Abstract
We report a case of bilateral superior altitudinal hemianopsia (BSAH) secondary to pituitary microadenoma related inferior optic chiasm damage. A 69-year-old-female developed a BSAH with macular involvement that was initially considered as malingering due to the obscurity of this symptom. The patient presents with multiple risk factors for ischemic disease to the ocular and occipital vessels, persistent migraine, hypothyroidism, and a stable pituitary microadenoma, yet no evidence of tissue ischemia or infarction was noted on imaging that could account for her visual field defects. A prior history of pituitary microadenoma is presumed to be the etiologic cause although the lesion had regressed by the time of presentation.
Keywords: bsah, hemianopsia, pituitary microadenoma, levothyroxine, bilateral superior altitudinal hemianopsia
Introduction
Visual defects can be caused by a multitude of cerebral, ocular, and optic pathway lesions with the pattern having localization value. Of these defects, bilateral altitudinal hemianopsias with macular (or central visual) involvement are rare. The etiologies for such conditions include bilateral pre-chiasmal lesions such as ischemic optic neuropathy [1-2], pituitary lesions [3-4], and cerebral vascular infarction involving the occipital lobe [5-14]. Herein we report on a patient with bilateral superior altitudinal hemianopsia (BSAH) without macular sparing and, to our knowledge, the first such case supported by multifocal visual evoked potential confirmation (mfVEP) of the field defects.
Case presentation
A 69-year-old female was referred to our visual electrophysiology clinic. The referring optometrist was concerned that the nature of her visual field defects, specifically a bilateral altitudinal hemianopsia with macular involvement, suggested a malingering disorder (Figures 1-2). The visual field changes were first noted 10 years prior, and magnetic resonance imaging (MRI) at the time revealed a pituitary microadenoma not affecting the optic chiasm. Yearly MRI examinations of the brain along with her visual fields remained stable without evidence of growth of the pituitary lesion. Her medical history was also significant for hyperlipidemia, hypertension, mitral valve prolapse, hypothyroidism treated with levothyroxine, and a 20-year history of migraine headaches without visual aura, treated with botulinum toxin injections and oral eletriptan hydrobromide. Her family history was notable for migraines in her mother and sister, the latter also having had a ruptured cerebral aneurysm.
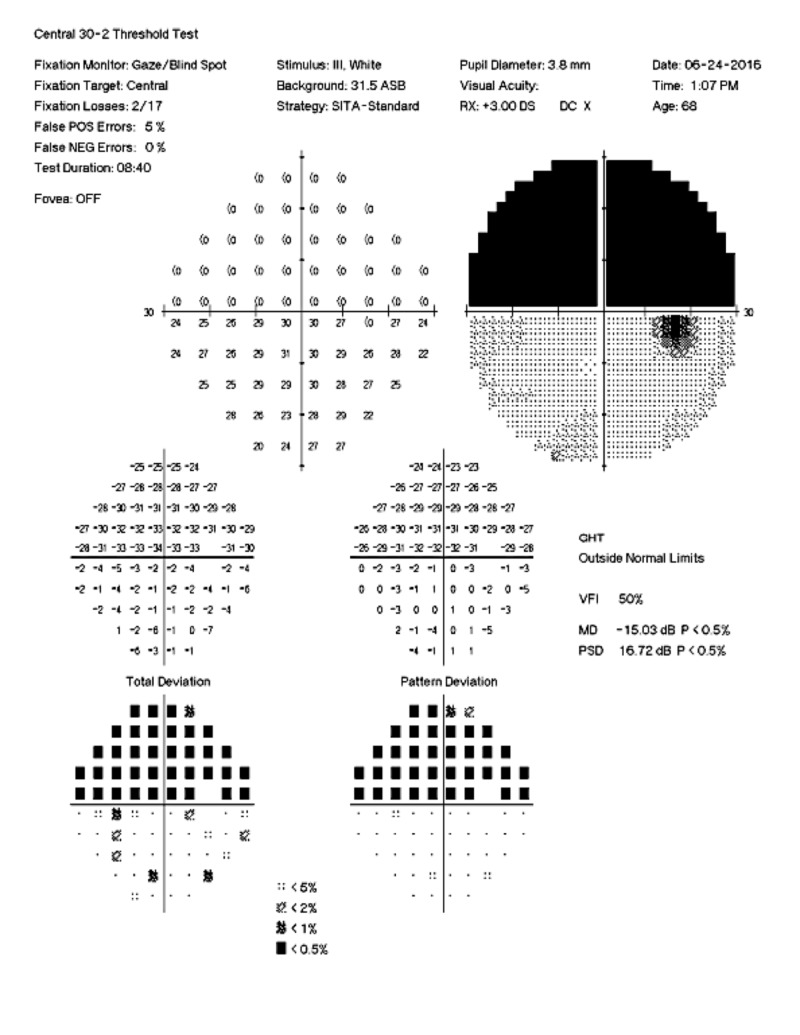
Figure 1. Left Visual Field.
Automated 30-2 visual field demonstrating complete superior hemifield defect with macular involvement in the left eye.
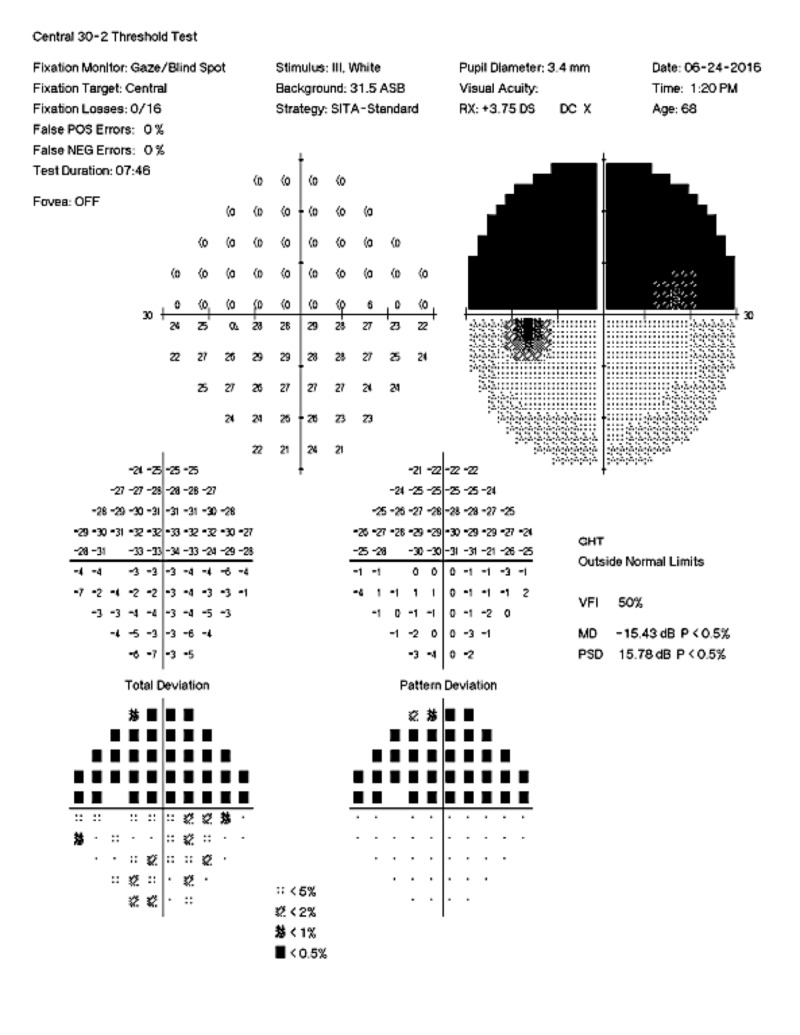
Figure 2. Right Eye Visual Field.
Automated 30-2 visual field demonstrating complete superior hemifield defect with macular involvement in the right eye.
Her physical examination was unremarkable. Ophthalmic examination revealed visual acuity of 20/20 in each eye. The anterior segments were normal. The cup to disc ratios were 0.3 with healthy appearing nerves bilaterally. Dilated funduscopic examination was normal in both eyes except for lattice degeneration in the inferior periphery of the right eye. Confrontational visual fields were absent superior to the midline. Optical coherence tomography testing of the optic nerve head and macula were normal. Color vision testing was normal in both eyes. A mfVEP was performed and confirmed bilateral superior altitudinal visual deficits with macular involvement (Figure 3). Repeated MRI and magnetic resonance angiogram (MRA) testing of the brain and orbit revealed a hypoenhancing lesion of the right side of the sella turcica, consistent with a pituitary microadenoma, measuring 7.8 x 3.6 mm. This was slightly smaller than the previous examination one year before.
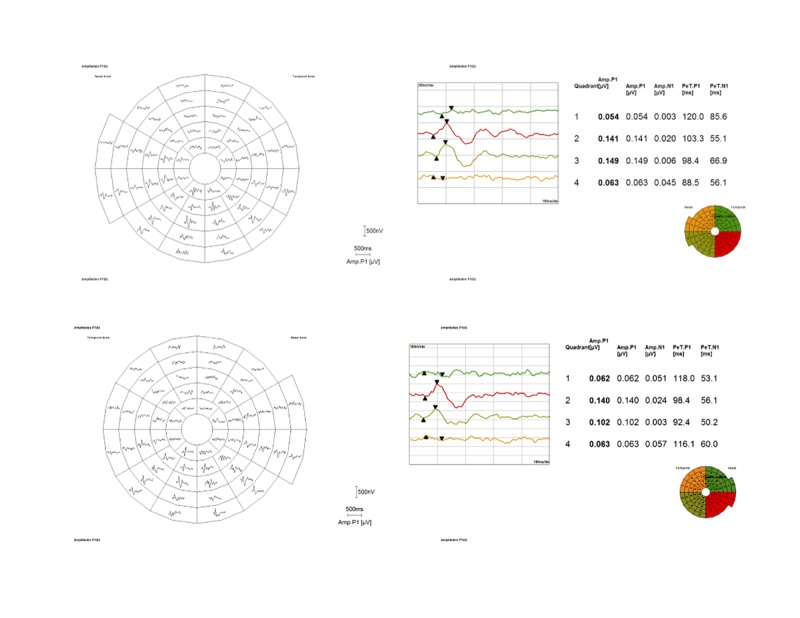
Figure 3. Multifocal Visual Evoked Potential.
Multifocal visual field (best of responses) of the right (top) and left (bottom) eye demonstrating complete superior visual field loss involving the macula as well. The image at the top is of the right eye, the bottom image is of the left eye.
Discussion
We present a patient with a BSAH with macular involvement confirmed by mfVEP testing. Such lesions are exceedingly rare due to the complex sequence of vascular lesions required to produce such defects [13]. The pathogenetic mechanism for reported lesions include bilateral pre-chiasmal lesions, neuropathy [1-2], pituitary lesions that involve only the inferior optic pathway fibers [3-4], bilateral occipital radiation lesions [15], and occipital lesions that respect the calcarine fissure bilaterally and involve the dual vascular supply to the occipital lobe provided by the posterior and middle cerebral [5-14]. One case has been reported of BSAH in a 61-year-old man who was found to have tentorial herniation secondary to a subdural hematoma. His MRI showed bilateral infarction of the inferior tip of the medial occipital lobe. The patient’s MRI results supported his visual field findings [13].
The etiology of the lesion in our patient remains unclear. Both MRI and MRA revealed normal cerebral anatomic and vascular findings thus excluding an occipital lesion. Funduscopic findings and ancillary retinal and optic nerve testing revealed normal anatomy making a pre-chiasmal lesion unlikely as well. Our patient reported a long-standing history of migraines controlled by medications. One reported case of bilateral altitudinal visual field with macular involvement lasting four months was found in a 25-year-old patient who had migraines with aura. The mechanism was attributed to decreased cerebral blood flow without infarction as a byproduct of cortical spreading depression (CSD). CSD is considered to cause aura in migraines; however, our patient had migraines without aura [1]. In addition, it is highly unlikely our patient’s migraine related visual field defects would have persisted over 10 years without further evaluation.
One plausible etiology for this patient’s BSAH with macular involvement is the patient’s history of a pituitary microadenoma. Injury to the optic chiasm is theorized to be the only single point lesion that can have this case presentation [4]. Compressive or infiltrative lesions in the intrasellar and suprasellar regions are considered one of the most common causes of bilateral superior or inferior anopia [3]. Despite the longitudinal MRI findings showing no compression of the optic chiasm, and no enlargement of the pituitary microadenoma, damage to the optic chiasm may have been caused by an enlargement that was not present from the point of her first MRI onwards. Primary hypothyroidism had caused a pituitary microadenoma with suprasellar extension in a previously reported patient, and long-term treatment with levothyroxine was reported to cause regression [16]. Our patient, similarly, was on levothyroxine therapy for hypothyroidism.
Conclusions
Of note, this patient was initially referred to us under the presumption of malingering. Unlike conventional visual field testing, which is patient dependent and prone to various errors, mfVEP is an objective measure of the patient’s electrical response at the occipital cortex when stimulated by an on/off checkerboard pattern stimulating the patient’s visual fields. A topographical correlation of the perceived visual field is mapped to the activity at the occipital cortex. In our patient, mfVEP unequivocally confirmed that the patient did have a BSAH with macular involvement. Medical providers should be aware of this unique visual defect and the role of mfVEP testing in the testing and diagnosis of presenting patients.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Migrainous prolonged and reversible bilateral inferior altitudinal visual field defect. Razeghinejad MR, Masoumpour M, Bagheri MH. Headache. 2009;49:773–776. doi: 10.1111/j.1526-4610.2008.01291.x. [DOI] [PubMed] [Google Scholar]
- 2.Bilateral simultaneous inferior altitudinal hemianopia due to ischemic optic neuropathy. Blundo C, Corsi FM, Di Battista G, Galgani S, Piazza G. https://link.springer.com/article/10.1007%2FBF02043349. Ital J Neurol Sci. 1982;3:65–69. doi: 10.1007/BF02043349. [DOI] [PubMed] [Google Scholar]
- 3.Bilateral altitudinal anopia caused by infarction of the calcarine cortex. Heller-Bettinger I, Kepes JJ, Preskorn SH, Wurster JB. https://www.ncbi.nlm.nih.gov/pubmed/1033487. Neurology. 1976;26:1176–1179. doi: 10.1212/wnl.26.12.1176. [DOI] [PubMed] [Google Scholar]
- 4.Altitudinal hemianopia: report of two cases. Berkley WL, Bussey FR. Am J Ophthalmol. 1950;22:593–600. [Google Scholar]
- 5.Bilateral altitudinal visual fields. Lakhanpal A, Selhorst JB. https://www.ncbi.nlm.nih.gov/pubmed/2331128. Ann Ophthalmol. 1990;22:112–117. [PubMed] [Google Scholar]
- 6.Correlation of CAT scan and visual field defects in vascular lesions of the posterior visual pathways. McAuley DL, Russell WR. https://pdfs.semanticscholar.org/8cca/f2e6fc0b5a6de49f31c2103e8d4d3a6d6463.pdf?_ga=2.165474554.495146885.1537805705-626331252.1535860820. J Neurol Neurosurg Psychiatry. 1979;42:298–311. doi: 10.1136/jnnp.42.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loss of color vision and Stiles’ ∏1mechanism in a patient with cerebral infarction. Young RSL, Fishman GA. J Opt Soc Am. 1980;70:1301–1305. doi: 10.1364/josa.70.001301. [DOI] [PubMed] [Google Scholar]
- 8.A case of integrative visual agnosia. Riddoch MJ, Humphreys GW. https://www.ncbi.nlm.nih.gov/pubmed/3427396. Brain. 1987;110:1431–1462. doi: 10.1093/brain/110.6.1431. [DOI] [PubMed] [Google Scholar]
- 9.“Associative” visual agnosia for objects, pictures, faces and letters with altitudinal hemianopia [Article in English, Japanese] Suzuki K, Nomura H, Yamadori A, Nakasato N, Takase S. https://www.ncbi.nlm.nih.gov/pubmed/9146070. Clin Neurol. 1997;37:31–36. [PubMed] [Google Scholar]
- 10.Radionecrosis of the inferior occipital lobes with altitudinal visual field loss after gamma knife radiosurgery. Monheit BE, Fiveash JB, Girkin CA. https://www.ncbi.nlm.nih.gov/pubmed/15348983. J Neuroophthalmol. 2004;24:195–199. doi: 10.1097/00041327-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Bilateral superior altitudinal hemianopia due to bilateral occipital lobe infarction. Ogawa K, Ishikawa H, Tamura M, Kamei S, Mizutani T. https://www.tandfonline.com/doi/abs/10.3109/01658100903226182 Neuroophthalmology. 2009;33:5–264. [Google Scholar]
- 12.Bilateral occipital lobe infarction with altitudinal field loss following radiofrequency cardiac catheter ablation. Luu ST, Lee AW, Chen CS. BMC Cardiovasc Disord. 2010;10:14. doi: 10.1186/1471-2261-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilateral homonymous superior quadrantanopia after traumatic attempts to remove a cockroach impacted in the external auditory canal. Ng WY, Chua BE, Hardy TA, Wechsler D, Reddel SW. https://www.ncbi.nlm.nih.gov/pubmed/21495947. Med J Aust. 2011;194:420–422. doi: 10.5694/j.1326-5377.2011.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 14.A case report of bilateral superior altitudinal hemianopia with cerebral infarction. Keklikoglu HD, Yoldas TK, Coruh Y. Neurologist. 2010;16:132–135. doi: 10.1097/NRL.0b013e3181cf867f. [DOI] [PubMed] [Google Scholar]
- 15.Bilateral superior altitudinal hemianopia: missing the goal, but hitting the stroke cause. Etgen T, Köhler M, Sander D. J Stroke Cerebrovasc Dis. 2010;19:165–166. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Regression of a pituitary adenoma following levothyroxine therapy of primary hypothyroidism. Valenta LJ, Tamkin J, Sostrin R, Elias AN, Eisenberg H. https://www.ncbi.nlm.nih.gov/pubmed/6411499. Fertil Steril. 1983;40:389–392. doi: 10.1016/s0015-0282(16)47307-3. [DOI] [PubMed] [Google Scholar]