Abstract
Background
Numerous pathologies of pregnancy originate from placental dysfunction. It is essential to understand the functions of key genes in the placenta in order to discern the etiology of placental pathologies. A paucity of animal models that allow conditional and inducible expression of a target gene in the placenta is a major limitation for studying placental development and function.
Results
To study the platelet‐derived growth factor receptor alpha (PDGFRα)‐directed and tamoxifen‐induced Cre recombinase expression in the placenta, PDGFRα‐CreER mice were crossed with mT/mG dual‐fluorescent reporter mice. The expression of endogenous membrane‐localized enhanced green fluorescent protein (mEGFP) and/or dTomato in the placenta was examined to identify PDGFRα promoter‐directed Cre expression. Pregnant PDGFRα‐CreER;mT/mG mice were treated with tamoxifen at various gestational ages. Upon tamoxifen treatment, reporter protein mEGFP was observed in the junctional zone (JZ) and chorionic plate (CP). Furthermore, a single dose of tamoxifen was sufficient to induce the recombination.
Conclusions
PDGFRα‐CreER expression is restricted to the JZ and CP of mouse placentas. PDGFRα‐CreER mice provide a useful tool to conditionally knock out or overexpress a target gene in these regions of the mouse placenta.
Keywords: inducible, recombination, tamoxifen, transgenic, trophoblast cells
Key Findings
Inducible PDGFRα‐directed Cre expression trophoblasts cells.
A single tamoxifen treatment is sufficient to induce the recombination.
Valuable tool to temporary knockout or over‐express a target gene in the placenta.
Do not require sophisticated system and suitable for ordinary laboratory setting.
Abbreviations
- CP
chorionic plate
- CreER
tamoxifen‐dependent Cre recombinase
- DB
decidua basalis
- E
embryonic day
- Epcam
epithelial cell adhesion molecule
- IF
immunofluorescence
- JZ
junctional zone
- La
labyrinth
- mEGFP
membrane‐enhanced green fluorescent protein
- mT
dTomato
- PDGFRα
platelet‐derived growth factor receptor alpha
1. INTRODUCTION
The placenta is a transient and autonomous organ, playing an essential role in fetal growth. The placenta provides a sole interface for nutrient and gas exchange between the fetus and the mother. Many other functions have been identified in placentas. For example, the placenta secretes hormones into maternal circulation, which regulate maternal metabolic and immune adaptations to pregnancy. Abnormality in placental development leads to pregnancy complications that affect not only fetal development but also maternal.1, 2 Therefore, elucidating the underlying mechanisms of placental development and its functions is essential for the prevention and treatment of placenta‐originated complications. Although genetic research has substantially improved our understanding of placental development, it is still limited by a lack of appropriate tools for gene manipulation in the placenta.3
Through conventional transgenic and gene‐targeted approaches, systemic gene manipulation has identified the functions of myriad genes. However, global manipulation of many genes results in embryonic lethality, precluding the postnatal study of these genes.4 In addition, the complex interaction between organs makes the identification of the key organ expressing the gene of interest challenging. Thus, methods for conditional tissue‐specific gene targeting have been developed, among which the most widely used is the Cre/loxP recombination.5 Cre recombinase, under the direction of a tissue‐specific promoter, catalyzes a directional DNA recombination between two loxP sites, allowing a tissue‐specific gain‐ or loss‐of function of the targeted gene. However, many genes are expressed in a stage‐specific and tissue‐specific manner, especially during development. Therefore, to temporally manipulate target gene expression, the inducible Cre/loxP system has been developed by adding exogenous ligand‐dependent Cre expression mechanisms. Among the inducible Cre/loxP systems, the tamoxifen‐dependent Cre recombinase (CreER) is the most successful and widely used, in which Cre expression construct was fused to the human estrogen receptor ligand‐binding domain.6
Using the promoter from a specific gene or a chimeric DNA construct to direct Cre expression in specific cells is the key component of the Cre/loxP system. Although several promoters have been used to target transgene expression in trophoblast lineages,7, 8, 9, 10, 11, 12, 13 to our knowledge very few studies have been conducted using inducible Cre/loxP specifically for placental research.14, 15 Furthermore, in both of these models, broadly expressed Cre transgenic animals were utilized, complicating the interpretation of the results.
Platelet‐derived growth factor receptor alpha (Pdgfrα) gene encodes a cell surface receptor tyrosine kinase,16 which is expressed in a tissue‐specific manner.17 In mouse embryogenesis, Pdgfrα is expressed in primitive mesoderm and endoderm precursors. At embryonic day (E) 6.5, the transcript was detected in mesenchyme precursors (somites, branchial arches, and limb bud), as well as in visceral endoderm.18 It is also expressed in both premigratory and migratory neural crest cell populations and is required for neural crest migration and palatogenesis.19 Interstitial cells of kidney have also been shown to express pdgfrα at E17.5 in mouse20 and in developing human kidney.21
Pdgfrα was first reported to be expressed in the mouse placenta by Ogura et al.22 They showed its expression in a primitive endoderm‐derived structure: the intraplacental yolk sac, which contributed to the chorioallantoic placenta and continued to strongly express PDGFRα until prenatal stages. In addition, intraplacental yolk sac was absent from mutant embryos not expressing pdgfrα, highlighting the primary role of this gene for the formation of this structure.22 In addition to its highest expression level23 and developmental role in mouse placenta22, 24; Pdgfrα may regulate trophoblast proliferation in human placenta.25 Therefore, we hypothesized that the PDGFRα promoter could direct Cre recombinase expression in the placenta. We anticipated that the expression profile of the PDGFRα‐Cre construct might differ from endogenous Pdgfrα gene described above; as in the absence of targeted knock‐in, the incorporation of a transgene into genomic DNA is random.26 Despite having the same promoter DNA, it is possible to observe differences in expression profiles between the endogenous gene and the gene directed by a manmade construct. Therefore, we undertook a careful characterization of the expression of Cre in the placenta of these transgenic mice.
The PDGFRα‐CreER transgenic mouse was first developed to study oligodendrocyte precursor cells in the forebrain and spinal cord of adult mice.27 The mT/mG mice are well characterized and commonly used as a dual‐fluorescent reporter mouse model, in which a membrane‐localized red fluorescent protein (dTomato [mT]) is constitutively expressed prior to Cre recombinase exposure.28 Expression of Cre recombinase permanently inactivates dTomato while activating a membrane‐localized enhanced green fluorescent protein (mEGFP), thus allowing the identification of all Cre‐expressing cells and their daughters. We crossed PDGFRα‐CreER and mT/mG mice to verify and characterize PDGFRα promoter‐directed Cre recombinase expression and activity in the placenta.
Our results showed that the reporter mEGFP was expressed at E12.5 in trophoblast cells in the junctional zone (JZ) and at E18.5 in the JZ and the chorionic plate (CP) after tamoxifen induction. However, tamoxifen‐induced recombination was also detected in fetal bones. Our study indicates that, despite some expression in fetal cells, the PDGFRα‐CreER mice can be a useful tool to temporally manipulate target gene(s) in the placenta.
2. RESULTS
2.1. Creation of the PDGFRα‐CreER;mT/mG mice
The line of PDGFRα‐CreER mice was created by coupling the mouse PDGFRα promoter‐directed Cre with mutated human estrogen receptor ligand‐binding domain.29, 30 The mT/mG mice are dual‐fluorescent reporter transgenic mice possessing loxP sites on either side of a membrane‐targeted mT tandem dimer followed by a strong stop codon. Prior to the Cre‐mediated excision, almost all cells express a membrane‐targeted red fluorescence. Following Cre‐mediated excision, the mT cassette is removed along with the stop codon, allowing the expression of the mEGFP driven by the CAG promoter (Figure 1A). We crossed PDGFRα‐CreER mice with mT/mG mice and produced transgenic mice with hemizygous PDGFR‐CreER allele and homozygous mT/mG (named as PDGFRα‐CreER Tg;mT/mGfl/fl). The resulting offspring are viable, with no significant alteration between the control and transgenic fetuses regarding the litter size (6.67 ± 0.33 vs 7.66 ± 0.88; P = 0.35), E18.5 placental weight (0.149 ± 0.01 vs 0.143 ± 0.01; P = 0.50), and E18.5 fetal weight (0.99 ± 0.04 vs 1.06 ± 0.07; P = 0.42).
Figure 1.
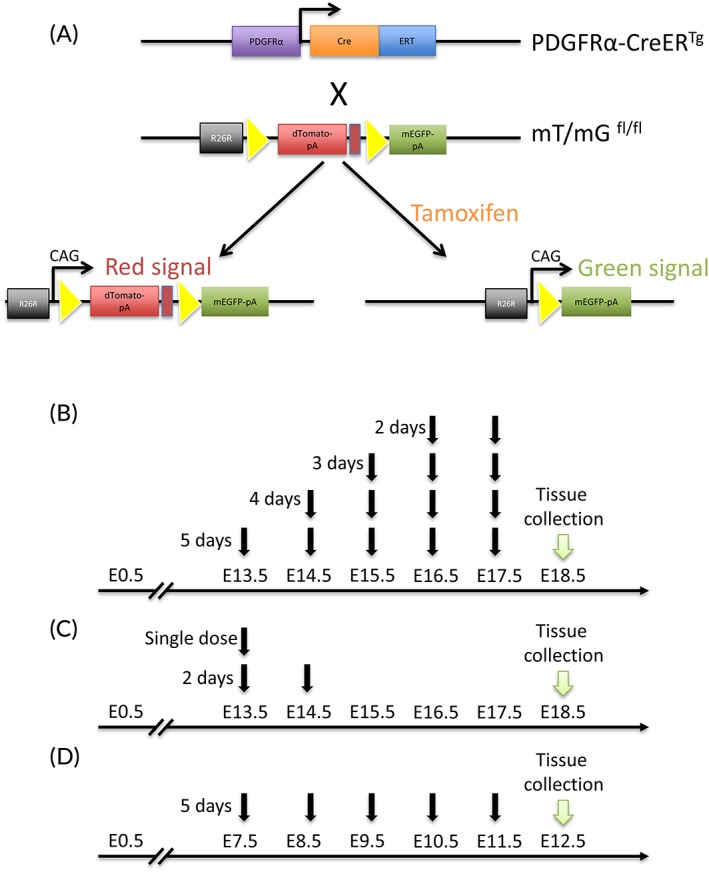
Transgenic animals design and tamoxifen treatments. A: The PDGFRα‐CreER mice were crossed with mT/mG reporter mice to produce PDGFRα‐CreER;mT/mG mice. Tamoxifen treatment induces Cre recombinase expression, then the dTomato transgene is deleted and allows the expression of the mEGFP. B: Pregnant mice were orally fed with tamoxifen (2 mg in 100 μL sunflower oil) for five, four, three, or two consecutive days starting at E13.5 and placentas collected at E18.5. C: Tamoxifen was administrated to transgenic mice at E13.5 and E14.5. Placentas were harvested at 18.5. D: A five‐day tamoxifen treatment was administrated to pregnant mice from E7.5 to E11.5; tissues were collected at E12.5. CreER, tamoxifen‐dependent Cre recombinase; E, embryonic day; mEGFP, membrane‐localized enhanced green fluorescent protein; PDGFRα, platelet‐derived growth factor receptor alpha
2.2. Tamoxifen‐induced mEGFP expression in the placentas of PDGFRα‐CreER Tg;mT/mGfl/fl mice
PDGFRα‐CreER Tg ;mT/mGfl/fl male breeders were mated with PDGFRα‐CreER Tg ;mT/mGfl/fl females. To assess the ability of the CreER to induce specific recombination in the placenta, pregnant PDGFRα‐CreER Tg ;mT/mGfl/fl dams were gavaged daily with tamoxifen for five consecutive days from E13.5 to E17.5, then placentas were collected at E18.5 (Figure 1B). mEGFP signals were observed in trophoblast cells in the placental JZ (Figure 2A, A1), as well as from the CP (Figure 2A,A2). In the control littermates with PDGFRα‐CreER − ;mT/mGfl/fl genotype, no mEGFP signal was observed in the placentas (Figure 2C). As expected, no mEGFP signal was detected in placentas of PDGFRα‐CreE Tg ;mT/mGfl/fl mice, which received vehicle treatment (Figure 2B). Interestingly, no mEGFP signal was seen in the labyrinth (La) layer. These results indicated that PDGFRα promoter‐directed Cre‐recombinase is expressed in the trophoblast cells in the JZ and CP only.
Figure 2.
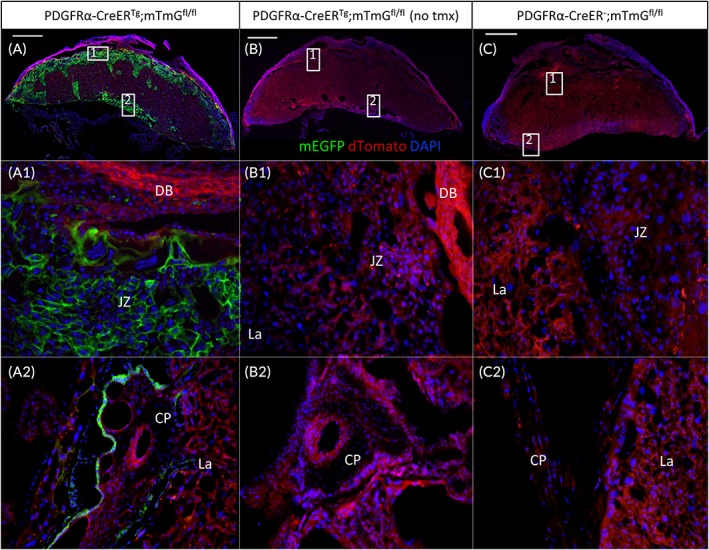
Tamoxifen‐induced Cre recombinase expression in mouse placentas. Placentas were collected at E18.5 after 5 consecutive days tamoxifen gavaging of pregnant PDGFRα‐CreER;mT/mG mice. Tissue were cryosectioned and stained with DAPI. Endogenous mEGFP signals were observed in trophoblast cells in the JZ and the CP (A,A1,A2). Only red dTomato fluorescent protein was observed in placenta without tamoxifen treatment (B,B1,B2) and fetuses without PDGFRα‐CreER transgene (C). Scale bar = 1 mm for top panel; 20X for two bottom panels. CP, chorionic plate; DB, decidua basalis; JZ, junctional zone; La, labyrinth; mEGFP, membrane‐enhanced green fluorescent protein; CreER, tamoxifen‐dependent Cre recombinase; E, embryonic day; PDGFRα, platelet‐derived growth factor receptor alpha
2.3. mEGFP colocalized with Tpbpα and Epcam
Since we detected mEGFP expression in the JZ, we assumed that the cells targeted by the PDGFRα promoter were spongiotrophoblast cells. To confirm this hypothesis, immunofluorescence (IF) was conducted using an antibody against trophoblast specific protein alpha (Tpbpα), a gene that is used to identify spongiotrophoblast and glycogen trophoblast in the JZ.13, 31 As expected, Tpbpα protein was observed only in the cells of JZ (Figure 3A). The areas of mEGFP and Tpbpα expression in the JZ were overlapped (Figure 3A1,A2), whereas the CP did not show Tpbpα‐positive cells (Fig. 3A3). These results confirmed that the cells tagged by PDGFRα promoter‐directed mEGFP in the JZ are spongiotrophoblast and/or glycogen trophoblast.
Figure 3.
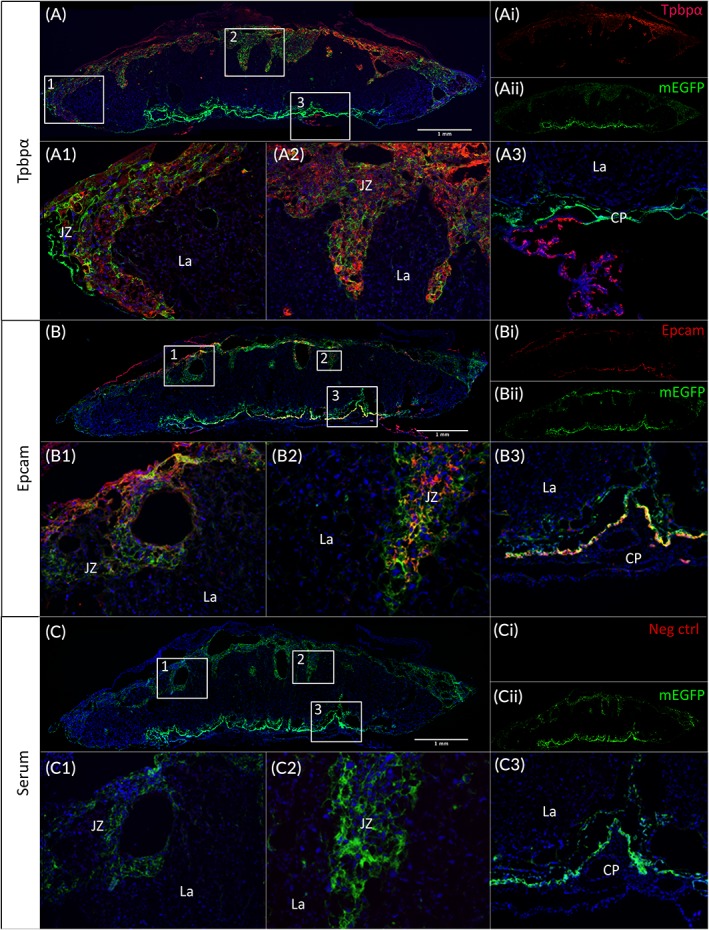
Colocalization of mEGFP and Tpbpα or Epcam protein in trophoblast cells. Placentas were collected at E18.5 after five consecutive days tamoxifen gavaging of pregnant PDGFRα‐CreER;mTmG mice, and then cryosectioned and stained. A: Tpbpα IF showed a strong signal overlapping with the mEGFP signal within the JZ (A1,A2,A3) of the placentas. B: Epcam signal is seen from the JZ as well (B1,B2), but also overlapped part of the mEGFP‐tagged cells in the CP (B3). No Tpbpα or Epcam signal is detected from the negative control (C1,C2,C3). A1,A2,A3,B1,B3,C1,C3: 10X high‐power view of insets in the upper panel. B2,C2: 20X magnification. CP, chorionic plate; CreER, tamoxifen‐dependent Cre recombinase; E, embryonic day; Epcam, epithelial cell adhesion molecule; IF, immunofluorescence; JZ, junctional zone; La, labyrinth; mEGFP, membrane‐enhanced green fluorescent protein; PDGFRα, platelet‐derived growth factor receptor alpha; Tpbpα, trophoblast specific protein alpha
The CP is part of the chorioamniotic membranes containing amniotic epithelial cells and mesenchymal cells.32 As a marker of epithelial cells, Epithelial cell adhesion molecule (Epcam) was used to target the CP cells. Figure 3B shows that part of the mEGFP‐tagged cells overlapped with the Epcam‐positive cells in the CP (Figure 3B3), as well as in the JZ (Figure 3B1,B2). No signal for either Epcam or Tpbpα was seen in the negative control (Figure 3C).
2.4. Effect of tamoxifen treatment duration on Cre expression
Tamoxifen is an estrogen receptor modulator, and its metabolites interact with the growing fetus. It has been reported that this molecule can be teratogenic and disrupt the maternal‐fetal interface,33 leading to adverse effects on the fetus in both animals34 and humans.35 Although a five‐day treatment is conventionally used in inducible Cre‐recombination systems, we assessed the ability of a shorter treatment to induce the recombination in order to reduce the adverse effects of tamoxifen on pregnancy and fetus. We then decreased the treatment duration in a stepwise manner, from five to two days (Figure 1B). Interestingly, the pattern of expression of mEGFP was the same, independent of the length of the tamoxifen treatment (Figure 4), with a signal present in the JZ and the CP. However, although the mEGFP signal intensity appeared similar in the CP, we did observe a decrease in the JZ signal that was proportional to shorter treatment times (Figure 4A–D).
Figure 4.
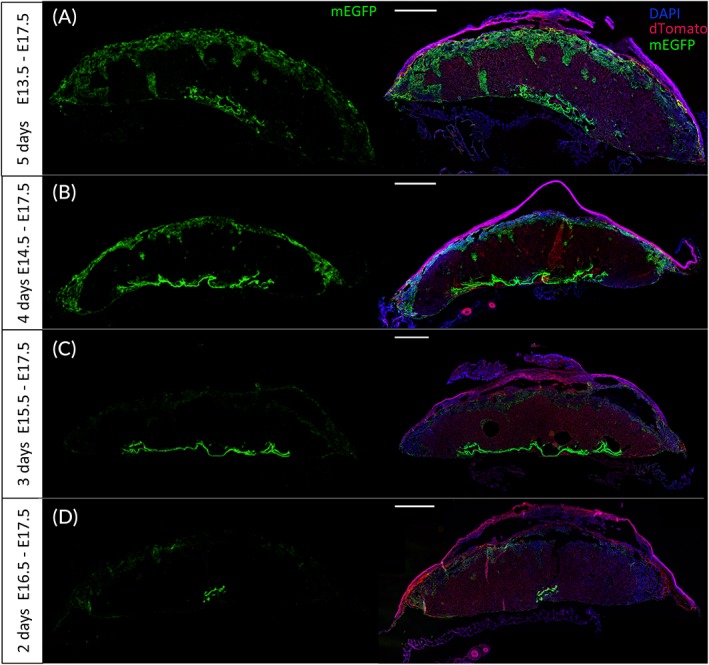
Effect of tamoxifen treatment duration on transgene expression. PDGFRα‐CreER Tg;mTmGfl/fl placentas were collected at E18.5 after five (A), four (B), three (C), or two (D) consecutive days of tamoxifen gavaging. Endogenous mEGFP and mTomato proteins were visualized. Left panels: mEGFP only; right panels: mEGFP + dTomato + DAPI. CreER, tamoxifen‐dependent Cre recombinase; E, embryonic day; mEGFP, membrane‐enhanced green fluorescent protein; mT, dTomato; PDGFRα, platelet‐derived growth factor receptor alpha. Scale bar = 1 mm
2.5. Stability of Cre expression and induction of EGFP at mid‐gestation
In the first treatment (Figure 1B), one day separated the last dose of tamoxifen from the tissue collection date (from E17.5 to E18.5), thereby not allowing us to estimate the efficiency of the recombination. Therefore, we next assessed if the recombination occurred temporarily or was stable within the tissue, through a single oral dose of tamoxifen at E13.5 or doses at E13.5 and E14.5 followed by the consecutive days without treatment until E18.5 (Figure 1C). The single‐dose treatment revealed a clear membrane‐localized EGFP signal in both the JZ (Figure 5A,A1) and the CP (Figure 5A,A2). If the treatment was extended to a second dose the next day (E13.5 + E14.5), the same pattern of expression was observed (Figure 5B,B1,B2) but the mEGFP signal intensity in JZ appeared to be increased (Figure 5B1 vs Figure 5A1).
Figure 5.
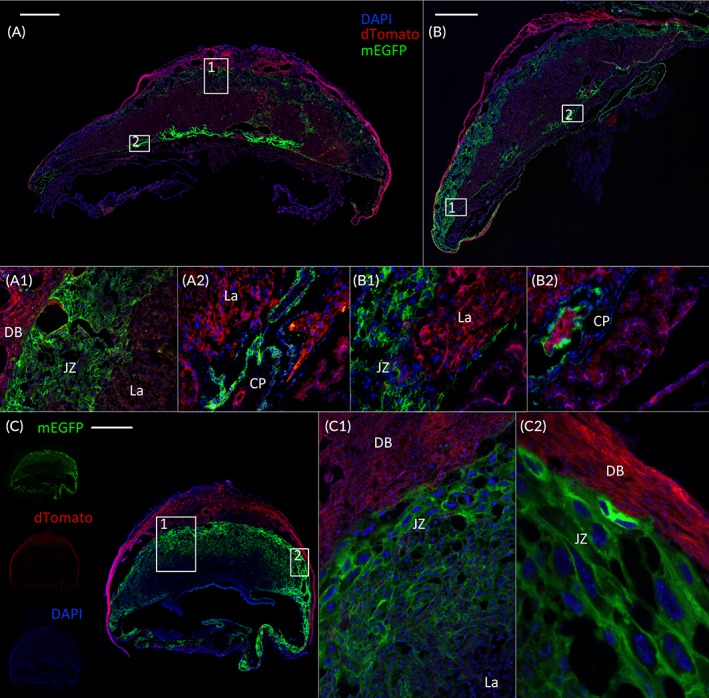
Single tamoxifen treatment during mid‐gestation induced Cre‐recombinase expression in placentas. Placentas were collected at E18.5 after a single dose of tamoxifen gavaging of pregnant PDGFRα‐CreER Tg;mTmGfl/fl mice at E13.5 (A) or E13.5/E14.5 (B), followed by consecutive days with no treatment before tissue collection. Tissue were then cryosectioned and stained with DAPI. mEGFP is detected in the JZ (A1,B1) as well as in the CP (B1,B2). C: E12.5 PDGFRα‐CreER Tg;mTmGfl/fl mice express the transgene in the JZ (C,C1,C2). A1,C1: 10X magnification. A2,B1,B2: 20X magnification. CP, chorionic plate; DB, decidua basalis; CreER, tamoxifen‐dependent Cre recombinase; E, embryonic day; JZ, junctional zone; La, labyrinth; PDGFRα, platelet‐derived growth factor receptor alpha. Scale bar = 1 mm
Finally, to assess if PDGFRα‐mediated Cre expression was inducible at earlier stages of pregnancy, we performed a tamoxifen treatment from E7.5 to E11.5 (Figure 1D). Figure 5C shows that this treatment exhibited a strong recombination, localized and restricted to the JZ of placentas collected at E12.5 from PDGFRα‐CreER Tg;mTmGfl/fl mice (Figure 5C,C1,C2).
2.6. PDGFRα‐directed Cre expression in fetal tissue
PDGFRα is known to be expressed in some cells during mouse embryogenesis.18 Its expression is particularly detected in the sclerotome (ie, at the origin of the formation of the vertebrae) and contributes to the development of the bones of the skull,36 as well as in tooth bud, developing lens, and choroid plexus.24
To investigate the PDGFRα promoter‐directed Cre expression in fetal tissue, median sagittal sections from E18.5 fetuses were analyzed. In PDGFRα‐CreER Tg;mTmGfl/fl fetuses, regardless of the length of tamoxifen treatment, mEGFP signal was observed only from the eye (Figure 6A1,B1,C1,D1) and bones (Figure 6A2,B2,C2,D2), corroborating PDGFRα gene expression during organogenesis.24 No recombination was detectable in fetuses from dams that received sunflower oil vehicle treatment (Figure 6E). As in the placenta, the mEGFP signal intensity seemed to decrease proportionally to the duration of tamoxifen treatment (Figure 6A–D).
Figure 6.
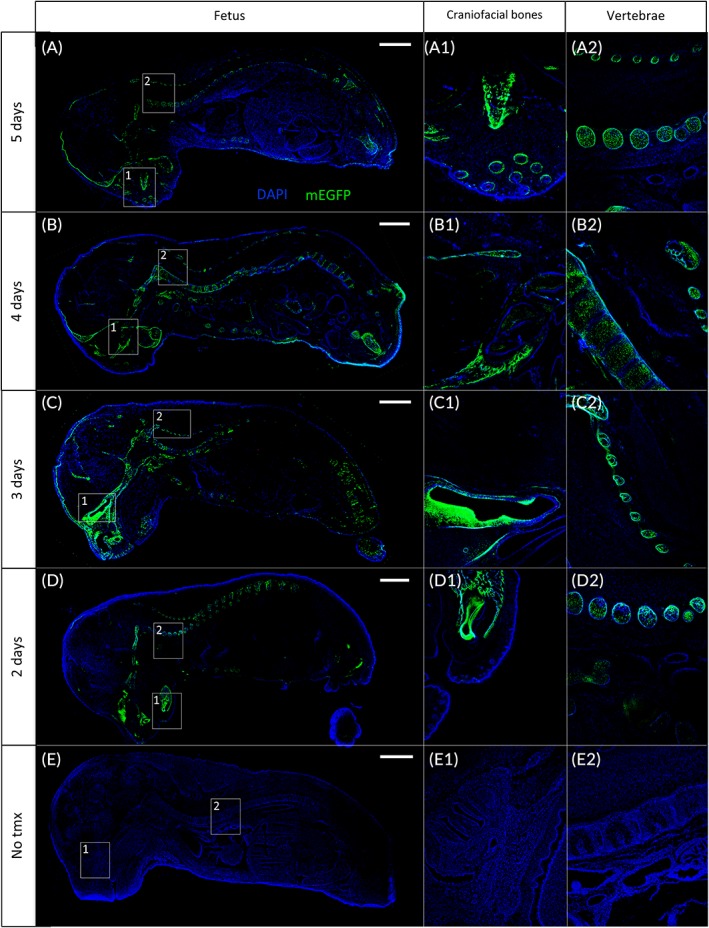
PDGFRα‐directed Cre recombinase expression in fetal tissues. PDGFRα‐CreER Tg;mTmGfl/fl fetuses were collected at E18.5 after five (A), four (B), three (C), or two (D) consecutive days of tamoxifen gavaging. Fetuses express mEGFP signal in craniofacial bones (A1,B1,C1,D1) and vertebrae (A2,B2,C2,D2). E: Absence of GFP expression in fetal tissue whether tamoxifen is not administrated, either in craniofacial bones (E1) or vertebrae (E2). The dTomato signal was removed intentionally to give a better visualization of the leaking mEGFP signal in fetal tissues (A,B,C,D) or the absence of mEGFP signal in the negative control (E). Scale bar, left panel = 2 mm. Craniofacial bones, vertebrae images: 4X magnification of insets in the left panel. CreER, tamoxifen‐dependent Cre recombinase; E, embryonic day; mEGFP, membrane‐localized enhanced green fluorescent protein; PDGFRα, platelet‐derived growth factor receptor alpha
3. DISCUSSION
Inducible conditional gene knockout or transgene animal model is a powerful tool in biomedical research. Unfortunately, this technique has not been able to be widely used in the research of placental biology due to the lack of appropriate transgenic models for these studies. In our efforts to establish an inducible placenta‐specific gene manipulation, we took advantage of previously created PDGFRα‐CreER mice due to high‐level PDGFRα expression in the placenta.23 By crossing mT/mG reporter mice with PDGFRα‐CreER mice, our study showed that tamoxifen treatment induced the target gene expression in the placenta. Although PDGFRα promoter–directed Cre recombinase expression can be detected in some fetal tissues, the PDGFRα‐CreER mice provide a useful tool to temporally manipulate target gene in trophoblast cells in JZ and CP for the studies that focus on placental development.
Genetic manipulation has been widely used to study target gene's function under both physiological and pathological conditions. Temporal and tissue‐specific transgene and gene deletion are especially important for research in dynamically developing organs and tissues. Unfortunately, the inducible Cre/LoxP recombination systems have not been used in placenta study due to a paucity of trophoblast‐specific promoter‐directed Cre transgenic tools. Alternatively, two other strategies have been developed for manipulating genes in the placenta: 1) cell‐based modulation of placental gene expression by intraplacental engraftment37 or chimerism,38 in which genetically modified cells are introduced into a placenta; and 2) viral vector‐mediated gene delivery using recombinant adenoviruses,39 retroviruses,40 or lentiviruses41, 42 for genetic modulation. However, these alternative approaches still cannot temporally control target gene expression. In addition, the requirement of sophisticated system and skills of these alternative approaches prevents their usage in an ordinary laboratory setting.
PDGFRα is highly expressed in the placenta.22 Our results showed that PDGFRα promoter directed Cre recombinase specifically in the trophoblast cells in JZ and CP. This finding was confirmed by the colocation (or overlap) of mEGFP reporter with Tpbpα, which is expressed in both spongiotrophoblast and glycogen trophoblast in the JZ.13, 31 More recently, La trophoblast progenitor cells, which are proliferative around mid‐gestation and differentiate into syncytiotrophoblast and sinusoidal trophoblast giant cells, have been identified in the CP.43 These cells support the development and growth of the La and express the cell adhesion protein Epcam.43 Given the pattern of mEGFP expression that we observed, and the fact that it was expressed in the region of the placenta where La trophoblast progenitors have been described, we examined co‐expression with Epcam to determine if mEGFP was expressed in these cells. Our results showed that Epcam overlapped with PDGFRα‐Cre activity in the CP. However, we did not observe mEGFP expression in trophoblast cells of the La following activation of Cre activity at any time beginning at E13.5. This result suggested that Epcam/mEGFP dual‐positive cells in the CP were not likely to be trophoblast progenitor cells. This conclusion was supported by the results of our experiments, in which PDGFRα‐Cre was activated between E7.5 and E11.5 and mEGFP was absent from the La at E12.5, clearly ruling out the possibility that PDGFRα‐Cre was active in Epcam‐positive trophoblast progenitors. Surprisingly, PDGFRα‐Cre activity was also absent from the CP at E12.5. We conclude, therefore, that in addition to the JZ, PDGFRα expression in the CP is primarily in the intraplacental yolk sac beginning at E13.5. In addition to expression in the intraplacental yolk sac, we also observed Epcam in the JZ at E18.5, which, to our knowledge, has not previously been reported.
Since mEGFP signal was not detected in the La layer, our results indicate that PDGFRα‐directed Cre is unable to induce recombination in the trophoblast cells of this layer. Therefore, PDGFRα‐CreER mice are not suitable to express Cre and manipulate target gene in La cells (ie, syncytiotrophoblasts and sinusoidal trophoblast giant cells). On the other hand, this model is of great benefit to studies focusing cells within the JZ and CP, which have previously relied on the use of Tpbpα‐Cre, the expression of which cannot be temporally controlled.13
Although PDGFRα‐directed Cre expression is abundant in the placenta, the recombination also occurred in the fetus, such as in craniofacial bones, vibrissae, and vertebrae (Figure 6). Therefore, caution should be taken for any study with an aim beyond placental development when using PDGFRα‐CreER mice.
Tamoxifen is known for adverse effects on placental/fetal development. Our model is not exempt from potential side effects. Therefore, tamoxifen treatment for control mice is recommended by doing littermates comparison using hemizygous PDGFRα‐CreER breeders. However, these side effects are not dependent on Cre expression and are a general shortfall of all models using tamoxifen to induce gene expression during pregnancy. Whereas not assessed specifically in our study, we did not observe any defects in placentation between tamoxifen‐treated and untreated pregnant mice; furthermore, we did not find any changes in placenta size, litter size, placenta weight, or fetal weight (see results, section 2.1 for values).
In summary, using the PDGFRα‐CreER Tg;mTmGfl/fl transgenic mice, our study demonstrated that Cre recombinase can be expressed in the trophoblast cells in JZ and CP under control of PDGFRα promoter and tamoxifen induction. Although a single tamoxifen treatment induced recombination in the placentas, demonstrating the high efficiency of this model, the longer the treatment, the stronger the signal (whether at E13.5‐E17.5 [Figures 1B and 4] or at E13.5‐E14.5 [Figures 1C and 5]). However, expression of Cre in fetal bone and other tissues must also be considered when interpreting the effect of placenta gene manipulation on fetal development. Together, our study indicates that PDGFRα‐CreER mice will be a valuable tool to temporally knock out or overexpress a target gene in the placenta and to study that gene's function in placental development.
4. EXPERIMENTAL PROCEDURES
4.1. Material
Tamoxifen was purchased from Sigma (#T5648, St Louis, MO). Rabbit anti‐Tpbpα (#ab104401) and rabbit anti‐Epcam (#ab71916) were both obtained from Abcam (Cambridge, MA). The goat anti‐rabbit Alexa Fluor 647 secondary antibody (#A21244) was purchased from Thermo Fisher Scientific. The ProLong Gold mounting medium (#P36935) was from Invitrogen (Carlsbad, CA). mT/mG and Cre primers and Reverse transcription polymerase chain reaction (RT‐PCR) probes were purchased from Integrated DNA Technologies (Coralville, IA).
4.2. Experimental animals
B6N.Cg‐Tg(pdgfrα‐cre/ERT)467Dbe/J mice (Jackson stock #018280) were cross‐mated with B6.129(Cg)‐Gt(ROSA)26Sor tm4(ACTB‐tdTomato,‐EGFP)Luo/J mice (Jackson stock #007676) reporter mice in order to generate PDGFRα‐CreER;mT/mG mice (Figure 1 A). Pregnancy was identified by the presence of a vaginal plug and was designated E0.5. Placentas and fetuses were collected via Caesarian section at E12.5 or E18.5. Animals were housed at 24°C with a 12‐hour light‐dark cycle (6 am/6 pm) with food and water provided ad libitum. Tail biopsies of mice were used to extract genomic DNA. Genotypes were determined by using regular PCR for Cre transgene or real‐time PCR protocols for mT/mG transgenes.24 All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health and carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the University of California San Diego Animal Care and Use Committee.
4.3. Tamoxifen induction of transgene expression
To induce expression of the Cre recombinase, pregnant PDGFRα‐CreER;mT/mG female mice were gavaged using a feeding cannula with 2 mg of tamoxifen (20 mg/mL in 100 μL sunflower oil per day). There were three treatments. The duration of the tamoxifen treatment was detailed in the Results section and figure legends. Briefly, treatment 1: daily gavaging for five, four, three, or two consecutive days and samples collected at E18.5 (Figure 1B). Treatment 2: gavaging tamoxifen for one or two consecutive days started at E13.5, then waited for four days and collected tissue at E18.5 (Figure 1C). Treatment 3: daily gavaging for five consecutive days from E7.5 to E11.5, then collected tissue at E12.5 (Figure 1D). Prior to tissue collection, mice were anesthetized with intraperitoneal injection of ketamine/xylazine and perfused intracardially with phosphate buffered saline (PBS).
4.4. Histology and immunofluorescence
Mouse placenta biopsy were fixed with 4% (vol./vol.) paraformaldehyde in PBS, cryopreserved in 10% sucrose, then 30%, and embedded in OCT compound (Tissue‐Tek). After cryosectioning samples, 4‐μm‐thick midsagittal frozen sections were rinsed in PBS for 10 minutes and slides were mounted in ProLong Gold containing DAPI and visualized by fluorescent optical microscopy (Nikon, Eclipse E800). For IF, cryosections were permeabilized in 0.1% phosphate buffered saline with Tween 20 (PBST) for five minutes and overlaid with 1% sodium dodecyl sulfate for five minutes to induce antigen retrieval. They were further blocked with 2% (vol./vol.) normal goat serum with 1% (wt./vol.) bovine serum albumin in PBS for two hours in a humid chamber at room temperature. Sections were then incubated with anti‐rabbit Tpbpα (1/500) or anti‐rabbit Epcam (1/100) primary antibody diluted in 2% goat serum/PBS or goat serum only (negative control) overnight at 4°C. After rinsing the slides three times for five minutes each in PBST, samples were overlaid with goat anti‐rabbit Alexa Fluor 647 secondary antibody (1/500 and 1/200 for Tpbpα and Epcam, respectively) for two hours at RT. After washing, sections were mounted in ProLong Gold/DAPI and visualized by fluorescent optical microscopy (Nikon, Eclipse E800).
4.5. Statistical analyses
Data are expressed as a mean ± SE of the mean (SEM). Statistical analyses were performed using the Student t‐test. Differences were considered significant at P < 0.05.
CONFLICT OF INTEREST
The authors wish to declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
J.S., J.‐S.W., and D.R.C.N. designed the study. J.‐S.W., S.L., and L.Q. contributed to data acquisition and analysis. J.‐S.W. and J.S. interpreted the data and wrote the manuscript, with a contribution from D.R.C.N. All authors approve the final version of this manuscript. J.S. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGMENTS
The authors would like to thank all past and current members of our laboratory. We appreciate Dr. Mana Parast (Department of Pathology, UC San Diego) for her suggestions in research design, and Dr. Thomas L. Brown (Wright State University) for reading and suggestions of the article.
Wattez J‐S, Qiao L, Lee S, Natale DRC, Shao J. The platelet‐derived growth factor receptor alpha promoter‐directed expression of cre recombinase in mouse placenta. Developmental Dynamics. 2019;248:363–374. 10.1002/dvdy.21
Funding information American Diabetes Association, Grant/Award Number: 1‐16‐IBS‐272; Canadian Institutes of Health Research, Grant/Award Number: 201203MOP‐275374‐CIA‐CBBA‐109624; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: HD069634; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: DK095132; UC San Diego
REFERENCES
- 1. Clifton VL, Stark MJ, Osei‐Kumah A, Hodyl NA. Review: The feto‐placental unit, pregnancy pathology and impact on long term maternal health. Placenta. 2012;33:S37‐S41. [DOI] [PubMed] [Google Scholar]
- 2. Cross JC. Adaptability and potential for treatment of placental functions to improve embryonic development and postnatal health. Reprod Fertil Dev. 2016;28:75‐82. [DOI] [PubMed] [Google Scholar]
- 3. Renaud SJ, Karim Rumi MA, Review SMJ. Genetic manipulation of the rodent placenta. Placenta. 2011;32(suppl 2):S130‐S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cross JC, Nakano H, Natale DRC, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation. 2006;74:393‐401. [DOI] [PubMed] [Google Scholar]
- 5. Sternberg N, Hamilton D. Bacteriophage P1 site‐specific recombination. I Recombination between loxP sites J Mol Biol. 1981;150:467‐486. [DOI] [PubMed] [Google Scholar]
- 6. Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand‐binding domains. Biochem Biophys Res Commun. 1997;237:752‐757. [DOI] [PubMed] [Google Scholar]
- 7. Jin Y, Lu SY, Fresnoza A, Detillieux KA, Duckworth ML, Cattini PA. Differential placental hormone gene expression during pregnancy in a transgenic mouse containing the human growth hormone/chorionic somatomammotropin locus. Placenta. 2009;30:226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamat A, Smith ME, Shelton JM, Richardson JA, Mendelson CR. Genomic regions that mediate placental cell‐specific and developmental regulation of human Cyp19 (aromatase) gene expression in transgenic mice. Endocrinology. 2005;146:2481‐2488. [DOI] [PubMed] [Google Scholar]
- 9. Kwon GS, Hadjantonakis A‐K. Eomes:GFP‐a tool for live imaging cells of the trophoblast, primitive streak, and telencephalon in the mouse embryo. Genesis. 2007;45:208‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin J, Linzer DI. Identification of trophoblast‐specific regulatory elements in the mouse placental lactogen II gene. Mol Endocrinol. 1998;12:418‐427. [DOI] [PubMed] [Google Scholar]
- 11. Nogués N, Del Río JA, Pérez‐Riba M, Soriano E, Flavell RA, Boronat A. Placenta‐specific expression of the rat growth hormone‐releasing hormone gene promoter in transgenic mice. Endocrinology. 1997;138:3222‐3227. [DOI] [PubMed] [Google Scholar]
- 12. Shi D, Winston JH, Blackburn MR, Datta SK, Hanten G, Kellems RE. Diverse genetic regulatory motifs required for murine adenosine deaminase gene expression in the placenta. J Biol Chem. 1997;272:2334‐2341. [PubMed] [Google Scholar]
- 13. Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567‐578. [DOI] [PubMed] [Google Scholar]
- 14. Home P, Kumar RP, Ganguly A, et al. Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Development. 2017;144:876‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kenchegowda D, Natale B, Lemus M, Natale D, Fisher S. Inactivation of maternal Hif‐1α at mid‐pregnancy causes placental defects and deficits in oxygen delivery to the fetal organs under hypoxic stress. Dev Biol. 2017;422:171‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsui T, Heidaran M, Miki T, et al. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989;243:800‐804. [DOI] [PubMed] [Google Scholar]
- 17. Betsholtz C. Role of platelet‐derived growth factors in mouse development. Int J Dev Biol. 1995;39:817‐825. [PubMed] [Google Scholar]
- 18. Orr‐Urtreger A, Lonai P. Platelet‐derived growth factor‐A and its receptor are expressed in separate, but adjacent cell layers of the mouse embryo. Development. 1992;115:1045‐1058. [DOI] [PubMed] [Google Scholar]
- 19. He F, Soriano P. A critical role for PDGFRα signaling in medial nasal process development. PLoS Genet. 2013;9:e1003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seifert RA, Alpers CE, Bowen‐Pope DF. Expression of platelet‐derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int. 1998;54:731‐746. [DOI] [PubMed] [Google Scholar]
- 21. Floege J, Hudkins KL, Seifert RA, Francki A, Bowen‐Pope DF, Alpers CE. Localization of PDGF alpha‐receptor in the developing and mature human kidney. Kidney Int. 1997;51:1140‐1150. [DOI] [PubMed] [Google Scholar]
- 22. Ogura Y, Takakura N, Yoshida H, Nishikawa SI. Essential role of platelet‐derived growth factor receptor alpha in the development of the intraplacental yolk sac/sinus of Duval in mouse placenta. Biol Reprod. 1998;58:65‐72. [DOI] [PubMed] [Google Scholar]
- 23. Su AI, Cooke MP, Ching KA, et al. Large‐scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465‐4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takakura N, Yoshida H, Ogura Y, Kataoka H, Nishikawa S, Nishikawa S. PDGFR alpha expression during mouse embryogenesis: immunolocalization analyzed by whole‐mount immunohistostaining using the monoclonal anti‐mouse PDGFR alpha antibody APA5. J Histochem Cytochem. 1997;45:883‐893. [DOI] [PubMed] [Google Scholar]
- 25. Kita N, Mitsushita J, Ohira S, et al. Expression and activation of MAP kinases, ERK1/2, in the human villous trophoblasts. Placenta. 2003;24:164‐172. [DOI] [PubMed] [Google Scholar]
- 26. Perucho M, Hanahan D, Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980;22:309‐317. [DOI] [PubMed] [Google Scholar]
- 27. Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double‐fluorescent Cre reporter mouse. Genesis. 2007;45:593‐605. [DOI] [PubMed] [Google Scholar]
- 29. Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang XW, Model P, Heintz N. Homologous recombination‐based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol. 1997;15:859‐865. [DOI] [PubMed] [Google Scholar]
- 31. Adamson SL, Lu Y, Whiteley KJ, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358‐373. [DOI] [PubMed] [Google Scholar]
- 32. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294‐1301. [DOI] [PubMed] [Google Scholar]
- 33. Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I. The impairment of metrial gland development in tamoxifen exposed rats. Exp Toxicol Pathol. 2012;64:121‐126. [DOI] [PubMed] [Google Scholar]
- 34. Sato T, Ohta Y, Okamura H, Hayashi S, Iguchi T. Estrogen receptor (ER) and its messenger ribonucleic acid expression in the genital tract of female mice exposed neonatally to tamoxifen and diethylstilbestrol. Anat Rec. 1996;244:374‐385. [DOI] [PubMed] [Google Scholar]
- 35. Tewari K, Bonebrake RG, Asrat T, Shanberg AM. Ambiguous genitalia in infant exposed to tamoxifen in utero. Lancet. 1997;350:183. [DOI] [PubMed] [Google Scholar]
- 36. McCarthy N, Liu JS, Richarte AM, et al. Pdgfra and Pdgfrb genetically interact during craniofacial development. Dev Dyn. 2016;245:641‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senut MC, Suhr ST, Gage FH. Gene transfer to the rodent placenta in situ. A new strategy for delivering gene products to the fetus. J Clin Invest. 1998;101:1565‐1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tam PPL, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155‐6163. [DOI] [PubMed] [Google Scholar]
- 39. Xing A, Boileau P, Caüzac M, Challier JC, Girard J, Hauguel‐de Mouzon S. Comparative in vivo approaches for selective adenovirus‐mediated gene delivery to the placenta. Hum Gene Ther. 2000;11:167‐177. [DOI] [PubMed] [Google Scholar]
- 40. Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci U S A. 2002;99:2140‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kunath T, Gish G, Lickert H, Jones N, Pawson T, Rossant J. Transgenic RNA interference in ES cell‐derived embryos recapitulates a genetic null phenotype. Nat Biotechnol. 2003;21:559‐561. [DOI] [PubMed] [Google Scholar]
- 42. Albers RE, Kaufman MR, Natale BV, et al. Trophoblast‐Specific Expression of Hif‐1α results in preeclampsia‐like symptoms and fetal growth restriction. Sci Rep. 2019;9:2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ueno M, Lee LK, Chhabra A, et al. c‐Met‐dependent multipotent labyrinth trophoblast progenitors establish placental exchange interface. Dev Cell. 2013;27:373‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]


