Abstract
To date, there is no reliable biomarker for the assessment or determination of cognitive dysfunction in Alzheimer’s disease and related dementia. Such a biomarker would not only aid in diagnostics, but could also serve as a measure of therapeutic efficacy. It is widely acknowledged that the hallmarks of Alzheimer’s disease, namely, amyloid deposits and neurofibrillary tangles, as well as their precursors and metabolites, are poorly correlated with cognitive function and disease stage and thus have low diagnostic or prognostic value. A lack of biomarkers is one of the major roadblocks in diagnosing the disease and in assessing the efficacy of potential therapies. The phosphorylation of cAMP Response Element Binding protein (pCREB) plays a major role in memory acquisition and consolidation. In the brain, CREB activation by phosphorylation at Ser133 and the recruitment of transcription cofactors such as CREB binding protein (CBP) is a critical step for the formation of memory. This set of processes is a prerequisite for the transcription of genes thought to be important for synaptic plasticity, such as Egr-1. Interestingly, recent work suggests that the expression of pCREB in peripheral blood mononuclear cells (PBMC) positively correlates with pCREB expression in the postmortem brain of Alzheimer’s patients, suggesting not only that pCREB expression in PBMC might serve as a biomarker of cognitive dysfunction, but that the dysfunction of CREB signaling may not be limited to the brain in AD, and that a link may exist between the regulation of CREB in the blood and CREB in the brain. In this Review we consider the evidence suggesting a correlation between the level of CREB signals in the brain and blood, the current knowledge about CREB in PBMC and its association with CREB in the brain, and the implications and mechanisms for a neuro-immune cross talk that may underlie this communication. This Review will discuss the possibility that peripheral dysregulation of CREB is an early event in AD pathogenesis, perhaps as a facet of immune system dysfunction, and that this impairment in peripheral CREB signaling modifies CREB signaling in the brain, thus exacerbating cognitive decline in AD. A more thorough understanding of systemic dysregulation of CREB in AD will facilitate the search for a biomarker of cognitive function in AD, and also aid in the understanding of the mechanisms underlying cognitive decline in AD.
Keywords: CREB, Alzheimer’s disease, biomarker, dementia, inflammation, neuroimmune, PBMC
1. INTRODUCTION
Alzheimer’s disease (AD) is a devastating neurological disorder affecting learning and memory. The incidence of AD is on the rise, and an anticipated 14 million persons will suffer from the disease by 2050 in the United States (Hebert et al., 2013). The early onset familial form of the disease is caused by mutations in amyloid precursor protein (APP) and presenilin-1,2 (PSEN-1,2). The cause of the sporadic disease remains unknown, but is hypothesized to be the result of dysfunction in processing of the amyloid precursor protein (APP) and hyperphosphorylation of the protein tau. These two processes result in the accumulation of beta amyloid (Aβ) plaques and neurofibrilarly tangles, which characterize the postmortem AD brain, and are considered to be the diagnostic hallmarks of the disease. For this reason, the search for a biomarker to aid in the prediction, diagnosis, and treatment of AD has been centered on forms of amyloid and tau. The current most successful biomarkers for AD based on amyloid and tau include measurements of total tau, phosphorylated tau, and Aβ42 in the cerebrospinal fluid (Olsson et al., 2016).
However, the severity of cognitive dysfunction in AD may be independent of the extent of amyloid or tau pathology, and indeed, the relationship between postmortem AD pathology and cognitive performance during life is notoriously poor, and therapies targeting amyloid or tau have thus far been largely unsuccessful (Morris et al., 2014). Therefore, a biomarker that can provide information on cognitive function in AD progression is essential, not only to compliment the current diagnostic biomarkers based on amyloid and tau, but also to provide a more sensitive means of measuring the efficacy of therapies meant to ameliorate cognitive dysfunction in AD.
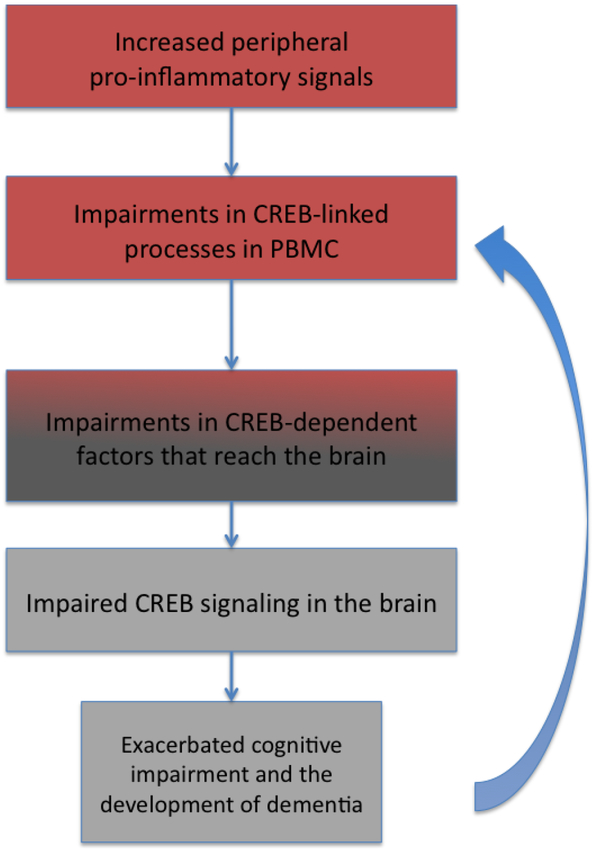
We, and others, have reported that the expression of an important factor in the formation of memory and its retrieval, Cyclic-AMP Response Element Binding Protein (CREB), is diminished in the postmortem AD brain. In this review, we first discuss the evidence supporting the involvement of CREB in cognitive decline in AD. Then we discuss the possibility that CREB signaling in the blood could be an indicator of CREB signaling in the brain, thus serving as a biomarker of cognitive function in AD. Finally, we propose a hypothesis that peripheral CREB deficits may be an early event in AD pathogenesis, perhaps as a result of immune system dysfunction and inflammation, and subsequently modifies CREB signaling in the brain, thereby contributing to cognitive decline in AD (Figure 1).
Figure 1.
Hypothesis for the mechanism by which CREB signaling defects in the periphery might affect CREB in the brain in AD. Following dysregulation of the immune system that accompanies AD, CREB signaling in PBMC is disrupted. This deficit may be imparted to the brain through mediators such as cytokines, neurotrophic factors, or hormones. Deficits in CREB signaling in the brain may accelerate cognitive decline in AD, and may in turn influence CREB signaling in the periphery through similar mediators, resulting in a cycle of CREB dysfunction and further cognitive decline.
2. CREB
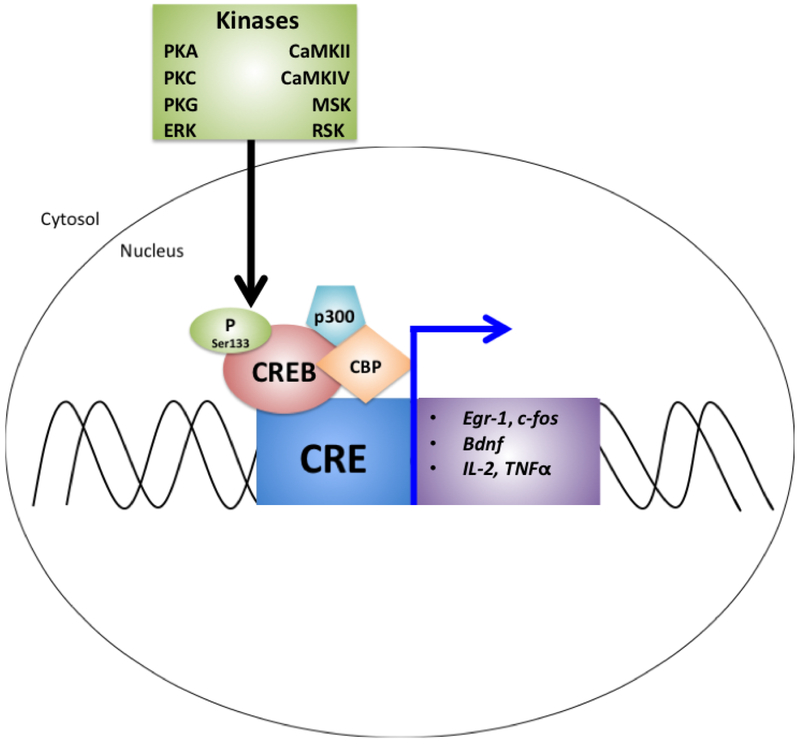
The exact mechanism underlying synaptic plasticity and memory formation, storage and retrieval remains a topic of investigation, but one of the prevailing hypotheses is that it is dependent on the regulation of key genes and proteins that are modulated as a result of neuronal activity. One of the signaling pathways implicated in this process is the CREB pathway. In the brain, the CREB pathway responds to the increased calcium that results from neuronal activity. CREB itself can be phosphorylated by a number of kinases that respond directly or indirectly to calcium, such as protein kinase A (PKA), protein kinase C (PKC), the calcium/calmodulin-dependent protein kinases CaMKII and CaMKIV, the extracellular signal-regulated kinase (ERK)-activated kinases mitogen- and stress-activated protein kinase (MSK) and the 90 kDa ribosomal S6 kinase (RSK) (Lonze and Ginty, 2002), as well as in response to nitric oxide (NO) through cGMP activation of PKG (Teich et al., 2015). Following phosphorylation at Ser133 and recruitment of cofactors, such as CREB binding protein (CBP) and p300, CREB can bind to a CRE sequence in the promoter region of downstream genes implicated in synaptic plasticity, including immediate early genes like Egr-1 (Figure 2) (Jones et al., 2001; Kandel, 2012; Lakhina et al., 2015; Yin et al., 1994). The critical nature of CREB for learning and memory has been demonstrated by studies showing that decreasing the expression or function of the CREB signaling pathway results in impairments in learning and memory. For example, CREB mutant mice that lack the major α and δ isoforms of CREB (though they retain small amounts of CREB activity due to the β isoform) have been generated (Lonze and Ginty, 2002). These CREB mutant mice exhibit impairments in LTP and in memory-based behavior tests, such as fear conditioning and water maze (Bourtchuladze et al., 1994). Importantly, CREB regulates formation of long-term memory in other species, as demonstrated by studies in olfaction long-term associative memory in C. elegans, indicating that this pathway is evolutionarily conserved (Lakhina et al., 2015; Yin et al., 1994). In addition, the effects of CREB signaling impairments may not be limited to the hippocampus, as CREB mutant mice also have impaired cortical plasticity (Glazewski et al., 1999). The importance of CREB in long term memory has been additionally demonstrated in mice by the use of genetic methods including a CREB repressor (Kida et al., 2002), and expression of a dominant negative form of CREB (Pittenger et al., 2002). Experiments using viruses to enhance CREB signaling in the rodent hippocampus have been shown to improve performance in a water maze task (Brightwell et al., 2007; Yu et al., 2017). However, indiscriminate upregulation of CREB, particularly of basal CREB, is problematic for memory formation and retrieval, and a dominant active form of CREB expressed in the mouse hippocampus has been reported to impair performance in a water maze task and cause neuronal death (Lopez de Armentia et al., 2007; Viosca et al., 2009). The difference in these outcomes may be partially due to the magnitude of increase in CREB activity, with a more moderate or transient enhancement in activity providing the optimal benefits for learning and memory, or the differences may depend on the mechanism of action of the enhancement of CREB activity, with forms that mimic the downstream effects of wild-type CREB conferring a greater benefit (Suzuki et al., 2011).
Figure 2.
The components of CREB-based signaling, a process that results in expression of CRE-driven genes thought to be important for the formation of memory. Egr-1, c-fos, brain derived neurotrophic factor (bdnf) and tumor necrosis factor alpha (TNFα) are examples of CRE-driven genes.
In addition to CREB itself, other elements of the CREB signaling complex have also been investigated in regards to learning and memory. CBP and p300 are transcriptional coactivators for CREB that are similar both in sequence and in function, and are necessary for CRE-based gene transcription (Arany et al., 1994; Chrivia et al., 1993). Mice deficient in CBP do not respond to an enriched environment (EE) paradigm in the same way as wild-type mice, in that they do not show enhanced neurogenesis or enhanced performance in spatial navigation and pattern separation tasks following EE (Lopez-Atalaya et al., 2011). Similarly, when p300 is conditionally knocked out in the mouse hippocampus and cortex, the performance of the mice in novel object recognition and contextual fear conditioning tasks is impaired (Oliveira et al., 2011). In addition to their role as a scaffold for the transcription complex, CBP and p300 may facilitate gene transcription through their intrinsic histone acetyltransferase (HAT) activity (Bannister and Kouzarides, 1996; Ogryzko et al., 1996). The HAT activity of CBP is essential for memory consolidation, as demonstrated by experiments in which the HAT activity of CBP is blocked, while the scaffolding function remains intact (Korzus et al., 2004). In addition, experiments using an activator of CBP/p300 HAT activity that resulted in increased histone acetylation in the hippocampus and cortex, also resulted in a greater duration of memory in a water maze task, suggesting that the HAT activity may be a critical feature of the importance of CBP/p300 in learning and memory (Chatterjee et al., 2013). In support of this idea, poor performance in the novel object recognition task by CBP mutant mice is improved by treatment with an HDAC inhibitor (Stefanko et al., 2009). On the other hand, HDAC inhibition in a CBP conditional knockout was reported not to be sufficient to restore the impairments these mice demonstrated in object recognition, fear conditioning, and spatial memory tasks, even when histone acetylation was rescued by treatment with and HDAC inhibitor (Chen et al., 2010). Therefore, CBP and p300 are likely important factors in learning and memory, but the exact mechanism of their involvement is yet to be fully unraveled.
While it is clear that direct manipulation of the CREB signaling pathway impairs the learning and memory process, the next question is whether CREB signaling plays a role in neurodegenerative diseases characterized by memory impairments, such as in the case of AD.
2.1. CREB IN THE AD BRAIN
The critical nature of CREB signaling for neural plasticity and cognitive performance suggests that this pathway may be dysfunctional in neurodegenerative diseases in which memory and cognitive function are impaired (Cowburn et al., 1992b; Schnecko et al., 1994b; Yamamoto-Sasaki et al., 1999a). Importantly, single nucleotide polymorphisms (SNPs) in CREB1 and CREBBP (which encodes CBP) have been associated with accelerated cognitive decline and impaired episodic memory, semantic memory and executive function (Barral et al., 2014; Wolf et al., 2017). These types of memory, and episodic memory in particular, are disrupted in AD, supporting a link between dysfunctional CREB and the types of memory and cognitive functions impacted in AD (Gold and Budson, 2008). In addition, rare mutations in the Snf2-related CREBBP activator protein (SRCAP) were identified in individuals with late onset Alzheimer’s disease (LOAD), suggesting a causative role for dysfunctional CREB signaling in AD (Vardarajan et al., 2017). However, the underpinnings of CREB dysfunction in persons with AD remain elusive. A few groups, including ours, have reported diminished levels of total and phosphorylated CREB in the postmortem AD brain in the prefrontal cortex (Bartolotti et al., 2016a) and hippocampus (Bartolotti et al., 2016a; Pugazhenthi et al., 2011; Yamamoto-Sasaki et al., 1999b), two brain structures that are thought to be particularly important in episodic and semantic memory (Gold and Budson, 2008). Our group also reported that CBP and p300 are reduced in the postmortem AD prefrontal cortex (Bartolotti et al., 2016a), an observation that is particularly intriguing in light of evidence that p300 may be overactive in the acetylation of tau in the AD prefrontal cortex (Aubry et al., 2015; Min et al., 2015; Min et al., 2010a). The level and activity of Type I adenylyl cyclase, an enzyme responsible for the generation of cAMP and therefore CREB activation, has been reported to be reduced in the postmortem AD hippocampus (Cowburn et al., 1992a; Schnecko et al., 1994a; Yamamoto et al., 1996; Yamamoto et al., 1997). Similarly, PKA, PKC, CamK, and ERK, which phosphorylate CREB, have been described to be abnormally expressed or activated in the postmortem AD prefrontal cortex or hippocampus (Battaini et al., 1999; Bonkale et al., 1999; Kim et al., 2001; Perry et al., 1999; Reese et al., 2011; Wang et al., 1994). This evidence suggests that there may be multiple points of failure in CREB signaling in the AD brain.
While much work remains to be done investigating the status of CREB in persons with AD, CREB signaling has been extensively studied in mouse models of genetically-linked AD (FAD), although the data from these studies is at times contradictory. For example, decreased basal levels of activated CREB have been reported in the hippocampus of 3-month-old female APPswe/PS1ΔE9 mice (Bartolotti et al., 2016b), 2-month-old male APPswe/PS1ΔE9 mice (Hu et al., 2013), and 6-month-old 3xTg-AD mice (sex unspecified; (Caccamo et al., 2010)). In addition, CREB activation is diminished in the hippocampus following a stimulus, such as environmental enrichment or a learning task such as fear conditioning or novel object recognition in 3-month-old female APPswe/PS1ΔE9 mice (Bartolotti et al., 2016b), or following training for a maze task in 10-week-old male and female TgCRND8 mice (Yiu et al., 2011). On the other hand, increased basal levels of activated CREB expression have been reported in the hippocampus of 4-month-old and 13-month-old Tg2576 (sex unspecified), though these authors observed lower levels of activated CREB in this mouse model at 20 months (Dineley et al., 2001). Similarly, increased basal levels of activated CREB have been observed in whole-brain homogenates from 4- to 6-week-old 3xTg-AD and M146V-PS1ki mice (sex unspecified; (Muller et al., 2011). These contradictory results may be due to a number of factors, including the type of memory tested, the mouse model, the age and sex of the mice, and whether the tissue was harvested at a basal state or following a learning and memory task since it is possible that deficits in CREB signaling may only become apparent as a failure of CREB activation following a learning task in some mouse models of AD. Careful consideration of these factors will be necessary in future experiments to determine the most appropriate mouse model for the study of CREB in AD. It is also important to consider the type of memory task utilized as a function of disease progression. For example, impairments in object recognition tasks [such as in (Dere et al., 2005)] may be apparent at a different disease stage than spatial memory tasks such as mazes. Selecting the appropriate cognitive test for the disease stage and understanding the role of CREB in the formation and retrieval of these types of memory will facilitate the translation of the role of CREB in AD, as well as in the development of therapies meant to restore CREB signaling and enhance cognitive function in AD.
Additional evidence supporting the role of CREB dysfunction in AD and in cognitive impairments comes from the improvements in learning in memory that are observed when CREB signaling is enhanced in FAD mice. For example, virus-mediated rescue of CREB signaling in the hippocampus of 10-week-old male and female TgCRND8 mice has been shown to ameliorate memory deficits in a maze task (Yiu et al., 2011). CREB can also be increased through the reduction of phosphodiesterases (PDE), a family of enzymes hydrolze cAMP (PDE4, PDE7, PDE8) or cGMP (such as PDE5, PDE6, PDE9) or both cAMP and cGMP (PDE1, PDE2, PDE3, PDE10, PDE11), thereby reducing signaling through these molecules (Garcia-Osta et al., 2012). Reducing the expression or activity of PDEs is one way in which CREB can be pharmacologically enhanced, and may serve as an effective therapy for cognitive dysfunction in AD. For example, downregulation of PDE4D in the hippocampus reversed memory deficits induced by Aβ42 in male mice, as measured by a novel object recognition and Morris water maze task, as well as rescued pCREB expression in the hippocampus (Zhang et al., 2014). Similarly, treatment of male rats with rolipram, a PDE4 inhibitor, rescued Aβ40–induced deficits in memory in rats, as measured by a passive avoidance task, and also rescued the Aβ40–induced reduction in hippocampal pCREB (Cheng et al., 2010). Peripheral administration of rolipram was also able to rescue deficits in performance in a contextual fear conditioning task in 3-month-old male and female APP/PS1 mice, and rescued diminished expression of pCREB while not significantly affecting Aβ40, Aβ42, expression or amyloid plaque burden in the hippocampus (Gong et al., 2004), again emphasizing that the CREB signaling pathway may be able to affect cognitive function independent of regulation of amyloid. Rolipram itself may be limited as a therapeutic due to its side effects (Zhu et al., 2001), but these studies have demonstrated the potential of PDE inhibition as a means of enhancing CREB signaling and cognitive function in AD. Additional support for this idea comes from studies of PDE5 inhibitors. PDE5 inhibitors can increase pCREB by increasing cGMP, which has been shown to be reduced as a result of Aβ-induced reductions in the nitric oxide signaling cascade (Puzzo et al., 2005). Indeed, inhibition of PDE5 has been shown to reverse memory deficits a contextual fear conditioning and water maze, as well as rescue pCREB expression in the hippocampus of male and female 3-month-old APPswe/PS1ΔE9 mice (Puzzo et al., 2009). Amelioration of cognitive dysfunction in a Y-maze task was also shown following treatment with a PDE5 inhibitor in 10-month-old male APPswe/PS1ΔE9 mice (Jin et al., 2014). PDE5 inhibitors are particularly attractive candidates for use in treating AD as data from clinical trials of extended use of PDE5 inhibitors in the treatment of erectile dysfunction suggests they are safe (Fusco et al., 2010). Therefore, in addition to highlighting the importance of CREB signaling in cognitive function in AD, the continued research and development of PDE inhibitors offer a promising means of enhancing this signaling and improving cognitive function in AD (Bischoff, 2004a; Fiorito et al., 2017; Garcia-Osta et al., 2012; Teich et al., 2015).
The cause of CREB impairments in AD is not clear. In FAD mouse models, dysregulation of CREB signaling may result from the mutations in PS1 and or APP that are commonly utilized to generate the model (Chen et al., 2012; Marambaud et al., 2003; Wang et al., 2006). For example, several studies suggest that PS1 regulates CREB expression and function, (Bonds et al., 2015; Marambaud et al., 2003; Muller et al., 2011; Watanabe et al., 2009). In addition, it is thought that normal presenilin may promote CBP-induced transcription, and that FAD mutant PS1 can interfere with this process (Francis et al., 2006; Saura et al., 2004). Mutations in APP may also contribute to CREB signaling dysfunction, possibly through the dysregulation of APP processing or by increasing Aβ toxicity (Dineley et al., 2010b; Dineley et al., 2001; Espana et al., 2010; Ma et al., 2007; Nishimoto et al., 1993). In support of this idea, 5-month-old male C57BL/6 mice treated with Aβ oligomers express lower levels of pCREB in the hippocampus and impaired performance in a fear conditioning task (Dineley et al., 2010a). Similarly, treatment of 11- to 12-month-old Tg2576 mice (sex unspecified) with anti-Aβ antibodies has been shown to rescue impairments in CREB activation (Ma et al., 2007). Interestingly, overexpression of BACE1, which is thought to be increased in FAD, reduces CREB phosphorylation, PKA activity, and cAMP levels in vitro, independent of its effects through Aβ, suggesting that CREB dysfunction may not be exclusively dependent on Aβ levels (Chen et al., 2012). Similarly, virus-mediated enhancement of CBP expression reduced memory impairments in a Morris water maze task without also affecting changes in amyloid or tau pathology in the hippocampus of 6-month-old 3xTg-AD mice (sex unspecified; (Caccamo et al., 2010)). Indeed, we have observed deficits in CREB signaling in the hippocampus of both male and female APPswe/PS1ΔE9 mice as early as 2-3 months of age (Hu et al., 2013), though these mice do not typically demonstrate wide-spread plaque deposition until 4 to 6 months of age (Jankowsky et al., 2004; Jankowsky et al., 2005). However, these mice do still have aberrant APP processing and increased levels of soluble Aβ at this age, indicating that more study is needed to determine the contribution of the different forms of Aβ on CREB signaling (Bonardi et al., 2011; Min et al., 2010b; Zhang et al., 2012). Therefore, while the mutations in APP and PS1 in mouse models of FAD are clearly the original source of CREB impairments, the proximate cause remains unclear and CREB may be regulated independently of amyloid and tau in FAD mice.
In sporadic AD, the mechanism underlying CREB dysfunction is even less clear. If CREB impairments are simply the result of increased plaque load or NFTs, as might be concluded from the FAD mouse data, a relationship between CREB expression and these pathological hallmarks should be observed. However, in our recent study in which we examined CREB signaling components in the postmortem AD prefrontal cortex, we did not observe a relationship between pCREB and the extent of plaque deposition or NFTs in the prefrontal cortex (Bartolotti et al., 2016a), suggesting that CREB dysfunction may not be simply a result of advanced neurodegeneration and AD pathology.
Aging is one of the primary risk factors for sporadic AD. It is therefore interesting that CREB decreases as a function of age in both the human (Yamamoto-Sasaki et al., 1999b) and rat hippocampus, both at a basal level (Foster et al., 2001), and following training on a contextual fear conditioning task in which the older rats exhibited poorer performance (Kudo et al., 2005). While the mechanism responsible for reduced CREB in the hippocampus during aging is unclear, reactive oxygen species may play a role (Bevilaqua et al., 1999; Ozgen et al., 2009; Ryu et al., 2005; Waldron and Rozengurt, 2000). Another hypothesis is that dysregulated inflammation during aging may underlie cognitive decline (Franceschi et al., 2007). This explanation is particularly attractive in that it provides a link between alterations in CREB signaling in the brain and the periphery, which we review next. However, it is important to note that the reductions in CREB signaling components observed in AD are typically age-matched, indicating that age alone is not responsible for the further reduction of CREB signaling observed in AD.
2.2. CREB IN PBMC
As CREB is a critical component of memory and cognitive function in the brain, its accessibility in the periphery could offer valuable and relevant insight into cognitive decline during AD, as well as serve as an indicator of the efficacy of therapies intended to enhance cognitive function. In addition, our work in APPswe/PS1ΔE9 mice suggests that CREB dysfunction in the hippocampus may precede plaque deposition (Bartolotti et al., 2016b; Hu et al., 2013), suggesting that CREB dysfunction is an earlier event than disease pathological hallmarks. In that regard, a peripheral marker would also aid in understanding the timescale of CREB impairments in AD, rather than depending on an end-state, postmortem analysis.
In our recent paper, we showed that levels of pCREB in PBMC samples isolated during life were temporally correlated with levels of pCREB in the postmortem AD prefrontal cortex (Bartolotti et al., 2016a). This observation suggests the interesting possibility that analysis of peripheral CREB signaling components, and pCREB in particular, could be used as a marker for CREB signaling in the brain. Others have previously investigated the potential of CREB signaling components in PBMC as markers for CREB signaling in the brain. For example, CREB and pCREB are reduced in the postmortem prefrontal cortex and hippocampus of individuals suffering from mood disorders (Dwivedi et al., 2003), and a reduction in pCREB has been observed in peripheral blood T lymphocytes of individuals suffering from depression (Koch et al., 2002), supporting the idea that abnormal CREB function may be apparent in the PBMC when it is occurring in the brain.
CREB plays important roles in regulating immune function and a detailed description of its roles in that regard are described elsewhere (Wen et al., 2010). In PBMC, CREB is primarily thought to be important fo survival, cell cycle and proliferation, and cytokine production (Wen et al., 2010). Here we will briefly review the current understanding of CREB signaling in different subtypes of PBMC (Table 1), and the evidence supporting the hypothesis that these processes are dysfunctional in AD.
Table 1.
Role of CREB in PBMC.
| Cell Type | Role of CREB |
|---|---|
| T cells | • Proliferation and survival. •Production of IL-2, IFN-γ, and IL-4 by CD4+ T cells. •Regulation of Th17 and Treg differentiation via TGFβ and IL-6. •Production of IL-17 by Th17 cells. |
| B cells | • Proliferation and survival. |
| NK cells | • Mediates response to IL-2. |
| Macrophages | • Production of IL-10. |
| Dendritic cells | •Maturation. •Production of IL-10. |
Lymphocytes make up the greatest percentage of PBMC and include T cells, B cells and natural killer (NK) cells. T cells, particularly CD4+ T cells, in turn make up the greatest percentage of lymphocytes. Therefore, it is likely that detectable changes in CREB expression in a pool of PBMC come from CD4+ T cells, but CREB may also be dysfunctional in other PBMC.
Several cytokines and immune-related factors, including interleukin 2 (IL-2), IL-6, IL-10, tumor necrosis factor alpha (TNF-r), cyclooxygenase-2, and macrophage migration-inhibitory factor possess a CRE element (Wen et al., 2010). In addition, CREB is thought to be important for the expression of interferon γ (IFN-γ), and interleukin 4 (IL-4) by CD4+ T cells (Zhang et al., 2000), and the expression of these cytokines is thought to be dysfunctional in, and perhaps contribute to the pathogenesis of, AD (Zheng et al., 2016). Data from CREB mutant mice suggests that CREB is important for regulating Th17 differentiation and regulatory T cell (Treg) differentiation, and may be a particularly important factor in maintaining balance between Th17 and Treg cells (Wang et al., 2017). While the role of Th17 and Treg cells in AD is controversial and remains an important topic for investigation (Baruch et al., 2015; Dansokho et al., 2016; Flego et al., 2015; He and Balling, 2013; Kim and Leonard, 2007; Larbi et al., 2009; Saresella et al., 2010; Tahmasebinia and Pourgholaminejad, 2017), there is some evidence that a dysfunction in CREB signaling in these cells could be present in AD. For example, an imbalance between Th17 and Treg cells has been reported in AD PBMC (Oberstein et al., 2018). In addition, CREB and CBP are important for the expression of interleukin-17 (IL-17) by Th17 cells (Hammitzsch et al., 2015; Hernandez et al., 2015), which has been shown to be altered in the serum of mice in response to hippocampal administration of Aβ1-42. Similarly, CBP and p300 are thought to be important in mediating Treg function, which is an important part of appropriate regulation of the inflammatory response (Klatzmann and Abbas, 2015), and deletion in CBP and p300 leads to aberrant expression of inflammatory genes (Liu et al., 2014).
In addition to T cells, other PBMC subsets may be impacted by dysfunctional CREB signaling in AD. For example, NK cells isolated from AD patients have been shown to be less responsive to stimulation including stimulation by IL-2 (Araga et al., 1991). In addition, numbers of NK cells have been reported to be diminished in AD, possibly as a result of increased apoptosis (Schindowski et al., 2006, but the important role of CREB signaling in NK cell function in response to IL-2 (Ponti et al., 2002) suggests that impaired CREB signaling may contribute to the dysregulation of NK cells in AD.
In addition to lymphocytes, macrophages and small numbers of dendritic cells (DCs) can also be found in PBMC. As in the case of lymphocytes, CREB may play a role in the survival of monocytes (Roach et al., 2005). CREB is thought to mediate interleukin 10 (IL-10) expression by macrophages (Ananieva et al., 2008) and DCs (Alvarez et al., 2009). Specific knockout of CREB in DCs leads to an impairment in immune processes related to DC function, specifically the expression of B cell lymphocytes in germinal centers (Ohl et al., 2018).
Finally, CREB is thought to be important in the proliferation of B cells (Yasuda et al., 2008). B cells have been reported to be decreased in AD PBMC (Richartz-Salzburger et al., 2007; Speciale et al., 2007), which might be interpreted as a result of impaired CREB signaling in AD PBMC, but further experiments are necessary to support this idea.
From this discussion it is clear that a more thorough understanding of the cell type responsible for CREB dysfunction in AD PBMC is critical to understanding this phenomenon. Future experiments should examine CREB signaling expression in the different subpopulations of PBMC. In addition, the cause of impaired phosphorylation of CREB in AD PBMC will be the topic of future study, but we will consider a few possibilities here. One possible mechanism for the impairments in CREB phosphorylation could be through kinase function. CREB in PBMC responds to many of the same kinases as neuronal CREB (for review (Kuo and Leiden, 1999)), suggesting the possibility of common regulatory pathways. For example, Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) activates CREB in leukocytes via RSK (Mitton et al., 2014), and peripheral administration of GM-CSF to 12-month-old male and female AβPPswe mice has been shown to improve cognitive function as measured by a radial-arm water maze (Boyd et al., 2010). Similarly, mice deficient in CaMKIV not only demonstrate memory impairments in fear conditioning and Barnes circular maze experiments (Takao et al., 2010), but also exhibit impaired phosphorylation of CREB in T cells (Anderson and Means, 2002). Mitogen and stress activated protein kinase (MSK) has also been shown to activate CREB in T cells, and is thought to have an anti-inflammatory role in the cells of the immune system (Kaiser et al., 2007; Reyskens and Arthur, 2016). Therefore, a common, system-wide impairment in the function of one or more kinase could be a mechanism underlying the diminished CREB phosphorylation in both brain and blood. We next consider the hypothesis that CREB dysfunction actually begins in the periphery and is transferred to the brain, thus contributing to cognitive decline in AD.
3. PERIPHERAL CREB DYSFUNCTION AS AN EARLY EVENT IN AD PATHOGENESIS
Dysregulation of the immune system is a hallmark of age-related cognitive decline and neurodegenerative diseases characterized by dementia (Franceschi et al., 2007). It is thought that the aged brain expresses higher levels of pro-inflammatory chemokines and cytokines like TNFα and IL-6, as well as lower levels of anti-inflammatory cytokines like IL-2 (Chung et al., 2009)). Aged individuals with greater expression of circulating pro-inflammatory cytokines are more likely to suffer from cognitive impairments, and this correlative observation leads to the hypothesis that pathological neuroinflammation may be playing a causative role in cognitive decline observed in aging (Marsland et al., 2015; Wyss-Coray, 2006). It has also been proposed that dysregulated inflammation during aging may act as a prodromal form of AD (Giunta et al., 2008). In support of this idea, it has been reported that increased expression of inflammatory proteins in the plasma precedes onset of AD (Engelhart et al., 2004). CREB signaling is a key component of appropriate regulation of the inflammatory response, particularly in its role of regulating cytokine production by PBMC (Raker et al., 2016), which suggests that peripheral CREB signaling may play a role in systemic aging. Intriguingly, exposure of old mice to young blood increases pCREB expression in the hippocampus of the old mice (Villeda et al., 2014), indicating that the effects of aging on peripheral blood may influence CREB signaling in the brain, and thus cognitive function. How altered CREB signaling in the aging peripheral blood might relate to altered CREB signaling in the brain remains unknown, but elucidating this connection may shed light on the connection between peripheral inflammation and cognitive decline in AD. Common indicators of inflammation, such as levels of cytokines and chemokines, have been repeatedly shown to be altered in AD compared to age-matched controls, both in the brain and in the peripheral fluids (for review see (Wyss-Coray and Rogers, 2012)), indicating that while increased inflammation may be a part of the aging process, the dysregulation of inflammation in AD may be more pathogenic. In our recent paper, we observed modest increases in pCREB, Total CREB and CBP in PBMC isolated from persons with mild cognitive impairment, the prodromal form of AD (Bartolotti et al., 2016a). We hypothesized that this increase could be an early attempt at compensating for impairments in CREB signaling in PBMC, or it could be indicative of increased CREB-dependent inflammation typical of MCI (Bonotis et al., 2008; Magaki et al., 2007). Indeed, peripheral expression of cytokines, including the CREB-driven cytokines IL-2 and IL-10, is reported to be especially high in MCI (King et al., 2018), suggesting that alterations in CREB signaling in PBMCs correspond to alterations in inflammation that occur with AD progression different from what is occurring in aging without AD.
While the issue of whether peripheral inflammation is a cause or effect of AD remains unclear, there is some evidence supporting a causative role. For example, stimulating an immune response through peripheral administration of LPS has been shown to cause cognitive impairment, as measured by a water maze task, and induce expression of Aβ1-42 in the hippocampus and cortex of male mice (Lee et al., 2008), supporting the idea that alterations in the periphery can influence cognitive function and AD pathology. In addition, a recent meta-analysis reported that increased peripheral inflammation increased risk for dementia (Koyama et al., 2013). These studies suggest that dysregulation of the immune system may be at least an early event in AD, and may even contribute to the disease in a causative way.
Importantly, clinical trials of non-steroid anti-inflammatory drugs (NSAIDs) have largely failed thus far as a therapy for AD (Aisen et al., 2003). While this failure may be due to many factors, such as initiation of treatment too late in the disease course, or lack of strength or specificity, this result does suggest that non-specific lowering of peripheral inflammation may not be sufficient to improve cognitive function in AD, and further work needs to be done to identify the mechanism of inflammatory dysfunction in AD. Recent evidence suggests that inflammasomes may respond to, as well as exacerbate, AD pathology, potentially resulting in a cycle of increased inflammation and increased pathology, thus emphasizing the contribution of the immune system to AD pathogenesis (Venegas et al., 2017). We propose that the dysregulated peripheral CREB signaling is a component of immune system dysfunction in AD, and that this dysfunction may be communicated to the brain, exacerbating cognitive decline in AD.
3.1. MOLECULAR MEDIATORS
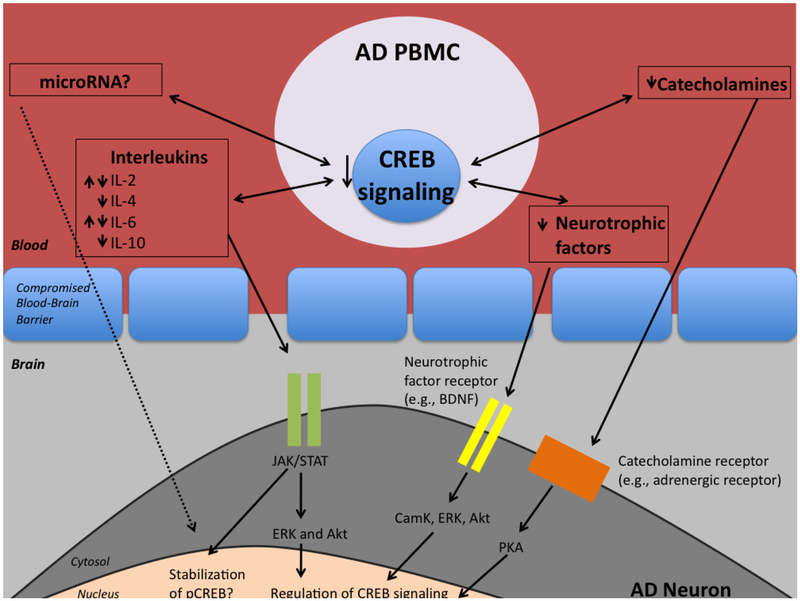
Perhaps one of the best-supported mechanisms for neuro-immune communication is via cytokines, particularly those expressed by PBMC. In AD, it is thought that the blood brain barrier (BBB) exhibits increased “leakiness”, which may allow abnormal trafficking of immune cells and increased communication between the blood and brain (Martorana et al., 2012). For example, activated T cells are able to cross the BBB from the periphery into the CNS, and increased levels of infiltrating T cells have been observed in the postmortem AD hippocampus (Togo et al., 2002). However, even in the absence of direct contact, brain cells and PBMC both produce and respond to cytokines (Bartfai and Schultzberg, 1993), offering another potential mechanism for transference of CREB-related signaling deficits (Figure 3).
Figure 3.
Hypothesis detailing possible mechanisms by which CREB signaling in PBMC affect CREB signaling in neurons. Deficits in CREB may cause dysfunction in the production of and response to interleukins, neurotrophic factors, catecholamines, or microRNAs. These factors may traffic across the compromised blood brain barrier and signal through neuronal receptors on vulnerable neurons, thus negatively affecting CREB activation and CRE-based transcription, important for learning and memory.
As mentioned above, CREB regulates both the production of and response to many interleukins in the PBMC, including IL-2, IL-4, IL-6, IL-10, and IL-17 (Acarin et al., 2000; Avni et al., 2010; Erta et al., 2012; Guyot et al., 1998; Hammitzsch et al., 2015; Hernandez et al., 2015; Jansky et al., 2003; Rigano et al., 1996; Zhang et al., 2000). A recent meta-analysis suggested that the peripheral expression of these and other cytokines is associated with AD and cognitive function (Lai et al., 2017). IL-2 has been reported to be both decreased (Beloosesky et al., 2002) and increased (Huberman et al., 1994) in AD PBMC, though more work needs to be done to determine the exact nature of this dysfunction. Similarly, the reports concerning production of IL-6 are also conflicting and has been reported to be both increased (Reale et al., 2005) and decreased (Bergman et al., 2002) in AD PBMC. IL-4 and IL-10 have both been reported to be reduced in AD PBMC (Reale et al., 2008; Speciale et al., 2007).
Interleukins are thought to have some permeance through the BBB (Alves et al., 2017; Banks et al., 2004; Waguespack et al., 1994). IL-2 has been reported to be decreased in the postmortem AD hippocampus (Alves et al., 2017), which is likely a result of decreased production from the resident cells of the brain, but could be partially due to decreased transfer from the periphery. IL-2 receptors are expressed on neurons and IL-2 knockout mice exhibit impaired performance on a Morris water maze task (Petitto et al., 1999). Importantly, specific knockout of brain-derived IL-2 does not worsen memory impairments as measured by a Morris water maze task, indicating a key role for peripherally-derived IL-2 on cognition (Petitto et al., 2015). Interestingly, treating 8-month-old male APPswe/PS1ΔE9 mice peripherally with IL-2 was found to rescue neurodegeneration and memory impairments in a Morris water maze task, as well as increasing the presence of IL-2 and Tregs in the brain (Alves et al., 2017). The expression of other CREB-driven interleukins in the AD brain also requires more study. The nature of IL-6 dysfunction in the AD brain, for example, remains unclear (Chakrabarty et al., 2010; Chong, 1997; Han et al., 2011; Hull et al., 1996a; Hull et al., 1996b; Ringheim et al., 1998). While IL-10 is thought to be reduced in AD PBMC, recent experiments in mice suggest that increased IL-10 in the brain of mice overexpressing APP may worsen cognitive impairments as demonstrated with a contextual fear conditioning task in 5-month-old male and female CRND8 mice (Chakrabarty et al., 2015). Similarly, a reduction in IL-10 was shown to be beneficial for cognitive function in 12- to 13-month-old male and female APP/PS1 mice, as demonstrated by improved performance on a novel object recognition task, and the same study reported that IL-10 may be increased in the AD brain (Guillot-Sestier et al., 2015). IL-10 may be differentially regulated depending on the disease course (Asselineau et al., 2015). Treatment with rolipram, a compound that increases phosphorylation of CREB, increases IL-10 levels and decreases TNF-α (a cytokine suppressed by CREB (Avni et al., 2010)) in the brain, while improving cognitive functioning in an animal model of diabetes, as measured by a Morris water maze task, (Miao et al., 2015), suggesting a link between these pathways.
It is important to note that the canonical interleukin signaling pathway acts via JAK/STAT, and though cytokine signaling via JAK/STAT has been implicated in the formation of LTM (Petitto et al., 2015), the relationship between this process and CREB signaling remains to be investigated and indicates that deficits in interleukin expression or activity resulting from CREB deficits in the periphery may indirectly affect CREB expression in the brain. One study demonstrated that JAK2 prevents degradation of pCREB, thus stabilizing it in adrenocortical cells, suggesting one potential mechanism by which JAK/STAT signaling might be linked to CREB signaling (Lefrancois-Martinez et al., 2011). In addition, the ERK and Akt pathways can respond to JAK/STAT signaling, and CREB could in turn respond to alterations in ERK or Akt (Kristiansen and Mandrup-Poulsen, 2005), which could lead to phosphorylation of CREB. In addition to CREB activation, interleukins may affect the expression or activity of CBP/p300. For example, IL-4 has been shown to increase the HAT activity of CBP in epithelial cells (Shankaranarayanan et al., 2001). Therefore, while interleukins are a promising candidate to mediate the communication of disrupted CREB signaling from the periphery to the brain, more work is needed to demonstrate a mechanistic link.
Other potential mediators of CREB deficits from the periphery from the brain include neurotrophic factors. PBMC can produce neurotrophic factors, which are BBB-permeable and are an important means of communication between the blood and brain (Otten et al., 2000). Production of neurotrophins by T cells is thought to be an important neuroprotective factor for injured neurons (Moalem et al., 2000), suggesting that communication from PBMC to the brain by neurotrophins is a plausible mechanism by which a dysfunction in CREB might be communicated. CREB is an important mediator both of the transcription of neurotrophic factors and of the response to neurotrophic factor signaling. Neurotrophic factors have been demonstrated to be dysfunctional in AD, both in peripheral fluids and postmortem tissue (Du et al., 2018), supporting the idea that the communication of CREB dysfunction may be mediated by neurotrophic factors. For example, brain-derived neurotrophic factor (BDNF), which possess a CRE region, has been reported to be decreased in AD peripheral blood samples (Qin et al., 2017), and higher levels of BDNF in serum have been linked to slower cognitive decline in AD (Laske et al., 2011). Therefore, it is possible that CREB dysfunction in PBMC could lead to reduced BDNF production by the PBMC, thereby reducing the contribution of circulating BDNF to signaling in the brain, a deficit that could presumably accelerate cognitive decline. In addition to BDNF, other neurotrophic factors are also potential candidates for the communication of CREB deficits from PBMC to the brain. For example, CREB also facilitates expression of NGF (McCauslin et al., 2006) and VEGF (Jeon et al., 2007), but the data concerning the expression of these neurotrophic factors in AD is conflicting (Du et al., 2018), and requires further study before these factors can be supported as a link between dysfunctional CREB in PBMC and in the brain.
Catecholamines (e.g., dopamine, epinephrine, and norepinephrine) are hormones that could also contribute to the communication of CREB deficits between the blood and brain. T cells can produce catecholamines (Flierl et al., 2008). CREB is important for tyrosine hydroxylase, which is a critical enzyme in the biosynthesis of catecholamines (Lewis-Tuffin et al., 2004; Piech-Dumas and Tank, 1999), and therefore disrupted CREB signaling could impair production of catecholamines. CREB is a mediator of the signaling response of catecholamines (Beck et al., 2004; Lorton and Bellinger, 2015) and could therefore be impacted by altered peripheral catecholamine signaling. Indeed, catecholamines are thought to be dysfunctional in the AD brain and may lead to neurodegeneration and cognitive dysfunction (Gannon et al., 2015). However, the role of CREB in the regulation of catecholamines in PBMC and the effect this alteration may have on CREB signaling in the brain still requires more study.
Though microRNAs (miRs) are typically thought of for their ability to regulate translation within a cell, evidence suggests that miRs can exist extracellularly and may be a form of cell-cell communication (Valadi et al., 2007). Indeed, trends of expression of miRs in peripheral circulation often mirror trends of expression in the brain, which has made them an attractive candidate for biomarkers. miRs known to regulate or be regulated by CREB, such as miR-9 (Tan et al., 2012a; Tan et al., 2012b), miR-124 (Rajasethupathy et al., 2009; Wu and Xie, 2006), miR-132 (Majer et al., 2012; Vo et al., 2005; Yi et al., 2014), miR-134 (Gao et al., 2010; Zhao et al., 2013), miR-212 (Hollander et al., 2010; Vo et al., 2005), could also serve as mediators of communicating CREB signaling dysfunction between the blood and the brain, and idea supported by evidence that these CREB-linked miRs are abnormally regulated in AD (An et al., 2017; Cogswell et al., 2008; Hebert et al., 2008; Lau et al., 2013; Lukiw, 2007). However, much work needs to be done to demonstrate not only that these miRs respond to CREB in PBMC, but also that the miRs are released from the PBMC, traffic to the brain, and impact CREB signaling in the brain and that this process is altered in AD for this to be a plausible mechanism of communication of CREB signaling dysfunction in AD.
4. CONCLUSIONS
Here we have discussed possible mechanisms underlying systemic and central CREB impairments. These mechanisms are important because they may underlie impaired CREB signaling as a cause of cognitive decline in AD and potentially other brain disorders characterized by cognitive deterioration. Focusing on mechanisms in AD, we have provided evidence that CREB signaling in PBMC is related to CREB signaling in the brain, and considered the possibility that the expression of CREB signaling components in PBMC may be a more faithful biomarker of cognitive function in AD than markers directly reliant on pathological hallmarks. We have proposed the hypothesis that CREB dysfunction in PBMC is an early event in AD pathogenesis, perhaps as a result of immune system dysfunction, and is communicated to the brain, causing dysregulation of CREB signaling in the brain and exacerbating cognitive dysfunction in AD. Finally, we have considered potential molecular mediators of this communication, with the acknowledgement that the data on immune to brain communication is still relatively underexplored and much more research is needed to fully elucidate the mechanism. However, a more thorough understanding of CREB in the immune system and CREB in the brain and the relationship between these two may shed light on the elusive nature of dementia and lead to more effective therapeutics.
Highlights:
There is no reliable biomarker for cognitive dysfunction in AD.
CREB signaling is critical to learning and memory and may be dysfunctional in AD.
Expression of CREB components in PBMC may provide a biomarker for CREB in the brain.
CREB dysfunction in PBMC may be a result of immune dysfunction.
PBMC CREB dysfunction may affect neuronal CREB, worsening cognitive decline in AD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acarin L, Gonzalez B, Castellano B, 2000. Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur J Neurosci 12, 3505–3520. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ, Alzheimer's Disease Cooperative, S., 2003. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289, 2819–2826. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Municio C, Alonso S, Sanchez Crespo M, Fernandez N, 2009. The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2. J Immunol 183, 1471–1479. [DOI] [PubMed] [Google Scholar]
- Alves S, Churlaud G, Audrain M, Michaelsen-Preusse K, Fol R, Souchet B, Braudeau J, Korte M, Klatzmann D, Cartier N, 2017. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer's disease mice. Brain 140, 826–842. [DOI] [PubMed] [Google Scholar]
- An F, Gong G, Wang Y, Bian M, Yu L, Wei C, 2017. MiR-124 acts as a target for Alzheimer's disease by regulating BACE1. Oncotarget 8, 114065–114071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, Wingate A, Monk CE, Toth R, Santos SG, Iversen L, Arthur JS, 2008. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol 9, 1028–1036. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Means AR, 2002. Defective signaling in a subpopulation of CD4(+) T cells in the absence of Ca(2+)/calmodulin-dependent protein kinase IV. Mol Cell Biol 22, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araga S, Kagimoto H, Funamoto K, Takahashi K, 1991. Reduced natural killer cell activity in patients with dementia of the Alzheimer type. Acta Neurol Scand 84, 259–263. [DOI] [PubMed] [Google Scholar]
- Arany Z, Sellers WR, Livingston DM, Eckner R, 1994. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell 77, 799–800. [DOI] [PubMed] [Google Scholar]
- Asselineau D, Benlhassan K, Arosio B, Mari D, Ferri E, Casati M, Gussago C, Tedone E, Annoni G, Mazzola P, Piette F, Belmin J, Pariel S, Bornand A, Beaudeux JL, Doulazmi M, Mariani J, Bray DH, 2015. Interleukin-10 Production in Response to Amyloid-beta Differs between Slow and Fast Decliners in Patients with Alzheimer's Disease. J Alzheimers Dis 46, 837–842. [DOI] [PubMed] [Google Scholar]
- Aubry S, Shin W, Crary JF, Lefort R, Qureshi YH, Lefebvre C, Califano A, Shelanski ML, 2015. Assembly and interrogation of Alzheimer's disease genetic networks reveal novel regulators of progression. PLoS One 10, e0120352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni D, Ernst O, Philosoph A, Zor T, 2010. Role of CREB in modulation of TNFalpha and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Molecular immunology 47, 1396–1403. [DOI] [PubMed] [Google Scholar]
- Banks WA, Niehoff ML, Zalcman SS, 2004. Permeability of the mouse blood-brain barrier to murine interleukin-2: predominance of a saturable efflux system. Brain, behavior, and immunity 18, 434–442. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T, 1996. The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643. [DOI] [PubMed] [Google Scholar]
- Barcia C Sr., Mitxitorena I, Carrillo-de Sauvage MA, Gallego JM, Perez-Valles A, Barcia C Jr., 2013. Imaging the microanatomy of astrocyte-T-cell interactions in immune-mediated inflammation. Frontiers in cellular neuroscience 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral S, Reitz C, Small SA, Mayeux R, 2014. Genetic variants in a 'cAMP element binding protein' (CREB)-dependent histone acetylation pathway influence memory performance in cognitively healthy elderly individuals. Neurobiol Aging 35, 2881 e2887–2881 e2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T, Schultzberg M, 1993. Cytokines in neuronal cell types. Neurochem Int 22, 435–444. [DOI] [PubMed] [Google Scholar]
- Bartolotti N, Bennett DA, Lazarov O, 2016a. Reduced pCREB in Alzheimer's disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Mol Psychiatry 21, 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolotti N, Segura L, Lazarov O, 2016b. Diminished CRE-Induced Plasticity is Linked to Memory Deficits in Familial Alzheimer's Disease Mice. Journal of Alzheimer's disease : JAD 50, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M, 2015. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nature communications 6, 7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaini F, Pascale A, Lucchi L, Pasinetti GM, Govoni S, 1999. Protein kinase C anchoring deficit in postmortem brains of Alzheimer's disease patients. Exp Neurol 159, 559–564. [DOI] [PubMed] [Google Scholar]
- Beck G, Brinkkoetter P, Hanusch C, Schulte J, van Ackern K, van der Woude FJ, Yard BA, 2004. Clinical review: immunomodulatory effects of dopamine in general inflammation. Critical care 8, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloosesky Y, Salman H, Bergman M, Bessler H, Djaldetti M, 2002. Cytokine levels and phagocytic activity in patients with Alzheimer's disease. Gerontology 48, 128–132. [DOI] [PubMed] [Google Scholar]
- Bergman M, Salman H, Beloosesky Y, Djaldetti M, Bessler H, 2002. Are peripheral blood cells from patients with Alzheimer disease more sensitive to apoptotic stimuli? Alzheimer disease and associated disorders 16, 156–160. [DOI] [PubMed] [Google Scholar]
- Bevilaqua LRM, Cammarota M, Paratcha G, de Stein ML, Izquierdo I, Medina JH, 1999. Experience-dependent increase in cAMP-responsive element binding protein in synaptic and nonsynaptic mitochondria of the rat hippocampus. Eur J Neurosci 11, 3753–3756. [DOI] [PubMed] [Google Scholar]
- Bischoff E, 2004a. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res 16 Suppl 1, S11–14. [DOI] [PubMed] [Google Scholar]
- Bischoff E, 2004b. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res 16, S11–S14. [DOI] [PubMed] [Google Scholar]
- Bonardi C, de Pulford F, Jennings D, Pardon MC, 2011. A detailed analysis of the early context extinction deficits seen in APPswe/PS1dE9 female mice and their relevance to preclinical Alzheimer's disease. Behavioural Brain Research 222, 89–97. [DOI] [PubMed] [Google Scholar]
- Bonds JA, Kuttner-Hirshler Y, Bartolotti N, Tobin MK, Pizzi M, Marr R, Lazarov O, 2015. Presenilin-1 Dependent Neurogenesis Regulates Hippocampal Learning and Memory. PloS one 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkale WL, Cowburn RF, Ohm TG, Bogdanovic N, Fastbom J, 1999. A quantitative autoradiographic study of [3H]cAMP binding to cytosolic and particulate protein kinase A in postmortem brain staged for Alzheimer's disease neurofibrillary changes and amyloid deposits. Brain Res 818, 383–396. [DOI] [PubMed] [Google Scholar]
- Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S, 2008. Systemic immune aberrations in Alzheimer's disease patients. Journal of neuroimmunology 193, 183–187. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ, 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79, 59–68. [DOI] [PubMed] [Google Scholar]
- Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H, 2010. GM-CSF Upregulated in Rheumatoid Arthritis Reverses Cognitive Impairment and Amyloidosis in Alzheimer Mice. Journal of Alzheimers Disease 21, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Neve RL, Colombo PJ, 2007. Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus. Learn Mem 14, 195–199. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S, 2010. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 107, 22687–22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P, 2010. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb J 24, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, DiNunno N, Rosario AM, Cruz PE, Verbeeck C, Sacino A, Nix S, Janus C, Price ND, Das P, Golde TE, 2015. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 85, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL, 2013. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci 33, 10698–10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Zou XY, Watanabe H, van Deursen JM, Shen J, 2010. CREB Binding Protein Is Required for Both Short-Term and Long-Term Memory Formation. J Neurosci 30, 13066–13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang X, Zhang YW, Rockenstein E, Bu G, Golde TE, Masliah E, Xu H, 2012. Alzheimer's beta-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of beta-amyloid. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 11390–11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YF, Wang C, Lin HB, Li YF, Huang Y, Xu JP, Zhang HT, 2010. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Abeta25–35 or Abeta1–40 peptide in rats. Psychopharmacology (Berl) 212, 181–191. [DOI] [PubMed] [Google Scholar]
- Chong Y, 1997. Effect of a carboxy-terminal fragment of the Alzheimer's amyloid precursor protein on expression of proinflammatory cytokines in rat glial cells. Life sciences 61, 2323–2333. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH, 1993. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365, 855–859. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C, 2009. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Research Reviews 8, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA, 2008. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. Journal of Alzheimer's disease : JAD 14, 27–41. [DOI] [PubMed] [Google Scholar]
- Cowburn RF, O'Neill C, Ravid R, Alafuzoff I, Winblad B, Fowler CJ, 1992a. Adenylyl cyclase activity in postmortem human brain: evidence of altered G protein mediation in Alzheimer's disease. J Neurochem 58, 1409–1419. [DOI] [PubMed] [Google Scholar]
- Cowburn RF, Oneill C, Ravid R, Alafuzoff I, Winblad B, Fowler CJ, 1992b. Adenylyl Cyclase Activity in Postmortem Human Brain - Evidence of Altered G-Protein Mediation in Alzheimers-Disease. J Neurochem 58, 1409–1419. [DOI] [PubMed] [Google Scholar]
- Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, Cagnard N, Holzenberger M, Piaggio E, Aucouturier P, Dorothee G, 2016. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA, 2005. Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiol Learn Mem 84, 214–221. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G, 2010a. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. Journal of neuroscience research 88, 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G, 2010b. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res 88, 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD, 2001. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 4125–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Wu HT, Qin XY, Cao C, Liu Y, Cao ZZ, Cheng Y, 2018. Postmortem Brain, Cerebrospinal Fluid, and Blood Neurotrophic Factor Levels in Alzheimer's Disease: A Systematic Review and Meta-Analysis. Journal of molecular neuroscience : MN 65, 289–300. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, Tamminga CA, Pandey GN, 2003. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Archives of general psychiatry 60, 273–282. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM, 2004. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 61, 668–672. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J, 2012. Interleukin-6, a major cytokine in the central nervous system. International journal of biological sciences 8, 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana J, Valero J, Minano-Molina AJ, Masgrau R, Martin E, Guardia-Laguarta C, Lleo A, Gimenez-Llort L, Rodriguez-Alvarez J, Saura CA, 2010. beta-Amyloid Disrupts Activity-Dependent Gene Transcription Required for Memory through the CREB Coactivator CRTC1. J Neurosci 30, 9402–9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito J, Vendome J, Saeed F, Staniszewski A, Zhang H, Yan S, Deng SX, Arancio O, Landry DW, 2017. Identification of a Novel 1,2,3,4-Tetrahydrobenzo[b][1,6]naphthyridine Analogue as a Potent Phosphodiesterase 5 Inhibitor with Improved Aqueous Solubility for the Treatment of Alzheimer's Disease. J Med Chem 60, 8858–8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flego D, Severino A, Trotta F, Previtero M, Ucci S, Zara C, Massaro G, Pedicino D, Biasucci LM, Liuzzo G, Crea F, 2015. Increased PTPN22 expression and defective CREB activation impair regulatory T-cell differentiation in non-ST-segment elevation acute coronary syndromes. Journal of the American College of Cardiology 65, 1175–1186. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA, 2008. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora's box? Mol Med 14, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A, 2001. Calcineurin links Ca2+ dysregulation with brain aging. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 4066–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panouraia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S, 2007. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development 128, 92–105. [DOI] [PubMed] [Google Scholar]
- Francis YI, Stephanou A, Latchman DS, 2006. CREB-binding protein activation by presenilin 1 but not by its M146L mutant. Neuroreport 17, 917–921. [DOI] [PubMed] [Google Scholar]
- Fusco F, Razzoli E, Imbimbo C, Rossi A, Verze P, Mirone V, 2010. A new era in the treatment of erectile dysfunction: chronic phosphodiesterase type 5 inhibition. BJU Int 105, 1634–1639. [DOI] [PubMed] [Google Scholar]
- Gannon M, Che P, Chen Y, Jiao K, Roberson ED, Wang Q, 2015. Noradrenergic dysfunction in Alzheimer's disease. Front Neurosci 9, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH, 2010. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Osta A, Cuadrado-Tejedor M, Garcia-Barroso C, Oyarzabal J, Franco R, 2012. Phosphodiesterases as therapeutic targets for Alzheimer's disease. ACS Chem Neurosci 3, 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J, 2008. Inflammaging as a prodrome to Alzheimer's disease. J Neuroinflammation 5, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Barth AL, Wallace H, McKenna M, Silva A, Fox K, 1999. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex 9, 249–256. [DOI] [PubMed] [Google Scholar]
- Gold CA, Budson AE, 2008. Memory loss in Alzheimer's disease: implications for development of therapeutics. Expert Rev Neurother 8, 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O, 2004. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest 114, 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J Jr., Leung BP, Rezai-Zadeh K, Town T, 2015. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85, 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot DJ, Newbound GC, Lairmore MD, 1998. Co-stimulation of human peripheral blood mononuclear cells with IL-2 and anti-CD3 monoclonal antibodies induces phosphorylation of CREB. Immunology letters 61, 45–52. [DOI] [PubMed] [Google Scholar]
- Hammitzsch A, Tallant C, Fedorov O, O'Mahony A, Brennan PE, Hay DA, Martinez FO, Al-Mossawi MH, de Wit J, Vecellio M, Wells C, Wordsworth P, Muller S, Knapp S, Bowness P, 2015. CBP30, a selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses. Proc Natl Acad Sci U S A 112, 10768–10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XM, Wang CH, Sima X, Liu SY, 2011. Interleukin-6 -174G/C polymorphism and the risk of Alzheimer's disease in Caucasians: a meta-analysis. Neurosci Lett 504, 4–8. [DOI] [PubMed] [Google Scholar]
- He F, Balling R, 2013. The role of regulatory T cells in neurodegenerative diseases. Wiley interdisciplinary reviews. Systems biology and medicine 5, 153–180. [DOI] [PubMed] [Google Scholar]
- Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, Sastre-y-Hernandez M, Schony W, Schratzer M, Soukop W, et al. , 1989. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry 22, 156–160. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B, 2008. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A 105, 6415–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JB, Chang C, LeBlanc M, Grimm D, Le Lay J, Kaestner KH, Zheng Y, Montminy M, 2015. The CREB/CRTC2 pathway modulates autoimmune disease by promoting Th17 differentiation. Nature communications 6, 7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ, 2010. Striatal microRNA controls cocaine intake through CREB signalling. Nature 466, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YS, Long N, Pigino G, Brady ST, Lazarov O, 2013. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3beta, neurotrophin-3 and CREB signaling. PloS one 8, e64460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman M, Shalit F, Roth-Deri I, Gutman B, Brodie C, Kott E, Sredni B, 1994. Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. Journal of neuroimmunology 52, 147–152. [DOI] [PubMed] [Google Scholar]
- Hull M, Fiebich BL, Lieb K, Strauss S, Berger SS, Volk B, Bauer J, 1996a. Interleukin-6-associated inflammatory processes in Alzheimer's disease: new therapeutic options. Neurobiol Aging 17, 795–800. [DOI] [PubMed] [Google Scholar]
- Hull M, Strauss S, Berger M, Volk B, Bauer J, 1996b. Inflammatory mechanisms in Alzheimer's disease. European archives of psychiatry and clinical neuroscience 246, 124–128. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR, 2004. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13, 159–170. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV, 2005. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. Journal of Neuroscience 25, 5217–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansky L, Reymanova P, Kopecky J, 2003. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiological research 52, 593–598. [PubMed] [Google Scholar]
- Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, Yie SW, Eom SH, Kim PH, 2007. The PKA/CREB pathway is closely involved in VEGF expression in mouse macrophages. Mol Cells 23, 23–29. [PubMed] [Google Scholar]
- Jin F, Gong QH, Xu YS, Wang LN, Jin H, Li F, Li LS, Ma YM, Shi JS, 2014. Icariin, a phosphodiesterase-5 inhibitor, improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signalling. Int J Neuropsychopharmacol 17, 871–881. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S, 2001. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci 4, 289–296. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Wiggin GR, Lightfoot K, Arthur JSC, Macdonald A, 2007. MSK regulate TCR-induced CREB phosphorylation but not immediate early gene transcription. Eur J Immunol 37, 2583–2595. [DOI] [PubMed] [Google Scholar]
- Kandel ER, 2012. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM, 2008. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7, 854–868. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ, 2002. CREB required for the stability of new and reactivated fear memories. Nat Neurosci 5, 348–355. [DOI] [PubMed] [Google Scholar]
- Kim HP, Leonard WJ, 2007. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 204, 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Nairn AC, Cairns N, Lubec G, 2001. Decreased levels of ARPP-19 and PKA in brains of Down syndrome and Alzheimer's disease. J Neural Transm Suppl, 263–272. [DOI] [PubMed] [Google Scholar]
- King E, O'Brien JT, Donaghy P, Morris C, Barnett N, Olsen K, Martin-Ruiz C, Taylor JP, Thomas AJ, 2018. Peripheral inflammation in prodromal Alzheimer's and Lewy body dementias. J Neurol Neurosurg Psychiatry 89, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D, Abbas AK, 2015. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nature reviews. Immunology 15, 283–294. [DOI] [PubMed] [Google Scholar]
- Koch JM, Kell S, Hinze-Selch D, Aldenhoff JB, 2002. Changes in CREB-phosphorylation during recovery from major depression. Journal of psychiatric research 36, 369–375. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M, 2004. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K, 2013. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. J Gerontol A Biol Sci Med Sci 68, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen OP, Mandrup-Poulsen T, 2005. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54 Suppl 2, S114–124. [DOI] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, Kanba S, 2005. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res 1054, 30–37. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Leiden JM, 1999. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol 17, 149–187. [DOI] [PubMed] [Google Scholar]
- Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M, Carvalho AF, Herrmann N, 2017. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 88, 876–882. [DOI] [PubMed] [Google Scholar]
- Lakhina V, Arey RN, Kaletsky R, Kauffman A, Stein G, Keyes W, Xu D, Murphy CT, 2015. Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron 85, 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Pawelec G, Witkowski JM, Schipper HM, Derhovanessian E, Goldeck D, Fulop T, 2009. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer's disease. J Alzheimers Dis 17, 91–103. [DOI] [PubMed] [Google Scholar]
- Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T, 2011. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol 14, 399–404. [DOI] [PubMed] [Google Scholar]
- Lau P, Bossers K, Janky R, Salta E, Frigerio CS, Barbash S, Rothman R, Sierksma AS, Thathiah A, Greenberg D, Papadopoulou AS, Achsel T, Ayoubi T, Soreq H, Verhaagen J, Swaab DF, Aerts S, De Strooper B, 2013. Alteration of the microRNA network during the progression of Alzheimer's disease. EMBO molecular medicine 5, 1613–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT, 2008. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois-Martinez AM, Blondet-Trichard A, Binart N, Val P, Chambon C, Sahut-Barnola I, Pointud JC, Martinez A, 2011. Transcriptional control of adrenal steroidogenesis: novel connection between Janus kinase (JAK) 2 protein and protein kinase A (PKA) through stabilization of cAMP response element-binding protein (CREB) transcription factor. J Biol Chem 286, 32976–32985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Quinn PG, Chikaraishi DM, 2004. Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Molecular and cellular neurosciences 25, 536–547. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Han R, Beier UH, Akimova T, Bhatti T, Xiao H, Cole PA, Brindle PK, Hancock WW, 2014. Two histone/protein acetyltransferases, CBP and p300, are indispensable for Foxp3+ T-regulatory cell development and function. Mol Cell Biol 34, 3993–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD, 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A, 2007. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 13909–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Ciccarelli A, Viosca J, Valor LM, Jimenez-Minchan M, Canals S, Giustetto M, Barco A, 2011. CBP is required for environmental enrichment-induced neurogenesis and cognitive enhancement. EMBO J 30, 4287–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorton D, Bellinger DL, 2015. Molecular mechanisms underlying beta-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. International journal of molecular sciences 16, 5635–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, 2007. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport 18, 297–300. [DOI] [PubMed] [Google Scholar]
- Ma QL, Harris-White ME, Ubeda OJ, Simmons M, Beech W, Lim GP, Teter B, Frautschy SA, Cole GM, 2007. Evidence of Abeta- and transgene-dependent defects in ERK-CREB signaling in Alzheimer's models. J Neurochem 103, 1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaki S, Mueller C, Dickson C, Kirsch W, 2007. Increased production of inflammatory cytokines in mild cognitive impairment. Experimental gerontology 42, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer A, Medina SJ, Niu Y, Abrenica B, Manguiat KJ, Frost KL, Philipson CS, Sorensen DL, Booth SA, 2012. Early mechanisms of pathobiology are revealed by transcriptional temporal dynamics in hippocampal CA1 neurons of prion infected mice. PLoS pathogens 8, e1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK, 2003. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114, 635–645. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB, 2015. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorana A, Bulati M, Buffa S, Pellicano M, Caruso C, Candore G, Colonna-Romano G, 2012. Immunosenescence, inflammation and Alzheimer's disease. Longevity & healthspan 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauslin CS, Heath V, Colangelo AM, Malik R, Lee S, Mallei A, Mocchetti I, Johnson PF, 2006. CAAT/enhancer-binding protein delta and cAMP-response element-binding protein mediate inducible expression of the nerve growth factor gene in the central nervous system. J Biol Chem 281, 17681–17688. [DOI] [PubMed] [Google Scholar]
- Miao Y, He T, Zhu Y, Li W, Wang B, Zhong Y, 2015. Activation of Hippocampal CREB by Rolipram Partially Recovers Balance Between TNF-alpha and IL-10 Levels and Improves Cognitive Deficits in Diabetic Rats. Cell Mol Neurobiol 35, 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Sohn PD, Schilling B, Cong X, Ellerby L, Gibson BW, Johnson J, Krogan N, Shamloo M, Gestwicki J, Masliah E, Verdin E, Gan L, 2015. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med 21, 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L, 2010a. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou YG, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L, 2010b. Acetylation of Tau Inhibits Its Degradation and Contributes to Tauopathy (vol 67, pg 953, 2010). Neuron 68, 801–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton B, Dutta R, Hsu YC, Sakamoto K, 2014. The Role of pp90rsk-Mediated CREB Phosphorylation in Acute Myelogenous Leukemia. Blood 124. [Google Scholar]
- Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, Schwartz M, 2000. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun 15, 331–345. [DOI] [PubMed] [Google Scholar]
- Morris GP, Clark IA, Vissel B, 2014. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun 2, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Cardenas C, Mei L, Cheung KH, Foskett JK, 2011. Constitutive cAMP response element binding protein (CREB) activation by Alzheimer's disease presenilin-driven inositol trisphosphate receptor (InsP3R) Ca2+ signaling. Proc Natl Acad Sci U S A 108, 13293–13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I, Okamoto T, Matsuura Y, Takahashi S, Murayama Y, Ogata E, 1993. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o). Nature 362, 75–79. [DOI] [PubMed] [Google Scholar]
- Oberstein TJ, Taha L, Spitzer P, Hellstern J, Herrmann M, Kornhuber J, Maler JM, 2018. Imbalance of Circulating Th17 and Regulatory T Cells in Alzheimer's Disease: A Case Control Study. Front Immunol 9, 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y, 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953–959. [DOI] [PubMed] [Google Scholar]
- Ohl K, Schippers A, Tenbrock K, 2018. CD11c-Specific Deletion Reveals CREB as a Critical Regulator of DC Function during the Germinal Center Response. J Immunol Res 2018, 8947230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Estevez MA, Hawk JD, Grimes S, Brindle PK, Abel T, 2011. Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learn Mem 18, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H, 2016. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol 15, 673–684. [DOI] [PubMed] [Google Scholar]
- Otten U, Marz P, Heese K, Hock C, Kunz D, Rose-John S, 2000. Cytokines and neurotrophins interact in normal and diseased states. Ann N Y Acad Sci 917, 322–330. [DOI] [PubMed] [Google Scholar]
- Ozgen N, Guo J, Gertsberg Z, Danilo P Jr., Rosen MR, Steinberg SF, 2009. Reactive oxygen species decrease cAMP response element binding protein expression in cardiomyocytes via a protein kinase D1-dependent mechanism that does not require Ser133 phosphorylation. Mol Pharmacol 76, 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X, Raina AK, Holbrook N, Siedlak SL, Harris PL, Smith MA, 1999. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport 10, 2411–2415. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Cushman JD, Huang Z, 2015. Effects of Brain-Derived IL-2 Deficiency and the Development of Autoimmunity on Spatial Learning and Fear Conditioning. J Neurol Disord 3, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto JM, McNamara RK, Gendreau PL, Huang Z, Jackson AJ, 1999. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. J Neurosci Res 56, 441–446. [DOI] [PubMed] [Google Scholar]
- Piech-Dumas KM, Tank AW, 1999. CREB mediates the cAMP-responsiveness of the tyrosine hydroxylase gene: use of an antisense RNA strategy to produce CREB-deficient PC12 cell lines. Brain research. Molecular brain research 70, 219–230. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER, 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34, 447–462. [DOI] [PubMed] [Google Scholar]
- Ponti C, Gibellini D, Boin F, Melloni E, Manzoli FA, Cocco L, Zauli G, Vitale M, 2002. Role of CREB transcription factor in c-fos activation in natural killer cells. Eur J Immunol 32, 3358–3365. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Wang M, Pham S, Sze CI, Eckman CB, 2011. Downregulation of CREB expression in Alzheimer's brain and in Abeta-treated rat hippocampal neurons. Mol Neurodegener 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, Arancio O, 2009. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 8075–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O, 2005. Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 6887–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Cao C, Cawley NX, Liu TT, Yuan J, Loh YP, Cheng Y, 2017. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N=7277). Mol Psychiatry 22, 312–320. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T, Kandel E, 2009. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63, 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raker VK, Becker C, Steinbrink K, 2016. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Frontiers in immunology 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Feliciani C, Gambi D, 2008. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease. Journal of Alzheimer's disease : JAD 14, 147–159. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D, 2005. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer's disease patients. Experimental gerontology 40, 165–171. [DOI] [PubMed] [Google Scholar]
- Reese LC, Laezza F, Woltjer R, Taglialatela G, 2011. Dysregulated phosphorylation of Ca(2+) /calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer's disease. J Neurochem 119, 791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyskens KM, Arthur JS, 2016. Emerging Roles of the Mitogen and Stress Activated Kinases MSK1 and MSK2. Front Cell Dev Biol 4, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richartz-Salzburger E, Batra A, Stransky E, Laske C, Kohler N, Bartels M, Buchkremer G, Schott K, 2007. Altered lymphocyte distribution in Alzheimer's disease. J Psychiatr Res 41, 174–178. [DOI] [PubMed] [Google Scholar]
- Rigano R, Profumo E, Teggi A, Siracusano A, 1996. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clinical and experimental immunology 105, 456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringheim GE, Szczepanik AM, Petko W, Burgher KL, Zhu SZ, Chao CC, 1998. Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Brain research. Molecular brain research 55, 35–44. [DOI] [PubMed] [Google Scholar]
- Roach SK, Lee SB, Schorey JS, 2005. Differential activation of the transcription factor cyclic AMP response element binding protein (CREB) in macrophages following infection with pathogenic and nonpathogenic mycobacteria and role for CREB in tumor necrosis factor alpha production. Infect Immun 73, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ, 2005. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. P Natl Acad Sci USA 102, 13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Calvo MG, Nemni R, Clerici M, 2010. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 21, 927–938. [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ 3rd, Kandel ER, Duff K, Kirkwood A, Shen J, 2004. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42, 23–36. [DOI] [PubMed] [Google Scholar]
- Schindowski K, Peters J, Gorriz C, Schramm U, Weinandi T, Leutner S, Maurer K, Frolich L, Muller WE, Eckert A, 2006. Apoptosis of CD4+ T and natural killer cells in Alzheimer's disease. Pharmacopsychiatry 39, 220–228. [DOI] [PubMed] [Google Scholar]
- Schnecko A, Witte K, Bohl J, Ohm T, Lemmer B, 1994a. Adenylyl cyclase activity in Alzheimer's disease brain: stimulatory and inhibitory signal transduction pathways are differently affected. Brain Res 644, 291–296. [DOI] [PubMed] [Google Scholar]
- Schnecko A, Witte K, Bohl J, Ohm T, Lemmer B, 1994b. Adenylyl-Cyclase Activity in Alzheimers-Disease Brain - Stimulatory and Inhibitory Signal-Transduction Pathways Are Differently Affected. Brain Research 644, 291–296. [DOI] [PubMed] [Google Scholar]
- Shankaranarayanan P, Chaitidis P, Kuhn H, Nigam S, 2001. Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase-1 gene. J Biol Chem 276, 42753–42760. [DOI] [PubMed] [Google Scholar]
- Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M, 2009. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A 106, 16877–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speciale L, Calabrese E, Saresella M, Tinelli C, Mariani C, Sanvito L, Longhi R, Ferrante P, 2007. Lymphocyte subset patterns and cytokine production in Alzheimer's disease patients. Neurobiol Aging 28, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA, 2009. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A 106, 9447–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, Mamiya N, Okano E, Hasegawa S, Mercaldo V, Zhang Y, Maeda R, Ohta M, Josselyn SA, Zhuo M, Kida S, 2011. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci 31, 8786–8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebinia F, Pourgholaminejad A, 2017. The role of Th17 cells in auto-inflammatory neurological disorders. Prog Neuropsychopharmacol Biol Psychiatry 79, 408–416. [DOI] [PubMed] [Google Scholar]
- Takao K, Tanda K, Nakamura K, Kasahara J, Nakao K, Katsuki M, Nakanishi K, Yamasaki N, Toyama K, Adachi M, Umeda M, Araki T, Fukunaga K, Kondo H, Sakagami H, Miyakawa T, 2010. Comprehensive behavioral analysis of calcium/calmodulin-dependent protein kinase IV knockout mice. PloS one 5, e9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Wang S, Yang B, Zhu L, Yin B, Chao T, Zhao J, Yuan J, Qiang B, Peng X, 2012a. The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PloS one 7, e49570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Wang S, Zhu L, Wu C, Yin B, Zhao J, Yuan J, Qiang B, Peng X, 2012b. cAMP response element-binding protein promotes gliomagenesis by modulating the expression of oncogenic microRNA-23a. Proc Natl Acad Sci U S A 109, 15805–15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich AF, Nicholls RE, Puzzo D, Fiorito J, Purgatorio R, Fa M, Arancio O, 2015. Synaptic therapy in Alzheimer's disease: a CREB-centric approach. Neurotherapeutics 12, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K, 2002. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol 124, 83–92. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9, 654–659. [DOI] [PubMed] [Google Scholar]
- Vardarajan BN, Tosto G, Lefort R, Yu L, Bennett DA, De Jager PL, Barral S, Reyes-Dumeyer D, Nagy PL, Lee JH, Cheng R, Medrano M, Lantigua R, Rogaeva E, St George-Hyslop P, Mayeux R, 2017. Ultra-rare mutations in SRCAP segregate in Caribbean Hispanic families with Alzheimer disease. Neurol Genet 3, e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, Vieira-Saecker A, Schwartz S, Santarelli F, Kummer MP, Griep A, Gelpi E, Beilharz M, Riedel D, Golenbock DT, Geyer M, Walter J, Latz E, Heneka MT, 2017. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer's disease. Nature 552, 355–361. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T, 2014. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viosca J, Malleret G, Bourtchouladze R, Benito E, Vronskava S, Kandel ER, Barco A, 2009. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learn Mem 16, 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S, 2005. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A 102, 16426–16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguespack PJ, Banks WA, Kastin AJ, 1994. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain research bulletin 34, 103–109. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Rozengurt E, 2000. Oxidative stress induces protein kinase D activation in intact cells. Involvement of Src and dependence on protein kinase C. J Biol Chem 275, 17114–17121. [DOI] [PubMed] [Google Scholar]
- Wang HY, Pisano MR, Friedman E, 1994. Attenuated protein kinase C activity and translocation in Alzheimer's disease brain. Neurobiol Aging 15, 293–298. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang YW, Sun P, Liu R, Zhang X, Xia K, Xia J, Xu H, Zhang Z, 2006. Transcriptional regulation of PEN-2, a key component of the gamma-secretase complex, by CREB. Mol Cell Biol 26, 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ni L, Chang D, Lu H, Jiang Y, Kim BS, Wang A, Liu X, Zhong B, Yang X, Dong C, 2017. Cyclic AMP-Responsive Element-Binding Protein (CREB) is Critical in Autoimmunity by Promoting Th17 but Inhibiting Treg Cell Differentiation. EBioMedicine 25, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Smith MJ, Heilig E, Beglopoulos V, Kelleher RJ, Shen J, 2009. Indirect Regulation of Presenilins in CREB-mediated Transcription. Journal of Biological Chemistry 284, 13705–13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R, 1987. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. The Journal of experimental biology 132, 43–57. [DOI] [PubMed] [Google Scholar]
- Wen AY, Sakamoto KM, Miller LS, 2010. The role of the transcription factor CREB in immune function. J Immunol 185, 6413–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, An Y, Tanaka T, Bilgel M, Gonzalez C, Kitner Triolo M, Resnick S, 2017. Cross-Sectional and Longitudinal Effects of CREB1 Genotypes on Individual Differences in Memory and Executive Function: Findings from the BLSA. Front Aging Neurosci 9, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xie X, 2006. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome biology 7, R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, 2006. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nature Medicine 12, 1005–1015. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J, 2012. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2, a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ozawa H, Saito T, Frolich L, Riederer P, Takahata N, 1996. Reduced immunoreactivity of adenylyl cyclase in dementia of the Alzheimer type. Neuroreport 7, 2965–2970. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ozawa H, Saito T, Hatta S, Riederer P, Takahata N, 1997. Ca2+/CaM-sensitive adenylyl cyclase activity is decreased in the Alzheimer's brain: possible relation to type I adenylyl cyclase. J Neural Transm 104, 721–732. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Sasaki M, Ozawa H, Saito T, Rosler M, Riederer P, 1999a. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Research 824, 300–303. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Sasaki M, Ozawa H, Saito T, Rosler M, Riederer P, 1999b. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res 824, 300–303. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, Ogata M, Kurosaki T, 2008. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity 28, 499–508. [DOI] [PubMed] [Google Scholar]
- Yi LT, Li J, Liu BB, Luo L, Liu Q, Geng D, 2014. BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. Journal of psychiatry & neuroscience : JPN 39, 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T, 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79, 49–58. [DOI] [PubMed] [Google Scholar]
- Yiu AP, Rashid AJ, Josselyn SA, 2011. Increasing CREB function in the CA1 region of dorsal hippocampus rescues the spatial memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 36, 2169–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XW, Curlik DM, Oh MM, Yin JC, Disterhoft JF, 2017. CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cheng Y, Wang H, Wang C, Wilson SP, Xu J, Zhang HT, 2014. RNA interference-mediated knockdown of long-form phosphodiesterase-4D (PDE4D) enzyme reverses amyloid-beta42-induced memory deficits in mice. Journal of Alzheimer's disease : JAD 38, 269–280. [DOI] [PubMed] [Google Scholar]
- Zhang F, Rincon M, Flavell RA, Aune TM, 2000. Defective Th function induced by a dominant-negative cAMP response element binding protein mutation is reversed by Bcl-2. J Immunol 165, 1762–1770. [DOI] [PubMed] [Google Scholar]
- Zhang W, Bai M, Xi Y, Hao J, Liu L, Mao N, Su CJ, Miao JT, Li ZY, 2012. Early memory deficits precede plaque deposition in APPswe/PS1dE9 mice: Involvement of oxidative stress and cholinergic dysfunction. Free Radical Bio Med 52, 1443–1452. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J, 2013. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochemical and biophysical research communications 435, 597–602. [DOI] [PubMed] [Google Scholar]
- Zheng C, Zhou XW, Wang JZ, 2016. The dual roles of cytokines in Alzheimer's disease: update on interleukins, TNF-alpha, TGF-beta and IFN-gamma. Transl Neurodegener 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mix E, Winblad B, 2001. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev 7, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]