Abstract
Respiratory failure is a significant problem within the pediatric population. A means of respiratory support that readily allows ambulation could improve treatment. The Pittsburgh Pediatric Ambulatory Lung (P-PAL) is being developed as a wearable pediatric pump-lung for long-term respiratory support and has previously demonstrated positive benchtop results. This study aimed to evaluate acute (4–6 hr) in vivo P-PAL performance as well as develop an optimal implant strategy for future long-term studies. The P-PAL was connected to healthy sheep (n = 6, 23–32 kg) via cannulation of the right atrium and pulmonary artery. Plasma free hemoglobin (PfHb) and animal hemodynamics were measured throughout the study. Oxygen transfer rates were measured at blood flows of 1–2.5 L/min. All animals survived the complete study duration with no device exchanges. Flow limitation due to venous cannula occlusion occurred in Trial 2 and was remedied via an altered cannulation approach. Blood exiting the P-PAL had 100% oxygen saturation with the exception of Trial 4 during which inadequate device priming led to intra-bundle clot formation. PfHb remained low (<20 mg/dL) for all trials. In conclusion, this study demonstrated successful performance of the P-PAL in an acute setting and established the necessary methods for future long-term evaluation.
Keywords: wearable artificial lung, pediatric ECMO
Introduction
Chronic lung diseases such as cystic fibrosis, pulmonary hypertension and pulmonary fibrosis continue to be sources of pediatric morbidity and mortality. These patients often require a heart or heart-lung transplant, however the waitlist duration is typically 1– 6 months.1 Mechanical ventilation (MV) and extracorporeal membrane oxygenation (ECMO) can be used to provide respiratory support to these patients as a bridge to transplant. Between 2009 and 2015, the number of pediatric extracorporeal respiratory support cases reported to the Extracorporeal Life Support Organization (ELSO) increased by 48% (569 cases in 2015).2 Both MV and ECMO however are often considered contraindications pre-transplant. MV can result in ventilator induced lung injury3 and the complex circuitry of current ECMO devices can lead to complications. In one study, up to 36% of the pediatric ECMO cases exhibited circuit complications.4 Recent advancements in ECMO therapy including polymethylpentene fibers, heparin coatings, centrifugal pumps and portability has led to the reevaluation of ECMO pre-transplant.5
Recent efforts have focused on improving the portability of ECMO circuits in order to improve patient mobility. The immobilization of patients on MV or ECMO can lead to muscular deconditioning and poor post-transplant outcomes. Several centers have demonstrated improved post-transplant outcomes in patients who were able to ambulate while on ECMO.6–8 One of the current barriers to ambulatory ECMO is the complexity of the ECMO circuit and need for a specialized, highly coordinated team effort during patient ambulation. Thus, current efforts aim to design ECMO units specific to pediatric needs that also allow for simpler and less risky patient ambulation within the hospital.
Our group is currently developing the Pittsburgh Pediatric Ambulatory Lung (P-PAL) as an ambulatory artificial lung for long-term respiratory support.9 The P-PAL improves compactness and decreases complexity by integrating the oxygenator and pump in to a single wearable unit. The device could be worn by either the patient or a caregiver. In the present study, we evaluated the in vivo performance of the P-PAL in an acute ovine model. In vivo gas transfer and hemolysis were measured in addition to animal hemodynamics. Additionally, we developed and refined our surgical and cannulation strategy in preparation for chronic, recovery studies.
Methods
Device Description
The P-PAL is an integrated pump-lung designed for respiratory support of pediatric patients and is ultimately intended to provide long-term (1 – 3 months) support. The device is intended to be operated at blood flow rates of 1 – 2.5 L/min and provide up to 90% of the respiratory support of 5 −25 kg children. This represents an oxygenation rate of 30 – 105 mL/min. Blood is drawn from the right atrium and pumped through a polymethylpentene hollow fiber membrane (HFM) bundle (0.3 m2) by an integrated centrifugal pump. Oxygenated blood is then returned to the pulmonary artery via an arterial cannula.
Two slightly different P-PAL design iterations were used during the acute trials. Trials 1–4 used the previously described P-PAL design (subsequently referred to as P-PAL v1).9 Trials 5 and 6 were conducted with a second generation P-PAL design (P-PAL v2 and shown in Figure 1). The P-PAL v2 centrifugal pump and HFM bundle are identical to that used in the P-PAL v1. The primary difference between the designs is a shortened blood flow channel leading from the pump compartment to the bundle compartment in the P-PAL v2. This change allows for easier priming and a more compact overall device. The priming volume of the P-PAL v2 circuit was 270 mL. Excess acrylic was also removed from the device housing in P-PAL v2. This reduced the mass of the device from 1157 g to 721 g. The in vitro pump performance, gas transfer and hemolysis results of the P-PAL v1 has been previously published.9 Benchtop pump and gas transfer performance were equivalent between the two versions.
Figure 1:
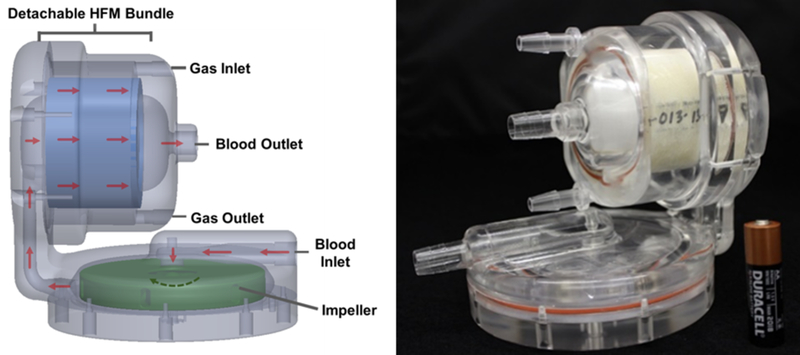
Picture of the P-PAL v2 and schematic demonstrating the gas and liquid flow pathways within the device. A schematic and picture of the P-PAL v1 has previously been published.9
Acute In Vivo Evaluation
Animal hemodynamics, gas exchange performance, and biocompatibility were evaluated in an acute (4 – 6 hours) ovine model. Sheep (n=6) weight ranged from 23.3 to 32.2 kg. All trials were completed in the Center for Preclinical Studies at the McGowan Institute and all animals received humane care. The University of Pittsburgh’s Institutional Animal Care and Use Committee approved the protocol for animal care and surgery.
Anesthesia was induced by a subcutaneous injection of atropine (0.05 mg/kg) followed by intravenous administration of ketamine (3.9 – 5.3 mg/kg). Animals were directly intubated with a cuffed Magill endotracheal tube. Anesthesia was maintained throughout the study via isoflurane inhalation (1.5 – 3 %) to effect. An arterial line was placed in the carotid artery to monitor mean arterial pressure (MAP). A venous line was placed in the jugular vein to monitor central venous pressure (CVP) and provided access for maintenance drips. Access to the right atrium and pulmonary artery was made via a right (Trials 1 – 2) or left (Trials 3 – 6) thoracotomy. A pressure monitoring line was placed in the pulmonary artery (Trials 2 – 6) to monitor pulmonary artery pressure (PAP). Heart rate, MAP, CVP and PAP were recorded every 15 minutes. Prior to cannula placement a heparin bolus (300 IU/kg) was administered to increase the activated clotting time (ACT) to greater than twice baseline to prevent clot formation in the cannulas prior to starting the pump. The venous cannula was placed in the right atrium and the arterial cannula was placed in the pulmonary artery. The ACT target for the duration of the study was 1.5 – 2 times baseline. Heparin was continuously administered through the venous line and the infusion rate periodically altered to maintain the target ACT range.
The cannulas were connected to a primed (1 U/mL heparinized saline) P-PAL device. After the P-PAL pump was started and flow initiated, a stabilization period of at least 10 minutes was permitted prior to the commencement of gas exchange sampling. A 5% CO2 95% O2 sweep gas mixture was set to 3 – 5 L/min. The P-PAL impeller rotation rate was varied in order to attain each targeted blood flow rate (1.0, 1.5, 2.0, 2.5 L/min) during three repeated measurements. Blood gas tensions and hematocrit were measured from device inlet and outlet samples. Plasma free hemoglobin was measured from blood samples taken from the arterial line. Methods for spectrophotometrically measuring plasma free hemoglobin have been previously published.10 Additional blood samples were routinely taken from the arterial line in order to monitor the animal’s arterial blood gas tensions, electrolytes and hematocrit. Ventilator settings (FiO2, PEEP and rate) were adjusted to maintain normal physiologic arterial blood gases. Pressure drop across the bundle was measured continuously (PCU-2000, Millar, Texas).
At the completion of the study, the heart and lungs were examined for gross abnormalities. The device was disconnected and saline was passively flowed through the device to wash away blood while preserving any intra-device thrombus. The device was disassembled, and any thrombus formation was noted. The inlet and outlet faces of the HFM bundle were photographed.
Statistics
Statistical analysis was conducted in SPSS (IBM, Armonk, NY). Results are reported as an average and standard deviation. The baseline and final MAP, CVP and heart rate were compared using a paired samples t-test per animal. The baseline MAP, CVP and heart rate are an average of vital signs recorded during the 30 minutes prior to initiation of device blood flow. The final MAP, CVP and heartrate are an average of the vital signs taken during the final hour of the study. Vital signs were recorded every 10–15 minutes. Statistical analysis was not performed on the PAP due to a lack of repeated measurements. A two-way ANOVA was used to evaluate the effect of device type (P-PAL v1 versus P-PAL v2). Differences were considered statistically significant for p < 0.05.
Results
All studies were electively terminated following the completion of data collection. Table 1 provides a comprehensive summary of each trial. The ACT range throughout the studies was 1.2 – 4.1 times baseline. The blood flow rate range for Trial 2 was limited to 1.0 – 1.4 L/min due to a venous cannula occlusion resulting in suction and limited blood flow at higher impeller rotation rates. The necropsy following Trial 2 revealed right atrium bruising indicative of cannula suction at the right atrium wall. Subsequent trials utilized a right thoracotomy to gain improved access to the heart and placement of the cannula at the main wall of the right atrium. In Trial 4, thrombus formed in the HFM bundle and subsequently led to decreased oxygen transfer and device blood flow rate. The bundle resistance increased from 11 mmHg/L/min to 120 mmHg/L/min by the end of the study. Visual inspection of the bundle showed thrombus formation within ~25% of the bundle (Figure 2). All other trials were completed without complication.
Table 1.
Summary of Acute Trials
| Trial No. |
Cannula Type | Implant Procedure |
Device | Flow Rate [L/min] |
Duration [hr] |
Comment |
|---|---|---|---|---|---|---|
| 1 | 18 Fr Straight (ven)1 | Left Thoracotomy | P-PAL v1 | 1.0–2.5 | 4.5 | - |
| 16 Fr Straight (art)2 | ||||||
| 2 | 22 Fr Right Angle (ven)3 | Left Thoracotomy | P-PAL v1 | 1.0–1.4 | 4 | Flow Limitation |
| 16 Fr Straight (art)2 | (ven cannula occlusion) | |||||
| 3 | 22 Fr Right Angle (ven)3 | Right Thoracotomy | P-PAL v1 | 1.0–2.5 | 6 | - |
| 16 Fr Straight (art)2 | ||||||
| 4 | 22 Fr Right Angle (ven)3 | Right Thoracotomy | P-PAL v1 | 1.0–2.4 | 6 | Device Failure |
| 16 Fr Straight (art)2 | (↑ bundle resistance) | |||||
| 5 | 22 Fr Right Angle Metal Tip (ven)4 | Right Thoracotomy | P-PAL v2 | 1.0–2.5 | 6 | - |
| 18 Fr Straight (art)5 | ||||||
| 6 | 22 Fr Right Angle Metal Tip (ven)4 | Right Thoracotomy | P-PAL v2 | 1.0–2.5 | 6 | - |
| 18 Fr Straight (art)5 | ||||||
Medtroni DLP Single Stage Venous Cannula (#66118)
Medtronic DLP One-Piece Arterial Cannula (#77016)
Edwards Lifesciences Thin-Flex Single Stage Venous Drainage Cannula (VCS02290)
Medtronic DLP Single Stage Venous Cannula (#69322)
Medtronic Elongated One-Piece Arterial Cannula (EOPA) (#77518)
Figure 2:
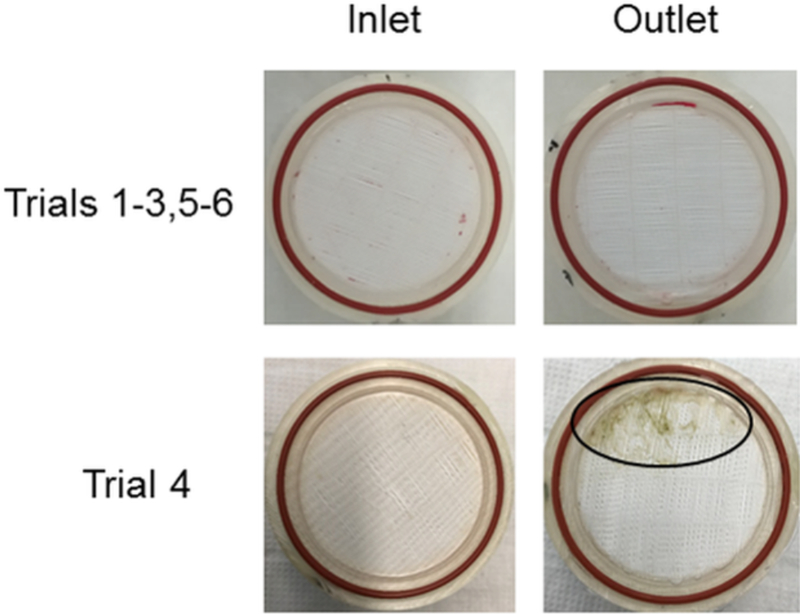
Photographs of a typical bundle inlet and outlet (top row) and that of Trial 4 (bottom row) at explant. Circle indicates area of clot formation
A maximum oxygen transfer rate of 83.7 ± 3.5 mL/min was achieved at 2.5 L/min (Figure 3). The average hemoglobin concentration across all trials was 6.2 ± 0.3 g/dL and the blood exiting the device was 100% saturated at all blood flow rates for all trials except Trial 4. There was no significant effect of device type (P-PAL v1 versus P-PAL v2) on oxygenation rate (p>0.05).
Figure 3:
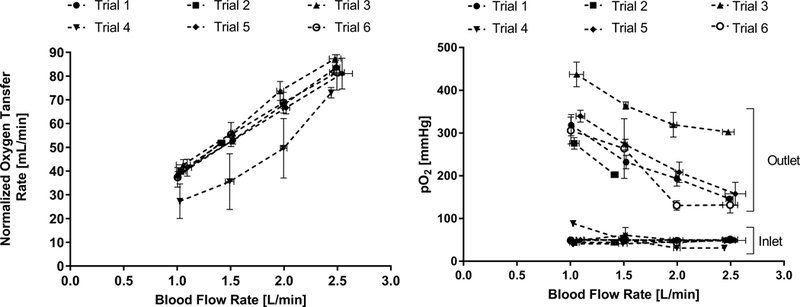
In vivo gas transfer of the P-PAL device
Plasma free hemoglobin remained below 20 mg/dL for all trials (Figure 4). Post-study macroscopic inspection of the device flow paths, bearings and bundle revealed an absence of any significant thrombus for all trials except Trial 4. Figure 2 provides representative post-study photographs of the bundle inlet and outlet faces.
Figure 4:
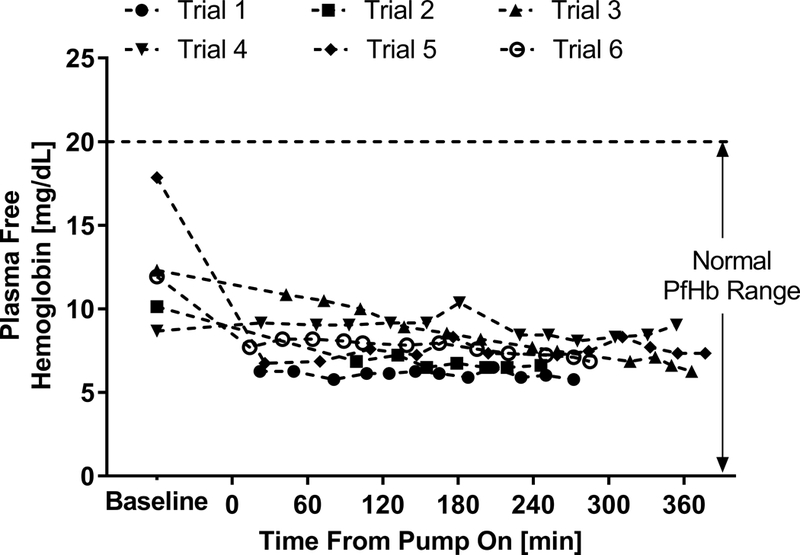
In vivo plasma free hemoglobin generated by the P-PAL device
Table 2 summarizes animal hemodynamics at baseline (prior to cannulation) and at the end of the study. Heart rate was not affected by the device (p > 0.05) during any of the trials. MAP statistically decreased (p < 0.05) in Trial 2 (89 ± 5 mmHg to 64 ± 10 mmHg) and increased in Trial 3 (52 ± 6 mmHg to 58 ± 4 mmHg). CVP statistically decreased (p < 0.05) in Trial 4 (11 ± 2 mmHg to 7 ± 1 mmHg) to the average CVP of this series of studies. With the exception of cannula-related bruising described above for Trial 2, no device-related damage to the heart or lungs was observed upon necropsy.
Table 2.
Baseline and Final Animal Hemodynamics
| MAP | CVP | Heart Rate | PAP | |||||
|---|---|---|---|---|---|---|---|---|
| [mmHg] | [mmHg] | [beats/min] | [mmHg] | |||||
| Trial No. |
Baseline | Final | Baseline | Final | Baseline | Final | Baseline | Final |
| 1 | 65 ± 5 | 54 ± 1.0 | 9 ± 2 | 9 ± 1 | 95 ± 13 | 88 ± 2 |
-- |
-- |
| 2 | 89 ± 5 | 64 ± 10* | 8 ± 1 | 5 ± 1 | 81 ± 5 | 80 ± 1 | 17 | 18 ± 1 |
| 3 | 52 ± 6 | 58 ± 4* | 6 ± 2 | 4 ± 1 | 94 ± 4 | 97 ± 2 | 18 | 21 ± 2 |
| 4 | 62 ± 14 | 53 ± 5 | 11 ± 2 | 7 ± 1* | 93 ± 5 | 95 ± 8 | 11 | 11 ± 1 |
| 5 | 68 ± 15 | 49 ± 6 | 7 ± 2 | 5 ± 1 | 89 ± 15 | 102 ± 5 | 14 | 17 ± 1 |
| 6 | 61 ± 21 | 41 ± 1 | 5 ± 4 | 10 ± 1 | 92 ± 19 | 95 ± 1 | 12 | 15 ± 2 |
p<0.05, considered significant between baseline and final values
MAP, mean arterial pressure; CVP, central venous pressure; PAP, pulmonary artery pressure
Discussion
MV and ECMO are the primary treatment options for pediatric patients with lung failure. These treatments, however, greatly restrict patient mobility and MV can further lung injury. The ability to mobilize ECMO patients and increase their participation in physical therapy pre-transplant has been shown to improve post-transplant outcomes.6–8 The P-PAL is specifically designed as a wearable, long-term respiratory assist device. The integration of the pump and oxygenator into a highly compact unit creates a less complex circuit to allow simplified, in-hospital patient ambulation. Previous in vitro work has demonstrated sufficient pumping, gas exchange, and hemolysis performance.9 The present study used a healthy ovine model to evaluate the acute in vivo performance of the P-PAL as well as develop an optimal implantation strategy. Results demonstrated successful performance of the P-PAL in an acute support setting and established the surgical and implantation techniques needed for chronic, recovery studies.
Current pediatric ECMO devices are multicomponent systems connected via lengths of tubing. These complex and cumbersome circuits require highly trained teams during patient ambulation and the risk of device or circuit malfunction is elevated. Thus, current research efforts have shifted toward the development of compact respiratory support systems that could be worn paracorporeally and enable patient mobility. The University of Maryland is developing the Pediatric Pump Lung (PediPL) as an ambulatory ECMO device.11,12 The PediPL integrates a centrifugal pump utilizing a magnetically levitated impeller with an annular HFM bundle. The PediPL has exhibited positive in vitro and in vivo results but has not progressed to clinical use as of yet. These efforts highlight the need for ambulatory respiratory support devices and the importance of the continued development necessary for their safe and effective use in patients.
The P-PAL demonstrated consistent oxygenation and pumping performance in an acute support setting. Blood flow limitation due to venous cannula inlet occlusion during Trial 2 led to a modified cannula placement technique via a right thoracotomy. In all subsequent trials, the P-PAL was able to generate the full range of targeted blood flow rates (1–2.5 L/min). The P-PAL device used during Trial 4 developed diffuse intra-bundle thrombus during the study duration. The thrombus formation resulted in progressive bundle resistance increase and oxygen transfer decrease during support. This was an isolated occurrence and is suspected to have originated from inadequate device priming prior to the implant. Previous computational work has predicted spatially uniform blood flow through the P-PAL HFM bundle9 and all other trials in the current work resulted in an HFM bundle remarkably free of thrombus. With the exception of Trial 4, the P-PAL achieved full blood oxygen saturation at all evaluated blood flow rates. In vivo oxygen transfer rates were limited by low hemoglobin concentrations and thus are not representative of the full oxygenation capability of the device. The intraoperative anemia observed in these acute studies is not uncommon and likely attributable to hemodilution and perioperative red blood cell sequestration. A return to more normal hemoglobin concentrations in the days following surgery is expected during longer-term P-PAL studies. As expected, there was no discernible difference in device performance between the two slightly different P-PAL design iterations used in this study. The evolution of the design was driven by a goal to attain a lighter device (P-PAL v2 was 436 g lighter) that was easier to prime.
P-PAL support was generally well tolerated and exhibited minimal effects on normal physiological function. Although statistically significant changes in MAP and CVP occurred in some trials, no consistent or concerning trends were observed. Hemolysis was low during P-PAL support with plasma free hemoglobin concentrations remaining generally constant throughout support and well below 20 mg/dL. Outside of one incident of slight bruising at the right atrium attributed to cannula suction, the native heart and lungs were free of gross abnormalities resulting from the device at the time of necropsy.
Surgical and cannulation methods were refined throughout this series of acute studies and led to an optimized implantation strategy to be used during longer-term recovery studies. Changing from a left to right thoracotomy allowed for better placement of the cannula into the main wall of the right atrium. The later trials using this approach (Trial 3 – 6) were free of complications arising from cannula suction within the right atrium. The cannulas used in Trials 5 and 6 enabled even further increased venous drainage and will ease tunneling of the cannulas during future chronic studies. Based on this series of acute studies, the cannulation strategy for upcoming long-term recovery studies will utilize a right thoracotomy to place a 22 Fr right angle venous cannula into the RA and an 18 Fr arterial cannula into the PA.
Although the Avalon DLC has popularized peripheral cannulation in adult ECMO patients, pediatric patients present unique challenges which may benefit from a central cannulation. A central cannulation is often preferred in pediatric patients due to concerns with patient cooperation, availability of appropriate cannula sizes and blood recirculation between the cannula inlet and outlet. Maeda et al. have recently published two cases where long-term pediatric ECMO patients initially cannulated peripherally required recannulation centrally.13
The P-PAL is a compact, integrated pump-lung designed for ambulatory respiratory support in children. Acute in vivo evaluation of the devices confirmed the sufficient gas transfer, pumping and hemolysis performance demonstrated during benchtop studies. Additionally, acute respiratory support with the P-PAL using the final implant strategy showed no adverse effects on animal hemodynamics or the native cardiopulmonary system. Future work includes 7- and 30-day sheep studies to further characterize the performance and hemocompatibility of the P-PAL v2 and its long-term effect on the cardiopulmonary system. A novel zwitterionic sulfobetaine coating14 will also be applied to the circuit in future studies to provide increased hemocompatibility.
Acknowledgments
Acknowledgements
The authors thank the Center for Preclinical Studies staff for their assistance with the in vivo studies. This work was supported by the National Institutes of Health (R01HL135482) and the McGowan Institute for Regenerative Medicine.
Source of Funding: This work was supported by NIH Grant R01HL135482–01. AM was supported by an NIH training grant (T32 HL076124) for the University of Pittsburgh Cardiovascular Bioengineering Training Program.
Footnotes
Conflicts of Interest William J. Federspiel chairs the Scientific Advisory Board and is a founder of ALung Technologies, in which he has an equity interest. No other authors have any conflicts of interest to disclose.
References
- 1.Valapour M, Paulson K, Smith J: OPTN/SRTR 2011 annual data report: lung Am J Transplant 13: 149–177, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Barbaro RP, Paden ML, Guner YS, et al. : Pediatric Extracorporeal Life Support Organization Registry International Report 2016: ASAIO J 63: 456–463, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slutsky A, Ranieri V: Ventilator-induced lung injury N Engl J Med 369: 2126–2136, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Barrett CS, Jaggers JJ, Cook EF, et al. : Pediatric ECMO Outcomes: Comparison of Centrifugal Versus Roller Blood Pumps Using Propensity Score Matching ASAIO J 59: 145–151, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Gulack B, Hirji S, Hartwig M: Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation J Thorac Dis 6: 1070–7079, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehr CJ, Zaas DW, Cheifetz IM, Turner DA: Ambulatory Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation Chest 147: 1213–1218, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Turner D, Rehder K, Bonadonna D, et al. : Ambulatory ECMO as a Bridge to Lung Transplant in a Previously Well Pediatric Patient With ARDS Pediatrics 134, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Rehder KJ, Turner DA, Hartwig MG, et al. : Active Rehabilitation During Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation Respir Care 58: 1291–1298, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orizondo RA, May AG, Madhani SP, et al. : In vitro characterization of the pittsburgh pediatric ambulatory lung device ASAIO J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svitek R, Frankowski B, Federspiel W: Evaluation of a pumping assist lung that uses a rotating fiber bundle ASAIO J 51: 773–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Sanchez P, Wei X, et al. : Effects of Cardiopulmonary Support With a Novel Pediatric Pump-Lung in a 30-Day Ovine Animal Model Artif Organs 39: 989–997, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Gellman B, Zhang T, Taskin M, Dasse K, Griffith B: Computational Fluid Dynamics and Experimental Characterization of the Pediatric Pump-Lung Cardiovasc Eng Technol 2: 276–287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda K, Ryan K, Conrad. CK, Yarlagadda V VV: An alternative cannulation approach for veno-venous extracorporeal membrane oxygenatioin in children for long-term ambulatory support J Thorac Cardiovasc Surg, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Malkin A, Ye S, Lee E, et al. : Development of zwitterionic sulfobetaine block copolymer conjugationstrategies for reduced platelet deposition in respiratory assist devices J Biomed Mater Res B Appl Biomater, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


