Abstract
Although the mechanism is unclear, it has been shown that genetically normal adult mice with a large wound form de novo morphogenesis of hair follicles in Wound Induced Hair Neogenesis (WIHN)(1). We focused on how tissues recognize damage signals and identified that double stranded RNA (dsRNA)-mediated toll like receptor 3 (TLR3) activation stimulates WIHN. Here we propose a hypothesis that TLR3 stimulates retinoic acid synthesis and signaling to allow for regeneration, suggesting that common clinical methods of facial rejuvenation in human subjects through damage (such as lasers or dermabrasion) and the use of topical retinoids reflect the same biologic pathway.
1. BACKGROUND
Although a rare example, damaged skin regenerates in rabbits and mice(1, 2), now known as wound-induced hair neogenesis (WIHN). Wounded skin induces the innate immune system including antimicrobial peptides, interferons, and interleukins to provide defense(3, 4). Danger-associated molecular patterns (DAMPS) signal through toll-like receptors (TLRs) to recognize harmful materials and activate the innate immune response early after a wound. Particularly, TLR3 is important as a damage sensing mechanism to induce regeneration(5–8).
Retinoic acid (RA) is a vitamin A derivative that exerts pleiotropic effects and orchestrates diverse physiological roles. During embryogenesis, retinoic acid (RA) acts as a potent morphogen in cell fate decision and organogenesis(9). Moreover, urodele amphibians regenerate their limbs and tails perfectly after amputation, partially due to a gradient of RA concentration in the proximodistal axis(10, 11). Abnormal RA signaling with disrupted localization of RA related factors alters feather development and epithelial cell morphology in chicken(12). In human, RA regulates the hematopoietic hierarchy by regulating cell cycle-mediated hematopoietic stem cell plasticity(13). Moreover, RA increases a population of CD161+ regulatory T cells, which facilitates the wound healing of epithelial cells in the gut(14). These results indicate that endogenous RA synthesis is critical for embryonic development and tissue homeostasis through repair of damaged tissues in adults.
On this basis, we query how RA synthesis and signaling are regulated in damaged tissues. Does TLR3 activation induce RA synthesis in a common pathway of regeneration? Do TLR3 and RA share signaling pathways during wound healing? In this Hypothesis Letter, we propose the functional relationship between TLR3 and RA signaling in damaged tissues to enhance regeneration.
2. PREMISES
Damage-induced TLR3 activation regulates regeneration.
TLR3 binds double stranded RNA (dsRNA) for the initial detection of microbes and viruses, to activate the innate immune responses. Although the activating dsRNA may be RNA viruses (15), it may also originate from transcription of inverted-repetitive sequences and transposable elements (16, 17). While TLR3 deficient mice display impaired wound healing, TLR3 activation enhances wound closure by upregulating the production of chemokines to recruit neutrophils and macrophages(18, 19). Previous reports demonstrate that ultraviolet (UVB)-damaged keratinocytes release the non-coding double stranded RNA (dsRNA) sequences of U1-snRNA to activate TLR3 signaling and stimulate inflammatory cytokines(20). Also, dsRNA released from damaged tissues triggers TLR3 downstream pathway to promote wound healing and WIHN in mice(6, 8). However, TLR3 does not appear to have particular dsRNA sequence avidity(21), and therefore even coding RNA or other noncoding RNA might act as ligands. Taken together, these data demonstrate that damage-induced dsRNA-TLR3 signaling regulate regeneration.
RA synthesis and signaling regulate hair follicle morphogenesis
Retinoids (vitamin A) obtained from dietary sources much be enzymatically metabolized to retinal by the alcohol dehydrogenases (ADHs) and then finally converted to all-trans RA by the retinal dehydrogenases (ALDH1A1, 2, and 3) to generate a functional molecule. Inhibition of RA signaling in epidermal and hair follicle keratinocytes causes aberrant differentiation of keratinocytes and a progressive alopecia(22). Also, RA is used for facial rejuvenation in humans, similar to that used by lasers clinically, suggesting a possible common pathway(23, 24). In addition, appropriate endogenous RA levels regulated by Cyp26b1, a RA degrading enzyme, are critical for hair follicle morphogenesis(25). These results highlight the important physiological roles of endogenous RA signaling in hair follicle biology.
Signaling proteins downstream of TLR3 control the RA synthesizing ALDH1A enzyme isoforms.
STAT3 and NF-κB-mediated signaling (normally downstream of TLR3) upregulate ALDH1A3 expression in chemoresistant and malignant pleural mesothelioma(26). Also, TLR3 and RA synergistically induce type I interferon and IRF3-dependent apoptosis in breast cancer cells(27, 28). Finally, in zebrafish, H6-homeobox 4 (hmx4) gene is required for proper expression of aldh1a2, which synthesizes RA to regulate sonic hedgehog (shh) and gli3 during forebrain patterning(29). Given that Shh/Gli3 and Stat3/ NF-κB are dependent on TLR3, these results indicate that RA and TLR3 signaling interact and TLR3-mediated signaling may induce RA synthesis and signaling.
5. HYPOTHESIS
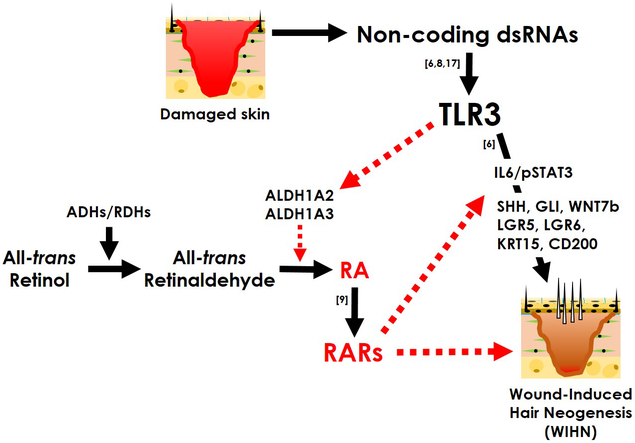
The regulatory mechanism of endogenous RA synthesis is not well established, certainly after wounding. To unite the above premises, we hypothesize that damage-induced TLR3 signaling stimulates intrinsic RA synthesis and signaling to enable WIHN (Figure 1).
Figure 1. Working model of dsRNA-mediated TLR3 signaling in WIHN.
Damage-tissues release non-coding dsRNA to activate TLR3 signaling. Besides, endogenous RA synthesis is induced by ALDH1A2/A3 upregulated by TLR3 dependent signaling, which triggers RA signaling via RARs. Eventually, TLR3 and RA signaling may work synergistically to facilitate WIHN.
6. HOW TO TEST THE HYPOTHESIS
ALDH1As are important enzymes to produce RA and our preliminary data show that ALDH1A2 and ALDH1A3 expression is dependent on TLR3 signaling. Previously, Nelson et al., reported that Tlr3 null mice fail to induce WIHN(6). Thus, this hypothesis can be examined by quantifying RA levels using analytical chemistry methods such as LC-MS between WT and Tlr3 null mice after wounding(30). This experiment will determine whether wound-induced TLR3 signaling stimulates intrinsic RA synthesis and any correlation between amount of RA and regeneration capacity. Since Kumar et al., demonstrated that defectives in Aldh1a-deleted mice are rescued by RA treatment(31), the hypothesis can be supported by checking if additional RA treatment in wounded skin of TLR3KO mice could rescue the defective regenerative ability.
Once RA binds to its receptors (RARα, RARβ, and RARγ) and heterodimerize with retinoid X receptors (RXRα, RXRβ, and RXRγ), the complex is recruited to retinoic acid response elements (RAREs) to initiate transcription(9). RARE-reporter (LacZ, GFP, and RFP) mice can be evaluated with or without Poly (I:C) (TLR3 ligand) after wounding to visualize the location of RA synthesis in skin. Since RARE reporter mice do not fully recapitulate RA responses, in vivo results must be confirmed by analyzing the gene expression of Aldh1a1–3 and the levels of RA in primary isolated cells such as keratinocytes and fibroblasts.
To investigate RA-induced mechanisms in WIHN, we suggest testing the requirement of endogenous RA signaling using tissue specific RAR-deleted mice. For example, epidermal functions of RA signaling can be evaluated by generating Krt5Cre tissue-specific constitutive RARs floxed mice in WIHN. The above experiments will test the hypothesis that TLR3-induced RA signaling supports WIHN.
7. RELEVANCE AND PERSPECTIVES
Although several medical aesthetic treatments with low levels of skin damage, such as microneedling, dermabrasion, and laser treatment, have been in clinical practice to enhance skin rejuvenation for decades, the exact mechanism is still unknown. The perspective to test is whether mechanisms of human rejuvenation overlap with mouse regeneration during WIHN. One key possible mechanism in these rejuvenation effects result from RA signaling stimulated by damage-induced TLR3 activation. This is logical since RA is already used for facial rejuvenation in humans. However, there is still important information about dsRNAs released by damaged tissues to explore. Future studies to define the exact sequence and the regulatory mechanisms of dsRNA will add to therapeutic drug development. Therefore, the study of RNA sensing to understand unknown mechanisms is important.
ACKNOWLEDGEMENTS
Both DK and LAG generated the ideas and wrote this Hypothesis Letter. Figure was created by both DK and LAG. LAG is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under R01AR064297 and AR068280 to LAG. This work was also supported by the Department of Defense, Armed Forces Institute of Regenerative Medicine, Extremities Regeneration (AFIRM2-ER11), CDMRMP W81XWH-16-C-0167 and 18–2-0055. DK is financially supported by Maryland Stem Cell Research Fund (2017-MSCRFF-3905).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicting interests to declare.
REFERENCES
- 1.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007: 447: 316–320. [DOI] [PubMed] [Google Scholar]
- 2.Breedis C Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer research 1954: 14: 575–579. [PubMed] [Google Scholar]
- 3.Fujisawa H, Kondo S, Wang B, et al. The expression and modulation of IFN-alpha and IFN-beta in human keratinocytes. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 1997: 17: 721–725. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LJ, Sen GL, Ward NL, et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-beta Production by Epidermal Keratinocytes during Skin Injury. Immunity 2016: 45: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 2009: 15: 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson Amanda M, Reddy Sashank K, Ratliff Tabetha S, et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell Stem Cell 2015: 17: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu AS, Li A, Ratliff TS, et al. After Skin Wounding, Noncoding dsRNA Coordinates Prostaglandins and Wnts to Promote Regeneration. The Journal of investigative dermatology 2017: 137: 1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkowski AW, Kuo IH, Bernard JJ, et al. Toll-like receptor 3 activation is required for normal skin barrier repair following UV damage. J Invest Dermatol 2015: 135: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhinn M, Dolle P. Retinoic acid signalling during development. Development (Cambridge, England) 2012: 139: 843–858. [DOI] [PubMed] [Google Scholar]
- 10.Monaghan JR, Maden M. Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev Biol 2012: 368: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen M, Singhal P, Piet JW, et al. Retinoic acid receptor regulation of epimorphic and homeostatic regeneration in the axolotl. Development (Cambridge, England) 2017: 144: 601–611. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Figueroa S, Jiang TX, et al. Diverse feather shape evolution enabled by coupling anisotropic signalling modules with self-organizing branching programme. Nature communications 2017: 8: ncomms14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabezas-Wallscheid N, Buettner F, Sommerkamp P, et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 2017: 169: 807–823 e819. [DOI] [PubMed] [Google Scholar]
- 14.Povoleri GAM, Nova-Lamperti E, Scotta C, et al. Human retinoic acid-regulated CD161(+) regulatory T cells support wound repair in intestinal mucosa. Nature immunology 2018: 19: 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar M, Carmichael GG. Antisense RNA: function and fate of duplex RNA in cells of higher eukaryotes. Microbiology and molecular biology reviews : MMBR 1998: 62: 1415–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamura K, Chung WJ, Ruby JG, et al. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature 2008: 453: 803–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghildiyal M, Seitz H, Horwich MD, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science (New York, NY) 2008: 320: 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Q, Fang D, Fang J, et al. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. J Immunol 2011: 186: 3710–3717. [DOI] [PubMed] [Google Scholar]
- 19.Lin Q, Wang L, Lin Y, et al. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid promotes wound healing in human and murine skin. J Invest Dermatol 2012: 132: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 20.Bernard JJ, Cowing-Zitron C, Nakatsuji T, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nature medicine 2012: 18: 1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Botos I, Wang Y, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 2008: 320: 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Chiba H, Warot X, et al. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development (Cambridge, England) 2001: 128: 675–688. [DOI] [PubMed] [Google Scholar]
- 23.Sendagorta E, Lesiewicz J, Armstrong RB. Topical isotretinoin for photodamaged skin. Journal of the American Academy of Dermatology 1992: 27: S15–18. [DOI] [PubMed] [Google Scholar]
- 24.Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. Journal of the American Academy of Dermatology 2008: 58: 719–737; quiz 738–740. [DOI] [PubMed] [Google Scholar]
- 25.Okano J, Levy C, Lichti U, et al. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. The Journal of biological chemistry 2012: 287: 39304–39315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canino C, Luo Y, Marcato P, et al. A STAT3-NFkB/DDIT3/CEBPbeta axis modulates ALDH1A3 expression in chemoresistant cell subpopulations. Oncotarget 2015: 6: 12637–12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardo AR, Cosgaya JM, Aranda A, et al. Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells. Cell death & disease 2013: 4: e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardo AR, Cosgaya JM, Aranda A, et al. Pro-apoptotic signaling induced by Retinoic acid and dsRNA is under the control of Interferon Regulatory Factor-3 in breast cancer cells. Apoptosis : an international journal on programmed cell death 2017: 22: 920–932. [DOI] [PubMed] [Google Scholar]
- 29.Gongal PA, March LD, Holly VL, et al. Hmx4 regulates Sonic hedgehog signaling through control of retinoic acid synthesis during forebrain patterning. Developmental biology 2011: 355: 55–64. [DOI] [PubMed] [Google Scholar]
- 30.Kane MA, Napoli JL. Quantification of endogenous retinoids. Methods in molecular biology (Clifton, NJ) 2010: 652: 1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Dollé P, Ghyselinck NB, et al. Endogenous retinoic acid signaling is required for maintenance and regeneration of cornea. Experimental eye research 2017: 154: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]