Abstract
Background:
Brain atrophy frequently occurs with Parkinson’s disease (PD) and relates to increased motor symptoms of PD. The predictive value of neuroimaging-based measures of global and regional brain volume on motor outcomes in deep brain stimulation (DBS) remains unclear but potentially could improve patient selection and targeting.
Objectives:
To determine the predictive value of preoperative volumetric MRI measures of cortical and subcortical brain volume on motor outcomes of subthalamic nucleus (STN) DBS in PD.
Methods:
Preoperative T1 3D MP-RAGE structural brain MRI images were analyzed for each participant to determine subcortical, ventricular, and cortical volume and thickness. Change in Unified Parkinson’s Disease Rating Scale (UPDRS) scores for subsection 3, representing motor outcomes, was computed preoperatively and postoperatively following DBS programming in 86 participants. A multiple linear regression analysis was performed to investigate the relationship between volumetric data and the effect of DBS on UPDRS 3 scores.
Results:
Larger ventricular and smaller thalamic volumes predicted significantly less improvement of UPDRS 3 scores after STN DBS.
Conclusions:
Our findings demonstrate in PD that regional brain volumes, in particular thalamic and ventricular volumes, predict motor outcomes after DBS. Differences in regional brain volumes may alter electrode targeting, reflect a specific disease trait such as postoperative progression of subclinical dementia, or directly interfere with the action of DBS.
Keywords: Parkinson’s disease, Deep brain stimulation, Magnetic resonance imaging
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves motor deficits in people with advanced Parkinson’s disease (PD), although the degree of benefit varies across individuals[1,2]. DBS surgery requires preoperative imaging for electrode targeting, and prior studies have established that regional brain atrophy accounts for some variability in DBS outcomes. In patients undergoing DBS, smaller mesencephalic surfaces predict decreased ADL improvement, thickness in the paracentral region predicts motor outcomes, and hippocampal atrophy corresponds with postoperative conversion to dementia[3–5]. However, no study to date has demonstrated a relationship between motor outcomes and global measures of brain atrophy which may be obtained from routine structural MRI, and little data exists regarding the predictive value of other cortical and subcortical regions. As DBS effectiveness varies and the procedure is not without risk, an additional tool to assess potential benefits from DBS may be useful in patient selection and improve clinical outcomes.
Brain atrophy occurs independent of DBS in PD and affects various cortical and subcortical structures, including lateral ventricles, sensorimotor, parietal and perisylvian cortex, hippocampus, and caudate[6–8]. While atrophy most strongly links to cognitive impairment in PD, the degree and pattern of brain atrophy also correlate with motor features of PD. In particular, sensorimotor cortex is among the regions most prone to atrophy in PD, and regional atrophy of sensorimotor cortex predicts increased motor symptoms of PD[7]. These areas are highly functionally connected to the common DBS targets of STN and internal globus pallidus, suggesting that atrophy affects regions that are modulated by STN DBS[9]. Additionally, STN not only anatomically connects to basal ganglia and thalamic regions but also anatomically connects to sensorimotor cortex[10,11]. As atrophy in PD affects regions with a high degree of anatomic and functional connectivity to the STN, the effect of STN DBS may be altered by changes in these regions.
We tested the predictive value of regional and global brain volumes measured by preoperative MRI on motor outcomes of STN DBS for PD. We hypothesized that reduced volumes of regions related to motor function like basal ganglia and thalamus and global brain volume would predict reduced motor benefit from DBS. We measured regional volumes and measures of global atrophy including ventricular and cortical volumes as well as global cortical thickness in preoperative MR images to determine which measure best predicts motor response to DBS.
Methods
Participants:
Participants were retrospectively selected from a consecutive series of PD patients that underwent DBS surgery at the Movement Disorders Center in Washington University School of Medicine and agreed to participate in preoperative DBS research imaging, separate from clinical imaging, and outcomes. A protocol was approved by the Washington University Institutional Review Board, and written consent was obtained from all participants. Basic criteria for study inclusion were: diagnosis of idiopathic PD by UK Brain Bank criteria[12], placement of bilateral STN DBS between 2006 and 2015, presence of a preoperative high-definition structural MRI (acquisition details described below), and available preoperative and postoperative OFF-medication (practically-defined) Unified Parkinson’s Disease Rating Scale Part 3 (UPDRS 3) data between 12 months prior to and 15 months after DBS implantation. Surgical exclusionary criteria included dementia (a score of ≤130 on the Mattis Dementia Rating scale), poor response to levodopa, and structural brain abnormalities that would increase surgical risk. 117 participants who fit inclusion criteria were identified by automated query of the Medical Automated Record System. MR images were inspected and 3 participants with missing or corrupted images and 6 participants with excessive motion artifact were excluded. Additionally, 22 participants were excluded due to insufficient clinical data to assess inclusion criteria, leaving a total of 86 participants.
Clinical evaluation:
All participants were evaluated at the Movement Disorders Center at Washington University School of Medicine. UPDRS 3 was measured during the presurgical inpatient ON/OFF evaluation and during postoperative DBS programming clinical visits. UPDRS 3 interrater reliability was validated for all UPDRS 3 raters on an annual basis by completing video-based testing of rating UPDRS 3. Preoperative scores were collected within 12 months prior to DBS and postoperative ON-stimulation scores were collected between initial DBS programming (median 21 days postoperative, range 6 to 57 days) and 15 months after DBS placement. All UPDRS 3 scores were in the practically-defined medication-OFF state, after at least 8 hours without medication for PD. The ON-stimulation ratings were performed after DBS settings were finalized at the end of each programming visit. We averaged preoperative and postoperative scores separately within each subject, and then calculated the change between preoperative OFF-medication and postoperative OFF-medication and ON-stimulation scores to determine the effect of DBS. Average scores within the entire postoperative period were used to mitigate random fluctuations and to represent the real-world performance of each subject after receiving DBS. Participants were classified as tremor dominant (TD), postural instability gait disorder dominant (PIGD) or mixed PD subtypes according to criteria described previously[13].
MRI technique and processing:
High-resolution structural MRI data was acquired preoperatively within 4 months of DBS using a Siemens 3T MAGNETOM Trio scanner. Scans included a T1-weighted sagittal MPRAGE, TR=2400 ms, TI=1000 ms, TE=3.14 ms, FA=8°, 0.9 mm3 voxels) and a T2-weighted fast spin echo (TR=3200 ms, TE=469 ms, 1.0 mm3 voxels). Images were inspected for significant motion and susceptibility artifacts and images with artifacts interfering with cortical measurements were excluded. Cortical reconstruction and volume segmentation were performed with Freesurfer v5.3 (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications[14–16]. Image analysis included skull stripping, parcellation, measurement of cortical volume, cortical thickness, and ventricular volume (including lateral and third ventricles). Images and parcellation maps were inspected for errors and areas of mislabeling were corrected by reviewer blinded to clinical outcomes. All volumes were normalized to total intracranial volume. Parcellation was performed using the Desikan atlas[16]. Specific regions of interest previously reported to atrophy in PD included lateral parietal (LP), sensorimotor (SM), perisylvian (PS), and hippocampal (HC) regions[7,17]. LP was defined as superior parietal, inferior parietal and supramarginal regions, SM was defined as precentral, postcentral and paracentral regions, PS was defined as superior temporal and transverse temporal regions, and HC was defined as hippocampal or parahippocampal regions in the Desikan atlas. Subcortical regions also including thalamus, caudate, putamen and pallidum were delineated in Freesurfer as previously validated[18].
Neurosurgical procedure:
All patients had bilateral STN DBS in accordance with standard clinical procedures at Washington University[19]. Patients were prescreened for medical and neuroanatomical comorbidities which would preclude safe electrode implantation and completed standard neuropsychological testing to exclude patients with dementia. Electrodes were stereotactically implanted into bilateral subthalamic nuclei. Accurate electrode placement was verified by intraoperative electrophysiological recordings, clinical responses to stimulation, and postoperative CT scans.
Statistical analysis:
Statistical analysis was performed in SPSS Statistics (IBM Corp, 2017). Multiple linear regression analysis was performed to control for clinical variables of age, years since PD onset, level of education, levodopa equivalent dose (LED) as calculated by standard criteria[20], and baseline UPDRS score. Analyses were performed to determine relationships between change in UPDRS score and regional and global cortical volumes, subcortical and ventricular volumes, and cortical thickness, as defined above. Checks on assumptions for multiple linear regression included inspection for linearity of data, normality of residuals, absence of autocorrelation and multicollinearity, homoscedasticity, and absence of outliers using Cook’s distance. For multiple linear regression results, multiple comparisons corrections were done with the Benjamini-Hochberg False Discovery Rate (FDR) method using a threshold of 0.05. Original p-values are reported with significance after FDR correction noted. For findings that were significant after multiple linear regression, scatterplots were generated and Pearson correlations were performed. A single apparent outlier was found in the inspection of the scatterplot for ventricular volume vs change in UPDRS 3. However, on further inspection this point had a small Cook’s distance, and removal of this point did not cause a significant change in the outcome of the multiple linear regression, therefore it was not considered an outlier. Student’s t-test was used to compare change in UPDRS 3 between upper and lower quartiles of thalamic and ventricular volume and volumes of thalamus and ventricles between TD and PIGD groups.
Results
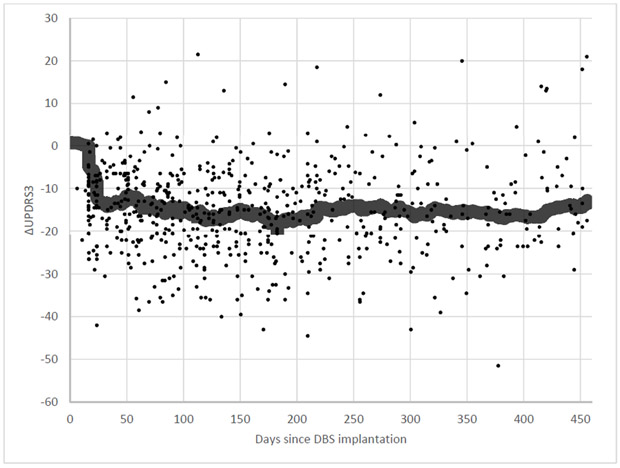
Patient demographics in our cohort are summarized in Table 1. Postoperatively, UPDRS 3 decreased 43% (36.7 ± 10.2 to 21.0 ± 7.10) and daily levodopa equivalents (LED) also decreased by 28% (1765 ± 775 to 1277 ± 741). UPDRS3 scores quickly decreased after DBS implantation and programming, and remained generally stable over the 15-month postoperative period (Figure 1).
Table 1:
Patient demographics at date of surgery
| Number of participants | 86 |
| Age | 62.9 ± 9.5 |
| Years since onset | 11.8 ± 4.4 |
| Sex | Male: 58 |
| Female: 28 | |
| Race | Caucasian: 78 |
| African American: 3 | |
| American Indian or Alaskan Native: 4 | |
| Any Hispanic: 1 | |
| Level of education: | Less than high school: 7 |
| Completed high school: 14 | |
| Some college or trade School: 25 | |
| Completed bachelor’s degree: 10 | |
| Completed advanced degree: 21 | |
| Did not specify: 9 | |
| PD motor subtype: | Tremor dominant: 15 |
| Postural instability gait disease dominant: 65 | |
| Mixed: 6 |
Relevant values reported as mean ± standard deviation
Fig. 1.
Change in UPDRS3 from baseline over time after DBS implantation, with running average.
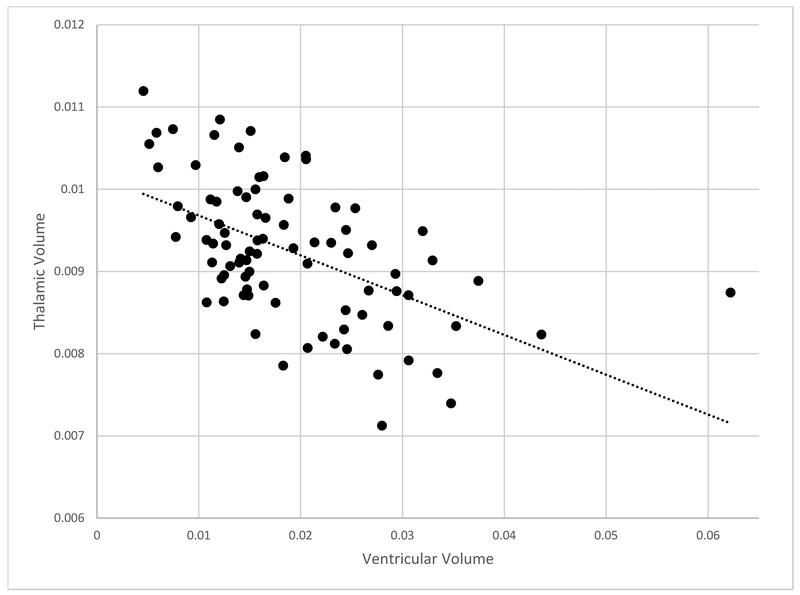
Regional volumes of thalamus and ventricles significantly predicted motor response to STN DBS. After multiple regression analysis (Table 2), thalamic volume predicted postoperative change in UPDRS 3 (β = −0.30, p < 0.001); smaller thalamic volumes related to less UPDRS 3 improvement postoperatively. Volume of the lateral and 3rd ventricles also predicted postoperative improvement of UPDRS 3 (β = 0.29, p < 0.001); increased ventricular volume related to less improvement in UPDRS 3 scores after DBS. Volume of caudate, putamen, and pallidum did not predict change in UPDRS 3. Thalamic volume correlated strongly with ventricular volume (R = −0.536, p < 0.001, Figure 2). Global cortical volume did not predict post-operative improvement of UPDRS 3 (β = −0.154, p = 0.067). Non-significant trends that did not meet multiple comparisons correction were observed with lateral parietal (β = −0.174, p = 0.039), sensorimotor (β = −0.174, p = 0.039) and hippocampal (β = −0.180, p = −0.043) volumes predicting change in UPRDS 3, while perisylvian volume (β = −0.093, p = 0.237) did not produce any notable trend. Global cortical thickness had no significant relationship with UPDRS 3 (β = −0.042, p = 0.607) or UPDRS 1 (β = −0.086, p = 0.308). Thickness of lateral parietal, sensorimotor, perisylvian and parahippocampal cortical regions did not significantly relate to change in UPDRS scores.
Table 2:
Volumetric measures vs change in UPDRS3 after STN DBS
| β | p | |
|---|---|---|
| Ventricle Volume | 0.29 | *<0.001 |
| Thalamus Volume | −0.30 | *<0.001 |
| Caudate Volume | 0.04 | 0.67 |
| Putamen Volume | −0.15 | 0.09 |
| Pallidum Volume | −0.06 | 0.43 |
| Cortical Volume | −0.15 | 0.07 |
| LP Volume | −0.17 | 0.04 |
| PS Volume | −0.09 | 0.24 |
| SM Volume | −0.17 | 0.04 |
| HC Volume | −0.18 | 0.04 |
| Cortical Thickness | −0.04 | 0.62 |
| LP Thickness | −0.05 | 0.51 |
| PS Thickness | 0.02 | 0.82 |
| SM Thickness | −0.02 | 0.77 |
| periHC Thickness | −0.14 | 0.10 |
Values are reported as beta values (standardized regression coefficient) followed by p-values.
indicates significance after correction for multiple comparisons at FDR <0.05
Fig. 2.
Ventricular vs thalamic volume.
Thalamic and ventricular volumes were separated by quartiles to quantify the difference between high and low atrophy in terms of motor response. A 30% reduction in UPDRS 3 was used as a threshold for a desirable response, as this approximates the minimum reduction in UPDRS 3 with levodopa typically considered to support candidacy for DBS[21]. UPDRS 3 reduction for 1st quartile thalamic volumes (corresponding to the most atrophy) averaged 28.4%, significantly less than 47.5% in the 4th quartile (p = 0.019). Similarly, UPDRS 3 reduction for 1st quartile ventricular volumes (corresponding to the least enlargement) averaged 47.9% compared to 4th quartile reduction of 35.2%, though this difference did not reach significance (p = 0.14). Of the 1st quartile thalamic volumes, 52% experienced at least a 30% reduction of UPDRS3 after DBS, while 90% of participants with 4th quartile thalamic volumes reached that mark. Among 1st quartile ventricular volumes, 90% had at least 30% reduction in UPDRS 3, while this was true for 67% of those in the 4th quartile. TD and PIGD groups did not significantly differ with regard to change in UPDRS 3 (p = 0.74), thalamic volume (p = 0.52), or ventricular volume (p = 0.59).
Discussion
We demonstrate, in a large consecutive cohort of PD patients undergoing STN DBS, that presurgical thalamic and ventricular volumes predicted degree of improvement in motor scores after DBS. Non-significant associations were seen for sensorimotor and lateral parietal regional volumes, while global measures of cortical volume and thickness did not predict outcomes after STN DBS. These data indicate that MRI volumetrics may contribute to PD patient selection for STN electrode implantation.
Thalamic and ventricular size had a strong relationship with postoperative motor outcomes in our data even after accounting for other relevant clinical variables. Smaller thalamic and increased ventricular volumes predicted a less robust motor benefit from DBS, while subjects in the quartiles corresponding with the most thalamic atrophy and most ventricular enlargement in these regions were less likely to benefit substantially from DBS. Ventricular volume is commonly used as a marker of global brain atrophy but may be particularly influenced by atrophy of adjacent subcortical structures such as caudate and thalamus, supported by the high degree of correlation between thalamic and ventricular volumes in our study, therefore it is not surprising that thalamic and ventricular volumes would predict motor outcomes to a similar degree[22]. The effect of thalamic and ventricular volume here was also much stronger than the effect of cortical volume and cortical thickness on either motor or non-motor outcomes. Cortical areas previously reported to atrophy in PD were more strongly associated with motor outcomes than was global cortical volume, although these associations did not reach significance.
Several possible explanations exist for the stronger relationship between thalamic and ventricular volumes and DBS motor outcomes compared to selected cortical regions. Technically, increased ventricular size may contribute to difficulty with surgical targeting of the STN and may predispose patients to electrode shift[23]. Additionally, thalamus is typically penetrated by electrodes en route to the STN, making this a possible contributor to structural causes of inaccuracy[24]. Furthermore, the ventricles are typically avoided in surgical planning, thus particularly large ventricles may add difficulty to accurate electrode placement. While each patient in this study received CT verification of electrode placement postoperatively, differences in accuracy sufficient to cause clinical change may occur on millimeter scale and therefore evade this quality check[25]. As the ventroanterior and ventrolateral nuclei of the thalamus are major downstream outputs of the STN and internal globus pallidus, structural changes in the thalamus may also hypothetically affect the functional pathways by which DBS exerts its therapeutic effects[26]. The mechanism by which STN DBS affects motor function in PD remains somewhat controversial, with modulation of pallidothalamic activity, antidromic stimulation of direct STN to cortex connections bypassing pallidothalamic connections (the “hyperdirect” pathway”), and alteration of functional connectivity in downstream networks all implicated in various studies[26–29]. The strong relationship between thalamic structure and clinical effects in DBS supports mechanistic models that include modulation of thalamic activity.
Other studies have previously demonstrated that brain volumes at the time of DBS surgery affect postoperative outcomes. Mesencephalic surfaces inversely correlated with improvement in activities of daily living (ADL) after STN DBS[3] whereas volumes of cortex, caudate and putamen did not predict motor outcomes, as we found. We did not include mesencephalic measures as this region is not isolated in our processing pipeline. Another group reported that small subregions of paracentral, superior frontal, and superior and inferior parietal cortex predicted motor outcomes and voltage required to achieve maximum effect[4]. We did not test whether such small isolated clusters as those seen in this study predict motor response to STN DBS, though we did not find that cortical thickness at the regional level can predict DBS outcomes. However, the significant clusters in their study largely fall within the sensorimotor and lateral parietal regions in our analysis, regions showing the strongest relationship with motor outcomes of any cortical area, although cortical thickness of these regions produced no significant results in our study. None of these other studies included analyses of thalamic or ventricular volumes, which we found predict motor outcomes. Furthermore, these MRI-based measurements of ventricular and thalamic volumes are easily performed and may be useful components of presurgical workup for DBS [30]. Although those in the quartiles corresponding to the highest degree of atrophy in thalamus and enlargement of ventricles were less likely to have a robust response to STN DBS, there was no degree of atrophy in our data that suggested a complete lack of benefit or a threshold to be used as a contraindication to DBS. If confirmed in another cohort, volumetric analysis of these regions may be useful for the decision-making process between clinicians and patients.
The main limitation of this study was its retrospective nature. This permitted analysis of a large sample but limited accounting for potentially confounding variables, such as socioeconomic factors and recruitment bias. However, most potentially confounding variables were addressed with multiple regression of clinical and demographic covariates. More importantly, these findings require replication in an independent cohort to confirm that these variables can be useful for predicting STN DBS outcomes, preferably using longitudinal data able to track volume measures over time to test the role of atrophy on DBS outcomes. Our population included relatively few TD compared to PIGD patients, although the groups were similar in atrophy and motor outcomes despite the more reliable historical response of tremor to STN DBS compared with gait and postural symptoms[21]. We limited this study to motor outcomes and did not address nonmotor manifestations. Future study of the effect of atrophy to predict outcomes for DBS of the internal globus pallidus may reveal differences from DBS targeting STN since the targets may have different side effect thresholds and cognitive effects[2].
Conclusion
We demonstrate here that thalamic and ventricular volumes predict degree of motor response to STN DBS for PD. Use of preoperative structural MRI to identify patients with high degrees of atrophy in these regions may be useful to inform physicians and patients during presurgical planning.
Supplementary Material
Highlights.
Smaller thalamic volumes predict a less robust motor response to STN DBS
Larger ventricular volumes also predict a less robust motor response to STN DBS
Neither global nor regional cortical volume and thickness predicted motor outcomes
Acknowledgements
This work was supported by NIH NINDS/NIA/NIBIB (NS075321 [JSP, MCC], NS41509 [JSP], NS097799 [MCC], NS097437 [MCC], R44AG054405 [MCC], T32EB021955-01A1 [JRY]), the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund); the American Parkinson Disease Association (APDA) Advanced Research Center for Parkinson Disease at Washington University in St. Louis; the Greater St. Louis Chapter of the APDA; the Barbara & Sam Murphy Fund; the McDonnell Center for Systems Neuroscience; and the Oertli Fund for Parkinson Disease Research.
Footnotes
Conflicts
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider G-H, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German NS Parkinson Study Group, A randomized trial of deep-brain stimulation for Parkinson’s disease., N. Engl. J. Med 355 (2006) 896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- [2].Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ Jr., Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease, N. Engl. J. Med 362 (2010) 2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- [3].Bonneville F, Welter ML, Elie C, du Montcel ST, Hasboun D, Menuel C, Houeto JL, Bonnet a. M., Mesnage V, Pidoux B, Navarro S, Cornu P, Agid Y, Dormont D, Parkinson disease, brain volumes, and subthalamic nucleus stimulation, Neurology. 64 (2005) 1598–1604. doi: 10.1212/01.WNL.0000160401.24880.83. [DOI] [PubMed] [Google Scholar]
- [4].Muthuraman M, Deuschl G, Koirala N, Riedel C, Volkmann J, Groppa S, Effects of DBS in parkinsonian patients depend on the structural integrity of frontal cortex, Sci. Rep 7 (2017) 6–11. doi: 10.1038/srep43571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aybek S, Lazeyras F, Gronchi-Perrin A, Burkhard PR, Villemure JG, Vingerhoets FJG, Hippocampal atrophy predicts conversion to dementia after STN-DBS in Parkinson’s disease, Park. Relat. Disord 15 (2009) 521–524. doi: 10.1016/j.parkreldis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [6].Camicioli R, Sabino J, Gee M, Bouchard T, Fisher N, Hanstock C, Emery D, Martin WRW, Ventricular dilatation and brain atrophy in patients with Parkinson’s disease with incipient dementia, Mov. Disord 26 (2011) 1443–1450. doi: 10.1002/mds.23700. [DOI] [PubMed] [Google Scholar]
- [7].Hwang KS, Beyer MK, Green AE, Chung C, Thompson PM, Janvin C, Larsen JP, Aarsland D, Apostolova LG, Mapping cortical atrophy in Parkinson’s disease patients with dementia., J. Parkinsons. Dis 3 (2013) 69–76. doi: 10.3233/JPD-120151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Y, Zhang J, Xu J, Wu X, Zhang Y, Feng H, Wang J, Jiang T, Cortical gyrification reductions and subcortical atrophy in Parkinson’s disease, Mov. Disord 29 (2014) 122–126. doi: 10.1002/mds.25680. [DOI] [PubMed] [Google Scholar]
- [9].Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A, Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases, Proc. Natl. Acad. Sci 111 (2014) E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Castle M, Aymerich MS, Sanchez-Escobar C, Gonzalo N, Obeso JA, Lanciego JL, Thalamic innervation of the direct and indirect basal ganglia pathways in the rat: Ipsi- and contralateral projections, J. Comp. Neurol 483 (2005) 143–153. doi: 10.1002/cne.20421. [DOI] [PubMed] [Google Scholar]
- [11].Nambu A, Tokuno H, Takada M, Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway, Neurosci. Res 43 (2002) 111–117. doi: 10.1016/S0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- [12].Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson ’ s disease : a clinico-pathological study of 100 cases, J. Neurol. Neurosurg. Psychiatry 55 (1992) 181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group., Neurology. 40 (1990) 1529–34. doi: 10.1212/WNL.40.10.1529. [DOI] [PubMed] [Google Scholar]
- [14].Fischl B, Dale AM, Measuring the thickness of the human cerebral cortex from magnetic resonance images, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM, Sequence-independent segmentation of magnetic resonance images, Neuroimage. 23 (2004) S69–S84. doi:DOI: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- [16].Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest, Neuroimage. 31 (2006) 968–980. doi:DOI: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [17].Pan PL, Shi HC, Zhong JG, Xiao PR, Shen Y, Wu LJ, Song YY, He GX, Li HL, Gray matter atrophy in Parkinson’s disease with dementia: Evidence from meta-analysis of voxel-based morphometry studies, Neurol. Sci 34 (2013) 613–619. doi: 10.1007/s10072-012-1250-3. [DOI] [PubMed] [Google Scholar]
- [18].Keller SS, Gerdes JS, Mohammadi S, Kellinghaus C, Kugel H, Deppe K, Ringelstein EB, Evers S, Schwindt W, Deppe M, Volume estimation of the thalamus using freesurfer and stereology: consistency between methods., Neuroinformatics. 10 (2012) 341–50. doi: 10.1007/s12021-012-9147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tabbal SD, Revilla FJ, Mink JW, Schneider-Gibson P, Wernle AR, de Erausquin GA, Perlmutter JS, Rich KM, Dowling JL, Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson’s disease., Neurosurgery. 61 (2007) 119–27; discussion 127–9. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- [20].Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE, Systematic review of levodopa dose equivalency reporting in Parkinson’s disease., Mov. Disord 25 (2010) 2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- [21].Rodriguez RL, Fernandez HH, Haq I, Okun MS, Pearls in patient selection for deep brain stimulation., Neurologist. 13 (2007) 253–60. doi: 10.1097/NRL.0b013e318095a4d5. [DOI] [PubMed] [Google Scholar]
- [22].Gaser C, Ph D, Hazlett EA, Ph D, Buchsbaum MS, Ventricular Enlargement in Schizophrenia Related to Volume Reduction of the Thalamus , Striatum , and Superior Temporal Cortex, 05 (2004) 154–156. [DOI] [PubMed] [Google Scholar]
- [23].Martinez-Ramirez D, Morishita T, Zeilman PR, Peng-Chen Z, Foote KD, Okun MS, Atrophy and other potential factors affecting long term deep brain stimulation response: a case series., PLoS One. 9 (2014) e111561. doi: 10.1371/journal.pone.0111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL, Deep brain stimulation for Parkinson’s disease: Surgical technique and perioperative management, Mov. Disord 21 (2006). doi: 10.1002/mds.20959. [DOI] [PubMed] [Google Scholar]
- [25].Welter M-L, Schüpbach M, Czernecki V, Karachi C, Fernandez-Vidal S, Golmard J-L, Serra G, Navarro S, Welaratne A, Hartmann A, Mesnage V, Pineau F, Cornu P, Pidoux B, Worbe Y, Zikos P, Grabli D, Galanaud D, Bonnet A-M, Belaid H, Dormont D, Vidailhet M, Mallet L, Houeto J-L, Bardinet E, Yelnik J, Agid Y, Optimal target localization for subthalamic stimulation in patients with Parkinson disease., Neurology. 82 (2014) 1352–61. doi: 10.1212/WNL.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL, Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat., Neuroscience. 99 (2000) 289–95. doi: 10.1016/S0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- [27].Lai H-Y, Younce JR, Albaugh DL, Kao YCJ, Shih YYI, Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus, Neuroimage. 84 (2014). doi: 10.1016/j.neuroimage.2013.08.026. [DOI] [PubMed] [Google Scholar]
- [28].Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K, Optical deconstruction of parkinsonian neural circuitry., Science. 324 (2009) 354–9. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saenger VM, Kahan J, Foltynie T, Friston K, Aziz TZ, Green AL, van Hartevelt TJ, Cabral J, Stevner ABA, Fernandes HM, Mancini L, Thornton J, Yousry T, Limousin P, Zrinzo L, Hariz M, Marques P, Sousa N, Kringelbach ML, Deco G, Uncovering the underlying mechanisms and whole-brain dynamics of deep brain stimulation for Parkinson’s disease., Sci. Rep 7 (2017) 9882. doi: 10.1038/s41598-017-10003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spinks R, Magnotta VA, Andreasen NC, Albright KC, Ziebell S, Nopoulos P, Cassell M, Manual and automated measurement of the whole thalamus and mediodorsal nucleus using magnetic resonance imaging, Neuroimage. 17 (2002) 631–642. doi: 10.1016/S1053-8119(02)91185-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.