Abstract
This study aimed to evaluate the effects of a source of dietary soluble (SF) and insoluble fiber (IF) without or with exogenous carbohydrases (xylanase, β-glucanase, and pectinase) on diarrhea incidence, selected immune responses, and growth performance in enterotoxigenic Escherichia coli (ETEC)-challenged pigs. Sixty weaned pigs (6.9 ± 0.1 kg BW, ~23 d of age) were blocked by initial BW and placed in individual pens. Pens were randomly assigned to one of six treatments (n = 10 per treatment), including a nonchallenged control (NC), a positive challenge control (PC), the PC + a soluble fiber diet (10% sugar beet pulp) without (SF−) or with carbohydrases (SF+), and PC + an IF diet (15% corn distillers dried grains with solubles) without (IF−) or with carbohydrases (IF+). The control diet was primarily based on corn and soybean meal with 13.5% whey powder. The two sources of fiber were added at the expense of cornstarch in the control diet. Pigs were orally inoculated with 6 mL hemolytic F18 ETEC (~3.5 × 109 cfu/mL) or sham infected with 6 mL phosphate-buffered saline on day 7 (0 d postinoculation, dpi) postweaning. All ETEC challenged pigs were confirmed to be genetically susceptible to F18 ETEC. Pigs had free access to feed and water throughout the 14-d trial. Pig BW and feed intake were recorded on dpi −7, 0, and 7 or 8. Fecal swabs were collected on dpi −7, 0, 1, 2, 3, 5, and 7 or 8 to evaluate hemolytic E. coli shedding. Fecal score was visually ranked daily postchallenge to evaluate diarrhea incidence. Blood samples were collected on dpi −1, 3, and 7 or 8 at necropsy and intestinal tissues were collected at necropsy. Pigs on PC had lower dpi 1 to 7 ADG and ADFI than those on NC (P < 0.05). Compared with PC pigs, SF+ pigs had greater ADG during both pre- and postchallenge period (P < 0.05). The IF− increased postchallenge diarrhea incidence compared with PC (P < 0.05). Pigs on SF− had lower ileal E. coli attachment than PC (P < 0.05). The SF+ reduced haptoglobin and IF+ reduced C-reactive protein on dpi 3 compared with PC (P < 0.05). Compared with PC pigs, SF+ pigs tended to have lower ileal tumor necrosis factor alpha and greater ileal occludin (OCLN) mRNA (P < 0.10) and had greater (P < 0.05) colonic OCLN mRNA levels. Collectively, IF− increased incidence of diarrhea and fecal E. coli shedding compared with PC. The SF+ pigs had improved growth compared with PC pigs, likely due in part to a reduction in inflammatory intermediates.
Keywords: diarrhea, dietary fiber, enterotoxigenic E. coli, enzymes, immune response, swine
Introduction
Postweaning diarrhea (PWD) associated with enterotoxigenic Escherichia coli (ETEC) usually occurs between 4 and 14 d postweaning and causes economic losses for pig producers worldwide (Fairbrother and Gyles, 2012). The ETEC, mainly F4 and F18 strains, adheres to specific receptors on enterocytes through fimbria followed by colonization and secretion of enterotoxins, which ultimately lead to secretory diarrhea in weaned pigs (Zhang et al., 2007; Luppi, 2017). With the use of antibiotics in animal production being restricted, alternative nutritional strategies are needed to control PWD and improve piglet health and performance. Dietary fiber is indigestible by pig endogenous enzymes but can be degraded by intestinal microbes. It is widely accepted that feeding dietary fiber may be beneficial to gut function (in terms of physiology, mucosal immunity, and barrier integrity) and microbioal population through microbial fermentation in the hindgut of pigs (Molist et al., 2014; Jha and Berrocoso, 2015).
There is growing interest in the inclusion of dietary fiber in pig diets to improve intestinal barrier function (e.g., increased tight junction protein mRNA; Chen et al., 2013) and enhance disease resistance through modulation of intestinal microbiota, microbial metabolites, and immune response (e.g., production of pro- and anti-inflammatory cytokines). However, results regarding the impact of dietary fiber on PWD caused by ETEC have been inconsistent, probably due to differences in the sources, chemical composition, inclusion level, and physiochemical properties of the fibers used, as well as individual differences in gut microbial populations and health status of pigs (Hopwood et al., 2004; Wellock et al., 2008; Molist et al., 2010).
Exogenous carbohydrases aid in fiber degradation and liberate entrapped nutrients, thus can be used in swine diets with high fiber contents to improve growth performance (Tsai et al., 2017; Li et al., 2018). Carbohydrases, particularly xylanase, have been shown to break down nonstarch polysaccharides in fibrous feed ingredients to release low molecular weight oligosaccharides or monosaccharides (Lærke et al., 2015; Pedersen et al., 2015). This results in the release of entrapped nutrients and subsequent improvements in energy and nutrient digestibility (Patience, 2017; Zeng et al., 2018) and an associated decrease in digesta viscosity (Lærke et al., 2015). A recent study in our research team showed that a blend of xylanase, β-glucanase, and cellulase improved small intestinal markers of barrier integrity (Li et al., 2018). Thus, it is reasonable to hypothesize that adding carbohydrases to fiber containing diets could further promote intestinal barrier function, beneficially modulate intestinal microbiota, and decrease the occurrence of diarrhea, which eventually leads to improved performance. Currently, limited information is available about the potential protective effect of carbohydrase on ETEC induced PWD in pigs.
Therefore, the objectives of this study were to evaluate the effects of a source of dietary soluble (SF) and insoluble fiber (IF) without or with exogenous carbohydrases (xylanase, β-glucanase, and pectinase) on the incidence of diarrhea, immune response, and growth performance in ETEC-challenged weaned pigs. It was hypothesized that the inclusion of a source of SF or IF would mitigate PWD and the addition of carbohydrases would provide additional benefits on alleviating PWD and improving performance.
MATERIALS AND METHODS
All procedures for this experiment adhered to guidelines for the ethical and humane care of animals used for research and were approved by the Institutional Animal Care and Use Committee at Iowa State University (IACUC #6-16-8306-S and #16-I-0027-A) prior to the start of this study.
Animals, Diets, and Experimental Design
Sixty newly weaned piglets (6.9 ± 0.1 kg; ~23 d of age; L337 × Camborough; PIC Inc., Hendersonville, TN) were blocked by initial BW and allocated to individual pens. Pens were randomly assigned to one of six treatments (n = 10 per treatment), including a nonchallenged control (NC), a positive challenge control (PC), the PC + a soluble fiber diet without (SF−) or with carbohydrases (SF+), and the PC + an IF diet without (IF−) or with carbohydrases (IF+). Three feed enzymes were added on top of the SF− and IF− diets to make the SF+ and IF+ diets, with 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet), based on the manufacturer’s recommendations (AB Vista, Plantation, FL). The control diet was primarily based on corn and soybean meal with 13.5% whey powder. Sugar beet pulp (SBP; 10%) or corn distillers dried grains with solubles (DDGS; 15%) were added to the control diet at the expense of cornstarch to supply the soluble or IF, respectively. Pelleted SBP was ground to a similar particle size as DDGS using a 2.5-mm screen to avoid the confounding effect of different particle sizes. All diets were formulated to meet or exceed NRC (2012) estimates of requirements of weaned pigs and did not contain antibiotics or pharmacological levels of copper or zinc (Table 1). Pigs had free access to feed presented in mash form and water throughout the 14-d experiment. Each pen (~4 ft2) was equipped with a four-space polyethylene dry feeder and one nipple drinker. Analyzed nutrient composition of SBP, DDGS, and diets is shown in Table 2.
Table 1.
Ingredient composition of the experimental diets (as-fed basis, %)
| Diets1 | |||
|---|---|---|---|
| Item | Control | SF | IF |
| Corn | 41.79 | 42.11 | 42.40 |
| Cornstarch | 15.00 | 5.00 | — |
| Soybean meal, 46.5% CP | 15.00 | 15.00 | 15.00 |
| DDGS2 | — | — | 15.00 |
| Sugar beet pulp | — | 10.00 | — |
| Fish meal, menhaden select | 5.00 | 5.00 | 5.00 |
| Whey powder, >61% lactose | 13.50 | 13.50 | 13.50 |
| Casein | 4.00 | 4.00 | 4.00 |
| Soybean oil | 2.50 | 2.50 | 2.50 |
| Monocalcium phosphate | 0.56 | 0.56 | 0.20 |
| Limestone | 0.95 | 0.73 | 1.10 |
| Sodium chloride | 0.25 | 0.25 | 0.25 |
| l-Lys HCl | 0.50 | 0.46 | 0.42 |
| dl-Met | 0.23 | 0.22 | 0.11 |
| l-Thr | 0.20 | 0.18 | 0.09 |
| l-Trp | 0.05 | 0.04 | 0.03 |
| l-Val | 0.07 | 0.05 | - |
| Vitamin premix3 | 0.25 | 0.25 | 0.25 |
| Trace mineral premix4 | 0.15 | 0.15 | 0.15 |
| Calculated nutrient levels, % | |||
| ME, Mcal/kg | 3.53 | 3.43 | 3.42 |
| NE, Mcal/kg | 2.68 | 2.53 | 2.50 |
| CP | 19.96 | 20.71 | 23.67 |
| Ether extract | 4.67 | 4.77 | 5.22 |
| Neutral detergent fiber | 5.04 | 9.56 | 10.16 |
| Acid detergent fiber | 2.00 | 4.35 | 4.55 |
| Total dietary fiber | 8.24 | 14.99 | 13.03 |
| Starch | 40.07 | 31.17 | 28.30 |
| Calcium | 0.80 | 0.80 | 0.80 |
| Total P | 0.60 | 0.61 | 0.64 |
| STTD P | 0.43 | 0.43 | 0.43 |
| SID Lys | 1.44 | 1.44 | 1.44 |
| SID Met + Cys | 0.79 | 0.79 | 0.79 |
| SID Thr | 0.85 | 0.85 | 0.85 |
| SID Trp | 0.26 | 0.26 | 0.26 |
1SF = soluble fiber diets without or with carbohydrases; IF = insoluble fiber diets without or with carbohydrases; the carbohydrases were added on top of the SF and IF diets, with 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet), based on the manufacturer’s recommendations (AB Vista, Plantation, FL).
2Distiller’s dried grains with solubles.
3Provided per kilogram of diet: 7,656 IU vitamin A, 875 IU vitamin D, 63 IU vitamin E, 4 mg vitamin K, 70 mg niacin, 34 mg pantothenic acid, 14 mg riboflavin, and 0.06 mg vitamin B12.
4Provided per kg of diet: 165 mg Zn (zinc sulfate), 165 mg Fe (iron sulfate), 39 mg Mn (manganese sulfate), 17 mg Cu (copper sulfate), 0.3 mg I (calcium iodate), and 0.3 mg Se (sodium selenite).
Table 2.
Analyzed nutrient composition of fibrous ingredients and experimental diets (as-fed basis, %)
| Item1 | Ingredient2 | Diets3 | |||||
|---|---|---|---|---|---|---|---|
| Beet pulp | DDGS | Control | SF− | SF+ | IF− | IF+ | |
| DM | 92.78 | 89.57 | 89.77 | 90.05 | 90.06 | 89.98 | 89.63 |
| GE, Mcal/kg | 3.82 | 4.48 | 4.02 | 4.05 | 4.06 | 4.17 | 4.17 |
| CP | 9.41 | 28.09 | 18.40 | 19.38 | 19.74 | 23.21 | 22.32 |
| aEE | 1.94 | 7.04 | 5.00 | 5.33 | 5.57 | 6.61 | 6.25 |
| NDF | 35.70 | 28.67 | 4.83 | 8.52 | 8.32 | 9.54 | 9.67 |
| ADF | 22.14 | 7.85 | 1.48 | 3.44 | 3.22 | 2.65 | 2.51 |
| Hemicellulose | 13.56 | 20.83 | 3.34 | 5.08 | 5.10 | 6.88 | 7.16 |
| SDF | 17.10 | 1.70 | 0.70 | 2.10 | 1.90 | 1.00 | 1.20 |
| IDF | 43.80 | 31.10 | 8.60 | 11.60 | 11.20 | 11.30 | 11.40 |
| TDF | 60.90 | 32.80 | 9.30 | 13.70 | 13.10 | 12.30 | 12.60 |
1GE = gross energy; aEE = acid ether extract; NDF = neutral detergent fiber; ADF = acid detergent fiber; SDF = soluble dietary fiber; IDF = insoluble dietary fiber; TDF = total dietary fiber; hemicellulose = NDF – ADF; TDF = SDF + IDF.
2Distillers dried grains with solubles.
3SF−: soluble fiber diet included 10% sugar beet pulp without carbohydrases; SF+: soluble fiber diet included 10% sugar beet pulp with carbohydrases; IF−: insoluble fiber diet 15% distiller’s dried grains with solubles without carbohydrases; IF+: insoluble fiber diet 15% distiller’s dried grains with solubles with carbohydrases; the carbohydrases were added on top of the SF and IF diets, with 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet), based on the manufacturer’s recommendations (AB Vista, Plantation, FL).
This experiment was conducted in a biosecurity level 2 facility at Iowa State University. Challenged pigs were housed in two rooms (25 pigs per room; five pigs per treatment) and nonchallenged pigs were housed in a separate room. Strict biosecurity procedures were followed to avoid ETEC contamination of the nonchallenged pigs. All pigs were given 6 mL of freshly grown F18 ETEC inoculum (~3.5 × 109 cfu/mL) or 6 mL of a sham inoculum of PBS via oral gavage on day 7 (0 d postinoculation, dpi) postweaning. Sows and piglets used in this experiment were not vaccinated against E. coli before this trial. None of the pigs shed hemolytic E. coli on dpi −7. All ETEC challenged pigs were confirmed to be genetically susceptible to F18 ETEC by genotype sequencing of the α (1,2) fucosyltransferase-1 gene according to Frydendahl et al. (2003).
Inoculum Preparation
A hemolytic F18 ETEC strain expressing heat-labile, heat-stable b, and enteroaggregative E. coli heat-stable enterotoxin 1 was recovered from the intestine of a nursery pig with enteric colibacillosis and used to prepare the bacterial inoculum. Briefly, a fresh bacterial culture grown (~18 h at 37 °C) on blood agar was used to inoculate two bottles (each having 50 mL) of sterile tryptic soy broth (TSB), which were then incubated overnight at 37 °C with shaking. The broth cultures were then transferred to two new bottles (each containing 450 mL fresh TSB) and incubated for an additional 5 h at 37 °C with shaking. The bacterial culture was centrifuged for 15 min at 3,000 × g and the pellet was suspended in sterile PBS. The OD600 of the culture in PBS was measured to be about 7.0 using a spectrometer, which had ~3.5 × 109 cfu/mL as determined using viable plate count.
Sample Collection
Pig BW and feed intake were recorded on dpi −7, 0, and 7 or 8 at the end of the trial to calculate ADG, ADFI, and G:F. Fresh fecal swabs were collected by rectal swabbing on dpi −7, 0 (immediately before ETEC inoculation), 1, 2, 3, 5, and 7 or 8 to evaluate hemolytic E. coli shedding. After collecting rectal swabs for feces, the rectal temperature was obtained from every pig via rapid-response digital electric thermometers (Model V911F/V912F, KAZ, Incorporated, Hudson, NY). Fecal score was visually ranked daily using the following scale: 1 = solid, 2 = semi-solid, 3 = semi-liquid, and 4 = liquid. Fecal score ≥3 was considered diarrhea (Liu et al., 2013). The incidence of diarrhea (%) = (total pig days with a fecal score ≥ 3)/(total pig days) × 100. Blood samples were collected from the jugular vein by venipuncture into 10-mL vacuum containers (Becton Dickinson, Franklin Lakes, NJ) on dpi −1 (as baseline), 3 (expected peak response), and 7 or 8 at necropsy (recovery). The blood was centrifuged at 2,000 × g for 10 min at 4 °C. The resulting sera were aliquoted into 1.5-mL microcentrifuge tubes and stored at −80 °C for later analysis of acute phase proteins (APPs).
Each half of the pigs (five pigs per treatment) was euthanized on dpi 7 and 8 by captive bolt stunning followed by exsanguination. For the convenience of result description, dpi 7 was used to indicate necropsy days and the end of this trial. Posteuthanasia, the abdomen was opened and the entire gastrointestinal tract was removed. The ileum (30 cm from the ileal–cecal junction) and mid-colon tissues were collected, rinsed with ice-cold PBS, snap-frozen in liquid N, and stored at −80 °C pending analysis of gene expression. Three 1-cm pieces of jejunum and ileum were fixed with 10% neutral buffered formalin for 24 h and then transferred to 75% alcohol for later microscopic assessment of E. coli attachment.
Chemical Analyses
Diets and the two fibrous ingredients were ground to 1 mm and analyzed in duplicate for DM (method 930.15; AOAC, 2007), nitrogen (method 990.03; AOAC, 2007; TruMac; LECO Corp., St. Joseph, MI), and acid-hydrolyzed ether extract (method 2003.06; AOAC, 2007). The EDTA was used (9.56% N; Leco Corporation) as a standard for N calibration and was determined to contain 9.56 ± 0.03% of N. CP was calculated as N × 6.25. Gross energy was determined in duplicate using an isoperibolic bomb calorimeter (Model 6200; Parr Instrument Co., Moline, IL). Benzoic acid (6,318 kcal GE/kg) was used as the standard for calibration and was determined to contain 6,316 ± 4 kcal GE/kg. Neutral and acid detergent fiber were analyzed in triplicate (Van Soest and Robertson, 1979). Soluble and IF were analyzed in duplicate (method 991.43; AOAC, 2007) using an Ankom TDF Fiber Analyzer (Ankom Technology, Macedon, NY).
Fecal Hemolytic E. coli Shedding
Fecal swabs were plated onto Remel Blood Agar (TSA with 5% sheep blood) followed by incubation at 35 °C for 24 h to determine hemolytic E. coli shedding using a semi-quantification method according to the growth of E. coli on the plates. The E. coli shedding was scored on a five-point scale from 0 to 4 with 0 representing no growth, 1 represens hemolytic colonies only in the primary streak, 2 represens compatible growth extending into the secondary streak, 3 represens growth into the tertiary streak, and 4 represents growth of hemolytic E. coli into the quaternary section of agar plate. The accumulative shedding score on each collection day was calculated by summing up the shedding scores of 10 pigs in each treatment. Identification of isolates as E. coli was confirmed by matching the mass spectrometry (MS) spectrum of the isolates with the MS spectra of E. coli in the open database using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS; Singhal et al., 2015) at the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL).
E. coli Attachment to Epithelial Cells in the Small Intestine
Formalin fixed jejunum and ileum were routinely processed and embedded in paraffin wax at the ISU VDL. Three transverse sections (5 μm) were cut from both jejunum and ileum, stained with hematoxylin and eosin, and mounted on glass slides. The E. coli attachment on epithelial cells was visualized using a microscope (OLYMPUS BX 53, Center Valley, PA) with a 40× power. Each section was scored as either 0 if there was no attachment (Supplemental Figure 1A) or 1 if there were ≥5 villi that had E. coli attached among all the villi of each section (Supplemental Figure 1B). The E. coli attachment frequency (%) of jejunum or ileum was calculated by summing up the scores of all three sections on each glass slide and then divided by 3.
Serum APP
Haptoglobin and C-reactive protein (CRP) concentration in the serum were analyzed in duplicate using commercially available porcine ELISA kits according to the manufacturer’s instructions (Immunology Consultants Laboratory, Inc., Portland, OR).
RNA Isolation and Quantitative PCR
Approximately 50 to 100 mg of ileal tissue was homogenized in 1 mL of Trizol (Invitrogen, Carlsbad, CA) using the Qiagen Tissuelyser II (Germantown, MD). Total RNA was then isolated according to the manufacturer’s recommendations. Concentration and RNA purity was measured using a spectrophotometer (ND-100; NanoDrop Technologies, Inc., Rockland, DE). All samples had 260:280 nm ratios above 1.8. Isolated RNA (1 μg) was used for cDNA synthesis using the QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. All cDNA samples were diluted 10-fold with nuclease free water for qPCR reactions.
Real-time quantitative PCR was performed in 20-µL reactions using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA). Each reaction included 10 µL of SYBR Green Supermix, 1 µL of each forward and reverse primer (10 µM), 2 µL of cDNA and 6 µL of nuclease free water. Gene-specific primer sequences are shown in Table 3. A no-reverse transcriptase control, a water control, and a pooled cDNA reference sample was included in each 96-well plate. Each sample was performed in triplicate. The PCR cycling conditions included 5-min initial denaturation at 95 °C followed by 40 PCR cycles (95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s) and a dissociation curve to confirm amplification of a single PCR product. Optical detection was performed at 55 °C. Fluorescence of SYBR Green was quantified with the iQ5 Real-Time PCR Detection System and cycle threshold (CT) value for each reaction was obtained by analysis of amplification plots with the iQ5 Optical System Software version 2.0 (Bio-Rad Laboratories Inc.). Ribosomal protein L19 (RPL19) was used as an endogenous reference gene. The mRNA abundance for each sample was normalized to RPL19 and the pooled sample using the 2−ΔΔCTmethod (Livak and Schmittgen, 2001).
Table 3.
Primer sequences used for quantitative PCR
| Gene | Primer sequence | Product size, bp | GenBank accession |
|---|---|---|---|
| TNFα | F: CACCACGCTCTTCTGCCTAC | 132 | X57321 |
| R: ACGGGCTTATCTGAGGTTTGAGACG | |||
| IL-1B | F: TGGCCCACACATGCTGAA | 84 | NM_214055 |
| R: CCTTGCACAAAGCTCATGCA | |||
| IL-6 | F: GGCTGTGCAGATTAGTACC | 124 | AF518322 |
| R: CTGTGACTGCAGCTTATCC | |||
| IL-8 | F: AGGACCAGAGCCAGGAA | 172 | NM_213867 |
| R: GTGGAATGCGTATTTATGC | |||
| IL-10 | F: TGGGTTGCCAAGCCTTGT | 61 | L20001 |
| R: GCCTTCGGCATTACGTCTTC | |||
| CLDN1 | F: GATTTACTCCTACGCTGGTGAC | 199 | AJ318102 |
| R: CACAAAGATGGCTATTAGTCCC | |||
| CLDN3 | F: TTGCATCCGAGACCAGTCC | 85 | NM_001160075 |
| R: AGCTGGGGAGGGTGACA | |||
| OCLN | F: AACTCCCGTCAGCAGATCC | 95 | NM_001163647 |
| R: ATCAGTGGAAGTTCCTGAACCA | |||
| ZO-1 | F: CTCTTGGCTTGCTATTCG | 197 | XM_003353439 |
| R: AGTCTTCCCTGCTCTTGC | |||
| TLR4 | F: CAGATAAGCGAGGCCGTCATT | 113 | AB232527 |
| R: TTGCAGCCCACAAAAAGCA | |||
| CD14 | F: CCTCAGACTCCGTAATGTG | 180 | AB267810 |
| R: CCGGGATTGTCAGATAGG | |||
| MYD88 | F: GCTGGAACAGACCAACTAT | 153 | NM_001099923.1 |
| R: TCCTTGCTTTGCAGGTAAT | |||
| CFTR | F: ACTATGGACCCTTCGAGCCT | 123 | NM_001104950 |
| R: CGCATTTGGAACCAGCGTAG | |||
| RPL19 | F: AACTCCCGTCAGCAGATCC | 147 | AF435591 |
| R: AGTACCCTTCCGCTTACCG |
TNFα = tumor necrosis factor alpha; CLDN1 = claudin-1; CLDN3 = claudin-3; OCLN = occludin; ZO-1 = zonula occludens-1; TLR4 = toll-like receptor 4; CD14 = cluster of differentiation 14; MYD88 = myeloid differentiation primary response 88; CFTR = cystic fibrosis transmembrane conductance regulator; RPL19 = ribosomal protein L19.
Statistical Analysis
Data were analyzed according to the randomized complete block design using PROC GLIMMIX of SAS (9.4; SAS Institute Inc., Cary, NC). Two pigs in PC treatment were removed due to excessive loss of weight (>1 kg). Probably due to weaning stress, a few pigs had negative G:F values during the prechallenge period. All G:F < −2 were removed from the analysis. Treatment, sex, and their interaction were fixed effects. Pen was the experimental unit. Block was a random effect.
Fecal score and E. coli shedding score data were analyzed using a multinomial model in PROC GENMOD. The frequency of occurrence of each score was identified in the FREQ statement. Diarrhea incidence data were analyzed by a χ2 test. The odds ratio of each treatment was reported. The E. coli attachment data were analyzed using PROC GLIMMIX with a binomial distribution. Preplanned contrasts were performed using the ESTIMATE statement to evaluate the effects of the ETEC challenge (NC vs. PC) and dietary treatment (SF−, SF+, IF−, or IF+ vs. PC), and to compare the effect of fiber sources (SF vs. IF), without or with enzymes, as well as fiber by enzyme interactions. Least square means of treatment were reported. Differences were considered significant if P ≤ 0.05 and a tendency if 0.05 < P ≤ 0.10.
RESULTS
Growth Performance
Pigs on the PC had lower (P < 0.05) postchallenge (dpi 1 to 7) ADG and ADFI than those on NC, resulting in lower (P < 0.05) final BW and overall ADG (Table 4). During the prechallenge period, SF− had no impact on pig performance parameters compared with PC, whereas SF+ improved (P < 0.05) pig BW and ADG, and tended (P < 0.10) to improve G:F. Growth performance of pigs fed IF, regardless of exogenous carbohydrases addition, was not different from those fed PC during the prechallenge period. During the postchallenge period, pigs on SF+ had greater (P < 0.05) ADG and a trend (P < 0.10) for greater ADFI than PC pigs. This resulted in improved (P < 0.05) final BW, ADG, and G:F in pigs fed SF+ compared with PC during the 14-d trial. The SF− pigs tended (P < 0.10) to have improved ADG during dpi 1 to 7 and had improved (P < 0.05) overall G:F compared with PC pigs. A tendency (P < 0.10) for greater ADG during dpi 1 to 7 and the overall period, but not ADFI or G:F, was observed in IF− pigs compared with PC pigs. Growth performance of pigs on IF+ did not differ from PC during the pre- and postchallenge or overall period.
Table 4.
Effect of soluble or IF without or with carbohydrases on growth performance and diarrhea incidence in weaned pigs challenged with F18 ETEC1
| Item | Treatment2 | Contrast P-value3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | SF | IF | SEM | ||||||
| SF− | SF+ | IF− | IF+ | Fiber | Enzyme | F × E | ||||
| Pig no. | 10 | 8 | 10 | 10 | 10 | 10 | ||||
| BW, kg | ||||||||||
| Dpi −7 | 6.86 | 6.84 | 7.03 | 6.89 | 6.93 | 6.84 | 0.25 | 0.416 | 0.220 | 0.812 |
| Dpi 0 | 7.87 | 7.36 | 8.07 | 8.40* | 8.22# | 7.52 | 0.40 | 0.201 | 0.520 | 0.078 |
| Dpi 7 | 9.90* | 8.57 | 9.82# | 10.34* | 9.93* | 9.17 | 0.58 | 0.211 | 0.777 | 0.136 |
| Dpi −7 to 0 | ||||||||||
| ADG, kg | 0.14 | 0.09 | 0.15 | 0.22* | 0.18 | 0.10 | 0.04 | 0.305 | 0.833 | 0.063 |
| ADFI, kg | 0.24 | 0.17 | 0.18 | 0.24 | 0.21 | 0.16 | 0.03 | 0.404 | 0.974 | 0.076 |
| G:F | 0.78 | 0.60 | 0.87 | 0.91# | 0.86 | 0.66 | 0.01 | 0.210 | 0.435 | 0.245 |
| Dpi 1 to 7 | ||||||||||
| ADG, kg | 0.29* | 0.17 | 0.25# | 0.28* | 0.244# | 0.236 | 0.03 | 0.397 | 0.731 | 0.527 |
| ADFI, kg | 0.45* | 0.31 | 0.39 | 0.43# | 0.37 | 0.36 | 0.05 | 0.294 | 0.806 | 0.590 |
| G:F | 0.69 | 0.55 | 0.53 | 0.64 | 0.62 | 0.69 | 0.08 | 0.341 | 0.196 | 0.775 |
| Overall | ||||||||||
| ADG, kg | 0.22* | 0.13 | 0.20 | 0.25* | 0.22# | 0.17 | 0.03 | 0.266 | 0.986 | 0.107 |
| ADFI, kg | 0.31# | 0.23 | 0.27 | 0.32# | 0.28 | 0.24 | 0.03 | 0.294 | 0.843 | 0.208 |
| G:F | 0.62 | 0.51 | 0.76* | 0.76* | 0.65 | 0.65 | 0.18 | 0.179 | 0.981 | 0.950 |
| Diarrhea, %4 | ||||||||||
| Dpi −7 to 0 | 0 | 0 | 5.71 | 2.86 | 5.71 | 1.43 | — | 0.622 | 0.133 | 0.622 |
| Dpi 1 to 7 | 7.14* | 40.00 | 31.43 | 27.14 | 57.14* | 40.00 | — | <0.001 | 0.075 | 0.337 |
*Significant difference compared with PC (P ≤ 0.05).
#Tendency for difference compared with PC (0.05 < P ≤ 0.10).
1 n = 10 pigs per treatment except for PC with eight pigs.
2SF = soluble fiber diets included 10% sugar beet pulp without or with carbohydrases; IF = insoluble fiber diets 15% distiller’s dried grains with solubles without or with carbohydrases; SF− = soluble fiber diet without carbohydrases; SF+ = soluble fiber diet with carbohydrases; IF− = insoluble fiber diet without carbohydrases; IF+ = insoluble fiber diet with carbohydrases; the carbohydrases contained 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet), based on the manufacturer’s recommendations (AB Vista, Plantation, FL).
3Fiber = dietary fiber type effect (SF vs. IF); Enzyme = carbohydrases effect (without vs. with); F × E = fiber type by carbohydrases interaction effect.
4Diarrhea incidence (%) = (total number of pigs with diarrhea score ≥ 3)/(total number of pigs) × 100; statistical analysis was conducted by a χ2 test.
Diarrhea Incidence, Fecal Score, and Rectal Temperature
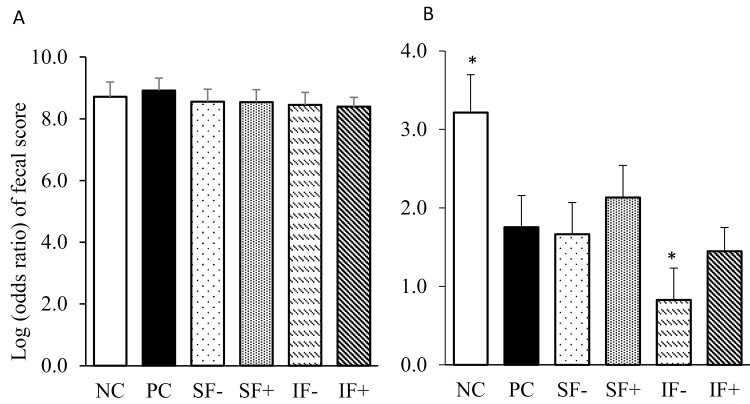
No mortality occurred during the experiment. During the prechallenge period (dpi −7 to 0), the incidence of diarrhea and the fecal scores were not different among treatments (Table 4; Figure 1A). During the postchallenge period, pigs on PC had a greater incidence of diarrhea than NC, but a lower incidence of diarrhea than IF− (P < 0.01). Meanwhile, PC decreased (P < 0.01) the odds ratio of the lower fecal score compared with NC, but increased the odds ratio of the lower fecal score compared with the IF− treatment (Figure 1B). In addition, the main effect of SF reduced (P < 0.05) the incidence of diarrhea compared with IF; enzyme supplementation tended (P < 0.10) to decrease the incidence of diarrhea compared with the two fiber diets without enzymes (SF− and IF−). There were no differences in rectal temperature among dietary treatments during the pre- or the postchallenge period (data not shown).
Figure 1.
Effects of soluble or IF without or with carbohydrases on the fecal score of pigs challenged with ETEC. (A) Prechallenge log (odds ratio) of fecal score, (B) postchallenge log (odds ratio) of fecal score. NC = nonchallenge control; PC = positive challenge control; SF− = soluble fiber diet included 10% sugar beet pulp without carbohydrases; SF+ = soluble fiber diet included 10% sugar beet pulp with carbohydrases; IF− = insoluble fiber diet 15% distiller’s dried grains with solubles without carbohydrases; IF+ = insoluble fiber diet 15% distiller’s dried grains with solubles with carbohydrases. The carbohydrases contained 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet) according to the manufacturer’s recommendations (AB Vista, Plantation, FL). P (IF vs. SF) < 0.001, P (enzyme) = 0.013, P (fiber × enzyme) > 0.10; *significant difference between NC vs. PC and IF− vs. PC (P < 0.05).
E. coli Shedding Score
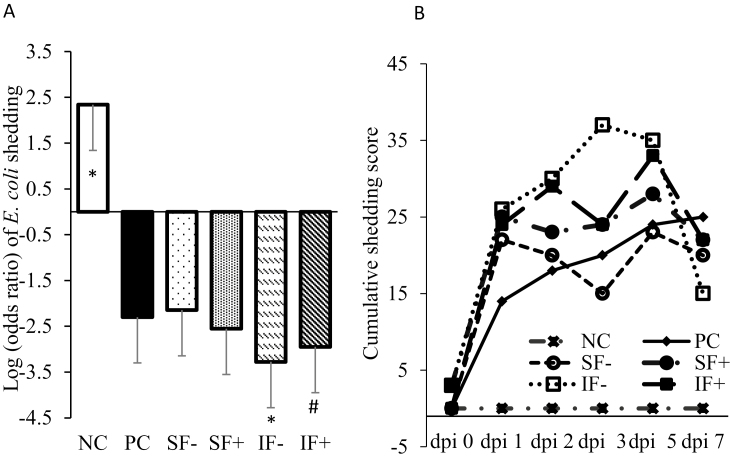
Pigs on NC had no hemolytic E. coli shedding and thus greater (P < 0.01) odds ratio of lower hemolytic E. coli shedding score compared with PC (Figure 2A). Pigs fed SF, regardless of enzyme addition, did not differ from PC in their postchallenge E. coli shedding score. The IF− reduced (P < 0.01) and IF+ tended (P < 0.10) to reduce the odds ratio of lower E. coli shedding score compared with PC. Pigs fed the SF diets had greater odds ratio of lower hemolytic E. coli shedding score than IF diets (P < 0.01). No hemolytic E. coli shedding was detected in NC pigs during dpi 1 to 7 before the ETEC challenge (Figure 2B).
Figure 2.
Effects of soluble or IF without or with carbohydrases on hemolytic E. coli shedding score of pigs challenged with ETEC. (A) Postchallenge log (odds ratio) of E. coli shedding score; (B) postchallenge cumulative E. coli shedding score. The accumulative shedding score on each collection day was calculated by summing up the shedding scores of 10 pigs in each treatment. NC = nonchallenge control; PC = positive challenge control; SF− = soluble fiber without carbohydrases; SF+ = soluble fiber with carbohydrases; IF− = insoluble fiber without carbohydrases; IF+ = insoluble fiber with carbohydrases. The carbohydrases contained 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet) according to the manufacturer’s recommendations (AB Vista, Plantation, FL). P (IF vs. SF) < 0.01, P (enzyme) > 0.10, P (fiber × enzyme) > 0.10. *Significant difference between NC vs. PC and IF− vs. PC (P < 0.01). #Trends between IF+ vs. PC (P < 0.10).
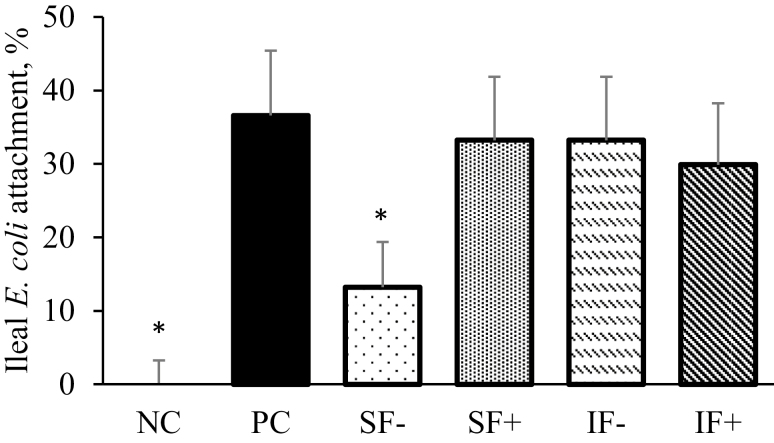
E. coli Attachment to Epithelial Cells
No differences were observed for E. coli attachment on jejunal epithelium among treatments (data not shown). As expected, pigs on NC had no E. coli attachment in the ileum (Figure 3). Compared with PC pigs, SF− pigs had decreased (P < 0.05) E. coli attachment in the ileum. No differences in E. coli attachment in the ileum were observed between pigs fed PC and those fed SF+, IF−, or IF+.
Figure 3.
Effects of soluble or IF diet without or with carbohydrases on ileal E. coli attachment of pigs challenged with ETEC. NC = nonchallenge control; PC = positive challenge control; SF− = soluble fiber without carbohydrases; SF+ = soluble fiber with carbohydrases; IF− = insoluble fiber without carbohydrases; IF+ = insoluble fiber with carbohydrases. The E. coli attachment frequency (%) was calculated by summing up the scores (E. coli attachment as 1 and no attachment as 0) of all three ileal sections on the glass slide and then divided by 3. The carbohydrases contained 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet) according to the manufacturer’s recommendations (AB Vista, Plantation, FL). *Significant difference between NC vs. PC and SF− vs. PC (P < 0.05).
Serum APPs
There were no differences in serum haptoglobin or CRP between PC pigs and NC pigs on any collection day (Table 5). Serum haptoglobin concentration was similar among treatments before challenge. There was a fiber × enzyme interaction for CRP on dpi −1, such that enzyme addition decreased (P < 0.01) CRP concentration in IF diets, but not in SF diets. On dpi 3, enzyme supplementation decreased (P < 0.05) haptoglobin concentration, regardless of fiber source. Serum haptoglobin on dpi 3 was lower (P < 0.05) in SF+ pigs, but not SF− pigs, compared with those on PC. The IF+ tended (P < 0.10) to decrease haptoglobin on dpi 7 and decreased (P < 0.05) CRP concentration on dpi 3 compared with PC. No diet effects were observed for CRP on dpi 7.
Table 5.
Effect of soluble or IF without or with carbohydrases on serum haptoglobin and C-reactive protein (CRP) in weaned pigs challenged with F18 ETEC1
| Item | Treatment2 | Contrast P-value3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | SF | IF | SEM | ||||||
| SF− | SF+ | IF− | IF+ | Fiber | Enzyme | F × E | ||||
| Haptoglobin, mg/mL | ||||||||||
| dpi −1 | 1.81 | 1.74 | 1.35 | 1.74 | 1.35 | 1.86 | 0.53 | 0.712 | 0.259 | 0.953 |
| dpi 3 | 0.52 | 0.65 | 0.29 | 0.34* | 0.52 | 0.29 | 0.21 | 0.035 | 0.042 | 0.287 |
| dpi 7 | 0.58 | 0.86 | 0.57 | 0.40 | 0.83 | 0.41# | 0.36 | 0.344 | 0.565 | 0.948 |
| CRP, μg/mL | ||||||||||
| dpi −1 | 304 | 382 | 328 | 379 | 485 | 190 | 116 | 0.579 | 0.136 | 0.012 |
| dpi 3 | 200 | 423 | 346 | 353 | 333 | 189* | 160 | 0.358 | 0.681 | 0.217 |
| dpi 7 | 184 | 149 | 153 | 150 | 385 | 122 | 183 | 0.947 | 0.246 | 0.184 |
*Significant difference compared with PC (P ≤ 0.05).
#Tendency for difference compared with PC (0.05 < P ≤ 0.10).
1 n = 10 pigs per treatment except for PC with eight pigs; P (day) < 0.05 and P (day × trt) > 0.10 for haptoglobin and CRP; least square means and pooled SEM were from data before log transformation.
2SF = soluble fiber diets included 10% sugar beet pulp without or with carbohydrases; IF = insoluble fiber diets 15% distiller’s dried grains with solubles without or with carbohydrases; SF− = soluble fiber diet without carbohydrases; SF+ = soluble fiber diet with carbohydrases; IF− = insoluble fiber diet without carbohydrases; IF+ = insoluble fiber diet with carbohydrases; the carbohydrases contained 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet), based on the manufacturer’s recommendations (AB Vista, Plantation, FL).
3Fiber = dietary fiber type effect (SF vs. IF); Enzyme = carbohydrases effect (without vs. with); F × E = fiber type by carbohydrases interaction effect.
Ileal and Colonic Gene Expression
Pigs on NC had lower mRNA abundance for IL-8, toll-like receptor 4 (TLR4), cluster of differentiation 14 (CD14), and greater mRNA abundance of claudin-1 (CLDN1) in the ileum than those on PC (P < 0.05; Table 6). There were no differences in mRNA levels of IL-1B, IL-6, claudin-3 (CLDN3), myeloid differentiation factor 88, and cystic fibrosis transmembrane conductance regulator in both ileum and colon among any treatments (data not shown). The ileal mRNA levels of tumor necrosis factor alpha (TNFα), IL-10, occludin (OCLN), and zonula occludens 1 (ZO-1) did not differ between NC and PC. The SF− pigs tended (P < 0.10) to have lower IL-10 and had greater (P < 0.05) CLDN1 mRNA in the ileum compared with PC pigs. A trend (P < 0.10) for lower TNFα and greater OCLN mRNA abundance in the ileum was observed in SF+ pigs than PC pigs. The IF+ tended (P = 0.052) to decrease TLR4 and decreased (P < 0.05) CD14 mRNA in the ileum compared with PC. In the colon, greater (P < 0.05) mRNA abundance of ZO-1 was observed in NC pigs than those on PC. Pigs fed SF+ tended (P = 0.051) to have greater ZO-1 and had greater (P < 0.05) mRNA abundance of OCLN than pigs on PC. The IF− diet did not impact mRNA levels of any genes in the ileum and colon. The main effect of the SF diet increased (P < 0.05) mRNA expression of CLDN1 in the ileum and OCLN and ZO-1 in the colon compared with IF diet. A fiber × enzyme interaction was observed for colonic IL-10 mRNA abundance; IF− increased (P < 0.05) IL-10 mRNA compared with SF−, but IF+ did not differ from SF+.
Table 6.
Effect of soluble or IF without or with carbohydrases on ileal and colonic gene mRNA abundance in weaned pigs challenged with F18 ETEC1
| Treatment3 | Contrast P-value4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SF | IF | |||||||||
| Gene2 | NC | PC | SF− | SF+ | IF− | IF+ | SEM | Fiber | Enzyme | F × E |
| Ileum | ||||||||||
| TNFα | 1.56 | 1.66 | 1.48 | 1.17# | 1.45 | 1.46 | 0.25 | 0.357 | 0.420 | 0.328 |
| IL-8 | 0.59* | 1.40 | 1.27 | 1.50 | 1.50 | 1.59 | 0.56 | 0.588 | 0.270 | 0.804 |
| IL-10 | 0.47 | 0.86 | 0.45# | 0.53 | 0.61 | 0.47 | 0.21 | 0.581 | 0.934 | 0.868 |
| CLDN1 | 2.32* | 0.96 | 3.11* | 1.14 | 1.22 | 0.77 | 0.93 | 0.026 | 0.086 | 0.757 |
| OCLN | 0.76 | 0.78 | 1.56 | 2.11# | 1.79 | 1.17 | 0.53 | 0.219 | 0.481 | 0.787 |
| ZO-1 | 1.76 | 1.32 | 1.47 | 1.41 | 1.45 | 1.21 | 0.27 | 0.423 | 0.497 | 0.466 |
| TLR4 | 1.35* | 1.68 | 1.62 | 1.42 | 1.49 | 1.38# | 0.18 | 0.471 | 0.314 | 0.934 |
| CD14 | 1.00* | 1.32 | 1.22 | 1.22 | 1.13 | 1.01* | 0.18 | 0.252 | 0.925 | 0.441 |
| Colon | ||||||||||
| TNFα | 1.30 | 1.30 | 1.15 | 1.38 | 1.35 | 1.28 | 0.23 | 0.711 | 0.367 | 0.323 |
| IL-8 | 1.49 | 0.90 | 1.07 | 1.40 | 1.04 | 1.43 | 0.32 | 0.724 | 0.167 | 0.978 |
| IL-10 | 1.21 | 1.36 | 1.05 | 1.59 | 2.04 | 1.31 | 0.34 | 0.237 | 0.797 | 0.038 |
| CLDN1 | 1.19 | 1.49 | 2.10 | 0.92 | 0.90 | 0.66 | 1.00 | 0.487 | 0.567 | 0.436 |
| OCLN | 1.16 | 0.92 | 1.18 | 1.29* | 0.88 | 0.93 | 0.18 | 0.011 | 0.385 | 0.615 |
| ZO-1 | 1.18* | 0.87 | 0.93 | 1.13# | 0.81 | 0.87 | 0.11 | 0.024 | 0.172 | 0.525 |
*Significant difference compared with PC (P ≤ 0.05).
#Tendency for difference compared with PC (0.05 < P ≤ 0.10).
1 n = 10 pigs per treatment except for PC with eight pigs; least square means of fold change were reported.
2 TNFα = tumor necrosis factor alpha; IL = interleukin; CLDN1 = claudin-1; OCLN = occludin; ZO-1 = zonula occludens-1; TLR4 = toll-like receptor 4; CD14 = cluster of differentiation 14.
3SF = soluble fiber diets included 10% sugar beet pulp without or with carbohydrases; IF = insoluble fiber diets 15% distiller’s dried grains with solubles without or with carbohydrases; SF− = soluble fiber diet without carbohydrases; SF+ = soluble fiber diet with carbohydrases; IF− = insoluble fiber diet without carbohydrases; IF+ = insoluble fiber diet with carbohydrases; the carbohydrases contained 0.01% pectinase (Pectinase ABE; 56 PE per kg of diet), 0.01% xylanase (Econase XT; 19,000 BXU per kg of diet), and 0.001% β-glucanase (Econase GT P; 23,200 BU per kg of diet), based on the manufacturer’s recommendations (AB Vista, Plantation, FL).
4Fiber = dietary fiber type effect (SF vs. IF); Enzyme = carbohydrases effect (without vs. with); F × E = fiber type by carbohydrases interaction effect.
Discussion
PWD induced by ETEC impairs growth performance of piglets due to decreased feed intake, intestinal villus atrophy, and an elevated inflammatory response. Previous research evaluating the impact of dietary fiber (different solubility, viscosity, and fermentability) in ETEC-challenged pigs have reported inconsistent results (Hopwood et al., 2004; Wellock et al., 2008; Molist et al., 2010). This study evaluated the effect of two fibrous ingredients (DDGS and SBP) that are commonly available and used in North American swine diets without or with carbohydrases in pigs challenged with F18 ETEC.
Postinoculation, pigs on PC presented an increased incidence of diarrhea, shedding of hemolytic E. coli, and adhesion of E. coli on ileal enterocytes compared with pigs on NC, confirming that the challenge model was successful. As expected, the ETEC challenge decreased pig BW and ADG during dpi 1 to 7 and the overall period, in agreement with previous research (Song et al., 2012; Liu et al., 2013). This was partly due to the reduction in ADFI and increased diarrhea postchallenge. Compared with PC pigs, while SF− pigs tended to increase ADG during dpi 1 to 7, SF+ pigs significantly improved ADG during both dpi 1 to 7 and the overall experimental period. This indicates that the inclusion of SF− alleviated the growth depression caused by ETEC infection and the addition of exogenous carbohydrases further mitigated the negative effects of ETEC infection on growth. Limited research has evaluated the effect of carbohydrase supplementation in diets containing SBP in pigs. Nevertheless, under nonchallenged conditions, carbohydrase addition in poultry diets containing 7.5% SBP improved ADG and ADFI compared with diets without carbohydrases (Abdel-Hafeez et al., 2018). Taken together, the data suggest that the addition of multiple carbohydrases degraded the fiber components (mainly pectin) in the SBP diet to liberate the cell wall entrapped nutrients (starch and protein) for digestion and absorption in the small intestine, which will be used by the pig with greater efficiency than if fermented in the hindgut (Patience, 2017). Furthermore, the released oligosaccharides from fiber degradation may enhance gut barrier function and reduce intestinal immune activation (Chen et al., 2012). It is also possible that enzyme supplementation in SBP-diets decreased endogenous losses of AA (from mucin secretion and sloughed epithelial cells) in the small intestine compared with SBP-diets without enzymes (Cowieson and Bedford, 2009).
Soluble fiber from SBP, irrespective of enzyme addition, did not reduce the incidence of diarrhea and the shedding of hemolytic E. coli compared with PC. Soluble fiber from pear barley and carboxymethylcellulose in nursery diets has previously been reported to increase digesta viscosity and stimulate ETEC proliferation in the intestine, thereby exacebating PWD (Hopwood et al., 2004; Montagne et al., 2004). However, Van Nevel et al. (2006) reported no increase in digesta viscosity by feeding 12% SBP in nursery diets. Because digesta viscosity was not measured, it remains unknown whether the 10% SBP used in this study resulted in increased viscosity compared with the control diet and whether the increase caused negative effects in the pigs. Despite the fact that the exact mechanisms of soluble fiber from SBP on performance and ETEC proliferation are not yet known, the inclusion of SBP in pig diets was reported to beneficially modulate intestinal microbial populations, such as reduces pathogenic bacteria and increases Bifidobacterium and Lactobacillus counts (Thomson et al., 2012; Laitat et al., 2015; Yan et al., 2017). Thus, the decreased attachment of E. coli on ileal epithelium in SF− group herein may result from increased beneficial bacteria (e.g., Lactobacillus) and decreased pathogenic bacteria in the small intestine (Roselli et al., 2007). It would also be possible that some soluble fibrous fractions in SBP directly bind to intestinal ETEC (González-Ortiz et al., 2014), leading to the decreased E. coli attachment in SF−.
In contrast, the inclusion of IF from DDGS, regardless of enzyme addition, increased hemolytic E. coli shedding compared with PC, suggesting increased pathogenic E. coli proliferation in the intestine. Furthermore, IF− increased the fecal score and the incidence of diarrhea compared with PC, in contradiction to results reported by Mateos et al. (2006) and Molist et al. (2010). This indicates the sources and physiochemical properties, in addition to solubility, of dietary fiber affect the pig’s response to bacterial infection (Molist et al., 2014). The exact reason for increased diarrhea and E. coli shedding in IF− remains unclear. One potential contributing factor for the diarrhea could be the increased CP content of the IF diet by 4.2% compared with the control diet (Kim et al., 2011; Opapeju et al., 2015). Additionally, feeding 30% DDGS decreased the relative abundance of Lactobacillus spp. in the colonic microbiota of pigs relative to pigs fed a standard corn–soy diet (Burrough et al., 2015). Such a reduction in Lactobacilli may lead to increased E. coli shedding because Lactobacillus can limit ETEC adhesion (Roselli et al., 2007).
Besides causing diarrhea, ETEC infection is also known to increase intestinal permeability through disruption of the tight junction structure (reduction of OCLN expression, delocalization of ZO-1, and dissociation of occludin from intercellular junctions; Berkes et al., 2003; Mukiza and Dubreuil, 2013). The decreased ileal CLDN1 and colonic ZO-1 mRNA abundance following an ETEC challenge observed herein agrees with previous findings (Gao et al., 2013). Tight junctions play a critical role in maintaining intestinal epithelial barrier integrity, which prevents bacteria and bacterial products (e.g., endotoxin) translocation across the intestinal tissues and the subsequent activation of an immune response (Roselli et al., 2007). The greater mRNA abundance of ileal and colonic OCLN and colonic ZO-1 in pigs fed SF+ compared with the PC suggests improved small and large intestinal paracellular barrier integrity, which in turn can reduce translocation of luminal pathogenic bacteria and bacterial products (e.g., endotoxins) and result in decreased markers of inflammation (ileal TNFα and serum haptoglobin; Cutler et al., 2007). The greater mRNA abundance for tight junction proteins in pigs fed SF compared with those fed IF may result from enhanced microbial fermentation and therefore increased production of fermentation products, especially acetate and butyrate (unpublished data in this study; Schiavon et al., 2004); these have been demonstrated to promote intestinal epithelial barrier integrity and regulate the inflammatory response (Wang et al., 2018).
APPs are liver-derived proteins and their concentrations in the blood are modulated by pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) after an immune challenge (Carroll et al., 2004; Jain et al., 2011). Elevated serum APP concentrations on day 3 and up to day 11 post-ETEC infection were previously reported (Houdijk et al., 2007; Liu et al., 2013; Kim et al., 2016). In contrast, the ETEC challenge in PC did not increase haptoglobin or CRP on dpi 3 and 7 compared with NC, suggesting a lack of systemic inflammation elicited by ETEC. The discrepancy in inflammatory response to an ETEC challenge is associated with the severity of the infection, the strain or dose of the inoculum, and type of enterotoxins expressed by the ETEC strain (Pavlova et al., 2008; Zhou et al., 2012; Loos et al., 2012). Another explanation might be that collecting blood on dpi 3 may have already missed the peak response because maximum serum APP concentration is typically reached within 24 to 48 h after the inflammation (Jain et al., 2011). Nevertheless, pigs on SF+ had lower haptoglobin and IF+ had lower CRP than PC pigs on dpi 3, which may indicate the addition of enzymes in fiber diets reduced systemic inflammation activation of pigs challenged with ETEC (Kiarie et al., 2009). This in turn potentially diverts more nutrients and energy toward growth production (Huntley et al., 2018; Li et al., 2018).
An experimental ETEC challenge induces not only systemic but also a local inflammatory response (Liu et al., 2013). In addition to recognition by antigen-presenting cells (M cells or dendritic cells) in the lamina propria, luminal bacteria can also be taken up by enterocytes through endocytosis, causing increased release of pro-inflammatory cytokines (Snoeck et al., 2005). The ETEC challenge in the current study increased mRNA levels of TLR4, CD14, and IL-8, indicating immune activation. These findings agree with Liu et al. (2014) who reported upregulated mRNA expression of genes related to immune activation after an ETEC infection. The increased IL-8 mRNA with ETEC infection was also reported by Roselli et al. (2007). Increased production of IL-8 was associated with pathogen-induced disruption of the epithelial barrier (Otte and Podolsky, 2004; Roselli et al., 2007). The decreased mRNA levels for ileal CLDN1 and colonic ZO-1 in pigs fed PC compared with those fed NC agrees with the notion of perturbed barrier function. Despite the observation that SF− enhanced CLDN1 and SF+ increased OCLN and ZO-1 compared with PC, no significant differences were observed in pro-inflammatory cytokines mRNA abundance except for TNFα (tended to be decreased by SF+). One possible explanation would be that the inclusion of dietary fiber accelerated the rate of recovery of pigs from an ETEC infection, thus resulting in no differences in cytokine expression levels when tissues were collected at the end of the trial (Correa-Matos et al., 2003).
In conclusion, the F18 ETEC challenge resulted in colonization of hemolytic E. coli as evidence by fecal shedding and adherence of E. coli to ileal epithelial cells, which consequently increased the incidence of diarrhea and caused decreases in ADFI and ADG during dpi 1 to 7. Compared with PC, SF+ improved ADG during both the pre- and postchallenge period. This may be partly due to increased ADFI and markers of gut barrier integrity (OCLN and ZO-1 mRNA), and reduced markers of inflammation (TNFα mRNA and serum haptoglobin). The SF− tended to have improved ADG during dpi 1 to 7, which was likely associated with decreased ileal E. coli attachment and increased ileal CLDN1 mRNA levels, compared with PC. Pigs on IF− had a greater incidence of diarrhea and E. coli shedding than pigs on PC without negatively affecting growth performance. Collectively, inclusion of SBP, a soluble and highly fermentable fiber combined with a carbohydrase complex, may be used to improve growth performance in pigs under moderate F18 ETEC challenge. The use of corn DDGS, an insoluble and less fermentable fiber, should be avoided in nursery pig diets if they are at risk of PWD. Future studies are warranted to explore the exact mechanisms whereby carbohydrase supplementation in SBP containing diets improved growth performance.
Conflict of interest statement. None declared.
Supplementary Material
Footnotes
The authors would like to thank the Attorney General of Iowa (Smithfield Foods funds) for financial support of this research, and AB Vista, Ajinomoto Heartland, DSM Nutritional Products, and Hamlet Protein for in-kind support. Appreciation is also expressed to Dr. Christopher Tuggle for assistance with the genotype sequencing to test the genetic sensitivity of weaned pigs to F18 ETEC.
LITERATURE CITED
- Abdel‐Hafeez H., Saleh E., Tawfeek S., Youssef I., and Abdel‐Daim A.. 2018. Utilization of potato peels and sugar beet pulp with and without enzyme supplementation in broiler chicken diets: effects on performance, serum biochemical indices and carcass traits. J. Anim. Physiol. Anim. Nutr. 102:56–66. doi:10.1111/jpn.12656 [DOI] [PubMed] [Google Scholar]
- AOAC 2007. Official methods of analysis. 18th rev. ed.Gaithersburg, MD:AOAC International. [Google Scholar]
- Berkes J., Viswanathan V. K., Savkovic S. D., and Hecht G.. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 52:439–451. doi:10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrough E. R., Arruda B. L., Patience J. F., and Plummer P. J.. 2015. Alterations in the colonic microbiota of pigs associated with feeding distillers dried grains with solubles. PLoS One. 10:e0141337. doi:10.1371/journal.pone.0141337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J., Fangman T., Hambach A., and Wiedmeyer C.. 2004. The acute phase response in pigs experimentally infected with Escherichia coli and treated with systemic bactericidal antibiotics. Livest. Prod. Sci. 85:35–44. doi:10.1016/S0301-6226(03)00115-5 [Google Scholar]
- Chen H. H., Chen Y. K., Chang H. C., and Lin S. Y.. 2012. Immunomodulatory effects of xylooligosaccharides. Food Sci. Technol. Res. 18:195–199. doi:org/10.3136/fstr.18.195 [Google Scholar]
- Chen H., Mao X., He J., Yu B., Huang Z., Yu J., Zheng P., and Chen D.. 2013. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 110:1837–1848. doi:10.1017/S0007114513001293 [DOI] [PubMed] [Google Scholar]
- Correa-Matos N. J., Donovan S. M., Isaacson R. E., Gaskins H. R., White B. A., and Tappenden K. A.. 2003. Fermentable fiber reduces recovery time and improves intestinal function in piglets following salmonella typhimurium infection. J. Nutr. 133:1845–1852. doi:10.1093/jn/133.6.1845 [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., and Bedford M. R.. 2009. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? World Poult. Sci. J. 65:609–624. doi:10.1017/S0043933909000427 [Google Scholar]
- Cutler S. A., Lonergan S. M., Cornick N., Johnson A. K., and Stahl C. H.. 2007. Dietary inclusion of colicin e1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob. Agents Chemother. 51:3830–3835. doi:10.1128/AAC.00360-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother J. M. and Gyles C. L. 2012. Colibacillosis. In: Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., Stevenson G. W., editors, Disease of swine. 10th ed.Ames, IA:Wiley-Blackwell; p. 723–47. [Google Scholar]
- Frydendahl K., Kåre Jensen T., Strodl Andersen J., Fredholm M., and Evans G.. 2003. Association between the porcine Escherichia coli F18 receptor genotype and phenotype and susceptibility to colonisation and postweaning diarrhoea caused by E. coli O138:F18. Vet. Microbiol. 93:39–51. doi:10.1016/S0378-1135(02)00348-6 [DOI] [PubMed] [Google Scholar]
- Gao Y., Han F., Huang X., Rong Y., Yi H., and Wang Y.. 2013. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J. Anim. Sci. 91:5614–5625. doi:10.2527/jas.2013-6528 [DOI] [PubMed] [Google Scholar]
- González-Ortiz G., Pérez J. F., Hermes R. G., Molist F., Jiménez-Díaz R., and Martín-Orúe S. M.. 2014. Screening the ability of natural feed ingredients to interfere with the adherence of enterotoxigenic Escherichia coli (ETEC) K88 to the porcine intestinal mucus. Br. J. Nutr. 111:633–642. doi:10.1017/S0007114513003024 [DOI] [PubMed] [Google Scholar]
- Hopwood D. E., Pethick D. W., Pluske J. R., and Hampson D. J.. 2004. Addition of pearl barley to a rice-based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post-weaning colibacillosis. Br. J. Nutr. 92:419–427. doi:10.1079/BJN20041206 [DOI] [PubMed] [Google Scholar]
- Houdijk J., Campbell F. M., Fortomaris P., Eckersall P., and Kyriazakis I.. 2007. Effects of sub‐clinical post‐weaning colibacillosis and dietary protein on acute phase proteins in weaner pigs. Livest. Sci. 108:182–185. doi:10.1016/j.livsci.2007.01.048 [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi:10.1186/s40104-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Gautam V., and Naseem S.. 2011. Acute-phase proteins: as diagnostic tool. J. Pharm. Bioallied Sci. 3:118–127. doi:10.4103/0975-7406.76489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., and Berrocoso J. D.. 2015. Review: dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 9:1441–1452. doi:10.1017/S1751731115000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Slominski B., Krause D., and Nyachoti C.. 2009. Acute phase response of piglets fed diets containing non-starch polysaccharide hydrolysis products and egg yolk antibodies following an oral challenge with Escherichia coli (k88). Can. J. Anim. Sci. 89:353–360. doi:10.4141/CJAS09008 [Google Scholar]
- Kim J., Heo J., Mullan B., and Pluske J.. 2011. Efficacy of a reduced protein diet on clinical expression of post-weaning diarrhoea and life-time performance after experimental challenge with an enterotoxigenic strain of Escherichia coli. Anim. Feed Sci. Technol. 170:222–230. doi:10.1016/j.anifeedsci.2011.08.012 [Google Scholar]
- Kim J. C., Mullan B. P., Black J. L., Hewitt R. J., van Barneveld R. J., and Pluske J. R.. 2016. Acetylsalicylic acid supplementation improves protein utilization efficiency while vitamin E supplementation reduces markers of the inflammatory response in weaned pigs challenged with enterotoxigenic E. coli. J. Anim. Sci. Biotechnol. 7:58. doi:10.1186/s40104-016-0118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lærke H. N., Arent S., Dalsgaard S., and Bach Knudsen K. E.. 2015. Effect of xylanases on ileal viscosity, intestinal fiber modification, and apparent ileal fiber and nutrient digestibility of rye and wheat in growing pigs. J. Anim. Sci. 93:4323–4335. doi:10.2527/jas.2015-9096 [DOI] [PubMed] [Google Scholar]
- Laitat M., Antoine N., Cabaraux J. F., Cassart D., Mainil J., Moula N., Nicks B., Wavreille J., and Philippe F. X.. 2015. Influence of sugar beet pulp on feeding behavior, growth performance, carcass quality and gut health of fattening pigs. Biotechnol. Agron. Soc. Environ. 19:20–31. [Google Scholar]
- Li Q., Gabler N. K., Loving C. L., Gould S. A., and Patience J. F.. 2018. A dietary carbohydrase blend improved intestinal barrier function and growth rate in weaned pigs fed higher fiber diets. J. Anim. Sci. 96:5233–5243. doi:10.1093/jas/sky383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T., Almeida J., Lee J., Bravo D., Maddox C., and Pettigrew J.. 2013. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 91:5294–5306. doi:10.2527/jas.2012–6194 [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T., Lee J., Bravo D., Maddox C., and Pettigrew J.. 2014. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection. J. Anim. Sci. 92:2050–2062. doi:10.2527/jas.2013–6422 [DOI] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Loos M., Geens M., Schauvliege S., Gasthuys F., van der Meulen J., Dubreuil J. D., Goddeeris B. M., Niewold T., and Cox E.. 2012. Role of heat-stable enterotoxins in the induction of early immune responses in piglets after infection with enterotoxigenic escherichia coli. PLoS One. 7:e41041. doi:10.1371/journal.pone.0041041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi A. 2017. Swine enteric colibacillosis: diagnosis, therapy and antimicrobial resistance. Porcine Health Manag. 3:16. doi:10.1186/s40813-017-0063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos G., Martin F., Latorre M., Vicente B., and Lazaro R.. 2006. Inclusion of oat hulls in diets for young pigs based on cooked maize or cooked rice. Anim. Sci. 82:57–63. doi:10.1079/ASC20053 [Google Scholar]
- Molist F., de Segura A. G., Pérez J., Bhandari S., Krause D., and Nyachoti C.. 2010. Effect of wheat bran on the health and performance of weaned pigs challenged with Escherichia coli K88+. Livest. Sci. 133:214–217. doi:10.1016/j.livsci.2010.06.067 [Google Scholar]
- Molist F., Van Oostrum M., Pérez J., Mateos G., Nyachoti C., and Van Der Aar P.. 2014. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim. Feed Sci. Technol. 189:1–10. doi:10.1016/j.anifeedsci.2013.12.013 [Google Scholar]
- Montagne L., Cavaney F. S., Hampson D. J., Lallès J. P., and Pluske J. R.. 2004. Effect of diet composition on postweaning colibacillosis in piglets. J. Anim. Sci. 82:2364–2374. doi:10.2527/2004.8282364x [DOI] [PubMed] [Google Scholar]
- Mukiza C. N., and Dubreuil J. D.. 2013. Escherichia coli STb enterotoxin impairs intestinal epithelial barrier function by altering tight junction proteins. Infect. Immun. 81:2819–2827. doi:10.1128/IAI.00455-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed.Washington, DC:National Academic Press. [Google Scholar]
- Opapeju F., Rodriguez-Lecompte J., Rademacher M., Krause D., and Nyachoti C.. 2015. Low crude protein diets modulate intestinal responses in weaned pigs challenged with Escherichia coli K88. Can. J. Anim. Sci. 95:71–78. doi:10.4141/CJAS-2014–071 [Google Scholar]
- Otte J. M., and Podolsky D. K.. 2004. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G613–G626. doi:10.1152/ajpgi.00341.2003 [DOI] [PubMed] [Google Scholar]
- Patience J. F. 2017. Meeting energy requirements in pig nutrition. In: Wiseman J. editor, Achieving sustainable production of pig meat. Cambridge, UK:Burleigh Dodds Science Publishing; p. 127–143. doi:10.19103/AS,2017.0013.07 [Google Scholar]
- Pavlova B., Volf J., Alexa P., Rychlik I., Matiasovic J., and Faldyna M.. 2008. Cytokine mRNA expression in porcine cell lines stimulated by enterotoxigenic Escherichia coli. Vet. Microbiol. 132:105–110. doi:10.1016/j.vetmic.2008.04.024 [DOI] [PubMed] [Google Scholar]
- Pedersen M. B., Yu S., Arent S., Dalsgaard S., Bach Knudsen K. E., and Lærke H. N.. 2015. Xylanase increased the ileal digestibility of nonstarch polysaccharides and concentration of low molecular weight nondigestible carbohydrates in pigs fed high levels of wheat distillers dried grains with solubles. J. Anim. Sci. 93:2885–2893. doi:10.2527/jas.2014-8829 [DOI] [PubMed] [Google Scholar]
- Roselli M., Finamore A., Britti M. S., Konstantinov S. R., Smidt H., de Vos W. M., and Mengheri E.. 2007. The novel porcine lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J. Nutr. 137:2709–2716. doi:10.1093/jn/137.12.2709 [DOI] [PubMed] [Google Scholar]
- Schiavon S., Tagliapietra F., Bailoni L., and Bortolozzo A.. 2004. Effects of sugar beet pulp on growth and health status of weaned piglets. Ital. J. Anim. Sci. 3:337–351. doi:10.4081/ijas.2004.337 [Google Scholar]
- Singhal N., Kumar M., Kanaujia P. K., and Virdi J. S.. 2015. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front. Microbiol. 6:791. doi:10.3389/fmicb.2015.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck V., Goddeeris B., and Cox E.. 2005. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 7:997–1004. doi:10.1016/j.micinf.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Song M., Liu Y., Soares J. A., Che T. M., Osuna O., Maddox C. W., and Pettigrew J. E.. 2012. Dietary clays alleviate diarrhea of weaned pigs. J. Anim. Sci. 90:345–360. doi:10.2527/jas.2010–3662 [DOI] [PubMed] [Google Scholar]
- Thomson L. W., Pieper R., Marshall J. K., and Van Kessel A. G.. 2012. Effect of wheat distillers dried grains with solubles or sugar beet pulp on prevalence of Salmonella enterica typhimurium in weaned pigs. J. Anim. Sci. 90(Suppl. 4):13–15. doi:10.2527/jas.53739 [DOI] [PubMed] [Google Scholar]
- Tsai T., Dove C., Cline P., Owusu-Asiedu A., Walsh M., and Azain M.. 2017. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livest. Sci. 197:46–52. doi:10.1016/j.livsci.2017.01.008 [Google Scholar]
- Van Soest P., and Robertson J.. 1979. Systems of analysis for evaluating fibrous feeds. In Standardization of analytical methodology for feeds: proceedings. Ottawa, ON, CA:IDRC. [Google Scholar]
- Van Nevel C. J., Dierick N. A., Decuypere J. A., and De Smet S. M.. 2006. In vitro fermentability and physicochemical properties of fibre substrates and their effect on bacteriological and morphological characteristics of the gastrointestinal tract of newly weaned piglets. Arch. Anim. Nutr. 60:477–500. doi:10.1080/17450390600 973659 [DOI] [PubMed] [Google Scholar]
- Wang C. C., Wu H., Lin F. H., Gong R., Xie F., Peng Y., Feng J., and Hu C. H.. 2018. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 24:40–46. doi:10.1177/1753425917741970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellock I. J., Fortomaris P. D., Houdijk J. G., Wiseman J., and Kyriazakis I.. 2008. The consequences of non-starch polysaccharide solubility and inclusion level on the health and performance of weaned pigs challenged with enterotoxigenic Escherichia coli. Br. J. Nutr. 99:520–530. doi:10.1017/S0007114507819167 [DOI] [PubMed] [Google Scholar]
- Yan C. L., Kim H. S., Hong J. S., Lee J. H., Han Y. G., Jin Y. H., Son S. W., Ha S. H., and Kim Y. Y.. 2017. Effect of dietary sugar beet pulp supplementation on growth performance, nutrient digestibility, fecal microflora, blood profiles and diarrhea incidence in weaning pigs. J. Anim. Sci. Technol. 59:18. doi:10.1186/s40781-017-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Li Q., Tian Q., Xu Y., and Piao X.. 2018. The combination of carbohydrases and phytase to improve nutritional value and non-starch polysaccharides degradation for growing pigs fed diets with or without wheat bran. Anim. Feed Sci. Technol. 235:138–146. doi:10.1016/j.anifeedsci.2017.11.009 [Google Scholar]
- Zhang W., Zhao M., Ruesch L., Omot A., and Francis D.. 2007. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 123:145–152. doi:10.1016/j.vetmic.2007.02.018 [DOI] [PubMed] [Google Scholar]
- Zhou C., Liu Z., Jiang J., Yu Y., and Zhang Q.. 2012. Differential gene expression profiling of porcine epithelial cells infected with three enterotoxigenic Escherichia coli strains. BMC Genomics 13:330. doi:10.1186/1471-2164-13-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.