Abstract
Despite the importance of hedonic reactions in pig’s intake, feed palatability has been typically inferred from preference or acceptance measures. However, these measures are influenced by factors beyond palatability, such as energy density and hunger. The aim of this study was to evaluate palatability responses in pigs to sweet and umami taste at different inclusions levels. Pigs (24 per experiment) were video recorded while exposed in pairs to different sucrose (Exp. 1) or monosodium glutamate (MSG, Exp. 2) solutions over seven consecutive 10 min tests (one concentration per day). In both experiments, palatability was estimated through consumption patterns (consumption time per approaches, CT/A), facial expressions (snout openings and tongue protrusions), and consumption during a brief 2 min period. Data were analyzed by sucrose or MSG concentration. Sucrose concentration affected total intake, producing an inverted-U function and a quadratic relationship with sucrose concentration (P = 0.012). In contrast, CT/A and snout openings showed a dose effect (P < 0.005) with a direct correlation between sucrose concentration and CT/A (R = 0.23, P = 0.033) but not for openings (R = 0.18, P = 0.105) where a quadratic relationship appears (P < 0.001). Tongue protrusions and brief consumption time were not affected by sucrose concentration (P = 0.144 and 0.205, respectively). MSG concentration affected consumption, CT/A, snout openings, and brief consumption time (P < 0.001), with significant (P < 0.001) positive correlations (R = 0.59, 0.56, 0.56, and 0.68), respectively. As with rats, CT/A appears to provide a novel and interesting measure reflecting the palatability of preferred ingredients in pigs. However, brief consumption time and orofacial reactions show less similarity between pigs and rodents. Thus further studies are necessary both to better understand the measurement methods themselves and relationship between hedonic reactions and simple consumption in pigs.
Keywords: brief intake, consumption pattern, palatability, pigs, reactivity test
Introduction
Understanding hedonic reactions in pigs may improve nutrition and welfare through helping to maximize intake and minimize stress by avoiding conflict between unpalatable feeds and nutrient demands. In pigs, the palatability of different feeds or solutions has typically been inferred indirectly from measurements of preference or acceptance (Forbes, 2010). However, these measures are influenced by factors beyond palatability such as satiety or hunger, providing weak evidence of pigs’ hedonism. Studies in rats, humans, and primates have demonstrated that the analysis of licking microstructure (Davis and Smith, 1992; Dwyer, 2012), orofacial responses (Grill and Norgren, 1978; Kringelbach et al., 2012), or consumption in a brief period of time (Anderson and Woodend, 2003) is related to the perceived palatability of solutions. For example, consumption of sucrose is highest at moderate concentrations, while lick cluster size (mean number of licks per bout of drinking) increases with sucrose concentration (Davis and Smith, 1992; Dwyer, 2012). Moreover, neonate’s appetitive facial expressions (tongue protrusions, lips licking, etc.) increase with sugars concentration (Mennella et al., 2004). Despite recent interest in pigs’ feeding behavior (Oostindjer et al., 2011; Figueroa et al., 2012; Clouard et al., 2014), there is little information about methods for estimating pigs’ hedonic reactions (Clouard et al., 2012). One recent study (Frías et al., 2016) provided preliminary evidence that consumption patterns, analogous to lick cluster size in rats, are related to sucrose palatability in pigs. Here, we evaluate three potential techniques adapted from rodent literature: consumption patterns, orofacial reactions (potentially analogous to taste reactivity test), and brief consumption time. Exp. 1 re-examines the sucrose consumption data from Frías et al. (2016), whereas Exp. 2 extends the analysis to umami taste produced with monosodium glutamate (MSG).
MATERIALS AND METHODS
Experiments were conducted at the weanling unit of the Universitat Autònoma de Barcelona (UAB) pig facilities. Experimental procedures were approved by Ethical Committee on Animal Experimentation of the UAB (CEAAH 1406) and by the Bioethics Committee of the “Facultad de Ciencias Veterinarias y Pecuarias”, Universidad de Chile, certificate Nº 252014.
Animals and Housing
A total of 48 male and female 42-d-old pigs [(Large White × Landrace) × (Pietrain)] were used in two consecutive experiments. Animals were individually identified by using a plastic ear-tag at the moment of birth, and were offered an unflavored creep-feed diet during the suckling period from day 10 using a commercial pan feeder. In both experiments, pigs were weaned at 28 d of life (weighing 7.6 ± 1.1 and 7.5 ± 0.9 kg, respectively) in a weaning room equipped with automatic, forced ventilation, and slatted floor. Animals were housed in group pens (3.2 m2 in floor area) that were equipped with a hopper feeder with three feeding spaces and an independent water supply to ensure ad libitum feeding and freshwater access. Pigs were fed with a commercial powder feed except for 1 h before and after each test. During the second week after weaning (35 to 41 d old), pigs were acclimated to solution tests by offering a dish of water for 1 h each morning (09:00 to 10:00 h) at the front of each pen. At the beginning of the third postweaning week (42 d old), animals were weighed (9.2 ± 1.5 and 9.0 ± 1.7 kg) and placed during experimental procedures in testing pens (1.6 m2 in floor area). After experiments, pigs returned to commercial production within the UAB facilities after the end of experiments.
Procedures
Pigs were exposed in pairs (12 pairs per experiment) to different sucrose solutions (0.5%, 1%, 2%, 4%, 8%, 16%, and 32%—(Exp. 1) or to different MSG solutions (0.1, 1, 10, 60, 100, 150, and 300 mM), adding ribonucleotides inosinate 5′-monophosphate and guanylate 5′-monophosphate at 2% of the MSG added to potentiate the effect of MSG (Exp. 2) over seven consecutive 10 min tests (one concentration per day). Pigs’ pairs were used as the experimental unit as these gregarious animals react aversively to isolation (e.g., attempting to escape or becoming lethargic). Half of the pig pairs in each experiments were tested with increasing concentrations and the rest with decreasing concentrations to counterbalance the design. Pigs were video recorded (four video cameras, IR Outdoor Cameras 700tvl 1/3 cmos Sony, SENKO SA, Santiago, Chile), video cameras were placed from the corridor towards the pens, forming an angle of view during testing to allow behavioral sampling over the experimental sessions.
Consumption time (total time drinking at the pan; CT) and approaches (number of times the pan was approached with a consumption result, A, were assessed from the video recordings by focal continuous sampling over the 10-min test period. Palatability was estimated through consumption patterns (CT/A) analogous to the licks/bout measure used in rats in lick cluster size analysis (Davis and Smith, 1992; Dwyer, 2012). Orofacial expressions were recorded as the number of snout openings and tongue protrusions [potentially analogous gaping and tongue protrusions exemplifying aversive and appetitive responses in the taste reactivity test (Grill and Norgren, 1978)]. Finally, to minimize satiation effects, consumption time during the first 2 min of solution exposure was also assessed as a palatability estimation (Baird et al., 2006). Consumption was measured by weighing pans at the beginning and end of each test. Because the assessment of consumption pattern measures of palatability in pigs is exploratory, it is not yet clear what time period is most informative. Thus our primary analysis focuses on the whole 10 min test period, and additional analyses of 0 to 5, and 6 to 10 min were performed to examine whether subperiods of the session would be more sensitive than the session as a whole.
Statistical Analysis
Variables (consumption, A, CT, CT/A, snout openings, tongue protrusions, and brief consumption time) were analyzed with ANOVA using statistical package SAS (SAS Inst. Inc., Cary, NC) with sucrose or MSG concentrations as the main factors. The experimental unit was the pig pair with results expressed as the average of both pigs’ data. Spearman correlation coefficients were used to evaluate relationships between sucrose/MSG concentration and all dependent variables by using the CORR procedure of SAS. Quadratic regressions were performed between sucrose/MSG concentration and dependent variables to explore the shape of their relationship in cases where spearman correlations were not present but a clear sucrose or MSG concentration effect existed. Although the primary analysis was over the whole testing period, we also performed indicative analyses at 0 to 5 and 6 to 10 min to explore whether the distribution of possible different effects depending on the testing period. Mean values are presented as least square means, and significance assessed at a criterion of 5%.
RESULTS
Exp. 1: Pig’s Palatability for Sucrose Solutions
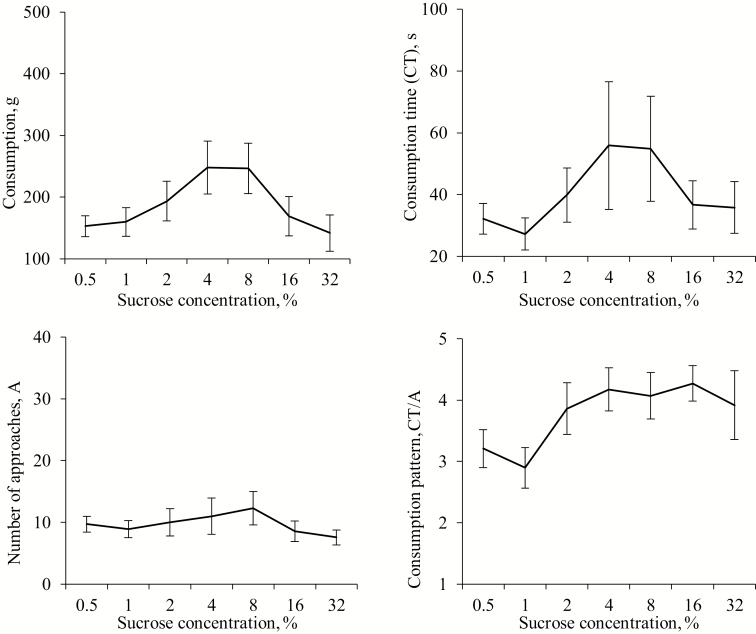
Consumption and number of approaches (A) were affected by sucrose concentrations during the total experimental time [F (6, 10) = 5.14, P = 0.012 and F (6, 10) = 3.91, P = 0.028, respectively]; meanwhile CT did not [F (6, 10) = 2.42, P = 0.104]. Numerically, these parameters showed inverted-U functions relative to sucrose concentration with the highest responses at intermediate levels of 4% and 8% of sucrose (Figure 1), although there was only a significant quadratic regression with concentration for consumption (P = 0.012) but not for A (P = 0.182) or CT (P = 0.141). The ANOVA revealed that CT/A was also influenced by sucrose concentration [F (6, 10) = 6.59, P = 0.005] which was positively related (R = 0.23, P = 0.033), indicating that unlike the other measures, palatability (CT/A) generally increased with sucrose concentration (Figure 1). Brief consumption time was not related with sucrose concentration [F (6, 10) = 1.76, P = 0.205]. The analysis for CT, A, and CT/A by periods is shown in Table 1. In relation to CT sucrose concentration did not had an effect in either the first or second period. The number of approaches only presented a positive correlation with sucrose concentration during the first period of the test. Nevertheless, it was observed that CT/A presented a sucrose concentration effect during both the 0 to 5 and 6 to 10 min periods with a positive correlations with sucrose concentration at 0 to 5 min period (P = 0.038) but a quadratic regression at the 6 to 10 min period (P = 0.005).
Figure 1.
Means (±SEM) of consumption, consumption time (CT), number of approaches (A), and consumption pattern (CT/A) over 10 min from nursery pig pairs (n = 12) exposed to different sucrose concentrations.
Table 1.
Consumption time (CT), number of approaches (A), and consumption pattern (CT/A) over 10 min from nursery pigs pairs (n = 12) exposed to different sucrose concentrations expressed by the first (0 to 5 min), last (6 to 10 min), and total (0 to 10 min) periods of the test
| Sucrose concentration (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT, s | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | P-value1 | 2Spearman’s r (P-value) |
| 0 to 2 min period | 17.46 | 17.5 | 19.83 | 25.13 | 23.67 | 23.5 | 23.5 | 0.205 | 0.105 (0.338) |
| 0 to 5 min period | 27 | 22.5 | 31.12 | 39.54 | 39.58 | 31.66 | 33.79 | 0.287 | 0.077 (0.483) |
| 6 to 10 min period | 5.12 | 4.75 | 7.7 | 16.37 | 15.25 | 5.04 | 2 | 0.056 | −0.005 (0.961) |
| 0 to 10 min period | 32.13 | 27.25 | 39.88 | 55.92 | 54.83 | 36.71 | 35.79 | 0.104 | 0.071 (0.515) |
| Number of approaches (A) | |||||||||
| 0 to 5 min period | 8.25 | 6.83 | 7.87 | 7.5 | 8.08 | 7.08 | 6.62 | 0.031 | −0.074 (0.500) |
| 6 to 10 min period | 1.45 | 2.04 | 2.12 | 3.5 | 4.2 | 1.5 | 0.95 | 0.104 | −0.018 (0.865) |
| 0 to 10 min period | 9.7 | 8.87 | 10 | 11 | 12.29 | 8.58 | 7.58 | 0.028 | −0.071 (0.517) |
| Consumption pattern (CT/A) | |||||||||
| 0 to 5 min period | 3.22 | 3.18 | 3.70 | 4.58 | 4.27 | 4.29 | 4.22 | <0.001 | 0.227 (0.038) |
| 6 to 10 min period | 1.72ab | 1.67ab | 2.59ab | 2.93ab | 3.5a | 2.35ab | 1.47b | 0.032 | 0.056 (0.610) |
| 0 to 10 min period | 3.21ab | 2.9a | 3.86ab | 4.17b | 4.07b | 4.27ab | 3.92ab | 0.005 | 0.234 (0.033) |
Values of CT for 0 to 2 min are also shown as an indication of consumption in a brief time. Different letters represent statistical differences between means of the same row after pairwise comparisons (P < 0.05).
1 P-value for the main effect of concentration.
2The r column represents a Spearman rank-order correlation between the mean value for each parameter concentration and the concentration with their respective significance.
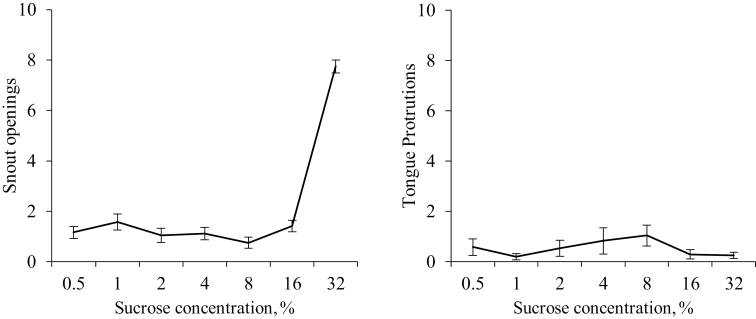
The orofacial data are shown in Figure 2. Snout openings were affected by concentration [F (6, 10) = 20.85, P < 0.001], with a non-uniform rate of increase suggested by a clear significant quadratic regression (P < 0.001), where the inclusions of sucrose at 32% caused more snouts openings than the other six sucrose concentrations (Ps < 0.01). However, tongue protrusions were not affected by sucrose concentration [F (6, 10) = 2.09, P = 0.144] considering the whole period. The analysis for periods is expressed in Table 2. It was observed that snout openings showed a dose effect during 0 to 5 and 6 to 10 min periods with a positive correlation with sucrose inclusion at the 6 to 10 min period but a quadratic regression on the 0 to 5 min period (P < 0.001). Tongue protrusions was affected by sucrose concentration during the first period [F (6, 10) = 3.54, P = 0.038].
Figure 2.
Means (±SEM) of snouts openings and tongue protrusions over 10 min from nursery pig pairs (n = 12) exposed to different sucrose concentrations.
Table 2.
Snout openings and tongue protrusions over 10 min
| Sucrose concentration (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Snouts openings | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | P-value1 |
2Spearman´s r (P-value) |
| 0 to 5 min period | 1.08a | 1.29ab | 0.95a | 1.00ab | 0.66a | 1.25a | 6.58b | <0.001 | 0.170 (0.122) |
| 6 to 10 min period | 0.08a | 0.29a | 0.08a | 0.12a | 0.08a | 0.16a | 1.16b | 0.033 | 0.221 (0.042) |
| 0 to 10 min period | 1.16a | 1.58a | 1.04a | 1.12a | 0.75a | 1.41a | 7.75b | <0.001 | 0.178 (0.105) |
| Tongue protrusions | |||||||||
| 0 to 5 min period | 0.12 | 0.20 | 0.29 | 0.70 | 0.79 | 0.2 | 0.25 | 0.038 | 0.042 (0.701) |
| 6 to 10 min period | 0.50 | 0.00 | 0.25 | 0.13 | 0.25 | 0.08 | 0.00 | — | −0.092 (0.404) |
| 0 to 10 min period | 0.58 | 0.20 | 0.54 | 0.83 | 1.04 | 0.29 | 0.25 | 0.144 | −0.016 (0.879) |
From nursery pigs pairs (n = 12) exposed to different sucrose concentrations expressed by the first (0 to 5 min), last (6 to 10 min), and total (0 to 10 min) periods of the test. Different letters represent statistical differences between means of the same row after pairwise comparisons (P < 0.05).
1 P-value for the main effect of concentration.
2The r column represents a Spearman rank-order correlation between the mean value for each parameter concentration and the concentration with their respective significance.
Exp. 2: Pig’s Palatability for MSG Solutions
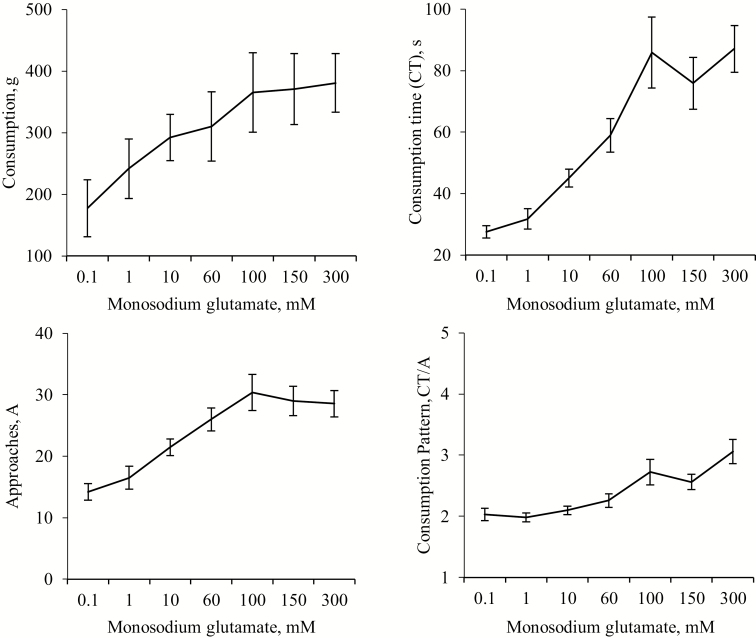
MSG concentration affected consumption [F (6, 10) = 12.84, P < 0.001], approaches [F (6, 10) = 22.42, P < 0.001], CT [F (6, 10) = 18.89, P < 0.001], and CT/A [F (6, 10) = 12.28, P < 0.001] (Figure 3), with positive correlations (P < 0.001) observed with all variables (R = 0.59, 0.59, 0.72, and 0.56, respectively). Brief consumption time was influenced by MSG concentration (F (6, 10) = 309, P < 0.001) also producing a positive correlation (R = 0.68; P < 0.001). The analysis for CT, A and CT/A by time periods is expressed in Table 3. All variables presented a dose effect on the first and second period of the test, with positive correlations observed in all cases.
Figure 3.
Means (±SEM) of consumption, consumption time (CT), number of approaches (A), and consumption pattern (CT/A) over 10 min from nursery pig pairs (n = 12) exposed to different monosodium glutamate concentrations.
Table 3.
Consumption time (CT), number of approaches (A), and consumption pattern (CT/A) over 10 min from nursery pigs pairs (n = 12) exposed to different monosodium glutamate concentrations expressed by the first (0 to 5 min), last (6 to 10 min), and total (0 to 10 min) periods of the test
| Monosodium glutamate concentration (mM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT, s | 0.1 | 1 | 10 | 60 | 100 | 150 | 300 | P-value1 | Spearman’s2 r (P-value) |
| 0 to 2 min period | 13.0a | 16.5ab | 21.5bd | 25.1cd | 30.0c | 26.9cd | 35.8e | <0.001 | 0.676 (<0.001) |
| 0 to 5 min period | 21.9a | 24.8a | 34.8b | 39.1b | 56.7bc | 46.8bc | 63.3c | <0.001 | 0.709 (<0.001) |
| 6 to 10 min period | 5.7a | 6.9a | 10.3a | 19.9ab | 29.3b | 29.1b | 23.8b | <0.001 | 0.559 (<0.001) |
| 0 to 10 min period | 27.6a | 31.8a | 45.1b | 59.0bd | 86.0cd | 75.9cd | 87.1c | <0.001 | 0.719 (<0.001) |
| Number of approaches (A) | |||||||||
| 0 to 5 min period | 10.7a | 12.5a | 16.5b | 17.5b | 20.6bc | 18.8bc | 20.8c | <0.001 | 0.572 (<0.001) |
| 6 to 10 min period | 3.5ac | 4.0ad | 5.0acd | 8.5c | 9.8b | 10.2b | 7.7d | <0.001 | 0.458 (<0.001) |
| 0 to 10 min period | 14.2a | 16.5a | 21.5c | 26.0d | 30.4d | 29.0d | 28.5d | <0.001 | 0.586 (<0.001) |
| Consumption pattern (CT/A) | |||||||||
| 0 to 5 min period | 2.1a | 2.1a | 2.1a | 2.3a | 2.7ab | 2.5ab | 3.0b | <0.001 | 0.506 (<0.001) |
| 6 to 10 min period | 1.5a | 1.8ab | 2.1ab | 2.2b | 2.7b | 2.7b | 2.8b | <0.001 | 0.440 (<0.001) |
| 0 to 10 min period | 2.0a | 2.0a | 2.1ab | 2.3ab | 2.7abc | 2.6bc | 3.1c | <0.001 | 0.561 (<0.001) |
Values of CT for 0 to 2 min are also shown as an indication of consumption in a brief time. Different letters represent statistical differences between means of the same row after pairwise comparisons (P < 0.05).
1 P-values for the main effect of concentration.
2The r column represents a Spearman rank-order correlation between the mean value for each parameter concentration and the concentration with their respective significance.
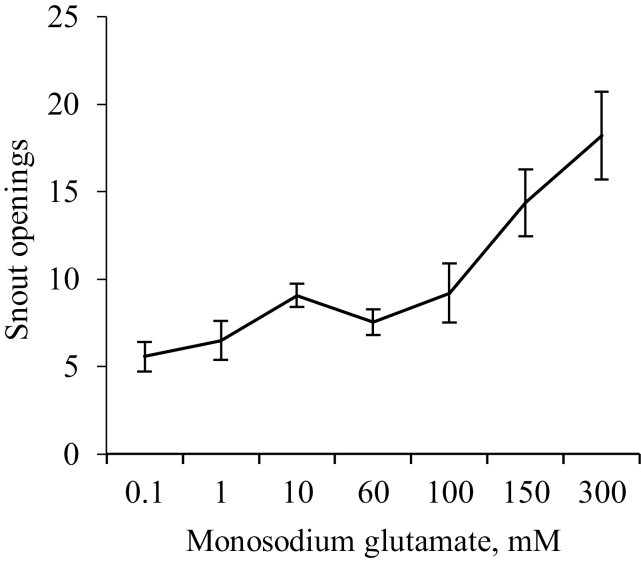
Taste reactivity test data in response to MSG solutions are shown in Figure 4. In terms of facial expressions, animals did not present tongue protrusions at any concentration or period. MSG concentration affected snout openings [F (6, 10) = 11.11, P < 0.001], with a positive correlation between them (R = 0.56, P < 0.001). By doing the analysis of the first (0 to 5 min) and the second (6 to 10 min) part of the experimental period (Table 4), it was observed that pigs presented positive correlations between snout openings at both periods.
Figure 4.
Means (±SEM) of snouts openings over 10 min from nursery pig pairs (n = 12) exposed to different monosodium glutamate concentrations.
Table 4.
Snout opening’s over 10 min from nursery pigs pairs (n = 12) exposed to different monosodium glutamate concentrations expressed by the first (0 to 5 min), last (6 to 10 min), and total (0 to 10 min) periods of the test
| Monosodium glutamate concentration, mM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Snouts openings | 0.1 | 1 | 10 | 60 | 100 | 150 | 300 | P-value1 | Spearman’s2 r (P-value) |
| 0 to 5 min period | 4.2a | 4.7ab | 7.1b | 5.8ab | 5.9ab | 9.2abc | 13.6c | <0.001 | 0.506 (<0.001) |
| 6 to 10 min period | 1.4ac | 1.8ac | 2.0abc | 1.7c | 3.3abc | 5.2bd | 4.6ad | <0.001 | 0.434 (<0.001) |
| 0 to 10 min period | 5.6a | 6.5ab | 9.1bd | 7.5ab | 9.2abd | 14.1cd | 18.2c | <0.001 | 0.561 (<0.001) |
Different letters represent statistical differences between means of the same row after pairwise comparisons (P < 0.05).
1ANOVA P-values for the main effect of concentration.
2The r column represents a Spearman rank-order correlation between the mean value for each parameter concentration and the concentration with their respective significance.
Discussion
The fact that consumption is attenuated at high concentrations of nutrient solutions due to satiety, while measures of hedonic reactions such as lick cluster size or taste reactivity are maintained at high concentrations in laboratory animals, strongly suggests that consumption alone is not a direct measure of food palatability. Nevertheless, within animal production systems, total intake is the main tool to estimate animal’s reaction in front of feed. The present experiments were performed to examine potential behavioral measures of hedonic reactions beyond simple consumption and preference measures in pigs.
The current results suggest that consumption pattern could represent an interesting and novel measure in feeding behavior reflecting palatability in pigs. In terms of overall intake measures such as consumption (initial – final pan weight) and CT (time spent drinking at the pan), pigs displayed inverted-U functions with sucrose concentrations, with the higher consumption and CT observed at intermediate concentrations (4% and 8%). However, for CT/A, adapted from lick cluster size analysis used previously in rats (Dwyer, 2012) and similar to what Clouard et al. (2014) described as the “duration of the drinking episodes,” the highest values were found as sucrose concentrations increased (Frías et al., 2016). Here, we extend this result to MSG: consumption and CT increase with concentration with the suggestion of a plateau at the higher concentrations (100, 150, and 300 mM), while CT/A presented the largest value at the highest MSG concentration (300 mM). This remained clear as the session progressed, with the increase in CT/A with concentration maintained in the 6 to 10 min period even as CT decreased relative to the 0 to 5 min period. That is, pigs spent longer periods drinking solutions in each approach to sweet and umami solutions as sucrose or MSG concentrations increased, producing a dissociation between consumption measures and hedonic measures as instantiated in consumption patterns (albeit that the dissociation between consumption measures and CT/A was greater for sucrose than MSG). These results are consistent with prior rodent work, where mean lick cluster size increases with the hedonic value of a solution (e.g., the sweet taste of sucrose) (Davis and Smith, 1992; Dwyer, 2012). Although the analysis of time periods was only indicative, there was a suggestion that the different time-periods analyses diverged between sucrose and MSG. For sucrose, where consumption was strongly biased towards the first half of the session (especially for the highest and lowest concentrations), the positive relationship between CT/A and concentration appears primarily driven by behavior in this first half (0 to 5 min). In contrast, for MSG, where the reduction in consumption over the second half of the session was less marked, the positive relationship between CT/A and concentration was maintained across the whole session. The contrast between the responses to sucrose and MSG is consistent with observations from rodents that consumption-pattern analysis is most reliable when there is a reasonably large sample of behavior to observe (Dwyer, 2008, 2012). Moreover, the fact that the sensitivity of CT/A consumption patterns to the palatability of the solution across may depended on the nature of the solution is a reminder that there is no single optimal observation window: parameters for future analysis should be chosen in light of potential ceiling or floor effects on consumption levels due to influences such as satiation or hunger.
For taste reactivity in rodents, the most diagnostic aversive orofacial reaction is gaping, which appears at high levels when unpalatable solutions (such as quinine) are exposed and is essentially absent for palatable solutions such as sucrose. Tongue protrusions are one clear appetitive reaction, seen at increasing levels as sucrose concentration increases (Grill and Norgren, 1978). The current data from pigs do not match these patterns of results—tongue protrusions were at generally low levels and unrelated to sucrose concentration, while snout openings (which we had thought might be analogous to gaping) were positively related to sucrose and MSG concentration (suggesting that snout opening might actually be an appetitive reaction in pigs). Thus, there does not appear to be an obvious relationship between the orofacial responses produced by rodents (and primates) and those produced by pigs (Berridge, 2000; Steiner et al., 2001). Therefore, rather than looking to the content of the rodent taste reactivity work for guidance, it may be worth considering the experimental process led to the development of the taste reactivity test: the initial characterization of orofacial responses as appetitive and aversive was performed by presenting categorically different solutions (e.g., unpalatable bitter quinine vs. palatable sweet sucrose) and looking for reactions which discriminated between these classes of solution (Grill and Norgren, 1978). Although there are studies of detection and preferences, for palatable (Frías et al., 2016) and unpalatable (Nelson and Sanregret, 1997) flavors in pigs, and studies of genetic differences between individuals related to those perceptions (Da Silva et al., 2014), no data exist on how differences in their hedonic perception of flavors manifest in different facial expressions. Further work on taste reactivity in pigs might apply the strategies to discover if there are orofacial responses that distinguish palatable from unpalatable solutions.
Finally, the use of brief consumption time measure (2 min) was based on the fact that short consumption times minimize sensory or postingestive satiety and thus that palatability can have the dominant effect on consumption (Kotlus and Blizard, 1998; Anderson et al., 2002; Anderson and Woodend, 2003; Sclafani and Ackroff, 2003). The current results were only inconsistently in line with this expectation: in Exp. 1, no relationship was observed between short-term consumption time and concentration levels of sucrose. However, in Exp. 2, concentration of MSG was directly correlated with brief consumption time. A comparison of the two experiments suggests that sucrose consumption at high concentrations might be limited by satiety even over a period of as short as 2 min. Indeed, brief contact tests in the rodent laboratory are typically shorter than 2 min, sometimes as little as seconds, although this requires the use of specialized equipment (Boughter et al., 2002).
As Forbes (2010) notes, although possible to define simply as “the pleasure of consumption”, palatability is a complex concept, which depends on several variables—both internal and external to the animal. Critically, consumption is not the same as palatability—something reinforced by the current data, especially where high nutrient concentrations can promote satiety and a reduction in consumption while palatability measures continue to increase. In addition, while preference tests can provide some valuable information, they too are limited by the possibility of interactions between options (combinations of nutrients can be preferred to the individual components) as well as by effects of choice time on observed preferences (Roura et al., 2008; Solà-Oriol et al., 2009; Clouard et al., 2012; Figueroa et al., 2012).
Comparing the three potential measures of hedonic reactions inspired by the laboratory rodent literature: there was clear evidence of dissociation between total intake and CT/A measures in response to changes in sucrose or MSG concentration, some evidence of dissociations between facial responses and consumption, and little consistent evidence for brief consumption time. This evidence demonstrates the limitations of using simple consumption measures as an indication of hedonic responses or palatability and suggests that CT/A might represent a particularly valuable measure for hedonic assessment in pigs. While the results for the facial response analysis and brief consumption time were less clear, the rodent literature offers strategies for future work aimed at refining these as measurement tools. That said, it should be acknowledged that the rate of increase in CT/A with sucrose concentration appeared to reduce at the highest concentrations. This might reflect a ceiling effect for the measure itself or a true plateau in the palatability of sucrose. In addition, the lack of a strong dissociation between consumption measures and CT/A in response to umami taste might relate to minimal of post-ingestive satiety effects of MSG in the absence of protein (albeit that the dissociation was more clear in the latter half of the session). Regardless, the current study suggests that the analysis of consumption patterns and facial expressions could represent an interesting and novel measure in feeding behavior reflecting palatability in pigs.
In terms of practical applications, the fact that palatability can be dissociated from consumption may be important for the formulation of diets in industry: low palatability of additives might be missed where other factors (e.g., hunger) maintain consumption, or consumption may be reduced by high-palatability additives that also contribute to satiety (e.g., meeting nutrient needs efficiently). Using laboratory testing as a screen for palatability assessment could allow accurate determination of the different factors contributing to the overall amount of consumption (e.g., palatability vs. hunger). Moreover, the fact that changes in the internal state of rodents through learning or stress (Myers and Sclafani, 2001; Forestell and LoLordo, 2003; Dwyer, 2009) can selectively influence these hedonic measures, also raises the possibility that the analysis of feeding consumption patterns in pigs may offer a particularly valuable tool for identification of hedonic changes after associative learning or hedonic dysfunctions related to welfare problems in animals kept in production systems. Again, laboratory testing using palatability assessment may help in identifying aspects of production systems that may impinge on animal welfare and about what changes could ameliorate or remove such problems, as well as providing evidence for where superficially averse treatments do not have long-term negative consequences.
Conflict of interest statement. None declared.
Footnotes
The present study was supported by the Chilean Government research project “FONDECYT Iniciación Nº 11140576 and the Animal Nutrition and Welfare group (SNIBA) of the Universitat Autònoma de Barcelona.
LITERATURED CITED
- Anderson G. H., and Woodend D.. 2003. Effect of glycemic carbohydrates on short-term satiety and food intake. Nutr. Rev. 61(5 Pt 2):S17–S26. doi:10.1301/nr.2003.may.S17-S26 [DOI] [PubMed] [Google Scholar]
- Anderson G. H., Catherine N. L., Woodend D. M., and Wolever T. M.. 2002. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am. J. Clin. Nutr. 76:1023–1030. doi:10.1093/ajcn/76.5.1023. [DOI] [PubMed] [Google Scholar]
- Baird J. P., Rios C., Gray N. E., Walsh C. E., Fischer S. G., and Pecora A. L.. 2006. Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291:R1265–R1274. doi:10.1152/ajpregu.00143.2006. [DOI] [PubMed] [Google Scholar]
- Berridge K. C. 2000. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 24:173–198. doi:10.1016/S0149-7634(99)00072-X [DOI] [PubMed] [Google Scholar]
- Boughter J. D. Jr, St John S. J., Noel D. T., Ndubuizu O., and Smith D. V.. 2002. A brief-access test for bitter taste in mice. Chem. Senses 27:133–142. doi:10.1093/chemse/27.2.133 [DOI] [PubMed] [Google Scholar]
- Clouard C., Chataignier M., Meunier-salaün M. C., and Val-Laillet D.. 2012. Flavour preference acquired via a beverage-induced conditioning and its transposition to solid food: sucrose but not maltodextrin or saccharin induced significant flavour preferences in pigs. Appl. Anim. Behav. Sci. 136:26–36. doi:10.1016/j.applanim.2011.11.007 [Google Scholar]
- Clouard C., Loison F., Meunier-Salaün M. C., and Val-Laillet D.. 2014. An attempt to condition flavour preference induced by oral and/or postoral administration of 16% sucrose in pigs. Physiol. Behav. 124:107–115. Doi:10.1016/j.physbeh.2013.10.025 [DOI] [PubMed] [Google Scholar]
- Davis J. D., and Smith G. P.. 1992. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav. Neurosci. 106:217–228. doi:10.1037/0735-7044.106.1.217 [PubMed] [Google Scholar]
- Dwyer D. M. 2008. Microstructural analysis of conditioned and unconditioned responses to maltodextrin. Learn. Behav. 36:149–158. doi:10.3758/LB.36.2.149 [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. 2009. Microstructural analysis of ingestive behaviour reveals no contribution of palatability to the incomplete extinction of a conditioned taste aversion. Q. J. Exp. Psychol. (Hove). 62:9–17. doi:10.1080/17470210802215152 [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. 2012. Licking and liking: The assessment of hedonic responses in rodents. Q. J. Exp. Psychol. 65:371–394. doi:10.1080/17470218.2011.652969 [DOI] [PubMed] [Google Scholar]
- Figueroa J., Solà-Oriol D., Borda E., Sclafani A., and Pérez J. F.. 2012. Flavour preferences conditioned by protein solutions in post-weaning pigs. Physiol. Behav. 107:309–316. doi:10.1016/j.physbeh.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Forbes J. M. 2010. Palatability: principles, methodology and practice for farm animals. J. Anim. Sci. 229–243. doi:10.1079/PAVSNNR20105052 [Google Scholar]
- Forestell C. A., and LoLordo V. M.. 2003. Palatability shifts in taste and flavour preference conditioning. Q. J. Exp. Psychol. B. 56:140–160. doi:10.1080/02724990244000232. [DOI] [PubMed] [Google Scholar]
- Frías D., Tadich T., Franco-Rosselló R., Dwyer D. M., and Figueroa J.. 2016. Consumption patterns: A proposed model for measurement of solution palatability in pigs. J. Anim. Sci. 94:103–105. doi:10.2527/jas2015-9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill H. J., and Norgren R.. 1978. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 143:263–279. doi:10.1016/0006-8993(78)90569-3 [DOI] [PubMed] [Google Scholar]
- Kotlus B. S., and Blizard D. A.. 1998. Measuring gustatory variation in mice: a short-term fluid-intake test. Physiol. Behav. 64:37–47. doi:10.1016/S0031-9384(98)00016-X [DOI] [PubMed] [Google Scholar]
- Kringelbach M. L., Stein A., and van Hartevelt T. J.. 2012. The functional human neuroanatomy of food pleasure cycles. Physiol. Behav. 106:307–316. doi:10.1016/j.physbeh.2012.03.023 [DOI] [PubMed] [Google Scholar]
- Mennella J. A., Griffin C. E., and Beauchamp G. K.. 2004. Flavor programming during infancy. Pediatrics 113:840–845. doi:10.1542/peds.113.4.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K. P., and Sclafani A.. 2001. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose. II. Taste reactivity analysis. Physiol. Behav. 74:495–505. doi:10.1016/S0031-9384(01)00596-0 [DOI] [PubMed] [Google Scholar]
- Nelson S. L., and Sanregret J. D.. 1997. Response of pigs to bitter-tasting compounds. Chem. Senses 22:129–132. doi:10.1093/chemse/22.2.129 [DOI] [PubMed] [Google Scholar]
- Oostindjer M., Bolhuis J. E., Mendl M., Held S., van den Brand H., and Kemp B.. 2011. Learning how to eat like a pig: effectiveness of mechanisms for vertical social learning in piglets. Anim Behav. 82:503–511. doi:10.1016/j.anbehav.2011.05.031 [Google Scholar]
- Roura E., Humphrey B., Tedó G., and Ipharraguerre I.. 2008. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can. J. Anim. Sci. 88:535–558. doi:10.4141/CJAS08014 [Google Scholar]
- Sclafani A., and Ackroff K.. 2003. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol. Behav. 79: 663–670. doi:10.1016/S0031-9384(03)00143-4 [DOI] [PubMed] [Google Scholar]
- da Silva E. C., de Jager N., Burgos-Paz W., Reverter A., Perez-Enciso M., and Roura E.. 2014. Characterization of the porcine nutrient and taste receptor gene repertoire in domestic and wild populations across the globe. BMC Genomics 15:1057. doi:10.1186/1471-2164-15-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solà-Oriol D., Roura E., and Torrallardona D.. 2009. Use of double-choice feeding to quantify feed ingredient preferences in pigs. Livest. Sci. 123(2-3):129–137. doi:10.1016/j.livsci.2008.10.015 [Google Scholar]
- Steiner J. E., Glaser D., Hawilo M. E., and Berridge K. C.. 2001. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 25:53–74. doi:10.1016/S0149-7634(00)00051-8 [DOI] [PubMed] [Google Scholar]