The HT–protein complex occurs ideally between pH 3 and 5, but also up to pH 7 (Murdiati et al., 1991; Osawa and Walsh, 1993), which may account for how the different sources of HT affected CP digestibility differently in the gut. It appears that TA decreased the ruminal degradation of dietary CP, as evidenced by a lower rumen NH3-N concentration, which led to decreased total-tract CP digestion and lower PUN concentration. However, the decreased ruminal degradation of dietary CP did not negatively affect estimated microbial N flow to the lower tract. The HT from CN may have decreased the digestion of dietary CP post-ruminally, as rumen NH3-N concentration and estimated microbial N flow were not affected even though total-tract CP digestibility and PUN decreased. Similarly, Hagerman et al. (1992) showed that both TA and HT extracted from forage differed in their protein binding abilities in the gut of sheep and deer. In contrast with the results for TA and CN in our study, the decrease in PUN for animals fed GA did not correspond to a decrease in apparent CP digestibility and ruminal NH3-N concentration, but a tendency for a decrease in isobutyrate proportion. Isobutyrate is formed through oxidative deamination and decarboxylation of the amino acid valine (Allison and Peel, 1971). As the gallotannin subunit is easily hydrolyzed in the rumen, it may be toxic to microbes that are not able to utilize its metabolites in contrast to CN and TA (Murdiati et al., 1992). Thus, decreased PUN concentration in animals fed a high CP diet supplemented with GA may have been due to inactivation of microbial deaminases by the toxic metabolites (Goel et al., 2005). Therefore, although the effect of complex forms of HT appears to be directed toward dietary CP, a component of the gallotannin may target the rumen microbes without affecting microbial N flow.
Nitrogen Retention, Excretion, and Urinary N Fraction
An increase in N intake can lead to increased N excreted in feces and urine (Dijkstra et al., 2013). Although N intake was not affected by the different forms of HT included in the diet, N excretion was altered. The observed 24% greater amount of N excreted in feces for animals fed TA and CN, compared with the control and GA diets, resulted from decreased CP digestibility as well as a shift in N excretion from urine to feces. The shift in route of N excretion is reflected by a 6% unit greater fecal N proportion and a 6% unit lower urinary N proportion. Similarly, other studies have shown that adding HT extract from CN at 1.53% dietary DM to a mixed diet increased fecal N excretion and decreased urinary N excretion in sheep (Wischer et al., 2014). Also, Yang et al. (2017) supplemented a mixed diet with TA at 1.3% and 2.6% dietary DM and reported decreased urinary N proportion (15% and 23%, respectively) and increased fecal N proportion (23% and 36%, respectively). However, contrary to the present study, GA added to a mixed diet increased the ratio of fecal N:urinary N in beef cattle fed at maintenance level (Wei et al., 2016). The authors attributed this shift in ratio to the binding effect of GA to protein and a possible decrease in protein digestibility, although CP digestibility was not measured. Gallic acid has been shown to be completely hydrolyzed in the rumen (Murdiati et al., 1992) and to lack effects on in vitro protein degradability (Getachew et al., 2008), which agrees with the findings from the present study where there was no effect of GA on apparent CP digestibility and estimated microbial N flow.
The decreased percentage of urea-N in urinary N for both the complex (TA and CN) and subunit (GA) forms of HT and the decreased amount of uric acid N in urine for GA indicate that the different sources of HT with the subunit of HT, affected the N fractions of the urine. The urinary N of cattle contains various compounds, but urea-N represents the greatest proportion (52% to 94%) as reviewed by Dijkstra et al. (2013). Urinary urea is hydrolyzed rapidly in the environment to form NH3 and later ammonium, which through nitrification and denitrification processes forms N2O, a potent GHG. The decrease in urea-N (% of urinary N) for all forms of HT treatments shows that HT, including a subunit of gallotannin, has the ability to reduce N2O emission from urine. The N in feces is in a less volatile form compared with that of urinary N because it is present mainly as indigestible organic N (proteins or nucleic acids; Hristov et al., 2011). The shift in route of N excretion from urine to feces for CN and TA suggests that these 2 sources of HT have the potential to reduce NH3 emissions, and eventually N2O emissions from manure, in contrast with control and GA.
Methane Emission Measurement
During feed fermentation, metabolic H2 is used for VFA production and also serves as an important substrate for reducing CO2 to CH4 by methanogens (Hegarty, 1999; Cottle et al., 2011). The metabolic H2 produced in the rumen is either gaseous H2 or dH2, but the dH2 is readily available for methanogens and influences fermentation pathways (Janssen, 2010; Wang et al., 2016). Thus, protozoa associated with methanogens have been reported to influence CH4 formation through interspecies transfer of dH2 from protozoa to CH4 (Morgavi et al., 2010). However, the decrease in CH4 due to GA was likely not because of the lack of effect of the different forms of HT inclusion on protozoa enumeration and dH2 concentration. Bhatta et al. (2013) showed that CH4 production may decrease without a decrease in protozoa with tannin addition, indicating that some tannins may directly affect methanogens that are not associated with protozoa. The tendency for GA to decrease CH4 yield and decrease CH4 relative to GE and DE intakes by 9% may have been partly due to a direct effect on methanogens. Similarly, supplementing a diet of 50% concentrate:50% corn silage with GA at 1%, 2%, and 4% linearly decreased in vitro CH4/substrate degraded (Wei et al., 2019). Contrary to the present study, adding TA to a mixed diet at 0.65%, 1.3%, and 2.6% dietary DM decreased CH4 production (L/kg DM intake) by 11.1%, 14.7%, and 33.6%, respectively, in beef cattle (Yang et al., 2017). Also, HT extract from CN added to a mixed diet at 0.5, 0.75, and 1.0 mg/mL decreased in vitro CH4 production quadratically (Jayanegara et al., 2015). Those studies (Jayanegara et al., 2015; Yang et al., 2017; Wei et al., 2019) showed that HT has the potential to decrease CH4 production; however, no study compared multiple sources of HT as in the present study. The mechanism by which tannins reduce CH4 production could be due to direct or indirect effects on methanogenesis. Tannins may bind to surface membranes of methanogens and decrease their growth (Naumann et al., 2017), decrease fiber degradation (Carulla et al., 2005), and act as a hydrogen sink (Becker et al., 2014). However, as total-tract NDF digestibility and dH2 in ruminal fluid were not affected by treatments in the present study, it appears that the rapid hydrolyzation of the HT component relative to the complex HT forms may have caused GA to be more toxic to methanogens than TA and CN. The metabolites of GA (pyrogallol, resorcinol, and phloroglucinol) can be hydrolyzed to acetate and butyrate by rumen microbes to generate energy (McSweeney et al., 2001). In the present study, GA tended to increase VFA compared with the control and TA and this may partly explain the 9% decrease in CH4 emission (% per DE intake) for animals fed the GA diet compared with animals fed the control and TA diets.
In summary, adding different complex forms of HT (TA or CN) and a component of HT (GA) to a high-protein alfalfa silage diet fed to heifers had no effects on apparent total-tract DM and fiber digestibilities, but TA and CN decreased apparent CP digestibility. Only TA decreased NH3-N concentration in rumen fluid, but all 3 forms of HT tended to reduce isobutyrate proportion in the rumen and decreased PUN concentration. Both complex forms of HT increased fecal N excretion and shifted N excretion from urine to feces compared with the control and GA treatments. However, regardless the form of HT, including its subunit from gallotannin, urea-N as a proportion of urinary N decreased compared with the control. Also, the results indicate that GA altered the N fractions in urine by decreasing the proportion of uric acid in urinary N compared with TA, CN, and control. Dietary supplementation with complex forms of HT (TA and CN) did not affect CH4 production but a subunit of HT applied as GA tended to decrease (g/kg DMI) or decreased CH4 (% GE and DE intakes) by 9% compared with the control.
In conclusion, this study demonstrates that the response to HT by beef cattle fed a high-protein forage diet depends on the composition of HT. Gallic acid, which is a component of HT, was more efficient in altering the N fractions of urine by reducing both urea and uric acid N without negatively affecting CP digestibility. However, because GA did not shift N excretion from feces to urine but regulated the urinary N fraction components, it may not reduce the overall NH3 emission in manure relative to TA and CN. On the contrary, GA reduced CH4 production from beef cattle, unlike TA and CN. We conclude that GA has the potential to lower CH4 and N2O emissions from beef cattle, without reducing feed digestibility in cattle consuming a high-protein forage diet.
Footnotes
This study was conducted with financial support from Agriculture and Agri-Food Canada. We thank K. Andrews, B. Farr, L. Holtshausen, D. Vedres, N. Johnson, J.-F. Coulombe, A. Romero-Perez, and E. Cassiano of the Lethbridge Research and Development Centre, AB, Canada, for their invaluable technical assistance. We also thank R. Merrill and the metabolism barn staff for their care of the experimental animals.
Lethbridge Research and Development Centre contribution number is 38718065.
LITERATURE CITED
- Aboagye I. A., Oba M., Castillo A. R., Koenig K. M., Iwaasa A. D., and Beauchemin K. A.. 2018. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 96:5276–5286. doi:10.1093/jas/sky352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., and Peel J. L.. 1971. The biosynthesis of valine from isobutyrate by Peptostreptococcus elsdenii and Bacteroides ruminicola. Biochem. J. 121:431–437. doi:10.1042/bj1210431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC). 2005. Official methods of analysis, 18th ed. AOAC Int., Gaithersburg, MD. [Google Scholar]
- Beauchemin K. A., Kreuzer M., O’Mara F., and McAllister T. A.. 2008. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 48:21–27. doi:10.1071/EA07199 [Google Scholar]
- Beauchemin K. A., and McGinn S. M.. 2006. Enteric methane emissions from growing beef cattle as affected by diet and level of intake. Can. J. Anim. Sci. 86:401–408. doi:10.4141/A06-021 [Google Scholar]
- Becker P. M., Wikselaar P. G., Franssen M. C. R., Vos R. C. H., Hall R. D., and Beekwilder J.. 2014. Evidence for a hydrogen-sink mechanism of (+)catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics 10:179–189. doi:10.1007/s11306-013-0554-5 [Google Scholar]
- Berard N. C., Wang Y., Wittenberg K. M., Krause D. O., Coulman B. E., McAllister T. A., and Ominski K. H.. 2011. Condensed tannin concentrations found in vegetative and mature forage legumes grown in western Canada. Can. J. Plant Sci. 91:669–675. doi:10.4141/cjps10153 [Google Scholar]
- Bhatta R., Saravanan M., Baruah L., Sampath K. T., and Prasad C. S.. 2013. Effect of plant secondary compounds on in vitro methane, ammonia production and ruminal protozoa population. J. Appl. Microbiol. 115:455–465. doi:10.1111/jam.12238 [DOI] [PubMed] [Google Scholar]
- Calsamiglia S., Ferret A., Reynolds C. K., Kristensen N. B., and van Vuuren A. M.. 2010. Strategies for optimizing nitrogen use by ruminants. Animal 4:1184–1196. doi:10.1017/S1751731110000911 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC). 2009. CCAC guidelines on: The care and use of farm animals in research, teaching and testing. CCAC, Ottawa, ON, Canada. [Google Scholar]
- Carulla J. E., Kreuzer M., Machmüller A., and Hess H. D.. 2005. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Austr. J. Agric. Res. 56:961–970. [Google Scholar]
- Chen X. B., and Gomes M. J.. 1992. Estimation of microbial protein supply to sheep and cattle based on purine derivatives: An overview of technical details. Int. Feed Res. Unit. Rowett Res. Inst., Bucksburn, Aberdeen, UK: p. 1–21. [Google Scholar]
- Chiarini A., Micucci M., Malaguti M., Budriesi R., Ioan P., Lenzi M., Fimognari C., Gallina Toschi T., Comandini P., and Hrelia S.. 2013. Sweet chestnut (Castanea sativa Mill.) Bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxid. Med. Cell. Longev. 2013:471790. doi:10.1155/2013/471790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle D. J., Nolan J. V., and Wiedemann S. G.. 2011. Ruminant enteric methane mitigation: A review. Anim. Prod. Sci. 51:491–514. doi:10.1071/AN10163 [Google Scholar]
- Dijkstra J., Oenema O., van Groenigen J. W., Spek J. W., van Vuuren A. M., and Bannink A.. 2013. Diet effects on urine composition of cattle and N2O emissions. Animal 7(Suppl. 2):292–302. doi:10.1017/S1751731113000578 [DOI] [PubMed] [Google Scholar]
- Djakpo O., and Yao W.. 2010. Rhus chinensis and Galla chinensis – folklore to modern evidence: Review. Phytother. Res. 24:1739–1747. doi:10.1002/ptr.3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos P., Raso M., Hervás G., Mantecón A., Pérez V., and Giráldez F. J.. 2004. Is there any detrimental effect when a chestnut hydrolysable tannin extract is included in the diet of finishing lambs? Anim. Res. 53:127–136. doi:10.1051/animres:2004001 [Google Scholar]
- Gerber P. J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., Falcucci A., and Tempio G.. 2013. Tackling climate change through livestock: A global assessment of emissions and mitigation opportunities. FAO, Rome, Italy. [Google Scholar]
- Getachew G., Pittroff W., Putnam D. H., Dandekar A., Goyal S., and DePeters E. J.. 2008. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim. Feed Sci. Technol. 140:444–461. doi:10.1016/j.anifeedsci.2007.03.011 [Google Scholar]
- Goel G., Puniya A. K., Aguilar C. N., and Singh K.. 2005. Interaction of gut microflora with tannins in feeds. Naturwissenschaften 92:497–503. doi:10.1007/s00114-005-0040-7 [DOI] [PubMed] [Google Scholar]
- Guyader J., Ungerfeld E. M., and Beauchemin K. A.. 2017. Redirection of metabolic hydrogen by inhibiting methanogenesis in the rumen simulation technique (RUSITEC). Front. Microbiol. 8:393. doi:10.3389/fmicb.2017.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman A. E. 2011. Tannin handbook Dept. Chem. Biochem., Miami Univ; http://www.users.muohio.edu/hagermae (Accessed 17 October 2017.) [Google Scholar]
- Hagerman A. E., Robbins C. T., Weerasuriya Y., Wilson T., and McArthur C.. 1992. Tannin chemistry in relation to digestion. J. Range Manag. 45:57–62. [Google Scholar]
- Hegarty R. S. 1999. Reducing rumen methane emissions through elimination of rumen protozoa. Aust. J. Agric. Res. 50:1321–1327. doi:10.1071/AR99008 [Google Scholar]
- Hristov A. N., Hanigan M., Cole A., Todd R., McAllister T. A., Ndegwa P. M., and Rotz A.. 2011. Review: Ammonia emissions from dairy farms and beef feedlots. Can. J. Anim. Sci. 91:1–35. doi:10.4141/CJAS10034 [Google Scholar]
- Janssen P. H. 2010. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 160:1–22. doi:10.1016/j.anifeedsci.2010.07.002 [Google Scholar]
- Jayanegara A., Goel G., Makkar H. P. S., and Becker K.. 2010. Reduction in methane emissions from ruminants by plant secondary metabolites: Effects of polyphenols and saponins. In: Odongo N. E., Garcia M., Viljoen G. J., editors, Sustainable improvement of animal production and health. FAO, Rome, Italy: p. 151–157. [Google Scholar]
- Jayanegara A., Goel G., Makkar H. P. S., and Becker K.. 2015. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 209:60–68. doi:10.1111/jpn.12531 [Google Scholar]
- Kawamoto H., Nakatsubo F., and Murakami K.. 1995. Quantitative determination of tannin and protein in the precipitates by high-performance liquid chromatography. Phytochemistry 40:1503–1505. doi:10.1016/0031-9422(95)00451-C [Google Scholar]
- Koenig K. M., McGinn S. M., and Beauchemin K. A.. 2013. Ammonia emissions and performance of backgrounding and finishing beef feedlot cattle fed barley-based diets varying in dietary crude protein concentration and rumen degradability. J. Anim. Sci. 91:2278–2294. doi:10.2527/jas.2012–5651 [DOI] [PubMed] [Google Scholar]
- Krueger W. K., Gutierrez-Bañuelos H., Carstens G. E., Min B. R., Pinchak W. E., Gomez R. R., Anderson R. C., Krueger N. A., and Forbes T. D. A.. 2010. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 159:1–9. doi:10.1016/j.anifeedsci.2010.05.003 [Google Scholar]
- Liu H., Vaddella V., and Zhou D.. 2011. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J. Dairy Sci. 94:6069–6077. doi:10.3168/jds.2011-4508 [DOI] [PubMed] [Google Scholar]
- McAllister T. A., Martinez T., Bae H. D., Muir A. D., Yanke L. J., and Jones G. A.. 2005. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J. Chem. Ecol. 31:2049–2068. doi:10.1007/s10886-005-6077-4 [DOI] [PubMed] [Google Scholar]
- McSweeney C. S., Palmer B., McNeill D. M., and Krause D. O.. 2001. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 91:83–93. doi:10.1016/S03778401(01)00232-2 [Google Scholar]
- Morgavi D. P., Forano E., Martin C., and Newbold C. J.. 2010. Microbial ecosystem and methanogenesis in ruminants. Animal 4:1024–1036. doi:10.1017/S1751731110000546 [DOI] [PubMed] [Google Scholar]
- Murdiati T. B., McSweeney C. S., and Lowry J. B.. 1991. Complexing of toxic hydrolysable tannins of yellow-wood (Terminalia oblongata) and harendong (Clidemia hirta) with reactive substances: An approach to preventing toxicity. J. Appl. Toxicol. 11:333–338. doi:10.1002/jat.2550110506 [DOI] [PubMed] [Google Scholar]
- Murdiati T. B., McSweeney C. S., and Lowry J. B.. 1992. Metabolism in sheep of gallic acid, tannic acid, and hydrolysable tannin from Terminalia oblongata. Aust. J. Agric. Res. 43:1307–1319. doi:10.1071/AR9921307 [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM) 2016. Nutrient requirements of beef cattle, 8th rev. ed. The National Academies Press, Washington, DC. doi:177226/19014 [Google Scholar]
- Naumann H. D., Tedeschi L. O., Zeller W. E., and Huntley N. F.. 2017. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. R. Bras. Zootec. 46:929–949. doi:10.1590/s1806-92902017001200009 [Google Scholar]
- Osawa R., and Walsh T. P.. 1993. Effects of acidic and alkaline treatments on tannic acid and its binding property to protein. J. Agric. Food Chem. 41:704–7. [Google Scholar]
- Patra A. K., and Saxena J.. 2011. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 91:24–37. doi:10.1002/jsfa.4152 [DOI] [PubMed] [Google Scholar]
- Penner G. B., Beauchemin K. A., and Mutsvangwa T.. 2006. An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. J. Dairy Sci. 89:2132–2140. doi:10.3168/jds.S0022-0302(06)72284-6 [DOI] [PubMed] [Google Scholar]
- Rhine E. D., Sims G. K., Mulvaney R. L., and Pratt E. J.. 1998. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci. Soc. Am. J. 62:473–480. doi:10.2136/sssaj1998.03615995006200020026x [Google Scholar]
- Tavendale M. H., Meagher L. P., Pacheco D., Walker N., Attwood G. T., and Sivakumaran S.. 2005. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 123:403–419. [Google Scholar]
- Veeramani A., Dias G. M., and Kirkpatrick S.. 2017. Carbon footprint of dietary patterns in Ontario, Canada: A case study based on actual food consumption. J. Clean. Prod. 162:1398–1406. doi:10.1016/j.jclepro.2017.06.025 [Google Scholar]
- Veira D. M., Ivan M., and Jui P. Y.. 1983. Rumen ciliate protozoa: Effects on digestion in the stomach of sheep. J. Dairy Sci. 66:1015–1022. doi:10.3168/jds.S0022-0302(83)81896-7 [DOI] [PubMed] [Google Scholar]
- Waghorn G. C. 2008. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production – progress and challenges. Anim. Feed Sci. Technol. 147:116–139. doi:10.1016/j.anifeedsci.2007.09.013 [Google Scholar]
- Wang M., Wang R., Janssen P. H., Zhang X. M., Sun X. Z., Pacheco D., and Tan Z. L.. 2016. Sampling procedure for the measurement of dissolved hydrogen and volatile fatty acids in the rumen of dairy cows. J. Anim. Sci. 94:1159–1169. doi:10.2527/jas.2015-9658 [DOI] [PubMed] [Google Scholar]
- Wei C., Guyader J., Collazos L., Beauchemin K. A., and Zhao G. Y.. 2019. Effects of gallic acid on in vitro rumen fermentation and methane production using rumen simulation (Rusitec) and batch-culture techniques. Anim. Prod. Sci. 59:277–287. doi:10.1071/AN17365 [Google Scholar]
- Wei C., Yang K., Zhao G., Lin S., and Xu Z.. 2016. Effect of dietary supplementation of gallic acid on nitrogen balance, nitrogen excretion pattern and urinary nitrogenous constituents in beef cattle. Arch. Anim. Nutr. 70:416–423. doi:10.1080/1745039X.2016.1214345 [DOI] [PubMed] [Google Scholar]
- White R. R., and Hall M. B.. 2017. Nutritional and greenhouse gas impacts of removing animals from US agriculture. Proc. Natl. Acad. Sci. USA 114:E10301–E10308. doi:10.1073/pnas.1707322114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischer G., Greiling A. M., Boguhn J., Steingass H., Schollenberger M., Hartung K., and Rodehutscord M.. 2014. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Animal 8:938–948. doi:10.1017/S1751731114000639 [DOI] [PubMed] [Google Scholar]
- Yang K., Wei C., Zhao G. Y., Xu Z. W., and Lin S. X.. 2017. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. J. Anim. Physiol. Anim. Nutr. (Berl.) 101:302–310. doi:10.1111/jpn.12531 [DOI] [PubMed] [Google Scholar]