Abstract
Pig is one of the major dietary protein sources for human consumption, from which muscle is the largest protein origin. However, molecular mechanisms concerning early porcine embryonic muscle development distinctions between pig breeds are still unclear. In this study, an integrated analysis of transcriptome and miRNAome was conducted using longissimus dorsi muscle of 4 early embryonic stages around the primary myofiber formation time (18-, 21-, 28-, and 35-d post coitus) from 2 pig breeds (Landrace [LR] and Wuzhishan [WZS]) differing in meat mass. The global miRNA/mRNA expression profile showed that WZS prepared for myogenic developmental processes earlier than LR. After identifying and analyzing the interaction network of top 100 up-/down-regulated miRNA and their target genes, we were able to find 3 gene clusters: chromatin modification-related (Chd2, H3f3a, Chd6, and Mll1), myogenesis-related (Pax3, Pbx1, Mef2a, and Znf423), and myosin component–related (Mylk, Myo5a, Mylk4, Myh9, and Mylk2) gene clusters. These genes may involve in miRNA-gene myogenic regulatory network that plays vital role in regulating distinct early porcine embryonic myogenic processes between LR and WZS. In summary, our study reveals an epigenetic-mediated myogenic regulatory axial that will help us to decipher molecular mechanisms concerning early porcine embryonic muscle development distinctions between pig breeds.
Keywords: epigenetic, miRNAome, myogenesis, porcine embryonic development, Sus scrofa, transcriptome
INTRODUCTION
Pig is one of the major dietary protein sources for human consumption and valuable model in biomedical research (Lunney, 2007; Patterson et al., 2008), from which skeletal muscle is the largest protein composition. In our previous study about skeletal muscle development between indigenous pig breed Lantang (LT, fat) and Landrace (LR, lean), we examined the longissimus dorsi muscle (LDM) by hematoxylin–eosin (HE) staining and found that LT with smaller size formed more primary myofibers on 35-d post coitus (dpc) (Zhao et al., 2011), and we also conducted microRNAome analysis by solexa sequencing and selected 18 novel candidate myogenic miRNAs in pig, which provided new insight into regulation mechanism mediated by miRNAs underlying muscle development (Qin et al., 2013). However, the mechanisms of this phenomenon are still remained to be further elucidated. Thus, to investigate the mechanisms of distinct embryonic muscle development mechanisms between pig breeds differing in meat mass, we used 4 stages (18, 21, 28, and 35 dpc): LR is characterized by high lean meat percentage, fast growing muscle, and high body weight (Li et al., 2003; Tang et al., 2007); the Wuzhishan miniature pig (WZS), a Chinese inbred miniature pig, whose mature adult weighs less than 40 kg (Wu et al., 2004). Our previous iTRAQ-based early embryonic muscle study of LR and WZS using same embryonic stages showed that the distinction of myofiber characteristics of these 2 pig breeds began in the early embryonic stages (Zhang et al., 2016). To gain further insight into the mechanism that regulates muscle development in pig breed with less meat production, we conducted integrated analysis of microRNAome and transcriptome data. Our study aims to uncover the mechanism underlying early porcine embryonic muscle development, which could contribute to the improvement in porcine meat production.
MATERIALS AND METHODS
Ethics Statement
We performed all the animal procedures according to China Council on Animal Care and the protocols we used were approved by the Animal Care and Use Committee of Guangdong Province, China. The approval ID or permit numbers are SCXK (Guangdong) 2011-0029 and SYXK (Guangdong) 2011-0112.
Preparation of Experimental Animals and Tissues
Twenty-four purebred sows with the same genetic background of both pig breeds (LR and WZS, 12 sows for each breed) were artificially inseminated with semen from the same purebred boars. For each breed in prenatal stages, 3 sows per time point was slaughtered at 18, 21, 28, and 35 after insemination, and embryos/fetuses were collected. The LDM tissues were dissected from all the embryos/fetuses. The whole embryos of the first 2 stages (18 and 21 dpc) and LDM tissues from fetuses of other stages were used as the experimental samples, at least 3 embryos or fetuses were collected for each breed at every stage. These samples were snap-frozen in liquid nitrogen and stored until further use.
RNA Extraction, Library Construction, and Small RNA/mRNA Sequencing
The RNA libraries were constructed and deep sequencing was performed by Beijing Genomics Institute (BGI, Shenzhen, China). We did independent extractions and sequencing of small RNA and mRNA and constructed 8 libraries of small RNA and mRNA each by utilizing pooled whole embryos or LDM tissue samples from 3 embryo/fetuses of each breed at each stage.
For small RNA library construction and sequencing, total RNA was extracted using miRNeasy Mini Kit (Cat#217004, QIAGEN, GmBH, Germany) according to the manufacturer’s protocol. Total RNA integrity was measured on an Agilent 2100 Bioanalyzer system (Agilent) for quality control. 16–35 nt RNA fragments were excised, purified from a PAGE gel, and ligated with 5′ and 3′ adaptors using T4 RNA ligase. Reverse transcription followed by PCR was used to create cDNA constructs based on the small RNA ligated with 3′ and 5′ adapters. Subsequently, the amplified cDNA constructs were purified from agarose gel, in preparation for sequencing analysis using the Illumina Genome Analyzer (Illumina, CA) according to the manufacturer’s instructions.
For mRNA library construction and sequencing, total RNA was extracted from frozen muscle tissues using TRIzol reagent (Invitrogen, CA) according to the manufacturer’s protocols. RNA integrity and concentration were evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Sequence tags were prepared using Illumina’s Digital Gene Expression Tag Profiling Kit according to the manufacturer’s protocols. The quality of all the sample solutions had RNA integrity numbers (RIN) ≥ 7.0 and 28S/18S ≥ 1.0. RNA libraries were constructed and Solexa sequencing was performed by the BGI on an Illumina Genome Analyzer.
Sequence tags were prepared using Illumina HiSeq 2000 (Illumina, San Diego, CA) according to the manufacturer’s protocols. Clean tags were obtained by filtering raw data to remove adaptor tags, low-quality tags, and tags of copy number= 1. The clean tags were classified according to their copy numbers in the library. The proportion of each categories in relation to total clean tags was determined. Similar analysis was carried out for clean distinct tags. The saturation analysis of the sequencing library was completed by BGI. For reads mapping, sequences were obtained from Sus Scrofa RefSeq (Sscrofa11.1) databases.
Differential Expression
To compare the differential expression of miRNAs across samples, the number of raw clean tags in each sample was normalized to Transcripts Per Million (TPM) to obtain normalized miRNA expression levels, as described before (Fahlgren et al., 2007). Differential expression of genes or tags across samples was detected according to previously described methods (Mortazavi et al., 2008). Genes were deemed significantly differentially expressed with |log2(FC)| ≥ 1(fold change (FC)) and FDR ≤0.05 in sequence counts across libraries.
Bioinformatic Analysis
We used Cluster 3.0 and TreeView software to analyze the systematic cluster of 8 libraries. Correlation analysis of 8 libraries were done by Pearson’s correlation coefficient (SPSS software 10.0).
The biological process (BP) of each differentially expressed (DE) miRNA was annotated by the Blast2GO software (http://www.blast2go.org/). Gene Ontology (GO) functional classification and enrichment analysis were also conducted to identify GO terms that were significantly enriched in DE miRNAs using DAVID analysis (http://david.abcc.ncifcrf.gov/). Heatmaps were drawn for the DE miRNAs using the R language package “Pheatmap.”
The miRNA–mRNA interaction network was drawn using the STRING (http://string-db.org/) and Cytoscape 3.1.0 (http://www.cytoscape.org/) programs. Venn diagram was drawn by online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
RNA-Seq Data Analysis
RNA-sequencing data were obtained from the same tissues as those used in miRNA sequencing. The reference genome index was built using the Bowtie2-build component of Bowtie2 (ver. 2.0) and SAMtools (ver. 0.1.18). Tophat2 (version 2.0.8) was applied to map the reads to the reference genome. The expression levels of each gene were normalized as FPKM (Reads per kilobase of exon model per million mapped reads) using the Refseq gene (Sus Scrofa RefSeq 11.1) databases model downloaded from the UCSC Browser gateway. Each FPKM was log2-transformed (Supplementary Table S1).
qPCR Analyses
Total RNA was extracted from cells or LDM tissues by TRIzol Reagent (Invitrogen, Shanghai, China), and then cDNA was synthesized using reverse-transcription Kit (Promega, Beijing, China). Real-time quantitative PCR reactions were performed using SYBR Green qPCR Kit (Genestar, Beijing, China) and detected in the LightCycler 480 II system (Roche, Basel, Switzerland). mRNA expression level results were normalized to that of Gapdh. Mir-17-5p was chosen as internal control of miRNAs’ expression level and all reactions were run in triplicate. The primers used for qPCR were given in Supplementary Table S2. The experimental data were analyzed using the 2−∆∆CT method.
Western Blot
For analyses of protein levels, embryos/muscle tissues at same stages from LR and WZS were homogenized in phosphosafe homogenizing buffer (Merk). Samples were run on an 11% SDS-PAGE gel and transferred onto a PVDF membrane. Then, immunoblotting for target proteins were carried out by specific antibodies (PAX3 Abcam, ab180754, 1:1000 dilution; MEF2A, Cell signaling, 9736s, 1:1000 dilution; H3F3A, Abcam, ab176840, 1:1000 dilution). GAPDH (ab8245, Abcam; 1:1000 dilution) and H3 (ab201456, Abcam; 1:2000 dilution) were used as the internal control. Secondary antibodies used were either anti-rabbit HRP-linked (#7074 S, CST, 1:1000 dilution) or anti-mouse HRP-linked (#7076 S, CST, 1:1000 dilution) antibodies. Results were visualized using a commercial enhanced chemiluminescence (ECL) detection Kit (Thermo Scientific).
Statistical Analysis
All data are presented as the mean ± s.e.m.; significance of differences in comparisons was determined by a Student’s t-test. Values of P < 0.05 were considered as statistically significant.
Data Availability Statement
The RNA-seq raw data from this study have been deposited in NCBI Sequence Read Archive with accession number SRR7369735-7369740, SRR7779832, and SRR7779834. The miRNA sequencing raw data from this study have been deposited in NCBI Sequence Read Archive with accession number SRR8168016-23. (http://www.ncbi.nlm.nih.gov/Traces/sra/).
RESULTS
WZS miRNA and mRNA Expression Profiles Prepared for Subsequent Developmental Processes Earlier Than LR
After trimming of adaptor sequences and removal of low-quality reads, ~94.79 million total clean reads were obtained from 8 libraries, of which the Q30 value of all the libraries was over 94.15% (Supplementary Tables S3 and S4). Therefore, the results demonstrate that the deep-sequencing data are able to accurately represent the miRNA transcriptome profiles of porcine skeletal muscle.
Of the obtained sequences, the most abundant size class in the small RNA sequences distribution was 22 nt, followed by 21 and 23 nt (Supplementary Figure S1), consistent with the known 21- to 23-nt range for mature miRNAs. In addition, the obtained sequences were analyzed referencing the data from miRbase (release 18.0), resulting in 302 known miRNAs and 1152 novel miRNAs (Supplementary Table S5). Next, the rest obtained sequences were subjected to Rfam (version 10) and noncoding RNA in Ensemble (Supplementary Table S6). Most obtained small RNAs were porcine miRNAs (ssc-miRNAs) of the unique sequence reads in 8 small RNA libraries. In summary, these results provide that the deep-sequencing data are highly enriched for known miRNA sequences, suggesting that the data are reliable for analyzing miRNA expression profiles. Additionally, miRNA expression profile analysis indicates that most miRNAs express at very low levels (Supplementary Figure S2).
For RNA-seq analysis, after filtering adaptor tags, empty reads, low-quality tags, and one-copy tags from 15 libraries of sequencing samples, 8.87 × 108 total clean tags (quantity of all the clean tags sequenced was controlled) were remained (Supplementary Table S4). For reads mapping, sequences were obtained from Sus Scrofa RefSeq and UniGene (NCBI36.1, 20090827) databases. Approximately 74.6% of the total clean reads were mapped to the reference database, whereas 68.3% of total clean reads were mapped uniquely (reads mapped to only one postion) to the reference database (Supplementary Table S5). Additionally, gene expression profile analysis suggests that most genes express at very low levels (Supplementary Table S1).
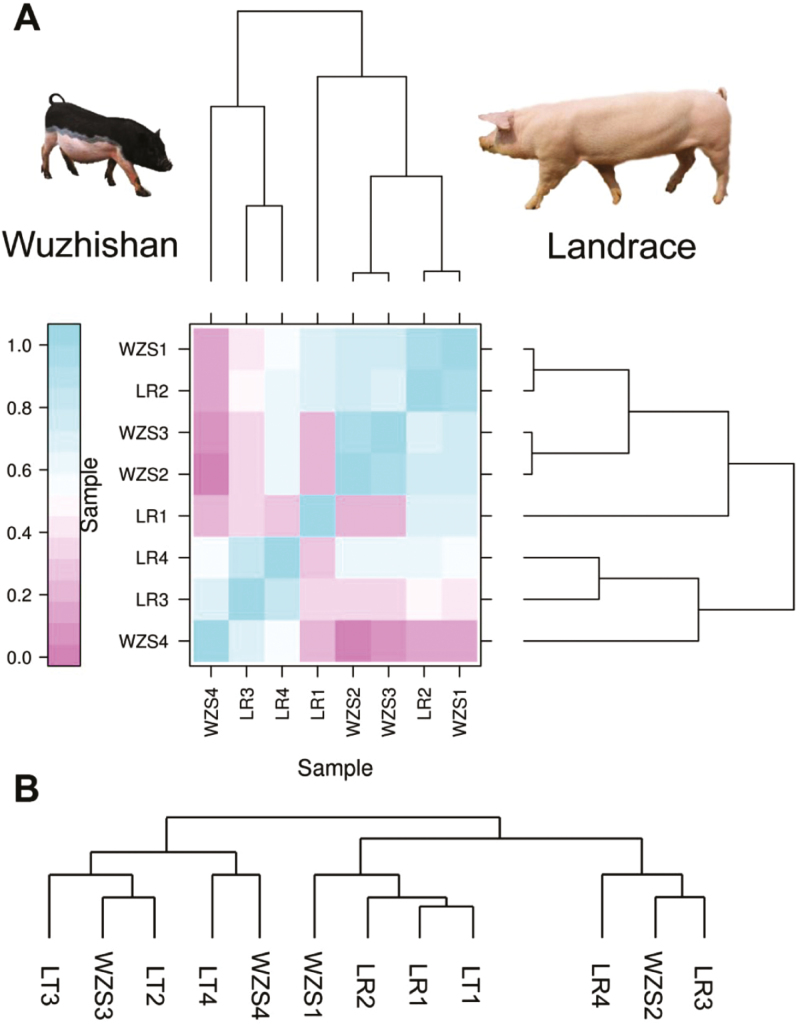
To understand the global miRNA and mRNA expression pattern of 8 libraries, cluster analysis (using all miRNAs/mRNA) were performed (Figure 1). The 8 different miRNA transcription profiles could be divided into several distinct classes, from which WZS (21 dpc) and WZS (28 dpc), LR (21 dpc) and WZS (18 dpc), and LR (28 dpc) and LR (35 dpc) tended to show most similar miRNA expression profile (Figure 1A). Similarly, the 8 different mRNA transcription profiles could be divided into several distinct classes, from which WZS (18 dpc) and LR (18&21 dpc) and WZS (21 dpc) and LR (28 dpc) tended to show most similar miRNA expression profile (Figure 1B). These results indicated that, during early embryonic development, miRNA expression profiles of WZS showed similarity with its early stages (miRNA expression profiles of WZS 28 dpc was similar to WZS 21 dpc), and miRNA/mRNA expression profiles of LR tended to cluster with the miRNA/mRNA expression profiles of WZS late stages (miRNA expression profiles of WZS 28 dpc were similar to LR 21 dpc, mRNA expression profiles of WZS 18 dpc were similar to LR 21 dpc, andmRNA expression profiles of WZS 21 dpc were similar to LR 28 dpc), thus leading us to suppose that WZS prepared for subsequent developmental processes earlier than LR.
Figure 1.
MicroRNA and mRNA expression profile cluster analysis of Landrace (LR) and Wuzhishan (WZS) pigs. Cluster analysis of miRNA (A) and mRNA (B) expression profiles from all the libraries was conducted. LR represents Landrace and WZS represents Wuzhishan. 1–4 represent 18, 21, 28, and 35 d post coitus (dpc), respectively.
WZS Showed More Up-Regulated DE miRNA During 18–21 dpc
Differentially expressed miRNAs may play important roles in porcine embryonic myogenesis (Cao et al., 2014). To determine miRNAs involved in porcine myogenesis, DE miRNAs between 2 pig breeds were identified by comparing the normalized expression data of all the 1457 miRNAs with the criteria of |log2(FC)| ≥ 1; FDR ≤ 0.05 (Supplementary Table S7).
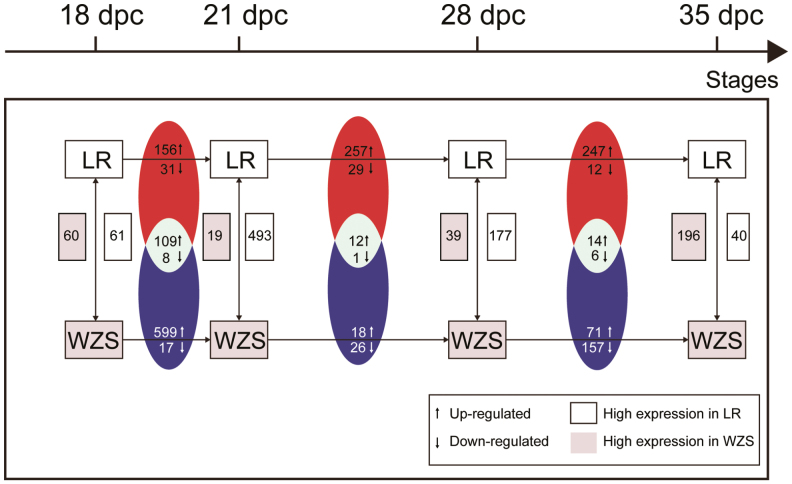
In comparison between 2 pig breeds at same stages, similar number of DE miRNAs was found at 18 dpc (61 highly expressed in LR and 60 highly expressed in WZS; Figure 2). While at 21 and 28 dpc, more DE miRNAs that showed higher expression in LR was found (493 highly expressed in LR and 19 highly expressed in WZS at 21 dpc, 177 highly expressed in LR, and 39 highly expressed in WZS at 28 dpc). Then at 35 dpc, more DE miRNAs that showed higher expression in WZS was found (40 highly expressed in LR and 196 highly expressed in WZS).
Figure 2.
Landscape of differentially expressed (DE) miRNAs. DE up-/down-regulated miRNAs of adjacent stages within the same breed or DE up-/down-regulated miRNAs between breeds at same stages were calculated. Shared up-/down-regulated miRNAs of WZS and LR were illustrated by Venn diagram. The number of miRNAs with higher expression in LR/WZS was also marked.
Shared up-/down-regulated DE miRNAs in comparison between adjacent stages within breeds during same period were also analyzed. In 21–18 comparison, 69.87% up-regulated DE miRNAs in WZS (109 out of 156 up-regulated DE miRNAs) were also up-regulated in LR, whereas in other comparison periods, less than 20 up-/down-regulated DE miRNAs were found.
In comparison between adjacent stages within breeds, the number of up-regulated DE miRNAs was more than that of down-regulated DE miRNAs in both breeds in 21-vs-18 comparison, and the number of up-regulated miRNAs in WZS was more than that of LR during this period (599 up-regulated DE miRNAs in WZS and 156 up-regulated DE miRNAs in LR; Figure 2). In 28-vs-21 comparison, LR showed greatly increase in the number of up-regulated DE miRNAs than that of WZS (257 up-regulated DE miRNAs in LR and 18 up-regulated DE miRNAs in WZS), whereas similar number of down-regulated DE miRNAs was found between 2 breeds (29 down-regulated DE miRNAs in LR and 26 down-regulated DE miRNAs in WZS). Furthermore, WZS had more down-regulated DE miRNAs than up-regulated DE miRNAs. In 35-vs-28 comparison, LR still showed more up-regulated DE miRNAs than down-regulated DE miRNAs (247 up-regulated DE miRNAs and 12 down-regulated DE miRNAs in LR), whereas WZS had more down-regulated DE miRNAs than up-regulated DE miRNAs (71 up-regulated DE miRNAs and 157 down-regulated DE miRNAs in LR).
These results revealed that WZS showed obviously more up-regulated miRNA during 18–21 dpc, whereas LR began to enter these processes in the period of 21–35 dpc. This is consistent with the cluster analysis that WZS may be earlier prepared for later developmental processes.
The Most Abundant Up/Down DE miRNAs Between Pig Breeds Showed Myogenic-Related Functional Enrichment
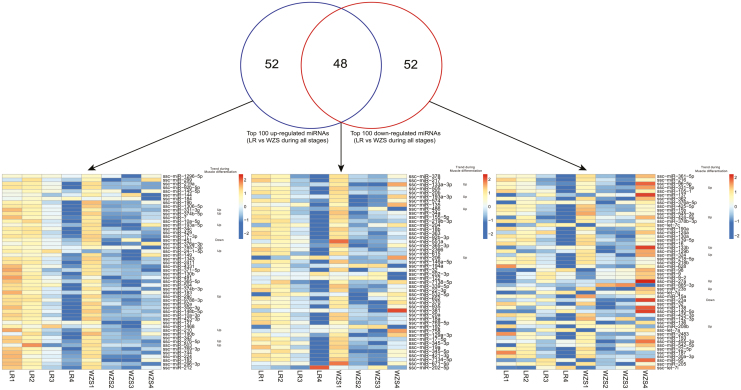
As the stages we studied is thought to be within the period of porcine primary myofiber formation, the DE miRNAs that up/down regulated significantly during these stages may play vital role in regulating myogenesis. Thus, 100 top up-/down-regulated DE miRNAs in comparison between LR and WZS during all stages were identified. We also utilized specific 52 top up-/down-regulated DE miRNAs and 48 shared up-/down-regulated DE miRNAs to conduct heatmap of their expression patterns (Figure 3).
Figure 3.
Analysis of top 100 up-/down-regulated DE miRNAs between WZS and LR. Top 100 up-/down-regulated DE miRNAs between WZS and LR during all stages were selected and Venn diagram was drawn. Heatmap of specific up-/down-regulated miRNAs or shared up/down miRNAs expression patterns were illustrated. Myogenic-related miRNAs and their expression trends during myogenesis from previous studies were marked.
As we can see in the heatmap of specific 52 top up-regulated DE miRNAs, most of the miRNAs in LR showed higher expression level than that of WZS during 18–28 dpc, whereas at 35 dpc, most of the miRNAs in WZS showed higher expression level than that of LR. Similarly, in the heatmap of specific 52 top down-regulated DE miRNAs, most of the miRNAs in LR showed higher expression level than that of WZS during 18–28 dpc. While at 35 dpc, most of the miRNAs in WZS showed higher expression level than that of LR. In the heatmap of 48 shared up-/down-regulated DE miRNAs, most miRNAs showed higher expression level in WZS at 18 and 35 dpc, whereas at 21 and 28 dpc, most of the miRNAs in LR showed higher expression level than that of WZS (Figure 3). In summary, top up-/down-regulated miRNAs showed distinct expression profiles between LR and WZS, most expression changes were found during 21–28 dpc in LR, whereas in WZS, most expression changes were found during 18–21 or 28–35 dpc.
A number of miRNAs that play vital roles during myogenesis (their expression trends during myogenesis were marked in Figure 3) were identified (Guller and Russell, 2010; Kovanda et al., 2014), such as miR-206 and miR-133a-3p, which was also identified in our previous LDM miRNAome analysis of LR (Qin et al., 2013). Of note, most of the myogenic-related miRNAs whose expression had up-regulated trend during myogenesis processes as previous reports described showed up-regulated trend in WZS during early embryonic stages than that of LR. Taken together, these DE miRNAs, especially myogenic DE miRNAs, may play an important role in regulating distinct myogenic processes between LR and WZS during early embryonic stages and these results indicate more intense myogenic processes in WZS during early embryonic stages.
Epigenetic-Mediated miRNA–mRNA Interaction Networks Were Involved in Regulating Porcine Embryonic Myogenesis
To further investigate the genes which were influenced by miRNA we identified before, target genes of myogenic miRNAs marked in Figure 3 were predicted by the shared results of Targetscan and miRDB (Supplementary Table S8). Among these target genes, we conducted GO analysis and identified 15 myogenic-related genes. By illustrating heatmap utilizing RNA-seq data from this study, we found that most myogenic target genes showed higher expression level in WZS during 28–35 dpc (Figure 4A). Although some target genes showed higher expression level in LR, most of them showed lower expression level at 28–35 dpc. Although the target genes showed higher expression level at 28 and 35 dpc in WZS, and just around this period, the primary myofibers form in pig. In addition, miRNA expression profiles of WZS 28 dpc clustered with its earlier stages 21 dpc, together with the findings in our previous study that WZS showed earlier and higher expression of myoblast differentiation marker MyHC (Zhang et al., 2016). Therefore, it was suggested earlier miRNA preparations for higher target genes expression at 28 and 35 dpc contributed to earlier progresses of myogenesis in WZS. Therefore, these results further prove that WZS and LR show distinct myogenic regulatory processes and WZS shows earlier progresses of myogenesis than that of LR.
Figure 4.
Identification of miRNA-gene interaction network during embryonic myogenesis. (A) Target genes of myogenic miRNAs marked in Figure 3. (B) Interaction network of target genes predicted by STRING and illustrated by Cytoscape. (C) Model of miRNA-gene regulatory network during early embryonic myogenesis proofed by previous studies or predicted in our study. Red fonts represent up-regulation and blue fonts represent down-regulation, in comparison between WZS and LR during stages we studied.
Interaction network was predicted by STRING website and visualized by Cytoscape software (Figure 4B and Supplementary Table S9). All the myogenic target genes showed regulatory interaction (Figure 4B). We then integrated the miRNA-gene regulatory prediction or previously reported interaction and illustrated the miRNA-gene regulatory network according to gene location, function, and their relative expression trends during developmental stages (WZS/LR) (Figure 4C). Three functional gene clusters could be found in Figure 4C, which are chromatin modification-related (Chd2, H3f3a, Chd6, and Mll1), myogenesis-related (Pax3, Pbx1, Mef2a, and Znf423), and myosin component-related (Mylk, Myo5a, Mylk4, Myh9, and Mylk2) genes. Of note, most of the genes involved in three functional clusters showed up-regulated expression trend in WZS during 18–35 dpc, which again proved that WZS had more intense myogenic processes than that of LR during early embryonic stages, and similar trend could also be found in the miRNAs regulating 3 gene clusters. These DE genes may interact with DE miRNAs to be involved in regulating distinct myogenic progressed between LR and WZS during early developmental stages.
Validation of the Sequencing Data
Real-time quantitative PCR was applied to validate the sequencing data. Although U6 snRNA is one of the most widely used internal control, Gu et al. demonstrated that the U6 snRNA was the least stable one compared with other candidate internal control miRNAs in all tissues (including muscle tissues) comparing with miR–17, –103, –107, and –23a (Gu et al., 2011). Hence, in our previous miRNAome study, the optimal internal control miRNA from above candidates was studied by calculating the standard deviation (Stdev.) of 5 miRNAs in all samples (Qin et al., 2013). As a consequence, miR-17-5p was actually the most stable one (Stdev.= 0.1), whereas the U6 was the least stable one (Stdev.= 4.9), consistent with the previous study (Gu et al., 2011). MiR-17-5p was therefore utilized as internal control and RT-qPCR was then performed on 3 myogenic miRNAs with different expression levels (ssc-miR-133b, -206, -424-5p) to validate the sequencing data (Figure 5) of all the samples studied. Gapdh was used as internal control of mRNA expression and RT-qPCR was performed on 3 myogenic genes with different expression levels (Mef2a, Mll1, and Myo5A) of all the samples studied to validate RNA-seq data (Figure 5). Primers were presented in Supplementary Table S2. The Pearson correlation coefficient of the real-time PCR and the Solexa sequencing was calculated and the r values ranged from 0.73 to 0.95 (Supplementary Table S10), indicating that there was a high consistency between the 2 methods.
Figure 5.
Validation of sequencing data. (A) Expression profiles of 3 genes and miRNAs’ sequencing results were validated by RT-qPCR. Pearson correlation coefficient was utilized to measure the correlation of 2 methods. R represents for Pearson correlation coefficient, P represents for P value, S represents for sequencing results, and Q represents for RT-qPCR results. Protein expression profiles were analyzed by (B) our previous iTRAQ proteomic data and (C) Western blot experiment. GAPDH and H3.3 were used as internal control.
As protein is the final executant of life functions, thus, in order to verify the relation between mRNA and protein level of 2 pig breeds, firstly, we utilized proteomic data from our previous study using same tissues of WZS and LR in this study during 21–35 dpc (Zhang et al., 2016). Only 3 target genes’ protein expression level could be found in the proteomic data (Supplementary Table S11) and the relative protein expression level for 3 target genes (Myo5a, H3f3a, and Pbx1) were shown in Figure 5B. H3F3A protein showed higher expression level in LR during 21–35 dpc, especially at 21 dpc, whereas H3f3a mRNA level also peaked in LR between 2 breeds at 21 dpc (Figure 4A). MYO5A protein changed slightly between 2 breeds during 21–35 dpc, whereas Myo5a mRNA level also changed slightly between 2 breeds during 18–28 dpc. However, at 35 dpc, WZS showed significantly up-regulated Myo5a mRNA level (Figure 5A). PBX1 protein changed slightly between 2 breeds during 21–35 dpc, whereas its mRNA was higher in LR at 18 dpc and higher in WZS at 28 dpc (Figure 4A). Secondly, we conducted Western Blot of Pax3, MEF2A, and H3F3A using same tissues of WZS and LR in this study (Figure 5C). Three proteins all showed similar expression trend with their mRNA level: expression level of H3F3A was all higher in LR, but not to the extent as iTRAQ data; expression level of MEF2A was higher in WZS since 21 dpc and expression level of PAX3 were high in both breeds at 18 and 21 dpc, and was higher in LR at 28 and 35 dpc. In brief, most protein level of target genes shows similar expression pattern with their mRNAs; the reason for some inconformity may be due to asynchronism of transcription and translation, posttranscriptional modification or other uncovered mechanisms.
DISCUSSION
In this study, an integrated analysis of transcriptome and miRNAome was conducted using LDM of 4 early embryonic stages (18, 21, 28, and 35 dpc) from 2 pig breeds (LR and WZS) differing in meat mass. The global miRNA/mRNA expression profile showed that WZS prepared for subsequent developmental processes earlier than LR. After identifying and analyzing the interaction network of top 100 up-/down-regulated miRNA and their target genes, we were able to find an epigenetic-regulated myogenesis regulatory axial that may play a vital role in regulating distinct early porcine embryonic myogenesis processes between LR and WZS.
Pigs With Less Meat Mass Showed More Intense Primary Myofiber Development at Early Embryonic Stages
Pig myogenesis is a biphasic phenomenon with primary myofibers forming from 35 to 55 dpc, followed by secondary myofibers forming around each primary myofiber between 50 and 90 dpc (Picard et al., 2010). The muscle growth is predominantly determined during prenatal skeletal muscle development (Ashmore et al., 1973; Picard et al., 2002). Zhao et al. conducted histomorphology and transcriptomic study of 11 developmental stages for both Tongcheng (Chinese indigenous pig, fat) and Yorkshire pigs (lean), including 30, 40, 55, 63, 70, 90, and 105 d of gestation. They found that the number and density of myoblasts in Tongcheng were more than that of Yorkshire pigs (Zhao et al., 2015). Recent study provided that myogenesis processes are more intense in Meishan (Chinese indigenous pig, fat) than that in Largewhite (LW, lean) pigs at 35 dpc (He et al., 2017), and they uncovered that the main functional miRNAs during muscle development were different between lean and fat pig breeds. These porcine muscle developmental studies all revealed that pigs with less meat mass showed more intense primary myofiber development at early embryonic stages.
The cluster analysis utilizing all the miRNA/mRNA expression profiles of LR and WZS during 18–35 dpc showed that miRNA/mRNA expression profiles of WZS showed similarity with early stages, and miRNA/mRNA expression profiles of LR tended to cluster with the late miRNA expression profiles of WZS. These findings suggested that WZS prepared for later developmental processes earlier than LR, which is consistent with our previous proteome study using same samples that WZS showed earlier embryonic myogenic development (Zhang et al., 2016).
Top Up-/Down-Regulated DE miRNAs Between LR and WZS Showed Myogenic-Related Fuctional Enrichment
After identification of DE miRNAs, we found that WZS showed more up-regulated DE miRNAs during 18–21 dpc, whereas LR showed more up-regulated DE miRNAs during 21–35 dpc in comparison between adjacent stages of each breed. We also found that at 28 dpc, more DE miRNAs that showed higher expression in WZS in comparison between 2 breeds at the same stage. Moreover, as the DE miRNAs that up-/down-regulated during these stages may play a vital role in regulating myogenesis, we identified 100 top up-/down-regulated DE miRNAs in comparison between LR and WZS during all stages. It was found that most top down-regulated miRNAs in comparison between LR and WZS were found during 18–28 dpc, whereas most top up-regulated miRNAs were found at 35 dpc. Taken together, these findings are consistent with the cluster analysis that WZS prepared for later developmental processes earlier than LR.
MicroRNAs (miRNAs) as regulators controlling gene expression at the post-transcriptional level (Bartel, 2004; Williams et al., 2009) are about 22 nt single-stranded RNAs, which promote mRNA degradation or inhibit translation by complementarily binding to the 3′-untranslated regions (UTRs) of specific target mRNAs. Among the top 100 up/down DE miRNAs, we identified a number of miRNAs that play vital roles during myogenesis. Most of the myogenic-related miRNAs with up-regulated trend during myogenesis processes as previous reports described also showed up-regulated trend in WZS during early embryonic stages than that of LR. Among which, miR-206 and miR-486 would cause myoblasts to exit from cell cycle, and subsequently to enter the differentiation phase by directly suppressing Pax7 expression (Dey et al., 2011). Muscle-specific miRNAs (MyomiRs), miR-206, miR-1, and miR-133 have many similar features in function and architecture (Naya and Olson, 1999). Moreover, these myomiRs were also found highly expressed in our previous LDM analysis of LR during 35–91 dpc and 2–180 d postnatal (dpn) (Qin et al., 2013), which further suggested that myomiRs also play an important role in regulating porcine myogenesis not only in both pig breeds, but also during early embryonic stages and adult stages.
Apart from these well-known myogenic miRNAs, several identified miRNAs also play a vital role in muscle development. miR-29c, participates in muscle development through targeting the YY1 gene and is associated with postmortem muscle pH in pigs (Zhang et al., 2015). miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting (Connolly et al., 2018). Dysregulation of a novel miR-23b/27b-p53 axis impairs muscle stem cell differentiation of humans with type 2 diabetes (Henriksen et al., 2017). The miR-23a-miR-27a-miR-24-2 cluster play roles in muscle hypertrophy (Hernandez-Torres et al., 2014). MiR-208b regulates cell cycle and promotes skeletal muscle cell proliferation by targeting CDKN1A (Wang et al., 2018). Porcine miR-208b is associated with microRNA biogenesis and expressions of SOX-6 and MYH7 with effects on muscle fiber characteristics and meat quality (Kim et al., 2015). Thus, these identified DE miRNAs may play a crucial role in regulating distinct muscle development progresses between LR and WZS during early embryonic stages.
Epigenetic-Mediated Myogenic Regulatory miRNA-gene Axial Was Identified in Regulating Porcine Embryonic Myogenesis
Further, we predicted target genes of these identified myogenic miRNAs and made an integrated analysis with miRNAome and RNA-seq data, as these genes may also play an important role in regulating muscle development in 2 pig breeds. We are glad to find that most myogenic target genes mRNA/protein showed higher expression level in WZS during 28–35 dpc. Although some target genes showed higher expression level in LR, most of them showed lower expression level at 28–35 dpc. Although the target genes showed higher expression level at 28 and 35 dpc in WZS, and just around this period, the primary myofibers form in pig. In addition, miRNA expression profiles of WZS 28 dpc clustered with its earlier stages 21 dpc, together with the findings in our previous study that WZS showed earlier and higher expression of myoblast differentiation marker MyHC (Zhang et al., 2016). Therefore, that the reason why WZS showed earlier progresses of myogenesis than that of LR is probably due to earlier miRNA preparations for higher target genes expression at 28 and 35 dpc. Thus, these results further proved that WZS showed earlier and more intense progresses of myogenesis than that of LR. After interaction network analysis, a gene-miRNA regulatory axial of muscle development was found, which consists of 3 gene clusters (chromatin modification-, myogenesis-, and myosin component-related clusters), and most of the genes involved in 3 clusters showed up-regulated expression trend in WZS during 18–35 dpc.
Of chromatin modification–related gene cluster, it is reported that myoblast determination protein (MYOD) determines cell fate and facilitates differentiation-dependent gene expression through chromodomain helicase DNA binding protein 2 (CHD2)-dependent deposition of H3f3a (H3.3) at myogenic loci prior to differentiation (Harada et al., 2012). Recent study found that selective H3.3 incorporation is essential for establishing specific modifications in myogenic genes, suggesting that the incorporation of specific histone H3 variants determines the lineage potential of myogenic potential (Harada et al., 2015). Of myogenesis-related gene cluster, Pax3 functions in muscle progenitor cells, which is an upstream factor of MRFs (Relaix et al., 2006). Pbx1 is a pioneer factor, which starts to make a suitable chromatin structure of myogenic genes for myogenic factors, such as MyoD to bind, in order to activate downstream myogenic progresses (Grebbin and Schulte, 2017). Of myosin component-related gene cluster, myosin light-chain kinase family members (Mylk, Mylk2, and Mylk4), myosin heavy-chain 9 (Myh9), and myosin VA (Myo5a) are all vital components of mature muscle structure (Giorgi et al., 2001; Velvarska and Niessing, 2013). Thus, our study revealed an epigenetic-mediated myogenic regulatory miRNA-gene axial that may play vital roles in regulating distinct muscle development progresses between LR and WZS during early embryonic stages, and the predicted miRNA genes related to myogenesis will benefit future studies.
In summary, our integrated miRNAome-transcriptome study proves that WZS shows earlier embryonic muscle development progress than that of LR and most importantly reveals an epigenetic-mediated myogenic regulatory miRNA-gene axial that may play vital roles in regulating distinct muscle development progresses between LR and WZS during early embryonic stages.
Conflict of interest statement. None declared.
Supplementary Material
Footnotes
This research was supported by the National Key Research and Development Program of China (2018YFD0501200, 2017YFD0501000, 2018YFD0500600), the National Natural Science Foundation of China (31772565), and Science and Technology Plan Project of Guangdong Province of China (2014B020202001). We thank the Beijing Genomics Institute (BGI) Shenzhen and gene denovo for providing us with technical assistance and bioinformatics analysis.
LITERATURE CITED
- Ashmore C. R., Addis P. B., and Doerr L.. 1973. Development of muscle fibers in the fetal pig. J. Anim. Sci. 36:1088–1093. doi:10.2527/jas1973.3661088x [DOI] [PubMed] [Google Scholar]
- Bartel D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi:10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Cao J., Huang T., Li X., and Zhao S.. 2014. Interactome mapping reveals important pathways in skeletal muscle development of pigs. Int. J. Mol. Sci. 15:21788–21802. doi:10.3390/ijms151221788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M., Paul R., Farre-Garros R., Natanek S. A., Bloch S., Lee J., Lorenzo J. P., Patel H., Cooper C., Sayer A. A.,. et al. 2018. Mir-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J. Cachexia. Sarcopenia Muscle 9:400–416. doi:10.1002/jcsm.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B. K., Gagan J., and Dutta A.. 2011. Mir-206 and -486 induce myoblast differentiation by downregulating pax7. Mol. Cell. Biol. 31:203–214. doi:10.1128/MCB.01009-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlgren N., Howell M. D., Kasschau K. D., Chapman E. J., Sullivan C. M., Cumbie J. S., Givan S. A., Law T. F., Grant S. R., Dangl J. L.,. et al. 2007. High-throughput sequencing of arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One 2:e219. doi:10.1371/journal.pone.0000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi D., Ferraz C., Mattéi M. G., Demaille J., and Rouquier S.. 2001. The myosin light chain kinase gene is not duplicated in mouse: partial structure and chromosomal localization of Mylk. Genomics 75:49–56. doi:10.1006/geno.2001.6571 [DOI] [PubMed] [Google Scholar]
- Guller I., and Russell A. P.. 2010. MicroRNAs in skeletal muscle: their role and regulation in development, disease and function. J. Physiol. 588(Pt 21):4075–4087. doi:10.1113/jphysiol.2010.194175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Li M., Zhang K., Chen L., Jiang A. A., Wang J., Lv X., and Li X.. 2011. Identification of suitable endogenous control microRNA genes in normal pig tissues. Anim. Sci. J. 82:722–728. doi:10.1111/j.1740-0929.2011.00908.x [DOI] [PubMed] [Google Scholar]
- Grebbin B. M., and Schulte D.. 2017. PBX1 as pioneer factor: a case still open. Front. Cell Dev. Biol. 5:9. doi:10.3389/fcell.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A., Maehara K., Sato Y., Konno D., Tachibana T., Kimura H., and Ohkawa Y.. 2015. Incorporation of histone H3.1 suppresses the lineage potential of skeletal muscle. Nucleic Acids Res. 43:775–786. doi:10.1093/nar/gku1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A., Okada S., Konno D., Odawara J., Yoshimi T., Yoshimura S., Kumamaru H., Saiwai H., Tsubota T., Kurumizaka H.,. et al. 2012. Chd2 interacts with H3.3 to determine myogenic cell fate. Embo J. 31:2994–3007. doi:10.1038/emboj.2012.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Zou T., Gai X., Ma J., Li M., Huang Z., and Chen D.. 2017. MicroRNA expression profiles differ between primary myofiber of lean and obese pig breeds. PLoS One 12:e0181897. doi:10.1371/journal.pone.0181897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T. I., Davidsen P. K., Pedersen M., Schultz H. S., Hansen N. S., Larsen T. J., Vaag A., Pedersen B. K., Nielsen S., and Scheele C.. 2017. Dysregulation of a novel mir-23b/27b-p53 axis impairs muscle stem cell differentiation of humans with type 2 diabetes. Mol. Metab. 6:770–779. doi:10.1016/j.molmet.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Torres F., Aranega A. E., and Franco D.. 2014. Identification of regulatory elements directing mir-23a-mir-27a-mir-24-2 transcriptional regulation in response to muscle hypertrophic stimuli. Biochim. Biophys. Acta 1839:885–897. doi:10.1016/j.bbagrm.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Kim J. M., Lim K. S., Hong J. S., Kang J. H., Lee Y. S., and Hong K. C.. 2015. A polymorphism in the porcine mir-208b is associated with microRNA biogenesis and expressions of SOX-6 and MYH7 with effects on muscle fibre characteristics and meat quality. Anim. Genet. 46:73–77. doi:10.1111/age.12255. [DOI] [PubMed] [Google Scholar]
- Kovanda A., Režen T., and Rogelj B.. 2014. MicroRNA in skeletal muscle development, growth, atrophy, and disease. Wiley Interdiscip. Rev. RNA 5:509–525. doi:10.1002/wrna.1227 [DOI] [PubMed] [Google Scholar]
- Li J.-Q., et al. 2003. Genetic effects of IGF-1 gene on the performance in LandraceXLantang pig resource population. Acta Genetica Sinica. 30:835–839. [PubMed] [Google Scholar]
- Lunney J. K. 2007. Advances in swine biomedical model genomics. Int. J. Biol. Sci. 3:179–184. doi:10.7150/ijbs.3.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A., Williams B. A., McCue K., Schaeffer L., and Wold B.. 2008. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods 5:621–628. doi:10.1038/nmeth.1226 [DOI] [PubMed] [Google Scholar]
- Naya F. J., and Olson E.. 1999. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol. 11:683–688. doi:10.1016/S0955-0674(99)00036-8 [DOI] [PubMed] [Google Scholar]
- Patterson J. K., Lei X. G., and Miller D. D.. 2008. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp. Biol. Med. (Maywood). 233:651–664. doi:10.3181/0709-MR-262 [DOI] [PubMed] [Google Scholar]
- Picard B., Berri C., Lefaucheur L., Molette C., Sayd T., and Terlouw C.. 2010. Skeletal muscle proteomics in livestock production. Brief. Funct. Genomics 9:259–278. doi:10.1093/bfgp/elq005 [DOI] [PubMed] [Google Scholar]
- Picard B., Lefaucheur L., Berri C., and Duclos M. J.. 2002. Muscle fibre ontogenesis in farm animal species. Reprod. Nutr. Dev. 42:415–431. doi:10.1051/rnd:2002035 [DOI] [PubMed] [Google Scholar]
- Qin L. J., et al. 2013. Integrative analysis of porcine microRNAome during skeletal muscle development. PLoS One. 8:e72418. doi:10.1371/journal.pone.0072418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F., Montarras D., Zaffran S., Gayraud-Morel B., Rocancourt D., Tajbakhsh S., Mansouri A., Cumano A., and Buckingham M.. 2006. Pax3 and pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 172:91–102. doi:10.1083/jcb.200508044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Li Y., Wan P., Li X., Zhao S., Liu B., Fan B., Zhu M., Yu M., and Li K.. 2007. Longsage analysis of skeletal muscle at three prenatal stages in tongcheng and landrace pigs. Genome Biol. 8:R115. doi:10.1186/gb-2007-8-6-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velvarska H., and Niessing D.. 2013. Structural insights into the globular tails of the human type v myosins myo5a, myo5b, and myo5c. PLoS One 8:e82065. doi:10.1371/journal.pone.0082065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., et al. 2018. MiR-208b regulates cell cycle and promotes skeletal muscle cell proliferation by targeting CDKN1A. J. Cell. Physiol. 234:3720–29. doi:10.1002/jcp.27146 [DOI] [PubMed] [Google Scholar]
- Williams A. H., Liu N., van Rooij E., and Olson E. N.. 2009. MicroRNA control of muscle development and disease. Curr. Opin. Cell Biol. 21:461–469. doi:10.1016/j.ceb.2009.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., et al. 2004. The study of new SLA classical molecules in inbreeding Chinese Wuzhishan pig. Transplant. Proc. 36:2483–2484. [DOI] [PubMed] [Google Scholar]
- Zhang W. Y., Wei W., Zhao Y. Y., Zhao S. H., and Li X. Y.. 2015. The microRNA, miR-29c, participates in muscle development through targeting the YY1 gene and is associated with postmortem muscle pH in pigs. Front. Agric. Sci. Eng. 2:311–317. doi:10.15302/J-FASE-2015075 [Google Scholar]
- Zhang X. M., et al. 2016. iTRAQ-based quantitative proteomic analysis reveals the distinct early embryo myofiber type characteristics involved in landrace and miniature pig. BMC Genomics 17:137. doi:10.1186/s12864-016-2464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li J., Liu H., Xi Y., Xue M., Liu W., Zhuang Z., and Lei M.. 2015. Dynamic transcriptome profiles of skeletal muscle tissue across 11 developmental stages for both Tongcheng and Yorkshire pigs. BMC Genomics 16:377. doi:10.1186/s12864-015-1580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Mo D., Li A., Gong W., Xiao S., Zhang Y., Qin L., Niu Y., Guo Y., Liu X.,. et al. 2011. Comparative analyses by sequencing of transcriptomes during skeletal muscle development between pig breeds differing in muscle growth rate and fatness. PLoS One 6:e19774. doi:10.1371/journal.pone.0019774 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq raw data from this study have been deposited in NCBI Sequence Read Archive with accession number SRR7369735-7369740, SRR7779832, and SRR7779834. The miRNA sequencing raw data from this study have been deposited in NCBI Sequence Read Archive with accession number SRR8168016-23. (http://www.ncbi.nlm.nih.gov/Traces/sra/).