Abstract
Objective:
Poor sleep quality and insufficient total sleep time have been shown to modify the relationship between college drinking and negative drinking consequences. This study aimed to examine whether prospective associations between risky drinking and negative drinking consequences similarly differ by sleep-related functional impairment, which is novel to the literature.
Method:
Data were obtained from a 2-month prospective study of 157 college drinkers (mean age = 19 years [SD = 1.11], 30% male, 76% White). Online questionnaires were administered at both Time 1 (T1) and Time 2 (T2) to measure sleep-related functional impairment (assessed by Insomnia Diurnal Impact Scale; Ruiz et al., 2011), and drinking behaviors and negative drinking consequences (assessed retrospectively over the past 2 months).
Results:
Prospective negative binomial regression analyses demonstrated that associations of both maximum drinks and binge drinking frequency at T1 with negative drinking consequences at T2 differed by T1 sleep-related functional impairment after controlling for covariates (sex, negative mood, total sleep time, insomnia symptoms, morning preference, and negative drinking consequences at T1). Students reporting lower sleep-related functional impairment experienced high levels of negative drinking consequences only at high levels of risky drinking, whereas students reporting higher sleep-related functional impairment experienced consistently high levels of negative drinking consequences regardless of their risky drinking levels.
Conclusion:
Findings indicate that sleep-related functional impairment may exacerbate negative drinking consequences of risky drinking. Thus, sleep-related functional impairment helps to explain individual differences in the association between risky drinking and negative drinking consequences in college students.
Keywords: sleep, functional impairment, alcohol, consequences, college students
1. Introduction
Risky college drinking remains highly prevalent and associated with substantial negative consequences, including academic and cognitive impairment, assault, injury, and death (for a review, see Merrill & Carey, 2016). Poor sleep quality and insufficient total sleep time have been shown to exacerbate associations between college drinking and negative drinking consequences. In a cross-sectional study, weekly drinking quantity was positively associated with negative drinking consequences only among college drinkers who reported poor sleep quality; in contrast, among students reporting better sleep quality, weekly alcohol consumption was not associated with experience of negative drinking consequences (Kenney et al., 2012). In a longitudinal study, drinking quantity was more positively associated with negative drinking consequences among college drinkers who reported subjective inadequate total sleep time concurrently and at one, three, and five month follow-ups (Miller et al., 2016).
A growing literature further indicates reciprocal associations between sleep and alcohol behaviors among college drinkers. For example, a daily study of heavy-drinking college students found that students that were more alert upon waking drank more heavily and heavier drinkers reported shorter sleep durations on average (Fucito et al., 2018). Further, intervention research suggests that targeting sleep problems among college drinkers may result in decreased alcohol use and related consequences (Fucito et al., 2015; Fucito et al., 2017). However, there remains a paucity of research considering the unique moderating role of sleep-related functional impairment on college drinking and negative drinking consequences.
The role of poor sleep quality and insufficient sleep duration in the association of college drinking with negative drinking consequences may actually be driven by sleep-related functional impairment. Sleep-related functional impairment is a sub-component of “sleep quality,” which is a complex construct encompassing both quantitative (e.g., total sleep time) and subjective (e.g., daytime impairment) aspects of sleep (Buysse et al., 1989). Sleep-related functional impairment represents the diverse daytime consequences of poor sleep, including sleepiness, irritability, and difficulties with concentration and socializing (Ruiz, Guilera, & Gomez-Benito, 2011). Sleep-related functional impairment may reduce one’s capacity to make safe decisions and avoid impulsive behaviors, such as risky drinking (Kenney et al., 2012). Thus, college students with high sleep-related functional impairment may be more susceptible to the impairing effects of alcohol (e.g., disinhibition) and experience more negative drinking consequences than peers with lower sleep-related functional impairment.
Sleep-related functional impairment may be a prime contributor to exacerbations of the college drinking and subsequent negative drinking consequences relationship given its striking prevalence among college students. Up to 71% of college students report falling asleep in class (DeMartini & Fucito, 2014) and 40 – 50% report frequent irritability, excessive drowsiness, and concentration problems due to insufficient sleep (Oginska & Pokorski, 2006). Further, college students who report difficulty initiating or maintaining sleep have reported daytime fatigue and concentration difficulties due to lack of sleep on at least four days per week (Alapin et al., 2000). However, sleep-related functional impairment overlaps considerably with other sleep constructs and sleep-related correlates. Specifically, sleep-related functional impairment has demonstrated moderate to high correlations with nighttime sleep difficulty (i.e., Athens Insomnia Scale, r = .65; Ruiz, Guilera, & Gomez-Benito, 2011). Thus, existing research on the moderating influences of poor sleep quality and insufficient sleep time on the college drinking and subsequent negative drinking consequences relationship (Kenney et al., 2012; Miller et al., 2016) may be due, at least in part, to sleep-related functional impairment. Research is needed to examine the unique moderating role of sleep-related functional impairment on college drinking and negative drinking consequences, after controlling for the influences of poor sleep, total sleep time, and other sleep-related constructs.
The current prospective study examined whether associations of college drinking with negative drinking consequences differed as a function of sleep-related functional impairment, after controlling for other aspects of sleep (i.e., insomnia symptoms, total sleep time, morning preference) and well-established sleep-related correlates (e.g., male sex and negative affect; Galambos et al., 2013; Taylor et al., 2013). This conservative approach allowed for isolation of the unique association of sleep-related functional impairment with drinking and negative drinking consequences. Based on previous findings (Kenney et al., 2012; Miller et al., 2016), it was hypothesized that drinking would be more strongly and positively associated with subsequent negative drinking consequences among college students with high levels of sleep-related functional impairment.
2. Materials and Methods
2.1. Participants and Procedure
Data were drawn from a two-wave longitudinal study of 171 college drinkers at a four-year university in the northeastern United States (Gellis et al., 2014; Goodhines et al., 2017). Participants were recruited from introductory psychology classes and compensated with credit for the course research component. Students who were at least 18 years of age and endorsed consuming one or more alcoholic drinks in the past 30 days were eligible to participate. This drinking-related eligibility criteria is consistent with a previous study validating a drinking consequences measure among college students (Earleywine, LaBrie, & Pedersen, 2008). Recruitment of a broad range of college drinkers (rather than exclusively heavy drinkers) was necessary given that even low-risk college drinkers have been shown to experience negative drinking consequences (Read et al., 2016) and as little as one drink can result in exacerbated insomnia symptoms during the night (Ebrahim et al., 2013) due to toxicity on sleep-related brain systems. The current sampling criteria was intended to capture a naturally-occurring cross-section of college sleepers, and thus sleep-related eligibility criteria was not implemented.
Participants completed two online surveys with an average interval of 68 days (SD = 10.22) between Time 1 (T1) and Time 2 (T2). Of the 171 participants at T1, 157 (92%) also participated at T2. Data from non-attriters who completed both T1 and T2 assessments (n = 157 students) were used for analyses. The final sample was, on average, 19 years old (SD = 1.11, range = 18 to 23) and 30% male. Students were 76% White, 10% Asian, 6% Black or African American, 6% multiracial, and 1% American Indian or Alaska Native. Multiple logistic regression demonstrated attrition was predicted only by T1 insomnia severity (OR = 1.19; 95% CI [1.03, 1.38]), suggesting that the current results based on complete data may slightly underestimate the effects of insomnia severity. All study procedures were approved by the university’s institutional review board.
2.2. Measures
2.2.1. Sleep-related functional impairment
The 6-item Insomnia Diurnal Impact Scale (Ruiz, Guilera, & Gomez-Benito, 2011) assessed the negative daytime effects of nighttime sleep difficulty over the “past month or longer” at T1 and T2. Participants indicated the degree to which they agreed with statements regarding daytime dysfunction (e.g., “I have generally felt sleepy throughout the day,” “I have found it difficult to concentrate during the day”) using a 4-point Likert scale ranging from 0 (totally disagree) to 3 (totally agree). Sum scores (possible range = 0 – 18; Cronbach’s α = 0.84) were used for analyses, with higher scores indicating greater sleep-related functional impairment.
This measure was selected in order to assess the varying domains of daytime functional impairment resulting from sleep problems. These sleep-related daytime functional impairments may result from an array of nighttime sleep symptoms, including but not limited to clinical insomnia or circadian rhythm sleep-wake disorders (American Psychiatric Association, 2013). This scale covers the broad range of sleep-related functional impairment relative to (a) measures of sleep problems which do not comprehensively assess the varied domains of daytime functional impairment (e.g., Pittsburgh Sleep Quality Index; Buysse et al., 1989) and (b) other measures assessing one specific domain such as fatigue (e.g., Fatigue Severity Scale, Krupp et al., 1989).
2.2.2. Drinking patterns
Four items were used at T1 and T2 to capture both typical drinking (i.e., alcohol use frequency and quantity) and risky drinking (i.e., maximum drinks and binge drinking frequency; Goodhines et al., 2017). The past-2-month timeframe was used to accommodate the 2-month time lapse between T1 and T2 assessments. For alcohol use and binge drinking frequency, students responded to “In the past 2 months, how often did you usually have any kind of drink containing alcohol?” and “In the past 2 months, how often did you have 5 (males) or 4 (females) or more drinks containing any kind of alcohol in within a two-hour period?” respectively, using a 9-point Likert scale ranging from 0 (I did not drink any alcohol in the past 2 months) to 8 (Every day). For alcohol quantity and maximum drinks consumed, students responded to “In the past 2 months, how many alcoholic drinks did you have on a typical day when you drank alcohol?” and “In the past 2 months, what is the largest number of drinks containing alcohol that you drank within a 24-hour period?” respectively, using an 11-point Likert scale ranging from 0 (I did not drink any alcohol in the past 2 months) to 10 (Twenty-five or more drinks). Items used to retrospectively report drinking are consistent with recommendations from the National Institute on Alcohol Abuse and Alcoholism (2003) and similar methodology is commonly used in college drinking studies (e.g., Keough, O’Connor, & Read, 2016), including national studies (e.g., Johnston et al., 2015).
2.2.3. Negative drinking consequences
The 23-item Rutgers Alcohol Problem Index assessed experience of diverse negative drinking consequences at T1 and T2 (White & Labouvie, 1989), such as “get into fights,” “have a bad time,” “neglect responsibilities,” and “black out.” The timeframe was modified from the past 12 months to accommodate the 2-month time lapse between T1 and T2 assessments. Participants responded to each item on a modified 7-point Likert scale ranging from 0 (Never in the past 2 months) to 6 (Six times or more in the past 2 months; Park et al., 2014). A sum score (possible range = 0 – 138; Cronbach’s α = .87) was used for analyses.
2.2.4. Insomnia severity
The 7-item Insomnia Severity Index assessed severity of insomnia symptoms at T1 and T2 (Bastien, Vallieres, & Morin, 2001). Participants indicated perceived severity of insomnia symptoms (e.g., difficulty falling asleep, waking up too early) using a 5-point Likert scale ranging from 0 (None) to 4 (Very severe). Sum scores (possible range = 0 – 28; Cronbach’s α = .86) were included as a covariate in all analyses, with scores ranging from 0 – 7 indicating no clinically significant insomnia, 8 – 14 indicating sub-threshold insomnia, 15 – 21 indicating moderate clinical insomnia, and 22 – 28 indicating severe clinical insomnia (Buysse et al., 2006; Smith & Wegener, 2003).
2.2.5. Total sleep time
Two investigator-developed items assessed average sleep duration in the past two weeks at T1 and T2. Participants identified times they typically went to bed or woke up on weekdays and weekends. Typical weekday and weekend sleep durations were calculated as the time elapsed between bed and wake times. By averaging typical weekday sleep duration (multiplied by five days) and weekend sleep duration (multiplied by two days) over a seven-day period, participants’ weighted average sleep duration (hours) was calculated and used as a covariate. While items are investigator-developed, this methodology and item wording is consistent with many past studies of college sleep (Singleton & Wolfson, 2009; Tavernier & Willoughby, 2015), as well as recommendations for sleep research (see Buysse et al., 2006).
2.2.6. Morning/evening preference
The 19-item Morningness-Eveningness Questionnaire assessed circadian preference at T1 (Horne & Ostberg, 1976). Participants identified their preferred sleep/wake times (e.g., “What time would you go to bed if you were entirely free to plan your evening?”) and functioning during the day (e.g., “How alert do you feel during the first half hour after you wake up in the morning?”). A sum score was used as a covariate in all analyses (possible range = 16 – 86; Cronbach’s α = .72), with higher scores indicating a morning (versus evening) preference.
2.2.7. Depression/anxiety
The 4-item Patient Health Questionnaire, which has demonstrated reliability and validity among college students (Khubchandani et al., 2016), assessed subjective depression and anxiety symptoms at T1 and T2 (Kroenke et al., 2009). Participants indicated how often during the past two weeks they experienced each symptom (e.g., “feeling nervous, anxious, or on edge,” “feeling down, depressed, or hopeless”) using a 4-point Likert scale ranging from 0 (not at all) to 3 (nearly every day). A sum score (possible range = 0 – 12; Cronbach’s α = .79) was used as a covariate in all analyses, given well-established associations of depression and anxiety with both sleep and drinking in college students (Kenney et al., 2013; Taylor et al., 2013).
2.2.8. Demographics
Participant age and sex (0 = female, 1 = male) were assessed at T1 and included as covariates in all analyses, given demonstrated associations with college drinking (LaBrie et al., 2011) and sleep (Lund et al., 2010).
2.3. Data Analytic Strategy
Data analyses were computed in SPSS Version 23. Univariate outliers on negative drinking consequences at T1 (i.e., scores greater than 42; n = 6) and T2 (i.e., scores greater than 45; n = 8) were winsorized (Hoaglin & Iglewicz, 1987), consistent with recommendations for handling over-dispersed data when logical cut-offs are not indicated (Spiegelhalter, 2005). No univariate outliers were detected on other study variables. Descriptive statistics and bivariate Pearson correlations were computed for all study variables.
Negative binomial regression was used to examine the moderating effect of T1 sleep-related functional impairment on prospective relationships between T1 drinking and T2 negative drinking consequences. Separate models were estimated for each of the four drinking predictors. The negative binomial distribution accounted for over-dispersion (i.e., variance > mean; Hilbe, 2011) in T2 negative drinking consequences (mean = 11.11; variance = 150.06; dispersion parameter = 0.67 – 0.70; skewness = 1.58; kurtosis = 1.76; observed range = 0 – 45). Incidence rate ratios (IRR) and 95% confidence intervals were used for an effect-size measure of predictors and covariates. Participant sex, T1 depression/anxiety symptoms, T1 insomnia severity, T1 total sleep time, T1 morning/evening preference, and T1 negative drinking consequences were included as covariates. For significant interactions, simple slopes analyses were conducted to examine associations of T1 drinking and T2 negative drinking consequences at low and high levels (±1 SD) of T1 sleep-related functional impairment.
Ancillary analyses were conducted to test the moderating effect of T1 sleep-related functional impairment on the cross-sectional (rather than prospective) relationships of T1 drinking with T1 negative drinking consequences using full data at T1 (N = 171).
3. Results
3.1. Descriptive Analyses
Descriptive statistics and bivariate correlation coefficients for all study variables are presented in Table 1. On average, participants reported moderate levels of sleep-related functional impairment (M = 7.82; SD = 3.37), sub-threshold insomnia (M = 8.57; SD = 5.37), eight hours (SD = 1.17) of sleep per night, and an evening (versus morning) circadian preference (M = 46.90; SD = 7.59) at T1. These mean levels of sleep-related variables are comparable to those in a prior college study (Ruiz, Guilera, & Gomez-Benito, 2011). Among this sample, 21 students (13%) screened positive for clinical insomnia at T1 according to the Insomnia Severity Index standard threshold (Bastien, Vallieres, & Morin, 2001; Buysse et al., 2006). Further, on average, participants reported drinking alcohol once or twice a week (M = 3.87; SD = 0.99), consuming 5 – 6 drinks on a typical drinking day (M = 3.75; SD = 1.59), consuming a maximum of 5 – 7 drinks within a 24-hour period (M = 5.04; SD = 1.85), and binge drinking two or three times per month (M = 2.68; SD = 1.72) at T1. Both T1 sleep-related functional impairment (r’s = .25; p’s < .01) and alcohol variables (r’s = .21 – .41; p’s < .01) were significantly and positively associated with negative drinking consequences at T1 and T2. Sleep-related functional impairment was significantly, negatively associated with total sleep time at T1 (r = −.22; p = .01), but T1 total sleep time was not significantly associated with negative drinking consequences at T1 or T2 (r’s = −.15 – −.11; p’s = .07 – .20).
Table 1.
Correlation Coefficients among Study Variables at Time 1 and Time 2
| r | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % or M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Time 1 Variables | |||||||||||||||||||||
| 1. Male Sex (vs. Female) | 30% | — | |||||||||||||||||||
| 2. Age | 18.87 (1.11) | .17 | — | ||||||||||||||||||
| 3. Sleep-Related Impairment | 7.82 (3.37) | −.17 | −.11 | — | |||||||||||||||||
| 4. Alcohol Frequency | 3.87 (0.99) | .15 | −.03 | .03 | — | ||||||||||||||||
| 5. Alcohol Quantity | 3.75 (1.59) | .37 | −.06 | −.05 | .51 | — | |||||||||||||||
| 6. Maximum Drinks | 5.04 (1.85) | .30 | −.10 | .07 | .62 | .79 | — | ||||||||||||||
| 7. Binge Drinking Frequency | 2.68 (1.72) | .12 | .03 | .08 | .65 | .69 | .67 | — | |||||||||||||
| 8. Depression/Anxiety | 3.58 (2.72) | −.07 | −.02 | .49 | −.03 | .01 | .09 | .06 | — | ||||||||||||
| 9. Insomnia Severity | 8.57 (5.37) | −.14 | .02 | .62 | .05 | .00 | .05 | .13 | .42 | — | |||||||||||
| 10. Total Sleep Time (hours) | 8.04 (1.17) | .09 | −.01 | −.22 | .09 | −.13 | −.17 | −.09 | −.18 | −.31 | — | ||||||||||
| 11. Morning Preference | 46.90 (7.59) | −.02 | .19 | −.12 | −.11 | −.13 | −.15 | −.15 | −.06 | −.04 | −.03 | — | |||||||||
| 12. Negative Drinking Consequences | 10.82 (10.75) | .09 | −.11 | .25 | .36 | .33 | .32 | .41 | .33 | .32 | −.15 | −.04 | — | ||||||||
| Time 2 Variables | |||||||||||||||||||||
| 13. Sleep-related impairment | 7.11 (3.62) | −.10 | .02 | .57 | −.00 | −.07 | −.02 | .02 | .34 | .44 | −.13 | −.09 | .20 | — | |||||||
| 14. Alcohol Frequency | 3.71 (1.06) | .18 | −.02 | −.05 | .55 | .34 | .40 | .39 | −.11 | −.03 | .10 | −.12 | .17 | −.10 | — | ||||||
| 15. Alcohol Quantity | 3.84 (1.72) | .22 | −.18 | −.08 | .41 | .60 | .58 | .43 | −.15 | −.07 | −.12 | −.30 | .19 | −.10 | .41 | — | |||||
| 16. Maximum Drinks | 4.96 (1.70) | .27 | −.15 | .00 | .41 | .58 | .70 | .39 | .04 | −.01 | −.15 | −.16 | .25 | −.10 | .42 | .60 | — | ||||
| 17. Binge Drinking Frequency | 2.50 (1.50) | .15 | −.12 | .01 | .49 | .51 | .57 | .55 | −.02 | .01 | −.05 | −.14 | .24 | −.01 | .54 | .57 | .57 | — | |||
| 18. Depression/Anxiety | 2.55 (2.69) | −.08 | −.00 | .31 | −.06 | −.13 | −.02 | −.03 | .42 | .33 | −.14 | −.04 | .22 | .53 | −.07 | −.09 | .03 | −.02 | — | ||
| 19. Insomnia Severity | 7.39 (5.30) | −.08 | .00 | .48 | .20 | .01 | .06 | .13 | .34 | .55 | −.13 | −.12 | .29 | .62 | .02 | .00 | −.05 | .04 | .51 | — | |
| 20. Total Sleep Time (hours) | 8.38 (1.19) | −.10 | −.21 | −.07 | −.04 | −.07 | −.02 | −.15 | −.14 | −.24 | .40 | .01 | −.17 | −.19 | −.10 | −.06 | .05 | −.09 | −.15 | −.25 | — |
| 21. Negative Drinking Consequences | 11.11 (12.25) | .13 | −.09 | .25 | .25 | .21 | .22 | .27 | .26 | .21 | −.11 | −.19 | .52 | .27 | .20 | .31 | .15 | .28 | .33 | .43 | −.14 |
Note. n = 157. Pearson’s correlation coefficient are reported for two continuous variables; Spearman’s coefficients are reported for continuous and dichotomous (i.e., Male Sex) variables. Significant correlation coefficients at p < .05 are highlighted in bold font.
3.2. Prospective Analyses Predicting T2 Negative Drinking Consequences
Results from main negative binomial regression analyses predicting T2 negative drinking consequences are presented in Table 2. There was a significant interaction between T1 maximum drinks and T1 sleep-related functional impairment on T2 negative drinking consequences (IRR = 0.98, 95% CI [0.96, 0.996], p = .02) after controlling for covariates. Likewise, there was a significant interaction between T1 binge drinking frequency and T1 sleep-related functional impairment on T2 negative drinking consequences (IRR = 0.97, 95% CI [0.95, .996], p = .03) after controlling for covariates. In contrast, T1 sleep-related functional impairment did not significantly moderate prospective associations of T1 drinking frequency (p = .09) or drinking quantity (p = .45) with T2 negative drinking consequences. Notably, significant moderation effects remained consistent when main analyses were re-run without the T1 insomnia severity covariate, thus illustrating that sleep-related functional impairment is responsible for observed moderation even after controlling for previously- identified moderators (i.e., nighttime sleep problems; Kenney et al., 2012; Miller et al., 2016).
Table 2.
Results from Negative Binomial Regression Analyses Predicting T2 Negative Drinking Consequences
|
IRR (95% CI) Predicting T2 Negative Drinking Consequences |
||||
|---|---|---|---|---|
| Predictors and Covariates | T1 Alcohol Frequency | T1 Alcohol Quantity | T1 Maximum Drinks | T1 Binge Drinking |
| Male Sex | 1.66 (1.21, 2.29)** | 1.64 (1.16, 2.33)** | 1.62 (1.17, 2.25)** | 1.58 (1.14, 2.18)** |
| T1 Depression/Anxiety | 1.02 (0.96, 1.10) | 1.03 (0.96, 1.10) | 1.04 (0.97, 1.11) | 1.03 (0.96, 1.10) |
| T1 Insomnia Severity | 1.02 (0.98, 1.05) | 1.01 (0.98, 1.05) | 1.02 (0.98, 1.05) | 1.01 (0.98, 1.05) |
| T1 Total Sleep Time | 0.97 (0.85, 1.11) | 0.96 (0.84, 1.10) | 0.94 (0.82, 1.08) | 0.96 (0.84, 1.09) |
| T1 Morning Preference | 0.97 (0.95, 0.99)*** | 0.97 (0.95, 0.99)** | 0.97 (0.95, 0.99)*** | 0.97 (0.95, 0.99)** |
| T1 Negative Drinking Consequences | 1.05 (1.03, 1.06)*** | 1.05 (1.03, 1.06)*** | 1.04 (1.03, 1.06)*** | 1.04 (1.03, 1.06)*** |
| T1 Alcohol Frequency, Quantity, Maximum, or Binge Drinking | 1.02 (0.85, 1.22) | 1.01 (0.89, 1.15) | 1.01 (0.92, 1.11) | 1.04 (0.94, 1.16) |
| T1 Sleep-related Functional Impairment | 1.04 (0.98, 1.11) | 1.05 (0.98, 1.11) | 1.04 (0.97, 1.10) | 1.05 (0.99, 1.11) |
| T1 Alcohol * T1 Sleep-related Functional Impairment | 0.96 (0.91, 1.01) | 0.99 (0.96, 1.02) | 0.98 (0.96, 1.00)* | 0.97 (0.95, 1.00)* |
Note. n = 157. IRR = incidence rate ratio; CI = confidence interval; T1 = Time 1; T2 = Time 2.
p < .05.
p < .01.
p < .001.
As shown in Figure 1, T1 maximum drinks and T1 binge drinking frequency were more strongly positively associated with T2 negative drinking consequences at lower levels of T1 sleep-related functional impairment (IRR = 1.10, 95% CI [0.99, 1.23], p = .08; IRR = 1.15, 95% CI [1.02, 1.31], p = .03) as compared to higher levels of T1 sleep-related functional impairment (IRR = 0.93, 95% CI [0.83, 1.05], p = .26; IRR = 0.94, 95% CI [0.82, 1.09], p = .43). Specifically, among students reporting lower (−1 SD) levels of T1 sleep-related functional impairment, greater T1 maximum drinks and binge drinking frequency was associated with greater T2 negative drinking consequences. However, among students reporting higher (+1 SD) levels of T1 sleep-related functional impairment, T2 negative drinking consequences were consistently high regardless of T1 maximum drinks and binge drinking frequency.
Figure 1.
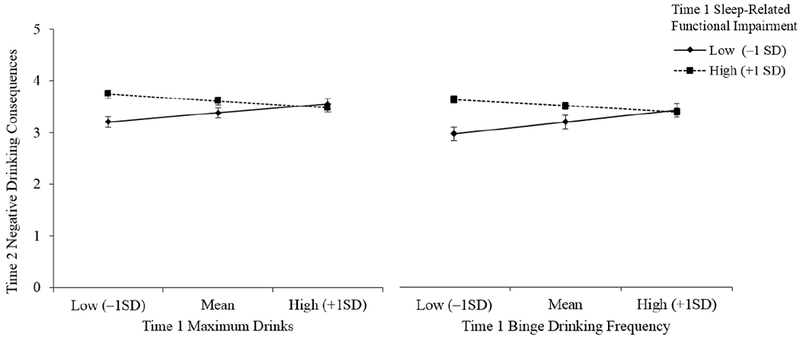
n = 157. Simple slopes for associations of Time 1 maximum drinks and binge drinking frequency with Time 2 negative drinking consequences, by Time 1 sleep-related functional impairment.
3.3. Ancillary Analyses Predicting T1 Negative Drinking Consequences
Consistent with prospective results, ancillary analyses using full cross-sectional data at T1 (N = 171) revealed a significant interaction between T1 maximum drinks and T1 sleep-related functional impairment on T1 negative drinking consequences (IRR = 0.98, 95% CI [0.96, 0.99], p = .01) after controlling for covariates. Simple slopes analyses also indicated that T1 maximum drinks was more strongly, positively associated with T1 negative drinking consequences at lower (IRR = 1.36, 95% CI [1.23, 1.49], p < .001) as compared to higher (IRR = 1.15, 95% CI [1.04, 1.27], p = .01) levels of T1 sleep-related functional impairment. No significant interactions of T1 alcohol frequency (IRR = 0.99, 95% CI [0.94, 1.03], p = .55), alcohol quantity (IRR = 0.98, 95% CI [0.96, 1.01], p = .20), or binge drinking frequency (IRR = 0.99, 95% CI [0.96, 1.01], p = .23) with T1 sleep-related functional impairment on T1 negative drinking consequences were found. Cross-sectional ancillary analyses highlight consistency of the observed moderation pattern both concurrently and prospectively, thereby increasing confidence in prospective findings.
4. Discussion
It is well-established that college students experience substantial sleep-related functional impairment and also frequently engage in risky drinking that leads to negative drinking consequences. The current two-month prospective study examined whether sleep-related functional impairment modified associations of T1 college drinking with T2 negative drinking consequences after accounting for T1 negative drinking consequences, depression/anxiety symptoms, sleep-related variables (i.e., insomnia severity, total sleep time, morning/evening preference) and sex. Students reporting higher sleep-related functional impairment experienced high levels of negative drinking consequences even at low levels of risky drinking. In contrast, students reporting lower sleep-related functional impairment experienced high levels of negative drinking consequences (at levels comparable to peers with higher sleep-related functional impairment) only at high levels of risky drinking. Thus, findings highlight sleep-related functional impairment as an important source of individual differences in the college drinking and negative drinking consequences relationship, over and above the effects of additional sleep constructs and sleep-related correlates.
Current results highlight college drinkers reporting higher sleep-related functional impairment as a high-risk group for unforeseen levels of negative drinking consequences, as these students experience consistently high levels of negative drinking consequences regardless of their drinking levels. In the presence of high sleep-related functional impairment, as opposed to nighttime sleep problems, failures in self-regulation resulting in negative drinking consequences may occur even at lower levels of risky drinking. For example, sleep-related functional impairment in young adults includes impulsive responding to negative stimuli (Anderson & Platten, 2011), weakened ability to inhibit aggression (Kahn-Greene et al., 2006), and impaired decision-making (Schnyer, Zeithamova, & Williams, 2009). Thus, sleep-related functional impairment may increase high-risk behavior and decrease protective behavior (e.g., pacing drinks) while drinking, resulting in greater experience of negative drinking consequences.
Previous research suggests students with poor sleep quality and subjective insufficient total sleep time experience greater negative drinking consequences only at high drinking quantity levels (Kenney et al., 2012; Miller et al., 2016). In contrast, the current study investigated associations of both general alcohol consumption (i.e., frequency, quantity) and risky drinking (i.e., maximum drinks, binge drinking frequency) with negative drinking consequences. Results indicate that sleep-related functional impairment may prospectively influence associations of risky drinking (but not general alcohol consumption) with negative drinking consequences, after accounting for insomnia severity, total sleep time, and morning/evening preference. This result may be explained by previous findings that risky drinking and general alcohol consumption are differentially associated with the impairing effects of alcohol (and by extension negative drinking consequences). For example, binge drinkers aged 16 – 20 years experienced more negative drinking consequences than their non-binge drinking peers, regardless of drinking frequency (Reboussin et al., 2006).
4.1. Clinical Implications
Current findings suggest college drinkers may benefit from integrated interventions targeting sleep and drinking. Evidence-based sleep interventions for college students include cognitive-behavioral therapy and sleep hygiene (for a review, see Friedrich & Schlarb, 2017), which might expand upon alcohol-specific content (Fucito et al., 2015). For example, limiting alcohol use before bed is recommended as a standard component of sleep hygiene education (Stepanski & Wyatt, 2003). Integrated interventions should include personalized feedback on drinking, moderate drinking recommendations, and drinking reduction strategies (Fucito et al., 2015). Findings also indicate specific targeting of sleep-related functional impairment within integrated interventions, such as monitoring of next-day functioning (as in cognitive therapy for insomnia; Harvey, 2005).
4.2. Limitations and Future Directions
Findings must be interpreted with regard to several limitations. First, data were drawn from a predominantly White, female, first-year sample of students recruited from psychology classes at a northeastern United States private university. Because drinking and sleep patterns vary by diverse individual and school characteristics (Johnston et al., 2015; Lichstein et al., 2004), replication in samples with greater heterogeneity is needed to investigate generalizability of our findings. Further, eligibility criteria required that all participants endorse alcohol use at least once in the past 30 days, so findings may not generalize to infrequent drinkers; however, 83% of students in a comparable sample without this eligibility criteria endorsed alcohol use in the past 30 days (Lowery, Merrill, & Carey, 2018), suggesting such sampling bias may be minimal. Likewise, findings might not generalize to heavy-drinking or clinical samples of young adults. Additionally, the current study did not assess specific sleep disorders that may cause functional impairment (e.g., insomnia disorder, circadian rhythm sleep-wake disorders, obstructive sleep apnea, etc.) and subjective retrospective assessments may have been vulnerable to self-reporting biases and memory errors. Lastly, although this prospective design allows for superior assessment of temporal relationships relative to cross-sectional designs, the current study remains correlational in nature and causal inferences are speculative.
Results of the current study have the potential to inform future research. Because this is the first study testing the moderating role of sleep-related functional impairment in associations of both risky and general alcohol use behaviors with negative consequences, replication is necessary among both general college samples and specific sub-samples of heavy-drinkers and poor sleepers. In order to optimize reporting accuracy, future investigations should use measures robust to retrospective reporting error for drinking (e.g., Timeline Follow-Back; Sobell & Sobell, 1992) and daily sleep behaviors (e.g., Consensus Sleep Diary; Carney et al., 2012). Further, in order to reconcile the current findings with previous findings (Kenney et al., 2012; Miller et al., 2016), research is needed to examine how roles of poor sleep quality and subjective total sleep time inadequacy in risky drinking and negative drinking consequences differ from the role of sleep-related functional impairment. Continued investigation might also assess specific factors (e.g., compromised decision making) that may mediate the relationship between sleep-related functional impairment and negative drinking consequences. Lastly, clinical research may investigate efficacy of integrating sleep-related functional impairment into college alcohol and sleep intervention programs (e.g., Fucito et al., 2015).
4.3. Conclusion
Despite these limitations, the current findings indicate that sleep-related functional impairment may exacerbate negative drinking consequences of risky drinking. Thus, sleep-related functional impairment helps to explain individual differences in the association between risky drinking and negative drinking consequences in college students.
Acknowledgments
This research was supported in part by NIH Grant R15AA022496 awarded to Aesoon Park.
Footnotes
Declarations of interest: None.
References
- Alapin I, Fichten CS, Libman E, Creti L, Bailes S, & Wright J (2000). How is good and poor sleep in older adults and college students related to daytime sleepiness, fatigue, and ability to concentrate? Journal of Psychosomatic Research, 49(5), 381–390. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental health disorders: Dsm-5 (5 ed.). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Anderson C & Platten CR (2011). Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behavioural Brain Research, 217(2), 463–466. doi: 10.1016/j.bbr.2010.09.020 [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, & Morin CM (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, & Morin CM (2006). Recommendations for a standard research assessment of insomnia. SLEEP, 29(9), 1155–1173. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: Standardizing prospective sleep self-monitoring. SLEEP, 35(2), 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini KS & Fucito LM (2014). Variations in sleep characteristics and sleep-related impairment in at-risk college drinkers: A latent profile analysis. Health Psychology, 33(10), 1164–1173. doi: 10.1037/hea0000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earleywine M, LaBrie JW, & Pedersen ER (2008). A brief rutgers alcohol problem index with less potential for bias. Addictive Behaviors, 33(9), 1249–1253. doi: 10.1016/j.addbeh.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, & Fenwick PB (2013). Alcohol and sleep i: Effects on normal sleep. Alcohol Clin Exp Res, 37(4), 539–549. doi: 10.1111/acer.12006 [DOI] [PubMed] [Google Scholar]
- Friedrich A & Schlarb AA (2017). Let’s talk about sleep: A systematic review of psychological interventions to improve sleep in college students. Journal of Sleep Research. doi: 10.1111/jsr.12568 [DOI] [PubMed] [Google Scholar]
- Fucito LM, Bold KW, Van Reen E, Redeker NS, O’Malley SS, Hanrahan TH, & DeMartini KS (2018). Reciprocal variations in sleep and drinking over time among heavy-drinking young adults. Journal of Abnormal Psychology, 127(1), 92–103. doi: 10.1037/abn0000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, DeMartini KS, Hanrahan TH, Whittemore R, Yaggi HK, & Redeker NS (2015). Perceptions of heavy-drinking college students about a sleep and alcohol health intervention. Behavioral Sleep Medicine, 13(5), 395–411. doi: 10.1080/15402002.2014.919919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, DeMartini KS, Hanrahan TH, Yaggi HK, Heffern C, & Redeker NS (2017). Using sleep interventions to engage and treat heavy‐drinking college students: A randomized pilot study. Alcoholism: Clinical and Experimental Research, 41(4), 798–809. doi: 10.1111/acer.13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos NL, Vargas Lascano DI, Howard AL, & Maggs JL (2013). Who sleeps best? Longitudinal patterns and covariates of change in sleep quantity, quality, and timing across four university years. Behavioral Sleep Medicine, 11(1), 8–22. doi: 10.1080/15402002.2011.596234 [DOI] [PubMed] [Google Scholar]
- Gellis LA, Park A, Stotsky MT, & Taylor DJ (2014). Associations between sleep hygiene and insomnia severity in college students: Cross-sectional and prospective analyses. Behavior Therapy, 45(6), 806–816. doi: 10.1016/j.beth.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Goodhines PA, Gellis LA, Kim J, Fucito LM, & Park A (2017). Self-medication for sleep in college students: Concurrent and prospective associations with sleep and alcohol behavior. Behavioral Sleep Medicine. doi: 10.1080/15402002.2017.1357119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2005). A cognitive theory and therapy for chronic insomnia. Journal of Cognitive Psychotherapy, 19(1), 41–59. doi: 10.1891/jcop.19.1.41.66332 [DOI] [Google Scholar]
- Hilbe JM (2011). Negative binomial regression (2 ed.). New York, New York: Cambridge University Press. [Google Scholar]
- Hoaglin DC & Iglewicz B (1987). Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association, 82(400), 1147. [Google Scholar]
- Horne JA & Ostberg O (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, & Miech RA (2015). Monitoring the future national survey results on drug use, 1975-2014: Volume ii, college students and adults ages 19-55. Ann Arbor: Institute for Social Research, The University of Michigan, 416. [Google Scholar]
- Kahn-Greene ET, Lipizzi EL, Conrad AK, Kamimori GH, & Killgore WDS (2006). Sleep deprivation adversely affects interpersonal responses to frustration. Personality and Individual Differences, 41(8), 1433–1443. doi: 10.1016/j.paid.2006.06.002 [DOI] [Google Scholar]
- Kenney SR, LaBrie JW, Hummer JF, & Pham AT (2012). Global sleep quality as a moderator of alcohol consumption and consequences in college students. Addictive Behaviors, 37(4), 507–512. doi: 10.1016/j.addbeh.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney SR, Lac A, LaBrie JW, Hummer JF, & Pham A (2013). Mental health, sleep quality, drinking motives, and alcohol-related consequences: A path-analytic model. Journal of Studies on Alcohol and Drugs, 74(6), 841–851. doi: 10.15288/jsad.2013.74.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough MT, O’Connor RM, & Read JP (2016). Replication and validation of the young adult alcohol consequences questionnaire in a large sample of canadian undergraduates. Alcoholism: Clinical and Experimental Research, 40(5), 1093–1099. doi: 10.1111/acer.13039 [DOI] [PubMed] [Google Scholar]
- Khubchandani J, Brey R, Kotecki J, Kleinfelder J, & Anderson J (2016). The psychometric properties of phq-4 depression and anxiety screening scale among college students. Archives of Psychiatric Nursing. doi: 10.1016/j.apnu.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, & Löwe B (2009). An ultra-brief screening scale for anxiety and depression: The phq-4. Psychosomatics, 50(6), 613–621. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, & Steinberg AD (1989). The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- LaBrie JW, Lac A, Kenney SR, & Mirza T (2011). Protective behavioral strategies mediate the effect of drinking motives on alcohol use among heavy drinking college students: Gender and race differences. Addictive Behaviors, 36(4), 354–361. doi: 10.1016/j.addbeh.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, & Bush AJ (2004). Epidemiology of sleep: Age, gender, and ethnicity. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Lowery AD, Merrill JE, & Carey KB (2018). How acceptable are intoxicated behaviors? Discrepancy between personal versus perceived approval. Addictive Behaviors, 76, 258–264. doi: 10.1016/j.addbeh.2017.08.021 [DOI] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, & Prichard JR (2010). Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal of Adolescent Health, 46(2), 124–132. doi: 10.1016/j.jadohealth.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Merrill JE & Carey KB (2016). Drinking over the lifespan: Focus on college ages. Alcohol Research : Current Reviews, 38(1), 103–114. [PMC free article] [PubMed] [Google Scholar]
- Miller MB, DiBello AM, Lust SA, Carey MP, & Carey KB (2016). Adequate sleep moderates the prospective association between alcohol use and consequences. Addictive Behaviors. doi: 10.1016/j.addbeh.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2003). Recommended alcohol questions. from http://www.niaaa.nih.gov/
- Oginska H & Pokorski J (2006). Fatigue and mood correlates of sleep length in three age-social groups: School children, students, and employees. Chronobiology International, 23(6), 1317–1328. doi: 10.1080/07420520601089349 [DOI] [PubMed] [Google Scholar]
- Park A, Kim J, Gellis LA, Zaso MJ, & Maisto SA (2014). Short-term prospective effects of impulsivity on binge drinking: Mediation by positive and negative drinking consequences. Journal of American College Health, 62(8), 517–525. doi: 10.1080/07448481.2014.929579 [DOI] [PubMed] [Google Scholar]
- Read JP, Haas AL, Radomski S, Wickham RE, & Borish SE (2016). Identification of hazardous drinking with the young adult alcohol consequences questionnaire: Relative operating characteristics as a function of gender. Psychological Assessment, 28(10), 1276–1289. doi: 10.1037/pas0000251 [DOI] [PubMed] [Google Scholar]
- Reboussin BA, Song EY, Shrestha A, Lohman KK, & Wolfson M (2006). A latent class analysis of underage problem drinking: Evidence from a community sample of 16-20 year olds. Drug and Alcohol Dependence, 83(3), 199–209. doi: 10.1016/j.drugalcdep.2005.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C, Guilera G, & Gomez-Benito J (2011). Development of a scale to assess the diurnal impact of insomnia. Psychiatry Research, 190(2-3), 335–341. doi: 10.1016/j.psychres.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Zeithamova D, & Williams V (2009). Decision-making under conditions of sleep deprivation: Cognitive and neural consequences. Military Psychology, 21(Suppl 1), S36–S45. doi: 10.1080/08995600802554607 [DOI] [Google Scholar]
- Singleton RA Jr. & Wolfson AR (2009). Alcohol consumption, sleep, and academic performance among college students. Journal of Studies on Alcohol and Drugs, 70(3), 355–363. [DOI] [PubMed] [Google Scholar]
- Smith MT & Wegener ST (2003). Measures of sleep: The insomnia severity index, medical outcomes study (mos) sleep scale, pittsburgh sleep diary (psd), and pittsburgh sleep quality index (psqi). Arthritis & Rheumatism, 49(5S), S184–S196. doi: 10.1002/art.11409 [DOI] [Google Scholar]
- Sobell LC & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported alcohol consumption In Litten RZ & Allen JP (Eds.), Measuring alcohol consumption: Psychosocial and biochemical methods. (pp. 41–72). Totowa, NJ, US: Humana Press. [Google Scholar]
- Spiegelhalter DJ (2005). Handling over-dispersion of performance indicators. Quality & Safety in Health Care, 14(5), 347–351. doi: 10.1136/qshc.2005.013755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanski EJ & Wyatt JK (2003). Use of sleep hygiene in the treatment of insomnia. Sleep Medicine Reviews, 7(3), 215–225. [DOI] [PubMed] [Google Scholar]
- Tavernier R & Willoughby T (2015). A longitudinal examination of the bidirectional association between sleep problems and social ties at university: The mediating role of emotion regulation. Journal of Youth and Adolescence, 44(2), 317–330. doi: 10.1007/s10964-014-0107-x [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bramoweth AD, Grieser EA, Tatum JI, & Roane BM (2013). Epidemiology of insomnia in college students: Relationship with mental health, quality of life, and substance use difficulties. Behavior Therapy, 44(3), 339–348. doi: 10.1016/j.beth.2012.12.001 [DOI] [PubMed] [Google Scholar]
- White HR & Labouvie EW (1989). Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol, 50(1), 30–37. doi: 10.15288/jsa.1989.50.30 [DOI] [PubMed] [Google Scholar]


