Abstract
Bleomycin is a redox-active drug with anticancer and other clinical applications. It is also frequently used as a tool in fundamental research on cellular responses to DNA double-strand breaks (DSBs). A conversion of bleomycin into its DNA-breaking form requires Fe, one-electron donors and O2. Here, we examined how a major biological antioxidant ascorbate (reduced vitamin C), which is practically absent in standard cell culture, impacts cellular responses to bleomycin. We found that restoration of physiological levels of vitamin C in human cancer cells increased their killing by bleomycin in 2D cultures and 3D tumor spheroids. Higher cytotoxicity of bleomycin occurred in cells with normal and shRNA-depleted p53. Cellular vitamin C enhanced the ability of bleomycin by produce DSBs, which was established by direct measurements of these lesions in three cell lines. Vitamin C-restored cancer cells also showed a higher sensitivity to killing by low-dose bleomycin in combination with inhibitors of DSB repair-activating ATM or DNA-PK kinases. The presence of ascorbate in bleomycin-treated cells suppressed a DSB-independent activation of the ATM-CHK2 axis by blocking superoxide radical. In vitro studies detected a greatly superior ability of ascorbate over other cellular reducers to catalyze DSB formation by bleomycin. Ascorbate was faster than other antioxidants in promoting two steps in activation of bleomycin. Our results demonstrate strong activation effects of vitamin C on bleomycin, shifting its toxicity further toward DNA damage and making it more sensitive to manipulations of DNA repair.
Keywords: bleomycin, vitamin C, ascorbate, DNA double-strand break, ATM, superoxide
Graphical Abstract
Introduction
Bleomycin (BLM) is a genotoxic cancer drug that also has other clinical uses. BLM is approved for treatment of various human malignancies such as lymphomas, head and neck carcinomas, testicular, cervical and other tumors [1–4]. Treatments with combination chemotherapies containing BLM in patients with germ cell cancers and Hodgkin’s lymphomas is curative in a large percentage of cases. For example, the use of BLM-containing regimens as the frontline treatments in Hodgkin’s lymphoma reaches complete response rates up to 94% in limited stage and 88% in advanced stage of this disease [2]. The ability of BLM to cause cancer cell death is linked to the production of DNA breaks, especially DNA double-strand breaks (DSBs) [1,5]. The use of BLM in cancer patients is frequently associated with serious side effects in the lung, which includes pneumonitis and the subsequent fibrotic response [6,7]. This dangerous lung injury impairs lung functions and can be lethal. Several recent studies from different countries have found very successful clinical applications of BLM as a frontline treatment for low flow lymphatic malformations in children [8–11]. The use of BLM has now largely replaced complications-associated surgery for treatment of this disfiguring abnormalities even when presented as large masses. In contrast to cancer patients, the administration of different forms of BLM for treatment of lymphatic abnormalities did not produce any short- or long-term systemic side effects and the BLM therapy is considered to be a safe alternative to surgery [8–11]. In addition to its clinical applications, BLM is also frequently used in fundamental research on the activation of DNA damage responses by DSBs. In fact, BLM is a “cleaner” inducer of DSBs in comparison to ionizing radiation, which is another common source of oxidatively produced DSBs. BLM produces no oxidized bases and generates 5–10-times higher ratios of DSBs to single-strand breaks than radiation [1,12,13].
The transition of BLM into its DNA-damaging form requires complexation with Fe(II) and the presence of one-electron donors and molecular oxygen. The role of one-electron reducers is to convert BLM-bound or free Fe(III) to Fe(II) and then to produce the DNA-attacking oxidant, BLM-Fe(II)-OOH hydroperoxide [1,12,13]. Both formation and decay of the activated BLM-Fe(II) complex can involve the production of reactive oxygen species. BLM activation and DNA cleavage reactions in vitro have been typically studied with biological and synthetic thiols as Fe(III) reducers. The main cellular thiol glutathione (GSH) was noted as a weak activator of DNA nicking by BLM-iron [5]. Ascorbate (Asc), the reduced form of vitamin C, is known as an excellent reducer of Fe(III) and its addition to BLM-Fe(III) reactions stimulated DNA cleavage [14,15]. Although BLM has been extensively used as a tool for the induction of DSBs in cultured cells, how different cellular reducers contribute to the activation of BLM is poorly understood. Variation in ROS-scavenging abilities of bioreducers of Fe(III) can potentially lead to their different impact on specific aspects of BLM toxicity. In light of its strong Fe(III)-reducing and radical scavenging activities and its millimolar concentrations in cells in the main tissues [16–18], Asc can be expected to play a significant role in BLM toxicity in vivo. Human and nonhepatic rodent cells in culture are severely deficient in Asc [16,19,20] due to the absence of vitamin C in the majority of standard cell culture media formulations and the typical addition of only 10% of serum, which is depleted of vitamin C during its preparation and storage. This raises a question how physiological levels of Asc in cells influence toxic effects of BLM, considering powerful antioxidant properties of reduced vitamin C and its excellent Fe(III)-reducing activity, with the latter being necessary for the initial step in the activation of BLM-Fe complex.
In this work, we investigated the effects of restoration of the physiological levels of vitamin C in human cancer cells on cytotoxic and genotoxic responses to BLM. We found that vitamin C enhanced killing of cancer cells by BLM in 2D cultures and 3D tumor spheroids, which was caused by a higher production of DSBs. Cellular vitamin C also altered BLM-induced signaling by the apical DNA damage-responsive kinase ATM and made cancer cells more sensitive to killing by low-dose BLM in the presence of inhibitors of DSB repair-activating kinases. Among the main biological reducers, Asc displayed the higher rates of two one-electron transfer reactions that are necessary for the full activation of iron-BLM complex.
Materials and Methods
Materials.
Bleomycin sulfate was purchased from LKT Laboratories. Dehydroascorbic acid dimer, L-ascorbic acid, proteinase K, N-lauroylsarcosine (≥98% pure), sodium deoxycholate (≥97% pure), PEG-SOD, p-benzoquinone and all salts and buffers were from Sigma-Aldrich. Doxorubicin and NU7441 were from Cayman Chemical, NU7026 from Calbiochem, KU60019 from Santa Cruz, imatinib from Enzo, mitomycin C from Tocris and 1,2-diamino-4,5-dimethoxybenzene dihydrochloride from Molecular Probes. Ultrapure DNA grade pulse field certified agarose was purchased from Bio-Rad and ultrapure LMP agarose was from Life Technologies. GelRed Nucleic Acid stain was from Phenix Research Products.
Cells.
H460 and A549 human lung carcinoma lines were obtained from ATCC and cultured in 10% fetal bovine serum (FBS)-supplemented RPMI1640 and F-12K media, respectively. Media for each cell line also included 1% PenStrep solution (100 Units/mL penicillin and streptomycin 100 μg/mL streptomycin) (Gibco). Both cell lines were maintained at 37°C in a 95% air/5% CO2 humidified atmosphere. IMR90 normal human lung fibroblasts (ATCC) were cultured in DMEM medium with 10% FBS and 1% PenStrep at 37°C in a 5% O2/5% CO2 humidified atmosphere. The construction of shRNA-mediated p53 knockdowns in H460 cells using infections with pSUPER-retro vectors has been described earlier [21].
Loading and quantitation of vitamin C.
Cells were allowed to grow overnight and then incubated for 90 min with dehydroascorbic acid (DHA) in Krebs-HEPES buffer [30 mM HEPES (pH 8.0), 130 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2] supplemented with 2% FBS and 0.5 mM glucose. Cellular vitamin C was extracted with 50 mM methanesulfonic acid/5 mM DTPA and quantified as a fluorescent product formed in the reaction with 1,2-diamino-4,5-dimethoxybenzene in the presence of ascorbate oxidase [22]. Protein content of cellular pellets formed after extractions was used for normalization purposes.
3D spheroids growth.
Cells (500/well) were seeded into 96-well Clear Black Round Bottom Ultra Low Attachment Spheroid Microplate (Corning) and grown for 2 days to form microscopically detectable spheroids. For Asc loading, spheroids were washed three times with 200 mL of Krebs buffer containing 2% FBS, 0.5 mM glucose and the indicated concentrations of DHA followed by the incubation for 90 min. Wells were then washed three times with standard growth media followed by 3 h treatment with BLM. DHA loading and BLM treatments were repeated four times at 2-day intervals. The size of spheroids was measured using Nikon Eclipse TS-100-F microscope equipped with PixeLink Megapixel FireWire Camera. Spheroid images were processed by PixeLink CaptureSE v.1.0 software using the Area Perimeter function.
Pulsed field gel electrophoresis (PFGE).
A previously described PFGE procedure for detection of DSBs was followed [23]. Cells were resuspended in 0.5 mL of Cell Suspension Buffer (10 mM Tris, pH 7.2, 50 mM EDTA, 20 mM NaCl), mixed with an equal volume of 1.6% Ultra Pure LMP agarose and immediately poured into CHEF Mapper XA System Plug Molds (3×105 cells per one plug) (Bio-Rad). Solidified agarose plugs were transferred to Proteinase K Reaction Buffer (10 mM Tris, pH 8.0, 100 mM EDTA, 1 % N-lauroylsarcosine, 0.2% sodium deoxycholate, 1 mg/mL proteinase K) and incubated overnight at room temperature with gentle mixing followed by five washes in Wash Buffer (20 mM Tris, pH 8.0, 50 mM EDTA). Cooled, solidified 1% agarose gel was placed into electrophoresis chamber containing 0.5×TBE buffer pre-chilled to 14°C. Electrophoresis was performed using Bio-Rad CHEF MAPPER system (Bio-Rad). DNA was stained for 1 h in 3× GelRed dye solution in 0.1 M NaCl.
Plasmid breakage assay.
Commercial ϕX174 RF1 plasmid DNA (Thermo Fisher Scientific) was purified from EDTA by precipitation with ethanol/sodium acetate and then dissolved in 25 mM HEPES, pH 7.4. Each reaction contained 25 mM HEPES (pH 7.4), 0.5 μg ϕX174 DNA, 0.1 μM BLM and 0.1 μM Fe(III) citrate. Reducers, BLM and Fe(III) citrate stock solutions were all made fresh. Reactions were set up by mixing HEPES buffer, BLM and Fe(III) followed by 5 min incubation on ice and then addition of ϕX174 DNA. Samples were incubated for 5 min on ice and then reducers were added. DNA breakage reactions were performed at 37°C and stopped by the addition of 100 μM deferoxamine and placement of samples on ice. Blue/Orange 6×Loading Dye was added to each sample and DNA were separated on 1% agarose gel in 1×TAE buffer (40 mM Tris-base, 20 mM acetic acid, 1 mM EDTA). Gels were incubated in 3×GelRed Nucleic Acid Stain (Phenix Research) for 30 min and DNA was visualized using Gel Doc XR+ Imaging System (Bio-Rad Laboratories).
Fe(II) formation.
Reduction of Fe(III) was measured the formation of Fe(II) ferrozine and Fe(II)-phenanthroline complexes [24,25]. Both Fe(II) probes were used at 300 μM final concentration. Two working solutions containing 25 mM HEPES (pH 7.4) were created: one with 2x concentrations of the reducers and ferrozine or o-phenanthroline, and the second with 2x concentrated Fe(III) ammonium citrate. Equal volumes of the solutions were rapidly mixed in a 96-well plate and the reactions were allowed to proceed at 37°C inside the SpectraMax M5 microplate reader. Absorbance readings of Fe(II) ferrozine (582 nm) and Fe(II)-phenanthroline (A512 nm) complexes were taken every 20 sec.
Western blotting.
Cells were collected by scraping, washed twice with ice-cold PBS and centrifuged at 1100×g for 5 min at 4°C. Total cellular proteins were extracted with a 2% SDS-containing buffer (2% SDS, 50 mM Tris, pH 6.8, 10% glycerol). Cells were boiled for 10 min, cooled to room temperature and then centrifuged at 10,000 ×g for 10 min. For analysis of chromatin-bound proteins, cells were first extracted with 100 μL of 0.3% NP40 buffer [20 mM HEPES, pH 7.0, 100 mM NaCl, Halt protease/phosphatase inhibitors (Thermo Scientific)] for 20 min on ice and then centrifuged 800g for 5 min at 4°C. Pellets were washed once in 50 μL of the lysis buffer and first and second supernatants were combined and used as a soluble fraction. Chromatin proteins were extracted from pellets by boiling in 100 μL of 2% SDS buffer. Protein concentrations were measured using DC Protein Assay Kit (Bio-Rad). Proteins were separated by SDS-PAGE and transferred onto Immun-Blot PVDF membranes (Bio-Rad). Antibodies for phospho-ATM (S1981), phospho-DNA-PK (S2056) and fibrillarin were from Abcam, phospho-KAP1 (S824) from Bethyl, γ-tubulin from Sigma, phospho-CHK2 (T68), phospho-p53 (S15), phospho-AMPK (T172), GAPDH (HRP-conjugated), histone H3 and p21 from Cell Signaling Technology, and p53 from Santa Cruz (sc-125). Fibrillarin, an abundant nucleolar protein, was used as a primary loading control for total cell lysates and as a specific loading control for the nuclear/chromatin fraction. GAPDH served as a loading control for soluble proteins. In some blots, γ-tubulin or ribosomal protein L7A were used as loading controls for total cell lysates, which was necessitated by specific electrophoresis/transfer conditions.
qRT-PCR.
H460 cells were seeded onto 100-mm dishes and on the next day preloaded with 0 or 0.5 mM DHA for 90 min followed by 1 h treatment with BLM (0 or 10 μg/mL). After 6 h recovery, cellular RNA was isolated with TRIzol Reagent (Life Technologies) and then reverse-transcribed using oligo-dT and RT First Strand Kit (Qiagen). cDNA was amplified using PCR primers from Qiagen and ABI PRISM 7900HT Sequence Detection System (Life Technologies). mRNA levels of three housekeeping genes (B2M, GAPDH, TBP) were used for normalization purposes. The fold changes in gene expression were analyzed by the 2−ΔΔCt method.
Redox-Sensitive Probes.
A previously described procedure for the use of the redox-sensitive dyes dihydrorhodamine (DHR) and dihydroethidium (DHE) in intact cells has been adapted [25]. Cells were grown in black 96-well optical bottom cell culture plates for these experiments. Control and DHA-treated cells were preloaded with 10 μM DHR or DHE in serum-free medium for 30 min and then treated with BLM for 1 h in complete growth medium. Cells were rinsed with warm PBS and then covered with a modified DPBS solution (Sigma-Aldrich, D4031) followed by measurements of specific fluorescence for the oxidized probes (DHR excitation/emission: 500/535 nm; DHE excitation/emission: 510/580 nm).
Cellular ATP measurements.
Cells (3000/well) were seeded into black, 96-well optical bottom cell culture plates and grown overnight. ATP levels in control and DHA-preincubated cells treated with various concentrations of BLM were measured with the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega).
Caspase-3/7 activity.
The effect of BLM on apoptotic responses was examined using the Caspase-Glo 3/7 Assay (Promega). H460 cells were seeded (3000 cells/well) into black, 96 well optical bottom cell culture plates and incubated overnight. Control and DHA-preloaded cells were treated with BLM for 3 h and luminescence readings of caspase 3/7 activity were recorded 24 h later.
Colony formation.
Cells were seeded on 60-mm dishes (400 cells/dish) and treated on the next day with DHA and drugs. Colonies were allowed to form during 7–8 days. Dishes were washed twice with PBS and cells were fixed with methanol (10 min at room temperature) and stained for 1 h with the Giemsa solution (Sigma-Aldrich). Groups of cells containing 30 or more cells were counted as colonies.
Statistics.
Statistical significance of differences between the groups were evaluated by two-tailed, unpaired t-test.
Results
Cellular Asc and BLM cytotoxicity.
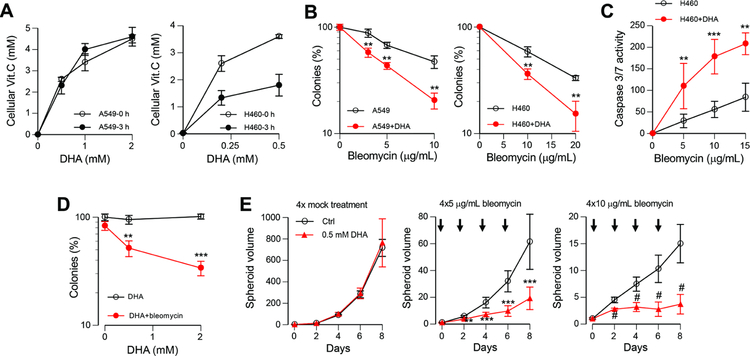
We choose H460 and A549 human lung carcinoma cell lines as our primary models for examination of potential biological interactions between BLM and cellular Asc. Both cell lines are frequently used for studies of DNA damage and are part of the NCI-60 panel of cancer cell lines. H460 cells have shown normal activation of DNA damage signaling in response to ionizing radiation [27] and in our experience, to other genotoxic agents [28,29]. In the preliminary studies, we found that similar to the majority of other transformed and primary cell lines, A549 and H460 cells did not show a biologically sufficient accumulation of the reduced form of vitamin C, Asc. In contrast, both cell lines rapidly accumulated millimolar concentrations of vitamin C when incubated in the presence of its oxidized form, DHA (Fig. 1A). The retention of cellular vitamin C at 3 h postloading was nearly 100% in A549 cells but only approximately 50% in H460 cells. Considering the loss of cellular Asc in prolonged incubations, we focused on studies of cellular responses to short-term-treatments with BLM (3 h or shorter). Examination of long-term cell viability by the colony formation assay showed that the restoration of the physiological levels of cellular Asc resulted in a significantly enhanced killing of A549 and H460 cells by BLM (Fig. 1B). Measurements of activity of the main executioner caspases 3 and 7 also found a higher cytotoxicity of BLM in DHA-preincubated cells (Fig. 1C). The enhancement of clonogenic lethality by DHA was concentration-dependent and observed for the BLM dose that was practically nontoxic in the standard Asc-deficient culture (Fig. 1D). To test the effects of cellular Asc on BLM activity in more in vivo relevant conditions, we examined growth of 3D tumor spheroids treated with several applications of DHA and BLM (Fig. 1E). We found that growth of H460 spheroids was not affected by multiple applications of 0.5 mM DHA alone. However, a combination of DHA and BLM was much more cytotoxic than BLM alone. For the higher dose of BLM, DHA-preincubated spheroids stopped growth after the second addition of the drug whereas mock-preincubated spheroids continued to expand through the entire treatment regimen (Fig. 1E, right panel). Thus, these results demonstrate a higher cytotoxic activity of BLM in Asc-restored cells.
Figure 1. Sensitization of Asc-restored human cancer cells to killing by BLM.
Data are means ±SD, n=3. Statistics: ** - p<0.01, *** - p<0.001, # - p<0.0001 relative to the no-DHA controls. All BLM treatments were for 3 h. (A) Asc levels in A549 and H460 cells immediately after incubation with DHA and following 3 h recovery. (B) Colony formation after BLM treatments of control and 0.5 mM DHA-preincubated cells. (C) Caspase 3/7 activity in control and 0.5 mM DHA-preincubated H460 cells at 24 h after BLM treatments. (D) Colony formation by BLM (3 μg/mL)-treated H460 cells preincubated with 0, 0.5 or 2 mM DHA. (E) 3D spheroid growth of H460 cells treated four times with BLM without or with 0.5 mM DHA preloading. Arrowheads indicate timing of BLM/DHA treatments.
Overloading of GLUT1-overexpressing cells with Asc can lead to oxidative and metabolic stress, impaired ATP production and a resulting loss of viability in cancer cells with a high dependence on aerobic glycolysis [30–33]. We found that our 90-min long preincubations of cells with 0.5 mM DHA did not cause significant changes in ATP levels in H460 or A549 cells after or before BLM treatments (Fig. 2A). Asc overload-induced ATP deficiency triggered activation of the metabolic stress-sensitive kinase AMPK [33,34]. Our tests of different dose combinations found no detectable effect of DHA on the activating phosphorylation of AMPK, which was elevated by BLM in two cell lines irrespective of cellular Asc (Fig. 2B,C). The ability of BLM to upregulate AMPK phosphorylation is probably related to the sensitivity of this kinase to oxidative stress. Finally, we asked a question whether potentiation of cytotoxicity by physiologically relevant concentrations of cellular Asc can be extended to other cancer drugs. We tested doxorubicin, which causes DSBs by inhibiting topoisomerase II, and mitomycin C, which forms toxic DNA interstrand crosslinks. We found that clonogenic toxicity of both doxorubicin and mitomycin C was not affected by DHA preincubation of either H460 or A549 cells (Fig. 2D,E). Overall, these results indicate that DHA-preincubated cells did not experience a metabolic stress and a higher toxicity of BLM in Asc-restored cells was a drug-specific phenomenon.
Figure 2. Lack of metabolic stress and sensitization to other DNA-damaging drugs after Asc loading.
Treatments with drugs were for 3 h in panels A, D, E and for 1 h in panels B and C. Data are means ±SD, n=3. (A) Normal levels of ATP in DHA-preincubated H460 and A549 cells. (B) AMPK phosphorylation in control and DHA-preincubated cells treated with high or (C) low doses of BLM. (D) Colony formation by H460 and A549 cells treated with doxorubicin or (E) mitomycin C without or with 0.5 mM DHA preincubation.
Activation of p53.
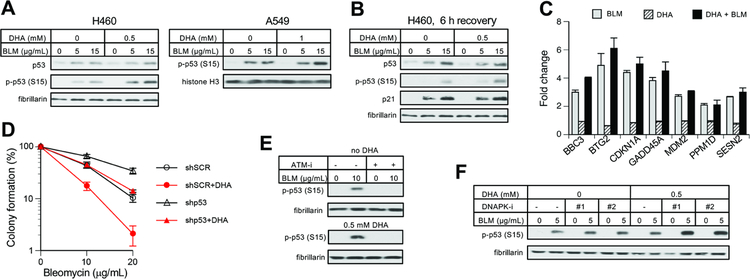
The transcription factor p53 plays a major role in cellular responses to DNA damage [35,36]. DSBs are known as potent activators of p53-mediated death in many cell types. The initial response to DSBs involves ATM-mediated phosphorylation of p53 at Ser15, resulting in protein stabilization. We found that DHA preincubation of cells led a moderately higher Ser15 phosphorylation by BLM but increases in p53 protein levels appeared similar in control and Asc-restored cells (Fig. 3A,B). Upregulation of the protein levels of the CDK inhibitor p21, which is the transcriptional target of p53, was also similar between mock and DHA-preincubated cells (Fig. 3B). RT-qPCR results for several p53-regulated genes showed their similar induction by BLM irrespective of cellular levels of Asc (Fig. 3C). A stable knockdown of p53 with shRNA led a higher long-term survival of BLM-treated cells, as assayed by the colony formation assay (Fig. 3D). However, the potentiating effect of DHA preincubation on BLM cytotoxicity was retained in p53-deficient cells. In both control and DHA-preincubated cells, Ser15-p53 phosphorylation in response to BLM was completely dependent on the apical DNA damage-sensitive kinase ATM (Fig. 3E). Interestingly, inhibition of another DSB-activated kinase, DNAPK, which controls a major DSB repair pathway (nonhomologous end-joining) [37,38], led to stronger increases in Ser15-p53 phosphorylation by BLM in DHA-preincubated cells (Fig. 3F), suggesting that cellular Asc may increase the DSB production by BLM.
Figure 3. BLM-activated responses in the p53 pathway.
Cells were treated with BLM for 1 h in panels A, B, C, E and for 3 h in panels D, F. Data are means ±SD, n=3. (A) Westerns for p53 protein and its Ser15 phosphorylation at Ser15 in control and DHA-preincubated H460 and A549 cells. (B) Ser15-p53 phosphorylation and protein levels of p21 (CDKN1A) and p53 at 6 h after BLM treatments. (C) mRNA levels of p53-regulated genes in control and 0.5 mM DHA-preloaded H460 cells at 6 h recovery after BLM treatments (10 μg/mL, 1 h). (D) Effect of DHA preincubation (0.5 mM) on BLM-induced clonogenic toxicity in H460 cells expressing scrambled (shSCR) or p53-targeting shRNA (shp53). (E) Loss of Ser15-p53 phosphorylation in BLM-treated H460 cells in the presence of the ATM kinase inhibitor KU60019 (ATM-i, 2 μM). (F) Impact of DNA-PK inhibitors on Ser15-p53 phosphorylation by BLM in H460 cells (DNAPK-i #1: 1 μM NU7441, DNA-PK-i #2: 30 μM NU7026).
Asc and DSB production.
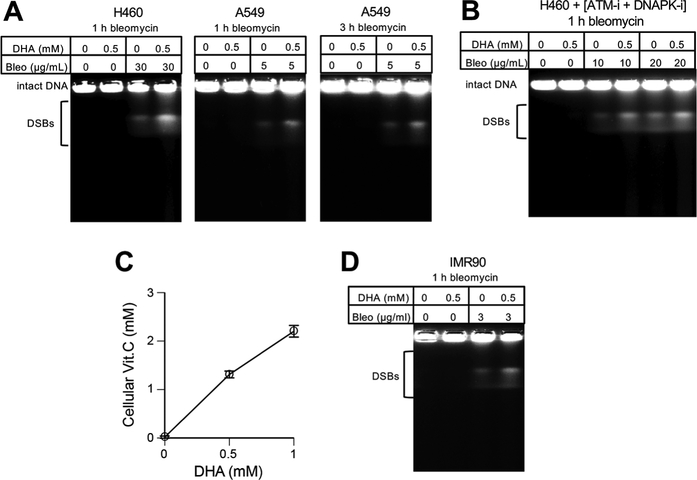
DSBs are highly toxic DNA lesions and alterations in their production would correspondingly change cytotoxic responses. To assess the DSB-generating activity of BLM in cells, we assayed the levels of chromosomal DSBs by their direct physical measurements using PFGE. We found that DHA preincubation of H460 and A549 cells increased the amounts of high-molecular weight DNA fragments after different BLM treatments (Fig. 4A). The amount of DSBs at a given time is determined by a balance of their production and repair, with later potentially becoming a major factor in prolonged incubations. To determine how Asc promotes the accumulation of DSBs, we performed PFGE analyses of cells that were treated with BLM in the presence of ATM and DNA-PK inhibitors. Both kinases, especially DNA-PK, are critically important for the activation of the major DSB repair pathways [37,38]. We found that DHA preincubation also increased the amounts of DSBs in BLM-treated cells with suppressed DNA repair (Fig. 4B), indicating that cellular Asc enhances DNA-breaking activity of BLM. Finally, we found that a delivery of physiological levels of Asc also increased the formation of DSBs by BLM in primary human cells (Fig. 4C,D).
Figure 4. Elevated production of DSBs by BLM in Asc-supplemented cells.
(A) PFGE of DNA from cells treated with BLM without or with 0.5 mM DHA preincubation. (B) DSBs in H460 cells treated with BLM in the presence of ATM (2 μM KU60019) and DNA-PK (10 μM NU7441) inhibitors. Both inhibitors were also present during 90 min incubations with DHA. (C) Vitamin C levels in IMR90 primary human cells immediately or 3 h later after incubation with DHA (means±SD, n=3). (D) PFGE analysis of DNA from IMR90 treated with BLM with and without 0.5 mM DHA preincubation.
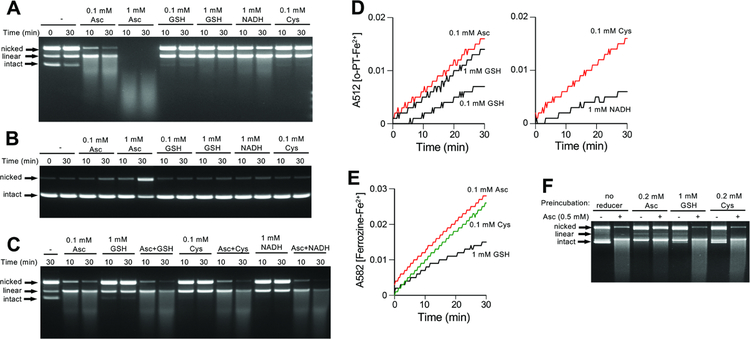
DNA-breaking activity of BLM-Fe(III) complex requires the presence of O2 and reducers of Fe(III) [1,12,13,39]. To better understand the effects of Asc on DNA breakage by BLM, we set up reactions with plasmid DNA in the presence of different cellular reducers of Fe(III). Specifically, we tested Asc, GSH, Cys and NADH at their physiological concentrations and additionally, Asc and GSH at the subphysiological 0.1 mM concentration. The reactions contained equimolar concentrations of BLM, Fe(III) and plasmid DNA. We found that Asc was dramatically more effective than other reducers in promoting DSB formation by BLM-Fe(III) complex in the plasmid DNA (Fig. 5A). In 1 mM Asc reactions, all plasmid DNA was broken into small fragments, indicating that each plasmid experienced multiple DSBs. At lower 0.1 mM Asc, the majority of plasmids migrated as linear molecules (a product of a single DSB) and a smear of smaller fragments (> one DSB per plasmid). Although all other reducers increased DSB formation by BLM-Fe(III), their effectiveness was much smaller than that of 0.1 mM Asc. Control studies showed that BLM was absolutely essential for the production of DSB in our in vitro reactions, as no linear plasmids or small fragments were observed when only Fe(III) and reducers were incubated together (Fig. 5B). The highest concentration of Asc at the longest exposure time clearly increased the levels of nicked DNA molecules, indicating the production of oxidants capable of forming single-stranded breaks. The activity of Asc in promoting DSBs in vitro was not affected by the addition of other cellular reducers (GSH, Cys or NADH), indicating that their weak potency in stimulating DNA breakage was a not a result of inactivation of reactive BLM-Fe forms (Fig. 5C). Since the first step in activation of BLM-Fe(III) complex involves reduction of Fe(III) to Fe(II) [1,12,13], one plausible explanation for the high DSB production in Asc reactions could be a much more rapid conversion of Fe(III) to Fe(II). A comparison of Fe(III) reduction by different reducers in two assays showed that the formation of Fe(II) by 0.1 mM Asc was close to that in reactions with 1 mM GSH or 0.1 mM Cys (Fig. 5D,E). Thus, relatively modest differences in Fe(II) formation cannot explain the dramatically higher production of DSBs in Asc-driven reactions relative to other reducers. A second step in the activation of BLM requiring one-electron reducers is the conversion of BLM-Fe(II)-O2•− peroxide into the final DNA-damaging form, BLM-Fe(II)-OOH hydroperoxide [1,12,13]. The fully activated BLM form is unstable in the absence of DNA and undergoes oxidative self-inactivation [12,39]. Thus, a loss of BLM in DNA-free reactions can assess the rate of the formation of its hydroperoxide form. We found that 5-min long preincubations without DNA resulted in a severe suppression of the DNA-breaking activity of BLM in Asc-containing reactions (Fig. 5F). The same preincubations with thiols did not cause noticeable losses of BLM, as the addition of 0.5 mM Asc into these reactions produced the same extent of DNA breakage as in the samples without any reducers (lanes 6 or 8 vs. lane 2, Fig. 5F). Thus, Asc is much more potent than cellular thiols in the formation of the final activated form of BLM.
Figure 5. Plasmid DNA breakage by BLM in the presence of different reducers.
Reactions were performed at 37°C and contained 25 mM HEPES, pH 7.4, 0.5 μg ϕX174 plasmid DNA, 0.1 μM BLM and 0.1 μM Fe(III) citrate. (A) Plasmid DNA breakage by BLM-iron(III) in the presence of different reducers. (B) DNA breakage by Fe(III) citrate in the absence of BLM. (C) DNA breakage by BLM-Fe(III) in the mixtures of Asc with other reducers. (D) Fe(II) formation measured by the o-phenanthroline (o-PT) assay in the reaction of Fe(III) citrate with cellular reducers (37°C reactions in 25 mM HEPES, pH 7.4). Shown are means of triplicate measurements. (E) Ferrozine assay for Fe(II) formation in the reaction of Fe(III) citrate with cellular reducers. Shown are means of triplicate measurements. (F) Self-inactivation of BLM in the absence of DNA. BLM-Fe(III) was preincubated with the indicated reducers for 5 min at 37°C prior to the addition of plasmid DNA and 0.5 mM Asc. The final mixtures were incubated for 10 min at 37°C.
ATM-dependent signaling.
ATM is the DSB-responsive kinase that coordinates multiple aspects of oxidant-induced stress signaling [40]. Oxidants can activate ATM as a result of the production of DSBs [39,40] or directly [43–45]. The DSB-independent stimulation of ATM directs its activity specifically toward nucleoplasmic substrates such as CHK2 kinase whereas the DSB-activated ATM phosphorylates both nucleoplasmic and chromatin-bound proteins such as KAP1. ATM autophosphorylation at Ser1981 is triggered by both DSBs and direct oxidation. We found that DHA preincubations of H460 and A549 cells did not cause major changes in ATM and KAP1 phosphorylation in response to BLM (Fig. 6A). However, phosphorylation of the soluble substrate CHK2 was diminished in DHA-preincubated cells. To test how general is the suppression of CHK2 phosphorylation by Asc restoration, we treated cells with the topoisomerase I inhibitor camptothecin, which induces ATM activation via the production of DSBs, and formaldehyde, which triggers ATM-mediated CHK2 phosphorylation as a result of chromatin damage in the absence of DSBs [46]. We found that CHK2 phosphorylation by both camptothecin and formaldehyde were not affected by DHA preincubation in two cell lines (Fig. 6B,C). ATM activation by DSBs is dependent on the c-ABL tyrosine kinase [47]. We found that inhibition of c-ABL strongly suppressed ATM-mediated phosphorylation of the chromatin-bound substrate KAP1 in both mock- and DNA-preincubated cells treated with BLM (Fig. 6D). In contrast, BLM-induced phosphorylation of the soluble substrate CHK2 was much more sensitive to the inhibition of c-ABL in DHA-preincubated cells. These results suggest that CHK2 phosphorylation by BLM in Asc-restored cells is dominated by the DSB-activated ATM whereas Asc-deficient cells have a larger fraction of ATM activated by BLM via a non-DSB mechanism, likely via oxidation of ATM. Consistent with this possibility, responses of the redox-sensitive probes DHR and DHE showed substantially lower levels of diffusible oxidants produced by BLM in DHA-preincubated cells (Fig. 6E). DHE is a relatively specific probe for detection of superoxide, which points to the diminished production of this oxidant by BLM in Asc-restored cells. This possibility is supported by a known ability of Asc to act as a very effective scavenger of superoxide [16,48]. Using paraquat to generate cellular superoxide [49,50], we found that Asc delivery via DHA preincubation completely abolished CHK2 phosphorylation by superoxide (Fig. 6F). Paraquat treatments did not induce KAP1 phosphorylation, which is consistent with a relatively weak reactivity of superoxide with DNA and its inability to produce DSBs in cells [51,52]. Addition of chemical (p-benzoquinone) or biological (cell-permeable SOD) scavengers of superoxide to cells prior to BLM treatments showed that CHK2 phosphorylation was diminished in Asc-deficient but not in Asc-restored (DHA-preincubated) cells (Fig. 6G). Overall, these results indicate that cellular Asc makes BLM a more specific activator of ATM via the DSB-dependent mechanism.
Figure 6. Impact of cellular Asc on ATM-dependent signaling in response to BLM.
(A) ATM-mediated protein phosphorylation in H460 and A549 cells treated for 1 h with BLM with and without DHA preincubation. (B) Normal CHK2 phosphorylation in DHA-preincubated cells in response to camptothecin (CPT, 1 h) and (C) formaldehyde (FA, 2h). (D) Sensitivity of CHK2 and KAP1 phosphorylation to inhibition of c-ABL kinase (1 μM imatinib, added for 3 h before BLM). H460 cells were treated with BLM for 1 h. (E) Production of diffusible oxidants in Asc-restored cells. Mock- and 0.5 mM DHA-preincubated H460 cells were loaded with the redox-sensitive dyes DHR and DHE for 30 min and then treated with 10 μg/mL BLM for 1 h. Data are means±SD, n=4–6, ** - p<0.01, *** - p<0.001. (F) CHK2 and KAP1 phosphorylation in A549 cells treated with paraquat for 3 h. (G) Effects of superoxide scavengers on CHK2 phosphorylation in H460 cells treated with 10 μg/mL BLM for 1 h. PEG-SOD1 (85 units/mL) and p-benzoquinone (p-BQ, 10 μM) were added during mock/0.5 mM DHA preincubations and were present during treatments with BLM (10 μg/mL, 1 h).
A higher formation of DSBs together with a suppression of a general oxidative stress in Asc-restored cells suggest that in these conditions, cell tolerance of BLM could become more dependent on the activity of DSB repair. ATM and DNA-PK are two DSB-responsive kinases that are needed for the activation of the major DSB repair processes in cells [37,40]. These kinases can also be targeted with highly selective inhibitors, which offers a possibility for exploration of potential synergistic drug interactions with BLM. First, we tested whether Asc enhances activation of these kinases by BLM in a DSB-related manner. For ATM, we looked at the levels of chromatin-bound autophosphorylated ATM and found them much more strongly elevated in DHA/BLM-treated cells relative to mock/BLM counterparts (Fig. 7A). The amounts of soluble phospho-ATM, which includes both DSB-activated ATM that dissociated from chromatin and direct oxidation-activated ATM, were not very different between mock- and DHA-preincubated groups of BLM-treated cells. For DNA-PK, we measured its autophosphorylation at Ser2056, which is specifically induced in response to the presence of DSBs. We found that Asc delivery into cells clearly increased DNA-PK autophosphorylation by BLM (Fig. 7B). Thus, for both ATM and DNA-PK kinases, biochemical readouts of their activation due to DSB formation was elevated by DHA preincubation. To test more directly the importance of both kinases in BLM tolerance by cells, we examined clonogenic survival of BLM-treated cells in the presence of ATM and DNA-PK inhibitors. We found that DHA preincubation of cells made them much more sensitive to killing by a combination of BLM and ATM or DNA-PK inhibitor (Fig. 7C). Thus, these results demonstrate a higher sensitivity of Asc-preloaded cells to a pharmacological inhibition of DSB repair-related kinases in combination with BLM.
Figure 7. ATM and DNA-PK in cellular resistance to BLM.
(A) Soluble and chromatin-bound fractions of autophosphorylated ATM in H460 cells (1 h BLM treatments). (B) Activating DNA-PK autophosphorylation in control and DHA-preincubated H460 and A549 cells treated with BLM for 1 h. (C) Clonogenic survival of A549 cells treated with BLM in the presence of inhibitors of ATM (1 μM KU60019) and DNA-PK (3 μM NU7441). Inhibitors were present during BLM treatments and for the next three days of growth. Means±SD, n=3, *- p<0.05, **-p<0.01, ***-p<0.001.
Discussion
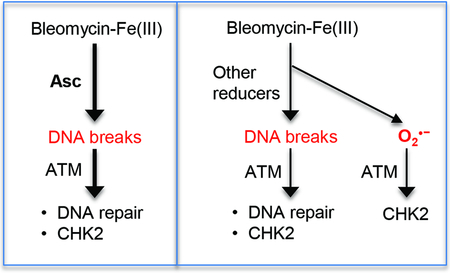
Our studies here found that restoration of physiological levels of vitamin C in human cells increased the production of toxic DSBs and altered DNA damage-related signaling in response to the redox-active drug BLM. Importantly, the elevated formation of DSBs by BLM in vitamin C-replete cells was established by direct physical measurements of these lesions. DSB formation triggers a rapid DNA damage response that involves activation of two apical kinases ATM and DNA-PK [37,38,40]. DNA-PK is a critical regulator of nonhomologous end-joining, a dominant DSB repair pathway in human cells, with a limited number of substrates outside this process. Activation of ATM triggers phosphorylation of numerous proteins that are involved in DSB repair, chromatin remodeling, cell cycle checkpoints, and cell death. Consistent with the measurements of DSBs, vitamin C-restored cells showed elevated levels of the DSB-activated forms of ATM and DNA-PK kinases in response to BLM treatments. The presence of vitamin C in cells also made them more sensitive to killing by BLM via inhibition of ATM and DNA-PK kinases. In agreement with the results in cells, in vitro DNA cleavage reactions found a much stronger ability of Asc to promote the BLM/Fe-mediated formation of DSBs in comparison to other cellular reducers of Fe(III), such as Cys, glutathione or NADH. Measurements of Fe(III)-reducing activity by the same electron donors showed that Asc was a much faster reducer of Fe(III) in comparison to glutathione or NADH. However, the rate of Fe(III) reduction alone could not explain the differences in the DSB production among biological reducers. At concentrations that produced approximate the same rates of Fe(III) reduction, Asc was still a much more potent promoter of DSBs by BLM-Fe(III) in comparison to thiols. Thus, Asc was the fastest electron donor in the last step of the BLM activation, which is a conversion of BLMFe(III) peroxide into BLM-Fe(III) hydroperoxide (Fig. 8) [1,12,13]. In the absence of DNA, a fully activated BLM is known to undergo self-inactivation [39], which is what we found after short incubations in reactions with Asc but not in those with other bioreducers. Asc is known as a very rapid scavenger of superoxide [16,46]. High reactivity of Asc with O2•− could be therefore responsible for its rapid one-electron transfer to BLM-Fe(III)-O2•− generating the fully activated, DSB-inducing form of BLM (Fig. 8).
Figure 8. Summary of Asc effects on BLM activation and ATM-dependent signaling.
Asc accelerates the formation of DSBs by BLM via high rates of Fe(III) reduction and the formation of the DNA-breaking hydroperoxide from the intermediate peroxide form. The speed of the latter reaction is important to minimize the release of free superoxide that can activate ATM via a DSB-independent mechanism. Asc further decreases the levels of O2•− through its direct scavenging. Inhibition of O2•− production in Asc-restored cells makes activation of ATM by BLM more completely dependent on the presence of DSBs.
The ability of high concentrations of thiols to reduce Fe(III) to Fe(II) but not being effective in the production of the fully activated BLM form is expected to result in a decay of the BLM-Fe(III)-O2•− intermediate, releasing free superoxide and BLM-Fe(III) (Fig. 8). Superoxide is not very reactive with DNA and is unable to produce DSBs in cells [51,52] or in vitro, as evidenced from our plasmid cleavage experiments with Fe(III) in the absence of BLM. However, O2•− is toxic to cells due to its direct protein damage and the formation of secondary oxidants [16,52]. Elevated release of superoxide in the reactions of BLM-Fe(III) with non-Asc electron donors can explain the observed differences between Asc-restored and Asc-deficient cells in the BLM-induced phosphorylation of ATM targets. Oxidants can activate ATM via its direct damage [43–45] and as a result of the formation of DSBs [41,42]. A spectrum of phosphorylation targets for the DSB-activated ATM is broader and includes both DNA repair/chromatin and checkpoint targets whereas directly activated ATM phosphorylates only checkpoint-related proteins. A suppression of the superoxide production by BLM in Asc-restored cells and the resulting loss of the direct activation of ATM helps explain our observations on the diminished levels of phosphorylation of its checkpoint mediator CHK2 kinase and a greater dependence of CHK2 phosphorylation on the DSB-sensing mechanisms. Another important checkpoint and apoptosis-promoting target of ATM is the transcription factor p53, which can be phosphorylated in response to both the DSB-dependent and direct oxidation-induced ATM activation [40,43]. We found that the overall activation of the p53-mediated gene expression and cell death was not significantly altered by Asc restoration prior to BLM treatments. Thus, it appears that a higher stress signaling originating from elevated DSBs was largely balanced by a diminished signaling from direct ATM oxidation (less superoxide), resulting in the overall comparable activation of p53 by BLM in the presence and absence of Asc.
Administration of pharmacological (supraphysiological) doses of Asc both in vivo and in cell culture showed evidence of a selective killing of cancer cells in different biological models [30–33]. This hypersensitivity to Asc has been linked to a heightened uptake of DHA by cancer cells due to their frequent overexpression of the DHA-transporting GLUT1, which is linked to specific oncogenic changes and the general increased dependency of transformed cells on aerobic glycolysis. Asc overloading and the resulting metabolic stress can also sensitize cancer cells to killing by some chemotherapeutic agents [17,34,53]. Our studies also found a stronger cytotoxicity of BLM in cells that were preincubated with DHA, however, this effect was unlikely linked to Asc overloading and the resulting metabolic/energy stress. In our experiments, DHA preincubations did not induce any significant changes in cellular ATP or activation of the metabolic stress-sensitive kinase AMPK, which are two biochemical indicators that are responsive to Asc overloading conditions [31,33,34]. Furthermore, our DHA preincubations delivered physiological levels of cellular vitamin C and did not change cytotoxicity of two other DNA-damaging drugs. It is possible that the use of pharmacological doses of DHA/Asc could further sensitize human cancer cells to killing by BLM.
Tumors frequently have low levels of Asc [54,55] and cancer patients are frequently vitamin C-deficient [56–58]. Thus, coadministration with Asc can enhance cancer cell killing activity of BLM and make it more dependent on the formation of DSBs. The latter is important for a preferential toxicity of BLM in transformed cells, which often contain mutations in DNA repair proteins resulting in their diminished tolerance of DNA damage. Inactivation of specific genome maintenance processes also renders cancer cells more dependent for viability on the activity of the remaining DNA repair mechanism. We found that a higher DSB production by BLM in Asc-restored cells made them more sensitive to inhibition of the DNA repair-activating kinases ATM and DNA-PK. ATM is mutated in a significant percentage of human cancers [59], which should make them more sensitive to killing with BLM/Asc, especially in combination with DNA-PK inhibitors. The heightened dependency of BLM activity in Asc-rich cells on the production of DSBs can also potentially help diminish the occurrence or severity of lung pneumonitis, which is the most dangerous side effect of BLM therapy [1,6,7]. Toxic effects of BLM in the lung were recently linked to its oxidative stress in the cytoplasm [60], which we found to be suppressed by cellular Asc. It is possible that the standard regimen of BLM is also overdosing many cancer patients since a lower-uptake derivative deglyco-BLM [61] was as effective as the standard BLM in three animal models of human cancer without a concomitant lung toxicity [60].
Conclusions.
Our findings demonstrate that cellular vitamin C increases the ability of bleomycin to produce DSBs, which makes cancer cells more dependent on functional DNA repair for survival. Ascorbate was faster than other small molecule antioxidants/Fe(III) reducers in two electron transfer reactions that are necessary for the full activation of iron-bleomycin complex.
Highlights.
Vitamin C enhances DNA double-strand breakage by bleomycin in cells and in vitro
Ascorbate is superior to other reducers in production of fully activated bleomycin
Vitamin C suppresses DNA breaks-independent activation of ATM by bleomycin
Increased role of DNA repair in bleomycin tolerance by vitamin C-restored cells
Acknowledgements.
This work was supported by the National Institute of Environmental Health Sciences [grant numbers ES008786 and ES028072].
Abbreviations:
- Asc
ascorbate
- BLM
bleomycin
- DHA
dehydroascorbic acid
- DHE
dihydroethidium
- DHR
dihydrorhodamine
- DSB
DNA double-strand break
- GSH
glutathione
- PFGE
pulsed field gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chen J, Stubbe J, Bleomycins: towards better therapeutics, Nat. Rev. Cancer 5(2) (2005) 102–112. DOI: 10.1038/nrc1547. [DOI] [PubMed] [Google Scholar]
- [2].Narkhede M, Sarraf Yazdy M, Cheson B, Determining the sequence of novel therapies in the treatment of relapsed Hodgkin’s lymphoma, Expert Rev. Hematol 11(10) (2018) 773–780. DOI: 10.1080/17474086.2018.1516135. [DOI] [PubMed] [Google Scholar]
- [3].Watson RA, De La Peña H, Tsakok MT, Joseph J, Stoneham S, Shamash J, Joffe J, Mazhar D, Traill Z, Ho LP, Brand S, Protheroe AS, Development of a best-practice clinical guideline for the use of bleomycin in the treatment of germ cell tumours in the UK, Br. J. Cancer 119(9) (2018) 1044–1051. DOI: 10.1038/s41416-018-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bröckelmann PJ, Sasse S, Engert A, Balancing risk and benefit in early-stage classical Hodgkin lymphoma, Blood 131(15) (2018) 1666–1678. DOI: 10.1182/blood-2017-10-772665. [DOI] [PubMed] [Google Scholar]
- [5].Povirk LF, Austin MJ, Genotoxicity of bleomycin, Mutat Res 257(2) (1991) 127–143. [DOI] [PubMed] [Google Scholar]
- [6].Sleijfer S, Bleomycin-induced pneumonitis, Chest 120(2) (2001) 617–624. [DOI] [PubMed] [Google Scholar]
- [7].Kawai K, Akaza H, Bleomycin-induced pulmonary toxicity in chemotherapy for testicular cancer. Expert Opin. Drug Saf 2(6) (2003) 587–596. [DOI] [PubMed] [Google Scholar]
- [8].Chen WL, Huang ZQ, Chai Q, Zhang DM, Wang YY, Wang HJ, L Wang S Fan, Percutaneous sclerotherapy of massive macrocystic lymphatic malformations of the face and neck using fibrin glue with OK-432 and bleomycin, Int. J. Oral Maxillofac. Surg 40(6) (2011) 572–576. DOI: 10.1016/j.ijom.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [9].Mohan AT, Adams S, Adams K, Hudson DA, Intralesional bleomycin injection in management of low flow vascular malformations in children, J. Plast. Surg. Hand Surg 49(2) (2015) 116–120. DOI: 10.3109/2000656X.2014.951051. [DOI] [PubMed] [Google Scholar]
- [10].Bhatnagar A, Upadhyaya VD, Kumar B, Neyaz Z, Kushwaha A, Aqueous intralesional bleomycin sclerotherapy in lymphatic malformation: Our experience with children and adult, Natl J. Maxillofac. Surg 8(2) (2017) 130–135. DOI: 10.4103/njms.NJMS_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee HJ, Kim TW, Kim JM, Kim GW, Ko HC, Kim BS, Kim MB, Kim HS, Percutaneous sclerotherapy using bleomycin for the treatment of vascular malformations, Int. J. Dermatol 56(11) (2017) 1186–1191. DOI: 10.1111/ijd.13733. [DOI] [PubMed] [Google Scholar]
- [12].Burger RM, Cleavage of Nucleic Acids by Bleomycin, Chem. Rev 98(3) (1998) 1153–1170. [DOI] [PubMed] [Google Scholar]
- [13].Hecht SM, Bleomycin: new perspectives on the mechanism of action, J. Nat. Prod 63(1) (2000) 158–168. [DOI] [PubMed] [Google Scholar]
- [14].Buettner GR, Moseley PL, Ascorbate both activates and inactivates bleomycin by free radical generation, Biochemistry 31(40) (1992) 9784–9788. [DOI] [PubMed] [Google Scholar]
- [15].Sugihara N, Kaneko A, Furuno K, Synergistic effects of flavonoids and ascorbate on enhancement in DNA degradation induced by a bleomycin-Fe complex, Free Radic. Res 39(3) (2005) 237–244. DOI: 10.1080/10715760500043058. [DOI] [PubMed] [Google Scholar]
- [16].Winterbourn CC, Reconciling the chemistry and biology of reactive oxygen species, Nat. Chem. Biol 4(5) (2008) 278–286. DOI: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- [17].Du J, Cullen JJ, Buettner GR, Ascorbic acid: chemistry, biology and the treatment of cancer, Biochim. Biophys. Acta 1826(2) (2012) 443–457. DOI: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smirnoff N, Ascorbic acid metabolism and functions: A comparison of plants and mammals, Free Radic. Biol. Med 122 (2018) 116–129. DOI: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reynolds M, Stoddard L, Bespalov I, Zhitkovich A, Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair, Nucleic Acids Res 35(2) (2007) 465–476. DOI: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Luczak MW, Green SE, Zhitkovich A, Different ATM signaling in response to chromium(VI) metabolism via ascorbate and nonascorbate reduction: implications for in vitro models and toxicogenomics, Environ. Health Perspect 124(1) (2016) 61–66. DOI: 10.1289/ehp.1409434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong VC, Cash HL, Morse JL, Lu S, Zhitkovich A, S-phase sensing of DNA-protein crosslinks triggers TopBP1-independent ATR activation and p53-mediated cell death by formaldehyde, Cell Cycle 11(13) (2012) 2526–2537. DOI: 10.4161/cc.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reynolds M, Armknecht S, Johnston T, Zhitkovich A, Undetectable role of oxidative DNA damage in cell cycle, cytotoxic and clastogenic effects of Cr(VI) in human lung cells with restored ascorbate levels, Mutagenesis 27(4) (2012) 437–443. DOI: 10.1093/mutage/ger095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zecevic A, Menard H, Gurel V, Hagan E, DeCaro R, Zhitkovich A, WRN helicase promotes repair of DNA double-strand breaks caused by aberrant mismatch repair of chromium-DNA adducts, Cell Cycle 8(17) (2009) 2769–2778. DOI: 10.4161/cc.8.17.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hirayama T, Nagasawa H, Chemical tools for detecting Fe ions, J. Clin. Biochem. Nutr 60(1) (2017) 39–48. DOI: 10.3164/jcbn.16-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Krawic C, Zhitkovich A, Toxicological antagonism among welding fume metals: inactivation of soluble Cr(VI) by iron, Chem. Res. Toxicol 31(11) (2018) 1172–1184. DOI: 10.1021/acs.chemrestox.8b00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].DeLoughery Z, Luczak MW, Zhitkovich A, Monitoring Cr intermediates and reactive oxygen species with fluorescent probes during chromate reduction, Chem. Res. Toxicol 27(5) (2014) 843–851. DOI: 10.1021/tx500028x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang D, Zaugg K, Mak TW, Elledge SJ, A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response, Cell 126(3) (2006) 529–542. DOI: 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- [28].DeLoughery Z, Luczak MW, Ortega-Atienza S, Zhitkovich A, DNA double-strand breaks by Cr(VI) are targeted to euchromatin and cause ATR-dependent phosphorylation of histone H2AX and its ubiquitination, Toxicol. Sci 143(1) (2015) 54–63. DOI: 10.1093/toxsci/kfu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Luczak MW, Zhitkovich A, Monoubiquitinated γ-H2AX: Abundant product and specific biomarker for non-apoptotic DNA double-strand breaks, Toxicol. Appl. Pharmacol 355 (2018) 238–246. DOI: 10.1016/j.taap.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M, Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues, Proc. Natl Acad. Sci. USA 102(38) (2005) 13604–13609. DOI: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ, Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality, Sci. Transl. Med 3(94) (2011). DOI: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, Sandhu S, Carlisle TL, Smith MC, Abu Hejleh T, Berg DJ, Zhang J, Keech J, Parekh KR, Bhatia S, Monga V, Bodeker KL, Ahmann L, Vollstedt S, Brown H, Shanahan Kauffman EP, Schall ME, Hohl RJ, Clamon GH, Greenlee JD, Howard MA, Schultz MK, Smith BJ, Riley DP, Domann FE, Cullen JJ, Buettner GR, Buatti JM, Spitz DR, Allen BG, O2 - and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate, Cancer Cell 31(4) (2017) 487–500. DOI: 10.1016/j.ccell.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC, Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350(6266) (2015) 1391–1396. DOI: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q, High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med 6(222) (2014) 222ra18 DOI: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- [35].Kruse JP, Gu W, Modes of p53 regulation, Cell 137(4) (2009) 609–622. DOI: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nikulenkov F, Spinnler C, Li H, Tonelli C, Shi Y, Turunen M, Kivioja T, Ignatiev I, Kel A, Taipale J, Selivanova G, Insights into p53 transcriptional function via genome-wide chromatin occupancy and gene expression analysis, Cell Death Differ 19(12) (2012) 1992–2002. DOI: 10.1038/cdd.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blackford AN, Jackson SP, ATM ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response, Mol. Cell 66(6) (2017) 801–817. DOI: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- [38].Pannunzio NR, Watanabe G, Lieber MR, Nonhomologous DNA end-joining for repair of DNA double-strand breaks, J. Biol. Chem 293(27) (2018) 10512–10523. DOI: 10.1074/jbc.TM117.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Burger RM, Peisach J, Horwitz SB, Activated bleomycin. A transient complex of drug, iron, and oxygen that degrades DNA, J. Biol. Chem 256(22) (1981) 11636–11644. [PubMed] [Google Scholar]
- [40].Shiloh Y, Ziv Y, The ATM protein kinase: regulating the cellular response to genotoxic stress, and more, Nat. Rev. Mol. Cell. Biol 14(4) (2013) 197–210. [PubMed] [Google Scholar]
- [41].Lee JH, Paull TT, ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex, Science, 308(5730) (2005) 551–554. DOI: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- [42].Falck J, Coates J, Jackson SP, Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage, Nature 434(7033) (2005) 605–611. DOI: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- [43].Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT, ATM activation by oxidative stress, Science, 330(6003), (2010) 517–521. DOI: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- [44].Lee JH, Mand MR, Kao CH, Zhou Y, Ryu SW, Richards AL, Coon JJ, Paull TT, ATM directs DNA damage responses and proteostasis via genetically separable pathways, Sci. Signal 11(512) 2018. DOI: 10.1126/scisignal.aan5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kozlov SV, Waardenberg AJ, Engholm-Keller K, Arthur JW, Graham ME, Lavin M. Reactive Oxygen Species (ROS)-Activated ATM-Dependent Phosphorylation of Cytoplasmic Substrates Identified by Large-Scale Phosphoproteomics Screen, Mol. Cell. Proteomics 15(3) (2016) 1032–1047. DOI: 10.1074/mcp.M115.055723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ortega-Atienza S, Wong VC, DeLoughery Z, Luczak MW, Zhitkovich A, ATM and KAT5 safeguard replicating chromatin against formaldehyde damage, Nucleic Acids Res 44(1) (2016) 198–209. DOI: 10.1093/nar/gkv957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kaidi A, Jackson SP, KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signaling, Nature, 498(7452) (2013) 70–74. DOI: 10.1038/nature12201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [48].Som S, Raha C, Chatterjee IB, Ascorbic acid: a scavenger of superoxide radical, Acta Vitaminol. Enzymol 5(4) (1983) 243–250. [PubMed] [Google Scholar]
- [49].Krall J, Bagley AC, Mullenbach GT, Hallewell RA, Lynch RE, Superoxide mediates the toxicity of paraquat for cultured mammalian cells, J. Biol. Chem 263(4) (1988) 1910–1914. [PubMed] [Google Scholar]
- [50].Huang TT, Yasunami M, Carlson EJ, Gillespie AM, Reaume AG, Hoffman EK, Chan PH, Scott RW, Epstein CJ, Superoxide-mediated cytotoxicity in superoxide dismutase-deficient fetal fibroblasts, Arch. Biochem. Biophys 344(2) (1997) 424–432. DOI: 10.1006/abbi.1997.0237. [DOI] [PubMed] [Google Scholar]
- [51].Cunningham ML, Peak JG, Peak MJ, Single-strand breaks in rodent and human cells produced by superoxide anion or its reduction products, Mutat. Res 184(3) (1987) 217–222. DOI: 10.1016/0167-8817(87)90019-8. [DOI] [PubMed] [Google Scholar]
- [52].Henle ES, Linn S, Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide, J. Biol. Chem 272, 199719095–19098. [DOI] [PubMed] [Google Scholar]
- [53].Blaszczak W, Barczak W, Masternak J, Kopczynski P, Zhitkovich A, Rubis B, Vitamin C as a modulator of the response to cancer therapy, Molecules 24(3) (2019) E453 DOI: 10.3390/molecules24030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC, Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer, Cancer Res 70(14) (2010) 5749–5758. DOI: 10.1158/0008-5472.CAN-10-0263. [DOI] [PubMed] [Google Scholar]
- [55].Kuiper C, Dachs GU, Munn D, Currie MJ, Robinson BA, Pearson JF, Vissers MC, Increased tumor ascorbate is associated with extended disease-free survival and decreased hypoxiainducible factor-1 activation in human colorectal cancer, Front. Oncol 4(10) 2014. DOI: 10.3389/fonc.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Anthony HM, Schorah CJ, Severe hypovitaminosis c in lung-cancer patients: The utilization of vitamin c in surgical repair and lymphocyte-related host resistance, Br. J. Cancer 46(3) (1982) 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Huijskens MJAJ, Wodzig WKWH, Walczak M, Germeraad WTV, Bos GMJ, Ascorbic acid serum levels are reduced in patients with hematological malignancies, Results Immunol 6 (2016) 8–10. DOI: 10.1016/j.rinim.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mayland CR, Bennett MI, Allan K, Vitamin C deficiency in cancer patients, Palliat. Med 19(1) (2005) 17–20. DOI: 10.1191/0269216305pm970oa. [DOI] [PubMed] [Google Scholar]
- [59].Kantidze OL, Velichko AK, Luzhin AV, Petrova NV, Razin SV, Synthetically Lethal Interactions of ATM, ATR, and DNA-PKcs, Trends Cancer 4(11) (2018) 755–768. DOI: 10.1016/j.trecan.2018.09.007. [DOI] [PubMed] [Google Scholar]
- [60].Burgy O, Wettstein G, Bellaye PS, Decologne N, Racoeur C, Goirand F, Beltramo G, Hernandez JF, Kenani A, Camus P, Bettaieb A, Garrido C, Bonniaud P, Deglycosylated bleomycin has the antitumor activity of bleomycin without pulmonary toxicity, Sci. Transl. Med 8(326) (2016) 326ra20 DOI: 10.1126/scitranslmed.aad7785. [DOI] [PubMed] [Google Scholar]
- [61].Schroeder BR, Ghare MI, Bhattacharya C, Paul R, Zaleski PA, Bozeman TC, Rishel MJ, Hecht SM, The disaccharide moiety of bleomycin facilitates uptake by cancer cells, J. Am. Chem. Soc 136(39) (2014) 13641–13656. DOI: 10.1021/ja507255g. [DOI] [PMC free article] [PubMed] [Google Scholar]