Abstract
Antibiotic use has been limited in U.S. swine production. Therefore, the objective was to determine whether supplementing l-glutamine at cost-effective levels can replace dietary antibiotics to improve piglet welfare and productivity following weaning and transport. Based on previous research, we hypothesized that withholding dietary antibiotics would negatively affect pigs while diet supplementation with 0.20% l-glutamine (GLN) would have similar effects on pig performance and health as antibiotics. Mixed sex piglets (N = 480; 5.62 ± 0.06 kg BW) were weaned (18.4 ± 0.2 d of age) and transported for 12 h in central Indiana, for 2 replicates, during the summer of 2016 and the spring of 2017. Pigs were blocked by BW and allotted to 1 of 3 dietary treatments (n = 10 pens/dietary treatment/replicate [8 pigs/pen]); antibiotics (A; chlortetracycline [441 ppm] + tiamulin [38.6 ppm]), no antibiotics (NA), or GLN fed for 14 d. On days 15 to 34, pigs were provided common antibiotic-free diets in 2 phases. Data were analyzed using PROC MIXED in SAS 9.4. Day 14 BW and days 0 to 14 ADG were greater (P = 0.01) for A (5.6% and 18.5%, respectively) and GLN pigs (3.8% and 11.4%, respectively) compared with NA pigs, with no differences between A and GLN pigs. Days 0 to 14 ADFI increased for A (P < 0.04; 9.3%) compared with NA pigs; however, no differences were detected when comparing GLN with A and NA pigs. Once dietary treatments ceased, no differences (P > 0.05) in productivity between dietary treatments were detected. On day 13, plasma tumor necrosis factor alpha (TNF-α) was reduced (P = 0.02) in A (36.7 ± 6.9 pg/mL) and GLN pigs (40.9 ± 6.9 pg/mL) vs. NA pigs (63.2 ± 6.9 pg/mL). Aggressive behavior tended to be reduced overall (P = 0.09; 26.4%) in GLN compared with A pigs, but no differences were observed between A and GLN vs. NA pigs. Huddling, active, and eating/drinking behaviors were increased overall (P < 0.02; 179%, 37%, and 29%, respectively) in the spring replicate compared with the summer replicate. When hot carcass weight (HCW) was used as a covariate, loin depth and lean percentage were increased (P = 0.01; 4.0% and 1.1%, respectively) during the spring replicate compared with the summer replicate. In conclusion, GLN supplementation improved pig performance and health after weaning and transport similarly to A across replicates; however, the positive effects of A and GLN were diminished when dietary treatments ceased.
Keywords: antibiotics, L-glutamine, nursery, pigs, transport, weaning
INTRODUCTION
Weaning is a complex stressor associated with social, environmental, and metabolic stress in pigs (Lallés et al., 2004). In newly weaned pigs, stress is induced by separation from the sow, relocation, and mixing piglet groups, and a radical change in diet that often reduces or eliminates feed intake in the first 48 h postweaning (Brooks et al., 2001). As a result, piglets undergo a variety of physiological and metabolic changes that can negatively affect welfare. Changes may result from elevated blood cortisol levels (Moeser et al., 2007; Van der Meulen, et al., 2010), compromised feed intake (Maenz et al., 1994), altered intestinal morphology (Lallés et al., 2004), and dehydration due to the switch from an all liquid (milk) to a solid diet (Berry and Lewis, 2001). Unfortunately, in commercial production systems, weaning stress may be compounded by transport stress, which can induce significant weight loss with as little as 4 h of travel time (Hicks et al., 1998), and ambient temperature likely plays a critical role in determining total stress load incurred by piglets (Lambooy, 1988). Therefore, it is imperative that effective recovery strategies are developed to improve the welfare and productivity of pigs following these stressful events.
Historically, swine producers used dietary antibiotics to help newly weaned pigs overcome the stress of weaning and other associated stressors (Chiba, 2010). However, due to increased consumer concern regarding the use of antibiotics in animal production, and legislative action promoting antibiotic-free diets, it has become increasingly important to develop alternatives that can help pigs recover from stressful events as effectively as dietary antibiotics. Previous research (Johnson and Lay, 2017) determined that inclusion of 0.20% l-glutamine (Ajinomoto North America, Inc., Raleigh, NC) in the diets of newly weaned and transported pigs could improve growth rate and well-being more effectively than dietary antibiotics [chlortetracycline (Aureomycin, Zoetis, Parsippany, NJ) + tiamulin (Denagard, Elanco Animal Health, Greenfield, IN)]. However, this study was conducted under controlled conditions utilizing simulated transport and individual housing. Therefore, study objectives were to evaluate the impact of replacing dietary antibiotics with 0.20% l-glutamine on swine welfare, growth performance, health status, and carcass characteristics of pigs in a production environment following weaning and transport. We hypothesized that withholding dietary antibiotics would negatively affect the overall well-being of piglets, and that diet supplementation with 0.20% l-glutamine would have a similar effect on piglet health and productivity as dietary antibiotics in a production environment.
MATERIALS AND METHODS
General
All procedures involving animal use were approved by the Purdue University Animal Care and Use Committee (protocol #1603001385), and animal care and use standards were based upon the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010). Mixed sex crossbred pigs [N = 480; 5.62 ± 0.06 kg initial BW; Duroc x (Landrace x Yorkshire)] were weaned and transported at 18.4 ± 0.2 d of age in central Indiana and replicated during July of 2016 (summer replicate) and April of 2017 (spring replicate). The terms summer replicate and spring replicate refer only to the time of year in which the pigs were weaned and transported. One day prior to weaning and transport, all pigs were individually weighed, blocked by body weight, and randomly allotted to pens, and pens of pigs within BW blocks were allotted to 1 of 3 dietary treatments with 10 pens per dietary treatment per replicate. Each pen, initially, contained 8 pigs. Dietary treatments were antibiotics [A; chlortetracycline (441 ppm) + tiamulin (38.6 ppm)], no antibiotics (NA), or 0.20% l-glutamine (GLN).
Sentinel Pigs
On 14.0 ± 1.9 d of age, calibrated thermochron temperature recorders (iButton model 1921H; accuracy ± 0.2 ºC; Dallas Semiconductor, Maxim, Irving, TX) were implanted intraabdominally into 12 selected mixed sex piglets (3 barrows and 3 gilts per replicate) to measure core body temperature (TC) in 10-min intervals and then the hourly mean was calculated. For thermochron temperature recorder implantation, pigs were anesthetized (1% to 4% isoflurane), and then an incision (6 cm) was made on the abdomen, 3 cm lateral to the linea alba. Sterile thermochron temperature recorders were inserted in between the peritoneum and abdominal muscle and the incision site was closed. Immediately following surgery, all piglets were administered a broad-spectrum antibiotic (5 mg/kg IM every 3 d; Ceftiofur; Zoetis; Florham Park, NJ) to prevent infection at the surgical site, as well as analgesia (2.2 mg/kg IM; flunixin meglumine; Merck Animal Health; Madison, NJ) immediately after surgery and 24 h postsurgery to control pain. Piglets were bandaged and then immediately returned to the sow after surgery where they remained until weaning.
Transportation
On the day of weaning and transport, selected pigs, including sentinel pigs, were removed from sows and herded up a ramp into a gooseneck livestock trailer (2.35 × 7.32 m; Wilson Trailer Company, Sioux City, IA) providing 0.07 m2 per pig and within the range of 0.060 to 0.084 m2 per pig required for 4.54 to 9.07 kg pigs, respectively (Federation of Animal Science Societies, 2010). The loading ramp to the trailer was 2.13 m in length providing an 11.0° incline, less than the recommended maximum of 20.0° (National Pork Board, 2015). Two data loggers (Hobo; data logger temperature/RH; Onset; Bourne, MA) were evenly spaced within the trailer to measure ambient temperature (TA) and relative humidity (RH) in 5-min intervals. During transport, the trailer TA and RH during the summer replicate was 29.4 ± 0.2 °C and 64.3 ± 0.8%, respectively, and during the spring replicate was 11.0 ± 0.2 °C and 63.1 ± 0.9%, respectively. Trailers were bedded with wood shavings and ventilation openings were adjusted based on the TA (National Pork Board, 2015).
Piglets were transported as a group in the trailer for approximately 12 h and 819 km without feed or water. Total transport time was determined by adding loading time, time spent in the trailer, unloading time, and the time it took to be sorted into their respective pens in the nursery facility. The average time to wean and load the trailer was 55 min. The drivers were the same and followed the identical route for the summer replicate and spring replicate. Attention was given when developing the transport route such that approximately 50% two-lane roads and 50% four-lane roads were utilized for transport. The route was 273 km in length and was completed 3 times during the transport phase for each replicate. The route took, on average 3 h 16 min to complete. The driver was switched, and the truck was refueled after each time the 273 km route was completed. At the conclusion of the 12-h transport, piglets were unloaded from the trailer, individually weighed, and placed into pens. The average time to unload the trailer, weigh the pigs, and place into pens was 1 h 10 min. All sentinel pigs were euthanized 24 h post-transport and body temperature recorders were removed.
Nursery Phase
Following transport, pigs were placed in their assigned pens and provided their respective dietary treatments for 14 d in 2 phases (days 0 to 14 postweaning; Table 1). Following the dietary treatment period, all pigs were fed common antibiotic-free diets from day 14 to the end of the nursery phase (day 34; Table 1). Diets were corn-soybean meal-based in meal form, fed in 4 phases, and were formulated to meet or exceed nutrient requirements (NRC, 2012) during the nursery period (Table 1). Pigs were weighed individually and feeders were weighed every 7 d during the nursery period to determine the response criteria of ADG, ADFI, and G:F.
Table 1.
Composition of nursery diets
| Phase 11 | Phase 22 | Phase 33 | Phase 44 | |||||
|---|---|---|---|---|---|---|---|---|
| Item | A5 | GLN6 | NA7 | A | GLN | NA | ||
| Ingredient, % as fed | ||||||||
| Corn | 30.81 | 31.18 | 31.38 | 37.52 | 37.89 | 38.09 | 51.63 | 57.38 |
| SBM, 48% CP | 13.95 | 13.95 | 13.95 | 18.00 | 18.00 | 18.00 | 25.65 | 30.70 |
| Dried distillers grain with solubles | – | – | – | – | – | – | – | 5.00 |
| Soybean oil | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 3.00 | – |
| Choice white grease | – | – | – | – | – | – | – | 3.00 |
| Limestone | 0.79 | 0.79 | 0.79 | 0.74 | 0.74 | 0.74 | 0.86 | 1.33 |
| Monocalcium phosphate | 0.40 | 0.40 | 0.40 | 0.49 | 0.49 | 0.49 | 0.49 | 0.74 |
| Vitamin premix8 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Trace mineral premix9 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 |
| Selenium premix10 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Phytase11 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Salt | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.30 | 0.35 |
| Plasma protein | 6.50 | 6.50 | 6.50 | 2.50 | 2.50 | 2.50 | – | – |
| Spray dried blood meal | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | – | – |
| Soy concentrate | 4.00 | 4.00 | 4.00 | 3.00 | 3.00 | 3.00 | 2.50 | – |
| Select menhaden fish meal | 5.00 | 5.00 | 5.00 | 4.00 | 4.00 | 4.00 | 4.00 | – |
| Dried whey | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 10.00 | – |
| Lactose | 5.00 | 5.00 | 5.00 | – | – | – | – | – |
| Lysine–HCL | 0.07 | 0.07 | 0.07 | 0.20 | 0.20 | 0.20 | 0.28 | 0.40 |
| dl-Methionine | 0.22 | 0.22 | 0.22 | 0.23 | 0.23 | 0.23 | 0.18 | 0.17 |
| l-Threonine | 0.04 | 0.04 | 0.04 | 0.09 | 0.09 | 0.09 | 0.12 | 0.14 |
| l-Tryptophan | – | – | – | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 |
| Zinc oxide | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 | – |
| Copper sulphate | – | – | – | – | – | – | – | 0.10 |
| Aureomycin 5012 | 0.40 | – | – | 0.40 | – | – | – | – |
| Denagard 1013 | 0.18 | – | – | 0.18 | – | – | – | – |
| l-Glutamine14 | – | 0.20 | – | – | 0.20 | – | – | – |
| Banminth 4815 | – | – | – | – | – | – | – | 0.10 |
| Clarifly, 0.67%16 | – | – | – | – | – | – | 0.08 | 0.07 |
| Calculated chemical composition | ||||||||
| ME, kcal/kg | 3536 | 3536 | 3536 | 3510 | 3510 | 3510 | 3418 | 3396 |
| Fat, % | 7.27 | 7.27 | 7.27 | 7.36 | 7.36 | 7.36 | 5.73 | 5.86 |
| CP, % | 24.62 | 24.62 | 24.62 | 22.87 | 22.87 | 22.87 | 22.29 | 21.28 |
| SID Lys, % | 1.55 | 1.55 | 1.55 | 1.45 | 1.45 | 1.45 | 1.35 | 1.25 |
| Ca, % | 0.90 | 0.90 | 0.90 | 0.85 | 0.85 | 0.85 | 0.80 | 0.75 |
| Total P, % | 0.75 | 0.75 | 0.75 | 0.71 | 0.71 | 0.71 | 0.64 | 0.57 |
| Avail. P, % | 0.60 | 0.60 | 0.60 | 0.55 | 0.55 | 0.55 | 0.45 | 0.36 |
| Analyzed chemical composition | ||||||||
| Summer replicate | ||||||||
| GE, kcal/kg | 4217 | 4251 | 4173 | 4172 | 4146 | 4184 | – | – |
| CP, % | 24.42 | 25.62 | 23.85 | 22.30 | 22.38 | 22.46 | 22.07 | 22.00 |
| Total Lys, % | 1.30 | 1.35 | 1.26 | 1.13 | 1.18 | 1.11 | – | – |
| Total Glu, %17 | 3.15 | 3.43 | 3.11 | 2.78 | 2.88 | 2.68 | – | – |
| Chlortetracycline, ppm18 | 467 | 0 | 0 | 468 | 0 | 0 | – | – |
| Spring replicate | ||||||||
| GE, kcal/kg | 4266 | 4199 | 4079 | 4174 | 4193 | 4129 | – | – |
| CP, % | 25.36 | 26.37 | 22.51 | 22.78 | 23.02 | 24.68 | 22.37 | 21.14 |
| Total Lys, % | 1.58 | 1.75 | 1.40 | 1.54 | 1.51 | 1.51 | – | – |
| Total Glu, % | 3.70 | 4.23 | 3.17 | 3.68 | 3.81 | 3.62 | – | – |
| Chlortetracycline, ppm | 436 | 0 | 0 | 436 | 0 | 0 | – | – |
1Fed days 0 to 7 postweaning and transport.
2Fed days 7 to 14 postweaning and transport.
3Fed days 14 to 21 postweaning and transport.
4Fed days 21 to 34 postweaning and transport.
5Pigs provided dietary antibiotics [chlortetracycline (441 ppm) + tiamulin (38.6 ppm)].
6Pigs provided 0.20% l-glutamine.
7Pigs provided no dietary antibiotics.
8Provided per kilogram of the diet: vitamin A, 6,615 IU; vitamin D3, 662 IU; vitamin E, 44 IU; vitamin K, 2.2 mg; riboflavin, 8.8 mg; pantothenic acid, 22 mg; niacin, 33 mg; B12, 38.6 mg.
9Provided available minerals per kilogram of the diet: iron, 121.3 mg; zinc, 121.3 mg; manganese, 15 mg; copper, 11.3 mg; iodine, 0.46 mg.
10Provided 0.3 ppm Se.
11Provided 600 FTU per kg of the diet.
12Aureomycin (Zoetis, Parsippany, NJ) provided 441 ppm chlortetracycline in the diet.
13Denagard (Elanco Animal Health, Greenfield, IN) provided 38.6 ppm tiamulin in the diet.
14Ajinomoto North America, Inc., Raleigh, NC.
15Banminth (Phibro Animal Health Corporation, Teaneck, NJ) provided 106 ppm pyrantel tartrate in the diet.
16Clarifly (Central Life Sciences, Schaumburg, IL) provided 5.4 ppm (Phase 3) and 4.7 ppm (Phase 4) diflubenzuron in the diet.
17Samples submitted to Ajinomoto for glutamic acid analysis.
18Samples submitted to Zoetis, Parsippany, NJ for chlortetracycline analysis.
Therapeutic antibiotic administration was recorded for the duration of the trial (weaning to market). The researchers and research farm staff were trained to identify pigs needing therapeutic injectable antibiotic treatment and were blinded to the study treatments. Pigs were treated when exhibiting clinical signs of illness. Treatment dose, product given, date given, pig and pen identification, and reason administered were recorded. Reason for therapeutic administration was then categorized for post hoc analysis. Categories were enteric challenge (e.g., scours or loose watery stool), respiratory challenge (e.g., coughing, thumping, or labored breathing), lameness (e.g., carrying a limb or difficulty walking or swollen joints), un-thriftiness (e.g., BW loss, poor gain, loss of body condition, or rough hair coat), and all other treatments (e.g., side paddling associated with Streptococcus suis infection, skin infection, and abscess).
The nursery facility where the initial 34 d of the trial was conducted contained pens (1.22 m × 1.37 m) that provided initially approximately 0.21 m2 per pig. All pens contained 1, 5-hole dry self-feeder and a cup waterer to allow for ad libitum access to feed and water. The nursery barn has a shallow pit for manure storage and completely slatted plastic floors. The nursery room operated on mechanical ventilation using a 4-stage digital controller (Airstream TC5-2V25A, Automated Production Systems, Assumption, IL). During days 0 to 14 postweaning, the nursery room average daily TA during the summer replicate was 31.48 ± 1.82 °C and during the spring replicate was 30.57 ± 0.68 °C. From days 14 to 34, the nursery TA was 28.70 ± 1.14 °C and 25.99 ± 0.84 °C for the summer and spring replicates, respectively.
Grow–Finish Phase
On day 34, all pigs were moved to the grow–finish facility for the remainder of the trial and pen integrity was maintained. Common antibiotic-free diets were corn-soybean meal-DDGS-based diets provided in meal form to meet or exceed nutrient requirements (NRC, 2012) in 6 phases during the grow–finish period (Table 2). Pigs and feeders were weighed every 21 d during the grow–finish period to determine the response criteria of ADG, ADFI, and G:F.
Table 2.
Composition of grow–finish diets
| Item | Phase 11 | Phase 22 | Phase 33 | Phase 44 | Phase 55 | Phase 66 |
|---|---|---|---|---|---|---|
| Ingredient, % as fed | ||||||
| Corn | 61.47 | 64.65 | 66.40 | 71.10 | 82.38 | 68.67 |
| SBM, 48% CP | 23.20 | 16.15 | 9.75 | 5.25 | 4.25 | 15.10 |
| Dried distillers grain with solubles | 10.00 | 15.00 | 20.00 | 20.00 | 10.00 | 10.00 |
| Choice white grease | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | 3.00 |
| Limestone | 1.37 | 1.35 | 1.39 | 1.32 | 1.16 | 1.26 |
| Monocalcium phosphate | 0.47 | 0.32 | 0.05 | 0.00 | 0.10 | 0.27 |
| Vitamin premix | 0.1507 | 0.1507 | 0.1258 | 0.1209 | 0.10010 | 0.1507 |
| Trace mineral premix | 0.1011 | 0.0912 | 0.0813 | 0.0714 | 0.0515 | 0.1011 |
| Selenium premix | 0.05016 | 0.05016 | 0.05016 | 0.05016 | 0.02517 | 0.05016 |
| Phytase18 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Salt | 0.35 | 0.35 | 0.30 | 0.30 | 0.25 | 0.30 |
| Lysine–HCL | 0.42 | 0.46 | 0.48 | 0.46 | 0.37 | 0.42 |
| dl-Methionine | 0.11 | 0.08 | 0.05 | 0.01 | 0.00 | 0.10 |
| l-Threonine | 0.130 | 0.130 | 0.120 | 0.105 | 0.095 | 0.160 |
| l-Tryptophan | 0.010 | 0.030 | 0.035 | 0.040 | 0.030 | 0.030 |
| Paylean 2.2519 | – | – | – | – | – | 0.15 |
| Availa Zn 12020 | – | – | – | – | – | 0.042 |
| Clarifly, 0.67% | 0.0721 | 0.0922 | 0.0721 | 0.0823 | 0.0922 | 0.1024 |
| Calculated chemical composition | ||||||
| ME, kcal/kg | 3373 | 3337 | 3351 | 3359 | 3371 | 3438 |
| Fat, % | 5.29 | 4.69 | 5.06 | 5.15 | 4.73 | 6.40 |
| CP, % | 19.34 | 17.59 | 15.99 | 14.18 | 11.90 | 16.01 |
| SID Lys, % | 1.10 | 0.98 | 0.85 | 0.73 | 0.60 | 0.90 |
| Ca, % | 0.70 | 0.65 | 0.60 | 0.55 | 0.50 | 0.60 |
| Total P, % | 0.50 | 0.47 | 0.41 | 0.38 | 0.35 | 0.42 |
| Avail. P, % | 0.32 | 0.30 | 0.26 | 0.24 | 0.20 | 0.26 |
| Analyzed chemical composition | ||||||
| Summer replicate | ||||||
| CP, % | 19.13 | 18.08 | 14.92 | 14.66 | 11.73 | 15.64 |
| Spring replicate | ||||||
| CP, % | 19.25 | 17.33 | 16.73 | 15.59 | 12.29 | 16.85 |
1Fed days 0 to 21 of the grow–finish phase.
2Fed days 21 to 42 of the grow–finish phase.
3Fed days 42 to 62 of the grow–finish phase.
4Fed days 62 to 83 of the grow–finish phase.
5Fed days 83 to 104 of the grow–finish phase.
6Fed days 104 to 125 of the grow–finish phase.
7Provided per kilogram of the diet: vitamin A, 3,969 IU; vitamin D3, 397 IU; vitamin E, 26 IU; vitamin K, 1.3 mg; riboflavin, 5.3 mg; pantothenic acid, 13 mg; niacin, 20 mg; B12, 23.2 mg.
8Provided per kilogram of the diet: vitamin A, 3,308 IU; vitamin D3, 331 IU; vitamin E, 22 IU; vitamin K, 1.1 mg; riboflavin, 4.4 mg; pantothenic acid, 11 mg; niacin, 17 mg; B12, 19.3 mg.
9Provided per kilogram of the diet: vitamin A, 3,175 IU; vitamin D3, 318 IU; vitamin E, 21 IU; vitamin K, 1.1 mg; riboflavin, 4.2 mg; pantothenic acid, 11 mg; niacin, 16 mg; B12, 18.5 mg.
10Provided per kilogram of the diet: vitamin A, 2,646 IU; vitamin D3, 265 IU; vitamin E, 18 IU; vitamin K, 0.9 mg; riboflavin, 3.5 mg; pantothenic acid, 9 mg; niacin, 13 mg; B12, 15.4 mg.
11Provided per available minerals kilogram of the diet: iron, 97 mg; zinc, 97 mg; manganese, 12 mg; copper, 9 mg; iodine, 0.37 mg.
12Provided per available minerals kilogram of the diet: iron, 87 mg; zinc, 87 mg; manganese, 11 mg; copper, 8 mg; iodine, 0.33 mg.
13Provided per available minerals kilogram of the diet: iron, 78 mg; zinc, 78 mg; manganese, 10 mg; copper, 7.2 mg; iodine, 0.29 mg.
14Provided per available minerals kilogram of the diet: iron, 68 mg; zinc, 68 mg; manganese, 8 mg; copper, 6.3 mg; iodine, 0.26 mg.
15Provided per available minerals kilogram of the diet: iron, 48.5 mg; zinc, 48.5 mg; manganese, 6 mg; copper, 4.5 mg; iodine, 0.18 mg.
16Provided 0.3 ppm Se.
17Provided 0.15 ppm Se.
18Provided 600 FTU per kg of the diet.
19Paylean (Elanco Animal Health, Greenfield, IN) provided 7.5 ppm ractopamine HCl in the diet.
20Zinpro Corporation, Eden Prairie, MN.
21Clarifly (Central Life Sciences, Schaumburg, IL) provided 4.7 ppm diflubenzuron in the diet.
22Clarifly (Central Life Sciences, Schaumburg, IL) provided 6.0 ppm diflubenzuron in the diet.
23Clarifly (Central Life Sciences, Schaumburg, IL) provided 5.4 ppm diflubenzuron in the diet.
24Clarifly (Central Life Sciences, Schaumburg, IL) provided 6.7 ppm diflubenzuron in the diet.
The grow–finish facility contained pens (1.68 × 4.27 m) that provided approximately 1.19 m2 per pig. All pens contained one 2-hole dry self-feeder and a nipple waterer to allow for ad libitum access to feed and water. The grow–finish barn had a shallow pit for manure storage and completely slatted concrete floors. The barn was mechanically ventilated. During days 0 to 62 of the grow–finish phase, the room average daily TA during the summer replicate was 22.35 ± 1.14 °C and during the spring replicate was 25.47 ± 2.64 °C. From days 62 to 125, the TA was 19.87 ± 0.83 °C and 25.74 ± 2.48 °C for the summer and spring replicates, respectively.
Blood Parameters
Blood samples were collected (BD vacutainers; Franklin Lakes, NJ; plasma; 5 mL) via jugular venipuncture immediately prior to transport, immediately post-transport, and 24 h post-transport from the sentinel animals. Blood samples were obtained at 0630 h on days 13 and 33 of the nursery phase from one randomly selected pig per pen. Sex of the selected pig was balanced across treatments within day and balanced within pen across collection days. Plasma was collected by centrifugation at 4 ºC and 1900 × g for 15 min, aliquoted and stored at −80 °C. Plasma cortisol concentrations were analyzed using a commercially available radioimmunoassay (RIA) kit (minimum detectable level: 0.9 ng/mL; Cortisol RIA, Tecan Trading AG, Mannedorf, Switzerland) according to manufacturer’s instructions. Plasma TNF-α concentrations were analyzed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Swine TNF-α ELISA Kit; InvitrogenTM; Thermo Fisher Scientific; Waltham, MA) according to manufacturer’s instructions. The intraassay coefficients of variation were 9.0% and 8.6%, for cortisol and TNF-α, respectively. The interassay coefficient of variation for TNF-α was 12.4%.
Animal Behavior
Piglets were video-recorded for 14 d immediately following weaning and transport using ceiling-mounted cameras (Panasonic WV-CP254H, Matsushita Electric Industrial Co. Ltd., Osaka, Japan) attached to a digital video recorder system (GeoVision VMS Software; GeoVision Inc., Tapei, Taiwan). Video was recorded both during the light and the dark periods (12 h: 12 h). Video files were later analyzed using Observer XT 11.5 behavioral analysis software (Noldus Information Technology B.V., Wageningen, The Netherlands) by 4 trained individuals that were blind to the treatments and maintained an agreement of 90% or greater. Individual behaviors were determined using an instantaneous scan sampling technique in 10-min intervals on days 2, 4, 8, and 12 postweaning for 3 periods each day (0800 to 1000, 1100 to 1300, and 1400 to 1600 h) for sickness and other behaviors. Sickness behavior include huddling and other behaviors included active, resting, aggressive, eating/drinking, and nonvisible. The percentage of pigs in each pen performing the specific behaviors was calculated for each timepoint. A definition for each behavior is defined in an ethogram (Table 3). The absolute temperature range measured on each day of behavior analysis was as follows: day 2 for summer and spring replicates (30.30 to 32.70 and 27.56 to 32.83 °C, respectively), day 4 for summer and spring replicates (29.97 to 36.32 and 30.60 to 33.61 °C, respectively), day 8 for summer and spring replicates (29.42 to 35.43 and 27.31 to 32.36 °C, respectively, and day 12 for summer and spring replicates (28.97 to 36.86 and 26.12 to 31.36 °C, respectively).
Table 3.
Ethogram used for behavioral observations
| Category | Behavior | Definition |
|---|---|---|
| Sickness Behavior | Huddling | When 3 or more pigs are touching while lying down and 50% of a pig’s body is touching another pig |
| Other | Active | Piglets are walking about or interacting in a nonaggressive manner with each other or their environment |
| Resting | Piglets are lying, either ventral or lateral, either alone or loosely in groups, with gaps of spaces between them | |
| Aggressive | Piglets are engaged in agonistic interactions | |
| Eating/drinking | The piglet has its nose in the feeder or its mouth on the waterer | |
| Nonvisible | When piglet moves out of view and cannot be observed |
Marketing
At the end of the 159-d experiment, pigs from each pen were individually tattooed with pen number and shipped approximately 48 km to Indiana Packers Corporation (Delphi, IN). Pigs were slaughtered under commercial conditions with carbon dioxide stunning. Standard carcass criteria of loin and backfat depth, hot carcass weight (HCW), fat-free lean index, and yield were collected. Fat depth and loin depth were measured with an optical probe (Fat-O-Meater, SFK Technology A/S, Herlev, Denmark) inserted between the third and fourth rib from the last rib (counting from the posterior of the carcass) and 7 cm from the dorsal midline of the hot carcass. Lean percentage was calculated according to the Indiana Packers Corporation (2015) formula and the fat-free lean percentage was calculated according to Schinckel et al. (2010) procedures.
Statistics
Data were analyzed as a randomized complete block design using the PROC MIXED procedure in SAS 9.4 (SAS Institute INC., Cary, NC), with pen as the experimental unit. The assumptions of normality of error, homogeneity of variance, and linearity were confirmed post hoc. All injectable antibiotic administration and behavioral data were log-transformed to meet assumptions of normality; however, all log-transformed data are presented as arithmetic means for ease of interpretation. All nontransformed data are presented as LS means. For repeated analyses for growth performance, each pen’s respective parameter was analyzed using repeated measures and covariance structure was selected based on goodness of fit criteria with week as the repeated effect. Statistical significance was defined as P ≤ 0.05 and a tendency was defined as 0.05 < P ≤ 0.10.
RESULTS
Sentinel Data
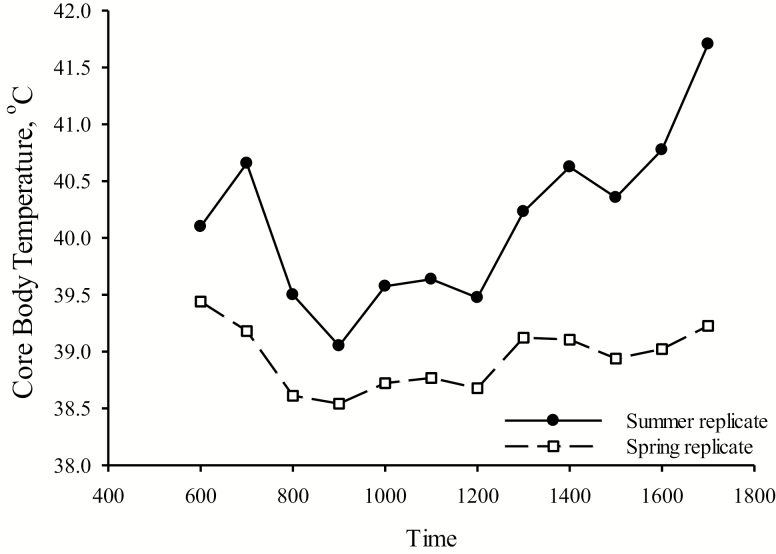
Due to the trailer being considered 1 experimental unit, all sentinel data are for descriptive purposes only. Core body temperature was 40.1 ± 0.2 and 38.9 ± 0.1 ºC during the summer replicate and spring replicate transport, respectively (Figure 1). Plasma cortisol and TNF-α concentrations during pretransport, post-transport, and 24 h post-transport are presented in Table 4.
Figure 1.
Descriptive data of core body temperature over time during weaning and transport in the summer of 2016 and the spring of 2017.
Table 4.
Effect of dietary treatment on blood plasma parameter concentrations
| Replicate | Diet | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Summer1 | Spring2 | A3 | GLN4 | NA5 | SE | D6 | R7 | D x R |
| Sentinel pigs8 | |||||||||
| Pretransport9 | |||||||||
| TNF-α10, pg/mL | 19.11 | 27.11 | – | – | – | 10.21 | – | – | – |
| Cortisol, μg/L | 25.24 | 54.80 | – | – | – | 13.91 | – | – | – |
| post-transport11 | |||||||||
| TNF-α, pg/mL | 3.27 | 12.58 | – | – | – | 10.24 | – | – | – |
| Cortisol, μg/L | 140.64 | 34.19 | – | – | – | 19.60 | – | – | – |
| 24 h post-transport12 | |||||||||
| TNF-α, pg/mL | 32.53 | 34.41 | – | – | – | 11.38 | – | – | – |
| Cortisol, μg/L | 37.01 | 19.06 | – | – | – | 9.96 | – | – | – |
| Experimental data13 | |||||||||
| Day 13 | |||||||||
| TNF-α, pg/mL | 47.88 | 46.02 | 36.73a | 40.92a | 63.19b | 6.94 | 0.02 | 0.82 | 0.14 |
| Cortisol, μg/L | 28.10 | 25.18 | 26.80 | 26.39 | 26.72 | 2.25 | 0.99 | 0.25 | 0.95 |
| Day 33 | |||||||||
| TNF-α, pg/ml | 45.33 | 77.32 | 62.03 | 54.78 | 67.16 | 5.84 | 0.31 | 0.01 | 0.92 |
| Cortisol, μg/L | 46.79 | 53.95 | 52.68 | 48.46 | 49.96 | 4.55 | 0.78 | 0.15 | 0.40 |
1Pigs weaned and transported for 12 h during July 2016.
2Pigs weaned and transported for 12 h during April 2017.
3Pigs provided dietary antibiotics [chlortetracycline (441 ppm) + tiamulin (38.6 ppm)] for 14 d postweaning and transport and then fed common antibiotic-free diets.
4Pigs provided 0.20% l-glutamine for 14 d postweaning and transport and then fed common antibiotic-free diets.
5Pigs provided no dietary antibiotics for 14 d postweaning and transport and then fed common antibiotic-free diets.
6Dietary treatment.
7Replicate.
8Six sentinel pigs per replicate was selected for blood parameter descriptive data.
9Blood samples were collected immediately prior to transport.
10Tumor necrosis factor alpha.
11Blood samples were collected immediately post-transport.
12Blood samples were collected 24 h post-transport.
13A total of 10 pens were used per dietary treatment per replicate with 1 pig per pen closest to the pen mean BW was selected for plasma cortisol concentration analysis.
a,bLetters indicate significant differences (P ≤ 0.05) within a row and dietary treatment.
Blood Parameters
On day 13, plasma TNF-α was reduced (P = 0.02; 38.6%) in A and GLN pigs vs. NA pigs, but no differences were detected between A and GLN pigs (Table 4). Tumor necrosis factor alpha was increased (P = 0.01; 70.6%) during the spring replicate compared with the summer replicate on day 33 (Table 4). No other plasma TNF-α differences were observed (P > 0.13) with any comparison (Table 4). No plasma cortisol differences were observed (P > 0.14) with any comparison (Table 4).
Growth Performance
Nursery phase.
When comparing the dietary treatments, ADG was greater overall (P = 0.01; 14.9%) from days 0 to 14 of the nursery period in A and GLN pigs compared with NA pigs, but no ADG differences were detected between A and GLN pigs (Table 5). Overall, from days 0 to 34 of the nursery period, ADG was increased (P = 0.01; 7.9%) in A compared with NA pigs, but no differences were detected between A and NA vs. GLN pigs (Table 5). An increase in ADFI was detected (P = 0.04) from days 0 to 14 of the nursery phase for A compared with NA pigs, but no differences were observed between A and NA vs. GLN pigs (Table 5). Average daily feed intake tended to be greater (P = 0.09) from days 0 to 34 of the nursery period in A compared with NA pigs, but no differences were observed between A and NA vs. GLN pigs (Table 5). Feed efficiency (G:F) was greater overall (P = 0.01; 7.7%) from days 0 to 14 of the nursery phase for A compared with NA and GLN pigs, but no differences were observed between NA and GLN pigs (Table 5). From days 0 to 34 of the nursery phase, G:F was increased (P = 0.01; 4.3%) in A compared with NA pigs, but no differences were observed for A and NA pigs compared with GLN pigs (Table 5). Day 14 BW was greater (P = 0.01) for A (5.6%) and GLN (3.8%) pigs compared with NA pigs; however, no differences were detected between A and GLN pigs (Table 5). Final BW was increased (P = 0.04; 4.8%) for A compared with NA pigs, but no differences were detected between A and NA vs. GLN pigs (Table 5). No other dietary treatment growth performance differences (P > 0.05) were detected during the nursery phase.
Table 5.
Effect of dietary treatment on nursery and grow-finish growth performance1
| Replicate | Diet | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Summer2 | Spring3 | A4 | GLN5 | NA6 | SE | D7 | R8 | D x R |
| Nursery period | |||||||||
| Days 0 to 14 | |||||||||
| Initial BW, kg | 5.64 | 5.51 | 5.58 | 5.59 | 5.57 | 0.29 | 0.99 | 0.70 | 0.99 |
| ADG, g | 210 | 206 | 224a | 210a | 189b | 10.19 | 0.01 | 0.56 | 0.82 |
| ADFI, g | 274 | 260 | 277a | 272ab | 253b | 13.21 | 0.04 | 0.08 | 0.92 |
| G:F | 0.80 | 0.80 | 0.84a | 0.79b | 0.77b | 0.01 | 0.01 | 0.91 | 0.17 |
| Day 14 BW, kg | 8.44 | 8.46 | 8.65a | 8.50a | 8.19b | 0.52 | 0.01 | 0.83 | 0.97 |
| Days 14 to 34 | |||||||||
| ADG, g | 439 | 455 | 458 | 447 | 436 | 12.05 | 0.21 | 0.09 | 0.43 |
| ADFI, g | 693 | 674 | 702 | 680 | 669 | 22.81 | 0.16 | 0.19 | 0.63 |
| G:F | 0.63 | 0.68 | 0.65 | 0.66 | 0.65 | 0.01 | 0.78 | 0.01 | 0.04 |
| Days 0 to 34 | |||||||||
| ADG, g | 347 | 355 | 364a | 352ab | 337b | 10.18 | 0.01 | 0.23 | 0.58 |
| ADFI, g | 525 | 509 | 532x | 517xy | 503y | 17.43 | 0.09 | 0.12 | 0.77 |
| G:F | 0.70 | 0.73 | 0.73a | 0.71ab | 0.70b | 0.01 | 0.03 | 0.01 | 0.07 |
| Day 34 BW, kg | 17.20 | 17.62 | 17.78a | 17.49ab | 16.96b | 0.74 | 0.04 | 0.11 | 0.69 |
| Grow–finish period | |||||||||
| Days 0 to 62 | |||||||||
| ADG, kg | 0.76 | 0.77 | 0.78 | 0.76 | 0.76 | 0.01 | 0.32 | 0.37 | 0.62 |
| ADFI, kg | 1.79 | 1.75 | 1.80 | 1.76 | 1.75 | 0.03 | 0.40 | 0.14 | 0.88 |
| G:F | 0.44 | 0.46 | 0.45 | 0.46 | 0.45 | 0.01 | 0.80 | 0.01 | 0.36 |
| Day 62 BW, kg | 64.72 | 65.50 | 65.99 | 65.02 | 64.31 | 0.96 | 0.22 | 0.32 | 0.76 |
| Day 62 to 125 | |||||||||
| ADG, kg | 0.82 | 0.96 | 0.88 | 0.89 | 0.90 | 0.02 | 0.41 | 0.01 | 0.36 |
| ADFI, kg | 2.83 | 2.96 | 2.87 | 2.91 | 2.90 | 0.05 | 0.72 | 0.01 | 0.42 |
| G:F | 0.29 | 0.33 | 0.30 | 0.31 | 0.31 | 0.01 | 0.17 | 0.01 | 0.62 |
| Days 0 to 125 | |||||||||
| ADG, kg | 0.79 | 0.87 | 0.83 | 0.83 | 0.83 | 0.01 | 0.95 | 0.01 | 0.58 |
| ADFI, kg | 2.31 | 2.35 | 2.33 | 2.33 | 2.32 | 0.03 | 0.97 | 0.21 | 0.60 |
| G:F | 0.37 | 0.39 | 0.38 | 0.38 | 0.38 | 0.01 | 0.54 | 0.01 | 0.56 |
| Final BW, kg | 117.37 | 127.19 | 122.77 | 121.73 | 122.34 | 1.23 | 0.83 | 0.01 | 0.64 |
1A total of 10 pens were used per dietary treatment per replicate.
2Pigs weaned and transported for 12 h during July 2016.
3Pigs weaned and transported for 12 h during April 2017.
4Pigs provided dietary antibiotics [chlortetracycline (441 ppm) + tiamulin (38.6 ppm)] for 14 d postweaning and transport and then fed common antibiotic-free diets.
5Pigs provided 0.20% l-glutamine for 14 d postweaning and transport and then fed common antibiotic-free diets.
6Pigs provided no dietary antibiotics for 14 d postweaning and transport and then fed common antibiotic-free diets.
7Dietary treatment.
8Replicate.
a,bLetters indicate significant differences (P ≤ 0.05) within a row and dietary treatment.
x,yLetters indicate tendencies (0.05 < P ≤ 0.10) within a row and dietary treatment.
Average daily feed intake tended to be reduced (P = 0.08; 5.1%) during the spring replicate compared with the summer replicate from days 0 to 14 of the nursery phase (Table 5). From days 14 to 34 of the nursery phase, ADG tended to be reduced (P = 0.09) and G:F was reduced (P = 0.01) during the summer replicate compared with the spring replicate (3.7% and 7.4%, respectively; Table 5). Overall, from days 0 to 34 of the nursery period, G:F was reduced (P = 0.04; 4.1%) during the summer replicate compared with the spring replicate (Table 5). No other replicate effects were observed during the nursery period (P > 0.05).
A diet x replicate interaction was detected (P = 0.04) from days 14 to 34 of the nursery phase where G:F was greater in the spring replicate in NA (0.69 ± 0.01) and GLN (0.68 ± 0.01) pigs compared with NA pigs (0.61 ± 0.01) during the summer replicate (data not presented). However, no differences were observed between A pigs (0.66 ± 0.01) during the spring replicate and A (0.64 ± 0.01) and GLN (0.63 ± 0.01) pigs during the summer replicate (data not presented). No other diet x replicate interactions were detected (P > 0.05; Table 5).
Grow–Finish Phase.
No dietary treatment differences were observed (P > 0.17) during the grow–finish period (Table 5). From days 0 to 62 of the grow–finish phase, G:F was reduced (P = 0.01; 4.3%) during the summer replicate compared with the spring replicate (Table 5). Average daily gain, ADFI, and G:F were reduced (P = 0.01; 14.6%, 4.4%, and 12.1%, respectively) in the summer replicate compared with the spring replicate from days 62 to 125 of the grow–finish phase (Table 5). Overall, from days 0 to 125 of the grow–finish period, ADG and G:F were reduced (P = 0.01; 9.2% and 5.1%, respectively) in the summer replicate compared with the spring replicate (Table 5). Final BW at the end of the grow–finish period was reduced (P = 0.01; 9.82 kg decrease) in the summer replicate compared with the spring replicate (Table 5). No other growth performance differences were observed (P > 0.05) during the grow–finish period with any comparison (Table 5).
Treatment Rate
Nursery phase.
A diet x replicate effect was detected (P = 0.04) where pigs treated for lameness from days 14 to 34 was greater in the spring replicate for GLN pigs (2.12 ± 1.00%) compared with all other treatments (data not presented). However, no differences were observed between A (0.56 ± 1.00%) and NA (0.00 ± 1.00%) pigs during the spring replicate, and A (0.48 ± 1.00%), GLN (0.00 ± 1.00%), and NA (0.00 ± 1.00%) pigs during the summer replicate (data not presented). There were no dietary treatment differences observed (P > 0.05) from days 0 to 14 (Table 6).
Table 6.
Effect of dietary treatment on therapeutic antibiotic treatment rate during the nursery period1
| Replicate | Diet | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Summer2 | Spring3 | A4 | GLN5 | NA6 | SE | D7 | R8 | D x R |
| Nursery period | |||||||||
| Days 0 to 14 | |||||||||
| Enteric9 | 4.59 | 5.29 | 3.13 | 5.97 | 5.72 | 2.31 | 0.31 | 0.38 | 0.07 |
| Lame10 | 1.67 | 0.88 | 1.26 | 1.64 | 0.94 | 1.02 | 0.73 | 0.27 | 0.89 |
| Unthrifty11 | 1.46 | 0.65 | 0.94 | 0.97 | 1.25 | 1.02 | 0.92 | 0.22 | 0.48 |
| Respiratory12 | – | – | – | – | – | – | – | – | – |
| Other13 | 0.00 | 1.06 | 0.63 | 0.35 | 0.63 | 0.86 | 0.86 | 0.02 | 0.86 |
| Days 14 to 34 | |||||||||
| Enteric | 0.48 | 0.19 | 0.00 | 0.48 | 0.52 | 0.66 | 0.36 | 0.33 | 0.37 |
| Lame | 0.16 | 0.89 | 0.52b | 1.06a | 0.00b | 1.00 | 0.08 | 0.06 | 0.04 |
| Unthrifty | 0.34 | 1.07 | 0.52 | 0.58 | 1.03 | 0.80 | 0.64 | 0.14 | 0.57 |
| Respiratory | 0.16 | 0.18 | 0.00 | 0.27 | 0.24 | 0.53 | 0.58 | 0.94 | 0.20 |
| Other | 0.00 | 0.18 | 0.00 | 0.27 | 0.00 | 0.53 | 0.31 | 0.27 | 0.31 |
| Grow–finish period | |||||||||
| Days 0 to 62 | |||||||||
| Enteric | 0.19 | 0.63 | 0.56 | 0.34 | 0.34 | 0.67 | 0.81 | 0.31 | 0.77 |
| Lame | 1.00 | 0.00 | 0.28 | 0.28 | 0.95 | 1.06 | 0.41 | 0.02 | 0.41 |
| Unthrifty | 0.82 | 0.19 | 0.89 | 0.00 | 0.62 | 0.99 | 0.24 | 0.17 | 0.16 |
| Respiratory | 9.96 | 1.30 | 5.84 | 5.95 | 5.11 | 2.92 | 0.60 | <0.01 | 0.77 |
| Other | 1.59 | 0.19 | 0.56 | 1.17 | 0.95 | 1.11 | 0.69 | 0.02 | 0.45 |
| Days 62 to 125 | |||||||||
| Enteric | 0.22 | 0.78 | 0.00y | 1.17x | 0.34y | 0.93 | 0.08 | 0.19 | 0.58 |
| Lame | 1.19 | 0.00 | 1.11 | 0.34 | 0.34 | 1.05 | 0.21 | 0.01 | 0.21 |
| Unthrifty | 0.19 | 0.56 | 1.12 | 0.00 | 0.00 | 0.93 | 0.01 | 0.28 | 0.30 |
| Respiratory | 10.24 | 7.17 | 8.83 | 7.81 | 9.48 | 4.20 | 0.81 | 0.49 | 0.86 |
| Other | 0.19 | 0.00 | 0.28 | 0.00 | 0.00 | 0.56 | 0.37 | 0.32 | 0.37 |
1A total of 10 pens were used per dietary treatment per replicate.
2Pigs weaned and transported for 12 h during July 2016.
3Pigs weaned and transported for 12 h during April 2017.
4Pigs provided dietary antibiotics [chlortetracycline (441 ppm) + tiamulin (38.6 ppm)] for 14 d postweaning and transport and then fed common antibiotic-free diets.
5Pigs provided 0.20% l-glutamine for 14 d postweaning and transport and then fed common antibiotic-free diets.
6Pigs provided no dietary antibiotics for 14 d postweaning and transport and then fed common antibiotic-free diets.
7Dietary treatment.
8Replicate.
9Percent of pigs within pen treated with therapeutic antibiotics for enteric challenge.
10Percent of pigs within pen treated with therapeutic antibiotics for lameness.
11Percent of pigs within pen treated with therapeutic antibiotics for unthriftiness.
12Percent of pigs within pen treated with therapeutic antibiotics for respiratory challenge.
13Percent of pigs within pen treated with therapeutic antibiotics for all other conditions.
a,bLetters indicate significant differences (P ≤ 0.05) within a row and dietary treatment.
x,yLetters indicate tendencies (0.05 < P < 0.10) within a row and dietary treatment.
Pigs treated for Other reasons were greater (P ≤ 0.02) from days 0 to 14 during the spring replicate compared with the summer replicate, regardless of dietary treatment (Table 6). No other replicate differences were observed (P > 0.05) for treatment rate (Table 6).
From days 0 to 14, GLN pigs tended (P = 0.07) to be treated for enteric challenges more often in the spring replicate (8.19 ± 2.31%) compared with A pigs (3.13 ± 2.31%), and A (3.13 ± 2.31%) and GLN (3.75 ± 2.31%) pigs during the summer replicate (data not presented). No other diet x replicate differences were detected (P < 0.05) during the nursery phase (Table 6).
Grow–finish phase.
From days 62 to 125, treatment for unthriftiness was reduced (P = 0.01) in GLN (0.00 ± 0.37%) and NA pigs (0.31 ± 0.37%) compared with A pigs (1.00 ± 0.37%), but no differences were observed between GLN and NA pigs (Table 6). During days 62 to 125, enteric disease treatments tended (P < 0.08) to be reduced by A (0.00 ± 0.93%) pigs and greatest for the GLN (1.17 ± 0.93%) pigs with NA (0.34 ± 0.93%) pigs being intermediate (Table 6). No other treatment rate differences for the main effect of dietary treatment were observed (P > 0.05) with any comparison (Table 6).
Pigs treated for lameness were greater (P < 0.02) from days 0 to 62 and days 62 to 125 during the summer replicate compared with the spring replicate, regardless of dietary treatment (Table 6). Treatment for respiratory challenges was greater (P < 0.01) from days 0 to 62 during the summer replicate compared with the spring replicate (Table 6). Pigs treated for other challenges were greater (P < 0.02) during the summer replicate compared with the spring replicate from days 0 to 62 (Table 6). No other replicate differences were observed (P > 0.05) for treatment rate (Table 6).
Behavior
Aggressive behavior tended to be reduced overall (P = 0.09; 26.4%) in GLN compared with A pigs, but no differences were observed between A and GLN vs. NA pigs (Table 7). No other diet differences were observed for behavior (P > 0.05) with any comparison (Table 7).
Table 7.
Effect of dietary treatment on behavior (% of time) from days 2 to 12 postweaning1
| Replicate | Diet | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Behavior | Summer2 | Spring3 | A4 | GLN5 | NA6 | SE | D7 | R8 | D x R |
| Huddling9, % | 5.52 | 15.38 | 10.30 | 8.58 | 11.20 | 1.46 | 0.92 | <0.01 | 0.84 |
| Active10, % | 9.14 | 12.49 | 10.90 | 10.64 | 10.71 | 0.55 | 0.78 | <0.01 | 0.14 |
| Resting11, % | 77.55 | 73.07 | 73.60 | 77.13 | 74.94 | 1.33 | 0.12 | 0.34 | 0.33 |
| Aggressive12, % | 1.39 | 1.57 | 1.74x | 1.28y | 1.41xy | 0.19 | 0.09 | 0.14 | 0.70 |
| Eat/Drink13, % | 8.70 | 11.26 | 10.70 | 9.96 | 9.14 | 0.51 | 0.17 | <0.01 | 0.18 |
| Nonvisible14, % | 0.75 | 0.34 | 0.83 | 0.41 | 0.36 | 0.37 | 0.26 | 0.04 | 0.67 |
1A total of 10 pens were used per dietary treatment per replicate.
2Pigs weaned and transported for 12 h during July 2016.
3Pigs weaned and transported for 12 h during April 2017.
4Pigs provided dietary antibiotics [chlortetracycline (441 ppm) + tiamulin (38.6 ppm)] for 14 d postweaning and transport and then fed common antibiotic-free diets.
5Pigs provided 0.20% l-glutamine for 14 d postweaning and transport and then fed common antibiotic-free diets.
6Pigs provided no dietary antibiotics for 14 d postweaning and transport and then fed common antibiotic-free diets.
7Dietary treatment.
8Replicate.
9When 3 or more pigs are touching while lying down and 50% of a pig’s body is touching another pig; collected independent of other behaviors.
10Piglets are walking about or interacting in a nonaggressive manner with each other or their environment.
11Piglets are lying, either ventral or sternal, either alone or loosely in groups, with gaps of spaces between them.
12Piglets are engaged in agonistic interactions.
13The piglet has its nose in the feeder or its mouth on the waterer.
14When piglet moves out of view and cannot be observed.
x,yLetters indicate tendencies (0.05 < P ≤ 0.10) within a row dietary treatment.
Huddling, active, and eating/drinking behaviors were increased overall (P < 0.02; 179%, 37%, and 29%, respectively) in the spring replicate compared with the summer replicate (Table 7; Supplementary Figure 1). Nonvisible behavior was greater (P < 0.04; 121%) in the summer replicate compared with the spring replicate (Table 7; Supplementary Figure 1F). No other replicate differences were observed for behavior (P > 0.05) with any comparison (Table 7; Supplementary Figure 1).
Huddling behavior was greater overall (P < 0.01) on days 2 and 4 compared with days 8 and 12 (Supplementary Figure 1A). Active behavior was greater overall (P < 0.01) on day 2 compared with days 4, 8, and 12 (Supplementary Figure 1B). In addition, active behavior was greater overall (P < 0.01) on days 8 and 12 compared with day 4 (Supplementary Figure 1B). Resting behavior was greater overall (P < 0.01) on days 4, 8, and 12 compared with day 2 (Supplementary Figure 1C). Aggressive behavior was greater overall (P < 0.01) on day 2 compared to days 4, 8, and 12 (Supplementary Figure 1D). In addition, aggressive behavior was greater overall (P < 0.01) on day 4 compared with day 12 but no differences were observed on days 4 and 12 vs. day 8 (Supplementary Figure 1D). Eating/drinking behavior was greater overall (P = 0.01) on days 8 and 12 compared with days 2 and 4 (Supplementary Figure 1E). No other day differences were observed for behavior (P > 0.05) with any comparison (Table 7; Supplementary Figure 1).
Active behavior was greater (P < 0.01) on days 2, 4, and 8 during the spring replicate compared with the summer replicate but was not different on day 12 (Supplementary Figure 1B). Resting behavior was increased (P < 0.01) on day 2 during the summer replicate compared with the spring replicate; however, on day 12, resting behavior was greater during the spring replicate compared with the summer replicate (Supplementary Figure 1C). Aggressive behavior tended to be greater (P = 0.07) on day 8 during the spring replicate compared with the summer replicate (Supplementary Figure 1D). Eating/drinking behavior was greater (P < 0.01) on days 2 and 4 during the spring replicate compared with the summer replicate, but no differences were detected on days 8 and 12 (Supplementary Figure 1E). No other behavioral differences were detected (P < 0.05) with any comparison (Table 7; Supplementary Figure 1).
Carcass Characteristics
No dietary treatment effects were observed (P > 0.60) on carcass characteristics (Table 8). Hot carcass weight and loin depth were increased (P < 0.01; 5.4% and 5.5%, respectively) and carcass yield was reduced (P < 0.01; 2.0%) for pigs weaned in the spring replicate compared with the summer replicate when HCW was not used as a covariate in the statistical model (Table 8). When HCW was used as a covariate in the statistical analysis, loin depth and lean percentage were increased (P = 0.01; 4.0% and 1.1%, respectively) and carcass yield was reduced (P = 0.01; 2.3%) for pigs weaned in the spring replicate compared with the summer replicate (Table 8). Fat-free lean percentage during the spring replicate tended to be greater (P = 0.07; 1.3%) compared with the summer replicate when HCW was included as a covariate (Table 8). No other carcass characteristic differences were observed (P > 0.05) with any comparison (Table 8).
Table 8.
Effect of dietary treatment on carcass characteristics1
| Replicate | Diet | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Summer2 | Spring3 | A4 | GLN5 | NA6 | SE | D7 | R8 | D x R |
| No HCW9 covariate | |||||||||
| HCW, kg | 92.42 | 97.44 | 95.32 | 95.54 | 93.93 | 1.32 | 0.60 | <0.01 | 0.70 |
| Loin depth, mm | 63.95 | 67.46 | 65.79 | 65.85 | 65.48 | 0.72 | 0.93 | <0.01 | 0.60 |
| Backfat, mm | 21.35 | 22.05 | 21.73 | 21.64 | 21.73 | 0.59 | 0.99 | 0.31 | 0.40 |
| Yield, % | 77.18 | 75.67 | 76.55 | 76.36 | 76.36 | 0.19 | 0.68 | <0.01 | 0.46 |
| Lean, %10 | 54.42 | 54.61 | 54.51 | 54.55 | 54.47 | 0.25 | 0.97 | 0.53 | 0.54 |
| Fat-free lean, %11 | 48.69 | 48.79 | 48.74 | 48.79 | 48.69 | 0.30 | 0.97 | 0.76 | 0.50 |
| HCW covariate | |||||||||
| Loin depth, mm | 64.43 | 66.99 | 65.72 | 65.74 | 65.68 | 0.69 | 0.99 | 0.01 | 0.66 |
| Backfat, mm | 22.02 | 21.41 | 21.64 | 21.49 | 22.00 | 0.49 | 0.75 | 0.33 | 0.57 |
| Yield, % | 77.33 | 75.52 | 76.52 | 76.33 | 76.42 | 0.17 | 0.69 | 0.01 | 0.63 |
| Lean, % | 54.20 | 54.82 | 54.54 | 54.60 | 54.38 | 0.23 | 0.78 | 0.04 | 0.71 |
| Fat-free lean, % | 48.41 | 49.06 | 48.77 | 48.85 | 48.58 | 0.27 | 0.77 | 0.07 | 0.68 |
1A total of 10 pens were used per dietary treatment per replicate.
2Pigs weaned and transported for 12 h during July 2016.
3Pigs weaned and transported for 12 h during April 2017.
4Pigs provided dietary antibiotics [chlortetracycline (441 ppm) + tiamulin (38.6 ppm)] for 14 d postweaning and transport and then fed common antibiotic-free diets.
5Pigs provided 0.20% l-glutamine for 14 d postweaning and transport and then fed common antibiotic-free diets.
6Pigs provided no dietary antibiotics for 14 d postweaning and transport and then fed common antibiotic-free diets.
7Dietary treatment.
8Replicate.
9Hot carcass weight.
10Equation used: 54.672154 − (0.412525 × backfat, mm) − (0.002982 × hot carcass weight, kg × 2.20462) + (0.1433242 × loin depth, mm) (Indiana Packers Corporation, 2015).
11Equation used: 51.2 - (0.510 × backfat, mm) + (0.131 × loin depth, mm) (Schinckel et al., 2010).
DISCUSSION
The need to wean and transport pigs is necessary to reduce the risk of infectious disease through multisite production (Harris, 2000). However, the resultant stress response can reduce growth performance and welfare in newly weaned pigs (Chambers and Grandin, 2001; Campbell et al., 2013), especially in the absence of dietary antibiotics (Heo et al., 2013). Despite this, the use of in-feed antibiotics has been reduced in swine production due to consumer preference, legislative action, and concerns about antibiotic resistance (Smith et al., 2010), putting the welfare and productivity of newly weaned and transported pigs at risk and necessitating the development of effective alternatives. Recent work has described improved welfare and productivity in piglets provided GLN compared with A and NA following weaning and simulated transport (Johnson and Lay, 2017). In accordance with the aforementioned study, piglets provided GLN after weaning and transport in the present study had improved growth performance compared with NA pigs during the 14-d dietary treatment period, regardless of replicate. However, no growth performance differences were detected between GLN and A pigs in the current study. Although reasons for this discrepancy are currently unknown, it may be due to differences in study design since the transport procedure was simulated and piglets were individually housed in the previous study (Johnson and Lay, 2017). While the mechanism(s) of action for improved growth performance has yet to be discerned, GLN can serve as energy source for enterocytes, thus reducing jejunal atrophy and intestinal epithelial damage (Wu et al., 1996; Yi et al., 2005; Wang et al., 2015a,b). Therefore, it is possible that piglets provided supplemental GLN had improved intestinal barrier function leading to greater pathogen resistance, reduced translocation of bacteria (Peng, 2004; Wang et al., 2015a,b), and subsequently an improvement in growth performance (Jiang et al., 2009; Johnson and Lay, 2017). Nevertheless, the advantages observed in early nursery growth performance may suggest that GLN supplementation could serve as an alternative to dietary antibiotics in production systems.
Although growth performance was improved in GLN and A pigs during the dietary treatment period and the advantage was maintained for the overall nursery period, no differences were detected when compared with NA pigs from day 14 to market when all pigs were fed a common antibiotic-free diet. However, these results were expected as previous studies have described a loss of growth performance differences once dietary antibiotic treatments (Skinner et al., 2014) or dietary formulation treatments (Dritz et al., 1996) cease. This may be due to pen to pen variability differences that diminished the growth rate advantages as the studies progressed or the performance advantages of feeding dietary treatments are limited only to the period when fed. Therefore, it could be suggested that feeding GLN to pigs for a longer duration could have extended the growth benefits. However, further work would be needed to confirm this hypothesis and any increase in growth performance would need to be balanced with the cost of including GLN in diets for a longer period of time.
Tumor necrosis factor alpha is a proinflammatory cytokine and elevated levels of plasma TNF-α can be indicative of systemic inflammation and immune system activation (Kalliolias and Ivashkiv, 2016). An activated immune system is energetically expensive to the pig as the glucose requirement increased (Kvidera et al., 2017). This increase in glucose requirement by the activated immune system consumes energy that could be used for growth. As a result, growth may be inhibited during an immune challenge. In the present study, the reduced plasma TNF-α concentrations of A and GLN compared with NA fed pigs could be indicative of reduced whole-body inflammatory response, which would decrease the immune system energy requirement as described previously (Kvidera et al., 2017). As a result, more energy would likely be available for growth in the A and GLN fed pigs and may partially explain the improved performance compared with the NA fed pigs. Although reasons for this reduction in TNF-α are currently unknown, it is possible that an improvement in intestinal health caused the reduction in TNF-α for A and GLN fed pigs since decreased intestinal barrier function is associated with an increase in bacterial translocation and systemic inflammation in pigs (Pearce et al., 2014). However, more research is needed to confirm this hypothesis.
Cortisol is often used by researchers as a physiological indicator of stress in pigs and is often increased during stress exposure (Marchant-Forde et al., 2012). One of the most stressful periods during a pig’s life is weaning and transport (Campbell et al., 2013). However, previous studies in weaned pigs transported under TN conditions have shown that although cortisol levels will increase during transport, they return to baseline or reduced levels at unloading (Bradshaw et al., 1996, Averós et al., 2009). In contrast, when pigs are weaned and transported under HS conditions, cortisol levels remain elevated post-transport and then are reduced to near baseline levels the next day (Johnson et al., 2018). In accordance with the aforementioned reports, although a 38% numerical reduction in post-transport cortisol levels were observed in spring replicate transported sentinel pigs, those transported during the summer replicate in the present study had a 457% numerical increase in circulating cortisol levels following transport. Despite the fact that the weaning and transport process appeared to be more stressful (as indicated by numerically elevated cortisol levels) during the summer replicate, no replicate or dietary differences were observed on days 13 and 33 posttransport. This is likely due to the fact that pigs had recovered from the acute stressor and cortisol levels had returned to near baseline as time progressed as described previously (Johnson et al., 2018).
Weaning and transport are stressful to piglets and may result in behavioral changes including increased aggression and activity that are indicative of distress (Lewis and Berry, 2006; Wamnes et al., 2008). As such, newly weaned and transported piglets in the present study exhibited behavioral signs of distress immediately following transport, which subsequently declined as time progressed following weaning and transport. These behaviors ranged from increased activity, which may be indicative of greater exploratory behavior and stress (Bøe, 1993), to greater huddling behavior that may have been due to greater subclinical illness (Hennessy et al., 2001), and an increase in aggressive behavior likely due to fighting and establishing a social hierarchy (Meese and Ewbank, 1973; Blackshaw et al., 1987; Colson et al, 2012). However, despite the improved growth performance, dietary A and GLN supplementation treatments did not appear to alleviate this postweaning and transport behavioral stress response relative to NA treated pigs. In addition, aggressive behavior tended to be greater in A compared with GLN pigs, which may be a sign of resource guarding (i.e., feed; Drake et al., 2008) in group-housed pigs. Therefore, potential mechanisms may have been that A pigs spent more time guarding feed as this was the only resource available in the pen or that they felt better and were therefore more capable of doing so. However, because GLN and A pigs had similar ADFI, but differ in levels of aggression, it is still unclear whether the increase in aggressive behavior was due to resource guarding and further research should be performed to determine the cause.
In addition to the impact of weaning and transport as well as dietary treatments on piglet behavior, replicate effects were also observed. Increased resting behavior was observed during the summer replicate on day 2 postweaning and transport compared with the spring replicate and this may have been due to greater exhaustion and dehydration during the summer replicate as reported previously (Berry and Lewis, 2001). Furthermore, pigs weaned and transported in the spring replicate exhibited greater huddling behavior compared with those weaned and transported in the summer replicate. Although a specific reason has yet to be elucidated, this response may have been related to TA and pigs’ need for supplemental heat (Hay et al., 2001). This is because the nursery TA during the summer replicate was at the upper end of the recommended thermoneutral zone and the spring replicate nursery TA was at the lower end of the recommended thermoneutral zone for nursery pigs (Federation of Animal Science Societies, 2010). Therefore, the increase in summer replicate nursery TA may have diminished the need for huddling (Hay et al., 2001). Furthermore, this nursery TA difference may have been responsible for a reduction in eating/drinking and active behavior during the summer replicate in an effort to reduce heat increment from feed consumption during the time of day when behavior was analyzed (Coffey et al., 1982; Nienaber et al., 1999).
Therapeutic injectable antibiotics are one of many options currently available to aid in the control of pathogens and disease in addition to good biosecurity practices, vaccinations, and dietary antibiotics (Maes, 2008). An increase in treatment rate with therapeutic antibiotics can be an indicator of illness in swine herds. In the present study, A pigs had fewer therapeutic antibiotic treatments for enteric challenges compared with GLN pigs during the spring replicate from days 0 to 14 postweaning, but no differences were detected during the summer replicate. Although this may indicate that dietary antibiotic treatments were more effective at reducing pathogen load compared with GLN, the lack of overall dietary treatment differences may suggest that the timing of weaning and transport throughout the year influences the impact of GLN on therapeutic treatments. Regardless, the increase in therapeutic treatments did not appear to coincide with a depression in growth performance and this may be due to differences in the mode of action between A and GLN treatments, whereby dietary antibiotics reduce pathogen colonization (Pluske et al., 2002) while GLN improves gut barrier function in pigs (Wang et al., 2015a,b). Further work is needed to explore the combined feeding of multiple nutraceuticals that have shown performance benefits independently to determine whether the effect of combining them is additive.
In the present study, no dietary treatment effects were observed for carcass trait differences, confirming previous reports that providing dietary additives (i.e., antibiotics) for a limited period in the nursery phase would have no impact on carcass composition (Skinner et al., 2014). Although the effects of providing GLN on carcass characteristics in pigs are unknown, previous reports in broilers reported that GLN supplementation during heat stress improves meat yield (Dai et al., 2011). However, because broilers were provided GLN until harvest in the aforementioned study and pigs in the present study were only provided GLN for the first 14 d postweaning, it is likely that the lack of carcass trait differences is related to the timing of dietary inclusion. Nevertheless, a lack of dietary treatment differences confirms that GLN would not have negative effects on carcass traits compared with A and NA diets.
Despite the lack of dietary treatment differences on carcass characteristics, pigs weaned in the spring replicate had greater HCW and loin depth and increased lean percentage and fat-free lean percentage when HCW was used as a covariate compared with summer replicate weaned pigs. Although the mechanism(s) for the improvement in carcass characteristics are unknown, we speculate that health status may have affected the carcass differences observed in the current study due to the differences in therapeutic antibiotic treatment rate between replicates. This response appears to be consistent with previous work by Holck et al. (1998) and Williams et al. (1997) who reported improved carcass characteristics when pigs were reared under higher health status. This suggests that poorer health status may have decreased growth rate and subsequently reduced lean tissue accretion rate. This potential advantage in health status during the spring replicate weaned pigs may have allowed the pigs to grow and deposit lean tissue at a rate closer to their genetic potential because previous studies determined that when pigs were exposed to chronic immune system activation in a health compromised environment, cytokine concentration was elevated (Williams et al., 1997), thereby suppressing lean growth. This is further explained by Zamir et al. (1994) where rats administered with an IL-1 receptor antagonist had reduced skeletal muscle catabolism when IL-1 was administered. Thus, based on these relationships, less environmental pathogens as indicated by reduced therapeutic antibiotic use could have decreased immune system and cytokine activation, thus allowing the potential for increased muscle accretion rate due to less skeletal muscle catabolism.
CONCLUSION
Weaning and transport is stressful to pigs and antibiotics have been routinely used to help young pigs overcome these challenges. Despite the advantages in growth performance and productivity found from the use of dietary antibiotics, alternatives to antibiotics are needed. It was proposed that l-glutamine supplementation could serve as an antibiotic alternative following weaning and transport and allow pigs to perform similarly to those given dietary antibiotics. We determined that l-glutamine supplemented at 0.20% improved pig health and productivity after weaning and transport similarly to antibiotics during the nursery phase; however, the positive effects of dietary antibiotics and l-glutamine were diminished during the grow–finish phase. However, pigs not provided dietary antibiotics had decreased growth rate during the nursery phase. Future work should address the mechanism(s) by which l-glutamine supplementation improves pig growth performance following weaning and transport.
Supplementary Material
Footnotes
This work was supported in part by the Pork Checkoff (grant no. 16–065), National Pork Board, Des Moines, IA.
We would like to thank the swine farm staff at Purdue University for daily animal care and the employees at the USDA-ARS Livestock Behavior Research Unit for assistance in animal care and data collection.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. No conflicts of interest, financial, or otherwise are declared by the author(s).
LITERATURE CITED
- Averós X., Herranz A., Sánchez R., and Gosálvez L. F.. 2009. Effect of duration of commercial journeys between rearing farms and growing-finishing farms on the physiological stress response of weaned pigs. Livestock Sci. 122:339–344. doi:10.1016/j.livsci.2008.09.019 [Google Scholar]
- Berry R. J., and Lewis N. J.. 2001. The effect of duration and temperature of simulated transport on the performance of early-weaned piglets. Can. J. Anim. Sci. 81:199–204. doi:10.4141/A00-069 [Google Scholar]
- Blackshaw J. K., Bodero D., and Blackshaw A. W.. 1987. The effect of group composition on behavior and performance of weaned pigs. Appl. Anim. Behav. Sci. 19:73–80. doi:10.1016/0168-1591(87)90204–8 [Google Scholar]
- Bøe K. 1993. The effect of age at weaning and post-weaning environment on the behaviour of pigs. Acta. Agric. Scand. 43:173–180. doi:10.1080/09064709309410162 [Google Scholar]
- Bradshaw R. H., Parrott R. F., Goode J. A., Lloyd D. M., Rodway R. G., and Broom D. M.. 1996. Behavioural and hormonal responses of pigs during transport: effect of mixing and duration of journey. Anim. Sci. 62:547–554. doi:10.1017/S1357729800015095 [Google Scholar]
- Brooks P. H., Moran C. A., Beal J. D., Demeckova V., and Campbell A.. 2001. Liquid feeding for the young piglet In: Varley M. A. and Wiseman J., editors, The weaner pig: nutrition and management. CABI Publishing, Wallingford, CT: p. 153–178. [Google Scholar]
- Campbell J. M., Crenshaw J. D., and Polo J.. 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi:10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers P. G., and Grandin T.. 2001. Guidelines for humane handling, transport and slaughter of livestock. RAP Publication. Bangkok, Thailand; p. 33–48. [Google Scholar]
- Chiba L. I. 2010. Swine production handbook. 13th Revision. Auburn University, Auburn, AL Section 6 62–75. [Google Scholar]
- Coffey M. T., Seerley R. W., Funderburke D. W., and McCampbell H. C.. 1982. Effect of heat increment and level of dietary energy and environmental temperature on the performance of growing-finishing swine. J. Anim. Sci. 54:95–105. doi:10.2527/jas1982.54195x [DOI] [PubMed] [Google Scholar]
- Colson V., Martin E., Orgeur P., and Prunier A.. 2012. Influence of housing and social changes on growth, behaviour and cortisol in piglets at weaning. Physiol. Behav. 107:59–64. doi:10.1016/j.physbeh.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Dai S. F., Gaoa F., Zhanga W. H., Songa S. X., Xub X. L., and Zhoub G. H.. 2011. Effects of dietary glutamine and gamma-aminobutyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. Anim. Feed Sci. Technol. 168:51–60. doi:10.1016/j.anifeedsci.2011.03.005 [Google Scholar]
- Drake A., Freaser D., and Weary D. M.. 2008. Parent–offspring resource allocation in domestic pigs. Behav. Ecol. Sociobiol. 62:309–319. doi:10.1007/s00265-007-0418-y [Google Scholar]
- Dritz S. S., Owen K. Q., Nelssen J. L., Goodband R. D., and Tokach M. D.. 1996. Influence of weaning age and nursery diet complexity on growth performance and carcass characteristics and composition of high-health status pigs from weaning to 109 kilograms. J. Anim. Sci. 74:2975–2984. doi:10.2527/1996.74122975x [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed.Fed. Anim. Sci. Soc., Champaign, IL: Chap. 11. [Google Scholar]
- Harris D. L. 2000. Multi-site pig production. Iowa State University Press, Ames, IA. [Google Scholar]
- Hay M., Orgeur P., Lévy F., Le Dividich J., Concordet D., Nowak R., Schaal B., and Morméde P.. 2001. Neuroendocrine consequences of very early weaning in swine. Physiol. Behav. 72:263–269. doi:10.1016/S0031-9384(00)00404-2 [DOI] [PubMed] [Google Scholar]
- Hennessy M. B., Deak T., and Schiml‐Webb P. A.. 2001. Stress‐induced sickness behaviors: an alternative hypothesis for responses during maternal separation. Dev. Psychol. 39(2):76–83. doi:10.1002/dev.1031 [DOI] [PubMed] [Google Scholar]
- Heo J. M., Opapeju F. O., Pluske J. R., Kim J. C., Hampson D. J., and Nyachoti C. M.. 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl). 97:207–237. doi:10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Hicks T. A., McGlone J. J., Wishant C. S., Kattesh H. G., and Norman R. L.. 1998. Behavioural, endocrine, immune and performance measures for pigs exposed to acute stress. J. Anim. Sci. 76:474–483. doi:10.2527/1998.762474x [DOI] [PubMed] [Google Scholar]
- Holck J. T., Schinckel A. P., Coleman J. L., Wilt V. M., Senn M. K., Thacker B. J., Thacker E. L., and Grant A. L.. 1998. The influence of environment on the growth of commercial finisher pigs. Swine Health Prod. 6(4):141–149. [Google Scholar]
- Indiana Packers Corporation 2015. Indiana Packers Corporation Procurement Program. Delphi, IN. [Google Scholar]
- Jiang Z. Y., Sun L. H., Lin Y. C., Ma X. Y., Zheng C. T., Zhou G. L., Chen F., and Zou S. T.. 2009. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J. Anim. Sci. 87:4050–4056. doi:10.2527/jas.2008-1120 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Aardsma M. A., Duttlinger A. W., and Kpodo K. R.. 2018. Early life thermal stress: impact on future thermotolerance, stress response, behavior, and intestinal morphology in piglets exposed to a heat stress challenge during simulated transport. J. Anim. Sci. 96:1640–1653. doi:10.1093/jas/sky107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., and Lay D. C. Jr.. 2017. Evaluating the behavior, growth performance, immune parameters, and intestinal morphology of weaned piglets after simulated transport and heat stress when antibiotics are eliminated from the diet or replaced with L-glutamine. J. Anim. Sci. 95:91–102. doi:10.2527/jas.2016.1070 [DOI] [PubMed] [Google Scholar]
- Kalliolias G. D. and Ivashkiv L. B.. 2016. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. 2016. Nat. Rev. Rheumatol. 12:49–62. doi:10.1038/nrrheum.2015.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Mayorga E. J., Sanz-Fernandez M. V., Abuajamieh M., Baumgard L. H.. 2017. Estimating glucose requirements of an activated immune system in growing pigs. J. Anim. Sci. 201795:5020–5029. doi:10.2527/jas2017.1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallés J. P., Boudry G., Favier C., Le Floc’h N., Luron I., Montagne L., Oswald I. P., Pié S., Piel C., and Séve B.. 2004. Gut function and dysfunction in young pigs: physiology. Anim. Res. 53:301–316. doi:10.1051/animres:2004018 [Google Scholar]
- Lambooy E. 1988. Road transport of pigs over a long distance: some aspects of behavior, temperature and humidity during transport and some effects of the last two factors. Anim. Prod. 46:257–263. doi:10.1017/S000335610004232X [Google Scholar]
- Lewis N. J., and Berry R. J.. 2006. Effects of season on the behavior of early-weaned piglets during and immediately following transport. Appl. Anim. Behav. Sci. 100:182–192. doi:10.1016/j.applanim.2005.12.006 [Google Scholar]
- Maenz D. D., Patience J. F., and Wolynetz M. S.. 1994. The influence of the mineral level in drinking water and the thermal environment on the performance and intestinal fluid flux of newly weaned pigs. J. Anim. Sci. 72:300–308. doi:10.2527/1994.722300x [DOI] [PubMed] [Google Scholar]
- Maes D., Segales J., Meyns T., Sibila M., Pieters M., and Haesebrouck F.. 2008. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 126:297–309. doi:10.1016/j.vetmic.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant-Forde J. N., Matthews D. L., Poletto R., McCain R. R., Mann D. D., DeGraw R. T., Hampsch J. M., Peters S., Knipp G. T., and Kissinger C. B.. 2012. Plasma cortisol and noradrenalin concentrations in pigs: automated sampling of freely moving pigs housed in the PigTurn® versus manually sampled and restrained pigs. Anim. Welfare. 21:197–205. doi:10.7120/09627286.21.2.197 [Google Scholar]
- Meese G. B. and Ewbank R.. 1973. The establishment and nature of dominance hierarchy in domesticated pigs. Anim. Behav. 21:326–34. doi:10.1016/S0003-3472(73)80074-0 [Google Scholar]
- Moeser A. J., Klok C. V., Ryan K. A., Wooten J. G., Little D., Cook V. L., and Blikslager A. T.. 2007. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver. Physiol. 292:G173–G181. doi:10.1152/ajpgi.00197.2006 [DOI] [PubMed] [Google Scholar]
- National Pork Board 2015. Transport Quality Assurance Handbook. 5th ed.Natl. Pork Board, Clive, IA. [Google Scholar]
- Nienaber J. A., Hahn G. L., and Eigenberg R. A.. 1999. Quantifying livestock responses for heat stress management: a review. Int. J. Biometeorol. 42:183–188. doi:10.1007/s004840050103 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pearce S. C., Sanz-Fernandez M. V., Hollis J. H., Baumgard L.H., and Gabler N. K.. 2014. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J.Anim. Sci. 92:5444–5454. doi: 10.2527/jas2014-8407 [DOI] [PubMed] [Google Scholar]
- Peng X., Yan H., You Z., Wang P., and Wang S.. 2004. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 30:135–139. doi:10.1016/j.burns.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Pethick D. W., Hopwood D. E., and Hampson D. J.. 2002. Nutritional influences on some major enteric bacterial diseases of pigs. Nutr. Res. Rev. 15:333–371. doi:10.1079/NRR200242 [DOI] [PubMed] [Google Scholar]
- Schinckel A. P., Wagner J. R., Forrest J. C., and Einstein M. E.. 2010. Evaluation of the prediction of alternative measures of pork carcass composition by three optical probes. J. Anim. Sci. 2010. 88:767–794. doi:10.2527/jas.2009–2286 [DOI] [PubMed] [Google Scholar]
- Skinner L. D., Levesque C. L., Wey D., Rudar M., Zhu J., Hooda S., and de Lange C. F. M.. 2014. Impact of nursery feeding program on subsequent growth performance, carcass quality, meat quality, and physical and chemical body composition of growing-finishing pigs. J. Anim. Sci. 92:1044–1054. doi:10.2527/jas.2013–6743 [DOI] [PubMed] [Google Scholar]
- Smith M. G., Jordan D., Chapman T. A., Chin J. J., Barton M. D., Do T. N., Fahy V. A., Fairbrother J. M., and Trott D. J.. 2010. Antimicrobial resistance and virulence gene profiles in multi-drug resistant enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea. Vet. Microbiol. 145:299–307. doi:10.1016/j.vetmic.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Van der Meulen J., Koopmans S. J., Dekker R. A., and Hoogendoom A.. 2010. Increasing weaning age of piglets from 4 to 7 weeks reduces stress, increases post-weaning feed intake but does not improve intestinal functionality. Animal 4:1653–1661. doi:10.1017/S1751731110001011 [DOI] [PubMed] [Google Scholar]
- Wamnes S., Lewis N. J., and Berry R. J.. 2008. The behaviour of early-weaned piglets following transport: effect of season and weaning weight. Can. J. Anim. Sci. 88(3):357–367. doi:10.4141/CJAS07083 [Google Scholar]
- Wang B., Wu G., Zhou Z., Dai Z., Sun Y., Ji Y., Li W., Wang W., Liu C., Han F., and Wu Z.. 2015a. Glutamine and intestinal barrier function. Amino Acids. 47:2143–2154. doi:10.1007/s00726-014-1773-4 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang C., Wu G., Sun Y., Wang B., He B., Dai Z., and Wu Z.. 2015b. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 145(1):25–31. doi:10.3945/jn.114.202515 [DOI] [PubMed] [Google Scholar]
- Williams N. H., Stahly T. S., and Zimmerman D. R.. 1997. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J. Anim. Sci. 75:2481–2496. doi:10.2527/1997.7592481x [DOI] [PubMed] [Google Scholar]
- Wu G., Meier S. A., and Knabe D. A.. 1996. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J. Nutr. 126:2578–2584. doi:10.1093/jn/126.10.2578 [DOI] [PubMed] [Google Scholar]
- Yi G. F., Carroll J. A., Allee G. L., Gaines A. M., Kendall D. C., Usry J. L., Toride Y., and Izuru S.. 2005. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J. Anim. Sci. 83:634–643. doi:10.2527/2005.833634x [DOI] [PubMed] [Google Scholar]
- Zamir O., O’Brien W., Thompson R., Bloedow D. C., Fischer J. E., and Hasselgren P.. 1994. Reduced muscle protein breakdown in septic rats following treatment with interleukin-1 receptor antagonist. Int. J. Biochem. 26:943. doi:10.1016/0020-711X(94)90088-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.