Abstract
Women with history of pregnancy loss (PL) have higher burden of cardiovascular disease (CVD) later in life, yet it is unclear whether this is attributable to an association with established CVD risk factors (RFs). We examined whether PL is associated with CVD RFs and biomarkers among parous postmenopausal women in the Women’s Health Initiative, and whether the association between PL and CVD RFs accounted for the association between PL and incident CVD. Linear and logistic regressions were used to estimate associations between baseline history of PL and CVD RFs. Cox proportional hazards regression models were used to estimate the associations between baseline history of PL and incident CVD after adjustment for baseline RFs. Of 79,121 women, 27,272 (35%) had experienced PL. History of PL was associated with higher BMI (p<0.0001), hypertension (p<0.0001), diabetes (p=0.003), depression (p<0.0001), and lower income (p<0.0001), physical activity (p=0.01), poorer diet (p<0.0001), smoking (p<0.0001) and alcohol use (p<0.0001). After adjustment for CVD RFs, PL was significantly associated with incident CVD over mean follow up of 16 years (HR 1.11, 95% CI 1.06-1.16). In conclusion, several CVD RFs are associated with PL, but they do not entirely account for the association between PL and incident CVD.
Keywords: Cardiovascular Disease, Miscarriage, Pregnancy Loss, Women
Introduction
Despite considerable advances in both the prevention and treatment of cardiovascular disease (CVD), it still remains the leading cause of mortality among women.1,2 Women have a different CVD risk profile compared with men, and key reproductive factors unique to women including pregnancy, complications of pregnancy, and infertility, have been previously associated with incident CVD.3–6 A history of pregnancy loss (PL), including spontaneous miscarriage and stillbirth, has been associated with up to a 2.7-fold increased odds of maternal CVD later in life.7–13 Among postmenopausal women in the Women’s Health Initiative (WHI) Study, we previously demonstrated a 19% and 27% increased odds of CVD associated with a history of miscarriage and stillbirth, respectively.12 The exact mechanisms underlying the association between PL and CVD are uncertain. Therefore, we sought to evaluate whether a history of PL is associated with either modifiable CVD risk factors (i.e. hypertension, dyslipidemia, tobacco use, and insulin resistance), or novel pathways including inflammation or hypercoagulability, 14,15 and whether the association between PL and CVD could be fully explained by these risk factors.
Methods
The design of WHI has been described previously.16,17 Briefly, WHI consists of a set of clinical trials and an observational study in which 161,808 women were enrolled from 1993 to 1998 at 40 clinical centers in the United States. We excluded women with preexisting CVD (n=27,247), including coronary heart disease (CHD), heart failure (HF), or stroke, or with incomplete risk factor and reproductive data (n=43,438) (Figure 1). We additionally excluded women with history of elective abortion (n=l,836) or ectopic pregnancy (n=45). In our primary analysis we chose to study only parous women (n=79,121), due to known associations between both nulliparity and infertility with CVD, and we studied nulliparous women in secondary analyses (n=10,121).5,18,19
Figure 1: Creation of the Study Sample.
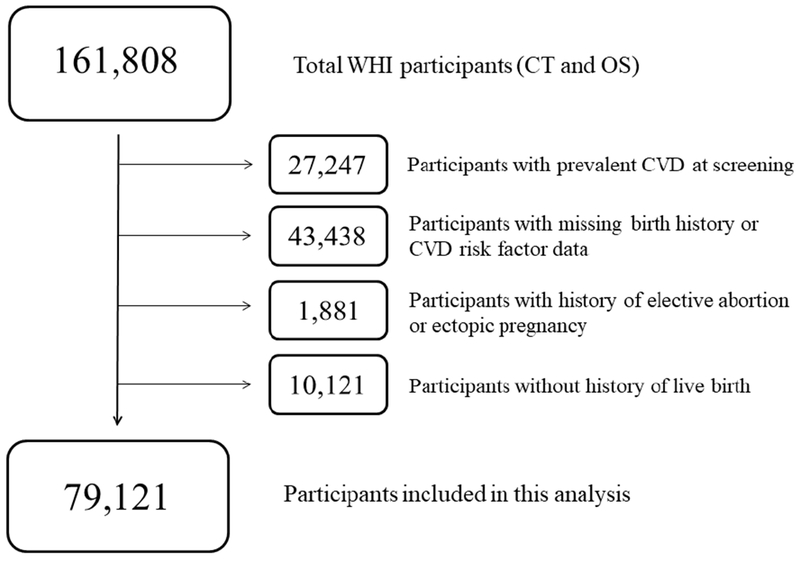
CT, clinical trial; OS, observational study
PL, the primary exposure, was defined as a self-reported history of either spontaneous miscarriage or stillbirth. Information on pregnancy history was obtained at baseline by questionnaires.16,17 Women were asked to report the number of live births, stillbirths from pregnancies lasting at least six months, and spontaneous miscarriages that they experienced. Covariates included age at screening, race/ethnicity, and age at first pregnancy lasting at least six months, which was documented as a categorical variable in WHI (<20, 20-24, 25-29, ≥30 years).
CVD RFs were assessed at baseline enrollment.16,17 For this analysis, RFs included body-mass index (BMI, kg/m2), systolic blood pressure (mm Hg), hypertension (defined by self-report, systolic blood pressure ≥140 mm Hg or diastolic blood pressure >90 mm Hg at screening, or use of antihypertensive medication), diabetes (defined by self-reported physician diagnosis or use of diabetes medication), hyperlipidemia (defined as self-reported high cholesterol requiring the use of medication, smoking status (current, previous, or never), physical activity (defined as metabolic equivalent hours (MET-hours) per week), alcohol use (current drinks per week, previous alcohol consumption, or never use), annual household income, education level, neighborhood socioeconomic status index (defined in WHI based on census tract data), psychosocial history of depression (defined using the Burnam screening algorithm, a short version of the Center for Epidemiologic Studies – Depression Scale [CES-D]), and diet quality (defined using the Healthy Eating Index [HEI-2005]).20,21 Serum levels of the biomarkers C-reactive protein (mg/dl), fibrinogen (mg/dl), interleukin-6 (pg/ml), and white blood cell (WBC) count (cells/μL) were available on subsets of the study population, having been measured as part of prior ancillary studies22.
Incident CVD was a cumulative outcome defined as acute myocardial infarction, stroke, venous thromboembolism, peripheral arterial disease, coronary revascularization, or death attributed to cardiovascular causes. CVD events were systematically adjudicated by physician members of the Cardiovascular Central Adjudication Committee, as described previously.17
Baseline characteristics by history of PL are shown as means and standard deviations for continuous variables and frequencies and proportions for categorical variables. For the subset of participants with biomarkers, the median and interquartile range are shown. Linear and logistic regression models were used to estimate the cross-sectional associations between a history of PL and CVD RFs individually, adjusting for age at enrollment, race/ethnicity, history of live birth, and age at first pregnancy lasting at least six months. Cox proportional hazards regression models were used to estimate associations between a history of PL and time to CVD event. We tested the assumption of proportional hazards with Kaplan-Meier survival plots. The baseline model was adjusted for age at enrollment, race/ethnicity, history of live birth, age at first pregnancy lasting at least six months, and WHI study enrollment (clinical trial or observational study component). For secondary analyses, Cox proportional hazards analyses were used to estimate associations between PL and the individual components of the combined CVD outcome. In the subset of participants with complete biomarker data, we used linear regression to estimate associations between PL and log-transformed biomarkers, and Cox models to estimate the association between PL and incident CVD adjusting for CVD RFs and log-transformed bio markers. We used Fine-Gray models to assess sensitivity of the results to the competing risk of death not due to cardiovascular causes during follow up. All analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Of the 79,121 women included in the analysis, 27,272 (35%) reported a history of PL, 25,573 (32%) reporting spontaneous miscarriage and 3,405 (4%) reporting stillbirth. Baseline demographic and reproductive characteristics of this sample are presented in Table 1. The mean age at screening was 63.1 years, and the average number of live births was 3.2.
Table 1:
Baseline Demographic and Reproductive Characteristics of the Study Sample (n-79,121), and the Association of Pregnancy Loss with CVD Risk Factors
| Patient Characteristics | Pregnancy Loss | ||
|---|---|---|---|
| Yes (n=27,272) |
No (n=51,849) |
P value* | |
| Age at screening (years) | 63.4 (±6.9) | 63.0 (±7.1) | |
| White (non-Hispanic) | 22,940 (84.1%) | 45,066 (86.9%) | |
| Black | 2,314 (8.5%) | 3,161 (6.1%) | |
| Hispanic/Latino | 1,211 (4.4%) | 1,826 (3.5%) | |
| Other | 807 (3.0%) | 1,796 (3.5%) | |
| Number of Pregnancies | 4.8 (±1.7) | 3.0 (±1.4) | |
| Pregnancy Loss | |||
| Miscarriage | 25,573 (93.8%) | 0 | |
| Stillbirth | 3,405 (12.5%) | 0 | |
| Hysterectomy | 12,179 (44.7%) | 22,149 (42.7%) | |
| Age at Menopause (years) | 47.2 (±6.7) | 47.6 (±6.5) | |
| Oral Contraceptive (ever use) | 12,128 (44.5%) | 22,432 (43.3%) | |
| Hormone Therapy (ever use) | 6,872 (25.2%) | 14,458 (27.9%) | |
| Body-mass index (kg/m2) | 28.2 (±5.9) | 27.7 (±5.7) | <0.0001 |
| Hypertension | 8,926 (32.7%) | 15,741 (30.4%) | <0.0001 |
| Systolic blood pressure (mm Hg) | 127.7 (±17.6) | 126.9 (±17.4) | 0.008 |
| Diastolic blood pressure (mm Hg) | 75.4 (±9.2) | 75.3 (±9.1) | 0.007 |
| Diabetes mellitus | 1,246 (4.6%) | 2,020 (3.9%) | 0.003 |
| Hyperlipidemia | 3,376 (12.4%) | 6,231 (12.0%) | 0.47 |
| Smoking status | <0.0001 | ||
| Current | 13,775 (50.5%) | 27,799 (53.6%) | |
| Former | 2,014 (7.4%) | 3,159 (6.1%) | |
| Never | 11,483 (42.1%) | 20,891 (40.3%) | |
| Socioeconomic Status Index | 75.7 (±8.7) | 76.2 (±8.1) | 0.01 |
| Psychosocial history of Depression | 6,461 (23.7%) | 11,478 (22.1%) | <0.0001 |
| Physical Activity, MET - hours/week | 12.3 (±13.5) | 12.7 (±13.7) | 0.01 |
| Healthy Eating Index | 64.2 (±10.8) | 64.7 (±10.7) | <0.0001 |
| Alcohol use (drinks/week) | <0.0001 | ||
| >7 | 3,163 (11.6%) | 6,140 (11.8%) | |
| 1-7 | 7,090 (26.0%) | 13,652 (26.3%) | |
| <1 | 9,160 (33.6%) | 17,204 (33.2%) | |
| Former | 5,022 (18.4%) | 8,778 (16.9%) | |
| Never | 2,837 (10.4%) | 6,075 (11.7%) | |
| Household Income ($/year) | <0.0001 | ||
| <$20,000 | 4,622 (17.0%) | 7,435 (14.3%) | |
| $20,000-$74,999 | 17,720 (65.0%) | 34,011 (65.6%) | |
| ≥$75,000 | 4,930 (18.1%) | 10,403 (20.1%) | |
| Education Level | 0.50 | ||
| ≤High School | 6,427 (23.6%) | 12,241 (23.6%) | |
| ≥Some College | 17,925 (65.7%) | 34,156 (65.9%) | |
SD, standard deviation
Linear or logistic regression adjusted for age at enrollment, race/ethnicity, and age at first pregnancy lasting ≥6 months
Baseline levels of CVD RFs are presented in Table 1, along with the corresponding tests for association with a history of PL. PL was associated with higher BMI (p<0.0001), systolic blood pressure (p=0.008), diastolic blood pressure (p=0.007), hypertension (p<0.0001), diabetes (p=0.003), depression (p<0.0001), poorer diet (p<0.0001), lower physical activity (p=0.01), lower SES index (p=0.01), and lower income (p<0.0001). PL was associated with lower prevalence of current smoking (p<0.0001) and alcohol use (p<0.0001). Table 2 presents the biomarkers, along with their tests for association with a history of PL. PL was associated with higher levels of interleukin-6 (p=0.02).
Table 2:
Association of Biomarkers with History of Pregnancy Loss
| Biomarkers | Pregnancy Loss | ||
|---|---|---|---|
| Yes | No | P-value* | |
| C-reactive protein (mg/dl) | n=8,436 2.80 (1.26-5.90) |
n=14,594 2.61 (1.19-5.49) |
0.11 |
| Fibrinogen (mg/dl) | n=2,436 284 (222-334) |
n=4,207 281 (229-331) |
0.64 |
| Interleukin-6 (pg/ml) | n=3,249 2.05 (1.13-4.25) |
n=5,923 1.83 (1.00-3.67) |
0.02 |
| White blood cell count (cells/μl) | n=27,091 57 (4.8-6.8) |
n=51,507 5.6 (4.8-67) |
0.93 |
Values expressed as medians with interquartile range
Linear or logistic regression adjusted for age at enrollment, race/ethnicity, and age at first pregnancy lasting ≥6 months
The mean and median follow-up duration were 16.6 years and 18.2 years, respectively. There were 8,882 adjudicated incident CVD events during follow up (11%). Table 3 presents the types of incident CVD event experienced during follow-up stratified by history of PL, as well as the hazard ratios and 95% confidence intervals for development of incident CVD based upon a history of PL. In the baseline model PL was associated with 14% higher hazards of incident CVD, (HR 1.14, 95% CI 1.10-1.19). After adjustment for CVD RFs, the association was only minimally attenuated (HR 1.11, 95% CI 1.06-1.16). In the subset of n=3,282 women with complete biomarkers, accounting for inflammation and coagulation biomarkers yielded a similar hazard ratio for the association between PL and CVD (1.19, 95% CI 1.05-1.35). Analyses accounting for competing risks of non-cardiovascular death were not materially different (data not shown).
Table 3:
Association of Pregnancy Loss with Incident CVD During Follow Up (n=79,121)
| Pregnancy Loss | |||
|---|---|---|---|
| Yes (n=27,272) |
No (n=51,849) |
Adjusted Hazard Ratio* (95% CI) | |
| Incident Cardiovascular Disease | 3,369 (12.4%) | 5,513 (10.6%) | 1.11 (1.06-1.16) |
| Cardiovascular Death | 427 (1.6%) | 629 (1.2%) | 1.20 (1.06-1.36) |
| Myocardial Infarction | 1,051 (3.9%) | 1,698 (3.3%) | 1.11 (1.03-1.20) |
| Stroke | 1,058 (3.9%) | 1,848 (3.6%) | 1.03 (0.96-1.11) |
| Pulmonary Embolism | 159 (0.6%) | 243 (0.5%) | 1.16 (0.95-1.42) |
| Peripheral Arterial Disease | 232 (0.9%) | 343 (0.7%) | 1.17 (0.99-1.38) |
| Coronary Revascularization | 1,445 (5.3%) | 2,338 (4.5%) | 1.13 (1.06-1.21) |
Cox Proportional Hazards regression of incident CVD based upon history of pregnancy loss, adjusted for age at enrollment, race/ethnicity, age at first pregnancy lasting at least six months, clinical trial/observational study status, systolic blood pressure, diabetes, hyperlipidemia, smoking status, body-mass index, income, education, socioeconomic status index, alcohol use, depression, physical activity, and Healthy Eating Index 2005.
In secondary analyses among n=10,121 nulliparous women, a history of PL was associated with increased BMI (p=0.02), diabetes (p=0.02), and lower education level (p=0.02), and also with lower prevalence of both smoking (p=0.0003) and heavy alcohol use (p=0.002) (Table 4). There were no significant associations between a history of PL and levels of C-reactive protein, interleukin-6, white blood cell count, or fibrinogen (Table 5). A history of PL was not associated with a statistically significant increased incidence of CVD during follow up (Table 6).
Table 4:
Baseline Demographic and Reproductive Characteristics of the Nulliparous Women (n=10,121)
| Patient Characteristics | Pregnancy Loss | ||
|---|---|---|---|
| Yes (n=l,074) |
No (n=9,047) |
P value* | |
| Age at screening (years) | 62.9 (±7.8) | 62.5 (±7.7) | |
| White (non-Hispanic) | 777 (72.4%) | 7,731 (85.5%) | |
| Black | 212 (19.7%) | 619 (6.8%) | |
| Hispanic/Latino | 45 (4.2%) | 331 (3.7%) | |
| Other | 40 (3.7%) | 366 (4.1%) | |
| Number of Pregnancies | 1.8 (±1.2) | 0 | |
| Pregnancy Loss | |||
| Miscarriage | 1,013 (94.3%) | 0 | |
| Stillbirth | 115 (10.7%) | 0 | |
| Hysterectomy | 512 (47.7%) | 3,542 (39.2%) | |
| Oral Contraceptive (ever use) | 375 (34.9%) | 2,650 (29.3%) | |
| Hormone Therapy (ever use) | 262 (24.3%) | 2,511 (27.8%) | |
| Age at menopause (years) | 46.3 (±7.0) | 47.1 (±6.3) | |
| Body-mass index (kg/m2) | 28.4 (±6.6) | 27.4 (±6.1) | 0.02 |
| Hypertension | 381 (35.5%) | 2,628 (29.1%) | 0.06 |
| Systolic blood pressure (mm Hg) | 128.0 (±18.2) | 125.9 (±17.7) | 0.10 |
| Diastolic blood pressure (mm Hg) | 75.4 (±9.2) | 75.3 (±9.1) | 0.15 |
| Diabetes mellitus | 72 (6.7%) | 350 (3.9%) | 0.02 |
| Hyperlipidemia | 3,376 (12.4%) | 6,231 (12.0%) | 0.47 |
| Smoking status | 0.0003 | ||
| Current | 496 (46.2%) | 4,818 (53.3%) | |
| Former | 92 (8.6%) | 612 (6.8%) | |
| Never | 486 (45.3%) | 3,617 (40.0%) | |
| Socioeconomic Status Index | 73.9 (±9.7) | 75.5 (±8.4) | 0.54 |
| Psychosocial history of Depression | 252 (23.5%) | 2,043 (22.6%) | 0.28 |
| Physical Activity, MET - hours/week | 12.0 (±13.5) | 12.7 (±13.7) | 0.33 |
| Healthy Eating Index | 63.9 (±11.0) | 64.5 (±11.0) | 0.47 |
| Alcohol use (drinks/week) | 0.002 | ||
| >7 | 131 (12.2%) | 1,325 (14.7%) | |
| 1-7 | 291 (27.1%) | 2,486 (27.5%) | |
| <1 | 332 (30.9%) | 2,940 (32.5%) | |
| Former | 236 (22.0%) | 1,439 (15.9%) | |
| Never | 84 (7.8%) | 857 (9.5%) | |
| Household Income ($/year) | 0.17 | ||
| <$20,000 | 194 (18.1%) | 1,357 (15.0%) | |
| $20,000-$74,999 | 683 (63.6%) | 6,058 (67.0%) | |
| ≥$75,000 | 197 (18.3%) | 1,632 (18.0%) | |
| Education Level | 0.02 | ||
| ≤High School | 166 (15.5%) | 1,185 (13.1%) | |
| ≥Some College | 799 (74.4%) | 7,211 (79.7%) | |
Table 5:
Association of Biomarkers with History of Pregnancy Loss Among Nulliparous Women
| Biomarkers | Pregnancy Loss | ||
|---|---|---|---|
| Yes | No | P-value* | |
| C-reactive protein (mg/dl) | n=369 2.60 (1.20-6.10) |
n=2,354 2.25 (0.97-4.80) |
0.85 |
| Fibrinogen (mg/dl) | n=113 292 (238-345) |
n=677 286 (222-334) |
0.94 |
| Interleukin-6 (pg/ml) | n=133 2.03 (1.23-4.77) |
n= 1,049 1.78 (0.97-3.59) |
0.55 |
| White blood cell count (cells/μl) | n=l,066 5.6 (4.7-6.7) |
n=8,965 5.6 (4.7-6.6) |
0.83 |
Values expressed as medians with interquartile range
Linear or logistic regression adjusted for age at enrollment, race/ethnicity, and age at first pregnancy lasting ≥6 months
Table 6:
Association of Pregnancy Loss with Incident CVD During Follow Up Among Nulliparous Women (n=10,121)
| Pregnancy Loss | |||
|---|---|---|---|
| Yes (n=1,074) |
No (n=9,047) |
Adjusted Hazard Ratio* (95% CI) | |
| Incident Cardiovascular Disease Event | 124 (11.6%) | 893 (9.9%) | 1.04 (0.85-1.28) |
| Cardiovascular Death | 20 (1.9%) | 125 (1.4%) | 1.37 (0.84-2.24) |
| Myocardial Infarction | 30 (2.8%) | 268 (3.0%) | 0.90 (0.61-1.35) |
| Stroke | 47 (4.4%) | 301 (3.3%) | 1.14 (0.81-1.60) |
| Pulmonary Embolism | 5 (0.5%) | 36 (0.4%) | 0.99 (0.38-2.58) |
| Peripheral Arterial Disease | 7 (0.7%) | 68 (0.8%) | 0.76 (0.34-1.68) |
| Coronary Revascularization | 58 (5.4%) | 329 (3.6%) | 1.29 (0.95-1.76) |
Cox Proportional Hazards regression of incident CVD based upon history of pregnancy loss, adjusted for age at enrollment, race/ethnicity, age at first pregnancy lasting at least six months, clinical trial/observational study status, systolic blood pressure, diabetes, hyperlipidemia, smoking status, body-mass index, income, education, socioeconomic status index, alcohol use, depression, physical activity, and Healthy Eating Index 2005.
Discussion
In this large prospective study of postmenopausal women, we extend prior findings that have demonstrated an association between PL and CVD, with four new and important novel findings. 7,8,10,12 First, we found that a history of PL was associated with several important CVD risk factors in mid-life, including hypertension, diabetes, body-mass index, depression, poor diet, and lower income level, lower physical activity as predicted, but also with lower prevalence of smoking or heavy alcohol use (>7 drinks/week). Second, we observed that CVD risk factors in mid-life do not fully explain the association between PL and CVD. Third, we demonstrated that a history of PL is associated with increased serum levels of interleukin-6 in mid-life. Fourth, we observed that among nulliparous women, PL was associated with diabetes, body-mass index, and lower education level, with lower prevalence of smoking or heavy alcohol use.
The previously demonstrated increased hazard of CVD related to PL was reproduced in this analysis, but with a relatively more modest effect size (11-14% excess hazard).4,7,10–13 Importantly, the estimated excess hazards associated with PL remained essentially unchanged after adjustment for CVD RFs and inflammatory bio markers, suggesting that important underlying mechanisms between PL and CVD may not be reflected in the several risk factors that we considered.
Investigating the association between PL and CVD is challenging, in part due to the heterogeneous causes of PL, and in part due to the significant time-lag between women’s reproductive period and the development of CVD. PL is estimated to occur in 20-30% of pregnancies, and multiple PLs are estimated to affect 1-2% of women.23,24 PL can be caused by maternal, placental, and fetal factors. Fetal factors, such as chromosomal abnormalities or other genetic defects, are estimated to cause about 35% of PL, with placental abnormalities estimated to cause about 27%.25 The remainder has been attributed to maternal factors such as cervical dysfunction, preterm labor, infection, or other underlying maternal diseases, especially those involving hypercoagulability or autoimmune pathology.25,26 Maternal factors such as obesity, hypercoagulability, polycystic ovarian syndrome, impaired glucose tolerance, gestational diabetes, and other endocrine abnormalities have each been associated with PL, and likely contribute to CVD risk later in life.27,28 Importantly, the causes underlying ~ 10% of PL are unknown.25,26 Our analysis included several of these important maternal factors, but detailed information on gestational diabetes, pregnancy-induced hypertension, preeclampsia, preterm delivery, hypercoagulability, and PCOS was not available in the cohort and may merit further investigation in future studies. Our finding that smoking was inversely associated with PL was somewhat surprising, as smoking has been previously associated with miscarriage, but smoking has also been associated with a reduced risk of preeclampsia.29,30 What is clear is that cigarette smoking did not underlie the increased risk of CVD with PL in the WHI cohort.
Strengths of our study include the large study population with a long follow up and information on a large number of CVD RFs. Our study also has several limitations. Importantly, all of the pregnancy history, risk factor assessment, and covariate ascertainment occurred at enrollment in WHI, when participants had already reached menopause. Information on CVD RFs during the reproductive period prior to pregnancy loss were not available, and pregnancy history was determined by self-report. Only 4% of the sample had complete inflammatory biomarker data, and 27% had missing reproductive or CVD RF data. We also did not have available biomarkers of hypercoagulability, which have been associated with pregnancy loss and may have contributed to the increased risk of CVD.27 Although this was a large population, our results should still be considered to be hypothesis-generating, and the underlying potential mechanisms warrant further investigation. However, this analysis did reproduce a significant association between PL and CVD, albeit with a relatively more modest effect size, which may be related to adjustment for additional RFs.
In conclusion, among postmenopausal women in WHI, a history of PL was significantly associated with incident CVD even after adjustment for CVD RFs, reproductive, sociodemographic, behavioral, and lifestyle factors, as well as inflammatory biomarkers. PL should be recognized as an independent, modest risk marker for CVD, and further investigation into potential underlying mechanisms is warranted.
Acknowledgement:
This work was supported by the American College of Cardiology ACC Merck Award (Philip Hall), American Heart Association Grant 13CRP17350002 (Nisha I. Parikh), NIH Grant Support provided by NIH grant 7R21HL115398 (Nisha I. Parikh). The WHI programs is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts, HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Agreement
This attached manuscript has been approved by all co-authors and by the Publications and Presentations committee of the Women’s Health Initiative. An early abstract was presented at the American Heart Association meeting in November 2017, but this has not been previously published and this manuscript is not currently under consideration for publication elsewhere.
References
- 1.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK, American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nabel EG. Heart Disease Prevention in Young Women: Sounding an Alarm. Circulation 2015;132:989–991. [DOI] [PubMed] [Google Scholar]
- 3.Hall PS, Nah G, Howard BV, Lewis CE, Allison MA, Sarto GE, Waring ME, Jacobson LT, Manson JE, Klein L, Parikh NI. Reproductive Factors and Incidence of Heart Failure Hospitalization in the Women’s Health Initiative. J Am Coll Cardiol 2017;69:2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas-Sun E, Ryckman KK, Waring ME, Schenken RS, Johnson KC, Edstedt-Bonamy A-K, Allison MA, Howard BV. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation 2016;133:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod Oxf Engl 2012;27:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rich-Edwards JW. The predictive pregnancy: what complicated pregnancies tell us about mother’s future cardiovascular risk. Circulation 2012;125:1336–1338. [DOI] [PubMed] [Google Scholar]
- 7.Maino A, Siegerink B, Algra A, Martinelli I, Peyvandi F, Rosendaal FR. Pregnancy loss and risk of ischaemic stroke and myocardial infarction. Br J Haematol 2016;174:302–309. [DOI] [PubMed] [Google Scholar]
- 8.Ranthe MF, Andersen EAW, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of atherosclerotic disease. Circulation 2013;127:1775–1782. [DOI] [PubMed] [Google Scholar]
- 9.Ranthe MF, Boyd HA. Miscarriage and cardiovascular disease. Heart Br Card Soc 2015;101:1933–1934. [DOI] [PubMed] [Google Scholar]
- 10.Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: a prospective population-based cohort study (EPIC-Heidelberg). Heart Br Card Soc 2011;97:49–54. [DOI] [PubMed] [Google Scholar]
- 11.Kharazmi E, Fallah M, Luoto R. Miscarriage and risk of cardiovascular disease. Acta Obstet Gynecol Scand 2010;89:284–288. [DOI] [PubMed] [Google Scholar]
- 12.Parker DR, Lu B, Sands-Lincoln M, Kroenke CH, Lee CC, O’Sullivan M, Park HL, Parikh N, Schenken RS, Eaton CB. Risk of cardiovascular disease among postmenopausal women with prior pregnancy loss: the women’s health initiative. Ann Fam Med 2014;12:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, Missmer SA, Rich-Edwards JW. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG Int J Obstet Gynaecol 2019;126:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Farina F, Raparelli V, Napoleone L, Guadagni F, Basili S, Ferroni P. Inflammation and Thrombophilia in Pregnancy Complications: Implications for Risk Assessment and Clinical Management. Cardiovasc Hematol Disord Drug Targets 2016;15:187–203. [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anon. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 17.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S, WHI Morbidity and Mortality Committee. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol 2003;13:S122–128. [DOI] [PubMed] [Google Scholar]
- 18.Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among U.S. women. Fertil Steril 2019;111:138–146. [DOI] [PubMed] [Google Scholar]
- 19.Niemczyk NA, Catov JM, Barinas-Mitchell E, McClure CK, Roberts JM, Tepper PG, Sutton-Tyrrell K. Nulliparity is associated with less healthy markers of subclinical cardiovascular disease in young women with overweight and obesity. Obes Silver Spring Md 2015;23:1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubowitz T, Ghosh-Dastidar M, Eibner C, Slaughter ME, Fernandes M, Whitsel EA, Bird CE, Jewell A, Margolis KL, Li W, Michael YL, Shih RA, Manson JE, Escarce JJ. The Women’s Health Initiative: The food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obes Silver Spring Md 2012;20:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res 2001;103:261–270. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan RC, McGinn AP, Baird AE, Hendrix SL, Kooperberg C, Lynch J, Rosenbaum DM, Johnson KC, Strickler HD, Wassertheil-Smoller S. Inflammation and hemostasis biomarkers for predicting stroke in postmenopausal women: the Women’s Health Initiative Observational Study. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2008;17:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–194. [DOI] [PubMed] [Google Scholar]
- 24.Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod Oxf Engl 1999;14:2868–2871. [DOI] [PubMed] [Google Scholar]
- 25.Korteweg FJ, Gordijn SJ, Timmer A, Erwich JJHM, Bergman KA, Bouman K, Ravise JM, Heringa MP, Holm JP. The Tulip classification of perinatal mortality: introduction and multidisciplinary inter-rater agreement. BJOG Int J Obstet Gynaecol 2006;113:393–401. [DOI] [PubMed] [Google Scholar]
- 26.Gordijn SJ, Korteweg FJ, Erwich JJHM, Holm JP, Diem MT van, Bergman KA, Timmer A. A multilayered approach for the analysis of perinatal mortality using different classification systems. Eur J Obstet Gynecol Reprod Biol 2009;144:99–104. [DOI] [PubMed] [Google Scholar]
- 27.Matjila MJ, Hoffman A, Spuy ZM van der. Medical conditions associated with recurrent miscarriage-Is BMI the tip of the iceberg? Eur J Obstet Gynecol Reprod Biol 2017;214:91–96. [DOI] [PubMed] [Google Scholar]
- 28.Smith GCS. Screening and prevention of stillbirth. Best Pract Res Clin Obstet Gynaecol 2017;38:71–82. [DOI] [PubMed] [Google Scholar]
- 29.Conde-Agudelo A, Althabe F, Belizán JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol 1999;181:1026–1035. [DOI] [PubMed] [Google Scholar]
- 30.Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 2014;179:807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]


