Abstract
Although atrial fibrillation/atrial flutter (AF/AFL) and heart failure with preserved ejection fraction (HFpEF) frequently coexist, the influence of AF/AFL on physical activity, NT-proBNP, and quality of life (QOL) in HFpEF is unclear and could have relevance to HFpEF trial design. We evaluated the association between AF/AFL and volitional physical activity, functional performance, NT-proBNP, and QOL in patients with HFpEF in the Nitrate’s Effect on Activity Tolerance (NEAT)-HFpEF trial. Of 99 patients with accelerometer data, 35 (35%) had AF/AFL. There were no differences between AF/AFL vs. no AF/AFL in baseline average daily accelerometer units (ADAUs; 9.06±0.54 vs. 9.06±0.48, P=0.75), hours active per day (9.7±2.3 vs. 9.2±2.2, P=0.86), or 6-minute walk distance (6MWD; 307±136m vs. 321±110m, P=0.85). AF/AFL status was associated with higher baseline NT-proBNP (586 [25th – 75th percentile: 291–1254] pg/mL vs. 154 [25th – 75th percentile: 92–288] pg/mL, P<0.001) and Kansas City Cardiomyopathy Questionnaire scores (69 [25th – 75th percentile: 46–88] vs. 48 [25th – 75th percentile: 37–70], P=0.01). While treatment responses to isosorbide mononitrate measured by change in ADAUs, hours active per day, or 6MWD did not vary by AF/AFL status (interaction P>0.05 for all), AF/AFL patients had greater reductions in NT-proBNP after isosorbide mononitrate than patients without AF/AFL (interaction P<0.001), possibly due to regression to the mean. In conclusion, baseline measures and treatment-related changes in volitional physical activity (ADAUs) and functional performance (6MWD) did not differ by AF/AFL in NEAT-HFpEF, whereas NT-proBNP did. In HFpEF—where AF/AFL prevalence is high—functional measures may be superior to natriuretic peptides as trial endpoints.
Keywords: atrial fibrillation, functional status, heart failure with preserved ejection fraction, clinical trials
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous clinical syndrome, and disease-modifying therapies to alter its natural progression are lacking.1 Indeed, several potential therapies for HFpEF which appeared promising in early-phase clinical trials based on surrogate endpoints ultimately have proved disappointing in later-phase trials.2 Challenges in identification of effective pharmacotherapies in HFpEF are related to the multiple comorbidities that associate with this clinical syndrome, of which atrial fibrillation and/or atrial flutter (AF/AFL) is common.3–5 AF/AFL may influence the endpoints utilized in early-phase HFpEF trials, thus complicating endpoint interpretation.6 To identify pharmacotherapies for drug development in HFpEF, reliable endpoints are needed that reflect functional status and disease severity and that can be broadly interpretable even in patients with multiple comorbidities, such as AF/AFL. We therefore aimed to evaluate the association between AF/AFL and physical activity, biomarkers, and quality of life among patients enrolled in the Nitrate’s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction (NEAT-HFpEF) trial.
Methods
The study design and primary results of the NEAT-HFpEF trial have been published previously.7,8 NEAT-HFpEF was a multicenter, double-blind, randomized, crossover study evaluating the effect of nitrate therapy on functional status. Inclusion criteria were adult ambulatory patients with heart failure (HF) with ejection fraction ≥50% and one of the following: HF hospitalization, hemodynamic testing indicating high filling pressures, elevated natriuretic peptides, or echocardiographic evidence of diastolic dysfunction. In the NEAT-HFpEF trial, HFpEF patients who received isosorbide mononitrate had significantly lower activity levels as measured by accelerometers than did patients who received placebo.
Each enrolled patient underwent baseline physical exam, electrocardiogram (ECG), core laboratory echocardiography, core laboratory N-terminal pro-B-type natriuretic peptide (NT-proBNP) measurement, Kansas City Cardiomyopathy Questionnaire (KCCQ) quality of life scores, and baseline activity levels via accelerometers and 6-minute walk distance (6MWD). Information regarding history of AF/AFL and type of AF/AFL was obtained. A baseline ECG was performed and presenting rhythm was adjudicated. All patients enrolled in the NEAT-HFpEF trial provided written, informed consent, and the clinical trial protocol was approved by the institutional review board at all enrolling institutions.
The protocol for the accelerometry in NEAT-HFpEF has been previously described.8 Daily activity was monitored by belts that contained 2 kinetic activity monitors (Kersh Health) with tri-axis accelerometers (KXUD9–2050, Kionix, Ithaca, NY).9 Patients were instructed to wear the belt for 24 hours each day. The accelerometers measure movement, which is expressed as accelerometer units. The kinetic activity monitors recorded accelerometer units continuously and stored values in 15-minute segments, corresponding to 96 values per day that were averaged to provide daily levels.9 Average daily accelerometer units (ADAUs) over specific time periods were calculated at baseline. Accelerometers also provided information regarding hours active per day, defined as the daily number of 15-minute cumulative accelerometer units >50. ADAUs and hours active per day were recorded continually during initiation of study drug (isosorbide mononitrate) until the maximally tolerated dose was reached. Trial protocol also outlined measurement of 6MWD, core lab NT-proBNP levels, and KCCQ scores at both baseline and 4 weeks after initiation and titration of isosorbide mononitrate or placebo to their maximally tolerated doses.
Data for analysis were obtained through the Biologic Specimen and Data Repository Information Coordinating Center of the National Heart, Lung, and Blood Institute. Depending on normality, continuous variables were expressed as median (25th – 75th percentiles) or mean (± standard deviation) and categorical variables were expressed as number (%). Wilcoxon rank sum tests or Student’s t-test and Pearson χ2 tests were used to compare continuous and categorical variables, respectively, by history of AF/AFL. Linear regression models assessed the association between AF/AFL history and baseline volitional physical activity (ADAUs and number of hours active per day), functional performance (6MWD), quality of life (KCCQ scores), and NT-proBNP levels. ADAUs and NT-proBNP levels were log-transformed for all baseline regression analyses due to skewed distribution. A sensitivity analysis was also performed to assess the association between AF/AFL on baseline ECG and ADAUs. Models were adjusted for baseline clinical variables that have been associated with reduced ADAUs: age, sex, height, and body mass index.9 Height and weight were tested for collinearity and demonstrated no significant correlation (r=0.08, P=0.41). To evaluate potential effect modification by AF/AFL status in treatment response, linear regression models were used to evaluate the change in all endpoints after administration of target dose isosorbide mononitrate using an interaction term for history of AF/AFL. Models were adjusted for their respective baseline endpoint values. All statistical analyses were performed using R version 3.5.0 (R Foundation for Statistical Computing). The secondary research protocol was approved by the Institutional Review Board of Northwestern University.
Results
Among the NEAT-HFpEF population (n=110), 11 patients did not have adequate baseline accelerometer data due to poor compliance (n=9), withdrawal of consent (n=1), and lost accelerometer (n=1). The remaining 99 patients with accelerometer data comprised the final study cohort. Over one-third of patients (n=35, 35%) had a history of AF/AFL, 16% (n=16) had persistent/permanent AF, and AF/AFL was the presenting rhythm on ECG in 14% (n=14). Patients with AF/AFL history were older, more likely to be men, and carried lower rates of sleep apnea, depression, and ischemic heart disease compared with those without AF/AFL (P<0.05 for all comparisons) (Table 1). Patients with AF/AFL had lower rates of New York Heart Association (NYHA) class III/IV symptoms (29% vs. 56%, P=0.02) compared with those without AF/AFL.
Table 1.
Clinical Profile of Patients by History of Atrial Fibrillation and/or Atrial Flutter.
| Atrial Fibrillation/Flutter | |||
|---|---|---|---|
| Characteristic | No (n=64) | Yes (n=35) | P-value |
| Age (years), median (IQR) | 67 (59 – 72) | 72 (68 – 81) | <0.001 |
| Women | 47 (73%) | 12 (34%) | <0.001 |
| White | 56 (87%) | 33 (94%) | |
| Black | 7 (11%) | 0 (0%) | 0.07 |
| Other | 1 (2%) | 2 (6%) | |
| New York Heart Association Class III/IV | 36 (56%) | 10 (29%) | 0.02 |
| Hypertension | 58 (91%) | 30 (86%) | 0.68 |
| Coronary heart disease | 46 (72%) | 15 (43%) | 0.009 |
| Diabetes mellitus | 26 (41%) | 8 (23%) | 0.12 |
| Chronic kidney disease | 11 (17%) | 7 (20%) | 0.94 |
| Chronic obstructive lung disease | 7 (11%) | 7 (20%) | 0.35 |
| Obstructive sleep apnea | 37 (61%) | 12 (34%) | 0.02 |
| Depression | 27 (42%) | 5 (14%) | 0.009 |
| AF/AFL on electrocardiogram | 0 (0%) | 14 (40%) | <0.001 |
| AF/AFL Type | - | ||
| New onset | - | 1 (3%) | |
| Paroxysmal | - | 13 (37%) | |
| Persistent/Permanent | - | 16 (46%) | |
| Unknown | - | 5 (14%) | |
| Systolic blood pressure (mmHg), median (IQR) | 127 (118 – 140) | 124 (118 – 141) | 0.76 |
| Heart Rate (bpm), median (IQR) | 68 (63 – 74) | 68 (62 – 75) | 0.48 |
| Body mass index (kg/m2), median (IQR) | 35.4 (30.5 – 42.5) | 30.7 (26.8 – 35.8) | 0.003 |
| Edema | |||
| None | 27 (42%) | 13 (38%) | 0.61 |
| Trace/Mild | 28 (44%) | 18 (53%) | |
| Moderate/Severe | 9 (14%) | 3 (9%) | |
| Elevated jugular venous pressure | 18 (28%) | 12 (35%) | 0.62 |
| Orthopnea | 34 (53%) | 18 (51%) | 0.99 |
| Sodium (mg/dL), median (IQR) | 140 (139 – 142) | 140 (137 – 141) | 0.08 |
| Creatinine (mg/dL), median (IQR) | 1.06 (0.86 – 1.30) | 1.09 (0.92 −1.29) | 0.68 |
| Hemoglobin (g/dL), median (IQR) | 13.3 (12.0 – 14.4) | 13.0 (12.2 −14.2) | 0.78 |
| N-terminal pro-B-type natriuretic peptide (pg/mL), median (IQR) | 154.1 (91.6 – 288.0) | 585.8 (291.1 – 1254.0) | <0.001 |
| Echocardiogram | |||
| Relative wall thickness, median (IQR) | 0.36 (0.30 – 0.44) | 0.38 (0.33 – 0.43) | 0.61 |
| Ejection Fraction (%), median (IQR) | 63 (56 – 65) | 61 (55 – 64) | 0.33 |
| Left atrial volume index (mL/m2), median (IQR) | 36.5 (29.6 – 43.2) | 41.4 (28.8 – 65.5) | 0.08 |
| Medial E/e’, median (IQR) | 12.6 (9.1 – 17.1) | 14.8 (10.6 – 18.3) | 0.27 |
| Right ventricular systolic pressure (mmHg) median (IQR) | 25 (21 – 29) | 27 (19 – 34) | 0.25 |
| Medications | |||
| Angiotensin converting enzyme inhibitor/ Angiotensin II receptor blocker | 43 (67%) | 18 (51%) | 0.19 |
| Mineralocorticoid antagonist | 15 (23%) | 10 (29%) | 0.75 |
| Loop diuretic | 38 (59%) | 27 (77%) | 0.12 |
| Beta-blocker | 44 69%) | 26 (74%) | 0.73 |
| Amiodarone | 1 (2%) | 1 (3%) | 0.99 |
| Anticoagulant | 5 (8%) | 23 66%) | <0.001 |
AF/AFL = atrial fibrillation and/or atrial flutter; IQR= interquartile range
The distributions of baseline metrics of volitional physical activity, functional performance, NT-proBNP, and quality of life by AF/AFL status are displayed in Figure 1. There was no difference in log-transformed ADAUs between patients by AF/AFL status (AF/AFL: 9.06±0.54, No AF/AFL: 9.06±0.48, P=0.97). Similarly, AF/AFL patients were active for a similar number of hours per day and had similar baseline 6MWD compared with those without AF/AFL (hours per day active: 9.7±2.3 vs. 9.2±2.2, P=0.32; 6MWD: 307±136 m vs. 321±110 m, P=0.58). NT-proBNP levels (585.8 pg/mL [25th−75th percentile: 291.1 – 1254.0] vs. 154.1 pg/mL [25th−75th percentile: 91.6 – 288.0], P<0.001) and KCCQ scores (69 [25th−75th percentile: 46 – 88], vs. 48 [25th−75th percentile: 37 – 70], P=0.003) were significantly higher compared with those without AF/AFL. After covariate adjustment, there remained no significant association between AF/AFL history and baseline log-transformed ADAUs (β coefficient: −0.04, 95% confidence interval [CI]: −0.26 – +0.19, P=0.75), hours active per day (β coefficient: +0.10, 95% CI: −0.92 – +1.12, P=0.86), or 6MWD (β coefficient: +5.4, 95% CI: −50.2 – +60.9, P=0.85). In sensitivity analysis, there was no significant association between AF/AFL on baseline ECG and log-transformed ADAUs (β coefficient −0.06, 95% CI: −0.33 – +0.21, P=0.64). AF/AFL history was significantly associated with higher log-transformed NT-proBNP levels (β coefficient +0.86, 95% CI: 0.38 – 1.35, P<0.001) and higher KCCQ scores (β coefficient +15.0, 95% CI: 3.4 – 27.0, P=0.01) after covariate adjustment.
Figure 1.
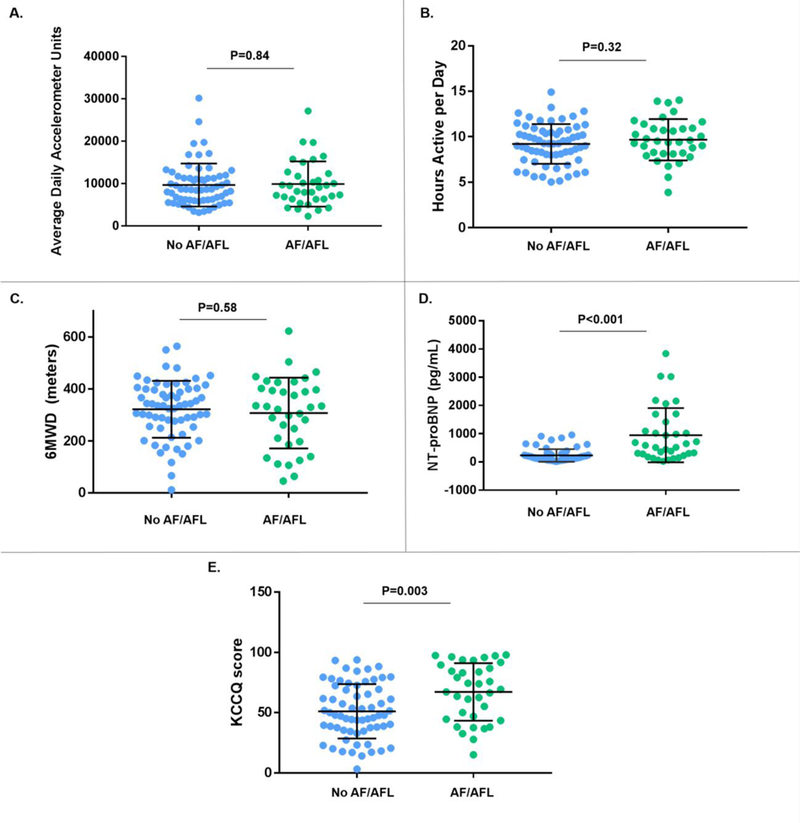
(A-E). Baseline levels of physical activity, natriuretic peptides, and quality of life scores by AF/AFL status. Bars signify mean values and standard deviation. 6MWD = 6-minute walk distance; AF/AFL: atrial fibrillation and/or atrial flutter; KCCQ= Kansas City Cardiomyopathy Questionnaire; NT-proBNP= N-terminal pro-B-type natriuretic peptide.
Endpoints after maximally tolerated doses of isosorbide mononitrate and placebo and their treatment difference by AF/AFL status are displayed in Figure 2. Regardless of AF/AFL history, there was a uniform decrease in median ADAUs (AF/AFL: −528.0 [25th−75th percentile: −1148.4 – +648.9], No AF/AFL: −468.6 [25th−75th percentile: −1174.8 – +280.1], P =0.54) and median hours active per day (AF/AFL: −0.21 [25th−75th percentile: −0.95 – +0.32], No AF/AFL: −0.37 [25th−75th percentile: −0.83 – +0.24], P=0.48) on isosorbide mononitrate therapy compared with placebo. Additionally, there were no significant differences in change in 6MWD or KCCQ scores between isosorbide mononitrate and placebo by AF/AFL status (Figure 2). After adjustment for baseline endpoint metrics, there remained no significant variation in change in ADAUs, hours active per day, 6MWD or KCCQ scores by AF/AFL status on highest tolerated isosorbide mononitrate dose (Table 2). Patients with AF/AFL history experienced significantly greater reduction in NT-proBNP concentrations after isosorbide mononitrate therapy compared with those without AF/AFL history (interaction P <0.001) (Table 2, Figure 2).
Figure 2.
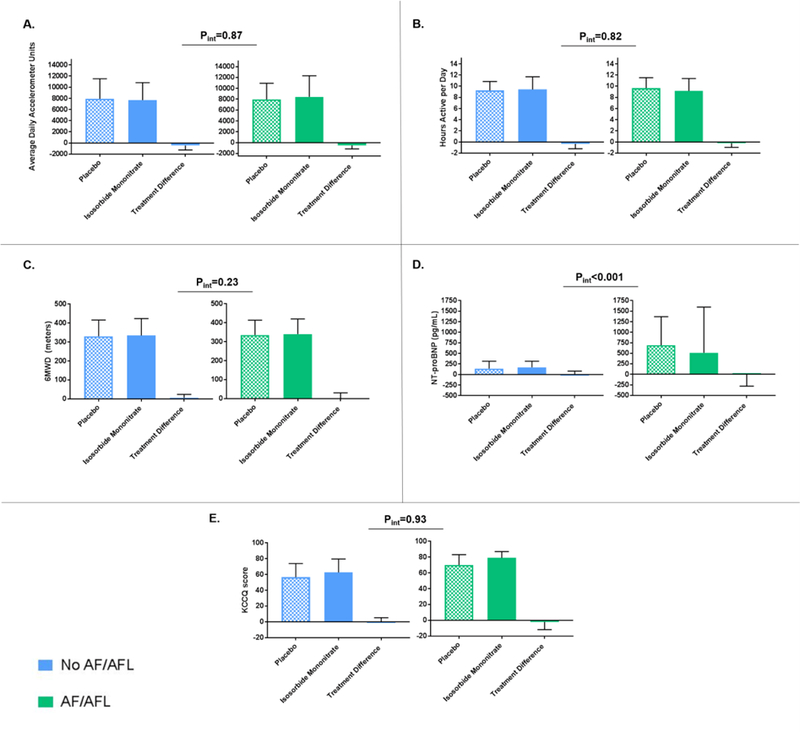
(A-E). Levels of physical activity, natriuretic peptides, and quality of life scores after maximally tolerated doses of isosorbide mononitrate and placebo and their treatment difference by AF/AFL status. Bars signify median and interquartile range. P-values represent interaction by AF/AFL status of change in endpoints from baseline after isosorbide mononitrate. 6MWD = 6-minute walk distance; AF/AFL: atrial fibrillation and/or atrial flutter; KCCQ= Kansas City Cardiomyopathy Questionnaire; NT-proBNP= N-terminal pro-B-type natriuretic peptide.
Table 2.
Subgroup Analysis: Change in Metrics of Volitional Physical Activity, Functional Performance, Quality of Life, and Natriuretic Peptides from Baseline after Isosorbide Mononitrate Therapy.
| Endpoint | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Change in ADAUs | P interaction | Change in hours active per day | P interaction | Change in 6MWD | P interaction | Change in KCCQ Score | P interaction | Change in NT-proBNP | P interaction |
| AF/AFL | ||||||||||
| Yes | −615.5 (−1976.4, −22.0) | 0.87 | −0.23 (−1.04, 0.47) | 0.82 | 2.8 (−48.0, 28.2) | 0.23 | −1.0 (−4.8, 4.4) | 0.93 | −5.5 (−135.0, 133.3) | <0.001 |
| No | −479.1 (−1768.7, 462.5) | −0.20 (−1.02, 0.46) | −8.1 (−41.3, 23.3) | 3.5 (−4.6, 15.9) | 14.3 (−14.5, 84.7) | |||||
Change in endpoints are displayed as median (interquartile range). All models are adjusted for baseline levels of respective endpoints.
6MWD = 6-minute walk distance; ADAUs= Average daily accelerometer units; AF/AFL: atrial fibrillation and/or atrial flutter; KCCQ= Kansas City Cardiomyopathy Questionnaire; NT-proBNP= N-terminal pro-B-type natriuretic peptide.
Discussion
In the NEAT-HFpEF trial, we found that a history of AF/AFL was not significantly associated with baseline volitional physical activity (ADAUs and hours active per day) or baseline functional performance (6MWD). In contrast, baseline NT-proBNP levels and KCCQ scores were significantly higher among the AF/AFL cohort after covariate adjustment. Regardless of AF/AFL status, there were uniform changes in ADAUs, hours active per day, 6MWD and KCCQ scores after treatment with isosorbide mononitrate. However, after isosorbide mononitrate, NT-proBNP levels were reduced significantly only among the AF/AFL subgroup, which could have reflected regression to the mean.
Important challenges are encountered in HFpEF clinical trial design and conduct in patients with comorbid HFpEF and AF/AFL. Certain challenges arise due to the influence of AF/AFL on several endpoints utilized in early phase trials. There are ongoing efforts to identify endpoints in early phase clinical trials of HFpEF without significant heterogeneity within the population.6 Changes in natriuretic peptide levels are common endpoints in early phase clinical trials, but vary significantly by clinical comorbidities common in HFpEF, including AF/AFL.10,11 In addition to influence on baseline NT-proBNP levels, AF/AFL status may result in differential natriuretic peptide change after certain therapies. In the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) trial, aliskiren reduced NT-proBNP levels significantly only among patients without AF/AFL.12 In our study of patients with HFpEF, significant heterogeneity in treatment response by AF/AFL status was also observed, as patients with AF/AFL experienced a more marked decrease in NT-proBNP levels with isosorbide mononitrate than those without AF/AFL. This signal of improvement in NT-proBNP after isosorbide mononitrate among the AF/AFL cohort contrasts with the overall negative results of NEAT-HFpEF. Given the high rate of comorbid AF/AFL-HFpEF, the role natriuretic peptides as surrogate endpoints may be limited in early-phase trials of investigational therapies. In less sick HFpEF patients, such as those enrolled in NEAT-HFpEF, any reduction in natriuretic peptide levels in the AF/AFL patients may be due to regression towards the mean. Indeed, a comprehensive review of endpoints in HFpEF revealed that short-term changes in natriuretic peptides may be variably associated with treatment effect.13
While endpoints of physical activity and quality of life are frequently employed in early phase clinical trials of HFpEF, they may also occasionally be problematic in the setting of AF/AFL. Cardiopulmonary exercise testing (CPET) has been frequently utilized as an endpoint due to its ability to predict survival in HFpEF,14 but AF/AFL may significantly influence baseline levels of maximal oxygen consumption during CPET.15,16 In our study, KCCQ scores were significantly higher among the AF/AFL cohort, confounding interpretability in HFpEF. Given lower rates of NYHA III/IV symptoms among the AF/AFL group, this finding may be due to the fact that symptoms among those with HFpEF and AF/AFL were driven more by arrhythmia than HF. This may be especially true if AF/AFL patients in NEAT-HFpEF were enrolled based on elevated natriuretic peptide levels, which can be elevated in the setting of AF/AFL alone; criteria for enrollment was not readily available at the time of analysis.
Our study has implications regarding the role of accelerometry measured activity and 6MWD as trial endpoints in HFpEF. Both ADAUs and 6MWD did not vary at baseline or with treatment by AF/AFL status, which allows for standardized endpoint interpretation in the heterogeneous HFpEF cohort. Accelerometry-measured activity levels and 6MWD are distinct metrics, and their use in combination may be more powerful to detect meaningful changes as opposed to either endpoint in isolation. While baseline values and changes in 6MWD did not differ significantly by AF/AFL in our study, 6MWD may not be sensitive to detect more subtle alterations in volitional physical activity. The sensitivity of accelerometer-related endpoints may allow for detection of drug effects that may be missed by routine spot measurements of functional performance. In contrast, 6MWD has demonstrated strong correlation with mortality among the HF with reduced ejection fraction cohort, which makes it a popular endpoint in early phase HF trials, regardless of ejection fraction.17 Accelerometer-based metrics of activity rely on patient desire to be active (i.e. volitional physical activity) in the case of improved symptoms, while 6MWD is an active measure of functional performance. The passive measurement of routine, physical activity by accelerometry may be confounded by sedentary lifestyle and the need for patient motivation to increase ADAUs.
This study has limitations. While the NEAT-HFpEF population was small, it remains one of the largest studies using accelerometer data, representing a unique study of patient-centered outcomes in HFpEF. Further research is necessary to confirm the variation in endpoints by AF/AF status and type (i.e. paroxysmal, persistent/permanent) in late phase trials of HFpEF of longer follow-up duration. The unique clinical profile of patients may limit the application of our results to other populations. Rhythm during follow-up was not available and it is not known if patients who presented in AF/AFL on baseline ECG converted to sinus rhythm during the trial, which could have influenced NT-proBNP levels during treatment.
In the NEAT-HFpEF trial, history of AF/AFL was not associated with baseline metrics of volitional physical activity as assessed by an accelerometer or functional performance as measured by 6MWD, while baseline levels of NT-proBNP and quality of life scores were significantly higher among patients with AF/AFL. There were no differences by AF/AFL status in accelerometer-based activity levels, 6MWD or quality of life after treatment with isosorbide mononitrate. In contrast, AF/AFL patients experienced significantly greater reductions in natriuretic peptides after isosorbide mononitrate therapy compared with those without AF/AFL, which could be due to regression to the mean. These findings suggest physical activity measured by accelerometer and functional performance measured by 6MWD may represent reliable and distinct endpoints in trials of HFpEF and do not appear to vary by background AF/AFL status.
Acknowledgements
Funding
Research reported in this manuscript was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number U10 HL084904 and award numbers U10 HL110297, U10 HL110342, U10 HL110309, U10 HL110262, U10 HL110338, U10 HL110312, U10 HL110302, U10 HL110336, U10 HL110337, and T32HL069771. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures:
Dr. Ravi B. Patel is supported by the NHLBI T32 postdoctoral training grant (T32HL069771).
Dr. Muthiah Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), and serves on advisory boards for AstraZeneca, Bayer AG, and Baxter Healthcare.
Dr. Javed Butler has received research support from the NIH and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, Relypsa, Vifor Pharma, and ZS Pharma.
Dr. Sanjiv Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, and United Therapeutics.
All other authors have reported that they have no other relationships relevant to the contents of this paper to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2.Vaduganathan M, Greene SJ, Ambrosy AP, Gheorghiade M, Butler J. The disconnect between phase II and phase III trials of drugs for heart failure. Nat Rev Cardiol 2013;10:85–97. [DOI] [PubMed] [Google Scholar]
- 3.Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, Depre C, Desvigne-Nickens P, Nkulikiyinka R, Lewis GD, Gomberg-Maitland M, O’Connor CM, Stockbridge N, Califf RM, Konstam MA, Januzzi JL Jr., Solomon SD, Borlaug BA, Shah SJ, Redfield MM, Felker GM. Heart Failure With Preserved Ejection Fraction Expert Panel Report: Current Controversies and Implications for Clinical Trials. JACC Heart Fail 2018;6:619–632. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol 2016;68:2217–2228. [DOI] [PubMed] [Google Scholar]
- 5.Patel RB, Vaduganathan M, Shah SJ, Butler J. Atrial fibrillation in heart failure with preserved ejection fraction: Insights into mechanisms and therapeutics. Pharmacol Ther 2017;176:32–39. [DOI] [PubMed] [Google Scholar]
- 6.Greene SJ, Mentz RJ, Fiuzat M, Butler J, Solomon SD, Ambrosy AP, Mehta C, Teerlink JR, Zannad F, O’Connor CM. Reassessing the Role of Surrogate End Points in Drug Development for Heart Failure. Circulation 2018;138:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, Network NHFCR. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakeri R, Levine JA, Koepp GA, Borlaug BA, Chirinos JA, LeWinter M, VanBuren P, Davila-Roman VG, de Las Fuentes L, Khazanie P, Hernandez A, Anstrom K, Redfield MM. Nitrate’s effect on activity tolerance in heart failure with preserved ejection fraction trial: rationale and design. Circ Heart Fail 2015;8:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snipelisky D, Kelly J, Levine JA, Koepp GA, Anstrom KJ, McNulty SE, Zakeri R, Felker GM, Hernandez AF, Braunwald E, Redfield MM. Accelerometer-Measured Daily Activity in Heart Failure With Preserved Ejection Fraction: Clinical Correlates and Association With Standard Heart Failure Severity Indices. Circ Heart Fail 2017;10:e003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen CW, Omland T, Clopton P, Westheim A, Wu AH, Duc P, McCord J, Nowak RM, Hollander JE, Storrow AB, Abraham WT, McCullough PA, Maisel A. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol 2005;46:838–844. [DOI] [PubMed] [Google Scholar]
- 11.Myhre PL, Vaduganathan M, Claggett BL, Anand IS, Sweitzer NK, Fang JC, O’Meara E, Shah SJ, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Association of Natriuretic Peptides With Cardiovascular Prognosis in Heart Failure With Preserved Ejection Fraction: Secondary Analysis of the TOPCAT Randomized Clinical Trial. JAMA Cardiol 2018;3:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene SJ, Fonarow GC, Solomon SD, Subacius HP, Ambrosy AP, Vaduganathan M, Maggioni AP, Bohm M, Lewis EF, Zannad F, Butler J, Gheorghiade M, Investigators A, Coordinators. Influence of atrial fibrillation on post-discharge natriuretic peptide trajectory and clinical outcomes among patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur J Heart Fail 2017;19:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira JP, Duarte K, Graves TL, Zile MR, Abraham WT, Weaver FA, Lindenfeld J, Zannad F. Natriuretic Peptides, 6-Min Walk Test, and Quality-of-Life Questionnaires as Clinically Meaningful Endpoints in HF Trials. J Am Coll Cardiol 2016;68:2690–2707. [DOI] [PubMed] [Google Scholar]
- 14.Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, Schairer JR, Ehrman JK, Velez M, Selektor Y, Lanfear DE, Keteyian SJ. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT-CPX) project. Am Heart J 2016;174:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, Deswal A, LeWinter M, Hernandez AF, Braunwald E, Redfield MM. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail 2014;7:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: Association With Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail 2017;5:92–98. [DOI] [PubMed] [Google Scholar]
- 17.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993;270:1702–1707. [PubMed] [Google Scholar]


