Abstract
Phacomatosis Pigmentovascularis (PPV) comprises a family of rare conditions that feature vascular abnormalities and melanocytic lesions that can be solely cutaneous or multisystem in nature. Recently published work has demonstrated that both vascular and melanocytic abnormalities in PPV of the cesioflammea and cesiomarmorata subtypes can result from identical somatic mosaic activating mutations in the genes GNAQ and GNA11.
Here we present three new cases of PPV with features of the cesioflammea and/or cesiomarmorata subtypes and mosaic mutations in GNAQ or GNA11. In order to better understand the risk of potentially occult complications faced by such patients we additionally reviewed 176 cases published in the literature. We report the frequency of clinical findings, their patterns of co-occurrence as well as published recommendations for surveillance after diagnosis. Features assessed include: capillary malformation; dermal and ocular melanocytosis; glaucoma; limb asymmetry; venous malformations; and central nervous system (CNS) anomalies such as ventriculomegaly and calcifications. We found that ocular findings are common in patients with phacomatosis cesioflammea and cesiomarmorata. Facial vascular involvement correlates with a higher risk of seizures (p=0.0066).
Our genetic results confirm the role of mosaic somatic mutations in GNAQ and GNA11 in phacomatosis cesioflammea and cesiomarmorata. Their clinical and molecular findings place these conditions on a clinical spectrum encompassing other GNAQ and GNA11 related disorders and inform recommendations for their management.
Keywords: Phacomatosis pigmentovascularis, GNAQ, GNA11, management, phacomatosis cesioflammea, phacomatosis cesiomarmorata
INTRODUCTION
Phacomatosis pigmentovascularis (PPV, ORPHA:2875) comprises a family of disorders featuring the co-presentation of vascular abnormalities and melanocytic lesions. Clinical findings may be solely cutaneous or multisystem in nature. Happle proposed that PPV can be phenotypically grouped into one of three categories on the basis of cutaneous findings: cesioflammea (blue spots and nevus flammeus), spilorosea (nevus spilus with a pale-pink telangiectatic nevus) and cesiomarmorata (blue spots and cutis marmorata telangiectatica congenita) (Happle, 2005).
Recently published work demonstrated that phacomatosis cesioflammea and phacomatosis cesiomarmorata can both result from identical, mosaic gain of function mutations in the guanine nucleotide-binding protein, Q polypeptide (GNAQ; OMIM [Online Mendelian Inheritance in Man] 600998) gene (p.Arg183Gln) and the highly homologous guanine nucleotide-binding protein, alpha-11 (GNA11; OMIM 139313) gene (p.Arg183Cys, p.Arg183Ser) (Thomas et al., 2016). The genetic basis of spilorosea has not yet been determined. While the mechanism by which a specific mutation in GNAQ and GNA11 can result in a range of phenotypes is incompletely understood, it appears to depend on when and in what cell lineage a somatic mutation arises (Thomas et al., 2016; Zhou, Han, Mao, & Chen, 2016a). The range of cutaneous and extracutaneous manifestations in individuals with PPV has been documented in the literature and reviewed by Fernandez-Guarino et al. 2008. However, the relative frequency of extracutaneous manifestations and patterns of their co-occurrence has not been systematically assessed. Such patterns of co-occurrence can potentially inform the screening of patients for complications of the condition. Similarly, while a variety of recommendations for surveillance after diagnosis have been published they have not been presented in summary form.
We present detailed clinical findings for three individuals with PPV of the cesioflammea and/or cesiomarmorata subtypes with somatic mutations in either GNAQ or GNA11. We also present a systematic literature review that documents the clinical spectrum of these disorders, explores relationships between readily observable findings and potentially occult complications and informs recommendations for management.
RESULTS
Case Summaries
Patient 1
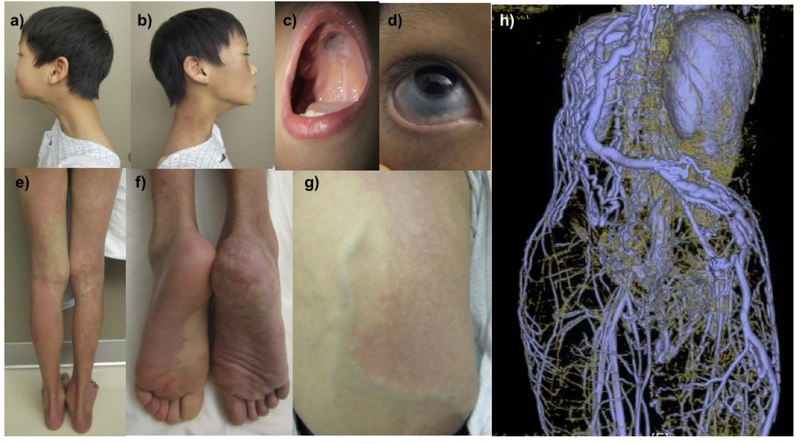
This is an 11-year-old boy who was born at term after an uneventful pregnancy and delivery. At birth, he was noted to have diffuse blanching vascular patches involving his right face, neck, shoulder, arm, flank and leg as well as his left foot (Figure 1). Dermal melanocytosis was noted in a similar, but non-identical distribution including the sclera of the right eye, right central palate, right ear, neck, chest, abdomen, arm, leg and left upper arm (Figures 1b–d). He had overgrowth of his right leg and prominent superficial collateral veins of his back and right thigh during childhood (Figures 1e–g). The findings were consistent with phacomatosis cesioflammea. MR body imaging demonstrated the absence of multiple deep veins including the infra-renal vena cava, bilateral iliac veins and the right axillary vein (Figure 1h).
Figure 1:
An 11-year-old boy with phacomatosis cesioflammea, characterized by dermal melanocytosis and nevus flammeus. Capillary malformation on his right face and neck (a,b). Hyperpigmentation noted on his central palate (c) and sclera of his right eye (d). Additional capillary malformations on his right flank, legs and feet (e-g). MR imaging demonstrated absence of multiple deep veins including bilateral iliacs, infrarenal vena cava and right axillary vein (h).
He also experienced abnormal jerking movements of his trunk, shoulder and neck as well as dysarthric and spasmodic speech that likely represented myoclonus. MRI with and without contrast including sequences T1, T2 and FLAIR was notable for left dominant transverse dural venous sinus and jugular bulb. He had an EEG, which was negative for epileptiform discharges. His cognitive development and linear growth have been typical for age. He does not take any medications. Ophthalmological evaluation revealed right-sided ocular melanocytosis and normal intraocular pressure. There is no report of similarly affected individuals within his family. He is of Vietnamese descent and there was no report of consanguinity.
Patient 2
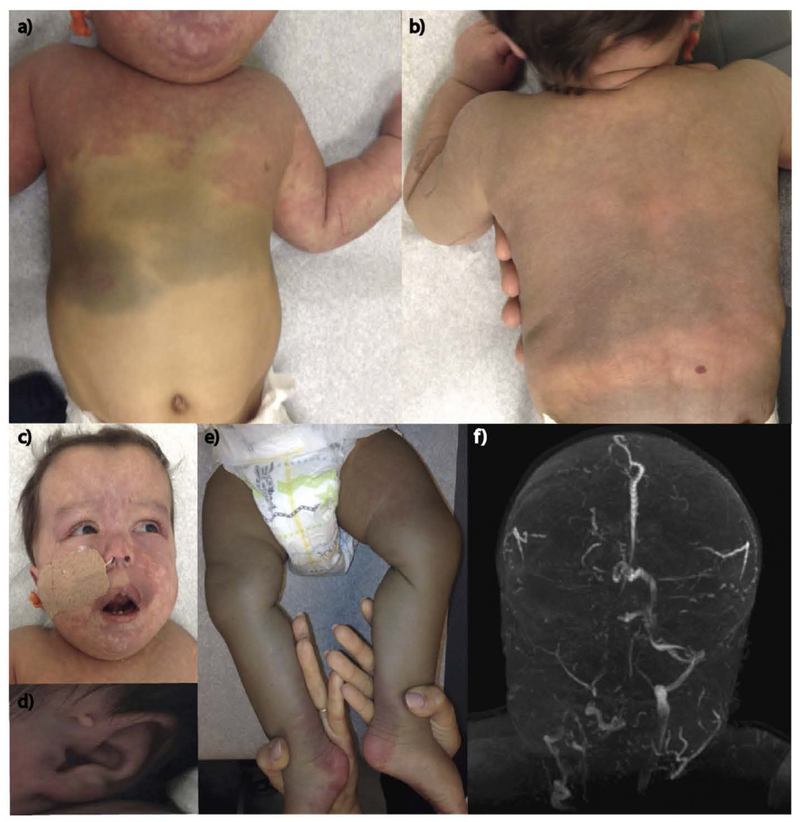
This is a 1-year-old boy delivered by Cesarean-section at 35 weeks gestation. The pregnancy was complicated by maternal diabetes requiring admission for glucose control and premature rupture of membranes. Fetal ultrasound showed hydrops with polyhydramnios, hepatic venous malformation of the lateral left lobe, and question of kidney and brain calcifications. At birth, diffuse edema required bilateral abdominal paracentesis and mechanical ventilation. Exam showed blanching vascular patches on the face, upper torso, arms and feet and dermal melanocytosis on the abdomen, back, and legs (Figure 2a–e). The findings were consistent with phacomatosis cesioflammea. The head circumference was initially normal (at birth 31 cm) and increased in size to macrocephalic (+3 standard deviations) by 9 months of age. He also had a prominent forehead, upturned nasal tip, tented upper lip, and a pre-auricular ear tag. Further assessment revealed bilateral ocular melanocytosis, corneal clouding and edema with increased intraocular pressure. Seizures occurred at 4 months of age. He had several EEGs with several abnormal findings. In particular, they showed disorganized, low amplitude, moderately slow background which is a non-specific indicator of diffuse encephalopathy. Abdominal ultrasound showed porto-systemic shunting and right renal calcifications. Brain and neck MRI/MRA/MRV with and without contrast showed unusual veins with diminutive sagittal, sigmoid and transverse sinuses, poor visualization of the right jugular vein and prominent lateral direct vein (Figure 2f). MRIs showed decreased cerebral volume. There was no report of similarly affected individuals within his family. He is of Mexican descent and there was no report of consanguinity.
Figure 2:
A 1-year-old male with phacomatosis cesioflammea, characterized by dermal melanocytosis and capillary malformation/nevus flammeus. Capillary vascular changes on the upper torso (a), face (c), and extremities (a, e). Hypermelanosis on the abdomen (a), back (b), and legs (e). Facial differences include prominent forehead, upturned nasal tip, tented upper lip (c), and a pre-auricular ear tag (d). MR imaging showed unusual veins with marked asymmetry, diminutive sagittal, sigmoid, and transverse sinuses. The right jugular vein was not seen (f).
Patient 3
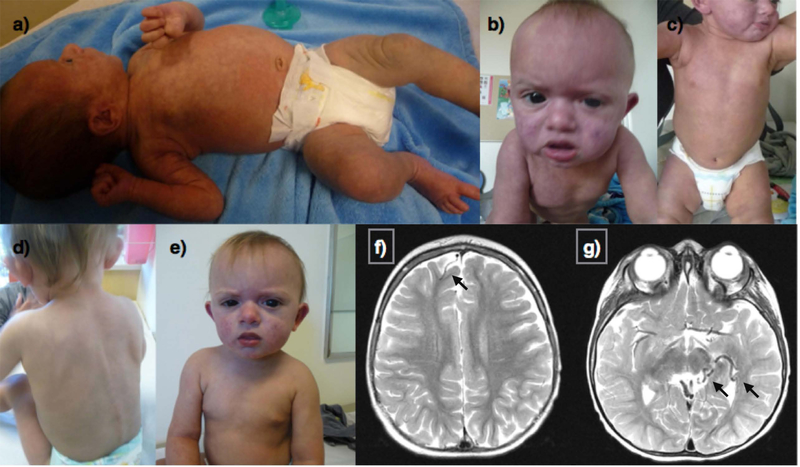
This is a 17-month-old boy born at term after an uneventful pregnancy and delivery. He was noted after birth to have diffuse reticulate vascular patches over multiple areas of his body with scattered dermal melanocytosis (primarily over the abdomen, back and upper thighs; Figure 3a–e) and ocular melanocytosis. He also had mild coronal hypospadias and chordae. He was evaluated by ophthalmology and found to have high myopia and bilateral glaucoma. An MRI without contrast and MRA of the brain was performed at 2 months of age and was reported as unremarkable. His cutaneous findings were considered to be most consistent with a clinical diagnosis of PPV with features overlapping with both cesioflammea and cesiomarmorata subtypes. At that time he was also noted to have mild global developmental delays. At 17 months his head circumference was in the 88th percentile and capillary malformations were less noticeable after several rounds of pulsed dye laser treatments. An MRI of the brain without contrast was repeated at 19 months of age and showed no obvious cortical malformations but a right frontal developmental venous anomaly and a prominent vessel in the left temporal region thought to also be a developmental venous anomaly were seen on FLAIR sequences, as well as diffuse abnormal marrow T1 and T2 signal in the visualized bones. Maternal ancestry is Hispanic and paternal ancestry is unknown. Consanguinity was denied.
Figure 3:
A 17 month old male with phacomatosis pigmentovascularis with features of cesioflammea and cesiomarmorata types. Cutis marmorata and diffuse capillary malformation with overlapping areas of dermal melanocytosis at initial evaluation at 2 weeks of age (a). Capillary malformation involving much of the face, torso and thighs with prominent melanocytosis of the upper thighs at 12 months of age (b, c) Faded appearance of capillary malformations after several rounds of pulsed dye laser treatments. Axial T2 weighted MRI of the brain showing a right front developmental venous anomaly (DVA) (f) as well as a likely DVA in the left temporal region and quadrigeminal cistern (g).
Genomic Analyses
Trio whole exome sequencing (WES) was performed on blood as part of the clinical evaluation of Patients 1 and 2. No pathogenic de novo events were detected by WES or SNP-microarray. No pathogenic or likely pathogenic variants were detected for either patient during analysis of trio WES data.
Subsequently, deep WES was performed on DNA directly extracted from a punch biopsy of affected skin of Patient 1, which revealed low-level mosaicism (5.7%) for a pathogenic GNAQ mutation, c.548G>A, p.Arg183Gln (Table 1; Supp. Table 1). Sanger sequencing after enrichment of the mutant sequence by restriction digest confirmed the result. The mutation was not detected in his mother’s or father’s blood samples, consistent with a somatic de novo event (Table 1). Given suspicion for a mosaic condition, Patient 2 also had DNA extracted directly from a punch biopsy of affected skin, and deep sequencing using a clinical gene panel of 479 cancer genes was performed through the University of California San Francisco. Patient 2 also harbored the c.548G>A (p.Arg183Gln) mutation in GNAQ, which was present with low-level mosaicism in DNA from skin (5%) and from buccal swab (3%) but not detectable in blood (Table 1). The GNAQ mutation identified in Patients 1 and 2 is a recurrent variant reported in phacomatosis cesioflammea (Thomas et al., 2016).
Table 1:
GNAQ and GNA11 Mutations identified in Patients 1–3
| Gene | Patient 1. GNAQ | Patient 2. GNAQ | Patient 3. GNA11 |
|---|---|---|---|
| Chromosome position | 9:80412493 | 9:80412493 | 19:3115012 |
| HGVS DNA Reference | NM_002072: c.548G>A | NM_002072: c.548G>A | NM_002067.2: c.547C>T |
| HGVS Protein Reference | p.Arg183Gln | p.Arg183Gln | p.Arg183Cys |
| Mutation Type | Single nucleotide alteration | Single nucleotide alteration | Single nucleotide alteration |
| Predicted Effect | missense | missense | missense |
| dbSNP/dbVar ID | rs397514698 | rs397514698 | NA |
| Genotype | Heterozygous, mosaic | Heterozygous, mosaic | Heterozygous, mosaic |
| ClinVar ID | 50853 | 50853 | NA |
| Number of reads (alternate:reference) | 12:199 skin | 62:1175 skin 8:260 buccal |
78:1713 skin |
| Clinical Significance | Pathogenic | Pathogenic | Pathogenic |
| Parent of Origin | de novo | de novo | de novo |
| Patient Blood Sample | Not detected | Not detected | Not detected |
| gnomAD Allele Frequency | Absent | Absent | Absent |
For Patient 3 skin biopsy and blood samples were obtained, DNA was extracted and molecular testing performed using a clinical Nevus Gene Panel (genes sequenced: FGFR3, GNA11, GNAQ, HRAS, KRAS, MAP3K3, NRAS, PIK3CA, and TEK) at Washington University Genomics and Pathology Services. Molecular testing results showed a non-synonymous pathogenic GNA11 c.547C>T, p.Arg183Gln variant from the skin biopsy at low allelic fraction (4.4%) consistent with somatic origin. Sanger sequence analysis of the peripheral blood was negative for the GNA11 variant (Thomas et al., 2016).
Literature Review
Phenotypic spectrum and frequency of extracutaneous findings
We performed a literature review to define the spectrum and frequency of extracutaneous manifestations associated with phacomatosis pigmentovascularis cesioflammea and cesiomarmorata types and how they relate to published recommendations for screening and surveillance. We conducted a systematic search of PubMed for case reports and case series from 1982 – 2017. Articles were identified by search terms “phacomatosis OR phakomatosis AND pigmentovascularis.” A total of 151 articles were screened, with 112 articles identified to have at least one case of PPV and 105 articles were ultimately assessed. As we reviewed this literature, we noted that 22 articles included recommendations for management.
We classified cases based on the appearance of corresponding pigmentary and vascular lesions in a similar fashion as Thomas et al with the exception of including a more recently described subtype melanorosea (Supp. Table 2). Of 176 cases, we classified 128 as the phacomatosis cesioflammea subtype using Happle’s criteria given dermal melanocytosis and nevus flammeus/capillary malformation. 13 were classified as having phacomatosis cesiomarmorata, seven were classified as having overlapping features of cesiomarmorata and cesioflammea (capillary malformation/nevus flammeus), ten individuals were classified as phacomatosis spilorosea (Happle, 2005) and two individuals were classified as having phacomatosis melanorosea (Tekin, 2016; Supp. Table 3). Sixteen individuals were not readily classifiable using Happle’s criteria. This was due to atypical pigmentary findings or other combinations of overlapping features. Extracutaneous findings in each of our classifications were tabulated, focusing on neurological, ophthalmologic, and vascular manifestations. Only individuals who had a relevant clinical evaluation (e.g. eye exam or MRI) were included in the summary statistic for the frequency of each finding.
A comparison of extracutaneous features from each subtype of phacomatosis can be found in Table 2. Across all subjects with the cesioflammea, cesiomarmorata or the cesioflammea-cesiomarmorata overlap subtype, recurrent extracutaneous manifestations were: ocular melanocytosis (85/131, 65%), glaucoma (42/121, 35%), hemihypertrophy (42/69, 61%), seizures (26/121, 21%), and ocular melanoma (14/95, 15%). Of those individuals with brain imaging (MRI: 33 individuals, CT scan: 21 individuals, both: 6 individuals) we tabulated the types of brain abnormalities seen. Anomalies on brain imaging were seen in 22/60 individuals with brain imaging (with the most common anomalies including brain calcifications (11 individuals, 18%), ventriculomegaly (10 individuals, 17%), and brain atrophy (10 individuals, 17%).
Table 2:
Frequency of extracutaneous manifestations in 176 previously reported cases of phacomatosis pigmentovascularis according to subtype.
| Subtype of disease | Phacomatosis Cesioflammea | Phacomatosis Cesiomarmorata +/− Cesioflammea overlap | Phacomatosis spilorosea | Phacomatosis Melanorosea | Unclassifiable | Total |
|---|---|---|---|---|---|---|
| Ocular melanocytosis | 78/115 (68%) | 7/16 (44%) | 0/2 (0%) | 0/1 (0%) | 5/15 (33%) | 90/149 (60%) |
| Glaucoma | 38/105 (36%) | 4/16 (25%) | 1/7 (14%) | 0/1(0%) | 1/12 (8%) | 44/141 (31%) |
| Seizures | 26/109 (24%) | 0/12 (0%) | 1/6 (17%) | 0/2 (0%) | 1/16 (6%) | 28/145 (19%) |
| Brain Abnormalities* | 19/51 (37%) | 3/9 (33%) | 2/6(33%) | 0/1 (0%) | ¼ (25%) | 25/71 (35%) |
| Choroidal melanoma | 14/80 (18%) | 0/15 (0%) | 0/5 (0%) | 0/1 (0%) | 0/9 (0%) | 14/110 (13%) |
| Leg length discrepancy | 18/54 (33%) | 2/16 (13%) | 5/6 (83%) | 0/1 (0%) | 3/8 (38%) | 28/85 (33%) |
| Limb Hypertrophy | 36/52 (69%) | 6/17 (35%) | 2/5 (40%) | NR | 3/9 (33%) | 47/83 (57%) |
NR: not reported;
These include ventriculomegaly, brain calcifications and atrophy seen on MRI or CT of the brain.
As they constitute all of the cases in the present series and the large majority of cases in the literature and share a molecular etiology, the remainder of our analyses focused on findings in subjects with the cesioflammea, cesiomarmorata or the cesioflammea-cesiomarmorata overlap subtypes. We investigated the co-occurrence of features in these conditions to determine if potentially occult complications were associated with specific observable findings (Table 3). Consistent with the literature on Sturge-Weber Syndrome/Port Wine Stain, individuals with phacomatosis cesioflammea, cesiomarmorata or the overlap of these subtypes with facial vascular involvement were more likely to have neurologic abnormalities including seizures (p=0.0066). Ocular melanosis was seen in all 14 individuals with ocular melanoma(Krema, Simpson, & McGowan, 2013; Shields, Di Nicola, Pellegrini, & Shields, 2017; Shields et al., 2011; Tran & Zografos, 2005; Zhou et al., 2016a), although in four individuals, ocular melanosis was not identifiable on external examination and required a dilated eye examination. (Shields et al., 2017; Shields et al., 2011). There was the suggestion of an association between limb asymmetry and venous malformations in individuals with vascular imaging (p=0.08).
Table 3:
Co-occurrence of observable findings and complications of PPV cesioflammea, cesiomarmorata or cesioflammea-cesiomarmorata overlap subtypes.
| Facial Vascular Involvement | |||
|---|---|---|---|
| Present | Absent | p-value |
|
| Seizures | 22/72 (31%) | 2/32 (6%) | 0.0066* |
| Developmental Delay | 8/54 (15%) | 2/24 (8%) | 0.43 |
| Glaucoma | 29/80 (36%) | 2/22 (9%) | 0.23 |
| Melanoma | 9/72 (13%) | 5/22 (23%) | 0.40 |
| Ocular Melanosis | |||
| Present | Absent | ||
| Ocular melanoma | 14/69 (20%) | NR | NA |
| Limb Asymmetry/Hypertrophy | |||
| Present | Absent | ||
| Venous malformations | 10/12 (83%) | 1/3 (33%) | 0.08 |
NR: Not reported;
Significant, by Z-test. References for this table are provided in supplementary table 3.
In order to put forward best practices for management, we identified the articles in our literature review that included recommendations for management of phacomatosis cesioflammea and cesiomarmorata. Twenty two articles were identified with recommendations. (Ajith, Narang, & Kanwar, 2006; Byrom, Surjana, Yoong, & Zappala, 2015; Carvalho, Batista, Bornemann, Acioly, & Tatagiba, 2011; Castori et al., 2011; Chiu, Chen, Wu, Ke, & Cheng, 2009; Finklea, Mohr, Warthan, Darrow, & Williams, 2010; Fischer & Trautinger, 2015; Gilliam, Ragge, Perez, & Bolognia, 1993; Goyal & Varshney, 2010; Hall, Cadle, Morrill-Cornelius, & Bay, 2007; Henry, Hodapp, Hess, Blieden, & Berrocal, 2013; Jun, Kim, Cho, Lee, & Kim, 2015; Nanda et al., 2016; Onsun, Inandirici, Kural, Teker, & Atilganoglu, 2007; Shields et al., 2017; Shields et al., 2011; Smith, Moore, & Stetson, 2012; Toelle, Weibel, Schiegl, & Boltshauser, 2011; Wobser, Goebeler, & Hamm, 2013; Yang et al., 2015; Zhou et al., 2016a). Recommendations varied substantially between reports and no specific recommendation was included in the majority of the articles reviewed. Authors’ recommendations included a detailed physical examination including careful examination of the face and skin and referral to specialists including neurology and ophthalmology as needed given high frequency of issues involving these organ systems. Ophthalmology examination was recommended for all individuals (independent of cutaneous and scleral findings) by some and only for individuals with conjunctival or other eye pigmentation by others. MRI with or without vascular imaging was recommended for all individuals by some and as needed based on clinical concern or facial vascular findings by other reports. One report recommended a hearing screen for all individuals with oculodermal melanocytosis.
DISCUSSION:
Molecular studies for the three patients presented here confirm that phacomatosis cesioflammea and phacomatosis cesiomarmorata result from somatic activating mutations in GNAQ and its paralog GNA11. The pathogenic mutations are typically detectable in affected skin, but not in blood. Recurrent GNAQ and GNA11 mutations have also been reported in other conditions including Sturge-Weber syndrome, isolated blue nevi, and congenital hemangioma.
Out of 12 patients (9 published, 3 in present series) with somatic mutations detected, four have identical amino acid substitutions: GNAQ p.Arg183Gln (Supp. Table 2). This amino acid change has also been previously associated with Sturge-Weber syndrome (Lian et al., 2014; Nakashima et al., 2014; Shirley et al., 2013; Thomas et al., 2016). Additionally, the remaining eight individuals have substitutions at the same amino acid residue p.Arg183 in GNAQ or GNA11, but differ with respect to the specific amino acid substitution. The p.Arg183 residue resides in the GTP binding site in the G alpha subunit of the G-protein signaling complex. Alterations at this site are predicted to abrogate GTP binding and lead to increased activity of downstream signaling pathways. This prediction is supported by experimental findings: Thomas et al. observed a pattern of increased phosphorylation of proteins in the MAPK, JNK and ERK pathways consistent with pathway activation.
It is possible that there are genotype-phenotype correlations that explain a portion of the clinical variability in these disorders. However, at this time there are insufficient numbers of genotyped patients to delineate any such relationships. The individuals in this study (Patients P1 and P2) shared features including ocular melanocytosis, venous anomalies and brain imaging anomalies as Patient L7 with the same GNAQ p.Arg183Gln variant. Another individual (Patient L8) has only a cutaneous capillary malformation and dermal melanocytosis. It appears that the variability in the phenotype of the disease is primarily driven by the tissue-specific mutation burden and accordingly the developmental timeframe during which a mutation arises. The effect of the specific amino acid substitution may be secondary in importance. As more individuals with these conditions are genotyped, it is possible that mutation burden or genotype could be used to stratify individuals into disease risk categories.
In caring for the three presented patients, we were interested to determine what surveillance for occult complications of phacomatosis cesioflammea and cesiomarmorata was indicated for newly diagnosed patients. While the spectrum of extracutaneous findings has been documented, their relative frequencies have not been systematically evaluated. We tabulated the reported frequency of extracutaneous findings to assess the potential utility of using associations with clinically observable features to help inform management. Our literature review examined 176 cases with phacomatosis cesioflammea emerging as the most common subtype, followed by phacomatosis cesiomarmorata, cases demonstrating a cesioflammea/cesiomarmorata overlap subtype, cases of spilorosea and melanorosea (Fernandez-Guarino et al., 2008). The most commonly reported extracutaneous complications were related to abnormalities of the eye (glaucoma, ocular melanoma) and of the brain (imaging abnormalities, developmental delay). Focusing on phacomatosis cesioflammea, cesiomarmorata, and the cesioflammea/cesiomarmorata overlap subtypes, we next assessed patterns of co-occurrence between readily observable clinical findings and less readily detectable complications of the condition that could potentially benefit from early detection through focused assessment. This analysis confirmed individuals with facial vascular involvement were more likely to have seizures and developmental delay. While all cases of ocular melanoma were seen in individuals with ocular pigmentation, in several cases this finding was not readily apparent on external eye exam(Shields et al., 2017). Finally, we found a potential correlation between limb asymmetry and either superficial or deep venous malformations. Limitations of our approach include the fact that as we reviewed findings from published literature, patients were not systematically assessed. Additionally, there is the potential bias towards more severe cases in published literature, which make it challenging to precisely estimate the frequency of various clinical features.
Our literature review found that published recommendations for the initial evaluation and surveillance of individuals with phacomatosis cesioflammea and cesiomarmorata vary widely. As phacomatosis cesioflammea and cesiomarmorata include features seen in disorders with shared molecular origins (specifically Sturge-Weber syndrome and ocular melanosis), we also reviewed recommendations of management for these related conditions specifically as they pertain to ocular and neurologic complications. Individuals with facial vascular involvement are at risk for neurologic abnormalities. Consensus is for these individuals to be evaluated by a neurologist (Bachur & Comi, 2013; Zallmann et al., 2018). Some proponents recommend brain imaging for all individuals before symptoms present (Waelchli et al., 2014), but the benefit of this approach is still under study (Zallmann et al., 2018). Individuals with ocular melanosis are at higher risk for glaucoma and ocular melanoma (Shields et al., 2013), and children with ocular melanocytosis should be seen by an ophthalmologist and followed closely – up to twice yearly (Shields et al., 2013).
Based on the shared underlying biology of the allelic disorders PPV cesioflammea and cesiomarmorata and findings of our literature review we find that initial evaluation of affected patients can reasonably include neurologic evaluation for patients with facial vascular involvement and brain imaging for patients with neurologic findings. Additionally, as some individuals who develop ocular melanoma have melanocytosis that is not visible on routine clinical exam, we concur with recommendations that all patients with cesioflammea, cesiomarmorata or the cesioflammea-cesiomarmorata overlap presentation should undergo an ophthalmologic exam. Those with ocular melanosis should have regular ophthalmologic follow-up.
Our study adds to the clinical and molecular evidence that phacomatosis cesioflammea and phacomatosis cesiomarmorata lie on a continuous phenotypic spectrum of disorders with associated pigmentary and vascular abnormalities. It is likely that the extent and severity of the clinical manifestations depend on the nature and timing of the mutation and the resultant cell populations affected. Our literature review assessed the frequency of extracutaneous manifestations including ocular, neuroimaging and developmental abnormalities and additionally assessed relationships between findings observable on routine clinical exam and those that would require additional assessment to detect. As in other disorders resulting from GNAQ and GNA11 mutation, the location of cutaneous findings and ocular melanosis were predictive of important extracutaneous complications. Finally, our findings provide an evidence base for surveillance for extracutaneous complications of phacomatosis cesioflammea, cesiomarmorata, and cesioflammea/cesiomarmorata overlap subtypes.
METHODS
Editorial Policies and Ethical Considerations
The study was reviewed by the Stanford IRB, subject 1 was additionally enrolled in the Undiagnosed Disease Network study. Written consent was obtained for all participants.
Whole Exome Sequencing (Patient 1)
For the paired-end pre-capture library procedure, genomic DNA from a lesional skin and blood was fragmented by sonication and ligated to the Illumina multiplexing paired-end (PE) adapters. The adapter-ligated DNA was then polymerase chain reaction (PCR) amplified using primers with sequencing barcodes (indexes). For target enrichment/exome capture procedure, the pre-capture library was enriched by hybridizing to biotin labeled VCRome 2.1 in-solution exome probes (Bainbridge et al., 2011) for 64–72 h at 47°C. Additional probes for over 3600 Mendelian disease genes were also included in the capture in order to improve the exome coverage. For massively parallel sequencing, the post-capture library DNA was subjected to sequence analysis on Illumina HiSeq platform for 100-bp paired-end reads. The following quality control metrics of the sequencing data are generally achieved: >70% of reads aligned to target, >95% target base covered at >20X, >85% target base covered at >40X, mean coverage of target bases >100X. Single-nucleotide polymorphism (SNP) concordance to genotype array: >99%. As a quality control measure, DNA was also analyzed by a SNP array: Illumina Human Exome-12v1 array and the Illumina CytoSNP-850K array. The SNP data are compared with the WES data to ensure correct sample identification and to assess sequencing quality.
Data Analysis and Interpretation by Mercury 1.0
The output data from Illumina HiSeq were converted from bcl file to FASTQ file by Illumina CASAVA 1.8 software, and mapped by BWA (Li & Durbin, 2009) to the reference haploid human genome sequence (Genome Reference Consortium human genome build 37, human genome 19). The variant calls are performed using Atlas-SNP and Atlas-indel developed in-house by the Baylor College of Medicine Human Genome Sequencing Center (BCM HGSC)(Yang et al., 2013). The variant annotations were performed using in-house developed software: HGSC-SNP-anno and HGSC-indel-anno. Synonymous variants, intronic variants not affecting splice site, and common benign variants were excluded from interpretation unless they were previously reported as pathogenic variants. The variants were interpreted according to American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015) and patient phenotypes. Positions were classified as having a de novo if the alteration was not seen in either of the patient’s parents. Variants related to patient phenotypes are confirmed by Sanger sequencing for patient and parents.
Literature Review
PubMed searches were performed to identify articles that contained case reports or case series on “phakomatosis OR phacomatosis AND pigmentovascularis”. We included case reports and case series from 1982–2017. The following criteria had to be met for studies to be included: Only English language papers which reported on original research were considered; samples had to include individuals with PPV. Reviews were excluded. Nine articles were not included as seven were not in English, two were repeated articles, one was not accessible and one had insufficient evidence to evaluate a diagnosis of PPV. Thus, a total of 176 cases from 105 articles were included in this review. The presence of a facial vascular finding was inferred either if it was described explicitly or if SWS was diagnosed. To use a consistent set of rules, cesioflammea, cesiomarmorata, spilorosea and melanorosea were used (Supp. Table 2). Of note the nomenclature reported in literature has historically used different terminology; these cases were reclassified (Supp. Table 3). (Adachi, Togashi, Sasaki, & Sekido, 2013; Ajith et al., 2006; Al Robaee, Banka, & Alfadley, 2004; Arnold, Kleine, & Happle, 2012; Ben Saif, AlShehab, & Almutawa, 2010; Bielsa, Paradelo, Ribera, & Ferrandiz, 1998; Boente Mdel et al., 2008; Brittain, Walsh, & Smidt, 2013; Byrom et al., 2015; Carvalho et al., 2011; Castori et al., 2008; Castori et al., 2011; Chang, Hsu, Chen, & Hsieh, 2007; Chen, Tsai, Lee, Tu, & Huang, 2013; Chhajed, Pandit, Dhawan, & Jain, 2010; Chiu et al., 2009; de las Heras, Boixeda, Ledo, & Happle, 1997; de Luna, Barquin, Casas, & Sidelsky, 1995; Di Landro, Tadini, Marchesi, & Cainelli, 1999; Diociaiuti et al., 2005; Dippel et al., 2003; Du, Delaporte, Catteau, Destee, & Piette, 1998; Fernandez-Guarino et al., 2008; Finklea et al., 2010; Fischer & Trautinger, 2015; Garg et al., 2013; Gilliam et al., 1993; Goyal & Varshney, 2010; Guiglia & Prendiville, 1991; Gupta, Dubey, & Agarwal, 2007; Ha et al., 2017; Hagiwara, Uezato, & Nonaka, 1998; Hall et al., 2007; Hasegawa & Yasuhara, 1985; Hayashi, Kaminaga, Tantcheva-Poor, Hamasaki, & Hatamochi, 2016; Henry et al., 2013; Huang & Lee, 2000; Jeon et al., 2013; Joshi, Garg, Agrawal, Agarwalla, & Thakur, 1999; Jun et al., 2015; Kaise, Watanabe, & Kobayashi, 1992; Kanaheswari, Hamzaini, Wong, & Zulfiqar, 2008; Karabudak, Dogan, Basekim, & Harmanyeri, 2007; Kaur, Sharma, Sethi, Kooner, & Banger, 2015; Kikuchi & Okazaki, 1982; Kim, Park, Yang, Park, & Lee, 2000; Kola, Mehrafza, & Kane, 2017; Kono et al., 2003; Krema et al., 2013; Kurokawa et al., 2013; Larralde, Santos-Munoz, Rodriguez Caceres, & Ciardiullo, 2008; Laureano, Carvalho, Amaro, Freitas, & Cardoso, 2014; Lee, Choi, Oh, Yoon, & Kim, 2005; Libow, 1993; Lo & Tzung, 2003; Ma et al., 2015; Mahroughan, Mehregan, & Mehregan, 1996; Mandal, Ghosh, Koley, & Roy, 2014; Mandt, Blume-Peytavi, Pfrommer, Krengel, & Goerdt, 1999; Moutray et al., 2010; Murdoch & Keefe, 2000; Namiki, Arai, Miura, & Yokozeki, 2016; Namiki et al., 2017; Nanda et al., 2016; Narchi, Santos, & Tunnessen, 2001; Okunola, Ofovwe, Abiodun, Isah, & Ikubor, 2012; Ono & Tateshita, 2000; Onsun et al., 2007; Park et al., 2003; Pathania & Kumar, 2015; Patil et al., 2015; Pradhan, Patnaik, Padhi, & Nayak, 2015; Qiao & Fang, 2011; Ruiz Villaverde, Galan Gutierrez, & Sierra Corcoles, 2013; Ruiz Villaverde, Viera Ramirez, Linares Solano, Naranjo Sintes, & Gutierrez Salmeron, 2003; Ruiz-Maldonado, Tamayo, Laterza, Brawn, & Lopez, 1987; Saricaoglu, Guven, Karakurt, Sengun, & Ziraman, 2002; Seckin, Yucelten, Aytug, & Demirkesen, 2007; Segatto, Schmitt, Hagemann, Silva, & Cattani, 2013; Sen, Bala, Halder, Ahar, & Gangopadhyay, 2015; Shields et al., 2017; Shields et al., 2011; Shimizu et al., 2015; Smith et al., 2012; Tadini et al., 1998; Teekhasaenee & Ritch, 1997; Tekin, Yucelten, & Happle, 2016; Thomas et al., 2016; Toelle et al., 2011; Torrelo, Zambrano, & Happle, 2003, 2006; Tran & Zografos, 2005; Tsuruta et al., 1999; Turk, Turkmen, Tuna, Karaarslan, & Ozdemir, 2011; Unger & Alsufyani, 2011; Unlu & Sahin, 2015; Uysal et al., 2000; Van Gysel, Oranje, Stroink, & Simonsz, 1996; Verma, Desai, Shah, & Happle, 2017; Vidaurri-de la Cruz, Tamayo-Sanchez, Duran-McKinster, Orozco-Covarrubias Mde, & Ruiz-Maldonado, 2003; Villarreal & Leal, 2016; Wobser et al., 2013; Woo et al., 2017; Yang et al., 2015; Yeung, 2010; Zhou et al., 2016a). Statistical analysis included a two sample Z-test of proportions with Bonferroni correction when applicable.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Intramural Research Program of the National Human Genome Research Institute and by the NIH Common Fund, through the Office of Strategic Coordination, Office of the NIH Director under Award Numbers U01 HG007708, U01 HG007709, U01 HG007703, U01 HG007530, U01 HG007942, U01 HG007690, U01 HG007674, U01 HG007672, U01 TR001395, U01 HG007943, and U54 NS093793. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding: National Institutes of Health awards U01 HG007708, U01 HG007709, U01 HG007703, U01 HG007530, U01 HG007942, U01 HG007690, U01 HG007674, U01 HG007672, U01 TR001395, U01 HG007943, and U54 NS093793.
REFERENCES
- Adachi K, Togashi S, Sasaki K, & Sekido M (2013). Laser therapy treatment of phacomatosis pigmentovascularis type II: two case reports. J Med Case Rep, 7, 55. doi: 10.1186/1752-1947-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajith C, Narang T, & Kanwar AJ (2006). Phakomatosis pigmentovascularis type II b associated with Sturge-Weber syndrome. Clin Exp Dermatol, 31(4), 590–592. doi: 10.1111/j.1365-2230.2006.02122.x [DOI] [PubMed] [Google Scholar]
- Al Robaee A, Banka N, & Alfadley A (2004). Phakomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome. Pediatr Dermatol, 21(6), 642–645. doi: 10.1111/j.0736-8046.2004.21605.x [DOI] [PubMed] [Google Scholar]
- Arnold AW, Kleine MU, & Happle R (2012). Phacomatosis melanorosea without extracutaneous features: an unusual type of phacomatosis pigmentovascularis. Eur J Dermatol, 22(4), 473–475. doi: 10.1684/ejd.2012.1780 [DOI] [PubMed] [Google Scholar]
- Bachur CD, & Comi AM (2013). Sturge-weber syndrome. Curr Treat Options Neurol, 15(5), 607–617. doi: 10.1007/s11940-013-0253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge MN, Wang M, Wu Y, Newsham I, Muzny DM, Jefferies JL, … Gibbs RA (2011). Targeted enrichment beyond the consensus coding DNA sequence exome reveals exons with higher variant densities. Genome Biol, 12(7), R68. doi: 10.1186/gb-2011-12-7-r68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Saif GA, AlShehab SA, & Almutawa A (2010). Unusual combination of pigmentary lesions. Int J Dermatol, 49(9), 1059–1062. doi: 10.1111/j.1365-4632.2009.04341.x [DOI] [PubMed] [Google Scholar]
- Bielsa I, Paradelo C, Ribera M, & Ferrandiz C (1998). Generalized nevus spilus and nevus anemicus in a patient with a primary lymphedema: a new type of phakomatosis pigmentovascularis? Pediatr Dermatol, 15(4), 293–295. [DOI] [PubMed] [Google Scholar]
- Boente Mdel C, Obeid R, Asial RA, Bibas-Bonet H, Coronel AM, & Happle R (2008). Cutis tricolor coexistent with cutis marmorata telangiectatica congenita: “phacomatosis achromico-melano-marmorata”. Eur J Dermatol, 18(4), 394–396. doi: 10.1684/ejd.2008.0458 [DOI] [PubMed] [Google Scholar]
- Brittain P, Walsh EJ, & Smidt AC (2013). Blotchy baby: a case of phakomatosis pigmentovascularis. J Pediatr, 162(6), 1293, 1293.e1291. doi: 10.1016/j.jpeds.2012.12.040 [DOI] [PubMed] [Google Scholar]
- Byrom L, Surjana D, Yoong C, & Zappala T (2015). Red-white and blue baby: a case of phacomatosis pigmentovascularis type V. Dermatol Online J, 21(6). [PubMed] [Google Scholar]
- Carvalho CH, Batista LM, Bornemann A, Acioly MA, & Tatagiba M (2011). Skull base tumor in a patient with phacomatosis pigmentovascularis. Brain Pathol, 21(6), 705–708. doi: 10.1111/j.1750-3639.2011.00528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castori M, Rinaldi R, Angelo C, Zambruno G, Grammatico P, & Happle R (2008). Phacomatosis cesioflammea with unilateral lipohypoplasia. Am J Med Genet A, 146a(4), 492–495. doi: 10.1002/ajmg.a.32165 [DOI] [PubMed] [Google Scholar]
- Castori M, Sarazani S, Binni F, Pezzella FR, Cruciani G, & Grammatico P (2011). Monozygotic twin discordance for phacomatosis cesioflammea further supports the post-zygotic mutation hypothesis. Am J Med Genet A, 155a(9), 2253–2256. doi: 10.1002/ajmg.a.34140 [DOI] [PubMed] [Google Scholar]
- Chang BP, Hsu CH, Chen HC, & Hsieh JW (2007). An infant with extensive Mongolian spot, naevus flammeus and cutis marmorata telangiectatica congenita: a unique case of phakomatosis pigmentovascularis. Br J Dermatol, 156(5), 1068–1071. doi: 10.1111/j.1365-2133.2007.07798.x [DOI] [PubMed] [Google Scholar]
- Chen LW, Tsai YS, Lee JS, Tu YF, & Huang CC (2013). Extensive subarachnoid venous angiomatosis with hydrocephalus in phacomatosis pigmentovascularis. Neurology, 81(11), 1020–1021. doi: 10.1212/WNL.0b013e3182a43ba6 [DOI] [PubMed] [Google Scholar]
- Chhajed M, Pandit S, Dhawan N, & Jain A (2010). Klippel-Trenaunay and Sturge-Weber overlap syndrome with phakomatosis pigmentovascularis. J Pediatr Neurosci, 5(2), 138–140. doi: 10.4103/1817-1745.76113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HH, Chen GS, Wu CS, Ke CL, & Cheng ST (2009). Phakomatosis cesioflammea with late-onset glaucoma and acquired nevus spilus-like lesion - 15 years of follow-up. Int J Dermatol, 48(4), 416–418. doi: 10.1111/j.1365-4632.2009.03885.x [DOI] [PubMed] [Google Scholar]
- de las Heras E, Boixeda JP, Ledo A, & Happle R (1997). Paired melanotic and achromic macules in a case of phacomatosis pigmentovascularis: a further example of twin spotting? Am J Med Genet, 70(3), 336–337. [DOI] [PubMed] [Google Scholar]
- de Luna ML, Barquin MA, Casas JG, & Sidelsky S (1995). Phacomatosis pigmentovascularis with a selective IgA deficiency. Pediatr Dermatol, 12(2), 159–163. [DOI] [PubMed] [Google Scholar]
- Di Landro A, Tadini GL, Marchesi L, & Cainelli T (1999). Phakomatosis pigmentovascularis: A new case with renal angiomas and some considerations about the classification. Pediatr Dermatol, 16(1), 25–30. [DOI] [PubMed] [Google Scholar]
- Diociaiuti A, Guidi B, Aguilar Sanchez JA, Feliciani C, Capizzi R, & Amerio P (2005). Phakomatosis pigmentovascularis type IIIb: a case associated with Sturge-Weber and Klippel-Trenaunay syndromes. J Am Acad Dermatol, 53(3), 536–539. doi: 10.1016/j.jaad.2005.01.091 [DOI] [PubMed] [Google Scholar]
- Dippel E, Utikal J, Feller G, Fackel N, Klemke CD, Happle R, & Goerdt S (2003). Nevi flammei affecting two contralateral quadrants and nevus depigmentosus: a new type of phacomatosis pigmentovascularis? Am J Med Genet A, 119a(2), 228–230. doi: 10.1002/ajmg.a.20128 [DOI] [PubMed] [Google Scholar]
- Du LC, Delaporte E, Catteau B, Destee A, & Piette F (1998). Phacomatosis pigmentovascularis type II. Eur J Dermatol, 8(8), 569–572. [PubMed] [Google Scholar]
- Fernandez-Guarino M, Boixeda P, de Las Heras E, Aboin S, Garcia-Millan C, & Olasolo PJ (2008). Phakomatosis pigmentovascularis: Clinical findings in 15 patients and review of the literature. J Am Acad Dermatol, 58(1), 88–93. doi: 10.1016/j.jaad.2007.08.012 [DOI] [PubMed] [Google Scholar]
- Finklea LB, Mohr MR, Warthan MM, Darrow DH, & Williams JV (2010). Two reports of phacomatosis pigmentovascularis type IIb, one in association with Sturge-Weber syndrome and Klippel-Trenaunay syndrome. Pediatr Dermatol, 27(3), 303–305. doi: 10.1111/j.1525-1470.2010.01144.x [DOI] [PubMed] [Google Scholar]
- Fischer K, & Trautinger F (2015). Phacomatosis pigmentovascularis. J Dtsch Dermatol Ges, 13(11), 1178–1179. doi: 10.1111/ddg.12834 [DOI] [PubMed] [Google Scholar]
- Garg A, Gupta LK, Khare AK, Kuldeep CM, Mittal A, & Mehta S (2013). Phacomatosis cesioflammea with Klippel Trenaunay syndrome: A rare association. Indian Dermatol Online J, 4(3), 216–218. doi: 10.4103/2229-5178.115522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam AC, Ragge NK, Perez MI, & Bolognia JL (1993). Phakomatosis pigmentovascularis type IIb with iris mammillations. Arch Dermatol, 129(3), 340–342. [PubMed] [Google Scholar]
- Goyal T, & Varshney A (2010). Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol, 76(3), 307. doi: 10.4103/0378-6323.62973 [DOI] [PubMed] [Google Scholar]
- Guiglia MC, & Prendiville JS (1991). Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol, 24(2 Pt 2), 359–363. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dubey S, & Agarwal M (2007). A case of Sturge-Weber syndrome in association with phacomatosis pigmentovascularis and developmental glaucoma. J aapos, 11(4), 398–399. doi: 10.1016/j.jaapos.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Ha JW, Hahm JE, Park SE, Lee JY, Kim CW, & Kim SS (2017). A Case of Phacomatosis Pigmentovascularis Type IIa in a Korean Infant. Ann Dermatol, 29(5), 638–639. doi: 10.5021/ad.2017.29.5.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Uezato H, & Nonaka S (1998). Phacomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome and pyogenic granuloma. J Dermatol, 25(11), 721–729. [DOI] [PubMed] [Google Scholar]
- Hall BD, Cadle RG, Morrill-Cornelius SM, & Bay CA (2007). Phakomatosis pigmentovascularis: Implications for severity with special reference to Mongolian spots associated with Sturge-Weber and Klippel-Trenaunay syndromes. Am J Med Genet A, 143a(24), 3047–3053. doi: 10.1002/ajmg.a.31970 [DOI] [PubMed] [Google Scholar]
- Happle R (2005). Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol, 141(3), 385–388. doi: 10.1001/archderm.141.3.385 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, & Yasuhara M (1985). Phakomatosis pigmentovascularis type IVa. Arch Dermatol, 121(5), 651–655. [PubMed] [Google Scholar]
- Hayashi S, Kaminaga T, Tantcheva-Poor I, Hamasaki Y, & Hatamochi A (2016). Patient with extensive Mongolian spots, nevus flammeus and nevus vascularis mixtus: A novel case of phacomatosis pigmentovascularis. J Dermatol, 43(2), 225–226. doi: 10.1111/1346-8138.13155 [DOI] [PubMed] [Google Scholar]
- Henry CR, Hodapp E, Hess DJ, Blieden LS, & Berrocal AM (2013). Fluorescein angiography findings in phacomatosis pigmentovascularis. Ophthalmic Surg Lasers Imaging Retina, 44(2), 201–203. doi: 10.3928/23258160-20130212-01 [DOI] [PubMed] [Google Scholar]
- Huang C, & Lee P (2000). Phakomatosis pigmentovascularis IIb with renal anomaly. Clin Exp Dermatol, 25(1), 51–54. [DOI] [PubMed] [Google Scholar]
- Jeon SY, Ha SM, Ko DY, Hong JW, Song KH, & Kim KH (2013). Phakomatosis pigmentovascularis Ib with left-sided hemihypertrophy, interdigital gaps and scoliosis: a unique case of phakomatosis pigmentovascularis. J Dermatol, 40(1), 78–79. doi: 10.1111/j.1346-8138.2012.01677.x [DOI] [PubMed] [Google Scholar]
- Joshi A, Garg VK, Agrawal S, Agarwalla A, & Thakur A (1999). Port-wine-stain (nevus flammeus), congenital Becker’s nevus, cafe-au-lait-macule and lentigines: phakomatosis pigmentovascularis type Ia--a new combination. J Dermatol, 26(12), 834–836. [DOI] [PubMed] [Google Scholar]
- Jun HJ, Kim SM, Cho SH, Lee JD, & Kim HS (2015). Phacomatosis Pigmentovascularis Type Vb in a Three-Year Old Boy. Ann Dermatol, 27(3), 353–354. doi: 10.5021/ad.2015.27.3.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaise M, Watanabe A, & Kobayashi Y (1992). A case of phacomatosis pigmentovascularis accompanied with esophageal varices due to hypoplasia of the portal veins. Gastroenterol Jpn, 27(4), 546–549. [DOI] [PubMed] [Google Scholar]
- Kanaheswari Y, Hamzaini AH, Wong SW, & Zulfiqar A (2008). Malignant hypertension in a child with phakomatosis pigmentovascularis type II b. Acta Paediatr, 97(11), 1589–1591. doi: 10.1111/j.1651-2227.2008.00971.x [DOI] [PubMed] [Google Scholar]
- Karabudak O, Dogan B, Basekim C, & Harmanyeri Y (2007). Phacomatosis spilorosea (phacomatosis pigmentovascularis type IIIb). Australas J Dermatol, 48(4), 256–258. doi: 10.1111/j.1440-0960.2007.00398.x [DOI] [PubMed] [Google Scholar]
- Kaur T, Sharma N, Sethi A, Kooner S, & Banger H (2015). Phacomatosis cesiomarmorata with hypospadias and phacomatosis cesioflammea with Sturge-Weber syndrome, Klippel-Trenaunay syndrome and aplasia of veins -- case reports with rare associations. Dermatol Online J, 21(9). [PubMed] [Google Scholar]
- Kikuchi I, & Okazaki M (1982). Congenital temporal alopecia in phakomatosis pigmentovascularis. J Dermatol, 9(6), 485–487. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park KB, Yang JM, Park SH, & Lee ES (2000). Congenital triangular alopecia in phakomatosis pigmentovascularis: report of 3 cases. Acta Derm Venereol, 80(3), 215–216. [DOI] [PubMed] [Google Scholar]
- Kola B, Mehrafza M, & Kane A (2017). Phacomatosis Pigmentovascularis: Simple Presentation of a Not So Simple Dermatological Condition. Clin Pediatr (Phila), 9922817737088. doi: 10.1177/0009922817737088 [DOI] [PubMed] [Google Scholar]
- Kono T, Ercocen AR, Chan HH, Kikuchi Y, Hori K, Uezono S, & Nozaki M (2003). Treatment of phacomatosis pigmentovascularis: a combined multiple laser approach. Dermatol Surg, 29(6), 642–646. [DOI] [PubMed] [Google Scholar]
- Krema H, Simpson R, & McGowan H (2013). Choroidal melanoma in phacomatosis pigmentovascularis cesioflammea. Can J Ophthalmol, 48(3), e41–42. doi: 10.1016/j.jcjo.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Kurokawa R, Kim P, Kawamoto T, Matsuda H, Hayashi S, Yamazaki S, … Kubota K (2013). Intramedullary and retroperitoneal melanocytic tumor associated with congenital blue nevus and nevus flammeus: an uncommon combination of neurocutaneous melanosis and phacomatosis pigmentovascularis--case report. Neurol Med Chir (Tokyo), 53(10), 730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larralde M, Santos-Munoz A, Rodriguez Caceres M, & Ciardiullo A (2008). Phacomatosis pigmentovascularis type Va in a 3-month old. Pediatr Dermatol, 25(2), 198–200. doi: 10.1111/j.1525-1470.2008.00633.x [DOI] [PubMed] [Google Scholar]
- Laureano A, Carvalho R, Amaro C, Freitas I, & Cardoso J (2014). Vascular malformation and common keratinocytic nevus of the soft type: phacomatosis pigmentovascularis revisited. Case Rep Dermatol Med, 2014, 437085. doi: 10.1155/2014/437085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Choi DY, Oh YG, Yoon HS, & Kim JD (2005). An infantile case of Sturge-Weber syndrome in association with Klippel-Trenaunay-Weber syndrome and phakomatosis pigmentovascularis. J Korean Med Sci, 20(6), 1082–1084. doi: 10.3346/jkms.2005.20.6.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, & Durbin R (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25(14), 1754–1760. doi: 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian CG, Sholl LM, Zakka LR, O TM, Liu C, Xu S, … Mihm MC Jr. (2014). Novel genetic mutations in a sporadic port-wine stain. JAMA Dermatol, 150(12), 1336–1340. doi: 10.1001/jamadermatol.2014.1244 [DOI] [PubMed] [Google Scholar]
- Libow LF (1993). Phakomatosis pigmentovascularis type IIIb. J Am Acad Dermatol, 29(2 Pt 2), 305–307. [DOI] [PubMed] [Google Scholar]
- Lo PY, & Tzung TY (2003). Phakomatosis pigmentovascularis type IIb with a patent umbilical vein and inferior vena cava hypoplasia. Br J Dermatol, 148(4), 836–838. [DOI] [PubMed] [Google Scholar]
- Ma H, Liao M, Qiu S, Luo R, Lu R, & Lu C (2015). The case of a boy with nevus of Ota, extensive Mongolian spot, nevus flammeus, nevus anemicus and cutis marmorata telangiectatica congenita: a unique instance of phacomatosis pigmentovascularis. An Bras Dermatol, 90(3 Suppl 1), 10–12. doi: 10.1590/abd1806-4841.20153466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahroughan M, Mehregan AH, & Mehregan DA (1996). Phakomatosis pigmentovascularis: report of a case. Pediatr Dermatol, 13(1), 36–38. [DOI] [PubMed] [Google Scholar]
- Mandal RK, Ghosh SK, Koley S, & Roy AC (2014). Sturge-Weber syndrome in association with Klippel-Trenaunay syndrome and phakomatosis pigmentovascularis type IIb. Indian J Dermatol Venereol Leprol, 80(1), 51–53. doi: 10.4103/0378-6323.125507 [DOI] [PubMed] [Google Scholar]
- Mandt N, Blume-Peytavi U, Pfrommer C, Krengel S, & Goerdt S (1999). Phakomatosis pigmentovascularis type IIa. J Am Acad Dermatol, 40(2 Pt 2), 318–321. [DOI] [PubMed] [Google Scholar]
- Moutray T, Napier M, Shafiq A, Fryer A, Rankin S, & Willoughby CE (2010). Monozygotic twins discordant for phacomatosis pigmentovascularis: evidence for the concept of twin spotting. Am J Med Genet A, 152a(3), 718–720. doi: 10.1002/ajmg.a.33232 [DOI] [PubMed] [Google Scholar]
- Murdoch SR, & Keefe M (2000). Phakomatosis pigmentovascularis type IIA in a Caucasian child. Pediatr Dermatol, 17(2), 157. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Miyajima M, Sugano H, Iimura Y, Kato M, Tsurusaki Y, … Matsumoto N (2014). The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge-Weber syndrome. J Hum Genet, 59(12), 691–693. doi: 10.1038/jhg.2014.95 [DOI] [PubMed] [Google Scholar]
- Namiki T, Arai M, Miura K, & Yokozeki H (2016). A case of phakomatosis pigmentovascularis type II: port-wine stain and dermal melanocytosis with cutis marmorata telangiectatica congenita-like lesions. Eur J Dermatol, 26(3), 302–303. doi: 10.1684/ejd.2016.2737 [DOI] [PubMed] [Google Scholar]
- Namiki T, Takahashi M, Nojima K, Ueno M, Hanafusa T, Tokoro S, & Yokozeki H (2017). Phakomatosis pigmentovascularis type IIb: A case with Klippel-Trenaunay syndrome and extensive dermal melanocytosis as nevus of Ota, nevus of Ito and ectopic Mongolian spots. J Dermatol, 44(3), e32–e33. doi: 10.1111/1346-8138.13505 [DOI] [PubMed] [Google Scholar]
- Nanda A, Al-Abdulrazzaq HK, Habeeb YK, Zakkiriah M, Alghadhfan F, Al-Noun R, & Al-Ajmi H (2016). Phacomatosis pigmentovascularis: Report of four new cases. Indian J Dermatol Venereol Leprol, 82(3), 298–303. doi: 10.4103/0378-6323.178905 [DOI] [PubMed] [Google Scholar]
- Narchi H, Santos M, & Tunnessen WW Jr. (2001). Picture of the month. Phakomatosis pigmentovascularis. Arch Pediatr Adolesc Med, 155(2), 191–192. [DOI] [PubMed] [Google Scholar]
- Okunola P, Ofovwe G, Abiodun M, Isah A, & Ikubor J (2012). Phakomatosis pigmentovascularis type IIb in association with external hydrocephalus. BMJ Case Rep, 2012 doi: 10.1136/bcr.12.2011.5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono I, & Tateshita T (2000). Phacomatosis pigmentovascularis type IIa successfully treated with two types of laser therapy. Br J Dermatol, 142(2), 358–361. [DOI] [PubMed] [Google Scholar]
- Onsun N, Inandirici A, Kural Y, Teker C, & Atilganoglu U (2007). Phakomatosis pigmentovascularis type II b with bilateral hearing impairment. J Eur Acad Dermatol Venereol, 21(3), 402–403. doi: 10.1111/j.1468-3083.2006.01880.x [DOI] [PubMed] [Google Scholar]
- Park JG, Roh KY, Lee HJ, Ha SJ, Lee JY, Yun SS, … Kim JW (2003). Phakomatosis pigmentovascularis IIb with hypoplasia of the inferior vena cava and the right iliac and femoral veins causing recalcitrant stasis leg ulcers. J Am Acad Dermatol, 49(2 Suppl Case Reports), S167–169. doi: 10.1067/mjd.2003.160 [DOI] [PubMed] [Google Scholar]
- Pathania V, & Kumar A (2015). Phacomatosis pigmentovascularis with Raynaud’s phenomena. Med J Armed Forces India, 71(Suppl 1), S119–121. doi: 10.1016/j.mjafi.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil B, Sinha G, Nayak B, Sharma R, Kumari S, & Dada T (2015). Bilateral Sturge-Weber and Phakomatosis Pigmentovascularis with Glaucoma, an Overlap Syndrome. Case Rep Ophthalmol Med, 2015, 106932. doi: 10.1155/2015/106932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S, Patnaik S, Padhi T, & Nayak BP (2015). Phakomatosis pigmentovascularis Type IIb, Sturge-Weber syndrome and cone shaped tongue: An unusual association. Indian J Dermatol Venereol Leprol, 81(6), 614–616. doi: 10.4103/0378-6323.168335 [DOI] [PubMed] [Google Scholar]
- Qiao J, & Fang H (2011). Birthmarks: phacomatosis pigmentovascularis. Med J Aust, 195(11–12), 709. [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, … Committee, A. L. Q. A. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17(5), 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Villaverde R, Galan Gutierrez M, & Sierra Corcoles C (2013). [Nevus flammeus, aberrant mongolian spot and neurological symptoms]. An Pediatr (Barc), 78(2), 125–126. doi: 10.1016/j.anpedi.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Ruiz Villaverde R, Viera Ramirez A, Linares Solano J, Naranjo Sintes R, & Gutierrez Salmeron MT (2003). Phakomatosis pigmentovascularis and Lisch nodules. Relationship between Von Recklinghausen and phakomatosis pigmentovascularis? J Eur Acad Dermatol Venereol, 17(1), 53–55. [DOI] [PubMed] [Google Scholar]
- Ruiz-Maldonado R, Tamayo L, Laterza AM, Brawn G, & Lopez A (1987). Phacomatosis pigmentovascularis: a new syndrome? Report of four cases. Pediatr Dermatol, 4(3), 189–196. [DOI] [PubMed] [Google Scholar]
- Saricaoglu MS, Guven D, Karakurt A, Sengun A, & Ziraman I (2002). An unusual case of Sturge-Weber syndrome in association with phakomatosis pigmentovascularis and Klippel-Trenaunay-Weber syndrome. Retina, 22(3), 368–371. [DOI] [PubMed] [Google Scholar]
- Seckin D, Yucelten D, Aytug A, & Demirkesen C (2007). Phacomatosis pigmentovascularis type IIIb. Int J Dermatol, 46(9), 960–963. doi: 10.1111/j.1365-4632.2007.03121.x [DOI] [PubMed] [Google Scholar]
- Segatto MM, Schmitt EU, Hagemann LN, Silva RC, & Cattani CA (2013). Phacomatosis pigmentovascularis type IIa--case report. An Bras Dermatol, 88(6 Suppl 1), 85–88. doi: 10.1590/abd1806-4841.20132248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Bala S, Halder C, Ahar R, & Gangopadhyay A (2015). Phakomatosis pigmentovascularis presenting with sturge-weber syndrome and klippel-trenaunay syndrome. Indian J Dermatol, 60(1), 77–79. doi: 10.4103/0019-5154.147801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CL, Di Nicola M, Pellegrini M, & Shields JA (2017). CHOROIDAL MELANOMA IN PHAKOMATOSIS PIGMENTOVASCULARIS WITH KLIPPEL-TRENAUNAY SYNDROME. Retina. doi: 10.1097/iae.0000000000001856 [DOI] [PubMed] [Google Scholar]
- Shields CL, Kaliki S, Livesey M, Walker B, Garoon R, Bucci M, … Shields JA (2013). Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes. JAMA Ophthalmol, 131(8), 993–1003. doi: 10.1001/jamaophthalmol.2013.129 [DOI] [PubMed] [Google Scholar]
- Shields CL, Kligman BE, Suriano M, Viloria V, Iturralde JC, Shields MV, … Shields J (2011). Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol, 129(6), 746–750. doi: 10.1001/archophthalmol.2011.135 [DOI] [PubMed] [Google Scholar]
- Shimizu N, Nakagawa K, Taguchi M, Okabayashi A, Kishida M, Kinoshita R, … Tsuruta D (2015). Unusual case of phakomatosis pigmentovascularis in a Japanese female infant associated with three phakomatoses: Port-wine stain, dermal melanocytosis and cutis marmorata telangiectatica congenita. J Dermatol, 42(10), 1006–1007. doi: 10.1111/1346-8138.13000 [DOI] [PubMed] [Google Scholar]
- Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, … Pevsner J (2013). Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med, 368(21), 1971–1979. doi: 10.1056/NEJMoa1213507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Moore MM, & Stetson CL (2012). JAAD grand rounds quiz. Red, purple, and brown skin lesions in a 2-month-old boy. Phakomatosis pigmentovascularis type V. J Am Acad Dermatol, 66(2), 341–342. doi: 10.1016/j.jaad.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Tadini G, Restano L, Gonzales-Perez R, Gonzales-Ensenat A, Vincente-Villa MA, Cambiaghi S, … Happle R (1998). Phacomatosis pigmentokeratotica: report of new cases and further delineation of the syndrome. Arch Dermatol, 134(3), 333–337. [DOI] [PubMed] [Google Scholar]
- Teekhasaenee C, & Ritch R (1997). Glaucoma in phakomatosis pigmentovascularis. Ophthalmology, 104(1), 150–157. [DOI] [PubMed] [Google Scholar]
- Tekin B, Yucelten D, & Happle R (2016). Phacomatosis Melanorosea: A Further Case of an Unusual Skin Disorder. Acta Derm Venereol, 96(2), 280–282. doi: 10.2340/00015555-2192 [DOI] [PubMed] [Google Scholar]
- Thomas AC, Zeng Z, Riviere JB, O’Shaughnessy R, Al-Olabi L, St-Onge J, … Kinsler V (2016). Mosaic Activating Mutations in GNA11 and GNAQ Are Associated with Phakomatosis Pigmentovascularis and Extensive Dermal Melanocytosis. J Invest Dermatol, 136(4), 770–778. doi: 10.1016/j.jid.2015.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toelle SP, Weibel L, Schiegl H, & Boltshauser E (2011). Phacomatosis pigmentovascularis and extensive venous malformation of brain vessels: an unknown association or a new vascular neurocutaneous syndrome? Neuropediatrics, 42(6), 234–236. doi: 10.1055/s-0031-1291243 [DOI] [PubMed] [Google Scholar]
- Torrelo A, Zambrano A, & Happle R (2003). Cutis marmorata telangiectatica congenita and extensive mongolian spots: type 5 phacomatosis pigmentovascularis. Br J Dermatol, 148(2), 342–345. [DOI] [PubMed] [Google Scholar]
- Torrelo A, Zambrano A, & Happle R (2006). Large aberrant Mongolian spots coexisting with cutis marmorata telangiectatica congenita (phacomatosis pigmentovascularis type V or phacomatosis cesiomarmorata). J Eur Acad Dermatol Venereol, 20(3), 308–310. doi: 10.1111/j.1468-3083.2006.01395.x [DOI] [PubMed] [Google Scholar]
- Tran HV, & Zografos L (2005). Primary choroidal melanoma in phakomatosis pigmentovascularis IIa. Ophthalmology, 112(7), 1232–1235. doi: 10.1016/j.ophtha.2005.02.019 [DOI] [PubMed] [Google Scholar]
- Tsuruta D, Fukai K, Seto M, Fujitani K, Shindo K, Hamada T, & Ishii M (1999). Phakomatosis pigmentovascularis type IIIb associated with moyamoya disease. Pediatr Dermatol, 16(1), 35–38. [DOI] [PubMed] [Google Scholar]
- Turk BG, Turkmen M, Tuna A, Karaarslan IK, & Ozdemir F (2011). Phakomatosis pigmentovascularis type IIb associated with Klippel-Trenaunay syndrome and congenital triangular alopecia. J Am Acad Dermatol, 65(2), e46–49. doi: 10.1016/j.jaad.2010.05.025 [DOI] [PubMed] [Google Scholar]
- Unger R, & Alsufyani MA (2011). Bilateral Temporal Triangular Alopecia Associated with Phakomatosis Pigmentovascularis Type IV Successfully Treated with Follicular Unit Transplantation. Case Rep Dermatol Med, 2011, 129541. doi: 10.1155/2011/129541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu E, & Sahin T (2015). A Neonatal Case of Phacomatosis Pigmentovascularis Type IIa. Balkan Med J, 32(1), 129–130. doi: 10.5152/balkanmedj.2015.15448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal G, Guven A, Ozhan B, Ozturk MH, Mutluay AH, & Tulunay O (2000). Phakomatosis pigmentovascularis with Sturge-Weber syndrome: a case report. J Dermatol, 27(7), 467–470. [DOI] [PubMed] [Google Scholar]
- Van Gysel D, Oranje AP, Stroink H, & Simonsz HJ (1996). Phakomatosis pigmentovascularis. Pediatr Dermatol, 13(1), 33–35. [DOI] [PubMed] [Google Scholar]
- Verma SB, Desai HK, Shah VN, & Happle R (2017). Phacomatosis Cesioflammea with Cutis Marmorata-like Lesions and Unusual Extracutaneous Abnormalities: Is It a Distinct disorder? Indian J Dermatol, 62(2), 207–209. doi: 10.4103/0019-5154.201760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurri-de la Cruz H, Tamayo-Sanchez L, Duran-McKinster C, Orozco-Covarrubias Mde L, & Ruiz-Maldonado R (2003). Phakomatosis pigmentovascularis II A and II B: clinical findings in 24 patients. J Dermatol, 30(5), 381–388. [DOI] [PubMed] [Google Scholar]
- Villarreal DJ, & Leal F (2016). Phacomatosis pigmentovascularis of cesioflammea type. An Bras Dermatol, 91(5 suppl 1), 54–56. doi: 10.1590/abd1806-4841.20164516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobser M, Goebeler M, & Hamm H (2013). Extensive red and blue patches in a young girl. Klin Padiatr, 225(1), 46–47. doi: 10.1055/s-0032-1329946 [DOI] [PubMed] [Google Scholar]
- Woo SH, Kwak HB, Park SK, Yun SK, Kim HU, & Park J (2017). An unusual case of phakomatosis pigmentovascularis type IIb with Becker’s nevus. Eur J Dermatol, 27(1), 83–85. doi: 10.1684/ejd.2016.2886 [DOI] [PubMed] [Google Scholar]
- Yang Y, Guo X, Xu J, Ye Y, Liu X, & Yu M (2015). Phakomatosis Pigmentovascularis Associated With Sturge-Weber Syndrome, Ota Nevus, and Congenital Glaucoma. Medicine (Baltimore), 94(26), e1025. doi: 10.1097/md.0000000000001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, … Eng CM (2013). Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med, 369(16), 1502–1511. doi: 10.1056/NEJMoa1306555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung HH (2010). Glaucoma associated with phacomatosis pigmentovascularis. J Pediatr Ophthalmol Strabismus, 47(1), 10, 33. doi: 10.3928/01913913-20100106-02 [DOI] [PubMed] [Google Scholar]
- Zallmann M, Mackay MT, Leventer RJ, Ditchfield M, Bekhor PS, & Su JC (2018). Retrospective review of screening for Sturge-Weber syndrome with brain magnetic resonance imaging and electroencephalography in infants with high-risk port-wine stains. Pediatr Dermatol, 35(5), 575–581. doi: 10.1111/pde.13598 [DOI] [PubMed] [Google Scholar]
- Zhou H, Han J, Mao R, & Chen M (2016a). Choroidal melanoma in phacomatosis pigmentovascularis cesio fl ammea. Indian J Dermatol Venereol Leprol. doi: 10.4103/0378-6323.174387 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.