Abstract
Background:
We conducted a phase II multi-center trial of induction chemotherapy followed by response-adapted, dose-reduced radiation therapy with radiosensitizing chemotherapy for patients with locally advanced, human papillomavirus (HPV)-associated squamous cell carcinoma of the oropharynx. The aim of this study was to determine the progression-free survival for patients treated using this de-escalated approach to determine its worthiness for further investigation in a phase III setting.
Methods:
Eligible patients presented with newly diagnosed, biopsy-proven stage III or IV squamous cell carcinoma of the oropharynx, p16-positivity, and Zubrod performance status 0 to 1. Smoking history was not used as a criterion for eligibility. Treatment was induction paclitaxel 175 mg/m2 and carboplatin area under the concentration-time curve (AUC) 6 for two cycles every 21 days followed by dose-reduced radiation of 54 Gy or 60 Gy for responders and non-responders, respectively, with weekly paclitaxel 30 mg/m2. Intensity-modulated radiotherapy with daily image-guidance was required. The primary endpoint was 2-year progression-free survival as determined by per protocol analysis. This study is registered with ClinicalTrials.gov, numbers NCT02048020 and NCT01716195.
Findings:
Between October 4, 2012 and March 3, 2015, forty-five patients (26 tonsil; 19 base of tongue) were registered, of which 44 were analyzable. Median age was 60 years old (IQR, 54 to 67). Sixty-nine percent of the patients were lifelong never smokers. Twenty-four patients (55%) exhibited a complete or partial response to induction chemotherapy; twenty patients (45%) had a less than partial response to induction chemotherapy. With a median follow up of 30 months (IQR, 26 to 37 months), three (7%) of 44 patients developed local-regional recurrence and another distant metastasis, yielding a 2-year progression-free survival of 92%. The most commonly observed grade 3+ toxicity was leukopenia (17 patients) with the most common grade 3+ non-hematologic toxicities being dysphagia (4 patients) and mucositis (4 patients). Seventeen (39%) of 44 patients and nine (20%) of 44 patients developed any grade 3+ and non-hematologic grade 3+ toxicity, respectively, during protocol therapy.. The proportion who were gastrostomy-tube dependent at 3- and 6-months post-therapy was 2% (1 of 44 patients) and 0%, respectively.
Interpretation:
The rates of disease control demonstrated with this de-escalation regimen for patients with locally advanced, HPV-associated oropharyngeal carcinoma were comparable to those of historical controls treated by standard dose chemoradiotherapy regimens. Additionally, long-term side effects appeared to be reduced. By decreasing toxicity while maintaining cure rates, de-escalation of radiation dose has the potential to improve the therapeutic ratio and optimize long-term function for patients with a disease that is rapidly increasing in incidence.
Keywords: head and neck cancer, HPV, radiotherapy, de-escalation, oropharynx
Introduction
The identification of human papillomavirus (HPV) as a causative agent for oropharyngeal carcinoma has suggested that not all head and neck squamous cell carcinoma behaves similarly. Studies have shown that HPV-associated head and neck cancer represents a unique entity with distinct clinical and molecular characteristics [1–3]. Moreover, recent data have demonstrated dramatic differences with respect to prognosis and treatment response between HPV-positive and HPV-negative head and neck cancer with the former having at least half the risk of death from cancer compared to the latter [4–6]. While many theories have been proposed to explain this observation, the lower risk in HPV positive cases is y thought to be attributable in part to differential sensitivity to therapeutic radiation [7–9].
This recognition that HPV-positive head and neck squamous cell carcinoma responds favorably to radiation therapy has prompted investigators to suggest that patients with these cancers might be “over-treated” with standard doses and unnecessarily subjected to the toxicity of intensive chemoradiotherapy with excessively high radiation doses. Indeed, current estimates of late dysphagia and xerostomia have been reported to be as high as 30% among survivors of head and neck cancer treated by radiation therapy [10]. We report the results of a phase II de-intensification trial using radiation doses approximately 15–20% less than historically used.
Methods and Materials
Study Design and Participants
The CCRO-022 study was conducted jointly at the University of California, Davis and the University of California, Los Angeles. The study protocol was approved by the human ethics committees of both universities, and all study participants provided written informed consent. All of the authors participated in the design of the study, collected the data, and contributed to the analysis and/or interpretation of the data. The trial is registered with clinicaltrials.gov (NCT02048020 and NCT01716195) and opened separately at the University of California, Davis and the University of California, respectively, before the data was centrally combined for aggregate analysis.
This phase II trial was designed as a de-intensification protocol in which induction chemotherapy was used to select patients for reduced dose radiation therapy. Based on encouraging single-institutional data, as well as a multi-institutional phase II study of 111 patients with stage III and IV squamous cell carcinoma of the head and neck conducted by the Eastern Cooperative Oncology Group (ECOG), a concurrent chemotherapy regimen in which 2 cycles of induction chemotherapy every 3 weeks with carboplatin (AUC, 6) and paclitaxel (175 mg/m2) followed by concurrent paclitaxel (30 mg/m2) weekly with definitive radiation therapy was chosen for the chemoradiotherapy backbone of the protocol [11].
Eligible participants were 18 years of age or older with Zubrod performance status of 0–1, and a histological diagnosis of newly diagnosed, stage III or IV, HPV-associated squamous cell carcinoma arising from the oropharynx. Smoking history was not used as a criterion for eligibility. HPV-positivity was defined as tumors that were p16-positive by immunohistochemistry. While central testing to confirm p16 status was not required for this study, the most commonly used antibody was the IgG mouse monoclonal antibody for the qualitative detection of the p16INK4a protein following the protocol supplied by the Ventana CINtec p16 histology kit (Ventana Medical Systems, Inc., Tucson, AZ, USA). Participants were ineligible if they were immunosuppressed; were pregnant or breast-feeding; had active lupus erythematosus or scleroderma; had impaired liver or kidney function; had current uncontrolled cardiac disease; had chronic obstructive pulmonary disease exacerbation or other respiratory illness requiring hospitalization; or had acute or fungal infection requiring antibiotic at registration. Participants were also excluded if they had distant metastasis, prior malignancy (except non-melanomatous skin cancer) unless disease free for a minimum of 3 years; or had received prior treatment for their oropharyngeal carcinoma. Laboratory studies obtained within 4 weeks of registration required patients to have adequate bone marrow function defined as absolute neutrophil count > 1,500 cells/mm3, platelets > 100,000 cells/mm3, and hemoglobin > 8.0 g/dl; adequate hepatic function defined as aspartate aminotransferase or alanine aminotransferase ≤ 2× the upper limit of normal; adequate renal function defined as serum creatinine ≤ 1.5 mg/dl or institutional upper limit of normal and creatinine clearance ≥ 50 ml/min determined by 24-hour collection or estimated by Cockcroft-Gault formula: male = [(140 − age) × (weight in kg)] [(Serum Cr mg/dl) × (72)]; female = 0.85 × (CrCl male). Women of childbearing potential were required to have a negative serum pregnancy test within 7 days prior to start of protocol therapy. Given the favorable prognosis generally connoted with the diagnosis of HPV-associated oropharyngeal carcinoma, the life expectancy of the patient population was believed to be excellent.
Procedures
All patients had measurable disease at registration. Tumors were staged according to the 2009 staging classification of the American Joint Committee on Cancer [12]. Computed tomography (CT) and/or magnetic resonance imaging (MRI) of the head and neck region, in addition to positron emission tomography (PET) were required prior to enrollment. Patients who had initial surgical treatment other than diagnostic biopsy of the primary site or nodal sampling of the neck disease were excluded.
Figure 1 illustrates the trial profile. Two cycles of induction chemotherapy (paclitaxel 175 mg/m2 infused over 3 hours followed by carboplatin AUC=6 as a 30 minute infusion) was administered to all patients primarily as a means to select HPV-positive squamous cell carcinoma patients, who may benefit from significant radiation dose de-intensification in the concurrent chemoradiotherapy phase of treatment. At least 2 weeks after completion of induction chemotherapy, concurrent chemoradiotherapy was initiated using single-agent paclitaxel infused at a dose of 30 mg/m2 over at least 1 hour given weekly with daily radiation for 5 total cycles. Dose modifications for induction chemotherapy were permitted based on neutropenia and/or thrombocytopenia observed during cycle 1 with reduction of paclitaxel and carboplatin doses to 150 mg/m2 and AUC=5, respectively. The development of high-grade hepatic, neuropathic, and/or myalgia allowed for similar reductions in dosing. During the concurrent chemoradiotherapy phase, paclitaxel was held in the presence of grade 2+ neutropenia, thrombocytopenia, and/or neuropathy, with resumption of weekly chemotherapy once toxicity resolved to grade 1 or less. Doses that were missed during weekly administration of chemotherapy with radiation therapy were documented but not made up. Granulocyte colony-stimulating factors were allowed to be used at the discretion of the treating physician.
Figure 1.
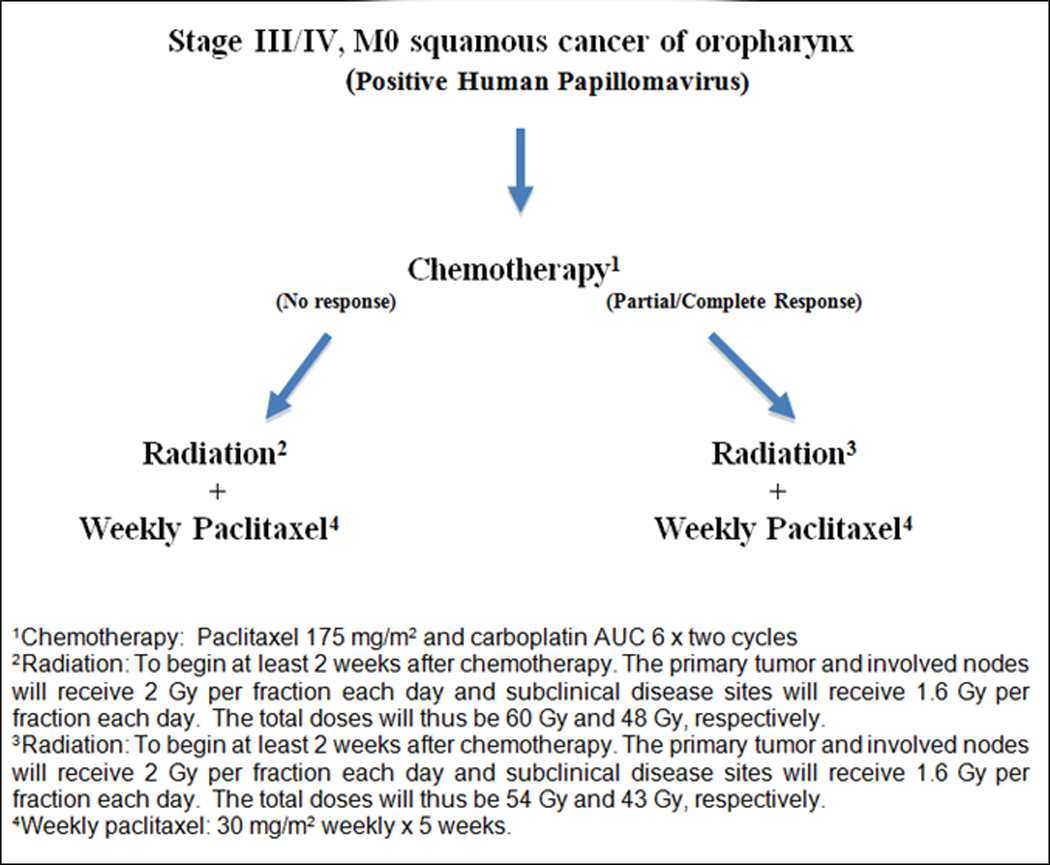
Trial schema/CONSORT diagram.
The radiation dose was determined by the clinical response to induction chemotherapy using RECIST criteria [13]. Complete and partial response was defined as 100% and at least 30% decrease in the sum of the longest diameter of target lesions compared to this value at baseline. All patients underwent CT after the initial 2 cycles of induction chemotherapy for response assessment. The timing of the CT was left to the discretion of the treating physician, but it was recommended that this be performed at approximately 2 weeks after induction chemotherapy. For patients with a complete response or partial response after induction chemotherapy, the total prescribed dose to the primary tumor and involved nodes were 54 Gy in 27 fractions. Uninvolved nodal areas of the neck were treated to 43 Gy. All other patients were classified as having minor response and received 60 Gy in 30 fractions, with a dose of 48 Gy delivered to uninvolved areas. Intensity-modulated techniques to perform simultaneous integrated boost were required on this protocol. Radiation target volumes were defined based on the pre-chemotherapy extent of disease derived from imaging and physical examination findings, including endoscopy, and adjusted to conform to post-chemotherapy anatomy and acknowledged anatomical boundaries to tumor spread. The planning goal was to encompass at least 95% of the planning target volume with the isodose corresponding to the prescription dose. Daily image-guidance was a requirement for treatment.
Laboratory studies consisting of complete blood counts with differential, metabolic panel with electrolytes, and liver function tests were obtained within 2 days prior to the administration of each induction chemotherapy cycle and then weekly during chemoradiotherapy. Patients were required to return for follow-up 2 to 4 weeks after completion of protocol therapy. Disease assessments, which included history taking, physical examination, and fiber-optic examination were then performed every 3 months for the first year, and then every 6 months thereafter. PET/CT was obtained at approximately 3 months after completion of chemoradiotherapy, with response rates reported using RECIST criteria. Treatment-related effects were assessed using the National Cancer Institute’s Common Toxicity Criteria [14]. Early and late toxicity was defined as those occurring within and after 90 days from the completion of protocol therapy. Serious adverse events were reviewed on an every other week basis by the principle investigator.
Surgical salvage at the primary site was required for all patients experiencing tumor progression at any time. All patients with N1 or N2 disease not obtaining a complete tumor response by 2 to 3 months after treatment were generally required to undergo surgical consultation for consideration of a post-treatment neck dissection.
Outcomes
The primary objective of the study was to estimate the 2-year progression-free survival of patients treated by reduced dose radiation therapy with failure defined as disease progression or death, with censoring of patients lost to follow-up. All patients meeting the eligibility criteria who signed a consent form and completed protocol treatment were considered evaluable for estimation of the 2-year progression-free survival rate. Those who died within 2 years were considered to have had disease progression unless documented evidence clearly indicated no progression had occurred. In the event that such evidence was obtained, or in the case of major treatment violation, the patients’ response data were considered censored at the date the patient was withdrawn from treatment. Secondary endpoints included overall survival, local-regional control, treatment-associated toxicity, protocol treatment delivery defined as completion of planned therapy, and death during or within 30 days of discontinuation of protocol treatment. Additionally, quality of life data was prospectively collected using the Functional Assessment of Cancer Therapy- Head and Neck survey and University of Washington Quality of Life (version 4) instrument at the time of registration and at various points during follow-up after completion of protocol therapy [15,16]. This information will be presented separately.
Statistical Analysis
A two-stage Simon design was adopted with a targeted accrual goal of 50 patients, which permitted early stopping of the trial if strong evidence occurred that the study regimen was inactive or was effective enough to warrant further investigation in a phase III trial, was employed to test the null hypothesis that the true 2-year progression-free survival was at greatest 72% [17]. A 2-year progression-free survival rate of 72% or lower was considered ineffective for the proposed dose de-intensified therapy in this population, and a 2-year progression-free survival rate of 86% or higher was considered to warrant further subsequent studies. Thus, statistical parameters were established with 2-year progression free survival rates of 72% and 86% serving as thresholds for termination or continuation of the trial. Interim analysis occurred after the first 25 patients in the study were enrolled in April 2014. Stage I evaluation required termination of the study due to inefficacy if 19 or fewer of these patients were without progression within 2 years; however, since only 1 event was recorded, the study proceeded to accrual with the stipulation that if 40 or fewer of the 50 patients enrolled were progression-free at 2 years, then it was accepted that the dose de-intensified therapy was ineffective in this population and not worth further investigation. If 41 or more patients were progression-free at 2 years, then this served as the statistical basis for the study regimen to be further evaluated in a phase III trial. The trial was closed after a second interim analysis in January 2016 recognized that at least 41 of the patients enrolled were disease-free at 2 years, thus meeting protocol requirement to proceed with a phase III comparison. The results presented herein represent the final analysis of the 44 analyzable patients. The overall significance level was 9% and the power was 81%. Assuming the significance level was at most 10%, if the 2-year progression-free survival rates were 78%, 81%, 85% and 87%, the statistical powers were 32%, 52%, 78%, 89%, respectively. A 95% confidence interval (CI) for all actuarial endpoints was constructed using the Duffy-Santner approach [18]. Time to event distributions was estimated using the Kaplan-Meier method [19]. Toxicity data, including the rate of 30 day mortality, was presented as descriptive statistics. Since the basis of this study was to show that de-intensified therapy is essentially non-inferior to historical treatments using full-dose radiation, a per protocol analysis was employed. All data, including the primary endpoint, was centrally reviewed for analysis. Statistical analysis was performed using SAS, Version 9.4 (SAS Institute, Cary, NC).
Role of the Funding Source
This work was supported by the Biostatistics Shared Resource of the University of California, Davis Comprehensive Cancer Center Support Grant, P30CA093373–11. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Patient Characteristics
From October 4, 2012 to March 3, 2015, a total of 45 patients (26 tonsil; 19 base of tongue) were registered and consented. All patients had biopsy-proven evidence of p16-positive squamous cell carcinoma of the oropharynx with measurable disease at registration. One patient was removed from the study after withdrawal of consent. The patient characteristics for the remaining 44 patients who comprised the primary subject population and was used for the analysis reported herein are outlined in Table 1. The median age was 60 years old (IQR, 54 to 67 years). Sixty-eight percent of the patients were lifelong never smokers. For former smokers, the intensity of tobacco use ranged from 2 to 60 pack-years (median, 20 pack-years). None of the patients were active smokers at registration.
Table 1.
Patient and disease characteristics.
| Characteristic | N | % |
|---|---|---|
| Race | ||
| Caucasian | 40 | 90.9 |
| Hispanic | 3 | 6.8 |
| Black | 1 | 2.3 |
| Zubrod | ||
| 0 | 34 | 77.3 |
| 1 | 10 | 22.7 |
| Smoking history | ||
| Never | 30 | 68.2 |
| ≤ 10 pack-years | 3 | 6.8 |
| 10–20 pack-years | 2 | 4.6 |
| 20–40 pack-years | 4 | 9.1 |
| >40 pack-years | 5 | 11.4 |
| Primary site | ||
| Tonsil | 26 | 59.1 |
| Base of tongue | 18 | 40.9 |
| T-stage | ||
| T1 | 16 | 36.4 |
| T2 | 18 | 40.9 |
| T3 | 3 | 6.8 |
| T4 | 7 | 15.9 |
| N-stage | ||
| N0 | 2 | 4.6 |
| N1 | 3 | 6.8 |
| N2a | 9 | 20.4 |
| N2b | 19 | 43.2 |
| N2c | 10 | 22.7 |
| N3 | 1 | 2.3 |
| AJCC stage | ||
| III | 2 | 4.6 |
| IV | 42 | 95.4 |
| ICON-S stage | ||
| I | 27 | 61.3 |
| II | 9 | 20.5 |
| III | 8 | 18.2 |
| NRG Risk Group* | ||
| Low | 16 | 36.4 |
| Other | 28 | 63.6 |
Abbreviations: AJCC, American Joint Committee on Cancer; ICON, International Collaboration on Oropharyngeal Cancer Network for Staging; *Defined as eligibility for NRG HN002 trial
Efficacy
After 2 cycles of induction chemotherapy, CT scan to assess response was obtained at a median of 14 days (IQR, 10 to 17 days). Five (11%) of 44 patients had a complete response at all disease sites and 19 (43%) of 44 patients had a partial response. These patients were stratified to receive 54 Gy. The remaining 20 patients (45%) had minor response or stable disease and subsequently received 60 Gy. No patient experienced disease progression at either local-regional or distant sites during induction chemotherapy. Initial evaluation after chemoradiotherapy obtained with PET/CT approximately 3 months post-treatment showed that overall tumor response at all disease sites were complete in 84%. The remaining 16% of patients with partial response had their disease resolve with subsequent follow-up imaging. No patient underwent post-therapy neck dissection for suspected residual disease.
With a median follow-up of 30 months, (IQR, 26 to 37 months), three patients experienced local-regional recurrences. The first was a 51-year-old never-smoking male with T1N2b poorly differentiated squamous cell carcinoma disease involving the right tonsil. He received 60 Gy after a minor response to induction chemotherapy and subsequently developed local recurrence at 25 months. The second was a 59-year-old male with 20 pack-year tobacco history who presented with T4N2b poorly differentiated disease involving the base of tongue and received 54 Gy after a partial response to induction chemotherapy. He developed regional recurrence at the left cervical neck approximately 17 months after completion of protocol therapy. The third was a 63-year-old never-smoking male with T2N2b moderately differentiated squamous cell carcinoma involving the right tonsil. He received 60 Gy after a minor response to induction chemotherapy and relapsed in the right cervical neck approximately 15 months after treatment. None of these patients had evidence of distant metastasis at the time of local-regional relapse, and all are currently without evidence of disease after surgical salvage. Three (7%) of 44 patients developed local-regional failure, resulting in the 2-year estimate of local-regional control being 95% (95% CI: 80%- 99%).
There was 1 case of distant metastasis observed during the follow-up period-- a 57 year-old male with T2N2a squamous cell carcinoma of the base of tongue who developed pulmonary metastasis at approximately 14 months. He had been previously treated with 60 Gy after a minor response to induction chemotherapy. The patient was referred for systemic therapy and currently has stable disease. As shown in Figure 2, four (9%) of 44 patients developed progression, resulting in the 2-year estimate of progression-free survival of 92% (95% CI: 77%- 97%). As shown in Figure 3, one (2%) of 44 patients died, leading to the 2-year overall survival rate of 98% (95% CI: 85%- 100%).
Figure 2.
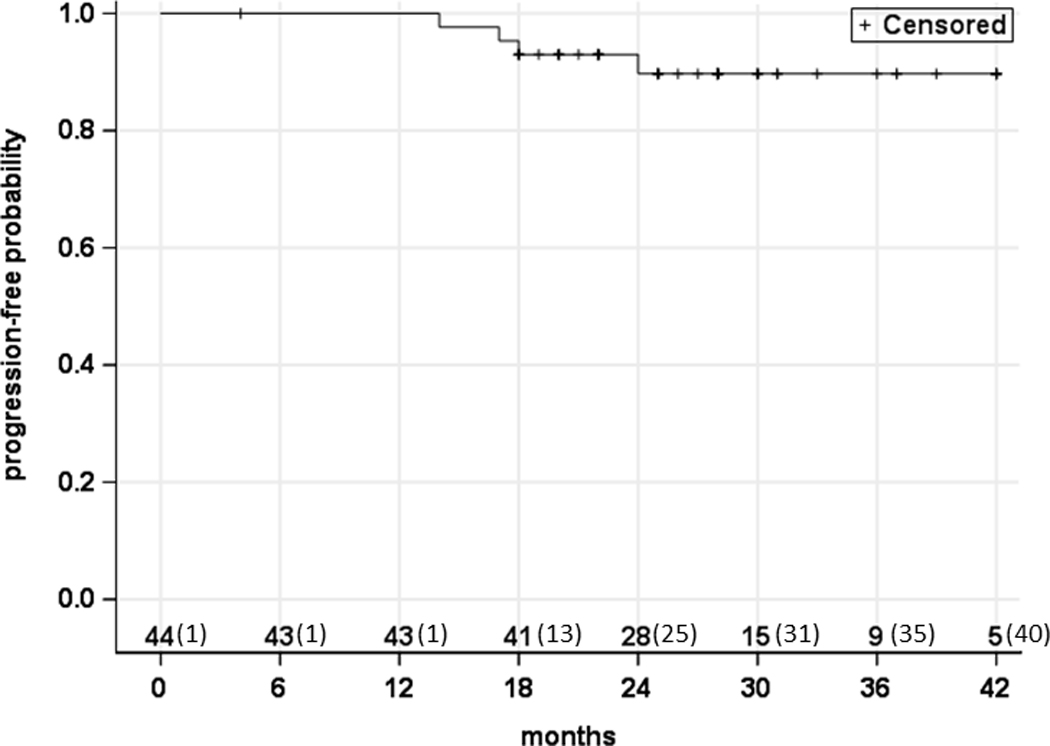
Progression-free survival. The numbers along the x-axis represent the number of patients at risk (and censured) in a cumulative fashion.
Figure 3.
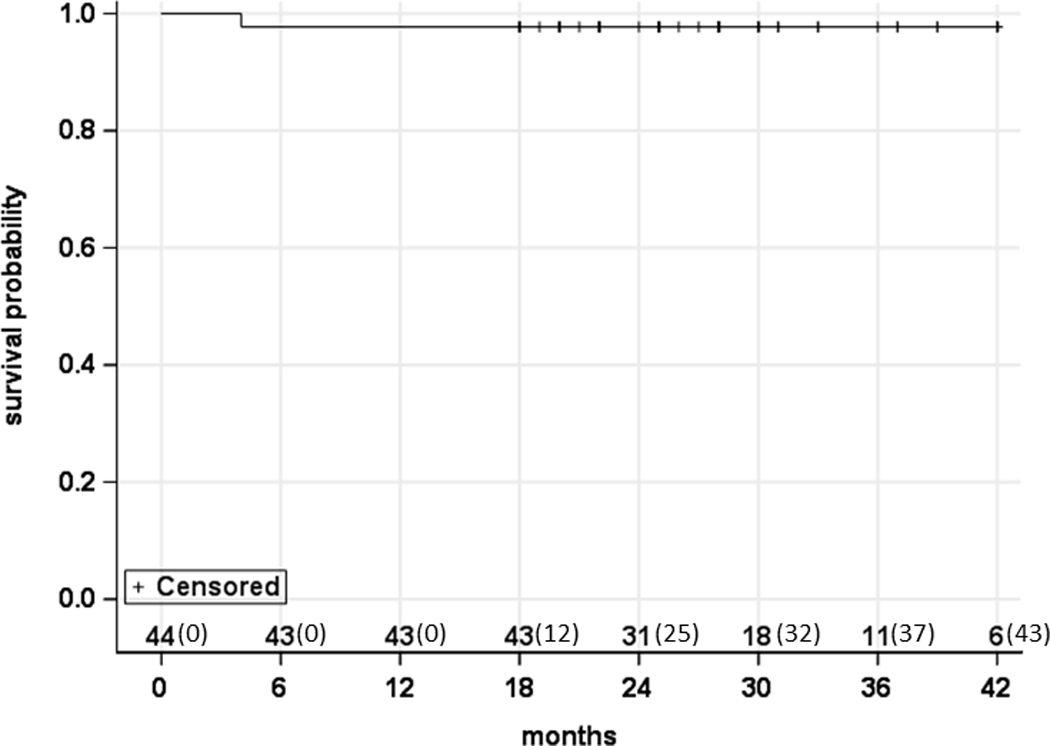
Overall survival. The numbers along the x-axis represent the number of patients at risk (and censured) in a cumulative fashion.
Toxicity
All patients completed protocol therapy except one who experienced an allergic reaction to paclitaxel infusion and was subsequently treated with carboplatin alone. No patient discontinued therapy at any time. None of the patients died during or within 30 days of completion of protocol therapy. Treatment-related adverse events during induction chemotherapy were generally infrequent and mild with no cases of grade 3+ non-hematologic toxicity reported. The proportion of patients who develop grade 3+ neutropenia and leukopenia was 11% and 39%, respectively. There were no cases of neutropenic fever. Three (7%) of 43 patients required dose reduction of cycle 2 of induction chemotherapy due to neutropenia with reduction of paclitaxel and carboplatin doses to 150 mg/m2 and AUC=5, respectively.
Two patients required hospitalization during concurrent chemoradiotherapy, the first for aspiration pneumonia which resolved with intravenous antibiotics, and the second for severe anxiety and panic attacks. Another patient, with a pre-existing history of depression, committed suicide approximately 2 months after completion of protocol therapy, accounting for the only death among the subject population. Thirty-seven (84%) of the 44 patients received all 5 planned cycles of concurrent chemotherapy. Adverse events occurring in the patient population are listed in Table 2. Seventeen (39%) of 44 patients and nine (20%) of 44 patients developed any grade 3+ and non-hematologic grade 3+ toxicity, respectively, during protocol therapy.
Table 2.
Adverse events.
| Toxicity | Induction chemotherapy | Concurrent chemoradiotherapy | ||||
|---|---|---|---|---|---|---|
| Grades 1–2 (%) | Grade 3 (%) | Grade 4 (%) | Grades 1–2 (%) | Grade 3 (%) | Grade 4 (%) | |
| Anemia | 39 (88.6) | 1 (2.3) | 0 (0) | 27 (61.3) | 1 (2.3) | 0 (0) |
| Anorexia | 4 (9.1) | 1 (2.3) | 0 (0) | 9 (20.4) | 2 (4.5) | 0 (0) |
| Anxiety | 7 (15.9) | 0 (0) | 0 (0) | 4 (9.1) | 1 (2.3) | 0 (0) |
| Arthralgia | 9 (20.4) | 1 (2.3) | 0 (0) | 4 (9.1) | 0 (0) | 0 (0) |
| Bone pain | 6 (13.6) | 0 (0) | 0 (0) | 2 (4.5) | 0 (0) | 0 (0) |
| Constipation | 3 (6.8) | 0 (0) | 0 (0) | 17 (38.6) | 0 (0) | 0 (0) |
| Cough | 2 (4.5) | 0 (0) | 0 (0) | 16 (36.4) | 0 (0) | 0 (0) |
| Dehydration | 4 (9.1) | 1 (2.3) | 0 (0) | 9 (20.4) | 1 (2.3) | 0 (0) |
| Diarrhea | 4 (9.1) | 0 (0) | 0 (0) | 3 (6.8) | 0 (0) | 0 (0) |
| Dysphagia | 20 (22.7) | 0 (0) | 0 (0) | 19 (43.2) | 4 (9.1) | 0 (0) |
| Fever | 3 (6.8) | 0 (0) | 0 (0) | 3 (6.8) | 0 (0) | 0 (0) |
| Headache | 3 (6.8) | 0 (0) | 0 (0) | 4 (9.1) | 0 (0) | 0 (0) |
| Hypokalemia | 8 (18.2) | 1 (2.3) | 0 (0) | 4 (9.1) | 0 (0) | 0 (0) |
| Hypomagnesemia | 5 (11.4) | 0 (0) | 0 (0) | 5 (11.4) | 0 (0) | 0 (0) |
| Hyponatremia | 20 (22.7) | 2 (4.5) | 0 (0) | 6 (13.6) | 2 (4.5) | 0 (0) |
| Increased creatinine | 18 (40.9) | 0 (0) | 0 (0) | 4 (9.1) | 0 (0) | 0 (0) |
| Leukopenia | 23 (52.3) | 17 (38.6) | 0 (0) | 37 (84.1) | 3 (6.8) | 0 (0) |
| Mucositis | 16 (36.4) | 1 (2.3) | 0 (0) | 34 (77.3) | 4 (9.1) | 0 (0) |
| Nausea | 8 (18.2) | 1 (2.3) | 0 (0) | 18 (40.9) | 1 (2.3) | 0 (0) |
| Neuropathy | 9 (20.4) | 0 (0) | 0 (0) | 3 (6.8) | 0 (0) | 0 (0) |
| Neutropenia | 18 (40.9) | 5 (11.4) | 0 (0) | 9 (20.4) | 0 (0) | 0 (0) |
| Pneumonia | 0 (0) | 0 (0) | 0 (0) | 1 (2.3) | 1 (2.3) | 0 (0) |
| Dermatitis | 0 (0) | 0 (0) | 0 (0) | 33 (75.0) | 3 (6.8) | 0 (0) |
| Thrombocytopenia | 20 (22.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Voice alteration | 0 (0) | 0 (0) | 0 (0) | 6 (13.6) | 0 (0) | 0 (0) |
| Vomiting | 5 (11.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Xerostomia | 1 (2.3) | 0 (0) | 0 (0) | 42 (95.4) | 1 (2.3) | 0 (0) |
Three patients (7%) had gastrostomy-tubes placed prophylactically within the first week of chemoradiation due to concerns regarding baseline alimentary status. An additional 3 patients (7%) required placement of a gastrostomy-tube during chemoradiation due to high-grade dysphagia and weight loss. Severe late effects related to radiation were uncommon with the incidence of grade 3+ late toxicity being 5% (2 of 44 patients). Esophageal stricture, stenosis, or aspiration pneumonia was not observed in the post-treatment setting. Only 1 patient (2%) was gastrostomy-tube dependent at 3-months post-treatment. The 2-year freedom from grade 3+ mucosal-esophageal toxicity, reported as post hoc analysis, was 85% and 86% for patients receiving 54 Gy and 60 Gy, respectively (p=0.47). The incidence of gastrostomy-tube dependence at 6 months was 0%. There were two observed cases of second-malignancy (prostate adenocarcinoma and cutaneous squamous cell carcinoma of the cheek), neither of which were considered to be related to protocol therapy.
Discussion
Our findings that patients with HPV-associated oropharyngeal squamous cell carcinoma can successfully be treated with reduced doses of radiation have the potential to alter practice paradigms for a disease in which treatment-related side effects have historically been unacceptably high. Although it has been long hypothesized that these cancers are more sensitive to both therapeutic irradiation as well as cytotoxic chemotherapy and could possibly be treated effectively with de-intensified chemoradiotherapy regimens, prospective data on how to do so have been lacking. Given the epidemic-like increase in the incidence of this disease worldwide, ongoing studies attempting to refine care for these patients are urgently needed. Current estimates are that approximately 60 to 70% of oropharyngeal carcinoma cases are attributable to HPV infection in developed countries and that the incidence continues to increase [20]. The recognition that the majority of HPV-associated cases occur in patients who are younger, healthier, and never-smoking compared to their HPV-negative counterparts also has significant implications regarding survivorship [1].
Currently used chemoradiotherapy regimens have been associated with significant toxicity. Given that the dose-limiting toxicity from prior chemoradiotherapy trials have been mucosal and esophageal, reducing the radiation dose in selected patients with more favorable biology (i.e. HPV-positive tumors) has the potential to improve the tolerability of treatment while preserving long-term function. As relevantly, data has demonstrated consistent dose-response relationships predicting toxicity for organs involved in salivary production, swallowing, and mucosal integrity, among others. For instance, normal tissue complication probability models have observed that for every 1 Gy in mean dose to the parotid gland, the likelihood of xerostomia increases by approximately 5% at 1 year after radiation therapy [21]. Data has similarly shown that dose to the pharyngeal constrictor muscles, larynx, and cricopharyngeal inlet affects the probability of late dysphagia, including gastrostomy-tube dependence, with a volume-dependent threshold for radiation-induced toxicity existing at approximately 55 to 60 Gy [22, 23]. Additionally, studies using patient-reported outcomes have demonstrated associations between dose and decreased quality of life and survivors of head and neck cancer [24].
Correlative biomarker studies have so convincingly established HPV status as the single most important predictor of outcome among patients treated for oropharyngeal carcinoma that HPV staining, typically assessed through the surrogate marker p16, is now routinely performed. In an analysis of 433 patients with oropharyngeal carcinoma treated by chemoradiotherapy to the standard dose of 70 Gy, the Radiation Therapy Oncology Group (RTOG) confirmed dramatic differences in 3-year rates of local-regional control (86% vs. 65%) and overall survival (82% vs. 57%) based on HPV status [4]. Interestingly, the risk of distant metastasis for HPV-positive and HPV-negative tumors was observed to be similar, affecting 10% and 13% of patients, respectively. Others have similarly shown that the HPV epidemic has resulted in a shift of disease failure patterns among patients treated such that the predominant mode of relapse is now distant [25]. These observations raise the question of whether a sequential treatment strategy utilizing induction chemotherapy to potentially address micrometastasis prior to chemoradiotherapy for patients with HPV-positive oropharyngeal carcinoma may be warranted. It is worthwhile to note that in the present study, only 1 case of distant metastasis was reported after treatment despite the inclusion of patients who might be deemed “high-risk,” based on T-stage and smoking history, using classification schemes proposed by others. Indeed, 23% and 25% of our subjects, respectively, presented with T3-T4 tumors and heavy (>10 pack-year) smoking histories.
While HPV staining is currently used only for prognostication purposes, data such as ours has the potential to individualize treatment for patients with newly diagnosed HPV-positive head and neck squamous cell carcinoma. Most importantly, the observed survival statistics compare favorably to historical controls of HPV-associated oropharyngeal carcinoma patients treated by chemoradiotherapy to the standard dose of 70 Gy. The most direct comparison is with ECOG 2399 who used the exact same chemoradiotherapy regimen (except to a dose of 70 Gy) showing 2-year overall survival and 2-year progression-free survival of 95% and 86%, respectively-- numbers nearly identical to those of our cohort treated to reduced doses of approximately 10 to 15% less [6]. Notably, the incidence of grade 3+ dysphagia and mucositis was 54% and 53% in comparison to the 9% and 9%, respectively, observed in the current trial. A markedly lower rate of gastrostomy tube placement was also seen with the de-intensification regimen compared to the historical ECOG control (7% versus 26%). In a subset analysis of 2 previously published prospective trials of chemoradiotherapy for locally advanced oropharyngeal cancer, the incidence of grade 3+ mucositis and 6-month gastrostomy tube-dependence was 56% and 17%, respectively, compared to 9% and 0% on the present study [26]. Although direct comparisons are potentially confounded by differences in patient and disease characteristics, it is quite apparent that the observed rates of late toxicity among patients treated by reduced dose radiation therapy in the current trial have been markedly lowered compared to historical controls (appendix, page 1).
The mechanism of HPV-mediated radioresponse is currently unclear, and there are limited studies that have addressed this topic in the setting of head and neck cancer. The most direct explanation is that HPV infection and the subsequent degradation of the p53 and retinoblastoma proteins by the viral products E6 and E7 somehow renders the host tumor cell more radiosensitive by interfering with such mechanisms as DNA repair, repopulation signaling, and cell cycle redistribution [27]. There is increasing evidence, however, attesting to the importance of the microenvironment in HPV-mediated radiation response. Indeed, several recent studies have suggested that radiation therapy enhances the host immune response to viral antigens, which are expressed on tumor [7, 8]. A higher number of tumor infiltrating lymphocytes and circulating white blood cells have been shown to be of favorable prognostic significance among HPV-positive head and neck cancer, suggesting that the adaptive immune system contributes to the suppression of tumor progression [28,29].
The optimal means of treatment de-intensification continues to be investigated for this population. Similar to the current trial, the ECOG completed a single-arm study of dose de-escalation for HPV-positive oropharyngeal cancer patients with a strategy of induction chemotherapy followed by reduced-dose radiation with cetuximab reserved for complete responders based on PET metrics [30]. Our trial, by contrast, de-intensified radiation dose for patients using response-adapted CT-based RECIST criteria without observing an excess of local-regional failures. Another important difference was that the present study was designed to ensure that all patients received lower doses of radiation with the degree of reduction determined by initial response to 2 cycles of induction chemotherapy. The rationale for this seemingly aggressive de-escalation strategy stemmed from pre-clinical data suggesting that even in the absence of selecting responders using induction chemotherapy, HPV-positive oropharyngeal carcinomas are exquisitely radiosensitive and characterized by robust and dramatically rapid responses to therapy [31,32]. For instance, Chera et al published the results of a phase II study of patients with HPV-positive oropharyngeal carcinoma treated by concurrent chemoradiotherapy to 60 Gy and reported an 86% pathological response rate based on post-treatment biopsy and neck dissection [33]. Based on data suggesting that radiation without chemotherapy may even be an appropriate strategy for HPV-positive oropharyngeal carcinoma, it seems that this approach may be a reasonable one to investigate in subsequent follow-up trials and may lead to further refinements in de-escalation strategies [34,35].
While acknowledging that the relatively small sample size of this single arm study leads to inherent challenges in drawing definitive conclusions and performing subset analysis, our findings demonstrating the feasibility of de-intensified radiation for HPV-associated oropharyngeal cancer nonetheless provides evidence that such a strategy warrants continued investigation. The heterogeneity of the study population with respect to eligibility criteria also has the potential to serve as a confounding factor. Others, for example, have suggested that such factors as smoking history, response to induction chemotherapy, and advanced T- and N-classification may be of prognostic significance [4,25,36]. This study was also not meant to comment on the acceptability of induction chemotherapy for oropharyngeal cancer but to use this approach as a possible means of selecting patients who might theoretically benefit the most from a dose de-intensified regimen.
Additional limitations relate to the lack of central review of 16 specimens. While pathologists generally score tumors as positive on the basis of the current standard of strong and diffuse nuclear and cytoplasmic staining in greater than 70% of the tumor, inter-observer variability in the analysis and interpretation of p16 status is possible. Although p16 expression has been shown to be a reliable surrogate for tumor HPV status with high concordance, it is not 100% accurate. Given that the false positive rate has been demonstrated to range from 2 to 7%, the possibility of inadvertently and unknowingly including patients with HPV-negative disease in the study needs to be considered [37,38]. Notably, it has been demonstrated that such patients, despite being p16 positive, do not retain the favorable prognostic benefits associated with those who are truly HPV-positive [39].
Lastly, it must be recognized that this trial was designed and/or initiated before the widespread adoption of risk classification schemes for HPV-positive oropharyngeal cancer proposed by Ang et al and O’Sullivan et al [4,25]. More recently, the International Collaboration on Oropharyngeal Cancer Network for Staging (ICON-S) developed a new classification system which has been both internally and externally validated [40–42]. The inclusion of HPV-positive patients with varying risk factors might lead to difficulty in identifying with certainty which subset might be best suited for this de-intensification approach. Indeed, on post hoc analysis, the proportion of patients with ICON-S Stage I, II, and II disease was 61%, 21%, and 18%, respectively, in our study.
The results of our prospective trial, representing one of the first to our knowledge to report on actual clinical endpoints among patients treated by reduced dose radiation for HPV-associated oropharyngeal carcinoma, hold considerable promise for a disease which is increasing in incidence. Our findings provide reassurance to patients participating in ongoing efforts of de-escalation by others. By decreasing toxicity while maintaining high rates of disease control and overall survival, we illustrate how de-intensification regimens have the potential to usher in a new standard of care for a disease in which the radiation dose has largely been the same for upwards of 50 years. A phase III study further investigating the efficacy of the de-escalation regimen used herein is currently being planned.
Acknowledgments
Funding: Supported by the Biostatistics Shared Resource of the University of California, Davis Comprehensive Cancer Center Support Grant, P30CA093373–11.
Footnotes
Declaration of interests: The authors declared no conflicts of interest
The authors indicate no disclosure of potential conflicts of interest.
References
- 1.Gillison ML, D’Souza G, Westra WH, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100: 407–420. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24: 736–747. [DOI] [PubMed] [Google Scholar]
- 3.Klussmann JP, Mooren JJ, Lehnen M, et al. Genetic signatures of HPV-related and unrelated oropharygneal carcinoma and their prognostic implications. Clin Cancer Res 2009; 15: 1179–1786. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassen P, Eriksen JG, Hamilton S, et al. Effect of HPV-associated p16 expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27: 1992–1998. [DOI] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008; 100: 261–269. [DOI] [PubMed] [Google Scholar]
- 7.Wansom D, Light E, Worden F, et al. Correlation of cellular immunity with human papillomavirus, p16 status, and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg 2010; 136: 1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg 2009; 135: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 9.Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res 2015; 21: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32: 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: Results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol 2007; 25: 3971–3977. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, (editors). American Joint Committee on Cancer staging manual, 7th edition France: Springer; 2010. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf. Updated June 14, 2010. Accessed January 3, 2016.
- 15.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11, 570–9 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Hassan SJ, Weymuller EA. Assessment of quality of life in head and neck cancer patients. Head Neck 1993; 15: 485–496. [DOI] [PubMed] [Google Scholar]
- 17.Duffy DE, Santner TJ. Confidence intervals for a binomial parameter based on multistage tests. Biometrics 1987; 43: 81–93. [Google Scholar]
- 18.Simon R Optimal two-stage designs for phase II clinical trials, Controlled Clinical Trials, 1989; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 547–581. [Google Scholar]
- 20.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013; 31: 4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 2010; 76(S): 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2010; 76: 403–409. [DOI] [PubMed] [Google Scholar]
- 23.Feng FY, Kim HM, Lyden, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007; 68: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 24.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008; 26: 3770–3776. [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metatasis. J Clin Oncol 2013; 31: 543–550. [DOI] [PubMed] [Google Scholar]
- 26.Dobrosotskaya IY, Bellile E, Spector ME, et al. Weekly chemotherapy with radiation versus high-dose cisplatin with radiation as organ preservation for patients with HPV-positive and HPV-negative locally advanced squamous cell carcinoma of the oropharynx. Head Neck 2014; 36: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dok R, Kalev P, Van Limbergen EJ, et al. P16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res 2014; 74: 1739–1751. [DOI] [PubMed] [Google Scholar]
- 28.Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer 2015; 121: 545–555. [DOI] [PubMed] [Google Scholar]
- 29.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer 2014; 110: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marur S, Li S, Cmelak AJ, et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN Cancer Research Group. J Clin Oncol 2016; 34: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen AM, Li J, Beckett LA, et al. Differential response rates to irradiation among patients with human papillomavirus positive and negative oropharyngeal cancer. Laryngoscope 2013; 123: 152–157. [DOI] [PubMed] [Google Scholar]
- 32.Gupta AK, Lee JH, Wilke WW, et al. Radiation response in two infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys 2009; 74: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chera BS, Amdur RJ, Tepper J, et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2015; 93: 976–985. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan B, Huang SH, Perez-Ordonez B, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol 2012; 103: 49–56. [DOI] [PubMed] [Google Scholar]
- 35.Chen AM, Zahra T, Daly ME, et al. Definitive radiation therapy without chemotherapy for human papiilomavirus-positive head and neck cancer. Head Neck 2013; 35: 1652–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Won HS, Lee YS, Jeon EK, et al. Clinical outcome of induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Anticancer Res 2014; 34: 5709–5714. [PubMed] [Google Scholar]
- 37.Holmes BJ, Maleki Z, Westra WH. The fidelity of p16 staining as a surrogate marker of human papillomavirus status in fine-needle aspirates and core biopsies of neck node metastases: implications for HPV testing protocols. Acta Cytologica 2015; 59: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol 2012; 36: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rietbergen NM, Brakenhoff RH, Bloemena E, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment de-escalation trials. Ann Oncol 2013; 24: 2740–2745. [DOI] [PubMed] [Google Scholar]
- 40.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol 2016; 17: 440–451. [DOI] [PubMed] [Google Scholar]
- 41.Husain ZA, Chen T, Corso CD, et al. A comparison of prognostic ability of staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. JAMA Oncology 2016; E1–E8 [DOI] [PubMed] [Google Scholar]
- 42.Dahlstrom KR, Garden AS, William WN, et al. Proposed staging system for patients with HPV-related oropharyngeal cancer based on nasopharyngeal cancer N categories. J Clin Oncol 2016; 16: 1848–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]


