Abstract
Background:
We have previously demonstrated that pre-scan salivary cortisol is associated with attentuated frontal-subcortical brain activation during emotion processesing and semantic list-learning paradigms in depressed subjects. Additionally, altered functional connectivity is observed after remission of acute depression symptoms (rMDD). It is unknown whether cortisol also predicts altered functional connectivity during remission.
Methods:
Participants were 47 healthy controls (HC) and 73 rMDD, 18-30 years old who provided salivary cortisol samples before and after undergoing resting-state fMRI. We tested whether salivary cortisol by diagnosis interactions were associated with seed-based resting connectivity of the default mode (DMN) and salience and emotion (SN) networks using whole-brain, cluster-level corrected (p<.01) regression in SPM8.
Results:
Pre-scan cortisol predicted decreased (HC) and increased (rMDD) cross-network connectivity to the dorsal anterior cingulate, dorso-medial and lateral- prefrontal cortex, brain stem and cerebellum (all seeds) and precuneus (DMN seeds). By and large, pre/post-scan cortisol change predicted the same pattern of findings. In network analyses, cortisol predominantly predicted enhanced cross-network connectivity to cognitive control network regions in rMDD.
Conclusions:
The association of cortisol with connections of default and salience networks to executive brain networks differs between individuals with and without a history of depression. Further investigation is needed to better understand the role of cortisol and related stress hormones as a potential primary and interactive driver of network coherence in depression.
Keywords: cortisol, connectivity, depression, remission, fMRI
1. Introduction
The hypothalamic-pituitary-adrenal axis (HPA-axis) is a major part of the neuroendocrine system (Bao et al., 2008; Sapolsky, 2000; Tsigos and Chrousos, 2002) that controls reactions to stress and regulates many psychophysiological responses including mood and emotions (Langenecker et al., 2012; Parker et al., 2003; Peters et al., 2016b), the immune system (Brown et al., 2004; Pariante, 2017), and energy storage and expenditure (Young et al., 1998). Major depressive disorder (MDD) is associated with HPA-axis alterations (Parker et al., 2003). Specifically, depression has been characterized by cortisol hyper-secretion (Parker et al., 2003), reduced glucocorticoid receptor mRNA expression (Hepgul et al., 2013; Pariante and Miller, 2001), and decreased glucocorticoid-induced inhibitory feedback to the HPA-axis (Rush et al., 1996).
MDD is also associated with alterations in resting-state functional magnetic resonance imaging (fMRI) networks (McEwen, 2004; Yehuda and LeDoux, 2007), including the default mode (DMN) and salience and emotion networks (SN). The DMN is involved in internally-driven mental processes, such as mind wandering, drawing on past experiences and envisioning future events (DMN; (Adler et al., 2004; Buckner and Carroll, 2007; Greicius et al., 2003; Gruberger et al., 2011). It includes mainly mid-line structures such as the posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), hippocampal formation, and precuneus, plus the angular gyrus, and lateral temporal cortices (Andrews-Hanna et al., 2014; Buckner et al., 2008). The SN is involved in detecting and filtering behaviorally relevant stimuli and coordinating integration of sensory, emotional, and cognitive information (Menon, 2011). It is primarily composed of the anterior insula, dorsal and subgenual anterior cingulate cortex (dACC; sgACC), amygdala, hypothalamus, thalamus, and striatum (Menon, 2011).
Enhanced cross-network DMN and SN connectivity has been observed to persist after remission from depression (rMDDJacobs et al., 2014; Lois and Wessa, 2016; Peters et al., 2016a)) and is associated with familial risk for depression (Posner et al., 2016). Specifically, increased connectivity of the DMN with the cingulate-frontal operculum system of the SN and the dorso-medial and lateral prefrontal and parietal regions of the cognitive control network (CCN) has been reported in both active and remitted depression (Drevets et al., 2008; Jacobs et al., 2014; Menon, 2011). Likewise, enhanced connectivity of the SN to lateral, parietal, and frontal regions of the CCN (Jacobs et al., 2014) and posterior midline structures of the DMN (Peters et al., 2016a) is present in rMDD.
These foci of MDD network alterations substantially overlap with the neural correlates of cortisol response to stress. For instance, enhanced cortisol response to stress induction is associated with hyperactivion of DMN regions in healthy adults, including the hippocampus, medial prefrontal cortex, and PCC. Cortisol response to stress is also associated with enhanced activation in limbic regions of the salience network (SN), including the amygdala, anterior cingulate cortex, insula, and hypothalamus, extending to the subgenual anterior cingulate (Kaiser et al., 2017; Pruessner et al., 2008; Quaedflieg et al., 2015; Vaisvaser et al., 2013; Wang et al., 2007). Moreover, we have previously illustrated that pre-scan cortisol elevations, relative to post-scan measurement and to a diurnal trend (Peters et al., 2016b; Weldon et al., 2015), are associated with attenuated frontal-subcortical activation during emotion perception (Peters et al., 2016b) and verbal list-learning in depression (Peters et al., 2018; Peters et al., 2016b; Weldon et al., 2015). This suggests that acute cortisol changes in anticipation of fMRI may relate broadly to regulatory brain system alterations in depression.
In line with these findings, other groups have also demonstrated that the psychological anticipation preceding fMRI procedures has been shown to evoke mild arousal of the sympathetic nervous system (Eatough et al., 2009; Lueken et al., 2012; Tessner et al., 2006), including elevated pre-scan cortisol. Elevated cortisol is present before but normalizes after the scan in both depressed (Peters et al., 2011) and healthy (Weldon et al., 2015) individuals. The extent of this arousal has also been shown to relate to neural functioning during emotion processing and decision-making tasks in both healthy (Keulers et al., 2015; Klimes-Dougan et al., 2014) and depressed subjects (Klimes-Dougan et al., 2014; Mareckova et al., 2017; Ming et al., 2017) and during remission from depression (Admon et al., 2015; Holsen et al., 2013; Ming et al., 2017). Hence, measuring and modeling whether pre-scan HPA-axis measures affect functional activation patterns differently between depressed and healthy individuals may yield valuable insights regarding adaptive and maladaptive functioning of the HPA-axis.
Beyond these initial findings using task-based fMRI, few studies have linked cortisol with altered resting-state networks. In healthy subjects, endogenous cortisol is associated with reduced amygdala-mPFC (Veer et al., 2012) and limbic (Kiem et al., 2013) connectivity, whereas cortisol awakening response predicts increased global mPFC connectivity (Wu et al., 2015). In MDD, one recent study demonstrated that morning serum cortisol was associated with reduced connectivity between the orbital-frontal cortex and cerebellum (Wang et al., 2018). To our knowledge, however, no existing studies have examined neural network associations with cortisol in rMDD, even though aberrant cross-network function may increase susceptibility to relapse (Dichter et al., 2015; Mulders et al., 2015). We were specifically interested in the effects of pre-scan cortisol seeing as fMRI scans have been shown to evoke anticipatory cortisol elevations that are present before but normalize after the scan. Thus, we assessed pre-scan cortisol-network associations in rMDD and healthy controls (HC), using resting-state fMRI. We hypothesized that in rMDD, pre-scan salivary cortisol would be associated with hyper-connectivity of the DMN and SN to CCN regions, and that a substantively similar pattern would be observed for pre/post-scan cortisol change.
2. Methods
2.1. Participants
rMDD (n = 73) and HC (n = 47), equivalent in terms of age, sex, and verbal IQ estimate (Table 1), were recruited for one of two NIMH-funded research studies (Research Grants: R01 MH091811, R01 MH101487) designed to assess neural networks of inhibitory control and RDoC models in mood disorders; cortisol samples were obtained for exploratory analyses. Participants were between the ages of 18-30 (M = 22.36, SD = 2.98). Trained M.A.-level clinical interviewers conducted systematic structured clinical interviews (DATA, 1997; Nurnberger et al., 1994) on all participants. rMDD previously met DSM-IV criteria for at least one historical, but not current major depressive episode, with a minimum remission duration of one month prior to enrollment. HCs had no current, past, or family history for MDD or any other psychiatric disorder. Additional exclusionary criteria included psychotherapy in the month prior to enrollment, substance abuse (past month) or dependence (past 6 months), active suicidal plan or serious attempt (past six months), serious medical illnesses or neurological illnesses, and standard contraindications to MRI (weighing >250 pounds, pregnancy, metallic implants, pacemakers, etc.).
Table 1.
Demographic and clinical characteristics of rMDD and HC participants
| Variable | rMDD (n = 73) M (SD) |
HC (n = 47) M (SD) |
t or X2 | p |
|---|---|---|---|---|
| Age | 22.04 (3.09) | 21.61 (2.66) | 0.78 | .43 |
| Female % (n) | 65.7% (48) | 65.9% (31) | <0.01 | .98 |
| Education in years | 15.40 (2.47) | 16.21 (2.91) | 1.63 | .11 |
| Verbal IQ Estimate | 107.70 (9.72) | 107.26 (7.44) | 0.26 | .79 |
| % UIC (n) | 78.1% (57) | 76.6% (36) | 0.04 | .84 |
| Body Mass Indexa | 25.13 (4.41) | 23.98 (2.88) | −1.49 | .14 |
| Pre-scan Cortisol (μg/dl) | 0.41 (0.46) | 0.45 (0.71) | −0.88 | .379 |
| Post-scan Cortisol (μg/dl)b | 0.31 (0.46) | 0.39 (0.56) | 0.88 | .379 |
| HDRS* | 4.03 (5.16) | 0.38 (.77) | 4.81 | <.001 |
| Age of MDE Onset | 15.53 (2.47) | -- | ||
| Current AD % (n) | 17.8% (13) | -- | ||
| Current AD Plusc% (n) | 4.1% (3) | -- |
Group differences at p <.05
Sample n = 107; rMDD n = 64, HC n = 43)
Sample n = 102; rMDD n = 65, HC n = 37
Refers to currently taking an antidepressant and additional psychiatric medications rMDD = remitted major depressive disorder; HC = Healthy Control; IQ = Intelligence Quotient; UIC = University of Illinois at Chicago; HDRS = Hamilton Depression Rating Scale; MDE = Major Depressive Episode; AD = Antidepressant medication
2.2. Procedures
Participants were enrolled at either the University of Michigan (UM) or the University of Illinois at Chicago (UIC) in accordance with respective Institutional Review Board approval. Both sites conducted an identical screening and enrollment protocol, including informed consent, diagnostic and symptom evaluation, salivary cortisol collection, and 3-Tesla neuroimaging procedures. Clinical diagnostic interviewers administered the Hamilton Depression Rating Scale (HDRS) and obtained a verbal IQ estimate (Shipley, 1982). Salivary cortisol and fMRI data were collected at a second visit. Pre-scan salivary cortisol samples were collected 10-15 minutes prior to fMRI, as typically there is a 10-15 minute window of participant preparation related to entering the scanner (metallic screening, participant instructions, placement, alignment). Post-scan salivary cortisol samples were collected immediately after exiting the scanner. During fMRI, participants completed an eyes-open resting-state scan acquired over 8 minutes, which was preceded by affect processing and working memory/executive functioning paradigms.
2.3. Saliva Sample Collection and Cortisol Assay
Saliva samples for cortisol assay were collected using Salivette Cortisol Tubes (Sarstedt AG & Co.) and stored at −80°C until they were processed at Clinical Ligand Assay Service Satellite Laboratory at the UM School of Public Health Department or the UIC Biorepository. Immunoassay was conducted using a Siemens Centaur automated analyzer via chemiluminescent technology. The assay range was 0.012-3.000 ug/dl: no subjects were out of range. The inter- and intra-assay coefficients of variation at 0.7 μg/dl were 12.4 and 3.6%, respectively. Average pre-scan cortisol was 0.43 μg/dl. Average post-scan cortisol was 0.34 μg/dl (post-scan: n = 102 due to qns or values below lower limit of detection). Natural log-transformed values were computed to adjust for right-skewed cortisol values and to allow for comparison to other studies; for descriptive purposes, actual cortisol values are reported. The log-transformed and raw cortisol values were highly correlated (prescan: r = .84, p <.001; post-scan: r = .81, p < .001); histograms indicated the log transformed data approximated a normal distribution.
fMRI scans were administered between 8:00 am and 4:00 pm, with the majority (n = 73, 61%) beginning and ending in the morning. Several strategies were employed to reduce the impact of the cortisol awakening response (Peters et al., 2016b). First, all participants were awake for at least one hour before the saliva collection (typical arrival time is 45 minutes before the scan). Second, the scanner start time was transformed into a 24-hour variable and included in imaging regression analyses as a covariate of non-interest to adjust for circadian variations in cortisol across the day. Third, we ensured that rMDD and HC participants did not differ in average time of scan (for detail see Results, Salivary Cortisol).
2.4. fMRI Acquisition
At UM (n = 11 HC, n = 16 rMDD), scans were collected with a 3.0 T GE Signa scanner (USA) using T2*-weighted single shot reverse spiral sequence with the following parameters: 90 degree flip, field-of-view 20, matrix size = 64 × 64, slice thickness 4mm, 30 ms echo time, 29 slices. At UIC (n = 36 HC, n = 57 rMDD) scans were collected with a 3.0 T GE Discovery Scanner (USA) using parallel imaging with ASSET and T2* gradient-echo axial echo planar imaging (EPI) with the following parameters: 90 degree flip, field-of-view 22, matrix size = 64 × 64, slice thickness = 3 mm, 22.2 ms echo time, 44 slices. Both sites used a repetition time (TR) of 2000 ms, with 240 total TRs collected and interleaved slice acquisition. High-resolution anatomic T1 scans were obtained for spatial normalization at both sites. Motion was minimized with foam pads, by instructing participants to gaze on a visual tracking line (UIC only) and/or crosshair (UIC and UM) on the display, and by conveying the importance of holding still to participants.
2.5. fMRI Preprocessing
Several steps were taken to reduce potential sources of noise and artifact as well as alignment with MNI template for uniform reporting. Slice timing was completed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/doc/, R4667) and motion detection algorithms were applied using FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, version 5.1). Coregistration of structural images to functional images was followed with spatial normalization of the coregistered T1-spgr to the MNI template. The resulting normalization matrix then was applied to the slice-time-corrected, physiologically corrected time series data. These normalized T2 time series data were spatially smoothed with a 5 mm Gaussian kernel resulting in T2 images with isotropic voxels, 2 mm on each side (Skudlarski et al., 1999). Gray matter volume was estimated following segmentation with DARTEL (VBM within SPM8) and application of a 8 mm Gaussian kernel and conversion to 2 mm isotropic voxels. All results were inspected for adverse effects of outliers.
2.6. Cross Correlation Analyses
Time series was de-trended and mean centered. Physiological correction was performed by regressing out the top five principal components of the masked white matter and cerebral spinal fluid signal (Behzadi et al., 2007). Motion parameters were regressed out (Jo et al., 2013) and all participants met strict motion criteria (TR to TR movement <1.5 degrees or 3 consecutive TR exceeding the same in any plane (Jacobs et al., 2014; Peters et al., 2016a)). Global signal was not regressed due to collinearity violations with gray matter signal, problematic misestimates of and introductions of anticorrelations (Fox et al., 2009), and effect on distance-micromovement relationships (Jo et al., 2013). Finally time-series were band-pass filtered over 0.01–0.10 Hz. Regions of Interest (ROIs; 2.9 mm radius, 19 voxels) were defined in MNI space. Seeds were overlaid on the average warped structural anatomy of the current sample to determine accuracy in seed location. Four bilateral seeds pairs were selected based on previous DMN and SN literature: anterior hippocampal formation (antHPF;(Schallmo et al., 2015)), PCC (Alexopoulos et al., 2012; Bluhm et al., 2011), amygdala (McCabe and Mishor, 2011; Pannekoek et al., 2013), and sgACC (Kelly et al., 2008; Margulies et al., 2007). Coordinates were: antHPF (+/−30, −12, −18), PCC (+/−5, −50, 36), amygdala (+/−23 −5 −19), and sgACC (+/−4, 21, −8). Correlation coefficients were calculated between mean time course for seed regions and all other voxels of the brain, resulting in three-dimensional correlation coefficient images (r images), transformed to Z scores using a Fisher transformation.
2.7. Data Analytic Approach
Clinical, demographic, and neuroendocrine measures for HC and rMDD were compared using independent samples t-tests or chi-square tests, as appropriate. A repeated measures ANOVA was used to assess change in cortisol from pre-to-post scan; residual change scores were calculated using linear regression.
For imaging analyses, z-images were used in multivariate linear regression analyses in SPM8 to assess whether the pre-scan cortisol by diagnosis interaction indicated differential cortisol-connectivity patterns in rMDD versus HC from the four bilateral seed regions (8 models). Sex, age, time of scan, HDRS, scan site, and movement parameters (roll, pitch, yaw) were covariates of no interest; main effects of cortisol that were not further qualified by a cortisol by diagnosis interaction are reported in the Supplement. All results surpassed whole-brain false-discovery rate correction of p <.05 by using 3dClustSim (AFNI version 16.2.19, with 1000 Monte Carlo simulations to determine the joint threshold). Our initial results were thresholded for an adjusted FWE with 4 bilateral seed analyses (2 sides by 2 networks by 2 seeds). However, given extensive results we further adjusted the threshold for simplicity in reporting (more conservative), resulting in a combined threshold for each analysis of p < .005 and k > 100. For each of eight regressions, this results in an adjusted p < .0001 for each regression, or a full experiment FWE of p < .008. Spatially averaged data for each contrast and for each participant was extracted with MARSBAR. Posthoc analyses evaluated whether pre-to-post scan cortisol change correlated with the same connectivity clusters.
We then computed tripartite resting-state network (Menon, 2011) scores (CCN, DMN, and SN) for the pre-scan cortisol x diagnostic group interaction. Network scores represent the proportion of cumulative voxels from the interaction for each bilateral seed pair that are spatially encompassed within CCN, DMN, and SN network masks (each lateral seed network score in Supplement). The CCN mask was created by combining the dorsal attention and frontoparietal network masks from an established seven-network parcellation (Yeo et al., 2011). The SN was created by combining ventral attention and limbic networks (Yeo et al., 2011), and the original DMN parcellation was retained (Yeo et al., 2011). Subsequently, for each bilateral seed pair, network scores were compared using chi-squared tests.
3. Results
3.1. Participants
Demographic and clinical characteristics of the sample are reported in Table 1. rMDD and HC participants were equivalent in age, sex, and verbal IQ. rMDD participants had higher scores on the HDRS relative to HCs, however these remained below the clinical cutoff for MDD. Participants enrolled at the UM (n = 27) did not significantly differ in sex distribution, χ2(1, 120) =.77, p =.512, or HAMD score, F(1, 119) = .29, p = .590 from those enrolled at UIC. UM participants were slightly younger (M = 20.59, SD = 22.91) than UIC (M = 22.91, SD = 3.13) participants, F(1, 119) = .12.48, p = .001, verbal IQ estimate was slightly higher in UM (M = 110.89, SD = 8.78) than UIC (M = 106.53, SD = 8.67) participants, F(1, 119) = 4.31, p = .040, and UM participants were of slightly lower educational attainment (M = 14.44, SD = 1.22) than UIC (M = 16.11, SD = 2.88) participants, F(1, 119) = 9.69, p = .002. However, there were no significant site by diagnosis interactions in age, F(2, 118) = .81, p = .369, verbal IQ, F(2, 118) = .49, p = .481, education, F(2, 118) = .71, p = .403, HAMD score, F(2, 118) = ..89, p = .346, or sex, χ2(1, 120) =.92, p =.556, indicating that participant characteristics were adequately stratified across groups.
3.2. Salivary Cortisol
Pre-scan salivary cortisol did not differ (t = −0.88, p = .379) between rMDD (M = 0.41, SD = 0.46 ) and HC participants (M = 0.45, SD = .71). Pre-scan cortisol did not correlate with HDRS scores (r = .02, p = .830). There was also no difference in pre-scan cortisol (t = −.38, p = .701) between females (M = 0.44, SD = 0.60) and males (M = 0.40, SD = 0.52). Pre-scan cortisol levels were inversely correlated with time of day (r = −.50, p < .001). Average scan start time did not differ (t = −0.41, p = .678) between rMDD (M = 11:19 am, SD = 215 minutes) and HC (M = 11:01 am, SD = 247 minutes).
Cortisol levels significantly decreased from pre-to-post scan in all subjects, F(1,100) = 9.38, p = .003. The rate of decrease from pre-to-post scan measurement did not differ between HC and rMDD, F(1,100) = .006, p = .94. Pre-scan cortisol levels were modestly correlated with the pre/post-scan residual change score (r = 0.56, p < .001).
3.3. Cortisol and DMN Connectivity
3.3.1. AntHPF (Table 2).
Table 2.
Foci of connectivity in pre-scan cortisol regression from bilateral anterior hippocampal formation seed
| Left antHPF Seed | Right antHPF Seed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | MNI coordinates | |||||||||||
| Contrast/Lobe | BA | x | y | z | Z | k | BA | x | y | z | Z | k |
| Cortisol by Diagnosis Interaction | ||||||||||||
| Positive Association in rMDD | ||||||||||||
| Frontal | ||||||||||||
| Superior Frontal | 10 | −24 | 56 | 14 | 3.63 | 103^ | 10 | 24 | 58 | 22 | 4.06 | 540 |
| Precentral | 9 | −42 | 8 | 40 | 4.08 | 165 | -- | -- | -- | -- | -- | -- |
| Middle Frontal | 6/9 | −38 | −80 | 24 | 3.71 | 179 | 9/10 | −28 | 54 | 16 | 3.45 | 512 |
| 9/46 | 48 | 22 | 24 | 3.13 | 133^ | 9/46 | 50 | 24 | 34 | 3.91 | 255 | |
| Inferior Frontal | 45/47 | 50 | 36 | 2 | 3.57 | 207 | 45/47 | −52 | 28 | 0 | 3.45 | 127 |
| Parietal | ||||||||||||
| Inferior Parietal Lobule | 40 | −60 | −32 | 30 | 3.25 | 110^ | -- | -- | -- | -- | -- | -- |
| Precuneus | 7 | −8 | −54 | 54 | 4.57 | 1597 | 7 | −10 | −58 | 54 | 3.99 | 220 |
| -- | -- | -- | -- | -- | -- | 7 | 10 | −58 | 58 | 3.56 | 18^ | |
| Temporal | ||||||||||||
| Inferior Temporal | 20 | −58 | −46 | −6 | 3.57 | 315 | -- | -- | -- | -- | -- | -- |
| Occipital | ||||||||||||
| Cuneus | 18 | 14 | −80 | 22 | 3.37 | 152 | -- | -- | -- | -- | -- | -- |
| Lingual Gyrus | 18 | 8 | −88 | −10 | 4.68 | 2288 | -- | -- | -- | -- | -- | -- |
| Subcortical | ||||||||||||
| Cerebellum, Culmen | −30 | −44 | −24 | 4.05 | 342 | -- | -- | -- | -- | -- | ||
| Cerebellum, Declive | -- | -- | -- | -- | -- | −46 | −76 | −16 | 3.23 | 109 | ||
Attenuated to trend effects and no longer reached the statistically significant threshold in sensitivity analyses excluding UM participants
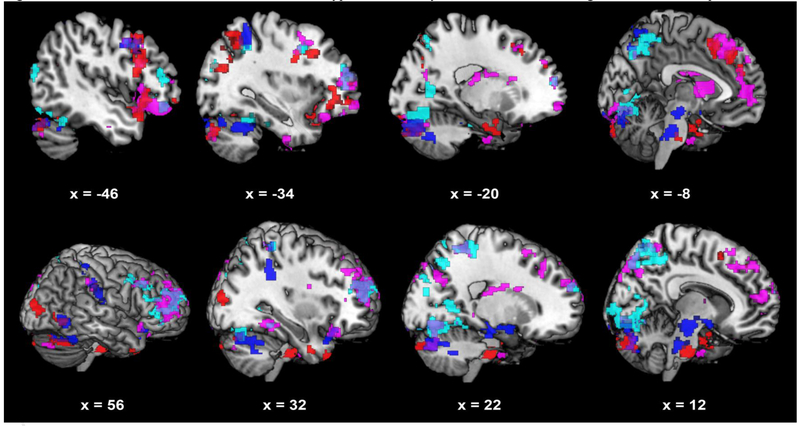
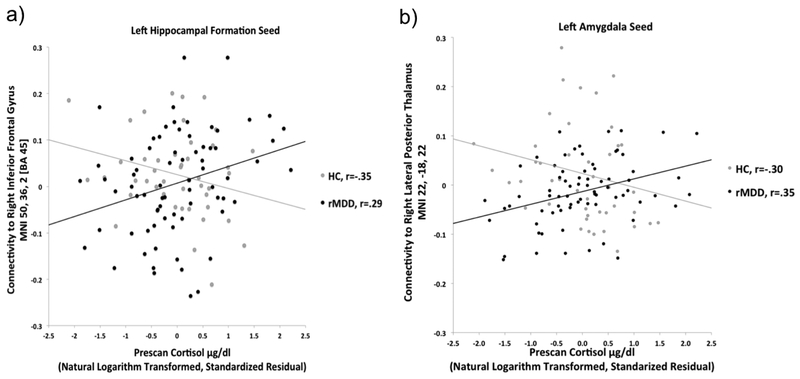
Pre-scan cortisol was associated with increased connectivity in rMDD but decreased connectivity in HC of the antHPF to the lateral and medial PFC, inferior parietal lobule, precuneus/cuneus, and the culmen and declive of the cerebellum (Figure 1, cyan). To illustrate, connectivity of the left antHPF to the right inferior frontal gyrus is plotted as a function of pre-scan cortisol in rMDD and HC separately (Figure 2a). In post-hoc analyses, the pre/post cortisol change by diagnostic group interaction was significant for 8/12 clusters from the left antHPF and 7/7 clusters from the right antHPF.
Figure 1.
Pre-scan cortisol is associated with relative hyper-connectivity of DMN and SN seed regions in rMDD compared to HC
*interactive Effects of Cortisol: Blue = Cortisol positively related to foci of connectivity from bilateral PCC seeds in rMDD but not HC; Cyan = Cortisol positively related to foci of connectivity from bilateral antHPF seeds in rMDD but not HC; Red = Cortisol positively related to foci of connectivity from bilateral sgACC seeds in rMDD but not HC; Violet = Cortisol positively related to foci of connectivity from bilateral amygdala seeds in rMDD but not HC. *In a multivariate analysis of covariance using extracted data for each cluster as dependent variables, all diagnosis x cortisol interactions remained significant after adjusting for BMI, F(19, 84) = 2.52,
p = .013. Additionally, there were no significant main effects of BMI in relation to brain clusters reported for diagnosis x cortisol interactions F(19, 84) = .77, p = .79, nor were there any significant diagnosis x cortisol x BMI interactions F(16, 84) = .79, p = .83.
Figure 2.
a) Differential association of pre-scan cortisol with connectivity of the left antHPF seed in rMDD vs. HC; b) Differential association of pre-scan cortisol with connectivity of the left amgydala seed in rMDD vs. HC
3.3.2. PCC (Table 3).
Table 3.
Foci of connectivity in pre-scan cortisol regression from bilateral posterior cingulate cortex seed
| Left PCC Seed | Right PCC Seed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | MNI coordinates | |||||||||||
| Contrast/Lobe | BA | x | y | z | Z | k | BA | x | y | z | Z | k |
| Cortisol by Diagnosis Interaction | ||||||||||||
| Positive Association in rMDD | ||||||||||||
| Frontal | ||||||||||||
| Inferior Frontal | -- | -- | -- | -- | -- | -- | 44 | −56 | 10 | 18 | 3.8 | 362 |
| Parietal | ||||||||||||
| Inferior Parietal Lobule | 40 | −60 | −34 | 34 | 3.23 | 128 | 40 | −62 | −32 | 32 | 3.39 | 236 |
| 40 | 68 | −22 | 24 | 3.48 | 251 | |||||||
| -- | -- | -- | -- | -- | -- | 40 | 44 | −36 | 56 | 3.43 | 141^ | |
| Precuneus | 7 | −10 | −46 | 54 | 3.43 | 253 | 7 | −10 | −46 | 54 | 3.31 | 102 |
| Temporal | ||||||||||||
| Superior Temporal | -- | -- | -- | -- | -- | -- | 38 | 36 | 16 | −28 | 4.17 | 169 |
| Occipital | ||||||||||||
| Middle Occipital | 37 | 58 | −62 | −4 | 3.64 | 223 | -- | -- | -- | -- | -- | -- |
| Subcortical | ||||||||||||
| Cerebellum, Tuber | −44 | −74 | −26 | 4.02 | 1113 | -- | -- | -- | -- | -- | ||
| Cerebellum, Culmen | 12 | −20 | −18 | 4.54 | 554 | -- | -- | -- | -- | -- | ||
| Cerebellum, Anterior Cerebellar | 42 | −52 | −30 | 4.5 | 275 | −34 | −54 | −28 | 4.25 | 334 | ||
| 44 | −52 | −30 | 4.75 | 1633 | ||||||||
| Cerebellum, Cerebellar Tonsil | -- | -- | -- | -- | -- | −34 | −48 | 50 | 4.28 | 300 | ||
Attenuated to trend effects and no longer reached the statistically significant threshold in sensitivity analyses excluding UM participants
Pre-scan cortisol was associated with increased connectivity in rMDD but decreased connectivity in HC of the PCC to the inferior frontal gyrus, inferior parietal cortex, parietal and occipital regions along the calcarine sulcus, and several clusters in the cerebellum (Figure 1, blue). The pre/post cortisol change by diagnostic group interaction was significant for all clusters from both the left and right PCC.
3.4. Cortisol and SN Connectivity
3.4.1. Amygdala (Table 4).
Table 4.
Foci of connectivity in pre-scan cortisol regression from bilateral amygdala seed
| Left Amygdala Seed | Right Amygdala Seed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | MNI coordinates | |||||||||||
| Contrast/Lobe | BA | x | y | z | Z | k | BA | x | y | z | Z | k |
| Cortisol by Diagnosis Interaction | ||||||||||||
| Positive Association in rMDD | ||||||||||||
| Frontal | ||||||||||||
| Superior Frontal | 10 | −28 | 54 | 14 | 4.29 | 318 | 9/10 | 14 | 62 | 26 | 3.39 | 489 |
| Precentral | -- | -- | -- | -- | -- | -- | 9/10 | −42 | 8 | 42 | 4.45 | 356 |
| -- | -- | -- | -- | -- | -- | 9/10 | −50 | 24 | 26 | 3.37 | 103^ | |
| Middle Frontal | 10 | −38 | 58 | −8 | 3.74 | 108 | 10 | −34 | 48 | 14 | 4.61 | 507 |
| 10 | 38 | 54 | 12 | 3.86 | 958 | 9/10 | 44 | 24 | 38 | 3.33 | 140 | |
| Medial Frontal | -- | -- | -- | -- | -- | -- | 8 | −6 | 32 | 48 | 4.40 | 1254 |
| Inferior | -- | -- | -- | -- | -- | -- | 47 | 44 | 18 | −16 | 3.91 | 230 |
| Parietal | ||||||||||||
| Inferior Parietal Lobule | 40 | 60 | −28 | 34 | 3.65 | 195^ | -- | -- | -- | -- | -- | -- |
| Precuneus | -- | -- | -- | -- | -- | -- | 7 | 14 | −52 | 58 | 3.54 | 167 |
| Temporal | ||||||||||||
| Parahippocampal Gyrus | 30 | 28 | −46 | −12 | 3.42 | 181 | 30/36 | −48 | 34 | −14 | 4.98 | 1732 |
| Inferior Temporal | -- | -- | -- | -- | -- | -- | 20 | −56 | −8 | −28 | 3.64 | 176 |
| Uncus | 36 | −28 | −8 | −40 | 3.60 | 111 | -- | -- | -- | -- | -- | -- |
| Occipital | ||||||||||||
| Precuneus | 11 | −12 | −66 | −26 | 3.15 | 138 | -- | -- | -- | -- | -- | -- |
| Cuneus | 17/18/19 | 6 | −78 | 46 | 4.33 | 661 | 17 | −10 | −82 | −18 | 3.39 | 164 |
| Subcortical | ||||||||||||
| Thalamus, Lateral Posterior | 22 | −18 | 22 | 3.99 | 222 | −16 | −20 | 20 | 3.52 | 150 | ||
| Nucleus/Pulvinar | ||||||||||||
| Caudate | -- | -- | -- | -- | -- | −12 | 10 | 10 | 3.75 | 297 | ||
| Cerebellum, Declive | 0 | −80 | −10 | 3.23 | 272 | -- | -- | -- | -- | -- | ||
| 20 | −76 | −12 | 3.52 | 142^ | -- | -- | -- | -- | -- | |||
| Cerebellum, Tuber | −44 | −82 | −28 | 4.00 | 100 | -- | -- | -- | -- | -- | ||
| Cerebellum, Pyramis | -- | -- | -- | -- | -- | 22 | −78 | −34 | 3.88 | 443 | ||
| Cerebellum, Inferior Semi Lunar Lobule | −26 | −82 | −38 | 4.01 | 305 | |||||||
Attenuated to trend effects and no longer reached the statistically significant threshold in sensitivity analyses excluding UM participants
Pre-scan cortisol was associated with increased connectivity in rMDD but decreased connectivity in HC of the amygdala to the lateral and medial PFC, the inferior parietal lobule, precuneus, parahippocampal gyrus, thalamus, and caudate (Figure 1, violet). To illustrate, connectivity of the left amygdala to the right lateral posterior thalamus is plotted as a function of pre-scan cortisol in rMDD and HC separately (Figure 2b). The pre/post cortisol change by diagnostic group interaction was significant for 9/12 clusters from the left amygdala and 15/15 clusters from the right amygdala.
3.4.2. sgACC (Table 5).
Table 5.
Foci of connectivity in pre-scan cortisol regression from bilateral subgenual anterior cingulate cortex seed
| Left SgACC Seed | Right SgACC Seed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | MNI coordinates | |||||||||||
| Contrast/Lobe | BA | x | y | z | Z | k | BA | x | y | z | Z | k |
| Cortisol by Diagnosis Interaction | ||||||||||||
| Positive Association in rMDD | ||||||||||||
| Frontal | ||||||||||||
| Anterior Cingulate | 32 | −12 | 26 | 42 | 3.61 | 495 | -- | -- | -- | -- | -- | -- |
| Middle Frontal | 6/8/9 | −42 | 20 | 24 | 3.97 | 631 | 6/8/9 | −4 | 34 | 40 | 3.90 | 300^ |
| Inferior Frontal | 47 | −42 | 28 | −6 | 3.60 | 478 | 47 | −44 | 24 | −4 | 3.66 | 334 |
| 45 | −38 | 34 | 6 | 3.50 | 119 | |||||||
| Parietal | ||||||||||||
| Precuneus | 7 | −26 | −74 | 58 | 3.42 | 569 | -- | -- | -- | -- | -- | -- |
| Temporal | ||||||||||||
| Superior Temporal | 38 | 38 | 14 | −36 | 3.66 | 172 | 39 | −56 | −56 | 26 | 3.47 | 150^ |
| Fusiform Gyrus | -- | -- | -- | -- | -- | -- | 37 | 54 | −60 | −8 | 3.66 | 121 |
| Uncus | 34 | −20 | 0 | −22 | 3.51 | 114 | -- | -- | -- | -- | -- | -- |
| Occipital | ||||||||||||
| Middle Occipital | 19 | −58 | −62 | 0 | 3.44 | 277^ | 19 | −58 | −62 | −2 | 3.55 | 218 |
| 19 | 36 | −90 | 10 | 3.40 | 175 | -- | -- | -- | -- | -- | -- | |
| Precuneus | -- | -- | -- | -- | -- | -- | 31 | −30 | −74 | 24 | 3.58 | 181 |
| -- | -- | -- | -- | -- | -- | 31 | 54 | −60 | −32 | 3.71 | 203^ | |
| Subcortical | ||||||||||||
| Anterior Insula | 13 | −46 | 14 | 16 | 3.72 | 319^ | ||||||
| Cerebellum, Tuber | −44 | −74 | −26 | 4.01 | 476 | -- | -- | -- | -- | -- | ||
| Cerebellum, Pyramis | -- | -- | -- | -- | -- | -- | −16 | −82 | −30 | 4.25 | 1055 | |
| Cerebellum, Inferior Semi Lunar Lobule | -- | -- | -- | -- | -- | -- | 12 | −78 | −36 | 3.80 | 302 | |
Attenuated to trend effects and no longer reached the statistically significant threshold in sensitivity analyses excluding UM participants
Pre-scan cortisol was associated with increased connectivity in rMDD but decreased connectivity in HC of the sgACC to the lateral PFC, ACC, temporal gyri, uncus, precueneus, occipital-parietal junction, anterior insula, and several clusters in the cerebellum (Figure 2, red). The pre/post cortisol change by diagnostic group interaction was significant for all clusters from the left sgACC and 11/12 clusters from the right sgACC.
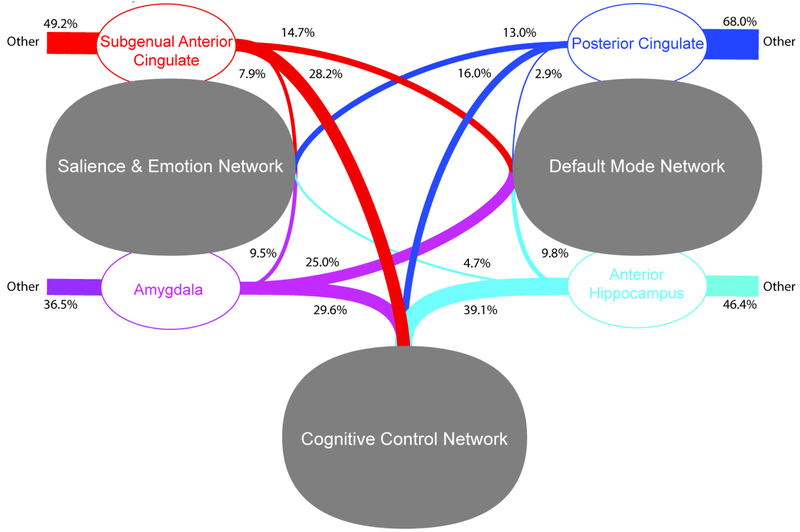
3.5. Voxel-Based Network Belongingness for Foci of Pre-scan Cortisol by Diagnostic Group Interactions (Figure 3).
Figure 3.
Voxel-Based Network Belongingness for Foci of Pre-scan Cortisol by Diagnostic Group Interactions in Seed-Based Connectivity Analyses
For the bilateral amygdala, pre-scan cortisol predicted greater connectivity in rMDD participants to the CCN relative to DMN (χ2=51.08, p<.001) and SN (χ2=1342.02, p<.001), and the DMN relative to SN (χ2=899.76, p<.001). For the bilateral sgACC; pre-scan cortisol predicted greater connectivity in rMDD participants to the CCN relative to DMN (χ2=362.00, p<.001) and SN (χ2=929.43, p<.001), and the DMN relative to SN (χ2=153.11, p<.001). For the bilateral antHPF, cortisol was associated with greater connectivity in rMDD participants to the CCN relative to DMN (χ2=1759.59, p<.001) and SN (χ2=2622.32, p<.001), and the DMN relative to SN (χ2=147.05, p<.001). For the bilateral PCC, cortisol was correlated with greater connectivity in rMDD participants of the CCN relative to the DMN (χ2=661.61, p<.001) and SN (χ2=566.67, p<.001), and the SN relative to the DMN (χ2=460.98, p<.001).
4. Discussion
Pre-scan cortisol is associated with segregation of network connectivity in HC, notably of the dorsal anterior cingulate, dorso-medial and lateral-PFC, brain stem and cerebellum (all seeds) and precuneus (DMN seeds), consistent with prior work (e.g., (Veer et al., 2012)). This study is the first to show inverted effects in rMDD where pre-scan cortisol predicts increased cross-network relationships, particularly from DMN and SN to CCN, consistent with our hypotheses. Because this occurs in a disease-specific manner, cortisol may selectively modify network function in rMDD. Alternatively, the inverted patterns may reflect compensation, or an adaptive process, which facilitates remission in rMDD.
Anatomically, the foci of connectivity associated with cortisol are broad and located throughout the brain in the medial PFC, lateral parietal lobe, visual cortex, cerebellum, medial temporal lobe, and brainstem. As cortisol exerts its influence through mineralocorticoid and glucocorticoid receptors, the latter of which are more ubiquitously located in the brain (de Kloet et al., 2006; Joels and Baram, 2009), cortisol-related alterations in connectivity could be a function of cortisol binding to glucocorticoid receptors in these areas. For instance, both DMN and SN seeds show robust connectivity to the medial PFC, where concentrations of glucocorticoid receptors are particularly high (Diorio et al., 1993). Molecular probes of glucocorticoid receptors, such as in positron emission tomography, should more formally test this hypothesis.
It is provocative that cortisol was associated with opposing effects on cross-network connectivity to the CNN in rMDD versus HC. Additionally, for convergence, the majority of the effects were retained when using pre/post scan change in cortisol as the regresssor. It has been proposed that segregation of networks (higher within-network coherence) may optimize the specialization of brain systems whose regions are distributed anatomically, but are in the service of similar functions (Chan et al., 2014). As cortisol was related to over-integration from the DMN and SN to the CCN in rMDD, speculatively, enhanced connectivity of networks to the CCN may challenge the functional specialization and efficiency of each network. Through this lens, higher cross-talk of the cognitive control with other networks could represent regulation (enhancement for remission) or interference (decreased efficiency as a disease marker) and may pose risk for depression or represent compensatory mechanisms.
There are some patterns of rMDD connectivity according to seed region that warrant additional discussion. First, PCC connectivity was somewhat attenuated to the CCN and enhanced to the SN, compared to other seeds, particularly the antHPF. Perhaps this pattern reflects that cortisol is differentially relevant to ventral and dorsal sub-components of the DMN (Bessette et al., 2018; Buckner et al., 2008). The antHPF seeds may more closely mirror results from the amygdala and sgACC because of density in MR and GR receptors, which are more sparsely distributed in the PCC (Sapolsky, 2000; Tsigos and Chrousos, 2002). Additionally, the PCC and antHPF showed largely bilateral effects, especially to the mid-brain and visual cortex, whereas connectivity of the sgACC and amygdala were somewhat lateralized, more so to the left PFC. Interestingly, the left PFC is a common target for antidepressant transcranial magnetic stimulation (Schutter, 2008) and its efficacy is associated with left dorsolateral PFC and sgACC anti-correlation (Fox et al., 2012). This gives rise to the possibility that anti-depressant effects of non-invasive stimulation could partly work through modulating disrupted neuroendocrine systems.
One limitation of this study is the cortisol awakening response represents a challenge in neuroendocrine assessment (Stalder et al., 2016) and we cannot rule out the influence of time of day on inter-subject variability, given a lack of a true baseline for comparison. Future studies should use fixed circadian time points for cortisol assessment and scanning, or alternatively, utilize the daily slope in cortisol across multiple measurements. Additionally, HPA-axis time scale is on the order of minutes; 10-30 minutes transpire between CRH surges and the cortisol response. Accordingly, cortisol may relate to more stable network relationships than short-term temporal modulations. Further, participants were recruited from two different sites and it was not possible to fully isolate the introduction of potentional effects or variability due to the scanners and site-specific settings. Third, basal cortisol levels and diurnal rhythm were not assessed in this exploratory study, so the exact meaning of the cortisol associations remains for future, more tightly controlled experiments. Specifically, inclusion of true pre/post stress tests and perceived stress measures will be essential. Last, resting state scans were preceded by cognitive and emotional tasks and we cannot rule out possible carryover effects related to the demands of these tasks on brain connectivity or cortisol.
Strengths of this study include a well-powered sample, conservative analytic threshold, co-measurement of salivary cortisol and neural networks, and a young sample, early in the course of illness, that is not confounded by the burden of chronicity and morbidity. This study is the first to demonstrate that the association of cortisol with cross-network connectivity differs in rMDD versus HC. Coupled with prior task-based work (Peters et al., 2016b; Weldon et al., 2015), resting emotional and introspective network connectivity to the CCN may occur at the expense of external task performance, by failing to disengage internal ones. Thus, cortisol may facilitate CCN regulation in rMDD, possibly reflecting compensatory mechanisms of resilience or trait markers of depression. Moreover, that pre-scan cortisol reflects prominent between group differences in connectivity underscores the methodological importance of including cortisol measurement in fMRI studies.
Highlights.
Cortisol predicted tripartite network segregation in healthy subjects
Cortisol predicted enhanced cross-network connectivity to the cognitive control network in remitted depression
Cortisol is implicated as a substrate of brain connectivity abnormalities in remitted depression
Footnotes
Conflict of Interests
None of the authors have conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM, 2004. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord 6, 540–549. [DOI] [PubMed] [Google Scholar]
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA, 2015. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biol Psychiatry 78, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM, 2012. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of affective disorders 139, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN, 2014. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF, 2008. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev 57, 531–553. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette KL, Jenkins LM, Skerrett KA, Gowins JR, DelDonno SR, Zubieta JK, McInnis MG, Jacobs RH, Ajilore O, Langenecker SA, 2018. Reliability, Convergent Validity and Time Invariance of Default Mode Network Deviations in Early Adult Major Depressive Disorder. Front Psychiatry 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA, 2011. Default network connectivity during a working memory task. Human brain mapping 32, 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Varghese FP, McEwen BS, 2004. Association of depression with medical illness: Does cortisol play a role? Biol Psychiatry 55, 1–9. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC, 2007. Self-projection and the brain. Trends Cogn Sci 11, 49–57. [DOI] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS, 2014. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A 111, E4997–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATA D, 1997. Structured clinical interview for DSM-IV axis I disorders. American Psychiatric Press, Washington. [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG, 2006. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40, 550–567. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, Smoski MJ, 2015. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord 172, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ, 1993. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13, 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML, 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213, 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD, 2009. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology 34, 1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A, 2012. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 72, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME, 2009. The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology 101, 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V, 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruberger M, Ben-Simon E, Levkovitz Y, Zangen A, Hendler T, 2011. Towards a neuroscience of mind-wandering. Front Hum Neurosci 5, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepgul N, Cattaneo A, Zunszain PA, Pariante CM, 2013. Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM, 2013. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience 250, 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Jenkins LM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, Verges A, Baker AM, Peters AT, Crane NA, Gotlib IH, Zubieta JK, Phan KL, Langenecker SA, Welsh RC, 2014. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One 9, e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS, 2013. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. Journal of applied mathematics 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Baram TZ, 2009. The neuro-symphony of stress. Nat Rev Neurosci 10, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, Olson DP, Teicher MH, Pizzagalli DA, 2017. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol Med, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP, 2008. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral cortex 19, 640–657. [DOI] [PubMed] [Google Scholar]
- Keulers EH, Stiers P, Nicolson NA, Jolles J, 2015. The association between cortisol and the BOLD response in male adolescents undergoing fMRI. Brain Res 1598, 1–11. [DOI] [PubMed] [Google Scholar]
- Kiem SA, Andrade KC, Spoormaker VI, Holsboer F, Czisch M, Samann PG, 2013. Resting state functional MRI connectivity predicts hypothalamus-pituitary-axis status in healthy males. Psychoneuroendocrinology 38, 1338–1348. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Eberly LE, Westlund Schreiner M, Kurkiewicz P, Houri A, Schlesinger A, Thomas KM, Mueller BA, Lim KO, Cullen KR, 2014. Multilevel assessment of the neurobiological threat system in depressed adolescents: interplay between the limbic system and hypothalamic-pituitary-adrenal axis. Dev Psychopathol 26, 1321–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Weisenbach SL, Giordani B, Briceno EM, Guidotti Breting LM, Schallmo MP, Leon HM, Noll DC, Zubieta JK, Schteingart DE, Starkman MN, 2012. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology 62, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois G, Wessa M, 2016. Differential association of default mode network connectivity and rumination in healthy individuals and remitted MDD patients. Soc Cogn Affect Neurosci 11, 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Muehlhan M, Evens R, Wittchen HU, Kirschbaum C, 2012. Within and between session changes in subjective and neuroendocrine stress parameters during magnetic resonance imaging: A controlled scanner training study. Psychoneuroendocrinology 37, 1299–1308. [DOI] [PubMed] [Google Scholar]
- Mareckova K, Holsen L, Admon R, Whitfield-Gabrieli S, Seidman LJ, Buka SL, Klibanski A, Goldstein JM, 2017. Neural - hormonal responses to negative affective stimuli: Impact of dysphoric mood and sex. J Affect Disord 222, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP, 2007. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37, 579–588. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, 2011. Antidepressant medications reduce subcortical–cortical resting-state functional connectivity in healthy volunteers. Neuroimage 57, 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 1032, 1–7. [DOI] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Ming Q, Zhong X, Zhang X, Pu W, Dong D, Jiang Y, Gao Y, Wang X, Detre JA, Yao S, Rao H, 2017. State-Independent and Dependent Neural Responses to Psychosocial Stress in Current and Remitted Depression. Am J Psychiatry 174, 971–979. [DOI] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I, 2015. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev 56, 330–344. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T, 1994. Diagnostic interview for genetic studies: rationale, unique features, and training. Archives of general psychiatry 51, 849–859. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol M-J, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ, 2013. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. Journal of affective disorders 145, 29–35. [DOI] [PubMed] [Google Scholar]
- Pariante CM, 2017. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharmacol 27, 554–559. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH, 2001. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49, 391–404. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM, 2003. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43, 60–66. [DOI] [PubMed] [Google Scholar]
- Peters AT, Burkhouse K, Feldhaus CC, Langenecker SA, Jacobs RH, 2016a. Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. J Affect Disord 200, 178–181. [DOI] [PubMed] [Google Scholar]
- Peters AT, Smith RA, Kassel MT, Hagan M, Maki P, Van Meter A, Briceno EM, Ryan KA, Weldon AL, Weisenbach SL, Starkman MN, Langenecker SA, 2018. A pilot investigation of differential neuroendocrine associations with fronto-limbic activation during semantically-cued list learning in mood disorders. J Affect Disord 239, 180–191. [DOI] [PubMed] [Google Scholar]
- Peters AT, Van Meter A, Pruitt PJ, Briceno EM, Ryan KA, Hagan M, Weldon AL, Kassel MT, Vederman A, Zubieta JK, McInnis M, Weisenbach SL, Langenecker SA, 2016b. Acute cortisol reactivity attenuates engagement of frontoparietal and striatal regions during emotion processing in negative mood disorders. Psychoneuroendocrinology 73, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Cleare AJ, Papadopoulos A, Fu CH, 2011. Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoneuroendocrinology 36, 737–741. [DOI] [PubMed] [Google Scholar]
- Posner J, Cha J, Wang Z, Talati A, Warner V, Gerber A, Peterson BS, Weissman M, 2016. Increased Default Mode Network Connectivity in Individuals at High Familial Risk for Depression. Neuropsychopharmacology 41, 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S, 2008. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 63, 234–240. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CW, van de Ven V, Meyer T, Siep N, Merckelbach H, Smeets T, 2015. Temporal dynamics of stress-induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PLoS One 10, e0124141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR Jr., Weissenburger JE, Crowley GT, Khatami M, Vasavada N, 1996. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry 57, 470–484. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, 2000. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57, 925–935. [DOI] [PubMed] [Google Scholar]
- Schallmo M-P, Kassel MT, Weisenbach SL, Walker SJ, Guidotti-Breting LM, Rao JA, Hazlett KE, Considine CM, Sethi G, Vats N, 2015. A new semantic list learning task to probe functioning of the Papez circuit. Journal of clinical and experimental neuropsychology 37, 816–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJLG, 2008. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological Medicine 39, 65–75. [DOI] [PubMed] [Google Scholar]
- Shipley WC, 1982. Shipley Institute of Living Scale: For Measuring Intellectual Impairment. Western Psychological Services. [Google Scholar]
- Skudlarski P, Constable RT, Gore JC, 1999. ROC analysis of statistical methods used in functional MRI: individual subjects. Neuroimage 9, 311–329. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wust S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A, 2016. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S, 2006. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp 27, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP, 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53, 865–871. [DOI] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, Fruchter E, Wald I, Pine DS, Tarrasch R, Bar-Haim Y, Hendler T, 2013. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci 7, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA, 2012. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology 37, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA, 2007. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci 2, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen G, Zhong S, Jia Y, Xia L, Lai S, Zhao L, Huang L, Liu T, 2018. Association between resting-state brain functional connectivity and cortisol levels in unmedicated major depressive disorder. J Psychiatr Res 105, 55–62. [DOI] [PubMed] [Google Scholar]
- Weldon AL, Hagan M, Van Meter A, Jacobs RH, Kassel MT, Hazlett KE, Haase BD, Vederman AC, Avery E, Briceno EM, Welsh RC, Zubieta JK, Weisenbach SL, Langenecker SA, 2015. Stress Response to the Functional Magnetic Resonance Imaging Environment in Healthy Adults Relates to the Degree of Limbic Reactivity during Emotion Processing. Neuropsychobiology 71, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang S, Li W, Qin S, He Y, Yang Z, Buchanan TW, Liu C, Zhang K, 2015. Cortisol awakening response predicts intrinsic functional connectivity of the medial prefrontal cortex in the afternoon of the same day. Neuroimage 122, 158–165. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J, 2007. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56, 19–32. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Lopez JF, Weinberg VM, Watson SJ, Akil H, 1998. The role of mineralocorticoid receptors in Hypothalamic-pituitary-adrenal axis regulation in humans. Journal of Clinical Endocrinology and Metabolism 83, 3339–3345. [DOI] [PubMed] [Google Scholar]