Abstract
Background and Objectives:
Fluorescence-guided surgery (FGS) is a rapidly advancing field that may improve outcomes in several cancer types. While screening has decreased colorectal cancer (CRC) mortality, it remains a common and often fatal malignancy. In this study we sought to identify an optical imaging agent for the application of FGS technology to CRC.
Methods:
We compared a panitumumab-IRDye800CW conjugate to an IgG-IRDye800CW isotype control. Mice were implanted with one of three CRC cell lines (LS174T, Colo205, and SW948) and imaged with open and closed-filed fluorescence imaging (FLI) systems. Fluorescent contrast was quantified by calculating the ratio between tumor and background fluorescence. After ten days the mice were sacrificed, and their tumors stained for microscopic imaging.
Results:
Panitumumab-IRDye800CW produced significantly greater (p < 0.05) fluorescent contrast in all three cell lines. Average tumor to background ratio (TBR) was 6.00 vs. 2.60 for LS174T, 5.78 vs. 2.52 for Colo205, and 4.31 vs. 1.70 for SW948. A 1 mg tumor fragment produced significantly greater fluorescent contrast in the Colo205 and SW948 cell lines in the panitumumab-IRDye800CW group. Western blotting for EGFR as well as a semi-quantitative analysis of EGFR expression noted strong expression in all three cell lines, however EGFR expression did not directly correlate to TBR.
Conclusion:
Panitumumab-IRDye800CW produces significantly greater fluorescent contrast than IgG-IRDye800CW in a murine model of CRC and is a suitable agent for the application of FGS technology to CRC.
INTRODUCTION
Fluorescence-guided surgery (FGS) is a burgeoning field that allows for precise visualization of diseased tissue, highlighting it from healthy background tissue through near-infrared fluorescence imaging. This technology is of considerable interest in oncologic surgery where it is primarily being evaluated as a way to enhance intraoperative assessment of tumor margins.1–3 Antibody-based FGS utilizes probes created by linking a fluorophore to an antibody that targets unique or constitutively overexpressed tumor proteins. After injection with an imaging probe, one of several fluorescence imaging systems are used to visualize disease specific fluorescent contrast. Monoclonal antibodies in clinical use for cancer chemotherapy are frequently utilized as the antibody portion of an imaging probe. A variety of fluorophores are used in FGS imaging probes, and they typically emit light in the 700-900 nm range to reduce background tissue auto-fluorescence.
Widespread adoption of screening has greatly decreased mortality from colorectal cancer (CRC), which remains the second leading cause of non-gender specific cancer mortality.4 The clinical utility of FGS in CRC has not been extensively investigated, and several attractive targets exist for the translation of this technology to this common cancer. These include the epidermal growth factor receptor (EGFR) and carcinoembryonic antigen (CEA) which are overexpressed in most colorectal tumors.5,6 EGFR in particular is of interest as the monoclonal antibody panitumumab is FDA approved for treatment of KRAS wild-type CRC.5 EGFR antibodies show promise as components of FGS imaging probes in several other cancer types, including head and neck squamous cell carcinoma, soft tissue sarcoma, and breast adenocarcinoma.1,2,7
In this study we evaluated a panitumumab-IRDye800CW probe targeting EGFR. IRDye800CW is a near-infrared dye (excitation 775nm, emission 795nm) that has been extensively studied in patients during FGS. The clinical use of this dye has been shown to be safe and capable of providing robust tumor-to-background contrast during surgery.8 To assess the potential of FGS using panitumumab-IRDye800CW in CRC, we tested the probe in a murine model of CRC using three cell lines and two fluorescence imaging systems to measure disease-specific fluorescent contrast.
METHODS
Reagents
Panitumumab (Vectibix, Amgen, Thousand Oaks, CA) is a fully humanized anti-EGFR antibody and IRDye800CW (IRDye800CW-N-hydroxysuccimide ester, LI-COR Biosciences, Lincoln, NE) is a near-infrared dye frequently used in FGS studies. Panitumumab was conjugated to IRDye800CW according to the manufacturer’s protocol by first diluting the antibody to 1mg/mL in phosphate buffered saline (PBS) and then incubating it with the dye in the dark at room temperature for 2 hours. The conjugate was then purified using spin columns included in the labeling kit and stored in single use vials in the dark at 4°C. A control agent was prepared using protein-A purified immunoglobulin G (IgG) (Innovative, Novi, MI) conjugated to IRDye800CW using the same protocol.
Cell lines and animal models
LS174T (KRAS mutant), Colo205 (KRAS wild type), and SW948 (KRAS wild type) (ATCC, Manassas, VA) human colon adenocarcinoma cells were maintained in Dulbecco’s modified Eagles medium with 10% fetal bovine serum and 1% Plasmocin (InvivoGen, San Diego, CA) at 37°C in 5% CO2.9–11 Cells were harvested at 70-90% confluence and cell numbers were determined using a hemocytometer. 2 x 106 cells suspended in PBS were injected subcutaneously into the flank of female athymic nude mice (Charles River Laboratories, Hartford, CT), aged eight weeks (n = 10 per cell line). Prior to panitumumab-IRDye800CW and IgG-IRDye800CW injection three weeks post implantation, mice were stratified into equal groups based on tumor volume (~10 mm3). The sub-cutaneous flank model has been used previously by our group in several studies evaluating fluorescence-guided surgery (FGS) in other cancer types.
Western blot analysis
Samples from each cell line were collected in RIPA lysis buffer (10 mM Tris-Cl [pH 8.0], 1mM ethylenediaminetetraacetic acid [EDTA], 0.5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid [EGTA], 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 140 mM NaCl) and a protease inhibitor tablet was added (Roche Life Sciences, Indianapolis, IN). The samples were centrifuged at 12,500 RPM at 4°C and protein concentration was determined using a bicinchoninic acid (BCA) protein assay (Thermo Scientific, Waltham, MA). Lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membrane was probed with β-actin horseradish peroxidase (HRP) (Santa Cruz, Santa Cruz, CA; 47778) and blocked with 5% nonfat dry milk prior to incubation with the primary rabbit anti-human EGFR antibody (Santa Cruz, 71034). An HRP-conjugated goat antirabbit IgG (Santa Cruz, 2004) was applied and after incubation the membrane was developed with an Amersham enhanced chemiluminescence (ECL) western blotting detection system (GE Healthcare, Buckinghamshire, UK).
Agent administration and imaging
Five mice from each cell line cohort were injected via the tail vein with 200 μg of the panitumumab-IRDye800CW conjugate, while the remaining five received 200 μg of the IgG-IRDye800CW control agent. The Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham approved all animal protocols (UAB IACUC 21023).
After injection the mice were imaged daily for 10 days with open and closed-field devices. Open-field images were captured using the LUNA fluorescence imaging system (Novadaq, British Columbia, Canada) by placing the anesthetized mouse 15 cm from the imaging head recording a 10 second acquisition. The LUNA is currently in clinical use, primarily for fluorescence angiography in plastic surgery and wound care. The device resembles a portable X-ray machine with its imaging head on a movable arm connected to a rolling workstation with a screen and keyboard. It was chosen due to its FDA approval and current clinical use as it or a similar device is what would likely be used in a clinical application of FGS in CRC. Closed-field images were captured using the Pearl Impulse fluorescence imaging system (LI-COR Biosciences) by placing the anesthetized mouse in the imaging tray and using on-board image acquisition software on the 800 nm channel. The Pearl is a preclinical tabletop device about the size of a miniature refrigerator with a small chamber where rodents are placed for imaging. It was chosen due to its optimization for use with our fluorophore, IRDye800CW. On day 10, the animals were sacrificed by cervical dislocation. The tumors were resected and the tumor in-situ and wound bed were imaged. To determine whether the imaging devices could detect fluorescent contrast in an amount of tissue that would be clinically silent, a 1 mg tumor fragment was returned the wound bed and images were obtained with both devices.
Closed-field images were analyzed using ImageStudio (LI-COR Biosciences). A hand drawn region on interest (ROI) was placed around the tumor border to calculate mean fluorescence intensity (MFI) within the tumor. A second ROI was drawn in a region of healthy tissue opposite the tumor to calculate background MFI. Tumor-to-background MFI ratio (TBR) was determined by dividing tumor MFI by background MFI. TBR is a measure of fluorescent contrast and the value of interest in evaluating agents for use in FGS. Values greater than one indicate greater fluorescence within the tumor, and higher numbers indicate a greater degree of fluorescent contrast between the tumor and background tissue. Open-field images were analyzed using the SPY-Q (Novadaq) image processing software included on-board the LUNA device in the same way as the closed-field images. Images of the tumor in-situ, wound bed, and a 1 mg tumor fragment taken on the tenth day were analyzed similarly, using hand drawn ROI around the tumor, wound bed, and tumor fragment.
Immunohistochemical analysis
Tumor samples were wrapped in resected dermal tissue and stored for 24 hours in formalin before transferring to 70% ethanol for storage until paraffin embedding. Each tumor was sectioned and stained with standard hematoxylin and eosin (H&E) as well as immunohistochemical stains for EGFR and cytokeratin 20 (CK20).
The tumors were deparaffinized and rehydrated in xylene, 95% ethanol, and 70% ethanol. They were placed in 1X Citrate Buffer, pH 6.0 (Thermo Scientific) for 10 minutes at 90°C and cooled for 20 minutes. The slides were blocked with 5% bovine serum albumin for 5 minutes at room temperature before application of the primary EGFR (Thermo Scientific RM-2111-R7) and CK20 (abcam EPR1622Y, Cambridge, MA) antibodies. After incubation in a humidified chamber for 1 hour at room temperature, goat anti-rabbit HRP-conjugated secondary antibody was applied. The samples were allowed to incubate an additional hour in the humidified chamber. 3,3′-diaminobenzidine (DAB) was applied afterwards and allowed to incubate at room temperature until appropriate color developed. Finally, the slides were dehydrated, mounted, coverslipped, and allowed to dry.
Five independent observers, blinded to the cohorts and each other (JMW, GDK, YEH, MTR, SEL), assessed relative EGFR expression in each cell line for semi-quantitative analysis. Two sections from each cell line were selected for comparison. Each section was compared against two sections from each of the remaining cell lines for a total of 18 comparisons. These comparisons were compiled into a document that was sent to the observers, along with an answer and instruction sheet. The observers were asked to select the section they felt had darker staining for EGFR and record their answer on the accompanying answer sheet. These results were then converted to a ten-point scoring scale. This was accomplished by totaling the instances each observer selected each cell line and dividing each by 18. The resulting percentages were averaged to determine the overall percentage a cell line was selected as having darker EGFR staining. Standard deviations were calculated and the results were graphed.
Statistical analysis
MFI and TBR were statistically compared using two-tailed unpaired T-tests. Statistical significance was considered p < 0.05.
RESULTS
Panitumumab-IRDye800CW produces significantly greater fluorescent contrast in a murine model of CRC
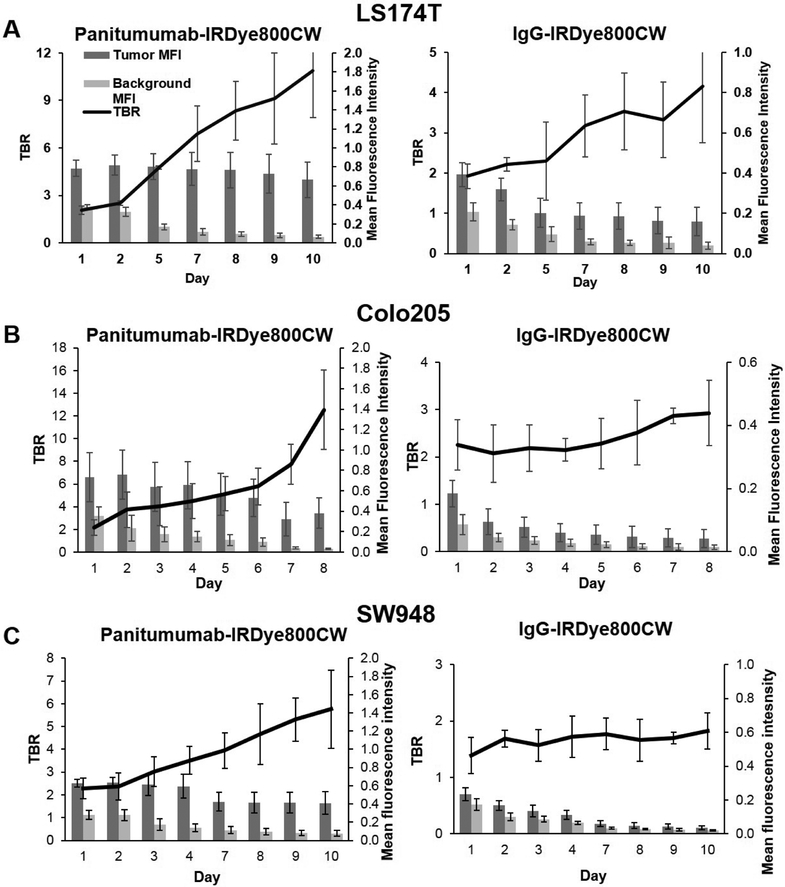
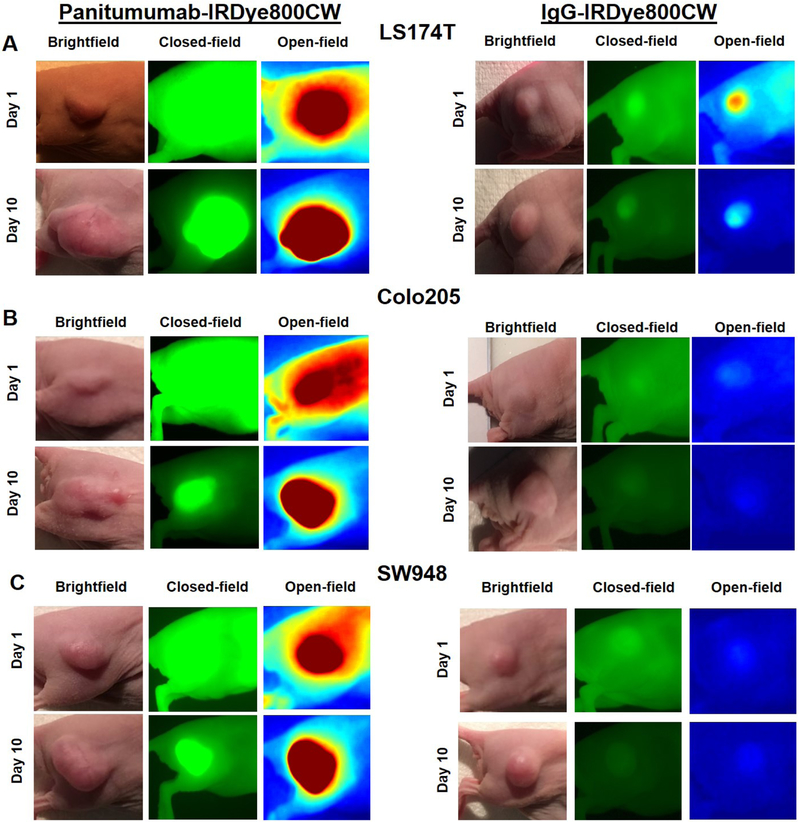
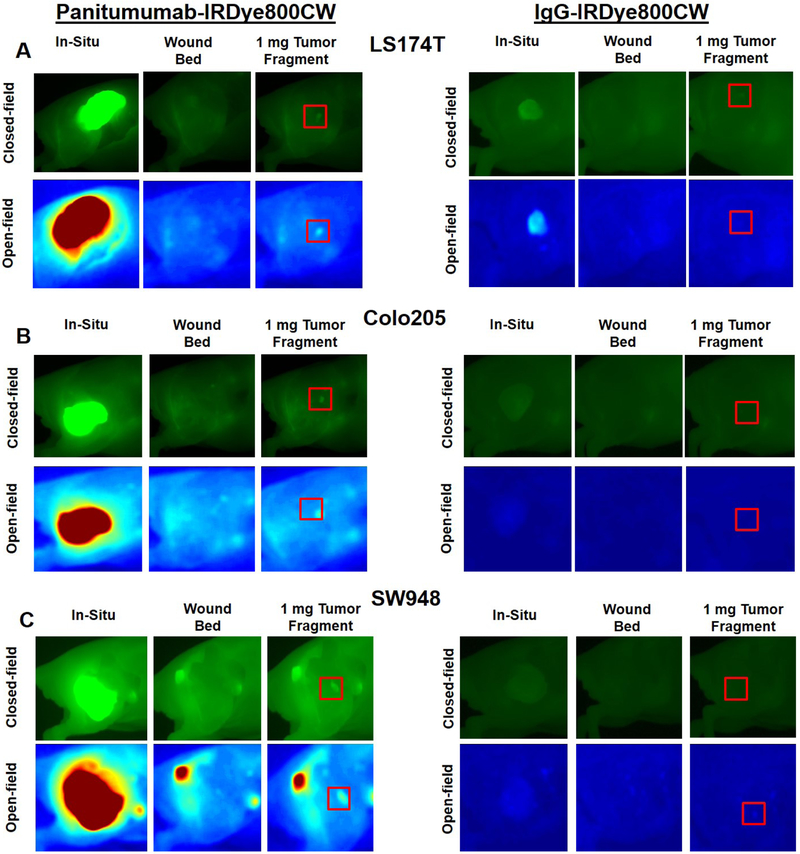
As seen in panels A, C, and E of figure 1, TBR increased each day of imaging, peaking on day 10 in all three cell lines treated with panitumumab-IRDye800CW (LS174T = 10.8 ± 2.94, Colo205 = 12.5 ± 3.51, SW948 = 5.76 ± 1.71) . Fluorescent contrast was significantly greater on day 10 in the panitumumab-IRDye800CW treated cell lines compared to the control group (p < 0.05 for all cell lines). Panels A, C and E of figure 2 demonstrate the fluorescent contrast created by the experimental and control agents with open and closed-field FLI on days 1 and 10 of imaging, with the mice treated with the panitumumab conjugate having more intense qualitative contrast enhancement. Panels A, C and E of figure 3 demonstrate open and closed-field FLI during the resection process on day 10. Of note, LS174T produced the most aggressive tumors with short doubling times and a high incidence of muscle invasion. Two mice in the LS174T group required early sacrifice due to tumor ulceration. SW948 however produced the most homogeneous tumors with no instances of muscle invasion and a doubling time of approximately 2 weeks in our experience. To determine whether the experimental agent allowed for detection of fluorescence in a subclinical tumor burden, a 1 mg tumor fragment placed in the wound bed was imaged with both devices. These results can be seen in the upper right image in panels A, C, and E of figure 3. Mice treated with treated with the panitumumab conjugate in the Colo205 and SW948 groups both exhibited a significantly greater TBR than the control group (p < 0.05 for both cell lines).
Figure 1:
(A-F) Average daily TBR with daily tumor and background MFI ± SD.
Figure 2:
(A-E) Brightfield, closed-field, and open-field FLI on days 1 and 10 of imaging.
Figure 3:
(A-F) Closed-field and open-field FLI from resection process on day 10 of imaging, from left to right: tumor in-situ, wound bed, wound bed with a 1 mg tumor fragment.
Results from open-field FLI mirror the closed-field FLI results with TBR peaking on day 10 and significantly greater in all three cell lines treated with the panitumumab conjugate (p < 0.05 for all cell lines). In line with the closed-field results, the panitumumab conjugate produced a significantly greater TBR when visualizing a 1 mg tumor fragment with open-field FLI in the Colo205 and SW948 groups (p < 0.05 for both cell lines).
In the panitumumab group, tumor MFI greatly exceeded background MFI in all cell lines, and background MFI decreased over the 10 days of imaging (0.38 day 1 vs. 0.06 day 10 for LS174T, 0.35 day 1 vs. 0.03 day 10 for Colo205, 0.28 day 1vs. 0.08 day 10 for SW948).
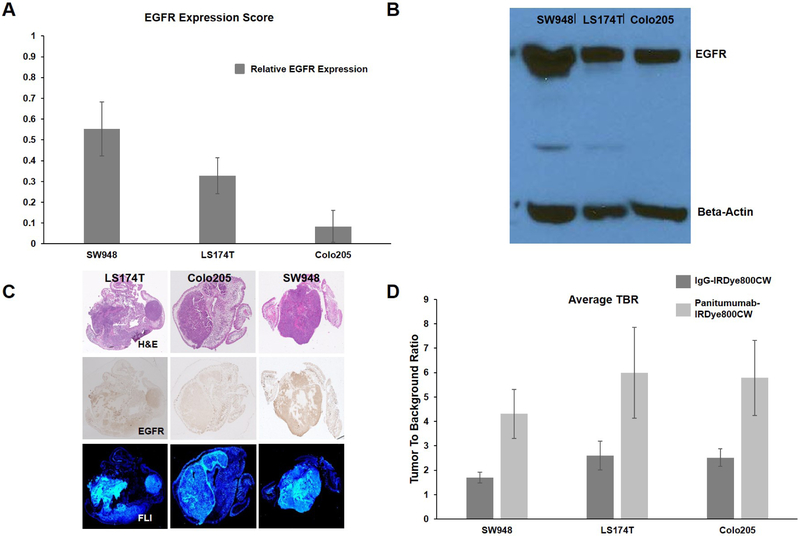
SW948 has greater EGFR expression than LS174T and Colo205; Panitumumab-IRDye800CW produces significantly greater fluorescent contrast in all cell lines
Figure 4 A shows that SW948 had the greatest EGFR expression based on objective ratings by five observers with an EGFR expression score of 0.55 ± 0.13. SW948 was also found to have greater EGFR expression on western blot analysis as seen in figure 4 B. Finally an EGFR stained section from each cell line is displayed in the middle row of figure 4 C, with SW948 having greater qualitative EGFR staining.
Figure 4-. Evaluation of EGFR expression and overall TBR:
(A) Results of semi-quantitative EGFR expression rating; EGFR expression score refers to the percentage each cell line was selected as having darker EGFR staining when compared against another cell line (n = 90 individual comparisons). (B) Western blot for EGFR with beta-actin control. (C) Representative tumor sections from each cell line with H&E staining, IHC for EGFR, and microscopic FLI. (D) Overall TBR for each cell line averaging TBR values from days one through ten.
To assess the overall effectiveness of panitumumab-IRDye800CW in producing fluorescent contrast in each cell line, the TBR values on each day of imaging were averaged. Figure 4 D illustrates these values for each cell line. For LS174T, average TBR for the IgG-IRDye800CW treated mice was 2.60 ± 0.58 versus 6.00 ± 1.86 for the panitumumab-IRDye800CW treated mice (p < 0.05). For Colo205, average TBR for the IgG-IRDye800CW treated mice was 2.52 ± 0.36 versus 5.78 ± 1.54 for the panitumumab-IRDye800CW treated mice (p < 0.05). For SW948, average TBR for the IgG-IRDye800CW treated mice was 1.70 ± 0.22 versus 4.31 ± 1.01 for the panitumumab-IRDye800CW treated mice (p < 0.05).
DISCUSSION
The results of this study are consistent with other studies evaluating panitumumab-IRDye800CW as an FGS imaging probe, and demonstrate the ability of panitumumab-IRDye800CW to create robust fluorescent contrast in a murine CRC model.7,1,3 These conclusions were also found consistent in previous studies in breast cancer and head and neck cancer (Korb et al, J Surg Res. 2014; 188 (1): 119-128, Prince et al, J Surg Oncol. 2017; November, Warram et al. J Path Clin Res. 2016; 2 (2): 104-112). Panitumumab has several advantages supporting its use in the application of FGS technology to CRC. Panitumumab is a fully human monoclonal antibody FDA approved for the treatment of KRAS wild-type CRC. This means it has been thoroughly tested in humans and does not require a test dose like cetuximab (a chimeric monoclonal antibody) requires. More importantly, an EGFR inhibitor-based agent has broad translatability to several different cancer types. Panitumumab and cetuximab-based agents have been studied in head and neck squamous cell carcinoma, breast adenocarcinoma, and soft tissue sarcoma with encouraging results.7,1,2 Ours is not the first study evaluating FGS of CRC, however these studies did not utilize an EGFR inhibitor.12-15 Criticisms of EGFR antibodies presented in these studies noted the often strong EGFR expression in background tissue and the variable degree of EGFR expression in tumor cells.12,6 In our study tumor MFI greatly exceeded background MFI, likely due to the agent’s long half-life leading to decreased background binding and increased tumor uptake over time. This is reflected in the continued rise in TBR in the panitumumab group over the 10 days of imaging.
IRDye800CW has been frequently studied in FGS, and our results are in line with prior studies where it produced robust fluorescence with both open and closed-field FLI systems.7,1 Indocyanine green (ICG) is an FDA-approved cyanine-based dye that has proven effective in FGS in several studies, but is not without problems.14,16 ICG links to proteins non-covalently which can lead to gradual dissociation from the antibody portion of the conjugate, and thus target tissues. This was mitigated by modification of ICG using short polyethylene-glycol linkers in a study by Sano et al.16 Compared to ICG, IRDye800CW has several advantages. Most importantly, it binds covalent to proteins allowing for strong interactions with an antibody. This increases a probe’s half-life and allows for longer systemic circulation greater target tissue uptake. In our study the half-life of the panitumumab-IRDye800CW conjugate was such that FLI of fixed tissue sections could be performed 12 weeks after the initial injection. While IRDye800CW is not FDA approved, it was safe in doses up to 20 mg/kg in a murine model.17 Compared to ICG, it is provided in a ready to use medium and does not require modification for improved performance.16
Western blotting and our semi-quantitative evaluation of EGFR expression both concluded that SW948 had the strongest EGFR expression of the cell lines we studied. This finding is unexpected considering this cell line had lower TBR values compared to the other cell lines, and a prior study found a linear association between EGFR density and MFI in head and neck squamous cell carcinoma (HNSCC).18 This study by De Boer et al assessed the relationship between EGFR density and MFI in patient derived HNSCC samples and found that well-differentiated tumors had lower MFI values than poorly differentiated tumors.18 They attributed this finding to a negative effect on MFI with increased tumor maturity and proposed that lack of vascular access in well differentiated tumors prevented robust uptake of imaging agents.18 Cell maturity’s effect on MFI in FGS has been previously discussed by Gusterson et al and this phenomenon may explain our results with the SW948 cell line, which formed the most consistent and homogenous tumors.19
FGS may not have the same impact on intraoperative margin assessment for oncologic resection of CRC compared to head and neck and breast cancer as colonic anatomy and preoperative imaging largely determines the extent of resection. Two areas where FGS may augment the current CRC management paradigm are the risk stratification of malignant colon polyps and selection for neoadjuvant chemotherapy. Assessment of malignant colon polyps lacks widely accepted guidelines and is currently accomplished through histologic classification systems such as the one devised by Haggitt et al.20 Depth of invasion of a microscopic foci of cancer determines a polyp’s Haggitt score, which then guides clinicians on the need for surgery versus surveilance.20,21 FGS could augment this process through microscopic FLI, which can detect fluorescent contrast in a single cell7. Administration of an imaging probe prior to colonoscopy in individuals at high risk for malignant colon polyps (prior history, hereditary cancer syndromes, ulcerative colitis) followed by microscopic FLI could allow for much more precise risk stratification and possibly improve outcomes. Improved ability to detect microscopic foci of cancer in malignant polyps could extend to more accurate selection of patients for neoadjuvant chemotherapy which is currently under investigation in the Fluoropyrimidine, Oxaliplatin and Targeted-Receptor pre-Operative Therapy for patients with high-risk, operable colon cancer (FOxTROT) trial. The rationale of FOxTROT is that patients with seemingly localized tumors develop recurrences due to unrecognized local spread and/or micro-metastasis, and these patients may benefit from pre-operative chemotherapy to clear these undetectable foci of cancer. The precise ability of FGS to detect even microscopic foci of cancer may be able to augment this selection process for neoadjuvant chemotherapy in the future once the results of FOxTROT are published and future studies can evaluate microscopic FLI of CRC.
A limitation of our study is the significant difference in the TBR values calculated from the two FLI systems (p < 0.05). This is likely due to IRDye800CW being optimized for the 800 nm wavelength channel of the Pearl (LI-COR Biosciences) FLI system. While the impact of this difference requires further investigation, effects on patient outcomes are unlikely given that both systems produced excellent fluorescent contrast.
CONCLUSION
Panitumumab-IRDye800CW proved superior to IgG-IRDye800CW in this proof of concept study evaluating the application of FGS to CRC. Application of FGS technology to CRC has the potential to improve the diagnosis, staging, and treatment of CRC.
Acknowledgements
Funding for this study obtained through:
1. National Institutes of Health (P30CA013148) – Suzanne E. Lapi, Ph.D.
2. Robert Grant Armstrong Fund ((P30CA013148) – Jason M. Warram, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korb ML, Hartman YE, Kovar J, Zinn KR Bland KI, Rosenthal EL. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res. 2014; 188(1):119–128. doi: 10.1016/j.jss.2013.11.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince AC, McGee AS, Siegel H, Rosenthal EL, Behnke NK, Warram JM. Evaluation of fluorescence-guided surgery agents in a murine model of soft tissue fibrosarcoma. J Surg Oncol. 2017;(November). doi: 10.1002/jso.24950 [DOI] [PubMed] [Google Scholar]
- 3.Tipirneni KE, Warram JM, Moore LS, et al. Oncologic Procedures Amenable to Fluorescence-guided Surgery. Ann Surg. 2017;266(1):36–47. doi: 10.1097/SLA.0000000000002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haggar F a, Boushey RP, Ph D. Colorectal Cancer Epidemiology : Incidence , Mortality , Survival , and Risk Factors. Clin Colon Rectal Surg. 2009;6(212):191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol. 2015;6(5):133–141. doi: 10.5306/wjco.v6.i5.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiernan JP, Perry SL, Verghese ET, et al. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br J Cancer. 2013;108(3):662–667. doi: 10.1038/bjc.2012.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warram JM, de Boer E, van Dam GM, et al. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J Pathol Clin Res. 2016;2(2):104–112. doi: 10.1002/cjp2.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal EL, Warram JM, de Boer E, et al. Safety and Tumor-specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2016;21(18):4062–4072. doi: 10.1158/1078-0432.CCR-15-0428.Bioactivity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toda K, Kawada K, Iwamoto M, et al. Metabolic Alterations Caused by KRAS Mutations in Colorectal Cancer Contribute to Cell Adaptation to Glutamine Depletion by Upregulation of Asparagine Synthetase. Neoplasia. 2016;18(11):654–665. doi: 10.1016/J.NEO.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalikaki A, Politaki H, Souglakos J, et al. KRAS genotypic changes of circulating tumor cells during treatment of patients with metastatic colorectal cancer. PLoS One. 2014;9(8):e104902. doi: 10.1371/journal.pone.0104902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn EF, Iida M, Myers RA, et al. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. 2011;30(5):561–574. doi: 10.1038/onc.2010.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutowski M, Framery B, Boonstra MC, et al. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg Oncol. 2017;26(2):153–162. doi: 10.1016/j.suronc.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Boogerd LSF, Hoogstins CES, Schaap DP, et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol. 0(0). doi: 10.1016/S2468-1253(17)30395-3 [DOI] [PubMed] [Google Scholar]
- 14.Hiroshima Y, Maawy A, Metildi CA, et al. Successful Fluorescence-Guided Surgery on Human Colon Cancer Patient-Derived Orthotopic Xenograft Mouse Models Using a Fluorophore-Conjugated Anti-CEA Antibody and a Portable Imaging System. J Laparoendosc Adv Surg Tech. 2014;24(4):241–247. doi: 10.1089/lap.2013.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberale G, Bourgeois P, Larsimont D, Moreau M, Donckier V, Ishizawa T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: A systematic review. Eur J Surg Oncol. 2017;43(9):1656–1667. doi: 10.1016/j.ejso.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 16.Sano K, Nakajima T, Miyazaki K, et al. Short PEG-Linkers Improve the Performance of Targeted, Activatable Monoclonal Antibody-Indocyanine Green Optical Imaging Probes. Bioconjug Chem. 2013;24(5):811–816. doi: 10.1016/j.fertnstert.2010.09.017.Development [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall MV, Draney D, Sevick-Muraca EM, Olive DM. Single-dose intravenous toxicity study of IRDye 800CW in sprague-dawley Rats. Mol Imaging Biol. 2010;12(6):583–594. doi: 10.1007/s11307-010-0317-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Boer E, Warram JM, Tucker MD, et al. In Vivo Fluorescence Immunohistochemistry: Localization of Fluorescently Labeled Cetuximab in Squamous Cell Carcinomas. Sci Rep. 2015;5(June):1–11. doi: 10.1038/srep10169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gusterson BA, Hunter KD. Should we be surprised at the paucity of response to EGFR inhibitors? Lancet Oncol. 2009;10(5):522–527. doi: 10.1016/S1470-2045(09)70034-8 [DOI] [PubMed] [Google Scholar]
- 20.Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: Implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89(2):328–336. doi: 10.1016/0016-5085(85)90333-6 [DOI] [PubMed] [Google Scholar]
- 21.Aarons CB, Shanmugan S, Bleier JIS. Management of malignant colon polyps: Current status and controversies. World J Gastroenterol. 2014;20(43):16178–16183. doi: 10.3748/wjg.v20.i43.16178 [DOI] [PMC free article] [PubMed] [Google Scholar]