Abstract
Relations between maternal baseline cortisol and infant cortisol reactivity to an emotion induction procedure at child ages 7, 15, and 24 months were analyzed using data from the Family Life Project (N=1,292). The emotion induction consisted of a series of standardized and validated tasks, including an arm restraint, toy removal, and mask presentation, intended to elicit responses of fear and frustration. Results revealed that at 7 and 15 months, maternal baseline cortisol was negatively related to child cortisol reactivity, such that children of mothers with lower cortisol exhibited steeper cortisol increases in response to the emotion induction. At 24 months, the association between mother and infant cortisol was moderated by socioeconomic risk, such that maternal baseline cortisol was associated with child cortisol reactivity only in dyads characterized by low socioeconomic risk. Furthermore, children of mothers with low baseline cortisol and low socioeconomic risk exhibited decreasing cortisol responses, whereas children of mothers with low baseline cortisol but high risk exhibited flat cortisol responses. Children in dyads characterized by high baseline maternal cortisol also exhibited flat cortisol responses regardless of socioeconomic risk. The role of caregiver physiology in the regulation of the child’s stress response in the context of adversity is discussed.
Keywords: cortisol, poverty, parenting, socioeconomic status, stress
1. Introduction
Exposure to early life stress, such as that associated with socioeconomic adversity, can affect the child’s stress response system, shaping developmental trajectories and impacting later-life health outcomes (Blair, 2010; Shonkoff, Boyce, & McEwen, 2009; Lupien, King, Meaney, & McEwen, 2001). A multitude of factors influences associations between environmental adversity and the child’s stress response. Extensive research shows that the caregiver plays a regulating role both as a mediator and a moderator of this association—for better or for worse—transmitting stress to the child or buffering the child from stress (Blair et al, 2008; Tang, Reeb-Sutherland, Romeo, & McEwen, 2014). The caregiver’s ability to regulate the infant, however, is dependent to some extent on the caregiver’s own level of stress (Barrett & Fleming, 2011). Most studies related to the maternal regulation of infant stress response have focused on the effects of proximal behavioral and psychosocial factors, such as parental sensitivity, depression, anxiety, parenting stress, intimate partner violence (IPV), and marital conflict (e.g., Gunnar & Donzella, 2002; Davis, West, Bilms, Morelen, & Suveg, 2018; Levendosky et al., 2016; Lawler et al., 2018; Sturge-Apple, Davies, Cicchetti, & Manning, 2012). However, environments of elevated stress, such as those characterized by poverty and low socioeconomic status (SES), can influence the caregiver’s stress physiology, primarily indicated by cortisol. In turn, caregiver stress physiology can affect the child’s stress physiology and compromise the caregiver’s ability to regulate the infant’s stress response (Blair & Raver, 2012; Hoff, Laursen, & Tardif, 2002; Kotchick & Forehand, 2002). Thus, the purpose of the present study was to investigate relations between maternal and infant cortisol activity in the context of socioeconomic risk.
1.1. Stress and Parenting in the Context of Socioeconomic Risk
A substantial literature demonstrates that environments of adversity, such as socioeconomic risk, can negatively affect psychological and physical health of adults and children (Kim, Evans, Chen, Miller, & Seeman, 2018; Lupien, McEwen, Gunnar, & Heim, 2009). The cumulative risks associated with socioeconomic adversity, such as financial strain, material hardship, diminished social support, neighborhood disadvantage, household crowding, and occupational status are associated with increased levels of psychological stress in parents (Evans & English, 2002; Baum, Garofalo, & Yali, 1999; Conger & Donnellan, 2007; Mills-Koonce & Towe-Goodman, 2012). Chronic exposure to stress can significantly influence a parent’s caregiving ability, reducing sensitive and responsive behaviors, and increasing harsh and intrusive ones (Masarik & Conger, 2017; Conger & Donnellan, 2007; Taylor et al., 2013). Relatedly, parenting in poverty is associated with increased risks for depression, marital discord, and IPV (Finegood & Blair, 2017).
In addition to psychological and behavioral effects, stress also alters the underlying physiology that supports the caregiver’s emotional and behavioral functioning. In particular, the environment within which caregiving occurs can influence the caregiver’s stress physiology (Barrett & Fleming, 2011). Research has shown that socioeconomic risk impacts stress physiology, most notably the hypothalamic-pituitary-adrenal (HPA) axis, resulting in dysregulation of the stress hormone cortisol (Kim et al., 2018). Importantly, relations between socioeconomic risk and cortisol are complex and not always consistent, with studies reporting conflicting findings (see Dowd, Simanek, & Aiellol, 2009; Miller, Chen, & Zhou, 2007 for reviews). However, several previous studies with mothers have shown that higher socioeconomic risk is associated with higher levels of cortisol (Clearfield, Carter-Rodriguez, Merali, & Shober, 2014; Thayer & Kuzawa, 2014; Ursache, Merz, Melvin, Meyer, & Noble 2017; Bosquet Enlow, et al., 2018; Tarullo, St. John, & Meyer, 2017).
1.2. Maternal Regulation of Child Stress Response
Theory and cross-species research from a psychobiological perspective have long recognized the role the caregiver plays in modulating offspring behavior and physiology, and especially the stress response (Field, 1985; Fogel, 1993; Hofer, 1994; Sullivan & Perry, 2015). Because the infant’s top-down neural and physiological regulatory mechanisms are not yet fully developed, the infant relies on the caregiver for regulation to support healthy development (Kolb et al., 2012; Gunnar & Donzella, 2002; Lewis & Todd, 2007). During parent-child interactions, the caregiver communicates information explicitly and implicitly to the infant about the external world and how to respond adaptively to environmental stressors and challenges to promote survival (Papousek 2007; Frith, 2008). Importantly, the caregiver serves a dual function in this regard: that of buffering against stressors or exacerbating their damaging effects (Perry, Blair, & Sullivan, 2017).
The extent to which a caregiver is able to regulate an infant’s stress physiology is dependent on many factors including not only behavioral and psychological characteristics, but also environmental and physiological factors (Hackman, O’Brien, & Zalewski, 2018). However, most research on caregiver regulation has focused on behavioral and psychological factors, such as maternal depression and anxiety, not environmental or physiological ones. Yet socioeconomic risk and parenting behaviors are associated with both psychological and physiological stress (Barrett & Fleming, 2011; Evans & English, 2002; Mills-Koonce et al., 2009). For instance, human and nonhuman studies have shown that sensitive caregiving behaviors can down-regulate a child’s cortisol response via social buffering (Gunnar and Donzella, 2002; Caldji, Diorio, & Meaney, 2000). But this ability to buffer can be impaired in contexts of adversity (Sanchez, McCormack, & Howell, 2015). Certainly, one potential factor disrupting caregiver regulation in adverse environments may be increased levels of psychological stress. Indeed, studies have shown that higher levels of caregiver’s self-reported stress are related to increased infant cortisol activity (Essex, Klein, Cho, & Kalin, 2002; Ursache et al., 2017; Leung et al., 2010).
Yet another potential factor disrupting the caregiver’s ability to regulate is the caregiver’s physiology (Leerkes, Su, Calkins, O’Brien, & Supple, 2017; Lorber & O’Leary, 2005). For instance, one study found that increases in mothers’ self-reported depressive symptomology was associated with increased infant cortisol responses to a stressor, but only if the mother also had high levels of cortisol (Khoury et al, 2016). This finding highlights the idea that maternal stress physiology may be a moderator of maternal regulation that is not necessarily detectable using only typical behavioral or psychosocial measures, such as global observations and subjective self-reports. This idea is consistent with Hofer’s “hidden regulators” hypothesis in which he identified mechanisms in the mother-child relationship that “were not evident when simply observing the ongoing mother-infant relationship,” but regulated child behavior and physiology (Hofer, 2010, p. 157). Indeed, other studies have found that maternal physiology may be unrelated to self-report and/or observed parenting measures, yet still related to the child’s physiology (Luecken, Crnic, Gonzales, Winstone, & Somer, 2018; Emery, McElwain, Groh, Haydon, & Roisman, 2014; Leerkes et al., 2017). Likewise, researchers have noted that much of the mother-infant relationship occurs outside of conscious awareness or intentional behavior (Bornstein, 2013; Papousek & Papousek, 2002). Thus, global observational and self-report measures may not be suitable methods to detect subtle cues or regulators in the parent-child relationship. Using this framework, we suggest that maternal cortisol can be understood as a hidden regulator of infant cortisol responses if the association between mother and child physiology occurs independently from global observational measures of behavior and self-report measures of psychosocial functioning. Thus, in the current analysis, to assess whether maternal cortisol may be a hidden regulator of infant cortisol responses, we controlled for measures of parenting behavior, maternal depression, IPV, and infant emotional reactivity, which may mediate mother-infant cortisol associations.
1.3. Associations between Caregiver and Child Physiology
Studying caregiver and infant cortisol activity together, while also controlling for potential proximal mediators, can provide insight into the co-regulatory dynamics of the mother-child relationship. Most studies, however, have investigated child and parent stress physiology separately, although a growing body of research indicates that caregiver and child stress physiology is co-regulated. Researchers have posited various terms to describe dyadic relations between caregiver-child bio-behavioral activity, such as “linkage”, “attunement”, “synchrony”, or “co-regulation” (Bornstein, 2013; Calkins, 2011; Harrist & Waugh, 2002; Feldman, 2007). Briefly, shared relations between caregiver-child behavior and physiology are thought to facilitate social and emotional communication to promote survival and healthy development (Butler, 2011; Feldman, 2012). According to bio-behavioral synchrony theory, the coordination or coupling of behavioral and physiological activity between a caregiver and child serves as the foundation for an affiliative bond and operates as a mechanism of maternal regulation (Feldman, 2012). Some also theorize that being physiologically attuned likely serves an evolutionary function promoting survival (Atkinson, Jamieson, Khoury, Ludmer, & Gonzalez, 2016; Feldman, 2017). For this reason, infants may be biologically predisposed to react readily to maternal cues, especially stress- and threat-related cues as infants are highly dependent on caregivers for protection (Bornstein, 2013; Papousek & Papousek, 2002; Feldman, 2017).
Similarly, parents are likely also biologically prepared to respond to infant cues (Fleming & Li, 2002; Bornstein, 2016). Studies have shown that a parent’s responsiveness to child cues is related to parent physiology (Lorber & O’Leary, 2005; Stallings et al., 2001). But the caregiver’s responsiveness is further dependent on child characteristics, specifically the infant’s ability to communicate signals such as crying that elicit responses from the caregiver. The infant’s emotional reactivity is a critical signal to the caregiver communicating the demand for protection and safety. In this regard, the infant regulates the caregiver by signaling distress and the need for comfort. In turn, the caregiver regulates the infant by responding sensitively and soothing the infant. Accordingly, the parent-child relationship is not a one-way exchange, but a dynamic, bidirectional, and reciprocal interaction during which the mother and the child co-regulate each other behaviorally and physiologically (Bornstein, 2013; Feldman, 2007). Thus, when studying relations between caregiver and child stress physiology it is important to consider both maternal as well as child contributions to physiological activity (Blair et al., 2008; Finegood et al., 2016; Bornstein, 2016).
Regarding stress physiology in particular, several studies have shown relations between caregiver and child cortisol levels at rest or baseline (Fuchs, Mohler, Resch, & Kaess, 2016; Thompson & Travathan, 2007; Clearfield et al., 2014; Granger et al., 1998; Hibel, Granger, Blair, Finegood, & Family Life Project Key Investigators, 2015), in response to stressors (Khoury et al., 2016; Hibel et al., 2015; Hibel, Granger, Blair, Cox, & Family Life Project Key Investigators, 2009), and across the day (Clearfield et al., 2014; LeMoult, Chen, Foland-Ross, Burley, & Gotlib, 2015). However, these relations are often moderated by behavioral and psychosocial factors, such as caregiving behavior and child emotional reactivity. For instance, some studies have shown that mother-child cortisol linkage is stronger for dyads characterized by higher maternal sensitivity (Sethre-Hofstad, Stansbury & Rice, 2002; van Bakel & Riksen-Walraven 2008; Atkinson et al., 2013). Similarly, Hibel and colleagues (2015), using the dataset analyzed here, found that cortisol attunement was greater for dyads characterized by higher maternal sensitivity and lower child emotional reactivity.
However, relations between caregiver and infant cortisol may occur for better or for worse. For example, if a mother is chronically stressed and has persistently high levels of cortisol, it may be damaging in the long-term for an infant to also have high levels of cortisol. In support of this idea, some research indicates that maternal risk factors are associated with relations between caregiver and child cortisol activity. For instance, among mothers with higher levels of depressive symptomatology, mother-child cortisol is more highly correlated than for mothers with lower levels (Laurent, Ablow, Measelle, 2011; Khoury et al., 2016). Additionally, Hibel et al. (2009), using a subsample from the dataset analyzed here, found that mothers who reported instances of IPV displayed cortisol responses that were more similar to their 7-month-old infants’ cortisol responses to a stressor.
1.4. Caregiver and Child Stress Physiology: The Role of Socioeconomic Risk
The studies described above indicate that positive as well as negative factors moderate associations between mother and child cortisol activity, but have focused mainly on the influence of proximal, behavioral and psychosocial factors. Few analyses have focused on distal, environmental factors, such as poverty and socioeconomic risk. Because the context within which caregiving occurs can affect both caregiving behavior and physiology, the environment likely moderates the association between caregiver and child stress physiology. Importantly, the context of socioeconomic risk can disrupt multiple aspects of the parent-child relationship. As such, here we test the hypothesis that this disruption occurs at the level of maternal stress physiology and ask whether the context of socioeconomic risk, net of a host of proximal covariates, disrupts the association between maternal and child cortisol.
Despite a substantial literature documenting associations between cortisol and SES, only two studies to our knowledge have explicitly investigated how SES affects the relation between mother and child cortisol (Clearfield et al., 2014; Thayer & Kuzawa, 2014). Of these, only Thayer and Kuzawa (2014) investigated the relation between infant cortisol reactivity and mother’s resting or baseline cortisol—the focus of the current analysis. More specifically, the authors found that higher levels of socioeconomic risk were associated with higher levels of maternal evening cortisol, and elevated infant cortisol reactivity to a stressor at 6 weeks of age. However, this study is limited by a relatively small and low-risk sample, and by evaluating the relation between mother and child cortisol at a single age in infancy. No prior study of which we are aware has investigated the extent to which mother-child cortisol associations vary as a function of SES. Understanding caregiver-child cortisol activity in contexts of socioeconomic risk is important because dysregulation of cortisol is likely a key factor by which environmental stress can “get under the skin” to impact development (Lupien et al. 2009; Kim et al. 2018).
1.5. Development of Caregiver and Child Stress Physiology
Another limitation of prior research is that most studies investigating caregiver-child cortisol have evaluated these relations at single ages. It is likely, however, that the association between caregiver and child physiology varies with development, especially early in childhood when the HPA axis and related top-down regulatory mechanisms, namely the prefrontal cortex, are going through rapid development, altering cortisol activity (Hostinar & Gunnar, 2013; Schore, 2015). For instance, it is well documented that over the first two years, infant cortisol reactivity decreases with age (Jansen, Beijers, Riksen-Walraven, & de Weerth, 2010; Gunnar, Talge, & Herrera, 2009; Davis & Granger, 2009). This shift may represent a normative stress hypo-responsive period analogous to that in nonhumans that is related to social buffering (Hostinar, Sullivan, & Gunnar, 2014). Similarly, the function and capacity of the caregiver as regulator changes as the child gets older and becomes relatively more autonomous and reliant on self- and mutual regulation rather than caregiver regulation alone (Sroufe, 1997; Bernier, Carlson, & Whipple, 2010). This gradual transition from external regulation to self-regulation coincides with the infant’s differentiating between self and other, and integrating of referential and symbolic social cues. These capacities, which emerge around 12 to 24 months of age, may be especially important in interpreting cues that facilitate social buffering (Tomasello, Carpenter, Call, Behne, & Moll, 2005; Feldman, 2007; Hostinar et al., 2014; Frith, 2008). In light of these early-life developments, one purpose of the present study was to investigate caregiver-child cortisol activity at three time points across infancy.
1.6. The Present Study
In the present study we used longitudinal data from a high-risk sample of mothers and infants to investigate how the association between maternal baseline cortisol and infant cortisol reactivity relates to socioeconomic risk across infancy, both from the perspective of the hidden regulators hypothesis and from a developmental psychobiological standpoint. Our analysis extends two previous studies with this dataset, which found that mother’s and infant’s baseline cortisol were related to each other (Hibel et al., 2015), and that mother’s cortisol was positively associated with socioeconomic risk during infancy (Finegood et al., 2016). Our first two aims are similar to these findings, but differ in two important ways. First, in contrast to Hibel et al. (2015), we included additional covariates (maternal depression, IPV, and socioeconomic risk), which yields a more conservative analysis and allows us to make claims regarding maternal cortisol as a hidden regulator. Second, in contrast to Finegood et al. (2016), we focused only on maternal baseline cortisol, prior to an emotion induction task, as the best indicator of non-intentionally stimulated, resting or basal cortisol. Conversely, Finegood et al. (2016) used an average of three maternal cortisol samples across the emotion induction, which includes baseline and reactivity cortisol.
Our first aim was to examine whether a baseline level of maternal cortisol was associated with socioeconomic risk. Our second and third aims, respectively, were to examine how maternal baseline cortisol related to the infant’s baseline cortisol level and cortisol response following an emotion induction at three ages (7, 15, and 24 months). Our fourth aim was to assess whether socioeconomic risk moderated the association between mother-infant cortisol activity.
First, we expected that maternal baseline cortisol levels would be positively related to socioeconomic risk. We also expected that maternal and infant baseline cortisol would be positively associated, suggesting maternal regulation of infant cortisol levels. We further hypothesized that mother’s baseline cortisol would be negatively related to infant cortisol reactivity, suggesting maternal regulation of infant cortisol responses. This negative relation indicates that higher levels of maternal baseline cortisol would be associated with slower rates of infant linear cortisol change. We further expected that the strength of the association between mother’s baseline cortisol and her infant’s cortisol response would be moderated by socioeconomic risk, such that at higher levels of risk, mother’s baseline cortisol would not be associated with her infant’s cortisol response, suggesting disruption of maternal regulation at high risk.
2. Methods
2.1. Participants
The Family Life Project (FLP) was designed to study families (N = 1,292) living in two areas of high poverty (Dill and Myers, 2004): three counties in North Carolina (NC) and three in Pennsylvania (PA). Data for this analysis come from the project’s home assessments when the infant was approximately 7, 15, and 24 months old. See Table 1 for descriptive statistics of the sample used in the present analysis. A comprehensive description of the sample is provided by Vernon-Feagans, Cox, & Family Life Project Key Investigators (2013).
Table 1.
Descriptive Statistics
| 7 Months |
15 Months |
24 Months |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M or % | SD | min | max | N | M or % | SD | min | max | N | M or % | SD | min | max | |
| Child Age (months) | 1133 | 7.72 | 1.48 | 5.04 | 15.36 | 1070 | 15.60 | 1.32 | 13.44 | 22.32 | 1014 | 24.84 | 1.94 | 22.2 | 35.4 |
| Child Race (% Black) | 1133 | 41.6% | — | — | — | 1070 | 43.2% | — | — | — | 1014 | 42.1% | — | — | — |
| Child Sex (% Female) | 1133 | 49.2% | — | — | — | 1070 | 49.0% | — | — | — | 1014 | 49.3% | — | — | — |
| Positive Parenting | 1088 | 2.90 | 0.79 | 1.00 | 4.80 | 1020 | 2.79 | 0.80 | 1.00 | 5.00 | 963 | 2.90 | 0.81 | 1.00 | 4.80 |
| Negative Parenting | 1088 | 2.41 | 0.77 | 1.00 | 5.00 | 1020 | 2.27 | 0.70 | 1.00 | 5.00 | 963 | 2.43 | 0.86 | 1.00 | 5.00 |
| Maternal Depression | 1129 | 0.37 | 0.54 | 0.00 | 3.33 | 1066 | 0.46 | 0.65 | 0.00 | 4.00 | 1001 | 0.41 | 0.64 | 0.00 | 4.00 |
| IPV (% in IPV Group) | 923 | 28.6% | — | — | — | 851 | 20.1% | — | — | — | 809 | 18.6% | — | — | — |
| Socioeconomic Risk | 1131 | 0.00 | 0.66 | −2.08 | 1.98 | 1067 | 0.01 | 0.67 | −2.01 | 2.00 | 1014 | 0.01 | 0.63 | −2.21 | 2.17 |
| Emotional Reactivity | 1018 | 0.10 | 0.21 | 0.00 | 0.96 | 843 | 0.37 | 0.31 | 0.00 | 1.00 | 769 | 0.37 | 0.34 | 0.00 | 0.98 |
| Child Cortisol T1 (ln μg/dl) | 1106 | −1.88 | 0.69 | −3.91 | 0.22 | 991 | −1.99 | 0.76 | −4.27 | 0.49 | 939 | −2.08 | 0.73 | −4.42 | 0.25 |
| Child Cortisol T2 (ln μg/dl) | 1002 | −1.77 | 0.73 | −4.27 | 0.38 | 943 | −1.83 | 0.81 | −4.34 | 0.72 | 942 | −2.07 | 0.77 | −4.20 | 0.40 |
| Child Cortisol T3 (ln μg/dl) | 934 | −1.87 | 0.67 | −3.86 | 0.21 | 878 | −1.83 | 0.82 | −4.40 | 0.80 | 917 | −2.13 | 0.75 | −4.27 | 0.48 |
| Mother Cortisol (ln μg/dl) | 1112 | −2.00 | 0.62 | −4.07 | 0.25 | 886 | −2.41 | 0.64 | −4.47 | −0.21 | 851 | −2.30 | 0.65 | −4.18 | −0.42 |
| Time of day (hh:mm) | 1133 | 13:31 | 2:52 | 8:11 | 20:08 | 1070 | 13:57 | 2:54 | 8:45 | 20:24 | 1014 | 13:48 | 3:12 | 8:20 | 20:46 |
IPV: Intimate Partner Violence; T1: baseline; T2: 20-min post-peak; T3: 40-min post-peak
2.2. Procedures
At each age families were visited in their homes for 2–3 hours. Mothers completed questionnaires and interviews and participated in a number of procedures, including a 10-min semi-structured interaction with her infant. At 7 and 15 months, the mother and infant engaged in a free-play task where mothers were given a standard set of toys and asked to play as they typically would. At 24 months, the mother and infant participated in a puzzle task, which involved providing the infant with a jigsaw puzzle and asking the mother to assist in any way she chose. Interactions were video-recorded and later coded for parenting behaviors. At each age infants also participated in a series of emotion induction tasks taken from the Laboratory Temperament Assessment Battery (LabTAB; Goldsmith & Rothbart, 1999). Saliva samples were collected at three time points before and after the emotion induction and later assayed for cortisol.
This study was reviewed and approved by the Institutional Review Board at Pennsylvania State University and the Office of Human Research Ethics at the University of North Carolina. Written informed consent was obtained from all adult participants and from the parents/legal guardians of all non-adult participants, in accordance with the Declaration of Helsinki. All participation was voluntary, with participants being informed prior to the study that they could remove their consent at any time.
2.3. Measures
2.3.1. Socioeconomic Risk
Based on previous work using these data (Vernon-Feagans et al., 2013), we created a composite index of socioeconomic risk comprised of 7 variables: maternal education, consistent partnership, household income-to-needs ratio, average weekly hours of employment, occupational prestige, household density, and neighborhood safety. Each variable was measured at each age. A continuous socioeconomic risk index was calculated by reverse scoring positively framed indicators, standardizing each measure, and averaging the standardized values. Socioeconomic risk scores were generated for each assessment age, with higher scores indicating higher levels of risk. For additional details regarding cumulative risk variables, we refer the reader to Vernon-Feagans, et al. (2013).
2.3.2. Positive and Negative Parenting
Global ratings of parenting behavior were made based on a scale adapted by Cox and Crnic (1999) from the NICHD Early Child Care Research Network (1999; Vernon-Feagans, et al., 2013; Hibel et al., 2009, 2015; Finegood et al., 2016; Blair et al., 2008). Videos of mother-infant interactions were coded for seven aspects of parenting: sensitivity, detachment, intrusiveness, stimulation, positive regard, negative regard, and animation in interacting with the child. At 7 and 15 months, ratings were made on a scale from 1 (“not at all characteristic”) to 5 (“highly characteristic”) and at 24 months on a scale from 1 (“not at all characteristic”) to 7 (“highly characteristic”). For consistency, scores at the 24-month visit were re-scaled to range from 1 to 5 (Mills-Koonce et al., 2011). Factor analyses conducted with an oblique rotation (i.e., Promax) at each age indicated distinct positive and negative dimensions of parenting. Positive parenting included five characteristics: sensitivity, detachment (reverse scored), positive regard, stimulation of development, and animation. Negative parenting included two characteristics: intrusiveness and negative regard. For each dimension, scores for respective characteristics were averaged to create indices of positive and negative parenting. Reliability was determined by calculating intra-class correlation coefficients (ICC) for ratings made by two trained coders for each dimension. ICCs for all dimensions of parenting ranged from .75 – .89 across the three time points. Approximately 30% of the mother-child interactions were double-coded independently.
2.3.3. Emotional Reactivity
At 7 months, three tasks were presented: the mask, barrier, and arm restraint tasks. At 15 and 24 months two tasks were presented: the mask and toy removal tasks. These tasks are designed to elicit emotional responses of fear (mask) and frustration (barrier, arm restraint, and toy removal). In the barrier task, the infant was given a toy to play with for 30 sec, after which it was removed and placed behind a clear Plexiglass barrier just outside the child’s reach for 30 sec. The experimenter then returned the toy to the child and the procedure was repeated two more times for a total of 3 trials. For the mask task, four different masks were worn one at a time for 10 sec each by the experimenter, who moved from side-to-side in front of the infant while calling the infant’s name. In the arm restraint task, the experimenter crouched behind the infant and gently restrained the infant’s arms for 2 min or until 20 sec of intense crying ensued. In the toy removal task, infants and their mothers were presented with an attractive toy and asked to play together for 2 min. The toy was then taken away from the child and placed out of reach (at 15 months of age) or in a clear plastic jar and handed back to them (at 24 months of age) for 2 min. The toy was returned to the child for 1 min. During all tasks, mothers watched while remaining out of their infant’s sight and were asked not to intervene, but could stop the task at any time. Tasks were video recorded and later coded for infant emotional reactivity at each age.
For each task, three levels of negative emotional reactivity were recorded: low, medium, and high. A score for each level of reactivity was created by summing the seconds of low, medium, and high reactivity. A proportion was then calculated by dividing the sum of each level of reactivity by the total task duration. A composite score for negative emotional reactivity for each task was created by summing the proportion of each reactivity level. For each time point, a total score for emotional reactivity was calculated by averaging the composite scores for each task. Coders were trained to achieve a Cohen’s k (reliability) of at least .75. Interrater reliability was calculated on at least 15% of the videos, resulting in kappas ranging from .85 – .94 for each task across the three visits.
2.3.4. Maternal Depression
At each visit, maternal depressive symptomatology was assessed using the depression subscale of the Brief Symptom Inventory-18 (BSI-18; Derogatis, 2001). The BSI-18 is a brief, highly sensitive self-report index of psychological distress. The depression subscale was comprised of 6 items, scored using a 5-point Likert-type scale from 0 (“not at all”) to 4 (“extremely”), targeting present feelings and symptoms (e.g., “Feeling no interest in things”, “Feeling lonely”). Raw item scores were summed to create a depression subscale total score. Reliability using Cronbach’s alpha was acceptable (6 months: α = .81; 15 months: α = .83; 24 months: α = .86).
2.3.5. Intimate Partner Violence (IPV)
To assess IPV, the Conflict Tactics Scale (CTS; Straus, Hamby, Boney-McCoy, & Sugarman, 1996) was administered at all ages to mothers regarding their partners, regardless of whether or not the partner lived in the household with the infant. The Couple Form of the CTS–Revised (Straus et al., 1996) consists of 19 items. Following Hibel et al. (2009), only items pertaining to physical or threatened physical aggression (e.g., “Kicked, bit, or hit you with a fist”, “Threatened to hit or throw something at you”) were used to determine membership in the IPV versus non-IPV group. Mothers who indicated that their partner used or threatened at least one violent act in the last 12 months were classified as the IPV group. Reliability using Cronbach’s alpha was acceptable (6 months: α = .77; 15 months: α = .79; 24 months: α = .81).
2.3.6. Salivary Cortisol
To assess baseline cortisol levels and cortisol responses to the emotion induction tasks, three saliva samples were collected from children and mothers. Saliva collection occurred near the end of the home visit at which time the data collectors had been in the home for at least one hour. This allowed for children’s and mothers’ cortisol levels ample time to return to baseline following the arrival of the data collectors. The first sample (baseline) was collected immediately prior to the administration of the emotion induction tasks. The second sample was collected approximately 20 min after completion of the tasks or peak emotional arousal, which occurred if the infant produced 20 sec of intense crying. The third sample was collected approximately 40 min after task completion or peak arousal. For the present analysis all three infant cortisol samples were used to estimate a cortisol response to the emotion induction task. For mothers, only the baseline sample was used to provide a reasonably accurate index of non-intentionally stimulated, basal or resting level of cortisol.
Unstimulated whole saliva was collected using either cotton or hydrocellulose absorbent material and expressed into 2 ml cryogenic storage vials using a needleless syringe (cotton) or by centrifugation (hydrocellulose). After collection, samples were immediately placed on ice and stored frozen (−20 °C). Because families were seen at times that were convenient for them, time of day of saliva collection varied between families, although most families were seen in the afternoon (see Table 1). Importantly, research shows that afternoon cortisol is more related to environmental factors, as opposed to genetic factors and thus may be a more reliable biomarker of the effects of environmental stress (Schreiber et al., 2006; Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012). Additionally, because the time to complete the stress tasks varied among children, the time between saliva sample collections also differed for each child. Both time of day of saliva collection and time between saliva samples were controlled for in all analyses.
All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μl of saliva, had a range of sensitivity from 0.007 to 3.0 μg/dl, and average intra- and inter-assay coefficients of variation less than 10% and 15%, respectively. Samples were assayed in duplicate and the average of duplicates was used in all analyses. Natural log transformations were applied to the cortisol values to correct for positive skew. Cortisol values greater than +/− 3 SD after transformation were excluded from analyses (n = 36, 34, and 38 saliva samples at 7, 15, and 24 months, respectively).
2.3.7. Control Covariates
We included demographic covariates in all our analyses, including infant’s race, gender, age, and mother’s age. We also assessed associations between mother and infant cortisol and several variables known to influence cortisol: pregnancy status, use of contraception, breastfeeding status and duration, tobacco use, parity status, if the mother or infant were taking medications, infant’s and mother’s body mass index, and mother’s and infant’s body temperatures. To ensure a parsimonious model, we only included covariates that were significantly related to at least one of the three infant cortisol samples and/or mother’s baseline cortisol. To test these associations, we evaluated correlations for continuous variables and independent samples t-tests for categorical variables. At 7 months, infant cortisol was associated with body temperature, pregnancy status, and medication use; maternal baseline cortisol was associated with tobacco use, breastfeeding duration and status, body mass index, and pregnancy status. At 15 months, infant cortisol was associated with breastfeeding status and duration; maternal baseline cortisol was associated with breastfeeding duration, mother’s body temperature, pregnancy status, contraception use, and tobacco use. At 24 months, infant cortisol was associated with body mass index and medication use; maternal baseline cortisol was associated with body mass index, pregnancy status, tobacco use, and parity status. These variables were initially included in models at the respective child age and removed from final models if they were not statistically significant. We also included time-varying covariates at each time point controlling for the time of day of saliva collection and time between each saliva collection.
2.4. Missing Data
Of the 1,292 families recruited, 1,204 were seen at 7 months, 1,169 at 15 months, and 1,144 at 24 months. Participants were included in the analyses if the child had at least one cortisol value at each age, resulting in an analytic sample of N = 1,133 at 7 months, N = 1,070 at 15 months, and N = 1,014 at 24 months. At 7 months 5.5% of children were missing one cortisol sample, 7.6% were missing two samples, and 6.1% of mothers were missing baseline cortisol. At 15 months 7.7% of children were missing one cortisol sample, 7.7% were missing two samples, and 18.3% of mothers were missing baseline cortisol. At 15 months 9.5% of children were missing one cortisol sample, 4.6% were missing two samples, and 21.0% of mothers were missing baseline cortisol. To test for systematic differences on analysis variables between cases with missing cortisol data versus not missing, we conducted independent samples t-tests for continuous variables and chi-square tests for categorical variables. At 7 months, mothers missing cortisol were more likely to be classified in the IPV group, χ2 (1, N = 978) = 5.60, p = .02, and had higher socioeconomic risk scores t(1,200) = 2.28, p = .02. At 7 months, infants missing at least one cortisol sample were more likely to be classified in the IPV group, χ2 (1, N = 978) = 5.69, p = .02, had higher socioeconomic risk scores t(1,200) = 2.13, p = .03, and were older t(1,202) = 3.71, p < .001. At 15 and 24 months, infants missing at least one cortisol sample were older, 15: t(1,167) = 2.25 p = .02; 24: t(1,142) = 3.10 p = .01. At 15 and 24 months, there were no differences for mothers missing versus not missing cortisol data. To control for potential bias due to missing data, we fitted all models using full information maximum likelihood estimation (Enders, 2010).
2.5. Analytic Strategy
To address our first hypothesis, we ran three linear regression models (one for each child age) to examine associations between maternal baseline cortisol levels and socioeconomic risk. To test our remaining hypotheses, for each age we used a 2-level mixed model with random intercepts, random linear slopes, and a fixed quadratic slope to estimate infant baseline cortisol and cortisol responses. At level 1, we modeled the three child cortisol samples (baseline, 20 minutes, and 40 minutes post emotion induction) and used the amount of time between samples as the within-person independent (time) variable. At level 2 we entered all between-person variables. Level 1 describes the within-person differences in cortisol responses (linear and fixed quadratic slopes) and level 2 describes the between-person differences in cortisol levels at baseline (intercepts) and between-person differences in cortisol responses (cross-level interactions). The time of the first saliva sample was coded as zero and thus, intercepts reflect cortisol at the baseline saliva collection. In this analysis, we use the term ‘slope’ to indicate the infant’s cortisol response to the emotion induction and the term ‘level’ to refer to baseline cortisol levels. All level 2 continuous independent variables were grand-mean centered for each time point.
Specifically, for the mixed models predicting infant cortisol, we first ran three unconditional growth models (one at each age) with only time as the independent variable to examine infant’s average cortisol levels, average linear and quadratic cortisol responses, and variation in the random intercepts and random linear slopes. Next, we added to each unconditional growth models the remaining independent variables and covariates at level 2, and re-ran the three mixed models. To address our second and third hypotheses regarding relations between mother’s baseline cortisol level and infant’s baseline cortisol and cortisol response, we regressed the intercept and linear slope of the three infant cortisol samples on mother’s baseline cortisol at level 2. We operationally defined maternal regulation as the statistically significant association between mother’s baseline cortisol levels and either infant baseline cortisol levels or infant cortisol responses. To address our fourth hypothesis regarding moderation, we also included in the same mixed models an interaction term between mother’s baseline cortisol and socioeconomic risk, and included this term in each of the three models. To interpret interactions we used simple slopes analysis to evaluate effects at high and low levels of the independent variable(s), which were defined, respectively, as one SD above and one SD below the mean (Aiken & West, 1991). Final models were determined by systematically including significant predictors and removing non-significant quadratic trends followed by linear ones. Best fitting models were evaluated successively based on AIC/BIC values, such that the model with the smaller values was chosen. Preliminary and descriptive analyses were computed using SPSS Statistics Version 22 (IBM). All models were estimated using Mplus v.7 (Muthén & Muthén, 1998–2012).
3. Results
3.1. Descriptive Statistics
Descriptive statistics for all key variables used in the analyses at each time point are displayed in Table 1 and zero-order correlations are shown in Tables 2–4. At each age all three infant cortisol samples were significantly associated with mother cortisol. There were small, significant positive relations between infant cortisol and socioeconomic risk: at 7 months baseline cortisol was positively correlated with socioeconomic risk; at 15 and 24 months, all three infant cortisol samples were positively correlated with socioeconomic risk. Higher levels of mother cortisol were associated with higher socioeconomic risk at each time point. Additionally, mother cortisol was related to positive parenting at each time point, such that higher levels of maternal cortisol were associated with lower levels of positive parenting. Infant emotional reactivity revealed small, significant positive correlations with the infant’s second cortisol sample at 7, 15, and 24 months, and the third cortisol sample at 24 months. Furthermore, positive parenting was negatively correlated with socioeconomic risk, whereas negative parenting was positively correlated with socioeconomic risk at each time point. Lastly, there were small but significant correlations between parenting and infant cortisol. At 7 and 15 months, infant baseline cortisol was negatively related to positive parenting. At 24 months, all three infant cortisol samples were negatively correlated with positive parenting and positively correlated with negative parenting.
Table 2.
Correlations for Analysis Variables at 7 Months
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Positive Parenting | 1 | ||||||||||
| 2. Negative Parenting | −.19** | 1 | |||||||||
| 3. Maternal Depression | −.03 | .10** | 1 | ||||||||
| 4. IPV | −.10** | .06 | .30** | 1 | |||||||
| 5. Socioeconomic Risk | −.46** | .34** | .19** | .14** | 1 | ||||||
| 6. Emotional Reactivity | .01 | −.03 | −.03 | .01 | −.04 | 1 | |||||
| 7. Child Cortisol T1 | −.13** | .07* | .01 | .01 | .11** | .01 | 1 | ||||
| 8. Child Cortisol T2 | −.01 | .04 | .04 | .04 | .05 | .11** | .47** | 1 | |||
| 9. Child Cortisol T3 | −.02 | .04 | .01 | .04 | .06 | .04 | .42** | .79** | 1 | ||
| 10. Mother Cortisol | −.09** | .04 | .06 | .02 | .12** | −.03 | .29** | .22** | .23** | 1 | |
| 11. Time of Day | .06* | −.01 | .02 | −.02 | −.13** | .05 | −.26** | −.27** | −.34** | −.49** | 1 |
IPV: Intimate Partner Violence; T1: Baseline; T2: 20-min post-peak; T3: 40-min post-peak
p< 0.05,
p< 0.01
Table 4.
Correlations for Analysis Variables at 24 Months
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Positive Parenting | 1 | ||||||||||
| 2. Negative Parenting | −.54** | 1 | |||||||||
| 3. Maternal Depression | −.10** | .15** | 1 | ||||||||
| 4. IPV | −.16** | .10** | .27** | 1 | |||||||
| 5. Socioeconomic Risk | −.51** | .40** | .20** | .16** | 1 | ||||||
| 6. Emotional Reactivity | −.09* | .07 | .04 | .05 | .06 | 1 | |||||
| 7. Child Cortisol T1 | −.13** | .13** | .04 | .02 | .14** | −.01 | 1 | ||||
| 8. Child Cortisol T2 | −.14** | .15** | .04 | .05 | .17** | .19** | .51** | 1 | |||
| 9. Child Cortisol T3 | −.10** | .13** | .09** | .07 | .17** | .11** | .45** | .77** | 1 | ||
| 10. Mother Cortisol | −.09* | .03 | .10** | .00 | .20** | −.01 | .30** | .27** | .31** | 1 | |
| 11. Time of Day | .14** | −.12** | −.05 | .02 | −.28** | −.07 | −.32** | −.28** | −.31** | −.50** | 1 |
IPV: Intimate Partner Violence; T1: Baseline; T2: 20-min post-peak; T3: 40-min post-peak
p< 0.05,
p< 0.01
3.2. Baseline Maternal Cortisol Levels and Socioeconomic Risk
The linear regressions predicting mother’s baseline cortisol from socioeconomic risk while controlling for all our covariates revealed that at all three ages, maternal cortisol was positively related to risk, such that higher levels of cortisol were associated with higher risk (7: b = 0.08, SE = 0.38, p = .03; 15: b = 0.11, SE = 0.39, p = .01; 24: b = 0.10, SE = 0.41, p = .02).
3.3. Unconditional Growth Models of Infant Cortisol
The unconditional growth models predicting infant’s cortisol response revealed a positive linear change in cortisol responses at 7 (b = 0.32, SE = 0.75, p < .001) and 15 months (b = 0.47, SE = 0.07, p < .001), indicating that infants displayed an average linear cortisol increase in response to the emotion induction tasks. There was also a significant negative effect for the fixed quadratic slope at 7 (b = −0.29, SE = 0.05, p < .001) and 15 months (b = −0.32, SE = 0.07, p < .001), indicating that infants displayed an average cortisol increase followed by a subsequent decrease. At 24 months there was no significant linear (b = −0.004, SE = 0.06, p = .95) or quadratic (b = −0.02, SE = 0.05, p = .73) change in cortisol. Additionally, there was significant variance at all three ages for random intercepts (7: σ2 = 0.36, SE = 0.02, p < .001; 15: σ2 = 0.46, SE = 0.03, p < .001; 24: σ2 = 0.41, SE = 0.03, p < .001) and random linear slopes (7: σ2 = 0.34, SE = 0.03, p < .001; 15: σ2 = 0.33, SE = 0.03, p < .001; 24: σ2 = 0.28, SE = 0.027, p < .001), indicating significant variation in child baseline cortisol levels and linear cortisol responses at all three ages. Thus, we included random intercepts and random linear slopes in all subsequent models.
3.4. Baseline Infant Cortisol Levels
Table 5 shows the results from our three mixed models predicting infant cortisol at 7, 15, and 24 months. To evaluate associations with baseline infant cortisol levels, we interpreted the coefficients for infant cortisol intercepts. Maternal baseline cortisol was positively associated with baseline infant cortisol at all three ages (7: b = 0.21, SE = 0.04, p < .001; 15: b = 0.21, SE = 0.06, p < .001; 24: b = 0.19, SE = 0.05, p < .001), indicating that higher levels of mother cortisol were related to higher levels of infant cortisol at baseline. Race predicted baseline infant cortisol levels at 7 (b = 0.14, SE = 0.05, p = .01), 15 (b = 0.22, SE = 0.06, p = .001), and 24 (b = 0.17 SE = 0.06, p = .01) months, such that African American infants exhibited higher baseline cortisol relative to White infants. At 24 months, emotional reactivity was related to infant baseline cortisol, such that children exhibiting higher levels of emotional reactivity had lower levels of baseline cortisol (b = −0.26, SE = 0.09, p = .01). There were no associations between baseline infant cortisol and parenting, depression, IPV, or socioeconomic risk at any time point.
Table 5.
Models Predicting Child Cortisol
| 7 Months |
15 Months |
24 Months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | S.E. | p | Estimate | S.E. | p | Estimate | S.E. | p |
| Intercept | −1.92 | 0.04 | <.001 | −1.90 | 0.05 | <.001 | −2.04 | 0.04 | <.001 |
| Time | 0.35 | 0.13 | .004 | 0.53 | 0.13 | <.001 | −0.19 | 0.11 | .09 |
| Time X Time | −0.27 | 0.10 | .03 | −0.38 | 0.12 | .002 | −0.09 | 0.09 | .30 |
| Time of day | −0.03 | 0.01 | <.001 | −0.02 | 0.01 | .06 | −0.06 | 0.01 | <.001 |
| Time of day X Time | −0.03 | 0.01 | <.001 | −0.03 | 0.01 | .002 | 0.04 | 0.02 | .07 |
| African American | 0.14 | 0.05 | <.01 | 0.22 | 0.06 | .001 | 0.17 | 0.06 | <.01 |
| African American X Time | −0.12 | 0.16 | .47 | −0.35 | 0.19 | .06 | −0.07 | 0.17 | .68 |
| Gender | 0.04 | 0.04 | .25 | 0.07 | 0.05 | .14 | 0.03 | 0.04 | .50 |
| Gender X Time | −0.07 | 0.13 | .58 | −0.22 | 0.15 | .13 | 0.04 | 0.13 | .77 |
| Positive Parenting | −0.04 | 0.03 | .17 | −0.05 | 0.04 | .15 | −0.03 | 0.04 | .43 |
| Positive Parenting X Time | 0.07 | 0.04 | .06 | 0.02 | 0.04 | .52 | 0.04 | 0.03 | .21 |
| Negative Parenting | 0.01 | 0.03 | .62 | −0.03 | 0.03 | .33 | 0.05 | 0.03 | .10 |
| Negative Parenting X Time | −0.001 | 0.03 | .97 | −0.03 | 0.04 | .39 | 0.02 | 0.04 | .64 |
| Socioeconomic Risk | −0.02 | 0.04 | .66 | 0.07 | 0.05 | .12 | −0.05 | 0.04 | .21 |
| Socioeconomic Risk X Time | −0.02 | 0.04 | .69 | −0.01 | 0.05 | .83 | 0.05 | 0.04 | .23 |
| Emo. Reactivity | 0.04 | 0.10 | .71 | −0.01 | 0.10 | .96 | −0.26 | 0.09 | <.01 |
| Emo. Reactivity X Time | 1.47 | 0.36 | <.001 | 0.83 | 0.30 | <.01 | 1.22 | 0.28 | <.001 |
| Emo. Reactivity X Time X Time | −1.24 | 0.29 | <.001 | −0.76 | 0.30 | <.01 | −0.69 | 0.21 | .001 |
| Maternal Depression | 0.01 | 0.04 | .86 | −0.004 | 0.04 | .90 | −0.003 | 0.04 | .93 |
| Maternal Depression X Time | 0.03 | 0.05 | .47 | −0.001 | 0.04 | .99 | 0.01 | 0.04 | .78 |
| Intimate Partner Violence | −0.02 | 0.05 | .68 | −0.01 | 0.06 | .81 | 0.05 | 0.06 | .43 |
| Intimate Partner Violence X Time | 0.07 | 0.06 | .22 | 0.07 | 0.07 | .31 | 0.04 | 0.06 | .51 |
| Mother Cortisol | 0.21 | 0.04 | <.001 | 0.21 | 0.06 | <.001 | 0.19 | 0.05 | <.001 |
| Mother Cortisol X Time | −0.12 | 0.04 | <.01 | −0.14 | 0.05 | <.01 | 0.03 | 0.04 | .49 |
| Mother Cortisol X Socioeconomic Risk | 0.06 | 0.05 | .21 | 0.10 | 0.06 | .11 | 0.01 | 0.05 | .89 |
| Mother Cortisol X Socioeconomic Risk X Time | −0.03 | 0.06 | .63 | −0.12 | 0.07 | .08 | −0.15 | 0.06 | .01 |
Emo. Reactivity: Emotional Reactivity
3.5. Infant Cortisol Responses
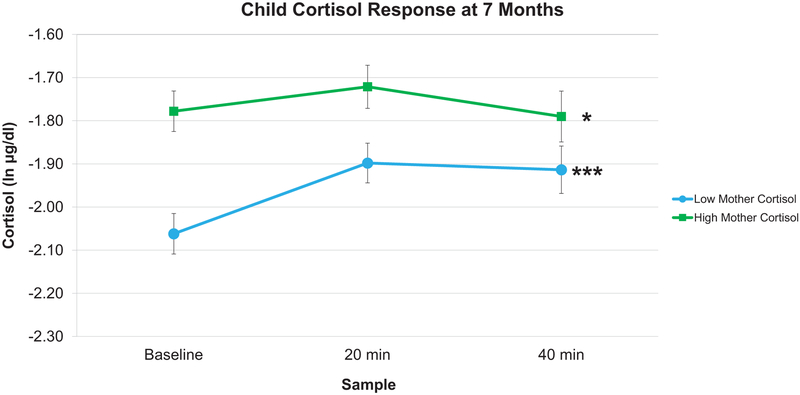
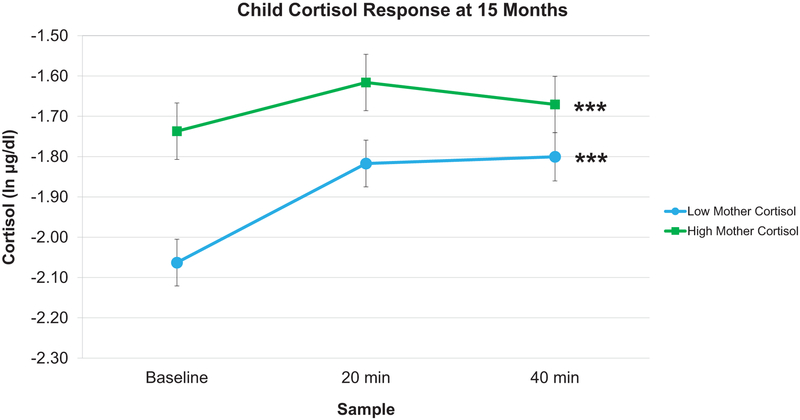
Next, to evaluate associations with infant cortisol responses to the emotion induction, we interpreted the coefficients for infant linear and fixed quadratic cortisol slopes. As shown in Table 5, mother cortisol negatively predicted infant linear slopes at 7 and 15 months (7: b = −0.12, SE = 0.04, p = .01; 15: b = −0.14, SE = 0.05, p = .01), but not at 24 months (b = 0.03, SE = 0.04, p = .49). To interpret the direction and magnitude of infants’ slopes as a function of maternal cortisol, we conducted a simple slopes analysis at high and low maternal cortisol. As can be seen in Figures 1 and 2, at 7 and 15 months, respectively, infants whose mothers had low levels of baseline cortisol displayed steeper increases in linear cortisol responses (7 months: b = 0.42, SE = 0.13, p = .001; 15 months: b = 0.63, SE = 0.14, p < .001) compared to infants whose mothers had high levels of baseline cortisol (7 months: b = 0.27, SE = 0.13, p = .04; 15 months: b = 0.53, SE = 0.16, p = .001). At all three ages, there were no associations between maternal cortisol and infant fixed quadratic cortisol slopes.
Figure 1.
Predicted infant cortisol responses at 7 months. In response to the stress task at 7 months, infant cortisol responses differed as a function of high or low (+/− 1 SD) maternal cortisol. Infants whose mothers had low baseline cortisol displayed steeper increases in cortisol responses compared to infants whose mothers had high baseline cortisol. Error bars represent Standard Error. (*** p < .001, * p < .05).
Figure 2.
Predicted infant cortisol responses at 15 months. In response to the stress task at 15 months, infant cortisol responses differed as a function of high or low (+/− 1 SD) maternal cortisol. Infants whose mothers had low baseline cortisol displayed steeper increases in cortisol responses compared to infants whose mothers had high baseline cortisol. Error bars represent Standard Error. (*** p < .001).
Positive parenting was marginally associated with linear infant cortisol slopes at 7 months (b = 0.07, SE = 0.04, p = .06), such that infants experiencing higher levels of positive parenting exhibited a cortisol increase in response to the emotion induction. Simple slopes analysis revealed that higher levels of positive parenting were associated with steeper linear increases in cortisol responses (b = 0.49, SE = 0.09, p < .001) compared to infants with lower levels (b = 0.38, SE = 0.09, p < .001). There were no concurrent associations between positive parenting at 15 and 24 months and linear infant cortisol slopes at 15 and 24 months, respectively. Child emotional reactivity was positively associated with linear cortisol slopes at all three ages (7: b = 1.47, SE = 0.36, p < .001; 15: b = 0.83, SE = 0.30, p = .01; 24: b = 1.22, SE = 0.28, p < .001). Additionally, emotional reactivity was associated with fixed quadratic cortisol slopes at all ages, such that increased emotional reactivity predicted a cortisol increase followed by a subsequent decrease (7: b = −1.24, SE = 0.29, p < .001; 15: b = −0.76, SE = 0.30, p = .01; 24: b = −0.69, SE = 0.21, p = .001). No other variables were associated with linear or fixed quadratic infant cortisol slopes.
3.6. Socioeconomic Risk as a Moderator of Mother-Infant Cortisol Associations
To address our fourth hypothesis regarding moderators of mother-infant cortisol associations, we evaluated interactions between mother cortisol and socioeconomic risk predicting infant cortisol linear slopes and intercepts at each age. Socioeconomic risk did not moderate the relation between mother cortisol and child cortisol at baseline at any age. Regarding infant cortisol slopes, the interaction of maternal baseline cortisol with socioeconomic risk was non-significant at 7 and 15 months, but was significant at 24 months (b = −0.15, SE = 0.06, p = .01). This interaction indicates that the association between mother baseline cortisol and infant cortisol responses depended on the level of risk.
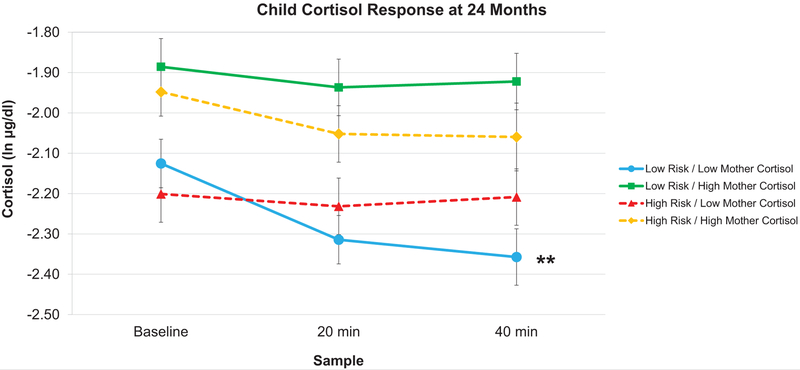
To interpret this three-way interaction of mother cortisol by socioeconomic risk by time at 24 months, we ran a simple slopes analysis at four points of interest: high risk/low mother cortisol, high risk/high mother cortisol, low risk/high mother cortisol, and low risk/low mother cortisol. As shown in Figure 3, at low risk/low mother cortisol, infants displayed decreasing cortisol slopes (b = −0.30, SE = 0.11, p = 0.01). Conversely, at low risk/high mother cortisol (b = −0.17, SE = 0.12, p = .16), high risk/low mother cortisol (b = −0.11, SE = 0.12 p = .37), and high risk/high mother cortisol (b = −0.17, SE = 0.12, p = .15) infants displayed no significant cortisol changes.
Figure 3.
Predicted infant cortisol responses at 24 months. In response to the stress task at 24 months, infant cortisol responses differed as a function of the interaction between levels (high or low: +/− 1 SD) of maternal baseline cortisol and socioeconomic risk. Infants in low risk/low mother cortisol dyads displayed significantly decreasing cortisol responses (** p < .01). Conversely, low risk/high mother cortisol, high risk/low mother cortisol, and high risk/high mother cortisol infants showed no significant changes in cortisol responses. Error bars represent Standard Error.
To further interpret the three-way interaction we assessed whether mother’s cortisol was associated with infant cortisol responses as a function of socioeconomic risk. Using the same three mixed models, we ran a simple slopes analysis at low and high socioeconomic risk, and evaluated the coefficient for the main effect of maternal baseline cortisol predicting infant cortisol slopes. We found that mother cortisol was associated with infant cortisol slopes at low risk (b = 0.14, SE = 0.06, p = .03), but not at high risk (b = −0.06, SE = 0.06, p = .33).
As a follow-up analysis, we evaluated differences in emotional reactivity to the task as a function of risk and maternal cortisol given that there were differences in cortisol reactivity as a function of socioeconomic risk and mother cortisol at 24 months. To do so, we included a three-way interaction (and the lower-order two-way interactions) between mother cortisol, socioeconomic risk, and emotional reactivity predicting infant cortisol slopes (technically creating a 4-way interaction by including time). Here, a significant interaction would indicate that the association between mother cortisol and socioeconomic risk predicting infant cortisol slopes depended on the level of emotional reactivity. This interaction was not significant (b = 0.02, SE = 0.26, p = .93).
4. Discussion
In this study, we investigated associations between maternal baseline cortisol levels and infant cortisol responses to emotion induction tasks in relation to socioeconomic risk at child ages 7, 15, and 24 months. The main contribution of this analysis was to test the hypotheses that maternal baseline cortisol would be associated with infant cortisol responses, and that this association would be moderated by socioeconomic risk. We found that the association between maternal-infant cortisol was not related to socioeconomic risk at 7 and 15 months. At both of these ages, regardless of risk, infants whose mothers had low levels of cortisol displayed steeper increases in cortisol responses compared to infants whose mothers had high cortisol levels. This is an important finding given the indication in prior analyses with these data that positive parenting and child emotional reactivity were the primary determinants of child cortisol reactivity (Blair et al., 2008, 2015). Our finding demonstrates that maternal cortisol is a substantive predictor of child cortisol over and above parenting and emotional reactivity. At 24 months, however, the association between maternal baseline cortisol and infant cortisol reactivity was moderated by socioeconomic risk, such that maternal cortisol predicted infant cortisol reactivity only for dyads characterized by low socioeconomic risk. Importantly, these effects were observed while controlling for potential mediators of the mother-infant cortisol association, including an observational measure of parenting, maternal depression, IPV, and infant emotional reactivity. By controlling for these variables, our results indicate that maternal cortisol is a robust predictor of infant cortisol reactivity and is distinct from these other proximal factors.
We also found that baseline maternal cortisol levels, prior to the emotion induction, were positively associated with socioeconomic risk. This finding is similar to that reported by Finegood et al. (2016) with this dataset, however, they used an average of three cortisol samples, which reflects cortisol activity across the emotion induction. In contrast, we were primarily interested in resting maternal cortisol and, as such, only used the baseline cortisol sample. Second, we found that baseline maternal cortisol was positively associated with baseline infant cortisol levels at 7, 15, and 24 months. Hibel et al. (2015) previously reported a similar finding with this dataset, however, we have included additional proximal covariates, namely, maternal depression, IPV, and socioeconomic risk.
To our knowledge, this is the first study to evaluate relations between maternal baseline cortisol and infant cortisol reactivity in the context of socioeconomic risk. Our findings have implications for understanding the role of caregiver stress physiology as a regulator of the child’s stress response, especially in contexts of risk. These results contribute to a growing body of research indicating that caregiver and child stress physiology are bi-directionally associated. Such coupling is thought to result from the dyad’s shared experiences and environments, and some have argued that being physiologically linked supports the caregiver’s ability to regulate the infant’s stress response (Atkinson et al., 2016; Hibel et al., 2015; Waters, West, Mendes, 2014). However, dyadic physiological relations may occur for better or for worse and, as our results show, are conditional upon characteristics of the environment, the caregiver’s stress physiology, and the child’s age.
4.1. Associations between Mother-Infant Cortisol at 7 and 15 Months
Our findings at child ages 7 and 15 months suggest that mothers and infants were physiologically “linked” and that this association was not affected by potentially adverse conditions, namely high levels of maternal cortisol and/or high socioeconomic risk. In other words, early in infancy maternal regulation via physiological linkage may be an experience-expectant process intended to support attachment to a caregiver—for better or for worse—to promote survival even at the cost of longer-term detriment. This is consistent with several studies in rodents showing that infant pups will form an attachment bond to their primary caregiver regardless of the quality of caregiving, even if the caregiver exhibits abuse and maltreatment (Perry et al., 2017; Perry & Sullivan, 2014; Rainecki, Moriceau, & Sullivan, 2010).
However, despite all dyads being physiologically linked at 7 and 15 months, the shape of infant cortisol responses depended on the level of maternal cortisol. At both ages, infants whose mothers had low levels of baseline cortisol displayed steeper cortisol increases compared to infants whose mothers had higher cortisol. In this case, low levels of maternal cortisol might facilitate the organization and regulation of a flexible infant cortisol response. Such flexible cortisol increases train the infant’s emotional and physiological stress response capacities, while scaffolding an infant’s learning to adaptively self-regulate stress physiology (Blair et al., 2008). In further support of this idea, we also replicated a previous finding from this dataset that positive parenting was positively associated with increases in cortisol at 7 months (Blair et al., 2008). Interestingly, also in the previous analysis, the authors found that at 15 months, infants who had larger increases in cortisol responses also had higher levels of attention (Blair et al., 2008).
On the other hand, infants of mothers with high baseline cortisol had attenuated cortisol responses, suggesting a lack of flexible physiological reactivity. Such a response may be related to the infant’s baseline cortisol levels. Specifically, the positive association between mother and infant baseline cortisol indicates that infants whose mothers had high baseline cortisol also had high baseline cortisol. Consequently, infants with high baseline cortisol may be showing a ceiling effect because they have less “room” to increase. Several other studies have found that higher basal levels of cortisol were associated with attenuated cortisol reactivity (e.g., Conradt et al., 2014; Sturge-Apple et al., 2012). However, the functional relation between baseline cortisol levels and cortisol reactivity has not been thoroughly investigated and thus, more research is needed to understand how these two aspects of stress physiology interact.
4.2. Associations between Mother-Infant Cortisol at 24 Months
At 24 months, we found that the association between maternal cortisol levels and infant cortisol responses was dependent on socioeconomic risk. Four important results are worth noting here. First, maternal cortisol levels were significantly associated with their infant’s cortisol responses only at low socioeconomic risk, potentially indicating these mothers were still regulating their infant’s response. This finding suggests that in environments of lower socioeconomic risk caregivers may be better able to regulate or buffer their child’s stress response. Conversely, mother and child cortisol was unrelated at higher levels of socioeconomic risk, suggesting these mothers were not regulating their infant’s cortisol responses. This finding is important given that we found that maternal baseline cortisol was positively related to socioeconomic risk, potentially suggesting a functional dependence between risk and maternal cortisol, which may disrupt the mother’s ability to regulate her infant when either risk or cortisol, or both, are high. On the other hand, the dissociation of mother-infant cortisol may be an adaptive response of the infant to de-couple herself from the mother’s high level of physiological stress or socioeconomic risk. In either case, it is plausible that there is a pathway connecting socioeconomic risk to caregiver stress, and caregiver stress to infant stress. Future research should test these relations using mediation analysis.
Second, infants in low-risk dyads whose mothers also had low cortisol levels displayed decreasing cortisol responses, whereas all other infants displayed flat cortisol responses. This decrease in reactivity may indicate an effect of social buffering (Hostinar et al., 2014). This result is consistent with cross-species research in both rodents and nonhuman primates showing that the caregiver buffers offspring stress response by regulating HPA axis activity (Moriceau & Sullivan, 2006; Sanchez et al., 2015). Moreover, social buffering may be especially important early in life for protecting the developing brain from the potentially damaging effects of elevated glucocorticoids and for scaffolding healthy attachment (Gunnar & Quevedo, 2007; Perry et al., 2017; Sullivan & Perry, 2015). The fact that we found a potential maternal buffering effect at 24 months, but not at 7 or 15, possibly indicates a developmental effect. That is, around 24 months, the infant may have sufficiently developed social cognitive skills and be better able to interpret subtle and indirect cues from the caregiver, thus facilitating social buffering (Frith, 2008; Tomasello et al, 2005; Feldman, 2007; Hostinar et al., 2014). On the other hand, at earlier ages, these skills may not yet be sufficiently developed to facilitate social buffering. However, as our results suggest, buffering may only occur if provided the right conditions—i.e., if the caregiver has a low level of physiological stress and is in a low-risk environment. Lastly, it is important to note that although at low risk/low maternal cortisol, infants had decreasing cortisol responses, the association between socioeconomic risk and maternal cortisol did not depend on emotional reactivity. That is, the decreasing cortisol responses were not due simply to these infants being less emotionally reactive to the tasks. Rather, the decreasing responses were distinctly related to the interaction of low socioeconomic risk and low maternal cortisol.
Third, at 24 months, the flat cortisol responses displayed by infants at low risk/high maternal cortisol, high risk/high maternal cortisol, and high risk/low maternal cortisol, potentially reflect “blunted” or dysregulated stress responses. Psychobiological research suggests that attenuated cortisol responses may result from chronic exposure to stress in which the HPA-axis attempts to compensate for elevated levels of glucocorticoids by down-regulating cortisol activity to protect the brain from dangerously high levels of cortisol (Fries, Hesse, Hellhammer, & Hellhammer, 2005). In our sample, flat responses may signify a sensitization of the infant’s HPA axis resulting from persistent exposure to high levels of maternal stress and/or environmental risk. In particular, given that we found a positive association between maternal and infant baseline cortisol, it is plausible that flat infant cortisol responses were a compensatory consequence of higher levels of maternal cortisol driving higher infant cortisol levels. In turn, because socioeconomic risk was associated with higher maternal cortisol levels, it may be the case that socioeconomic risk was indirectly affecting infant cortisol activity through the caregiver’s stress physiology. Several studies have found flat or blunted cortisol responses in infants and toddlers who experienced early-life stress (e.g., Sturge-Apple et al., 2012; Koss, Mliner, Donzella & Gunnar, 2016; Cordero et al., 2017), and in particular among low-income children (Raffington et al., 2018). Importantly, flat or blunted cortisol responses to stressors may be an adaptive response in the short term, however, they may negatively impact later cognitive, behavioral, and physical health outcomes (Blair, Granger, & Razza, 2005; Taylor, 2010; Ginty, Phillips, Roseboom, Carroll, & Derooij, 2012).
Fourth, our findings at 24 months may indicate that the caregiver’s ability to regulate child cortisol is influenced by environmental adversity in later infancy but not at 7 and 15 months. Cross-species research has shown that caregiver regulation is specific to early childhood and infancy (Gee, 2016; Moriceau & Sullivan, 2006; Perry et al., 2017). Moreover, the sensitive period for environmental influence via caregiving on the child’s stress response may close early or be delayed in contexts of adversity (Gee, 2016; Sullivan & Holman, 2010; Santiago, Lim, Opendak, Sullivan, & Aoki, 2018; Furukawa et al., 2017). That is, in environments of high risk and elevated stress, the caregiver’s ability to regulate the child’s stress response may be limited to a smaller window of time. Our results further support this idea. We found that at 24 months, but not 7 or 15 months, for dyads at higher levels of socioeconomic risk there was no association between mother cortisol and infant cortisol responses, suggesting that maternal regulation was disrupted at this later age, perhaps (partially) due to socioeconomic risk and/or maternal stress. Conversely, at low risk, maternal cortisol was associated with infant cortisol responses, perhaps indicating that these mothers were still regulating their infant’s stress response at 24 months. However, at 7 and 15 months, mother’s cortisol levels were associated with infant cortisol responses regardless of socioeconomic risk, possibly indicating that the window for maternal regulation had not yet closed. By 24 months, this sensitive period may have closed for high-risk dyads, but not low-risk dyads.
Lastly, it is worth noting that unlike at 7 and 15 months, at 24 months there was no average increase in infants’ cortisol responses, which may indicate a normative developmental effect. Studies have shown that infants display less cortisol reactivity to stressors with age in the first two years (Jansen et al., 2010; Gunnar et al., 2009; Davis & Granger, 2009). Some have suggested that this decreased reactivity reflects the infant’s growing behavioral repertoire that facilitates the down-regulation of physiological activity even when behavioral reactivity is still displayed (Jansen et al., 2010). Importantly, as previously reported by Ursache et al. (2013) using this dataset, on average, emotional reactivity to the fear task at 24 months was no different from 15 months. However, emotional reactivity to the frustration task at 24 months was lower than at 15 months, but higher than at 7 months. Thus, it seems that at 24 months infants did display significant emotional reactivity to the tasks. Furthermore, it is important to note that only at 24 months, when there was no significant average increase in cortisol responses, did we find that socioeconomic risk moderated the mother-infant cortisol association. Thus, it is possible that this moderation was present precisely because there was no average cortisol increase. Conversely, at 7 and 15 months it is possible that we did not find moderation because there were significant increases in infant cortisol. Unfortunately, we are not able to evaluate whether this moderation would be present if the infants had displayed increasing physiological reactivity at 24 months.
4.3. Maternal Stress Physiology as a “Hidden” Regulator
An important feature of our analysis is that we also controlled for psychosocial and behavioral effects on mother-infant cortisol associations. Specifically, the associations between maternal cortisol levels and infant cortisol responses were present over and above parenting behavior, maternal depression, IPV, or infant emotional reactivity. This suggests that maternal cortisol was a more robust predictor than the other proximal, observational and self-report variables. We interpret these findings within the framework of Hofer’s (1994, 2010) “hidden regulators” hypothesis, which claims that there are regulatory components of the mother-infant relationship that are not immediately apparent when simply observing behavior. We suggest that caregiver stress physiology can operate as an indirect non-conscious, covert regulating mechanism of child stress response physiology. Our results support the idea that dyadic physiological activity operates outside of conscious awareness and apart from overt behavior, and that the effects of elevated cortisol may be transmitted via non-conscious cues from the mother to her child (Harrist and Waugh, 2002; Papousek & Papousek, 2002). Thus, in contexts of increased stress, the association between caregiver-child physiology may serve as a hidden pathway of stress transmission between a parent and child. Although behaviors certainly play a role in co-regulatory physiological processes, these may often be subtle or “hidden” behaviors, such as touch, gaze, smell, tone of voice, posture, gesture, or facial expression, which are not detectable using global observational or self-report measures, but may be more easily detected using physiological methods. Three recent studies provide support for this idea, having shown that the effects of stress exposure on a child’s physiology were mediated by the mother’s physiological stress (Waters et al., 2014; Halevi et al., 2017; Hibel & Mercado, 2017). Importantly, in all three studies stress transmission was at least partially independent of observed measures of caregiving behavior, highlighting the significance of physiology as a more robust indicator of transmission. Thus, the factors that operate as co-regulators in the mother-child relationship may not always be overt, conscious, or readily observable behaviors. Consequently, future research should combine both physiological and behavioral measures in studies of caregiver-child relationships in order to advance our understanding of their dynamics.
4.4. Limitations
One limitation to the current study is that we used only one sample of maternal cortisol adjusted for time of day as an index of maternal stress physiology. Although this method of sampling is not optimal, it is necessary with large sample field-based studies such as ours. Nonetheless, cortisol levels are known to vary from day-to-day and multiple measures across days are ideal for obtaining a reliable measure of resting or basal cortisol. We suggest that this limitation is somewhat minimized by the fact that we included numerous covariates known to affect cortisol, the fact that we collected saliva after data collectors had been in the home for more than one hour, and the fact that we replicated similar results at multiple time points. That is, we found that maternal baseline cortisol was related to infant baseline cortisol at all three ages. Furthermore, the fact that maternal cortisol was related to socioeconomic risk at three different ages provides support for this metric as a reliable indicator of maternal stress physiology.
An additional limitation of our study is that based on prior literature, we have interpreted our findings as maternal cortisol regulating infant cortisol. This interpretation suggests a directionality in the effect, however, we cannot definitively claim that maternal physiology is driving child physiology. We acknowledge that such dyadic activity is bidirectional and that the child’s cortisol response may also influence maternal cortisol levels. However, given that the emotion induction was targeted at the infant, we had no hypotheses regarding how the infant’s cortisol response might influence the mother’s pre-task cortisol level. Nonetheless, future research should take into account the reciprocal processes operating within the mother-child relationship. Relatedly, our results are merely correlational and thus, we cannot make any causal inferences with respect to our findings.
Finally, cortisol activity is determined in part by genetic and epigenetic factors (Gerritsen et al., 2017; Lee & Sawa, 2014), which we cannot rule out as possible explanations for our results. Further research is needed to disentangle the relative contributions of environmental versus genetic factors in studies of mother-infant physiological relations.
4.5. Conclusions
Overall, our study provides support for the idea that a caregiver’s physiological stress can “get under the skin” and influence her child’s physiological stress response in contexts of environmental adversity. Importantly, the associations between caregiver-infant cortisol were independent of behavioral and psychosocial factors, suggesting that caregiver physiology is a significant regulator of the infant stress response. Considerable work is needed to better delineate how dyadic physiological activity mediates and moderates psychosocial and environmental factors, and shapes the child’s regulatory capacities. As such, future research should investigate the mechanisms that facilitate or constrain caregiver-child physiological associations. Such research has implications for understanding the dynamic and subtle processes by which the consequences of risk compromise caregiver-infant interactions and impact child development. Additionally, our findings draw attention to the need for early interventions targeted at reducing the burden of socioeconomic risk and its concomitant stress on the caregiver as a means of supporting healthy child development. By enhancing our comprehension of the dynamics of caregiver-child physiological activity, we will be better positioned to understand its contribution to parent-child relationships and developmental processes and, ultimately, to inform programs and practices for improving parent and child outcomes, especially for families at risk.
Table 3.
Correlations for Analysis Variables at 15 Months
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Positive Parenting | 1 | ||||||||||
| 2. Negative Parenting | −.30** | 1 | |||||||||
| 3. Maternal Depression | −.11** | .13** | 1 | ||||||||
| 4. IPV | −.10** | .06 | .33** | 1 | |||||||
| 5. Socioeconomic Risk | −.51** | .32** | .21** | .18** | 1 | ||||||
| 6. Emotional Reactivity | .04 | .07* | −.01 | −.03 | .04 | 1 | |||||
| 7. Child Cortisol T1 | −.11** | .023 | .03 | .02 | .13** | .01 | 1 | ||||
| 8. Child Cortisol T2 | −.06 | −.01 | .02 | −.01 | .09** | .13** | .64** | 1 | |||
| 9. Child Cortisol T3 | −.06 | −.01 | .02 | .02 | .11** | .05 | .55** | .81** | 1 | ||
| 10. Mother Cortisol | −.13** | .01 | .04 | −.01 | .18** | −.06 | .26** | .20** | .18** | 1 | |
| 11. Time of Day | .07* | −.01 | −.04 | −.05 | −.21** | .01 | −.19** | −.18** | −.26** | −.48** | 1 |
IPV: Intimate Partner Violence; T1: Baseline; T2: 20-min post-peak; T3: 40-min post-peak
p< 0.05,
p< 0.01
Acknowledgments
This work was supported by the National Institutes of Child Health and Human Development grant numbers R01 HD51502 and P01 HD 39667 with co-funding from the National Institute on Drug Abuse. The role of the first author was also supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE1342536. This material is also based upon work supported by the National Science Foundation under Grant No. 1810208 awarded to RP. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. No authors have any conflicts of interest with regards to the work presented in this manuscript.
References
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J, . . . Levitan R (2013). Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology, 38(12), 2943–2951. doi: 10.1016/j.psyneuen.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Atkinson L, Jamieson B, Khoury J, Ludmer J, & Gonzalez A (2016). Stress physiology in infancy and early childhood: Cortisol flexibility, attunement and coordination. Journal of Neuroendocrinology, 28(8), n/a-n/a. doi: 10.1111/jne.12408 [DOI] [PubMed] [Google Scholar]
- Barrett J, & Fleming AS (2011). Annual research review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry, 52(4), 368–397. doi: 10.1111/j.1469-7610.2010.02306.x [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, & Yali AM (1999). Socioeconomic status and chronic stress: Does stress account for SES effects on health? Ann N Y Acad Sci, 896(1), 131–144. doi:doi: 10.1111/j.1749-6632.1999.tb08111.x [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev, 81(1), 326–339. doi: 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Blair C (2010). Stress and the development of self-regulation in context. Child Dev Perspect, 4(3), 181–188. doi: 10.1111/j.1750-8606.2010.00145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, & Peters Razza R (2005). Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Dev, 76(3), 554–567. doi: 10.1111/j.1467-8624.2005.00863.x [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, . . . Family Life Project, I. (2008). Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Dev Psychol, 44(4), 1095–1109. doi: 10.1037/0012-1649.44.4.1095 [DOI] [PubMed] [Google Scholar]
- Blair C, & Raver C (2012). Child development in the context of adversity: experiential canalization of brain and behavior. Am Psychol, 67(4), 309–318. doi: 10.1037/a0027493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein M (2016). Determinants of parenting In Cicchetti D (Ed.), Developmental Psychopathology (Vol. 4, pp. 180–270.): John Wiley & Sons, Inc. [Google Scholar]
- Bornstein MH (2013). Mother–infant attunement: A multilevel approach In The infant mind: Origins of the social brain (pp. 266–300). New York, NY: The Guilford Press. [Google Scholar]
- Bosquet Enlow M, Sideridis G, Chiu YM, Nentin F, Howell EA, Le Grand BA, & Wright RJ (2018). Associations among maternal socioeconomic status in childhood and pregnancy and hair cortisol in pregnancy. Psychoneuroendocrinology, 99, 216–224. doi: 10.1016/j.psyneuen.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA (2011). Temporal interpersonal emotion systems: The “TIES” that form relationships. Personality and Social Psychology Review, 15(4), 367–393. doi: 10.1177/1088868311411164 [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, & Meaney MJ (2000). Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry, 48(12), 1164–1174. doi: 10.1016/S0006-3223(00)01084-2 [DOI] [PubMed] [Google Scholar]
- Calkins SD (2011). Caregiving as coregulation: Psychobiological processes and child functioning In Booth A MS., Landale N (Ed.), Biosocial Foundations of Family Processes (pp. 49–59). New York, NY: Springer. [Google Scholar]
- Clearfield MW, Carter-Rodriguez A, Merali AR, & Shober R (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behav Dev, 37(3), 298–304. doi: 10.1016/j.infbeh.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Conger RD, & Donnellan MB (2007). An interactionist perspective on the socioeconomic context of human development. Annu Rev Psychol, 58, 175–199. doi: 10.1146/annurev.psych.58.110405.085551 [DOI] [PubMed] [Google Scholar]
- Conradt E, Abar B, Lester BM, LaGasse LL, Shankaran S, Bada H, . . . Hammond JA (2014). Cortisol reactivity to social stress as a mediator of early adversity on risk and adaptive outcomes. Child Dev, 85(6), 2279–2298. doi: 10.1111/cdev.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MI, Moser DA, Manini A, Suardi F, Sancho-Rossignol A, Torrisi R, . . . Schechter DS (2017). Effects of interpersonal violence-related post-traumatic stress disorder (PTSD) on mother and child diurnal cortisol rhythm and cortisol reactivity to a laboratory stressor involving separation. Horm Behav, 90, 15–24. doi: 10.1016/j.yhbeh.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Davis EP, & Granger DA (2009). Developmental differences in infant salivary alpha-amylase and cortisol responses to stress. Psychoneuroendocrinology, 34(6), 795–804. doi: 10.1016/j.psyneuen.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, West K, Bilms J, Morelen D, & Suveg C (2018). A systematic review of parent-child synchrony: It is more than skin deep. Dev Psychobiol. doi: 10.1002/dev.21743 [DOI] [PubMed] [Google Scholar]
- Derogatis LR (2001). BSI 18, Brief Symptom Inventory 18 : Administration, scoring and procedures manual. Minneapolis, MN: NCS Pearson, Inc. [Google Scholar]
- Dill BT, & Myers SL (2004). Rediscovering rural America In Blau JR (Ed.), Blackwell companion to sociology (pp. 196–210). Oxford, UK: Blackwell Publishing Ltd. [Google Scholar]
- Dowd JB, Simanek AM, & Aiello AE (2009). Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol, 38(5), 1297–1309. doi: 10.1093/ije/dyp277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery HT, McElwain NL, Groh AM, Haydon KC, & Roisman GI (2014). Maternal dispositional empathy and electrodermal reactivity: Interactive contributions to maternal sensitivity with toddler-aged children. J Fam Psychol, 28(4), 505–515. doi: 10.1037/a0036986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. New York: Guilford Press. [Google Scholar]
- Essex MJ, Klein MH, Cho E, & Kalin NH (2002). Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry, 52(8), 776–784. doi: 10.1016/S0006-3223(02)01553-6 [DOI] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev, 73(4), 1238–1248. doi: 10.1111/1467-8624.00469 [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48(3‐4), 329–354. doi: 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Parent-infant synchrony: A bio-behavioral model of mutual influences in the formation of affiliative bonds. Monogr Soc Res Child Dev, 77(2), 42–51. doi: 10.1111/j.1540-5834.2011.00660.x [DOI] [Google Scholar]
- Feldman R (2017). The neurobiology of human attachments. Trends Cogn Sci, 21(2), 80–99. doi: 10.1016/j.tics.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Field T (1985). Attachment as psychobiological attunement: Being on the same wavelength In Reite TFM (Ed.), The psychobiology of attachment and separation (pp. 415–454). New York: Academic Press. [Google Scholar]
- Finegood ED, & Blair C (2017). Poverty, parent stress, and emerging executive functions in young children In Deater-Deckard K & Panneton R (Eds.), Parental Stress and Early Child Development: Adaptive and Maladaptive Outcomes (pp. 181–207). Cham: Springer International Publishing. [Google Scholar]
- Finegood ED, Blair C, Granger DA, Hibel LC, Mills-Koonce R, & Family Life Project Key, I. (2016). Psychobiological influences on maternal sensitivity in the context of adversity. Dev Psychol, 52(7), 1073–1087. doi: 10.1037/dev0000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, & Li M (2002). Psychobiology of maternal behavior and its early determinants in nonhuman mammals. Handbook of parenting, 2, 61–97. [Google Scholar]
- Fogel A (1993). Developing through relationships. Chicago: University of Chicago Press. [Google Scholar]
- Fries E, Hesse J, Hellhammer J, & Hellhammer DH (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30(10), 1010–1016. doi: 10.1016/j.psyneuen.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Frith CD (2008). Social cognition. Philos Trans R Soc Lond B Biol Sci, 363(1499), 2033–2039. doi: 10.1098/rstb.2008.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Mohler E, Resch F, & Kaess M (2016). Sex-specific differences in adrenocortical attunement in mothers with a history of childhood abuse and their 5-month-old boys and girls. J Neural Transm (Vienna), 123(9), 1085–1094. doi: 10.1007/s00702-016-1525-6 [DOI] [PubMed] [Google Scholar]
- Furukawa M, Tsukahara T, Tomita K, Iwai H, Sonomura T, Miyawaki S, & Sato T (2017). Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem Biophys Res Commun, 493(3), 1243–1249. doi: 10.1016/j.bbrc.2017.09.143 [DOI] [PubMed] [Google Scholar]
- Gee DG (2016). Sensitive periods of emotion regulation: Influences of parental care on frontoamygdala circuitry and plasticity. New Dir Child Adolesc Dev, 2016(153), 87–110. doi: 10.1002/cad.20166 [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Milaneschi Y, Vinkers CH, van Hemert AM, van Velzen L, Schmaal L, & Penninx BW (2017). HPA axis genes, and their interaction with childhood maltreatment, are related to cortisol levels and stress-related phenotypes. Neuropsychopharmacology, 42(12), 2446–2455. doi: 10.1038/npp.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Phillips AC, Roseboom TJ, Carroll D, & Derooij SR (2012). Cardiovascular and cortisol reactions to acute psychological stress and cognitive ability in the Dutch Famine Birth Cohort Study. Psychophysiology, 49(3), 391–400. doi: 10.1111/j.1469-8986.2011.01316.x [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, & Rothbart MK (1999). The laboratory temperament assessment battery. Locomotor version, 3. [Google Scholar]
- Granger DA, Serbin LA, Schwartzman A, Lehoux P, Cooperman J, & Ikeda S (1998). Children’s salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia longitudinal risk project. International Journal of Behavioral Development, 22(4), 707–728. doi:doi: 10.1080/016502598384135 [DOI] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annu Rev Psychol, 58, 145–173. doi: 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. doi: 10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, & Herrera A (2009). Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34(7), 953–967. doi: 10.1016/j.psyneuen.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, O’Brien JR, & Zalewski M (2018). Enduring association between parenting and cortisol: A meta-analysis. Child Dev. doi: 10.1111/cdev.13077 [DOI] [PubMed] [Google Scholar]
- Halevi G, Djalovski A, Kanat-Maymon Y, Yirmiya K, Zagoory-Sharon O, Koren L, & Feldman R (2017). The social transmission of risk: Maternal stress physiology, synchronous parenting, and well-being mediate the effects of war exposure on child psychopathology. J Abnorm Psychol, 126(8), 1087–1103. doi: 10.1037/abn0000307 [DOI] [PubMed] [Google Scholar]
- Harrist AW, & Waugh RM (2002). Dyadic synchrony: Its structure and function in children’s development. Developmental Review, 22(4), 555–592. doi: 10.1016/S0273-2297(02)00500-2 [DOI] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ, & Family Life Project Key, I. (2009). Intimate partner violence moderates the association between mother-infant adrenocortical activity across an emotional challenge. J Fam Psychol, 23(5), 615–625. doi: 10.1037/a0016323 [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Finegood ED, & Family Life Project Key, I. (2015). Maternal-child adrenocortical attunement in early childhood: continuity and change. Dev Psychobiol, 57(1), 83–95. doi: 10.1002/dev.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, & Mercado E (2017). Marital conflict predicts mother-to-infant adrenocortical transmission. Child Dev. doi: 10.1111/cdev.13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA (1994). Early relationships as regulators of infant physiology and behavior. Acta Paediatrica, 83(s397), 9–18. [DOI] [PubMed] [Google Scholar]
- Hofer MA (2010). Hidden regulators within the mother–infant interaction. In Lester BM & Sparrow JD (Eds.), Nurturing children and families. [Google Scholar]
- Hoff E, Laursen B, & Tardif T (2002). Socioeconomic status and parenting In Bornstein M (Ed.), Handbook of parenting Volume 2: Biology and ecology of parenting (Vol. 8, pp. 231–252). London: Lawrence Erlbaum Associates. [Google Scholar]
- Hostinar CE, & Gunnar MR (2013). The developmental psychobiology of stress and emotion in childhood (2 ed. Vol. 2). Hoboken, NJ: Wiley. [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull, 140(1), 256–282. doi: 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Beijers R, Riksen-Walraven M, & de Weerth C (2010). Cortisol reactivity in young infants. Psychoneuroendocrinology, 35(3), 329–338. doi: 10.1016/j.psyneuen.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Khoury JE, Gonzalez A, Levitan R, Masellis M, Basile V, & Atkinson L (2016). Maternal self-reported depressive symptoms and maternal cortisol levels interact to predict infant cortisol levels. Infant Ment Health J, 37(2), 125–139. doi: 10.1002/imhj.21554 [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Chen E, Miller G, & Seeman T (2018). How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan. In Handbook of life course health development (pp. 463–497). [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, & Gibb R (2012). Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A, 109 Suppl 2, 17186–17193. doi: 10.1073/pnas.1121251109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, Mliner SB, Donzella B, & Gunnar MR (2016). Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology, 66, 31–38. doi: 10.1016/j.psyneuen.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchick BA, & Forehand R (2002). Putting parenting in perspective: A discussion of the contextual factors that shape parenting practices. Journal of Child and Family Studies, 11(3), 255–269. doi: 10.1023/a:1016863921662 [DOI] [Google Scholar]
- Laurent HK, Ablow JC, & Measelle J (2011). Risky shifts: how the timing and course of mothers’ depressive symptoms across the perinatal period shape their own and infant’s stress response profiles. Dev Psychopathol, 23(2), 521–538. doi: 10.1017/S0954579411000083 [DOI] [PubMed] [Google Scholar]
- Lawler JM, Bocknek EL, McGinnis EW, Martinez-Torteya C, Rosenblum KL, & Muzik M (2018). Maternal postpartum depression increases vulnerability for toddler behavior problems through infant cortisol reactivity. Infancy. doi: 10.1111/infa.12271 [DOI] [PubMed] [Google Scholar]
- Lee RS, & Sawa A (2014). Environmental stressors and epigenetic control of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology, 100(4), 278–287. doi: 10.1159/000369585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Su J, Calkins SD, O’Brien M, & Supple AJ (2017). Maternal physiological dysregulation while parenting poses risk for infant attachment disorganization and behavior problems. Dev Psychopathol, 29(1), 245–257. doi: 10.1017/S0954579416000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Chen MC, Foland-Ross LC, Burley HW, & Gotlib IH (2015). Concordance of mother-daughter diurnal cortisol production: Understanding the intergenerational transmission of risk for depression. Biol Psychol, 108, 98–104. doi: 10.1016/j.biopsycho.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E, Tasker SL, Atkinson L, Vaillancourt T, Schulkin J, & Schmidt LA (2010). Perceived maternal stress during pregnancy and its relation to infant stress reactivity at 2 days and 10 months of postnatal life. Clin Pediatr (Phila), 49(2), 158–165. doi: 10.1177/0009922809346570 [DOI] [PubMed] [Google Scholar]
- Levendosky AA, Bogat GA, Lonstein JS, Martinez-Torteya C, Muzik M, Granger DA, & von Eye A (2016). Infant adrenocortical reactivity and behavioral functioning: relation to early exposure to maternal intimate partner violence. Stress, 19(1), 37–44. doi: 10.3109/10253890.2015.1108303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, & Todd RM (2007). The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Development, 22(4), 406–430. doi: 10.1016/j.cogdev.2007.08.004 [DOI] [Google Scholar]
- Lorber MF, & O’Leary S G (2005). Mediated paths to over-reactive discipline: mothers’ experienced emotion, appraisals, and physiological responses. J Consult Clin Psychol, 73(5), 972–981. doi: 10.1037/0022-006X.73.5.972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Crnic KA, Gonzales NA, Winstone LK, & Somer JA (2018). Mother-infant dyadic dysregulation and postpartum depressive symptoms in low-income Mexican-origin women. Biol Psychol. doi: 10.1016/j.biopsycho.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2001). Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology, 13(3), 653–676. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Masarik AS, & Conger RD (2017). Stress and child development: a review of the Family Stress Model. Curr Opin Psychol, 13, 85–90. doi: 10.1016/j.copsyc.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull, 133(1), 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Garrett-Peters P, Barnett M, Granger DA, Blair C, & Cox MJ (2011). Father contributions to cortisol responses in infancy and toddlerhood. Dev Psychol, 47(2), 388–395. doi: 10.1037/a0021066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper C, Gariepy JL, Barnett M, Moore GA, Calkins S, & Cox MJ (2009). Psychophysiological correlates of parenting behavior in mothers of young children. Dev Psychobiol, 51(8), 650–661. doi: 10.1002/dev.20400 [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, & Towe-Goodman N (2012). Poverty and hpa functioning in young children In King R & Maholmes V (Eds.), The Oxford handbook of poverty and child development. New York: Oxford University Press. [Google Scholar]
- Moriceau S, & Sullivan RM (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci, 9(8), 1004–1006. doi:http://www.nature.com/neuro/journal/v9/n8/suppinfo/nn1733_S1.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998-2012). Mplus user’s guide. Seventh edition. Los Angeles, CA: Muthén & Muthén [Google Scholar]
- NICHD. (1999). Child care and mother-child interaction in the first three years of life. Developmental Psychology, 35(6), 1399–1413. doi: 10.1037/0012-1649.35.6.1399 [DOI] [PubMed] [Google Scholar]
- Papoušek H, & Papoušek M (2002). Intuitive parenting In Handbook of parenting: Biology and ecology of parenting, Vol. 2, 2nd ed. (pp. 183–203). Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Papousek M (2007). Communication in early infancy: an arena of intersubjective learning. Infant Behav Dev, 30(2), 258–266. doi: 10.1016/j.infbeh.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Perry R, & Sullivan RM (2014). Neurobiology of attachment to an abusive caregiver: Short‐term benefits and long‐term costs. Dev Psychobiol, 56(8), 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Blair C, & Sullivan RM (2017). Neurobiology of infant attachment: attachment despite adversity and parental programming of emotionality. Current Opinion in Psychology, 17, 1–6. doi: 10.1016/j.copsyc.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffington L, Prindle J, Keresztes A, Binder J, Heim C, & Shing YL (2018). Blunted cortisol stress reactivity in low–income children relates to lower memory function. Psychoneuroendocrinology, 90, 110–121. doi: 10.1016/j.psyneuen.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, & Howell BR (2015). Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci, 10(5), 512–526. doi: 10.1080/17470919.2015.1087426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago AN, Lim KY, Opendak M, Sullivan RM, & Aoki C (2018). Early life trauma increases threat response of peri-weaning rats, reduction of axo-somatic synapses formed by parvalbumin cells and perineuronal net in the basolateral nucleus of amygdala. J Comp Neurol. doi: 10.1002/cne.24522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore AN (2015). Affect regulation and the origin of the self: The neurobiology of emotional development: Routledge. [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, . . . Goldsmith HH (2006). Environmental influences on family similarity in afternoon cortisol levels: twin and parent-offspring designs. Psychoneuroendocrinology, 31(9), 1131–1137. doi: 10.1016/j.psyneuen.2006.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, & Rice MA (2002). Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology, 27(6), 731–747. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce W, & McEwen BS (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA, 301(21), 2252–2259. doi: 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- Sroufe LA (1997). Emotional development: The organization of emotional life in the early years: Cambridge University Press. [Google Scholar]
- Stallings J, Fleming AS, Corter C, Worthman C, & Steiner M (2001). The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpostpartum women. Parenting, 1(1–2), 71–100. doi: 10.1080/15295192.2001.9681212 [DOI] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, & Sugarman DB (1996). The revised conflict tactics scales (CTS2) development and preliminary psychometric data. Journal of Family Issues, 17(3), 283–316. [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, & Manning LG (2012). Interparental violence, maternal emotional unavailability and children’s cortisol functioning in family contexts. Dev Psychol, 48(1), 237–249. doi: 10.1037/a0025419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, & Holman PJ (2010). Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neurosci Biobehav Rev, 34(6), 835–844. doi: 10.1016/j.neubiorev.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, & Perry RE (2015). Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Soc Neurosci, 10(5), 500–511. doi: 10.1080/17470919.2015.1087425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, & McEwen BS (2014). On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol, 35(2), 245–251. doi: 10.1016/j.yfrne.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Tarullo AR, St John AM, & Meyer JS (2017). Chronic stress in the mother-infant dyad: Maternal hair cortisol, infant salivary cortisol and interactional synchrony. Infant Behav Dev, 47, 92–102. doi: 10.1016/j.infbeh.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE (2010). Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A, 107(19), 8507–8512. doi: 10.1073/pnas.1003890107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ZE, Spinrad TL, VanSchyndel SK, Eisenberg N, Huynh J, Sulik MJ, & Granger DA (2013). Sociodemographic risk, parenting, and effortful control: relations to salivary alpha-amylase and cortisol in early childhood. Dev Psychobiol, 55(8), 869–880. doi: 10.1002/dev.21079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer ZM, & Kuzawa CW (2014). Early origins of health disparities: material deprivation predicts maternal evening cortisol in pregnancy and offspring cortisol reactivity in the first few weeks of life. Am J Hum Biol, 26(6), 723–730. doi: 10.1002/ajhb.22532 [DOI] [PubMed] [Google Scholar]
- Thompson LA, & Trevathan WR (2008). Cortisol reactivity, maternal sensitivity, and learning in 3-month-old infants. Infant Behav Dev, 31(1), 92–106. doi: 10.1016/j.infbeh.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, & Moll H (2005). Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences, 28(5), 675–691. doi: 10.1017/S0140525X05000129 [DOI] [PubMed] [Google Scholar]
- Ursache A, Blair C, Stifter C, & Voegtline K (2013). Emotional reactivity and regulation in infancy interact to predict executive functioning in early childhood. Developmental Psychology, 49(1), 127–137. doi: 10.1037/a0027728 [DOI] [PubMed] [Google Scholar]
- Ursache A, Merz EC, Melvin S, Meyer J, & Noble KG (2017). Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology, 78, 142–150. doi: 10.1016/j.psyneuen.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel HJ, & Riksen-Walraven JM (2008). Adrenocortical and behavioral attunement in parents with 1-year-old infants. Dev Psychobiol, 50(2), 196–201. doi: 10.1002/dev.20281 [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, & Goldsmith HH (2012). Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior, 62(1), 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox M, & Family Life Project Key Investigators. (2013). The Family Life Project: an epidemiological and developmental study of young children living in poor rural communities. Monogr Soc Res Child Dev, 78(5), 1–150, vii. doi: 10.1111/mono.12046 [DOI] [PubMed] [Google Scholar]
- Waters SF, West TV, & Mendes WB (2014). Stress contagion: physiological covariation between mothers and infants. Psychol Sci, 25(4), 934–942. doi: 10.1177/0956797613518352 [DOI] [PMC free article] [PubMed] [Google Scholar]