Abstract
Women are more likely to develop Post Stroke Depression (PSD) than men and generally do not respond well to anti-depressants with age. This study investigated the effect of microRNA mir363-3p treatment on PSD using a physiologically-relevant animal model. Our previous work showed that mir363-3p treatment, delivered post-stroke, effectively reduces infarct volume in the acute phase of stroke in middle-aged females but not males. Middle-aged female Sprague Dawley rats were tested for baseline sensory motor function and depressive-like behaviors, and then subjected to ischemic stroke via middle cerebral artery occlusion (MCAo) or sham surgery. Animals received either control oligos (MCAo+scrambled, Sham+scrambled) or mir363-3p (MCAo+mir363-3p, Sham+mir363-3p) treatment 4h later. Sensory motor function and depressive-like behaviors were reassessed up to 100d after stroke, and circulating levels of IL-6, TNF-alpha and Brain-Derived Neurotrophic Factor (BDNF) were quantified at regular intervals. Prior to termination, Fluorogold was injected into the striatum to assess meso-striatal projections. MCAo+scrambled animals had impaired sensorimotor performance in the acute phase (5days) of stroke and developed anhedonia, decreased sociability and increased helplessness in the chronic phase. MCAo+mir363-3p animals showed significantly less sensory motor impairment and fewer depressive-like behaviors. IL-6 and TNF-alpha were elevated transiently at 4weeks after MCAo in both groups. BDNF levels decreased progressively after stroke in the MCAo+scrambled group, and this was attenuated in the mir363-3p group. The number of retrogradely-labeled SNc and VTA cells was reduced in the ischemic hemisphere of the MCAo+scrambled group. In contrast, there was no interhemispheric difference in the number of retrogradely-labeled SNc and VTA cells of MCAo+mir363-3p treated animals. Our results support a therapeutic role for mir363-3p for long-term stroke disability.
Keywords: T maze Cost/benefit test, Forced swim test, social interaction test, cytokines, BDNF, mesostriatal pathway
INTRODUCTION
In addition to physical and cognitive impairments, post-stroke depression (PSD) is a critical, long-term consequence of stroke. PSD is characterized by anxiety, anhedonia, social dysfunction and feelings of despair[1–3]. About one-third of stroke survivors develop PSD[4], which not only affects their quality of life but also adversely influences their recovery[5, 6]. Women are more likely to develop PSD than men[7]. Although the mechanistic explanation for this sex difference is poorly understood, it may be related to greater disability after stroke in women, which makes them less independent in their daily activities and consequently more likely to be in assisted-living facilities[8, 9]. Social isolation and lower quality of life resulting from institutional living also contributes to the development of PSD.
Depression is associated with several central and peripheral biochemical markers, including changes in monoamine neurotransmitter levels [10, 11], inflammatory cytokines [12–14], and neurotrophic factors [15, 16]. Anti-depressant therapies therefore include tricyclic drugs (TCA) that increase levels of norepinephrine and serotonin, or newer drugs such as the serotonin reuptake inhibitors (SSRI). However, the SSRI fluoxetine is not efficacious for PSD until at least a year of administration[17] and the TCA family of drugs such as imipramine, amitriptyline and amoxapine are not as effective for PSD in older (65+ years) women compared to age-matched men or young females[18]. This resistance to conventional anti-depressants suggests the likelihood of a unique pathophysiology of PSD.
In animal models, occlusion of the middle cerebral artery (MCAo), which is the most commonly affected artery in ischemic stroke, can result in a depressive phenotype[19–21]. MCAo damages the striatum and overlying cortex and loss of striatal growth factors such as BDNF may contribute to the depressive phenotype[20, 22]. Accordingly, BDNF treatment after stroke has been shown to reduce post stroke depression [23]. The striatum also receives projections from midbrain dopaminergic neurons, and loss of these ‘reward’ pathways may constitute another substrate for post stroke depression.
We hypothesize that if ischemic cell loss contributes to the pathology of PSD, then drugs that reduce infarct volume in the acute stage of stroke may also reduce PSD. Our recent studies show that a small non-coding RNA mir363-3p is inversely correlated with infarct size and stroke outcome [24], and a single IV injection of mir363-3p, delivered 4h after MCAo, reduces infarct volume and improves sensory-motor performance in middle-aged female rats [25]. Remarkably, mir363-3p treatment to age-matched males had no effect on infarct volume or sensory motor impairment, adding to the growing literature on stroke neuroprotectants that display sex-specific effects. Hence this study focused on the long-term effects of mir363-3p treatment in females only. Middle-aged female rats were subject to MCAo and treated 4h later with mir363-3p and assessed for motor impairment and depressive behaviors for a period of 3 months. We report that MCAo leads to sensory motor impairment in the acute stage of ischemia, which resolves over time, and depressive-like behaviors, which are observed between 30 days and 100+ days post stroke. Treatment with mir363-3p attenuates both acute motor impairment and long-term depressive behaviors in middle-aged females. These data are consistent with the hypothesis that ischemic pathology contributes to PSD and that neuroprotectants, that reduce infarct volume, may act as anti-depressant therapies for PSD.
Methods and Materials
Animals and estrous cycle determination:
Middle-aged female rats (10-12 months, 230-340g) were purchased from Envigo (IN). All animals were housed in a temperature (22°C) and humidity (45-55%) controlled AAALAC-accredited vivarium facilities on a 12/12 h light and dark cycle. Water and food were available ad libitum. Three weeks after arrival, the rats underwent daily vaginal smearing for up to 21 days to determine estrous status as described previously [26–29]. Cotton swabs were used to obtain vaginal smears and cells were deposited on glass slides. When the animals displayed cell cytology indicating constant diestrus for at least 7 days, they were included in the study. Our previous studies have shown that females in constant diestrus have low estradiol levels and elevated FSH levels, which resembles salient hormonal aspects of the post-menopausal stage in women ([26, 30]). A total of 40 animals were used in these studies. A timeline of the procedures and behavioral assays is shown in Fig 1. All procedures were performed under approved protocols in accordance with institutional guidelines for humane treatment of animals. Animals were randomly assigned to scrambled control or mir363-3p treatment groups after middle cerebral artery occlusion and sham surgeries. All behavioral tests were performed between 8:00h and 12:00h, and experimenters were blind to the treatment conditions while performing and analyzing tests.
Fig 1:
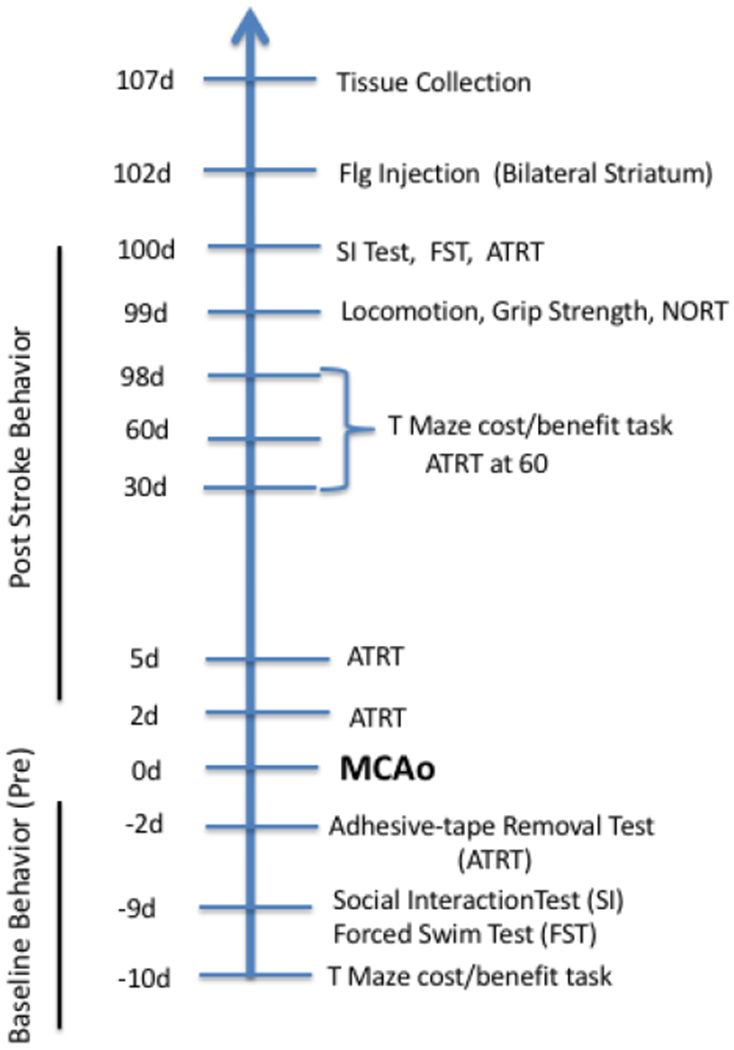
Experimental timeline of behavioral tests in relation to stroke (MCAo): MCAo is demarcated as 0d (day 0). All animals were injected with Fluorogold (Flg) after the last test and terminated 5 days later. ATRT: Adhesive Tape Removal Test; FST: Forced Swim Test; SI: MCAo: Middle Cerebral Artery occlusion; NORT: Novel Object Recognition Test; Social Interaction test.
Middle Cerebral Artery Occlusion (MCAo):
Animals were subjected to MCAo by stereotaxic injection of a vasoconstrictor, Endothelin-1 (ET-1), using our previously established procedures[24, 25, 31, 32]. Animals were anesthetized (20mg/ml/kg xylazine and 100 mg/kg ketamine) and placed in a stereotaxic equipment. Endothelin-1 (ET-1) (American Peptide Company INC; 0.5ug/ul, 600 pmol; 3 uls) was microinjected to occlude the left middle cerebral artery at the following coordinates relative to bregma: AP: +0.9, ML: −3.4, DV: −8.5. Body temperature was maintained at 37 °C throughout the surgery and in the home cage until recovery from anesthesia. For Sham surgeries, animals were subject to all other surgical procedures (such as anesthetic, scalp incision, drilling of the skull), but did not receive ET-1.
Four hours after ET-1 or Sham surgery, animals received either control oligos (scrambled) or miR-363-3p delivered via tail vein injection. Scrambled and Mir363-3p mimic (AAUUGCACGGUAUCCAUCUGU) oligonucleotide sequences were purchased from Thermo Fisher, Grand Island, NY and packaged in In-vivo RNA-LANCEr II (Bio-Scientific, Austin, TX).
Behavioral Assays:
All the behavior tests were performed between 8:00 am-12:00 pm in the light cycle. A timeline of these tests is shown in Fig 1 and described here. For assessing sensorimotor function, adhesive-tape removal test was done before (−2d) and after (2d, 5d, 60d and 100d) stroke. Similarly, assays for depressive-like phenotype were performed before and after stroke. Forced Swim Test and Social Interaction: −9d and 100d. T Maze cost/benefit task: −10d, 30d, 60d and 98d. Grip strength, Locomotion and Novel object recognition was performed at 99d after stroke. All the tests were performed and scored blinded.
Adhesive-tape removal test was performed prior to and after MCAo using our previously published methods[24, 25, 31, 33]. Adhesive-backed foam tape (12.7 × 12.7 mm) was attached to the palmar surface of the paw of each forelimb. For each limb, the time taken to remove the tape was recorded for three trials. Animals were allowed to rest for 5 min between trials and each trial had a maximum time limit of 120s.
Forced Swim Test:
This test was used to determine helplessness[20]. Rats were placed in an open cylinder (diameter 30 cm, height 60cm) filled with water (23-25°C) to a height of 40 cm for 5 min. Sessions were video-recorded with an overhead camera and the duration of immobility was scored by a blind observer. A rat was judged to be immobile if it was passively floating in water with only movements necessary to keep its head above water.
Social Interaction Test:
A three-chambered Plexiglass box was used for the social interaction test. Each chamber had a dimension of 40”×13”and all chambers had open access to each other. For habituation, the three chambers were closed off from each other, and the test rat was placed in each chamber for 2 min. Thereafter, the rat was allowed to freely explore the 3 chambers for additional 10 min and then returned to the home cage. For the testing session, a conspecific (stranger) rat was placed within a plastic mesh cylinder in one of the end chambers, while the test rat was placed back in the middle chamber and allowed to explore for 10 min. The time spent in each chamber was recorded and sociability was scored as the total time (in seconds) spent by the test rat in the chamber with the stranger rat.
T-maze cost/benefit task:
This test was used to determine anhedonia. The physical apparatus consisted of a T-maze, where the 2 end arms were 20” long and 5” wide, and the central limb was 6” long and 6” wide. Each arm was baited with sucrose pellets during the test session. For habituation, the rats were allowed to freely explore the maze for 2 consecutive days for 30 min each day and sucrose pellets placed on the floor of the home-cage for 2 days. For training (daily for 6 weeks), rats were deprived of food for 12 hours before the training on each alternate day for the first 2 weeks and were given 10 trials to choose an arm. After each trial, the rat was placed in its home cage, for one minute, and then placed back to the start arm of the maze. For the first week, a small barrier (6 cm rectangular box) was placed midway on one arm of the T-maze and a sucrose pellet placed in the far corner of the arm. The rats were trained for to go over the small barrier to retrieve the sucrose pellet. From the second week, a large barrier (12 cm rectangular box) was also added midway down the other arm of the T-maze and 4 sucrose pellets placed in the far corner of this arm. Arm choice was recorded for each trial up to 10 trials. After 6 weeks of training, the test was repeated 10 days before the stroke as a pre-stroke baseline measure. Following stroke, the test was repeated at 30d, 60d and 98d after MCAo.
Open Field Test:
Locomotor activity was assessed using the Open Field Arena (Kinder Scientific, CA). Rats were placed in the middle of a clear acrylic box (16” by 16”) and allowed to freely explore for 30 min. General locomotor activity as indicated by the total number of beam breaks over 30 min as well as the percent of beam breaks in the center of the field was quantified using MotorMonitor Software.
Novel Object Recognition Test:
This test was used to assess cognition. This test consisted of three phases: habituation, familiarization, and test phase. In the habituation phase, the rat was placed in 16”×16” open field and allowed to freely explore the arena for 10 min each day for 2 days. On the third day (familiarization phase), the animal was again placed in the open-field apparatus, which now contained two identical objects (A + A) placed diagonally from each other. The rat was allowed to explore the arena and the objects for 10 minutes. The rat was then returned to its home cage for 1h (retention interval) and then placed again in the open-field arena for the test phase. For the test phase, the arena contained two objects in the same location, one that was previously available (A) and the other that was novel (B). The rats’ behavior was recorded for 5 mins and the amount of time spent exploring the novel object was determined from these recordings by an investigator blind to the experimental condition. Exploration of an object was defined as the animal’s snout directed to the object, sniffing or touching the object with its snout at a distance <2 cm to the object and/or touching it with the nose. Running around the object, sitting or climbing on it was not recorded as exploration. The Novel object preference (%) was calculated as: (Time spent exploring Novel object)/(Time spent exploring Novel + Familiar object) *100.
Grip Strength Test:
Grip strength was measured to ensure that MCAo-induced physical disability did not contaminate performance on the depressive behaviors. Rats were held by the tail and lowered towards the rod attached to the grip strength meter. Once the animal grabbed the bar, it was pulled backwards in a horizontal plane. The force applied to the bar just before it loses grip was recorded as the peak tension. Three such trials were performed, and the mean peak tension was normalized to body weight in grams.
Cytokine Assay:
Rat cytokine/chemokine assay kit (MAP kit, Millipore, CA) was used to quantify a panel of inflammatory cytokines/chemokines in serum, using manufacturer’s instructions and our previously established procedures [29]. Plates were read on a Bio-Plex suspension array system (Bio-Rad Laboratory, CA).
BDNF expression:
BDNF levels were quantified in serum samples using a Rat BDNF ELISA Kit (ThermoScientific, MA) and manufacturer’s instructions. BDNF was detected by a sandwich ELISA and a colorimetric readout. Absorbance was measured on ELISA microplate reader set to 450nm. Sample unknowns were interpolated from a standard curve.
Assessment of the meso-striatal pathway:
Retrograde labeling by fluorogold was used to assess meso-striatal pathway 100d+ after stroke. Animals were anesthetized and placed in a stereotaxic frame. Fluorogold (0.2ul of 2% (dissolved in de-ionized water), Santa Cruz Biotechnology, TX) was injected into both the left and right striatum at 2 depths (0.1 ul in each depth) using 1-ul Hamilton microsyringe. The coordinates for the injection from bregma were as follows: 1mm anterior, 3mm lateral, 4.5mm and 5.5mm ventral from dura. The needle was slowly retracted 5 minutes after injection to prevent backflow. Five days later, rats were deeply anesthetized and perfused transcardially with saline followed by 4% formaldehyde. The brain was removed and submerged in 4% paraformaldehyde overnight. Thereafter brains were prepared for block embedding and sectioning (30um) (NeuroScience Asssociates, TN). Sections through the striatum were inspected for Flg label and the rostro caudal extent of the Flg injection was calculated for each hemisphere for each animal. Animals where the injections did not cover 75% of the rostrocaudal extent of the striatum would be excluded from further analysis. No animal met this criterion and thus all injected animals were analyzed. Subsequently, sections through the SNc and VTA were imaged under fluorescent illumination (4× magnification) for Flg and photographed using Q-capture (QImaging, BC, Canada). Three sections per animal, 240 micrometers apart, were selected and brightly fluorescent neurons in the SNc and VTA region was counted in both hemispheres using ImageJ. The total number of cells in each region was added together for each hemisphere.
Statistical Analyses:
For all assays, group mean ±SEM are reported. Group differences were determined by a two-way ANOVA performed for surgery (Sham/MCAo) and treatment (Scrambled/Mir363-3p), with planned comparisons. For analyses where only 2 groups were compared (mesostriatal projections), a student t-test was used. All group differences were considered significant at p<0.05. Statistics were analyzed using Prism GraphPad (GraphPad, San Diego, CA) and SPSS (IBM Corporation).
RESULTS:
Mir363-3p improves sensory-motor deficit after stroke:
Sensory motor performance was evaluated by the Adhesive-tape removal test, which was performed at 5 different time points: prior to MCAo (baseline/pre), 2d, 5d, 60d and 100d after MCAo. As shown in Fig 2, animals removed the tape rapidly at the pre-stroke time point but were significantly delayed in the early acute stroke phase (2-5d) in both the MCAo+scrambled and MCAo+mir363-3p group as compared to pre-stroke performance (F(4,68): 5.189, p=0.0010). At 5d post stroke, planned comparison showed that the MCAo+scrambled group was significantly delayed compared to its pre-stroke timings (p=0.0101), while the mir363-3p treated group was not different from baseline. Both groups recovered substantially in the chronic phase where their performance was not different from baseline at 60d (p=0.7518), or 100d (p>0.9999) after stroke. Thus, mir363-3p treatment accelerates the rate of sensory motor recovery as compared to controls. This is consistent with previous work that showed that mir363 treatment reduced sensorimotor deficit and reduced infarct volume in the acute phase following MCAo[25].
Fig 2:
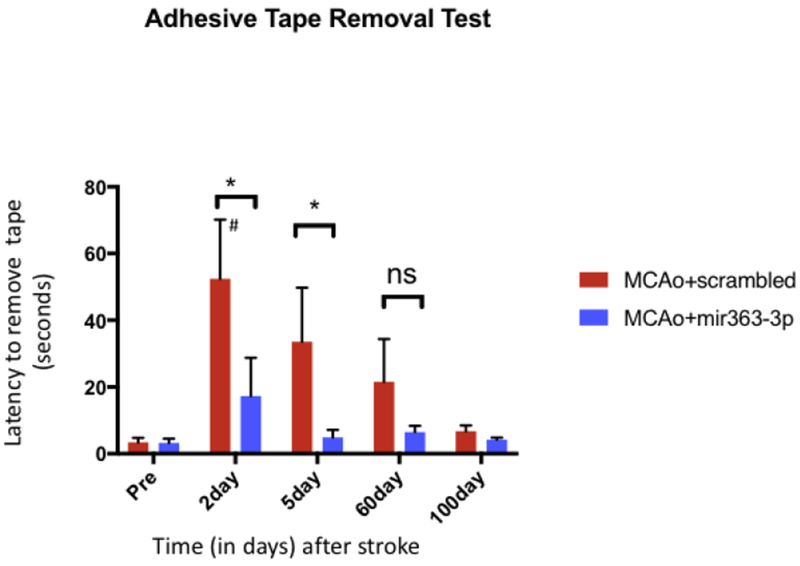
Assessment of stroke-associated sensorimotor impairment: Histogram depicts mean±SEM of the latency (in seconds) to remove an adhesive tape from the forepaw of the limb contralateral to the ischemic hemisphere. This test was performed before (Pre) and at 2, 5, 60 and 100d after stroke. Key: #: p<0.05, comparison of the group with its baseline levels; *: p<0.05, comparison of treated vs control groups at the same time point. ns: not significant.
Mir363-3p treatment abrogates the development of depressive-like behaviors after stroke
Depressive behaviors were assessed by a battery of tests including T-maze cost/benefit task, social interaction and forced swim test (Fig 1) to capture the following domains of depressive-like behaviors: anhedonia, reduced sociability and vulnerability to helplessness in stressful events, respectively.
T-maze cost/benefit task:
Anhedonia was assessed using a T-maze cost/benefit task, which gives rats two options: Climb a high barrier for high reward (4 sucrose pellets) or climb a low barrier for low reward (1 sucrose pellet). This test was performed at 4 different time points: Before MCAo (baseline/pre), 30d, 60d and 98d after MCAo. At baseline, animals displayed a preference for the high barrier-high reward over 80% of the time (Fig 3). After MCAo, both groups (MCAO+scrambled and MCAo+mir363-3p) showed a gradual decrease in their choice of high barrier-high reward (main effect of time, F(1,27): 77.82, p<0.0001), compared to sham controls. Moreover there was a significant effect of treatment (F(1,27): 4.35, p=0.047). In the MCAo+scrambled group, this preference dropped from 87% at baseline to 22% at 30d, 14% at 60d and 5.5% at 98d. In the MCAO+mir363-3p group, high barrier preference decreased from 90% at baseline to 44% at 30d, 40% at 60d and 30% at 98d. At each time point after stroke, the MCAo+mir363-3p group showed a greater preference for the high barrier/high reward than MCAo+scrambled (30d: p=0.08, 60d: p=0.024 and 98d: p=0.034). Since the behavioral assay spanned 3 months, sham surgery groups, treated with scrambled oligos or mir363-3p were also compared to assess if time/age would affect performance. As shown in Fig 3, sham groups treated with either mir363 or scrambled oligos did not show any decrease in high barrier/high reward choice up to 98 days.
Fig 3:
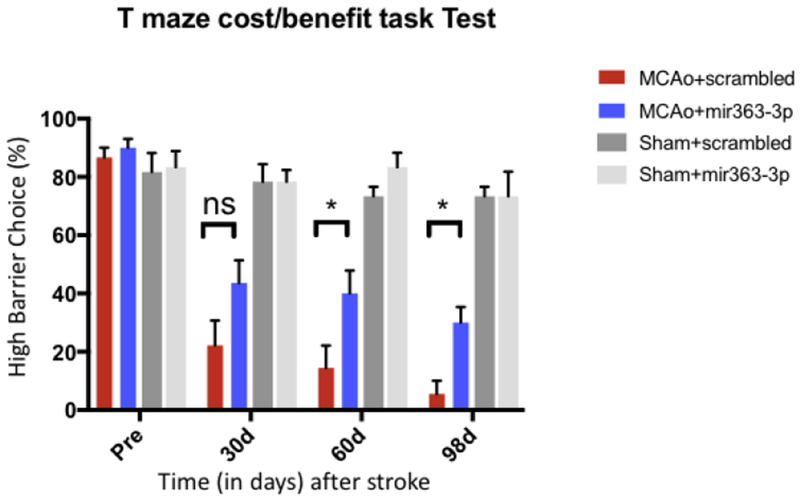
T-maze cost/benefit task Test: Stroke and sham animals, treated with either scrambled oligos or mir363-3p, were assessed on this test prior (Pre) to stroke (or sham) surgery and 30d, 60d, 98d after stroke. Histogram depicts mean±SEM % preference for the high barrier/high reward high. Key: *: p<0.05, comparison of treated vs control groups at the same time point. ns: not significant.
Social Interaction (SI) test:
The total amount of time spent in the chamber containing the conspecific stranger rat was recorded as a measure of sociability. This test was performed at 2 time points: Before MCAo (Pre) and 100d after MCAo and at equivalent time points for the Sham (no stroke) group. There was an overall effect of stroke and drug treatment (F(3,28): 7.46, p=0.001) Before MCAo, the test rats interacted with the stranger rat for over 400 seconds out of 600 seconds. At 100d after stroke, sociability decreased by 67% in the MCAo+scrambled group as compared to baseline, while the MCAo+mir363 group was not different from baseline (Fig 4). Social interaction was therefore significantly higher in the MCAo+mir363 group than the MCAo+scrambled group at 100d (p<0.0001). Sham groups treated with either mir363-3p or scrambled oligos had similar baseline values and did not show any reduction in sociability at 100d.
Fig 4:
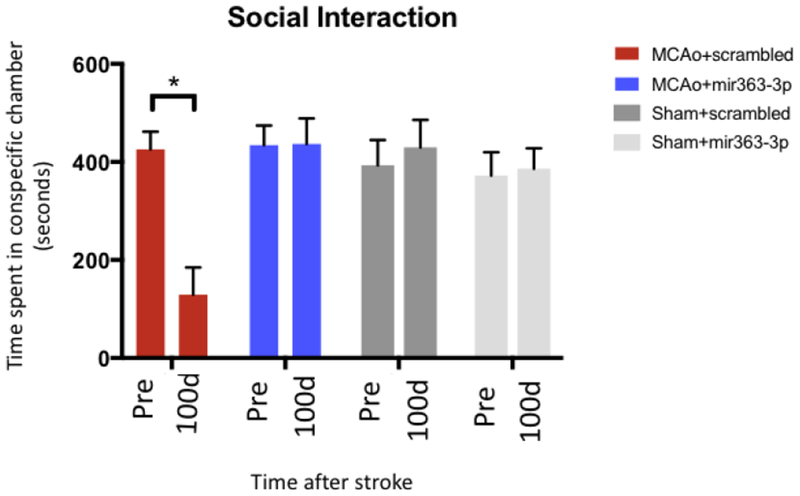
Social Interaction test: Stroke and sham animals, treated with either scrambled oligos or mir363-3p, were tested for social interaction with a conspecific stranger prior (pre) and 100d after stroke. Histogram depicts mean±SEM time spent with the conspecific. Key: *: p<0.05, comparison of the group with its baseline levels.
Forced Swim Test (FST):
Vulnerability to helplessness was assessed using the Forced Swim Test (Fig 5), defined as the total time a rat spends passively floating in the water. Greater immobility time indicates that the rat has ‘given up’ in the stressful environment. This test was performed before MCAo (Pre) and 100d after MCAo and at equivalent time points for the Sham (no stroke) group. There was an overall effect of MCAo and treatment (F(3,28): 3.48, p=0.0290). Sham groups treated with either scrambled oligos or mir363-3p had similar baseline values and showed no significant changes in immobility at 100d (p=0.6891). In groups subject to MCAo, the duration of immobility in the scrambled group was 185.5 secs and was elevated to 246.33 secs after MCAo, an increase in 36% over baseline (p=0.0026). In contrast, the mir363-3p treated group had a higher immobility at baseline (224.27 secs) which rose after stroke to 238.8 secs, which was not significantly different from baseline (p=0.385).
Fig 5:
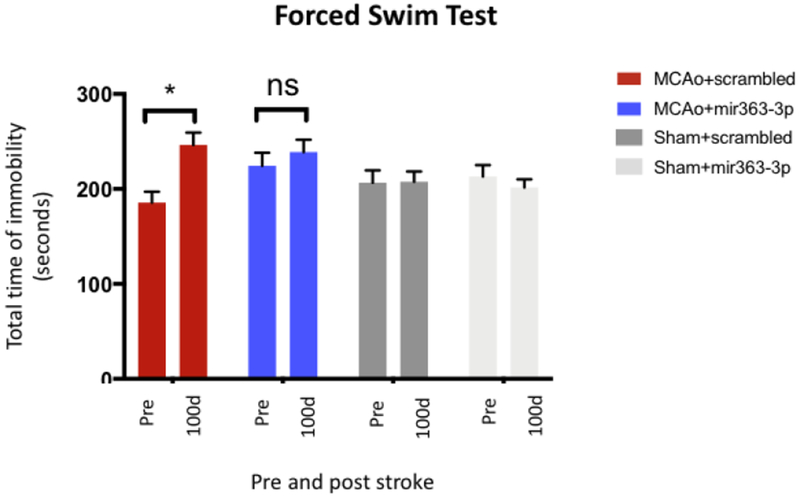
Forced Swim Test: Stroke and sham animals, treated with either scrambled oligo or mir363-3p, were tested for immobility in the Forced Swim Test. Histogram depicts the mean±SEM amount of time spent immobile. *:p<0.05, comparison of the group with its baseline levels; ns: not significant.
Locomotion, motor strength and cognition at 3+ months after stroke:
Animals were tested for locomotion, motor strength and cognition at 3+ months (99d) after stroke to ensure that performance on the tests of depression were not confounded by motor or memory impediments. Locomotion was assessed by total X/Y beam breaks in the open field chamber during a 30 min test period. There were no differences in the number of beam breaks in the Sham+scrambled, Sham+mir363-3p, MCAo+scrambled and MCAo+mir363-3p groups over the 30 min testing period (Fig 6A; F(3,25): 1.616, p=0.211). There was also no difference in the proportion of time spent in the center between the groups at 3+ months (F(3,25): 0.2579, p=0.8550). Similarly, the Grip Strength Test showed that there was no difference in motor strength in the Sham or MCAo groups treated with either scrambled or mir363-3p (Fig 6B; F(3,26): 0.295, p=0.82). The force of the grip normalized to the body weight ranged from 2.47 - 2.65 g/g for all the groups, indicating that, similar to sensory motor function (Fig 2), motor strength is also completely recovered by 3 months after stroke. The Novel Object Recognition Test revealed that Sham+scrambled (80.04%), Sham+mir363-3p (70.63%), MCAo+scrambled (69.24%) and MCAo+mir363-3p (78.3%) groups all showed a greater preference for the novel object compared to the familiar object, and there were no group differences in novel object preference (Fig 6C; F(3,26): 0.513, p=0.67). Collectively, these data suggest that impaired performance on the depressive-behavior tests was not contaminated by impaired locomotion, motor deficits or cognitive decline at the time of the test.
Fig 6:
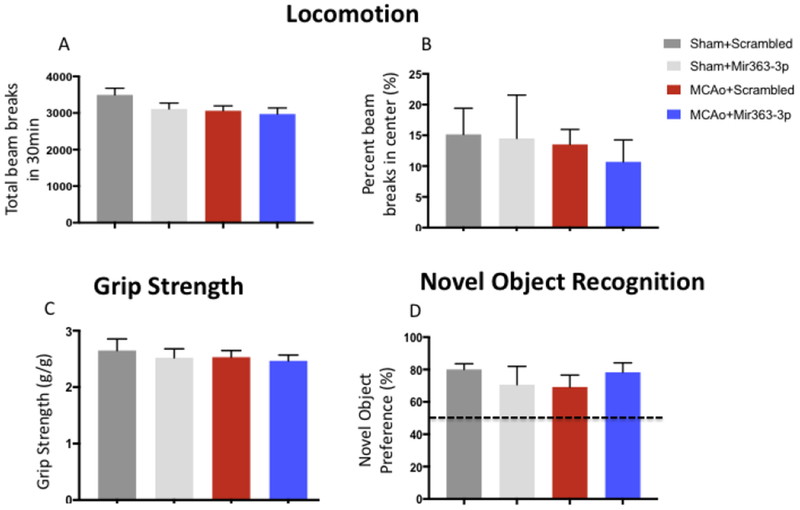
Locomotion, motor strength and cognitive changes at 3+ months (99d) after stroke: (A) Locomotor impairment was assessed by the total number of beam breaks and beam breaks in the center in an open field apparatus. (A) The total number of beam breaks was no different between sham or MCAo groups treated with either scrambled oligos or MCAo+mir363-3p at 99d after stroke. (B) The percent time spent in the center of the open field was not different in any of the groups. (C) Motor impairment was tested using the grip strength meter. The peak tension force for forelimb grip was no different between the sham or MCAo groups treated with either scrambled oligos or MCAo+mir363-3p at 99d after stroke. (D) Novel Object Recognition: Novel object recognition test was used to assess loss of cognitive capacity. All groups showed a greater preference (>50%; dotted line) for the novel object than the familiar object and were statistically no different from each other.
Growth factor and inflammatory changes after stroke:
Low levels of BDNF and elevated levels of inflammatory cytokines IL-6 and TNF-a have been implicated in depression in both preclinical and clinical studies[20, 34, 35].
Cytokines: IL-6:
As shown in Fig 7A, there was a time effect (F(3,27): 7.805, p=0.0007) and treatment effect (F(1,27): 6.273, p=0.0186). IL-6 levels were no different in each group at baseline but were significantly elevated above baseline at 30d after stroke (p=0.0018). This increase was mainly due to elevated levels of IL-6 in the MCAo+scrambled group (planned comparisons, p=0.0023). At 60d and 100+d there were no differences between the groups. A similar pattern was seen in TNF-alpha (Fig 7B, time effect F(3,65): 6.915, p=0.0004), with a significant elevation over baseline at 30d in the MCAo+scrambled group (p=0.0160). At 60d and 100d there were no differences over baseline or between the groups. BDNF: Due to limited volume of serum samples, pre-stroke levels of BDNF are not available. BDNF levels were measured at 30, 60 and 100d after stroke. As shown in Fig 7C, BDNF expression decreased over this time course in the MCAo+scrambled group, such that neurotrophin expression at 100+d after stroke was one third of the level seen at 30d levels (p=0.0009). There was a time X treatment interaction effect F(2,34): 5.4, p=0.0092. By contrast, BDNF expression in the MCAo+mir363-3p group showed a scalloped pattern; BDNF levels decreased at 60d compared to 30d after stroke, and then recovered to 30d levels when measured at 100d post stroke. At 100d post stroke, BDNF levels were significantly higher in the MCAo+mir363-3p group as compared to the MCAo+scrambled group (p=0.0170).
Fig 7:
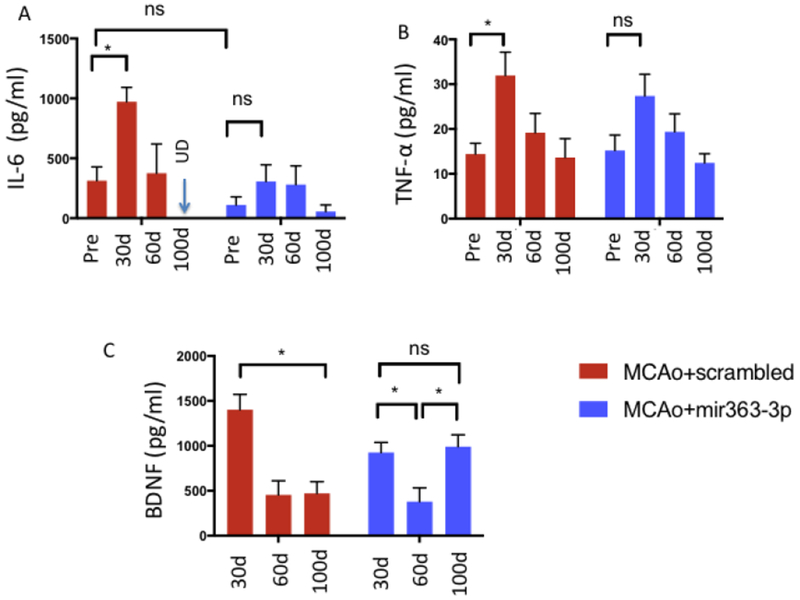
Time course of cytokine and neurotrophin expression after stroke: Circulating levels of IL-6 (A) and TNF-alpha (B) were significantly elevated at 30d after MCAo as compared to baseline in the MCAo+scrambled group, but not the MCAo+mir363-3p treated animals. By 100d after stroke, both cytokines returned to baseline levels. C) Circulating BDNF levels decreased at 60 and 100d after stroke compared to 30d after stroke in the MCAo+scrambled group. In the MCAo+mir363-3p treated group, BDNF levels decreased at 60d compared to 30 days after stroke but returned to 30d levels by 100d after stroke. *: p<0.05; ns: not significant.
Mesostriatal projections after stroke:
Since BDNF levels and inflammatory cytokines are both known to affect neuroplasticity, we next investigated whether striatal infarction would, in the long term, affect reward circuitry. FluoroGold (Flg) was injected bilaterally into the striatum at 102 days after stroke to detect changes in the mesostriatal pathway. Shown in Fig 8A is a representative striatal injection site from the MCAO+scrambled and MCAo+mir363-3p groups. Visual inspection indicated that Flg label was detected throughout the striatum, spanning a rostrocaudal extent 1.7-1.9 mm (Paxinos Bregma interaural 10.70 mm to 8.70 mm). There were no differences in the mean rostrocaudal extent of tracer distribution between the ischemic and non-ischemic hemispheres, or between the MCAO+scrambled and MCAo+mir363-3p groups (Fig 8B). Fig 8C shows retrogradely-labeled neurons in the VTA and SNc in the ischemic and non-ischemic hemisphere of MCAo+scrambled and MCAo+mir363-3p animals. The proportion of retrogradely-labeled cells in these regions was significantly lower in the ischemic hemisphere (F(1,15): 5.068; p=0.04), compared to the non-ischemic hemisphere. Furthermore, there was a significant hemisphere X treatment interaction (F(1,15): 9.042, p=0.009), indicating lower numbers of retrogradely-labeled neurons in the ischemic hemisphere of the MCAo+scrambled group compared to the MCAo+mir363-3p group. These data indicate that the mesolimbic reward pathway undergoes stroke-induced plasticity, in association with depressive behaviors.
Fig 8:
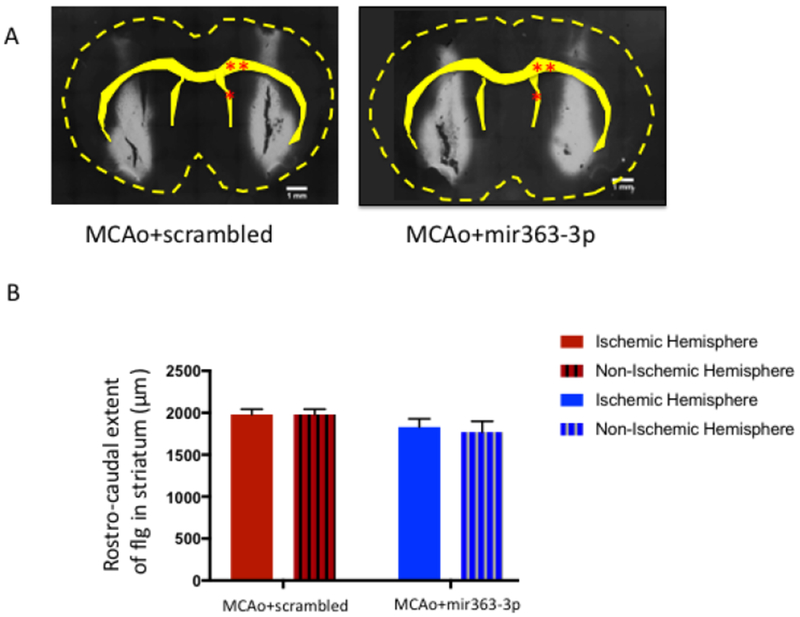
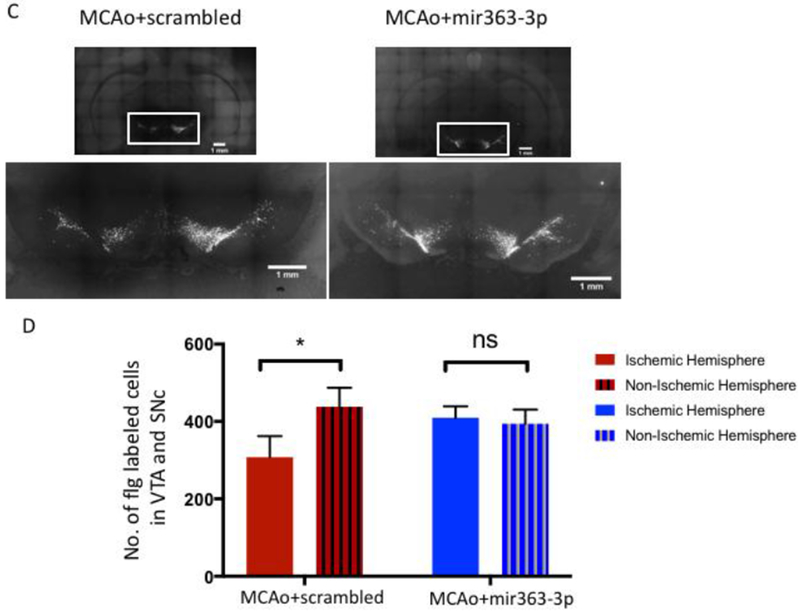
Assessment of the mesostriatal pathway at 3+ months (107d) after stroke: A) Photomicrograph depicting bilateral injections of Fluorogold into the striatum. The outline of the brain and key landmarks are shown in stippled yellow. B) The rostrocaudal extent of Fluorogold dye was similar in both hemispheres and in both groups, indicating similar amount of dye at the target site. C) Fluorogold labeling of SNc and VTA neurons: Photomicrograph of a coronal section depicting Flg-labeled neurons in the VTA and SNC from the MCAo+scrambled group (Ci) and MCAo+mir363-3p treated group (Cii). Boxed area in each image is shown in higher magnification below. D) Histogram depicts mean (±SEM) number of Flg-labeled cells in SNc and VTA in the ischemic and non-ischemic hemisphere. *: p<0.05, ns: not significant. Single red asterisk: Lateral ventricle, double red asterisk: Corpus callosum
Discussion
The present studies employed middle-aged female rats to assess the development of post stroke depression. Post stroke depression occurs in a subset of stroke patients, and disproportionately occurs in older female stroke patients. Preclinical studies thus far have not focused on this demographic. Our studies have three major findings: first, MCAo causes an early loss of sensory motor function that resolves in the chronic phase. Second, stroke results in depressive-like behavioral changes as measured by a cluster of tests, that are observed between 30d and 3+ months after MCAo. Finally, treatment with mir363-3p, administered i.v. 4h after MCAo, reduces sensory motor impairment in the acute phase, and attenuates depressive-like behaviors in the chronic phase. Moreover, mir363-3p treatment attenuates the loss of BDNF in the chronic phase. MCAo also resulted in decreased numbers of retrogradely-labeled cells in the mesostriatal reward pathway, which was attenuated in the mir363-3p tested group. Overall, these results indicate that a microRNA that is neuroprotective for middle-aged females in the acute phase of stroke [25] also improves PSD among the same population. In contrast, early sensory motor deficits and post-stroke depressive-like symptoms are worse in scrambled controls, suggesting that early neuroprotection (by mir363-3p) improves later consequences of stroke.
MiRNAs are important regulators of mRNA transcript stability[36] and gene translation[37] and play a significant role in disease processes. MiRNA profiles are altered with stroke in both human[38, 39] and experimental[40–43] models, and specific miRNA are associated with pathogenic processes that contribute to or exacerbate stroke, such as hyperlipidemia (mir33), hypertension (mir155), atherosclerosis (mir21, mir126) plaque rupture (mir222) (reviewed in[44]). We were among the first to show that miRNA can serve as stroke neuroprotectant. ICV injections of antagomirs to Let7f and mir1, which are predicted/validated targets for IGF-1, a neuroprotective factor, reduced infarct volume in adult female rats[32]. Mir181 and mir29 have been shown to reduce ischemia-induced cell death[45, 46], by targeting members of the Bcl-2 family of pro- and anti-apoptotic proteins. Subsequent studies have shown that exosomes from mesenchymal stromal cells [47] enriched with mir133b[48], mir124[49], mir17-92 [50]or their upregulation in neural progenitor cells are neuroprotective for stroke and leads to functional recovery in rodents[42, 47–49, 51]. Expression of mir363-3p, identified through miRNA profiling analysis[25], was negatively correlated with infarct volume. Subsequently, we reported that iv injections of mir363-3p mimics effectively reduced infarct volume and behavioral impairment in middle-aged females, but not males. Only a handful of validated targets are known for mir363-3p, which includes caspase-3, a cell death effector, [52], and genes associated with tumor suppression such as proliferating cell nuclear antigen (PCNA) [53], high mobility group AT-Hook 2 (HMGA2) [54], and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) [55]. Our studies showed that mir363-3p treatment after stroke reduces caspase-3 expression and activation in middle-aged females but not males. This is consistent with previous studies that have defined caspase-3 pathway as a sex-specific stroke pathway [56, 57], and underscores the importance of sex-specific therapies for stroke. Few studies have examined the effect of miRNA treatment on long-term consequences of stroke. An exception to this is a recent study which showed that mir137 decreased depressive behaviors caused by stroke combined with chronic mild stress at 3 weeks, although it is not clear if mir137 is also neuroprotective[58]. The current studies show that mir363-3p, which exerts a neuroprotective effect in the early phase of stroke, also attenuates long-term consequences of stroke.
The present studies add to a growing literature showing that depressive behaviors develop after stroke and social isolation in a rodent model[19–21]. While it is a challenge to study depression in a rodent model, some inferences are possible using a cluster of tests that assess cardinal signs of the disease. A clinical (DSM-V) diagnosis of depression requires at least 5 symptoms over 2 weeks that show impaired social or occupational functioning. Anhedonia, reduced interest in social interactions and propensity for negative mood are some of the major hallmarks of depression and these attributes were assessed in our behavioral tests. Reduced social interaction and despair behavior were measured by the social interaction test[19] and by immobility in the FST[59, 60] respectively. Anhedonia is usually assessed by the sucrose preference test, although recent work suggests that this test per se, is not as sensitive a measure of depressive behavior as “effort based” behaviors for palatable rewards[61]. As a refinement to the anhedonia test[62], we adapted the T-maze cost/benefit task where the test subject has to expend effort (climbing over a barrier) to obtain a food-reward, a measure of diminished interest and low motivation. On these tests, performance after stroke (in the non-drug treated group) was worse when compared to baseline. The mir363-3p treated group either showed no change from baseline (as in the social interaction test and the FST) or a blunted response (as in the T-maze cost/benefit task). We further ensured that impairment on these depressive-behavior tests were not contaminated by loss of motor strength or dexterity or sensory-motor loss at the time of the tests. Moreover, it is possible that performance on the T-maze cost/benefit task may be impaired at 3+ months due to cognitive decline rather than affective decline. However, in the novel object recognition test, both the scrambled and the mir363-3p treated stroke groups displayed a greater interest in the novel object to a similar extent, indicating that group differences in the T-maze cost/benefit task is likely indicative of anhedonia and not loss of memory. Thus, by modeling three different components of depression, our data shows that depressive-like behavior develops in the long term in middle-aged female rats, which can be improved by mir363-3p treatment.
Multiple mechanisms have been proposed to explain the pathogenesis of depression. These include biochemical alterations, such as low levels of the monoamine neurotransmitters serotonin and catecholamine[10, 11], low expression of the neurotrophin BDNF in the frontal cortex and hippocampus[15, 16], or elevated expression of pro-inflammatory cytokines[14, 63]. Depression has also been linked to disruption of neural circuits that mediate forebrain reward pathways, including midbrain-striatum-cortex circuits[64, 65]. The present study shows partial support for several of these theories. Middle-aged female rats who received scrambled oligo treatment after stroke had increased levels of pro-depressant[66–69] cytokines (IL-6, TNF-alpha) at 30d post stroke, lower levels of circulating BDNF and greater disruption of meso-striatal projection in the ischemic hemisphere by 100d. Loss of trophic support may lead to aberrant plasticity in neural circuitry[70] and the gradual development of depressive-like behavior. Most of these measures were alleviated in animals that received mir363-3p injections, including higher levels of circulating BDNF, less IL-6 at 30d and virtually no disruption of meso-striatal projection in the ischemic hemisphere. Our present study design does not allow us to define which events are primary and which events are secondary. It is possible that ongoing degeneration of the neural circuitry occurs first, which primes inflammation. Alternately, loss of trophic support from the ischemic striatum may be the primary event that leads to retrograde degeneration in mesostriatal circuits. Glial-cell derived neurotrophic factor (GDNF), for example, is retrogradely transported from the striatum to the VTA and SNc and protects dopaminergic neurons against injury[71, 72]. Similarly, BDNF is also retrogradely transported by dopaminergic projections from the midbrain to the striatum, and loss of BDNF-trkB signaling leads to progressive degeneration of the nigrostriatal pathway[73]. This loss of meso-striatal projection develops gradually after stroke, such that it is not observed in the acute phase of stroke (5d after stroke) but is evident as early as 45 days after stroke (unpublished observations). Our previous work showed that administration of mir363-3p mimics localizes to neurons and reduces the expression and activation of Caspase-3 in middle-aged female brain, suggesting an anti-apoptotic role for this miRNA [25]. We hypothesize that the changes in the level of the trophic factors and inflammatory cytokines over time may be a direct effect of infarction, and that mir363-3p stabilizes these changes by reducing the initial infarct volume. Future imaging studies would be important in evaluating initial infarction and consequent changes in meso-striatal projections at 3+ months.
In conclusion, these studies and others [74–76] indicate that post stroke depression may be more tractable to neuroprotectant therapy as compared to conventional anti-depressants. Our previous work showed that a single dose of mir363-3p injected intravenously after stroke reduced infarct volume in the cortex and striatum and attenuated the early loss of sensory motor behavior in middle-aged females[25]. The present study shows that this early treatment may be sufficient to alleviate post stroke depression. While the precise mechanism underlying this outcome needs further study, we propose that neuroprotectants may be a better op t ion for older women after stroke instead of agonist/transmitter-based therapies, possibly due to their pleitrophic actions on other stroke-induced changes in the body.
Highlights.
This study focuses on post stroke depression in acyclic middle-aged female rats
Ischemic stroke causes depressive-like behaviors in the chronic phase
Depressive-like behaviors are accompanied by a reduction in mesostriatal projection neurons
Mir363-3p attenuates depressive-like behaviors, preserves mesostriatal projections and elevates BDNF levels
Acknowledgements:
This work was supported by AG042189 and NS074895 to FS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to declare.
References
- 1.Robinson RG, Neuropsychiatric consequences of stroke. Annu Rev Med, 1997. 48: p. 217–29. [DOI] [PubMed] [Google Scholar]
- 2.Robinson RG and Jorge RE, Post-Stroke Depression: A Review. Am J Psychiatry, 2016. 173(3): p. 221–31. [DOI] [PubMed] [Google Scholar]
- 3.Whyte EM, et al. , Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc, 2004. 52(5): p. 774–8. [DOI] [PubMed] [Google Scholar]
- 4.Hackett ML, et al. , Frequency of depression after stroke: a systematic review of observational studies. Stroke, 2005. 36(6): p. 1330–40. [DOI] [PubMed] [Google Scholar]
- 5.Williams LS, Ghose SS, and Swindle RW, Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry, 2004. 161(6): p. 1090–5. [DOI] [PubMed] [Google Scholar]
- 6.Glader EL, et al. , Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke, 2003. 34(8): p. 1970–5. [DOI] [PubMed] [Google Scholar]
- 7.Poynter B, et al. , Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics, 2009. 50(6): p. 563–9. [DOI] [PubMed] [Google Scholar]
- 8.Whitson HE, et al. , Chronic medical conditions and the sex-based disparity in disability: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci, 2010. 65(12): p. 1325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargano JW, Reeves MJ, and I. Paul Coverdell National Acute Stroke Registry Michigan Prototype, Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke, 2007. 38(9): p. 2541–8. [DOI] [PubMed] [Google Scholar]
- 10.Schildkraut JJ, The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry, 1965. 122(5): p. 509–22. [DOI] [PubMed] [Google Scholar]
- 11.Randrup A and Braestrup C, Uptake inhibition of biogenic amines by newer antidepressant drugs: relevance to the dopamine hypothesis of depression. Psychopharmacology, 1977. 53(3): p. 309–314. [DOI] [PubMed] [Google Scholar]
- 12.Miller AH, Maletic V, and Raison CL, Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry, 2009. 65(9): p. 732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howren MB, Lamkin DM, and Suls J, Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med, 2009. 71(2): p. 171–86. [DOI] [PubMed] [Google Scholar]
- 14.Dunn AJ, Swiergiel AH, and de Beaurepaire R, Cytokines as mediators of depression: what can we learn from animal studies? Neuroscience & Biobehavioral Reviews, 2005. 29(4): p. 891–909. [DOI] [PubMed] [Google Scholar]
- 15.Martinowich K, Manji H, and Lu B, New insights into BDNF function in depression and anxiety. Nature neuroscience, 2007. 10(9): p. 1089–1093. [DOI] [PubMed] [Google Scholar]
- 16.Duman RS and Monteggia LM, A neurotrophic model for stress-related mood disorders. Biological psychiatry, 2006. 59(12): p. 1116–1127. [DOI] [PubMed] [Google Scholar]
- 17.Fruehwald S, et al. , Early fluoxetine treatment of post-stroke depression--a three-month double-blind placebo-controlled study with an open-label long-term follow up. J Neurol, 2003. 250(3): p. 347–51. [DOI] [PubMed] [Google Scholar]
- 18.Shima S, The efficacy of antidepressants in post-stroke depression. Keio J Med, 1997. 46(1): p. 25–6. [DOI] [PubMed] [Google Scholar]
- 19.Verma R, et al. , Pair housing reverses post-stroke depressive behavior in mice. Behav Brain Res, 2014. 269: p. 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Keefe LM, et al. , Social isolation after stroke leads to depressive-like behavior and decreased BDNFlevels in mice. Behav Brain Res, 2014. 260: p. 162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronenberg G, et al. , Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry, 2012. 72(4): p. 273–81. [DOI] [PubMed] [Google Scholar]
- 22.Altar CA, et al. , Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature, 1997. 389(6653): p. 856–60. [DOI] [PubMed] [Google Scholar]
- 23.Harris NM, et al. , Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol Biochem Behav, 2016. 150-151: p. 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvamani A, et al. , Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clinical science, 2014. 127(2): p. 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvamani A and Sohrabji F, Mir363-3p improves ischemic stroke outcomes in female but not male rats. Neurochemistry international, 2017. 107: p. 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jezierski M and Sohrabji F, Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiology of aging, 2001. 22(2): p. 311–321. [DOI] [PubMed] [Google Scholar]
- 27.Selvamani A and Sohrabji F, The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. Journal of Neuroscience, 2010. 30(20): p. 6852–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okoreeh AK, Bake S, and Sohrabji F, Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia, 2017. 65(7): p. 1043–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bake S, et al. , Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS One, 2014. 9(3): p. e91427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvamani A and Sohrabji F, Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging, 2010. 31(9): p. 1618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balden R, Selvamani A, and Sohrabji F, Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology, 2012. 153(5): p. 2420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvamani A, et al. , An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One, 2012. 7(2): p. e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bake S, et al. , Fetal Alcohol Exposure Alters Blood Flow and Neurological Responses to Transient Cerebral Ischemia in Adult Mice. Alcohol Clin Exp Res, 2017. 41(1): p. 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levada OA and Troyan AS, Poststroke Depression Biomarkers: A Narrative Review. Front Neurol, 2018. 9: p. 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma R, et al. , Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain, behavior, and immunity, 2017. 66: p. 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denli AM and Hannon GJ, RNAi: an ever-growing puzzle. Trends Biochem Sci, 2003. 28(4): p. 196–201. [DOI] [PubMed] [Google Scholar]
- 37.Ambros V, microRNAs: tiny regulators with great potential. Cell, 2001107(7): p. 823–6. [DOI] [PubMed] [Google Scholar]
- 38.Jickling GC, et al. , microRNA Expression in Peripheral Blood Cells following Acute Ischemic Stroke and Their Predicted Gene Targets. PLoS ONE, 2014. 9(6): p. e99283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jickling GC, et al. , Hemorrhagic transformation after ischemic stroke in animals and humans. Journal of Cerebral Blood Flow & Metabolism, 2014. 34(2): p. 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dharap A, et al. , Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab, 2009. 29(4): p. 675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dharap A, Nakka VP, and Vemuganti R, Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke, 201142(4): p. 1105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeyaseelan K, Lim KY, and Armugam A, MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke, 2008. 39(3): p. 959–66. [DOI] [PubMed] [Google Scholar]
- 43.Sepramaniam S, et al. , Circulating microRNAs as biomarkers of acute stroke. Int J Mol Sci, 201415(1): p. 1418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rink C and Khanna S, MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics, 2011. 43(10): p. 521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouyang YB and Giffard RG, MicroRNAs affect BCL-2 family proteins in the setting of cerebral ischemia. Neurochem Int, 2014. 77: p. 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouyang YB, et al. , miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis, 201245(1): p. 555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xin Η, Li Y, and Chopp M, Exosomes/miRNAs as mediating cell-based therapy of stroke. Frontiers in cellular neuroscience, 2014. 8: p. 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xin H, et al. , MiR - 133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome - enriched extracellular particles. Stem cells, 2013. 31(12): p. 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu XS, et al. , MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol Exp Neurol, 2013. 72(8): p. 718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XS, et al. , MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem, 2013. 288(18): p. 12478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan KS, et al. , Expression profile of MicroRNAs in young stroke patients. PLoS One, 2009. 4(11): p.e7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Floyd DH, et al. , Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS One, 2014. 9(5): p. e96239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, et al. , miR-363-3p inhibits tumor growth by targeting PCNA in lung adenocarcinoma. Oncotarget, 2017. 8(12): p. 20133–20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang C, et al. , microRNA-363-3p inhibits cell growth and invasion of nonsmall cell lung cancer by targeting HMGA2. Mol Med Rep, 201817(2): p. 2712–2718. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, et al. , MicroRNA-363-3p inhibits papillary thyroid carcinoma progression by targeting PIK3CA. American journal of cancer research, 2017. 7(1): p. 148–158. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Liu F, et al. , Sex differences in caspase activation after stroke. Stroke, 2009. 40(5): p. 1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Renolleau S, et al. , Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem, 2007100(4): p. 1062–71. [DOI] [PubMed] [Google Scholar]
- 58.Zhao L, et al. , miR-137, a new target for post-stroke depression? Neural Regen Res, 20138(26): p. 2441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahmoud R, et al. , Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology, 2016107: p.278–293. [DOI] [PubMed] [Google Scholar]
- 60.Gobinath AR, et al. , Voluntary running influences the efficacy of fluoxetine in a model of postpartum depression. Neuropharmacology, 2018128: p. 106–118. [DOI] [PubMed] [Google Scholar]
- 61.Pardo M, et al. , Selection of sucrose concentration depends on the effort required to obtain it: studies using tetrabenazine, D 1, D 2, and D 3 receptor antagonists. Psychopharmacology, 2015. 232(13): p. 2377–2391. [DOI] [PubMed] [Google Scholar]
- 62.Salamone JD, et al. , Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. Journal of Pharmacology and Experimental Therapeutics, 2003. 305(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 63.Zhu CB, Blakely RD, and Hewlett WA, The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology, 2006. 31(10): p. 2121–31. [DOI] [PubMed] [Google Scholar]
- 64.Gong L, et al. , Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res, 2017. 84: p. 9–17. [DOI] [PubMed] [Google Scholar]
- 65.Nestler EJ and Carlezon WA Jr., The mesolimbic dopamine reward circuit in depression. Biol Psychiatry, 2006. 59(12): p. 1151–9. [DOI] [PubMed] [Google Scholar]
- 66.Maes M, et al. , Increased serum 1L-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine, 1997. 9(11): p. 853–858. [DOI] [PubMed] [Google Scholar]
- 67.Basterzi AD, et al. , IL - 6 levels decrease with SSRI treatment in patients with major depression. Human Psychopharmacology: Clinical and Experimental, 2005. 20(7): p. 473–476. [DOI] [PubMed] [Google Scholar]
- 68.Elomaa A-P, et al. , Elevated levels of serum IL-5 are associated with an increased likelihood of major depressive disorder. BMC psychiatry, 201212(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suarez EC, Krishnan RR, and Lewis JG, The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosomatic medicine, 2003. 65(3): p. 362–368. [DOI] [PubMed] [Google Scholar]
- 70.Calabrese F, et al. , Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Frontiers in cellular neuroscience, 2014. 8: p. 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gash DM, Gerhardt GA, and Hoffer BJ, Effects of glial cell line-derived neurotrophic factor on the nigrostriatal dopamine system in rodents and nonhuman primates. Adv Pharmacol, 1998. 42: p. 911–5. [DOI] [PubMed] [Google Scholar]
- 72.Barroso-Chinea P, et al. , Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci, 2005. 21(7): p. 1815–27. [DOI] [PubMed] [Google Scholar]
- 73.Baydyuk Μ, Nguyen MT, and Xu B, Chronic deprivation of TrkB signaling leads to selective late-onset nigrostriatal dopaminergic degeneration. Experimental neurology, 2011. 228(1): p. 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maes M, et al. , Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2, 3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro endocrinology letters, 2011. 32(3): p. 264–273. [PubMed] [Google Scholar]
- 75.Pang C, et al. , The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axis. Neuropharmacology, 2015. 97: p. 447–456. [DOI] [PubMed] [Google Scholar]
- 76.Hurley LL and Tizabi Y, Neuroinflammation, neurodegeneration, and depression. Neurotoxicity research, 2013. 23(2): p. 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]


