Introduction
Dysregulation of lipid metabolism is pathologically associated to metabolic diseases such as atherogenic dyslipidemia, hepatic steatosis, obesity and type 2 diabetes. Up-regulation of lipid metabolism has been demonstrated to result in lipid droplets biogenesis within the cells. Furthermore, there is increasing evidences linking elevated numbers of lipid droplets with cancer biology.
Acyl-CoA:diacylglycerol acyltransferase (DGAT1 and DGAT2) proteins control lipid synthesis by catalyzing the last step involved in the formation of triacylglycerol (TAG) synthesis. Our group has recently unveiled cross-talk between glycerolipid and sphingolipid metabolism through DGAT2-dependent synthesis of 1-O-acylceramide from ceramide and fatty acyl-coA in the endoplasmic reticulum (ER)- lipid droplet interface (LD). Sequestration of pro-apoptotic ceramide by 1-O-acylceramide formation was associated with chemotherapy resistance in colon cancer cells.
Here, we review the function of DGAT enzyme and focus on the role of DGAT in ceramide homeostasis, as well as the potential therapeutic of pharmacological inhibition of DGAT protein in the treatment of metabolic diseases and cancer; LD biogenesis and its relationship with DGAT proteins. We explore the emerging roles of DGAT enzymes and LDs in tumor carcinogenesis, cancer aggressiveness, chemotherapy resistance and cancer stem cells invasiveness.
1. Sphingolipid metabolism
Sphingolipids, are fundamental constituents of all eukaryotic membranes and their metabolism is carried out by a broad array of anabolic and catabolic reactions with ceramide as their hub (for a review, (1)). Ceramide can be formed by multiple pathways: the de novo pathway where ceramide is synthesized from non-sphingolipid precursors, the multi-step hydrolysis of complex sphingolipids and the salvage pathway where sphingosine can be re-acetylated to form ceramide.
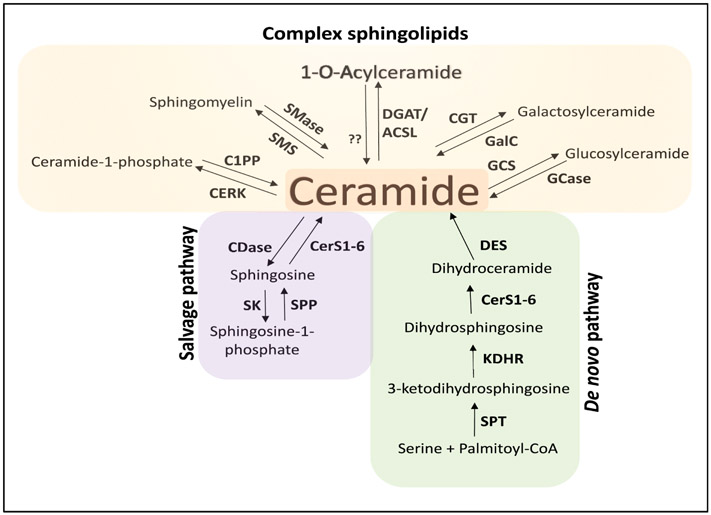
The de novo biosynthesis of sphingolipids begins with the condensation of serine and palmitoyl-CoA, a reaction catalyzed by the ER-located enzyme serine palmitoyltransferase (SPT) (reactions are depicted in Figure 1). Its product, 3-ketodihydrosphingosine, is converted to dihydrosphingosine by the enzyme 3-ketodihydrosphingosine reductase (KDHR). Ceramide synthases (CerS1-6) N-acetylate the dihydrosphingosine backbone to form dihydroceramide, which can be then transformed to ceramide by the enzyme dihydroceramide desaturase (DES). Interestingly, ceramide can be further modified at the 1-hydroxyl position to form more complex sphingolipids (Figure1). For instance, ceramide kinase (CERK) catalyzes its phosphorylation to generate ceramide-1-phosphate. Sphingomyelin is the product of the sphingomyelin synthases activity (SMS1-2), which transfer a phosphocholine moiety from phosphatidylcholine to the 1-O-position. Glucosylceramides are produced by the addition of UDP-galactose (galactosyltransferase, CGT) or UDP-glucose (glucosylceramide, GCS) at the ceramide backbone. More recently, we discovered the formation of O-acylceramides by the addition of a fatty acyl (FA) chain at the 1 or 3-O-hydroxyl and catalyzed by the ceramide O-acyltransferase activity of diacylglycerol acyltransferase, DGAT 1 or 2 enzymes (see in detail below) (2). Interestingly, CerS1-6, fatty acyl-CoA synthase (ACSL) and DGAT2 were found to form a multi-enzyme complex on the ER-LD interface (2) (Figure 2).
Figure 1. Sphingolipid metabolism.
Ceramide is the hub of the sphingolipid metabolism and can be synthesized by several pathways. Ceramide can be generated by the de novo pathway (green box) from the condensation of serine and palmitoyl-CoA to generate 3-ketodihydrosphingosine, which is then reduced to dihydrosphingosine and further N-acylated to form dihydroceramide by (dihydro)ceramide synthases (CerS). Dihydroceramide desaturase (DES) then forms ceramide.
Ceramide can be hydrolyzed to sphingosine and then re-acylated back to ceramide in the salvage/recycling pathway (purple box) or phosphorylated to sphingosine-1-phosphate by sphingosine kinase (SK).
Ceramide can be also modified to form more complex sphingolipids (yellow box) such as ceramide-1-phosphate, sphingomyelin, hexosylceramdies (glucosylceramide and galactosylceramide) and O-acylceramides. Serine palmitoyltransferase (SPT); 3-ketodihydrosphingosine reductase (KDHR); ceramidase (CDase); sphingosine kinase (SK); sphingosine-1-phosphate phosphatases (SPP); ceramide kinase (CERK); ceramide-1-phosphate phosphatase (C1PP) sphingomyelinases (SMase); sphingomyelin synthase (SMS); glucosylceramide synthase (GCS); glucosylceramidase (GCase); ceramide galactosyltransferase (CGT); galactosylceramidase; fatty acyl-CoA synthase (ACSL); acyl-CoA:diacylglycerol acyltransferase (DGAT2)
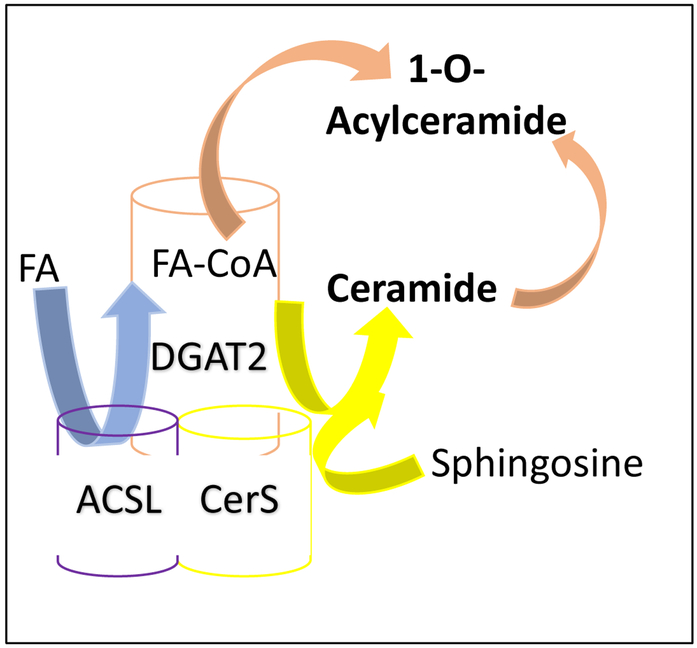
Figure 2. Synthesis of O-acylceramide by an ACSL-CerS-DGAT2 enzymatic complex.
Synthesis of O-acylceramide from ceramide is carried out by the enzymatic complex ACSL-CerS-DGAT2 localized to the ER/lipid droplet contact sites. Fatty acyl-CoA synthase (ACSL) catalyzes the synthesis of the activated fatty acid (FA) as fatty acyl-CoA (FA-CoA). Ceramide synthase (CerS) synthesize ceramide by N-acylating the sphingosine backbone. Acyl-CoA:diacylglycerol acyltransferase (DGAT2) transforms ceramide to O-acylceramide by transferring a FA group from FA-CoA to the hydroxyl group likely in the carbon-1 of ceramide.
The salvage pathway is regulated by several enzymes, such as sphingomyelinases (neutral, acid or alkaline; SMase) or by the cleavage of glycosphingolipids, controlled by β-glucosidases (GCases) and galactosidases (GalC). Likewise, ceramidases (CDases) breakdown ceramide generating sphingosine, which can either be phosphorylated to sphingosine-1-phosphate by the action of sphingosine kinases (SK1-2) or re-acylated to ceramide by ceramide synthases (CerS1-6). Hydrolysis of acyl-ceramides may also contribute to the generation of ceramides. However, the enzyme(s) that catalyze(s) this reaction remains unknown.
2. Diacylglycerol acyltransferase (DGAT) enzymes
Triacylglycerides (TAG) are the principal source of energy storage in eukaryotic cells. TGs are neutral lipids formed by a glycerol backbone and three molecules of fatty acids (FA) covalently linked by ester bonds. FAs need to be activated to acyl-CoA FAs by the ACSL enzyme. Because of their hydrophobic nature, TGs are stored in plasma lipoproteins or in cytosolic LDs. TGs can be synthesized within the cell by two pathways: the glycerol phosphate or Kennedy pathway and the monoacylglycerol pathway. Both pathways converge into the last reaction of TAG synthesis, where diacylglycerol (DG) is converted to TAG by the action of the acyl-CoA:diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) enzyme (3).
Mammals have two DGAT enzymes, DGAT1 and DGAT2. However, they are members of two different gene families and differ in their tissue distribution. Recent studies have concluded that DGAT1 and DGAT2 differ in their substrate affinities, topology, protein partners and cellular functions.
Several lines of evidence suggested that DGAT2 is involved in the bulk of TAG synthesis. For example, overexpression of DGAT2 in rat hepatoma cells showed significantly more TAG mass accumulation than DGAT1 overexpressing cells (4). Conversely, deletion of the yeast ortholog of DGAT2 (Dga1) resulted in higher reduction of TAG than the ortholog of DGAT1 (Are1 or Are2) (5) (6). Likewise, DGAT2-deficient mice (Dgat2(−/−)) had deficient lipid body (lipopenia) and died soon after birth due to impaired permeability barrier function in the skin (4), whereas DGAT1-deficient mice (Dgat1(−/−)) showed less TAG in the liver and mammary gland (7). Mutations in DGAT1 associated with decreased levels of DGAT1 protein were identified in patients with congenital diarrheal disorders. Patient-derived organoids showed altered TAG metabolism, such as reduced TAG levels and lack of LD formation when cells were loaded with oleic acid. In addition, DGAT1-deficient cells were sensitized to lipid stress upon oleate treatment which resulted in increased caspase3/7 activation as compared to control. Overexpressed DGAT1 or DGAT2 reversed the phenotype observed in the organoids derived from mutant DGAT1 patients (8).
2.1. Identification of human DGAT genes
Identification of DGAT1 gene
The enzymatic synthesis of TG was first described in 1960 (9). Nevertheless, the genes encoding murine and human acyl-CoA:diacylglycerol acyltransferase (DGAT1) were first identified by the Farese laboratory in 1998 (10). In this study, human and murine expressed sequence tags (EST) with similarity to acyl-CoA: cholesterol acyltransferase (ACAT) were identified. One cDNA was found to encode a 500-aminoacids protein with a 20% sequence similarity to mouse ACAT. Then, H5 insect cells were infected with baculovirus containing this cDNA. The authors found that infected cells highly expressed an approximately 50Kda protein in the membrane fraction, as well as showed increased TAG mass. In addition, DGAT activity was more than 5-fold higher in membranes isolated from insect cells expressing the cDNA as compared to wild-type virus-infected cells. Taken together, these data confirmed the identification of a DGAT cDNA clone.
The human DGAT1 gene (Dgat1) has been located to chromosome 8 and comprises 17 exons (3,10). DGAT1 protein is ubiquitously expressed, with the highest expression levels in the small intestine, followed by testis, adipose tissue, mammary gland, pancreas and liver. Orthologues of DGAT1 have been found in plants and yeast (3).
Identification of human DGAT2 gene
Several lines of evidence pointed at the existence of more than one DGAT activity. Smith et al. reported that DGAT1-deficient (Dgat−/−) mice shown decreased body mass, whereas they had normal plasma TG levels (7). At the same time, Lardizabal et al. purified and identified a couple of peptides with DGAT enzymatic activity (36 and 36.5 kDa, respectively) from the lipid bodies of the fungus Mortierella ramanniana (11). Sequencing of the peptides and later cloning of the cDNA, showed that the novel DGAT (designated as DGAT2) expressing genes were unrelated to the previously identified DGAT1.
Farese’s group cloned and characterized the mammalian DGAT2 gene thorough its homology to the fungal DGAT2 identified in M. ramanniana (12). DGAT2 homologs were identified in fungi, plants, and mammals. In 2002, the acyl-CoA:monoacylglycerol acyltransferase (MGAT, EC 2.3.1.22) was identified as a DGAT2 gene family member through its homology (13).
The human DGAT2 gene (Dgat2) has been located to chromosome 11 and comprises 8 exons (3). In most species, the DGAT2 gene encodes a 350–400 amino acid protein, with a predicted molecular weight of 40-45 kDa. The DGAT2 protein is expressed majorly in liver and adipose tissue, followed by mammary gland and testis (12,14).
2.2. Biochemistry of DGAT enzymes
Multiple lines of evidence have concluded that both DGAT1 and DGAT2 enzymes catalyze the synthesis of TAG. First, isolated membranes from insect cells overexpressing mouse DGAT1 or DGAT2 cDNA resulted in elevated TAG mass. Second, increasing in vitro synthesis of TAG in those membranes correlated with increasing substrate concentration of either DAG or (C18:1) oleoyl-CoA (10,12). Third, mice lacking DGAT1 or DGAT2 show defective tissue-specific synthesis of TAG.
Because of the challenges associated to the purification and crystallization of membrane proteins, DGAT enzymes have not been successfully purified yet. Nevertheless, Cases et al utilized similar amounts of overexpressed DGAT1 and DGAT2 enzymes in insect cells to study substrate specificity of DGAT1 and DGAT2 enzymes. The two enzymes showed similar maximal rates of TAG synthesis when incubated with DAG and oleoyl-CoA up to 200μM. However, DGAT1 overexpressing cells retained the enzyme activity at higher oleoyl-CoA than DGAT2, suggesting different Km values, with DGAT2 enzyme being more active at lower oleoyl-CoA concentrations. In addition, in vitro competition assays of distinct unlabeled FA-CoAs with [14C]oleoyl-CoA were performed. Interestingly, (C18:2) linoleyl-CoA, (C16) palmitoyl-coA and (C20:4) arachidonyl-coA competed similarly. Taken together, the authors concluded that both DGAT1 and DGAT2 enzymes have comparable specificity for FA-CoA substrates (12).
2.3. Non-canonical acyltransferase activities
DGAT1 catalyzes the synthesis of diacylglycerols, retinyl esters and waxes
Additional acyltransferase activities have been probed for both DGAT1 and DGAT2 enzymes. DGAT1 has been reported to possess acyl-CoA:retinol acyltransferase (ARAT) activity both in vitro assays with DGAT1-overexpressing insect cells and in vivo assays in murine and mammalian cells (15-17). Additionally, DGAT1 also possesses acyl-CoA:monoacylglycerol acyltransferase (MGAT) activity (17), suggesting that the DGAT1 enzyme might be able to catalyze the two-step esterification of MAG to TAG. Overexpressed DGAT1 in COS-7 cells exhibited wax synthase activity, whereas the control cells did not (17).
DGAT2 catalyzes the synthesis of 1-O-acylceramide
As mentioned above, our group discovered that human DGAT2 displays in vitro acyl-CoA:ceramide acyltransferase activity resulting in the formation of O-acylceramide (2). By using a proteomic approach, Senkal et al. found that ACSL5 interacts with several CerS (CerS 1, 2, 4, 5 and 6) in HCT-116 colon cancer cells. The authors hypothesized that the FA-CoA generated by ACSL5 would be utilized by CerS to generate ceramide. However, ACSL5 down-regulated resulted in an increase in total ceramide mass although CerS activity remained unchanged. Inhibition of SPT by myriocin or CerS by fumonisin B1 abrogated the accumulation of ceramide in cells lacking ACSL5; thus, suggesting that ACSL5 down-regulation accumulates ceramide synthesized by the de novo pathway at the level of CerS. On the other hand, overexpression of ACSL5 decreased the cellular levels of ceramide, implying that ACSL5 enzyme might direct ceramide to the formation of O-acylceramide (2).
To test the new hypothesis, cells were subjected to a base hydrolysis reaction that resulted in the selective hydrolysis of ester-linked FAs at carbon 1 position and therefore producing ceramide and free FA. Levels of O-acylceramide can be quantified by measuring the different in ceramides levels upon base hydrolysis (18). Interestingly, the levels of C16-O-acylceramide were significantly increased in ACSL5-overexpressing cells. Conversely, downregulation of ACSL5 resulted in the reduction of C16-O-acylceramide. Thus, ACSL5 modulates the cellular levels of ceramide and O-acylceramide (2).
Synthesis of O-acylceramides was first characterized in vivo in rat brain in 1977 (19). Three different radio-labeled (3H and 14C-labeled) ceramides were injected into the brains of 18-day-old rats. After 2h, the animals were sacrificed, and the sphingolipids were isolated and purified, which led to the discovery of an unknown group of lipids that were identified as ceramide fatty acid esters. Almost twenty years later, it was found that a novel enzyme catalyzed the esterification of ceramide at the hydroxyl group at the C1-position in Madin-Darby canine kidney (MDCK) cells and mouse tissue homogenates (20). In 2002, a novel enzyme with lysosomal phospholipase A2 and transacylase activity was purified and characterized from bovine brain (21). In vitro activity assays unveiled its capability to transfer the acyl group at the sn-2-position from glycerophospholipids (such as, phosphatidylethanolamine (PE) and phosphatidylcholine (PC)) to ceramide. The new enzyme was named 1-O-acylceramide synthase (ACS).
Most recently, two yeast acyl-CoA:sterol acyltransferases (Lro1p and Dga1p) were found to acylate ceramide at the C1 hydroxy group (22). LRO1 is homologous with the mammalian LCAT (lecithin cholesterol acyltransferase) (6). Lro1p enzyme was known to transfer a FA from PE (phosphatidylethanolamine) or PC (phosphatidylcholine) to DAG. Voynova et al demonstrated that NBD–ceramides were acylated in vitro and in vivo by Lro1p in the presence of oleic acid. Likewise, purified Lro1p–GFP enzyme catalyzed the O-acylation of NBD–ceramide to produce O-acylceramides. Conversely, S. cerevisiae lro1Δ cells showed reduced levels of O-acylceramides (22). Interestingly, when the capability of Dga1p to acylate ceramides with different acyl-CoAs as a donor was tested in vitro (microsomal fraction), no changes in O-acylceramide levels were observed. However, when metabolic labelling experiments were carried out in vivo, Dga1p-dependent synthesis of O-acylceramides was detected, suggesting that the Dga1p may act in a different location than in the ER. The authors speculated that synthesis of O-acylceramides might occurr at the LDs, where Dga1p is known to catalyze the formation of TAG. Furthermore, mice lacking DGAT2 do not survive and show impaired permeability barrier function in the skin associated to reduced levels of acylceramide (4).
Considering the above evidence, our group tested whether modulation of DGAT2 activity could affect ceramide levels in HCT-116 colon cancer cells. Several approaches were examined. First, silencing of DGAT2 resulted in increased ceramide levels and concomitant decrease of O-acylceramides. On the other hand, gain-of-function experiments induced accumulation of O-acylceramide within the cells. Second, acyl-CoA:ceramide acyltransferase activity was assayed in vitro from DGAT2 overexpressing cells. Thus, microsomal fraction was incubated with palmitoyl-CoA and NBD-C12-ceramide which resulted in the formation of NBD-acylceramide, Interestingly, the levels of acylceramide remained unchanged when catalytically inactive DGAT2 was used. Third, in vitro synthesis of O-acylceramide was significantly reduced by the DGAT2 specific inhibitor PF-06424439 in a dose-dependent fashion (2). Taken together, these results suggested that DGAT2, in collaboration with CerS and ACSL5, is involved in the formation of cellular O-acylceramides.
The bioactive role of ceramide in cell death has been extensively studied since we demonstrated that exogenous ceramide treatment induced apoptosis in leukemic cells (23). Thus, Senkal et al focused on the biological significance of the formation of O-acylceramide from ceramide in the context of cancer cell death. As expected, the levels of ceramide were increased in HCT-116 cells treated with the chemotherapeutic 5-fluorouracil (5-FU). Interestingly, knock-down of ACSL5 basally decreased cell viability and induced caspase3/7 activation (measured by activity assay and by western blot), resembling the effects of CerS1 enzyme overexpression. When cells lacking ACSL5 or DGAT2 were challenged with 5-FU, caspase activation was synergistically elevated and ceramide increase was also observed. On the other hand, overexpression of ACSL5 protected cells from 5-FU induced cell death. Thus, inhibition of O-acylceramide generation resulted in accumulation of ceramide levels and sensitized cells to chemotherapy.
2.4. Topology of DGAT enzymes
Both DGAT1 and DGAT2 enzymes are integral membrane proteins that localize mainly to the microsomal fraction, hence ER localization, where TAG synthesis takes place. Shockey et al investigated the production of TAG by DGAT1 and DGAT2 enzyme in seeds of the tung tree (Vernicia fordii). Myc epitope-tagged tung DGAT1 or DGAT2 was overexpressed in tobacco (Nicotiana tabacum) suspension cells and then examined their immunofluorescence staining patterns by confocal laser-scanning microscopy (CLSM). As previously published, both DGAT1 and DGAT2 proteins localized to the ER and were enriched in subdomains of the ER. When myc-DGAT1 and GFP-DGAT2 were co-expressed in tobacco cells, the authors found that DGAT1 and DGAT2 do not co-localize but rather were juxtaposed at different subdomains of the ER, thus, suggesting that DGAT1 and DGAT2 might be involved in different subcellular pools of TAG (24).
In the yeast Saccharomyces cerevisiae, the highest DGAT (homolog of DGAT) enzyme activity was found in lipid particles, followed by the microsomal and mitochondrial factions, respectively (5). DGAT2 but not DGAT1 translocates to LDs when cells are treated with oleate (a known inducer of LD formation) in Drosophila S2 cells (25), Saccharomyces cerevisiae (26) as well as mammalian cells (27). Furthermore, overexpressed murine DGAT2 partially co-localized with mitochondria in COS-7 cells. When cells were loaded with oleate, mitochondria re-organized around LDs which did not occur in DGAT1-overexpressing cells. Subcellular fractionation showed DGAT2 enrichment in the mitochondria-associated-membranes (MAMs), correlated with higher DGAT2 enzymatic acyl-CoA:diacylglycerol acyltransferase activity as compared to the microsomal fraction. A mitochondrial targeting sequence was identified in the N terminus between amino acids 61 and 66 of murine DGAT2 (28). Taken together, these findings suggest that reorganization of organelles such as LDs, ER and MAMs might maximize lipid synthesis, and that DGAT2 protein (through its N-terminus) may favor this reorganization by localizing to the contact sites between the different organelles (bridging function) and promoting more efficient lipid synthesis.
2.5. Inhibition of DGAT enzymes as a therapeutic target
DGAT1 Inhibitors
Pharmacological inhibition of DGAT protein has been proposed to have therapeutic potential in the treatment of metabolic diseases such as atherogenic dyslipidemia, hepatic steatosis, obesity and type 2 diabetes. Small-molecules inhibitors for DGAT1 and DGAT2 enzymes have been developed by several pharmaceutical companies and have entered clinical trials (29). For instance, JTT-553 compound trans-5'-(4-amino-7,7-dimethyl-2-trifluoromethyl-7H-pyrimido[4,5-b][1,4]oxazin-6-yl)-2',3'-dihydrospiro(cyclohexane-1,1'-inden)-4-yl]acetic acid monobenzenesulfonate, a selective inhibitor of DGAT1, was shown to increase glucose uptake in adipose tissues, reduce body weight gain and fat weight in diet-induced obesity (DIO) mouse models. It also suppressed plasma TAG in a dose-dependent fashion upon olive oil loading in Sprague-Dawley (SD) rats (30,31). The small molecule T863 2-((1,4-trans)-4-(4-(4-Amino-7,7-dimethyl-7H-pyrimido[4,5-b][1,4]oxazin-6-yl)- phenyl)cyclohexyl)acetic acid, has been tested in vitro and in vivo. When orally administered, T863-treated mice showed delayed fat absorption. Likewise, T863 compound caused weight loss, improved insulin sensitivity and lessened hepatic steatosis in a diet-induced obese C57/BL6 mouse model. T863 enhanced insulin-stimulated glucose uptake in differentiated 3T3-L1 adipocytes (32).
Several DGAT1 inhibitors have been evaluated in clinical trials. The compound LCQ908 2-[4-[4-[5-[[6-(trifluoromethyl)pyridin-3-yl]amino]pyridin-2-yl]phenyl]cyclohexyl]acetic acid has been extensively study in vitro and in vivo. Also, named Pradigastat, this selective and potent DGAT1 inhibitor entered a Phase 2 evaluation for the treatment of diabetes mellitus 2 in 2010 (www.clinicaltrials.gov identifier NCT00901979). Although no results have yet been published in a peer-reviewed journal. Pradigastat is currently in Phase 3 evaluation for the treatment of familial chylomicronemia syndrome, where it showed to decreased TAG in patients (www.clinicaltrials.gov identifier NCT01514461). Pradigastat has been demonstrated to reduced postprandial TAG levels and glucose after high-fat meal in a single-dose study in overweight or obese but healthy subjects. Mild gastrointestinal adverse effects were reported, such as diarrhea, nausea, and abdominal pain (33,34) (35).
The compound PF-04620110 trans-4-[4-(4-Amino-7,8-dihydro-5-oxopyrimido[5,4-f][1,4]oxazepin-6(5H)-yl)phenyl]-cyclohexaneacetic acid is an orally bioactive, selective DGAT1 inhibitor. In vivo experiments showed decreased TAG synthesis in HT-29 human cells as well as rats (36). In 2009, PF-04620110 was selected for clinical studies for the treatment of obesity as reported on www.clinicaltrials.gov (identifier NCT00959426). To our knowledge, the results of the Phase 1 Clinical Trials have not been reported in the literature. Likewise, PF-04620110 entered a Phase 1 Clinical Trials for the treatment of type 2 diabetes patients (www.clinicaltrials.gov identifier NCT01298518). The results of the study are publicly available on the website. AstraZeneca developed the compound AZD7687, which showed a potent and selective inhibition against recombinant DGAT1 as well as the enzyme isolated from human liver microsomal, adipose and intestinal tissues (29,37). A single-dose clinical study in humans with AZD7687 reported decreased levels of postprandial TAG levels following a high-fat meal challenge in a dose-dependent manner. AZD7687 can be administered orally and no major safety concerns were observed. However, gastrointestinal adverse events (such as nausea, diarrhea and vomiting) was reported in the subjects treated with the highest concentrations of AZD7687, thus, limiting the dose escalation (38). Nevertheless, several Phase 1 clinical trials with AZD7687 have been reported in www.clinicaltrials.gov for the treatment of obesity (identifier NCT01119352) and Type 2 Diabetes (identifier NCT01217905).
DGAT2 Inhibitors
The use of DGAT2 tissue-specific antisense oligonucleotides in rodents has unveiled the beneficial effects of regulating DGAT2 enzymatic activity. Suppression of DGAT2 expression, but not DGAT1, reduced TAG levels in the liver and improved hepatic insulin sensitivity in a diet-induced hepatic steatosis rat model (39). Knockdown of DGAT2 in mouse liver showed reduced TAG synthesis, VLDL TAG, Apo B secretion and plasma cholesterol as compared to wild-type animals (40). In addition, liver and adipose knockdown of DGAT2 reduced hepatic lipogenesis, hepatic steatosis, and attenuated hyperlipidemia in both high-fat diet-induced obese and ob/ob mouse models (41). These data have led to the development of DGAT2 inhibitors (14) (42), whereby several specific DGAT2 inhibitors are commercially available for research purposes, such as JNJ DGAT2-A (chemical name: 3-Bromo-4-[2-fluoro-4-[[4-oxo-2-[[2-(pyridin-2-yl)ethyl]amino]-1,3-thiazol-5-(4H)ylidene]methyl]phenoxy]benzonitrile) and JNJ DGAT2-B (chemical name: 5-Fluoro-2-(3-phenoxyphenyl)-1,2-benzisothiazol-3(2H)-one that were tested in vitro with purified recombinant DGAT2 expressed in insect cells or HepG2 cells lysates (43). To our knowledge, these compounds have not been tested in animals. An imidazopyridine-based inhibitor, namely PF-06424439 (chemical name: [(3R)-1-[2-[1-(4-Chloro-1H-pyrazol-1-yl)cyclopropyl]-3H-imidazo[4,5-b]pyridin-5-yl]-3-piperidinyl]-1-pyrrolidinyl-methanone methanesulfonate), selectively inhibits DGAT2 and has been demonstrated to decrease circulating and hepatic lipids when orally administered to dyslipidemic rodent models (44,45).
Most recently, our group has reported the effects of PF-06424439 on the levels of ceramide and O-acylceramide in the liver and LDs from 8-week old mice fed a high-fat diet enriched with oleate. Mice loaded with oleate had increased LDs and steatosis as compared to the control group. Furthermore, accumulation of ceramide and O-acylceramides were significantly increased in both the liver and LDs from oleate high-fat diet fed mice. However, when PF-06424439 was added to the drinking water, levels of total ceramides and O-acylceramides were unchanged in the control mice group, but decreased in the high-fat diet fed mice group. These data suggested that DGAT2 is indeed responsible for synthesis of O-acylceramide in mice (2).
Unpublished data from our group point at DGAT2 as a new therapeutic target in the treatment of breast cancer. We found that the percentage of dead cells (as quantified by the lactate dehydrogenase (LDH) released in the media assay) were synergistically increased in MCF-7 cells co-treated with the DGAT2 inhibitor PF-06424439 and cisplatin. Furthermore, whereas cisplatin alone increased total levels of ceramide 2-fold, the combination of PF-06424439/cisplatin resulted in a 5-fold elevation of ceramides as compared to untreated cells. PF-06424439 alone did not significantly affect ceramide levels or cells death, although it did decrease basal levels of O-acylceramide by half. Moreover, we also found synergistic effects in cell death using DGAT2 inhibition in several other conditions we tested, as depicted in Table1.
Table 1.
Various breast cancer cell lines were either left untreated or treated with PF-06424439 (100μM) alone or with the indicated chemotherapeutic drug after 1 hour of PF-06424439. Treatments were performed as follows: MCF-7 cells were treated with either 20μM of cisplatin (24h) or 1μM of doxorubicin (48h). MDA-MB-231 cells were treated with 1μM of doxorubicin or 25μM of cisplatin (48h). SK-BR-3 cells were treated with 25μM of cisplatin (24h). At the end of the treatment period, plasma membrane permeabilization was measured by LDH release into the medium, using a colorimetric assay kit commercially available (Biovision, Milpitas, CA) following the manufacturer's instructions. Briefly, 50μL of reaction mixture was transferred to a 96-well plate. Same volume of sample medium was added to the wells. The plate was incubated for 30min at 37°C. Absorvance was measured at 490nm and 680nm. Cell death was quantified by subtracting the absorbance at 680nm from the absorbance at 490nm. Percentage of cell death was calculated as compared to total cell lysis.
Data are the average of three independent experiments.
| Cancer Cell Line | Percentage of cell death (as compared to total lysis) | |||
| Untreated | Cisplatin | PF-06424439 | PF-06424439+ Cisplatin | |
| MCF-7 | 6 | 46 | 5 | 82 |
| MDA-MB-231 | 10 | 40 | 11 | 55 |
| SK-BR-3 | 21 | 40 | 29 | 63 |
| Cancer Cell Line | Percentage of cell death (as compared to total lysis) | |||
| DMSO | Doxorubicin | PF-06424439 | PF-06424439+ Doxorubicin | |
| MCF-7 | 15 | 39 | 25 | 63 |
| MDA-MB-231 | 13 | 44 | 18 | 55 |
3. DGAT proteins, lipid droplets and cancer cells
3.1. Lipid droplets accumulate in distinct cancers
Lipid droplets biogenesis
For a long time, LDs were solely considered as fat storage compartments and only recently has the scientific community begun to appreciate the pathobiological role of LDs. Currently, LDs are considered dynamic and functional organelles involved in a myriad of cellular processes such as lipid metabolism, cellular membrane biosynthesis, cell signaling, inflammation and cancer (for a review, see (46)). LDs are found in almost all human cells, but are especially abundant in hepatocytes, enterocytes, and adipocytes (47). Ultrastructural analysis demonstrated that LDs are cytoplasmic organelles formed from the budding of the ER outer lipid monolayer, and are often in contact with mitochondria and the ER. LDs have also been reported to be present in nuclei (48).
Due to FA lipotoxicity (excessive FAs within the cells triggers β-oxidation, which in turn is associated with increased ROS levels, ER stress, mitochondria failure and subsequent cell death), increased FAs levels within the cells are stored in the LDs as neutral lipids (TAG), where DGAT proteins catalyze the last step of the TAG synthesis (49). Several models for the formation of LDs have been reported. Wilfling et al, proved that two distinct populations of LDs coexist in the cell. Smaller LDs resulted from ER budding (named growing LDs), where DGAT1 is involved in TAG synthesis. Larger LDs (named expanding LDs) result from the in situ enzymatic activity of DGAT2, that translocate to the LDs from the ER (25,50). The Walther group also showed that downregulation of DGAT1 greatly affects the abundance of smaller LDs, whereas the lack of DGAT2 affects the larger population of LDs in oleate-induced lipid droplet formation in Drosophila cells. In agreement with these results, our group has observed a dramatic decrease in the formation of LDs when a specific DGAT1 (T863 or PF-04620110) inhibitor was used in MCF-7 breast cancer cells loaded with oleate, stained with BODIPY-493/503, and visualized using Leica confocal microscope. However, a DGAT2 (PF-06424439) inhibitor modestly affects the number of formed LDs, suggesting that DGAT2 function may not be at the level of LD synthesis but rather at the expansion level. We also observed that formation of LDs is completely blocked when DGAT1 and DGAT2 inhibitors are simultaneously added to the cells (unpublished data).
Besides TAG, the LDs are enriched in a variety of lipids such as FAs, DAG, cholesterol, ceramides and O-acylceramides (49). Proteins derived from the cytosol of the ER are also present in the LDs, which play an important role in the formation, growth, trafficking and catabolism of the LDs (46). The protein coating of the LDs varies between different LDs in the same cell, in response to metabolic conditions or between different cell types (49). Proteomic analysis has concluded that the proteins present in the LDs can be classified into three groups: 1) proteins involved in the metabolism and catabolism of the LDs, such as DGAT2, adipose tissue triacylglycerol lipase (ATGL), monoacylglycerol lipase (MGL) and hormone-sensitive lipase (HSL) (51); 2) structural proteins, such as the PAT family formed by 5 different members (perilipin, adipose differentiation-related protein (ADRP), tail-interacting protein of 47 kiloDaltons (TIP47), S3-12, and OXPAT). Perilipins are the most abundant proteins in the LD surface and control the access of lipases to the lipid core (52); 3) proteins involved in membrane trafficking, such as Rab GTPases (for instance, Rab8a), caveolins, cavins or the CIDE family proteins (including Cidea, Cideb, and Fsp27). Fsp27 has been reported to be enriched at LD-LD contact site and be involved in LD fusion (53,54).
Lipid droplets and cancer cell proliferation and aggressiveness
Cancer cells are characterized by energy metabolism reprogramming that favors an uncontrolled growth, which represents the basis of neoplasia (55). Increased glucose uptake followed by glycolysis and lactate acid fermentation in aerobic conditions (Warburg effect) favor the synthesis of ATP and de novo generation of nucleotides, FAs, and proteins necessary as building blocks for cell proliferation (46,56,57). In 1963, Aboumrad et al reported a class of mammary carcinoma characterized by abundant intracytoplasmic neutral lipid storage stained with Sudan IV (58). A decade later, Ramos et al clinically and morphologically examined several lipid-rich carcinomas of the breast and concluded that those tumors had a more aggressive behavior (59). Since then, increased numbers of LDs were observed in various cancers such as breast (60-65), colon adenocarcinomas (66-69), pancreatic cancer (70-72), prostate cancer (73), glioblastoma (74,75), liver tumor (76,77), cervical carcinoma cells (78,79), lung carcinoma cells (80,81) and ovarian cancer (82,83).
Accioly et al, reported increased numbers of lipid bodies (LDs) in human colon adenocarcinoma cell lines (CACO-2, LOVO, HT-29, and HCT-116 cells) and in a H-rasV12-transformed intestinal epithelial cell line (IEC-6 H-rasV12) as compared with non-transformed IEC-6 cells. COX-2 and prostaglandin E2 (PGE2) synthase (which is believed to promote tumor growth) were increased in the CACO-2 cells as compared to the IEC-6 cells. Furthermore, they were found to localize to the LDs. Inhibition of LDs formation by either aspirin or FAS inhibitor, correlated to inhibition of PGE2 generation and cell proliferation (67).
Nieva et al. used Raman microspectroscopy (RS) to examine the lipid phenotype associated with cancer malignancy in several breast cancer cell lines: MDA-MB-435 (lung metastasis), MDA-MB-468 (non-metastatic breast cancer cell line), MDA-MB-231 (bone metastasis), SK-BR-3 (non-metastatic, epithelial morphology cell line), MCF-7 (breast ductal carcinoma) and MCF-10A (benign breast tumor) cells. The Raman spectra allowed the quantification of total fatty acid (TFA, 2845 cm−1 band) and total unsaturated fatty acid (TUFA, 3015 cm−1 band) levels in the different cell lines. The TFA band intensity was significantly higher in the MDA-MB-435 as compared to the MCF-10A cell line, in agreement with the Ramos’ observation that lipid accumulation is a characteristic of aggressive cancer cells. No significant differences were observed in the TUFA bands. Interestingly, the authors developed an algorithm to predict the metastatic ability of breast cancer cells (84). Most recently, Abramczyk et al. utilized RS to analyze the composition of the LDs in non-malignant and malignant human breast epithelial cell lines. It was found that the number of LDs in MCF-10A cancer cells was 2-fold lower than in MCF-7 cells and 4-fold lower than in MDA-MB-231 cancer cells. Thus, increased levels of LDs correlated with increased aggressiveness (85).
Lipid droplets and chemotherapy resistance
Multidrug resistance is a major impairment for the treatment of cancer cells. Several lines of evidence have pointed at LDs as a factor involved in the survival of cancer cells upon a cell stressor such as chemotherapy.
When human colorectal cancer (CRC) cell lines were treated with 5-FU or oxaliplatin alone or in combination, increased number of LDs were present within the cells, as stained with Nile red. LD formation was observed to depend on acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT2) activity. When LPCAT2 was overexpressed, higher number of LDs was present within cells, which conferred chemotherapy resistance as compared to wild-type cells. Conversely, inhibition of LD biogenesis reversed the resistance phenotype (86). Moreover, progesterone receptor (PR)-positive cells T47D showed increased lipid accumulation and LD formation upon progestin treatment, whereas PR-negative MDA-MB-231 cells did not. Increased number of LDs correlate with docetaxel resistance (65). Differences in metabolism were studied in two ovarian cancer cell lines: cisplatin-sensitive (2008) and cisplatin-resistant (C13) cells. Higher lipid accumulation mainly in the LDs were found in the C13 cells (87).
Lipid droplets and cancer stem cells
Altered lipid metabolism has also been reported in cancer stem cells. For example, FA synthase was found to be upregulated in glioma stem cells (GSCs) as compare to non- GSCs. The expression of fatty acid synthase (FASN) was upregulated in human glioblastoma cells. The same study demonstrated that inhibition of FASN activity by cerulenin decreased cell survival of GSCs, as well as lipogenesis and invasiveness. Together, these data suggested that de novo FA synthesis is essential for the GSC stemness (88).
Kashuba et al, reported that overexpression of mitochondrial ribosomal protein MRPS18-2 (S18-2) resulted in immortalization of primary rat embryonic fibroblast (REFs) with stem cell phenotype (89). Years later, the same group observed that LD formation was increased in the tumorigenic S18-2 clone as compared to REFs. S18-2 clone but not REFs showed anchorage-independent growth in soft agar and formed tumors in SCID mice, suggesting that LDs may play a role in cancer tumorigenicity and cancer stem cell phenotype (90).
Other lines of evidence that outline the role of LDs and cell in stem-like cells are: 1) resveratrol-induced cell death in breast cancer stem-like cells by decreasing the levels of FASN upstream of apoptosis (91), 2) circulating tumor cells are an indicator of metastasis and are associated with a poor prognosis. However, their detection presents technical difficulties. By RS microscopy, lipid-rich prostate circulating tumor cells were detected in peripheral blood of patients with metastatic prostate cancers, which could serve as a more sensitive biomarker for prostate metastasis (92).
3.2. DGATs are overexpressed in cancer cells
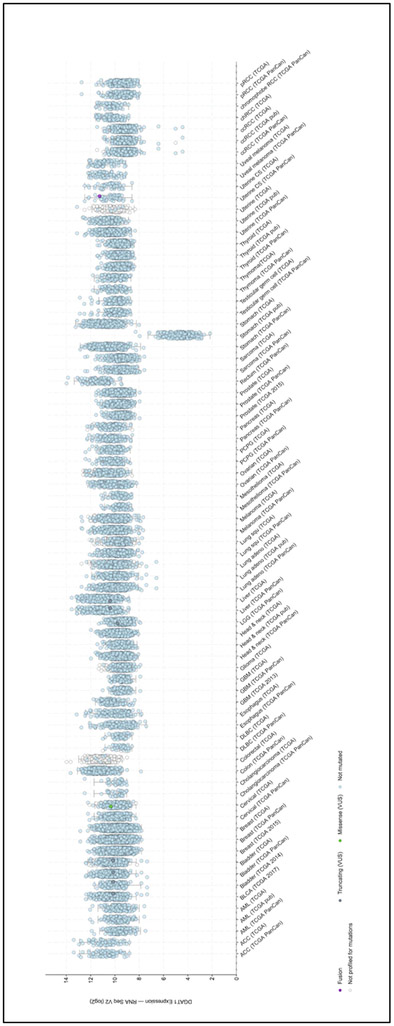
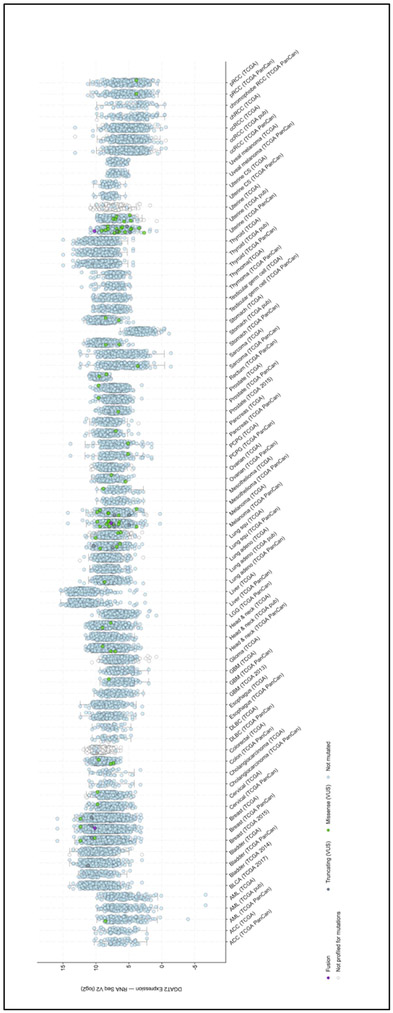
Numerous genes involved in normal function of the cell, play a role in cancer. When dysregulated, due to up or down-regulation, these genes are involved in uncontrolled growth. When performing cross-cancer queries at the cBio Cancer Genomics Portal (http://cbioportal.org) for the DGAT gene expression (93,94), our group found that both DGAT1 and DGAT2 were overexpressed. As depicted in Figure 3A, DGAT1 is overexpressed in liver, colon, breast, bladder and ovarian cancers. DGAT2 (Figure 3B) is highly expressed in liver, breast, thyroid, prostate and pancreatic cancers.
Figure 3. DGAT proteins are overexpressed in multiple cancers.
Analysis of the genetic alterations of DGAT1 (A) and DGAT2 (B) by cBioPortal (http://cbioportal.org). Every spot represents a single study. Purple spots represent fusion genes, grey sports represent truncating genes, green dot represent missense mutations, blue spots represent normal results of gene sequencing of DGAT1 or DGAT2 and white spots represent those analyzed without gene sequencing. Gene expression is represented as a logarithm in base 2.
As stated above, metabolic reprogramming and subsequent lipid accumulation favor cancer cell proliferation and play a role in chemotherapy resistance. Elucidating and identifying cellular modifications that favor lipid storage pathways, such as overexpression of DGAT proteins, can open new therapeutic targets. Although very few peer-review articles have been published on this matter, their results look promising. For instance, DGAT1, ABHD5, ACAT1 and ATGL genes were found to be overexpressed in prostate cancer cells. Knock-down of DGAT1 in prostate cancer LNCaP cells resulted in decreased cell growth and autophagy (73). Moreover, in breast cancer studies overexpression of the protooncogene HER2 in the normal breast cell line HB4a led to lipogenic reprogramming and overexpression of DGAT and other lipid related genes upon treatment by trustuzumab (Herceptin) (95). These studies are beginning to implicate DGAT enzymes and lipogenesis in oncogenic transformation in cancer.
Conclusions and future directions.
Our and others studies are giving us novel insights into the role of DGATs, LDs, and acylated ceramides in the regulation of cancer biology. Interestingly DGATs, their inhibitors and LDs albeit extensively studied in metabolic and cardiovascular related diseases, have not previously been significantly implicated in cancer biology. New insights into fat storage and relative resistance to cell death pathways have begun to advance our knowledge about altered lipid metabolic pathways as potential targets for cancer therapeutics. Our work on the role of DGATs in ceramide metabolism to acylceramide and its sequestration into LDs thus making it “inaccessible” for cell death opened up a novel avenue of investigation for us. We show for the first time that DGATs are overexpressed in many cancers and that inhibiting DGATs appears to synergize with chemotherapy to increase ceramide levels and enhance cancer cell death. These observations will hopefully lead to future preclinical and clinical studies on targeting DGATs as potential novel approaches to cancer chemotherapy.
Acknowledgements.
This work was supported by NIH grants GM062887, P01CA097132, and Veterans Affairs Merit Award to LMO.
Abbreviations:
- DGAT1 and DGAT2
Acyl-CoA:diacylglycerol acyltransferase
- MGAT
Acyl-CoA:monoacylglycerol acyltransferase
- ACAT
acyl-CoA:cholesterol acyltransferase
- ARAT
Acyl-CoA:retinol acyltransferase
- LPCAT2
Acyl-CoA:lysophosphatidylcholine acyltransferase
- CerS
Ceramide synthase
- DAG
Diacylglycerol
- FASN
Fatty acid synthase
- HSL
Hormone-sensitive lipase
- LDs
Lipid droplets
- MAG
Monoacylglycerol
- MGL
Monoacylglycerol lipase
- TAG
Triacylglycerol
- ATGL
Triacylglycerol lipase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Hannun YA, and Obeid LM (2018) Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19, 175–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senkal CE, Salama MF, Snider AJ, Allopenna JJ, Rana NA, Koller A, Hannun YA, and Obeid LM (2017) Ceramide Is Metabolized to Acylceramide and Stored in Lipid Droplets. Cell Metab 25, 686–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen CL, Stone SJ, Koliwad S, Harris C, and Farese RV Jr. (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. Journal of lipid research 49, 2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, and Farese RV Jr. (2004) Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. The Journal of biological chemistry 279, 11767–11776 [DOI] [PubMed] [Google Scholar]
- 5.Sorger D, and Daum G (2002) Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J Bacteriol 184, 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oelkers P, Cromley D, Padamsee M, Billheimer JT, and Sturley SL (2002) The DGA1 gene determines a second triglyceride synthetic pathway in yeast. The Journal of biological chemistry 277, 8877–8881 [DOI] [PubMed] [Google Scholar]
- 7.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, and Farese RV Jr. (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nature genetics 25, 87–90 [DOI] [PubMed] [Google Scholar]
- 8.van Rijn JM, Ardy RC, Kuloglu Z, Harter B, van Haaften-Visser DY, van der Doef HPJ, van Hoesel M, Kansu A, van Vugt AHM, Thian M, Kokke FTM, Krolo A, Basaran MK, Kaya NG, Aksu AU, Dalgic B, Ozcay F, Baris Z, Kain R, Stigter ECA, Lichtenbelt KD, Massink MPG, Duran KJ, Verheij J, Lugtenberg D, Nikkels PGJ, Brouwer HGF, Verkade HJ, Scheenstra R, Spee B, Nieuwenhuis EES, Coffer PJ, Janecke AR, van Haaften G, Houwen RHJ, Muller T, Middendorp S, and Boztug K (2018) Intestinal Failure and Aberrant Lipid Metabolism in Patients With DGAT1 Deficiency. Gastroenterology 155, 130–143 e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss SB, Kennedy EP, and Kiyasu JY (1960) The enzymatic synthesis of triglycerides. The Journal of biological chemistry 235, 40–44 [PubMed] [Google Scholar]
- 10.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, and Farese RV Jr. (1998) Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proceedings of the National Academy of Sciences of the United States of America 95, 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, and Hawkins DJ (2001) DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. The Journal of biological chemistry 276, 38862–38869 [DOI] [PubMed] [Google Scholar]
- 12.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, and Farese RV Jr. (2001) Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. The Journal of biological chemistry 276, 38870–38876 [DOI] [PubMed] [Google Scholar]
- 13.Yen CL, Stone SJ, Cases S, Zhou P, and Farese RV Jr. (2002) Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proceedings of the National Academy of Sciences of the United States of America 99, 8512–8517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naik R, Obiang-Obounou BW, Kim M, Choi Y, Lee HS, and Lee K (2014) Therapeutic strategies for metabolic diseases: Small-molecule diacylglycerol acyltransferase (DGAT) inhibitors. ChemMedChem 9, 2410–2424 [DOI] [PubMed] [Google Scholar]
- 15.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, and Palczewski K (2004) Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. The Journal of biological chemistry 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen CL, Brown C. H. t., Monetti M, and Farese RV Jr. (2005) A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. Journal of lipid research 46, 2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen CL, Monetti M, Burri BJ, and Farese RV Jr. (2005) The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. Journal of lipid research 46, 1502–1511 [DOI] [PubMed] [Google Scholar]
- 18.Rabionet M, Bayerle A, Marsching C, Jennemann R, Grone HJ, Yildiz Y, Wachten D, Shaw W, Shayman JA, and Sandhoff R (2013) 1-O-acylceramides are natural components of human and mouse epidermis. Journal of lipid research 54, 3312–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okabe H, and Kishimoto Y (1977) In vivo metabolism of ceramides in rat brain. Fatty acid replacement and esterification of ceramide. The Journal of biological chemistry 252, 7068–7073 [PubMed] [Google Scholar]
- 20.Abe A, Shayman JA, and Radin NS (1996) A novel enzyme that catalyzes the esterification of N-acetylsphingosine. Metabolism of C2-ceramides. The Journal of biological chemistry 271, 14383–14389 [DOI] [PubMed] [Google Scholar]
- 21.Hiraoka M, Abe A, and Shayman JA (2002) Cloning and characterization of a lysosomal phospholipase A2, 1-O-acylceramide synthase. The Journal of biological chemistry 277, 10090–10099 [DOI] [PubMed] [Google Scholar]
- 22.Voynova NS, Vionnet C, Ejsing CS, and Conzelmann A (2012) A novel pathway of ceramide metabolism in Saccharomyces cerevisiae. The Biochemical journal 447, 103–114 [DOI] [PubMed] [Google Scholar]
- 23.Obeid LM, Linardic CM, Karolak LA, and Hannun YA (1993) Programmed cell death induced by ceramide. Science 259, 1769–1771 [DOI] [PubMed] [Google Scholar]
- 24.Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, and Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18, 2294–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiam AR, Farese RV Jr., and Walther TC (2013) The biophysics and cell biology of lipid droplets. Nature reviews. Molecular cell biology 14, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, and Schneiter R (2011) Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. Journal of cell science 124, 2424–2437 [DOI] [PubMed] [Google Scholar]
- 27.Kuerschner L, Moessinger C, and Thiele C (2008) Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 28.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, and Farese RV Jr. (2009) The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. The Journal of biological chemistry 284, 5352–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeVita RJ, and Pinto S (2013) Current status of the research and development of diacylglycerol O-acyltransferase 1 (DGAT1) inhibitors. J Med Chem 56, 9820–9825 [DOI] [PubMed] [Google Scholar]
- 30.Tomimoto D, Okuma C, Ishii Y, Kobayashi A, Ohta T, Kakutani M, Imanaka T, and Ogawa N (2015) JTT-553, a novel Acyl CoA:diacylglycerol acyltransferase (DGAT) 1 inhibitor, improves glucose metabolism in diet-induced obesity and genetic T2DM mice. J Pharmacol Sci 129, 51–58 [DOI] [PubMed] [Google Scholar]
- 31.Tomimoto D, Okuma C, Ishii Y, Akiyama Y, Ohta T, Kakutani M, Ohkuma Y, and Ogawa N (2015) Pharmacological characterization of [trans-5'-(4-amino-7,7-dimethyl-2-trifluoromethyl-7H-pyrimido[4,5-b][1,4]oxazin-6 -yl)-2',3'-dihydrospiro(cyclohexane-1,1'-inden)-4-yl]acetic acid monobenzenesulfonate (JTT-553), a novel acyl CoA:diacylglycerol transferase (DGAT) 1 inhibitor. Biol Pharm Bull 38, 263–269 [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Zhou Y, Peng H, Huang X, Stahler S, Suri V, Qadri A, Gareski T, Jones J, Hahm S, Perreault M, McKew J, Shi M, Xu X, Tobin JF, and Gimeno RE (2011) Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J Biol Chem 286, 41838–41851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyers CD, Amer A, Majumdar T, and Chen J (2015) Pharmacokinetics, pharmacodynamics, safety, and tolerability of pradigastat, a novel diacylglycerol acyltransferase 1 inhibitor in overweight or obese, but otherwise healthy human subjects. J Clin Pharmacol 55, 1031–1041 [DOI] [PubMed] [Google Scholar]
- 34.Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, and Gaudet D (2015) Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis 14, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung M, Tangirala RS, Bethi SR, Joshi HV, Ariazi JL, Tirunagaru VG, and Kumar S (2018) Discovery of Tetralones as Potent and Selective Inhibitors of Acyl-CoA:Diacylglycerol Acyltransferase 1. ACS Med Chem Lett 9, 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dow RL, Li JC, Pence MP, Gibbs EM, LaPerle JL, Litchfield J, Piotrowski DW, Munchhof MJ, Manion TB, Zavadoski WJ, Walker GS, McPherson RK, Tapley S, Sugarman E, Guzman-Perez A, and DaSilva-Jardine P (2011) Discovery of PF-04620110, a Potent, Selective, and Orally Bioavailable Inhibitor of DGAT-1. ACS Med Chem Lett 2, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox BM, Sugimoto K, Iio K, Yoshida A, Zhang JK, Li K, Hao X, Labelle M, Smith ML, Rubenstein SM, Ye G, McMinn D, Jackson S, Choi R, Shan B, Ma J, Miao S, Matsui T, Ogawa N, Suzuki M, Kobayashi A, Ozeki H, Okuma C, Ishii Y, Tomimoto D, Furakawa N, Tanaka M, Matsushita M, Takahashi M, Inaba T, Sagawa S, and Kayser F (2014) Discovery of 6-phenylpyrimido[4,5-b][1,4]oxazines as potent and selective acyl CoA:diacylglycerol acyltransferase 1 (DGAT1) inhibitors with in vivo efficacy in rodents. J Med Chem 57, 3464–3483 [DOI] [PubMed] [Google Scholar]
- 38.Denison H, Nilsson C, Kujacic M, Lofgren L, Karlsson C, Knutsson M, and Eriksson JW (2013) Proof of mechanism for the DGAT1 inhibitor AZD7687: results from a first-time-inhuman single-dose study. Diabetes Obes Metab 15, 136–143 [DOI] [PubMed] [Google Scholar]
- 39.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, and Shulman GI (2007) Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282, 22678–22688 [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Millar JS, Cromley DA, Graham M, Crooke R, Billheimer JT, and Rader DJ (2008) Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim Biophys Acta 1781, 97–104 [DOI] [PubMed] [Google Scholar]
- 41.Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, Kelly S, Chen S, McKay R, Monia BP, and Bhanot S (2005) Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 42, 362–371 [DOI] [PubMed] [Google Scholar]
- 42.Imbriglio JE, Shen DM, Liang R, Marby K, You M, Youm HW, Feng Z, London C, Xiong Y, Tata J, Verras A, Garcia-Calvo M, Song X, Addona GH, McLaren DG, He T, Murphy B, Metzger DE, Salituro G, Deckman D, Chen Q, Jin X, Stout SJ, Wang SP, Wilsie L, Palyha O, Han S, Hubbard BK, Previs SF, Pinto S, and Taggart A (2015) Discovery and Pharmacology of a Novel Class of Diacylglycerol Acyltransferase 2 Inhibitors. J Med Chem 58, 9345–9353 [DOI] [PubMed] [Google Scholar]
- 43.Qi J, Lang W, Geisler JG, Wang P, Petrounia I, Mai S, Smith C, Askari H, Struble GT, Williams R, Bhanot S, Monia BP, Bayoumy S, Grant E, Caldwell GW, Todd MJ, Liang Y, Gaul MD, Demarest KT, and Connelly MA (2012) The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J Lipid Res 53, 1106–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Futatsugi K, Kung DW, Orr ST, Cabral S, Hepworth D, Aspnes G, Bader S, Bian J, Boehm M, Carpino PA, Coffey SB, Dowling MS, Herr M, Jiao W, Lavergne SY, Li Q, Clark RW, Erion DM, Kou K, Lee K, Pabst BA, Perez SM, Purkal J, Jorgensen CC, Goosen TC, Gosset JR, Niosi M, Pettersen JC, Pfefferkorn JA, Ahn K, and Goodwin B (2015) Discovery and Optimization of Imidazopyridine-Based Inhibitors of Diacylglycerol Acyltransferase 2 (DGAT2). J Med Chem 58, 7173–7185 [DOI] [PubMed] [Google Scholar]
- 45.Pabst B, Futatsugi K, Li Q, and Ahn K (2018) Mechanistic Characterization of Long Residence Time Inhibitors of Diacylglycerol Acyltransferase 2 (DGAT2). Biochemistry [DOI] [PubMed] [Google Scholar]
- 46.Tirinato L, Pagliari F, Limongi T, Marini M, Falqui A, Seco J, Candeloro P, Liberale C, and Di Fabrizio E (2017) An Overview of Lipid Droplets in Cancer and Cancer Stem Cells. Stem Cells Int 2017, 1656053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walther TC, and Farese RV Jr. (2012) Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 81, 687–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohsaki Y, Kawai T, Yoshikawa Y, Cheng J, Jokitalo E, and Fujimoto T (2016) PML isoform II plays a critical role in nuclear lipid droplet formation. J Cell Biol 212, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petan T, Jarc E, and Jusovic M (2018) Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, Liu X, Buhman KK, Coleman RA, Bewersdorf J, Farese RV Jr., and Walther TC (2013) Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell 24, 384–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schittmayer M, and Birner-Gruenberger R (2009) Functional proteomics in lipid research: lipases, lipid droplets and lipoproteins. J Proteomics 72, 1006–1018 [DOI] [PubMed] [Google Scholar]
- 52.Bickel PE, Tansey JT, and Welte MA (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791, 419–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu L, Xu D, Zhou L, Xie B, Yu L, Yang H, Huang L, Ye J, Deng H, Yuan YA, Chen S, and Li P (2014) Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev Cell 30, 378–393 [DOI] [PubMed] [Google Scholar]
- 54.Wu L, Zhou L, Chen C, Gong J, Xu L, Ye J, Li D, and Li P (2014) Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci China Life Sci 57, 107–116 [DOI] [PubMed] [Google Scholar]
- 55.Hanahan D, and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 56.Huang C, and Freter C (2015) Lipid metabolism, apoptosis and cancer therapy. Int J Mol Sci 16, 924–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vander Heiden MG, Cantley LC, and Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aboumrad MH, Horn RC Jr., and Fine G (1963) Lipid-secreting mammary carcinoma. Report of a case associated with Paget's disease of the nipple. Cancer 16, 521–525 [DOI] [PubMed] [Google Scholar]
- 59.Ramos CV, and Taylor HB (1974) Lipid-rich carcinoma of the breast. A clinicopathologic analysis of 13 examples. Cancer 33, 812–819 [DOI] [PubMed] [Google Scholar]
- 60.Lettiero B, Inasu M, Kimbung S, and Borgquist S (2018) Insensitivity to atorvastatin is associated with increased accumulation of intracellular lipid droplets and fatty acid metabolism in breast cancer cells. Sci Rep 8, 5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sevinsky CJ, Khan F, Kokabee L, Darehshouri A, Maddipati KR, and Conklin DS (2018) NDRG1 regulates neutral lipid metabolism in breast cancer cells. Breast Cancer Res 20, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright HJ, Hou J, Xu B, Cortez M, Potma EO, Tromberg BJ, and Razorenova OV (2017) CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc Natl Acad Sci U S A 114, E6556–E6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abramczyk H, Surmacki J, Kopec M, Olejnik AK, Kaufman-Szymczyk A, and Fabianowska-Majewska K (2016) Epigenetic changes in cancer by Raman imaging, fluorescence imaging, AFM and scanning near-field optical microscopy (SNOM). Acetylation in normal and human cancer breast cells MCF10A, MCF7 and MDA-MB-231. Analyst 141, 5646–5658 [DOI] [PubMed] [Google Scholar]
- 64.Goswami S, and Sharma-Walia N (2016) Crosstalk between osteoprotegerin (OPG), fatty acid synthase (FASN) and, cycloxygenase-2 (COX-2) in breast cancer: implications in carcinogenesis. Oncotarget 7, 58953–58974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlaepfer IR, Hitz CA, Gijon MA, Bergman BC, Eckel RH, and Jacobsen BM (2012) Progestin modulates the lipid profile and sensitivity of breast cancer cells to docetaxel. Mol Cell Endocrinol 363, 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bozza PT, and Viola JP (2010) Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids 82, 243–250 [DOI] [PubMed] [Google Scholar]
- 67.Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, and Viola JP (2008) Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68, 1732–1740 [DOI] [PubMed] [Google Scholar]
- 68.Liang YS, Qi WT, Guo W, Wang CL, Hu ZB, and Li AK (2018) Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr Res 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, Potze L, Marotta R, Ruffilli R, Rajamanickam VP, Malerba M, De Angelis F, Falqui A, Carbone E, Todaro M, Medema JP, Stassi G, and Di Fabrizio E (2015) Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells 33, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sunami Y, Rebelo A, and Kleeff J (2017) Lipid Metabolism and Lipid Droplets in Pancreatic Cancer and Stellate Cells. Cancers (Basel) 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saison-Ridinger M, DelGiorno KE, Zhang T, Kraus A, French R, Jaquish D, Tsui C, Erikson G, Spike BT, Shokhirev MN, Liddle C, Yu RT, Downes M, Evans RM, Saghatelian A, Lowy AM, and Wahl GM (2017) Reprogramming pancreatic stellate cells via p53 activation: A putative target for pancreatic cancer therapy. PLoS One 12, e0189051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaida MM, Mayer C, Dapunt U, Stegmaier S, Schirmacher P, Wabnitz GH, and Hansch GM (2016) Expression of the bitter receptor T2R38 in pancreatic cancer: localization in lipid droplets and activation by a bacteria-derived quorum-sensing molecule. Oncotarget 7, 12623–12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitra R, Le TT, Gorjala P, and Goodman OB Jr. (2017) Positive regulation of prostate cancer cell growth by lipid droplet forming and processing enzymes DGAT1 and ABHD5. BMC Cancer 17, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spence AM, and Rubinstein LJ (1975) Cerebellar capillary hemangioblastoma: its histogenesis studied by organ culture and electron microscopy. Cancer 35, 326–341 [DOI] [PubMed] [Google Scholar]
- 75.Laurenti G, Benedetti E, D'Angelo B, Cristiano L, Cinque B, Raysi S, Alecci M, Ceru MP, Cifone MG, Galzio R, Giordano A, and Cimini A (2011) Hypoxia induces peroxisome proliferator-activated receptor alpha (PPARalpha) and lipid metabolism peroxisomal enzymes in human glioblastoma cells. J Cell Biochem 112, 3891–3901 [DOI] [PubMed] [Google Scholar]
- 76.Zhong L, Kong JN, Dinkins MB, Leanhart S, Zhu Z, Spassieva SD, Qin H, Lin HP, Elsherbini A, Wang R, Jiang X, Nikolova-Karakashian M, Wang G, and Bieberich E (2018) Increased liver tumor formation in neutral sphingomyelinase-2-deficient mice. J Lipid Res 59, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Straub BK, Herpel E, Singer S, Zimbelmann R, Breuhahn K, Macher-Goeppinger S, Warth A, Lehmann-Koch J, Longerich T, Heid H, and Schirmacher P (2010) Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Mod Pathol 23, 480–492 [DOI] [PubMed] [Google Scholar]
- 78.Than NG, Sumegi B, Bellyei S, Berki T, Szekeres G, Janaky T, Szigeti A, Bohn H, and Than GN (2003) Lipid droplet and milk lipid globule membrane associated placental protein 17b (PP17b) is involved in apoptotic and differentiation processes of human epithelial cervical carcinoma cells. Eur J Biochem 270, 1176–1188 [DOI] [PubMed] [Google Scholar]
- 79.Sharma A, Jha AK, Mishra S, Jain A, Chauhan BS, Kathuria M, Rawat KS, Gupta NM, Tripathi R, Mitra K, Sachdev M, Bhatt MLB, and Goel A (2018) Imaging and Quantitative Detection of Lipid Droplets by Yellow Fluorescent Probes in Liver Sections of Plasmodium Infected Mice and Third Stage Human Cervical Cancer Tissues. Bioconjug Chem [DOI] [PubMed] [Google Scholar]
- 80.Tomin T, Fritz K, Gindlhuber J, Waldherr L, Pucher B, Thallinger GG, Nomura DK, Schittmayer M, and Birner-Gruenberger R (2018) Deletion of Adipose Triglyceride Lipase Links Triacylglycerol Accumulation to a More-Aggressive Phenotype in A549 Lung Carcinoma Cells. J Proteome Res 17, 1415–1425 [DOI] [PubMed] [Google Scholar]
- 81.Chowdhury R, Amin MA, and Bhattacharyya K (2015) Intermittent Fluorescence Oscillations in Lipid Droplets in a Live Normal and Lung Cancer Cell: Time-Resolved Confocal Microscopy. J Phys Chem B 119, 10868–10875 [DOI] [PubMed] [Google Scholar]
- 82.Roy D, Mondal S, Khurana A, Jung DB, Hoffmann R, He X, Kalogera E, Dierks T, Hammond E, Dredge K, and Shridhar V (2017) Loss of HSulf-1: The Missing Link between Autophagy and Lipid Droplets in Ovarian Cancer. Sci Rep 7, 41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishida T, Okagaki T, Tagatz GE, Jacobson ME, and Doe RP (1977) Lipid cell tumor of the ovary: an ultrastructural study. Cancer 40, 234–243 [DOI] [PubMed] [Google Scholar]
- 84.Nieva C, Marro M, Santana-Codina N, Rao S, Petrov D, and Sierra A (2012) The lipid phenotype of breast cancer cells characterized by Raman microspectroscopy: towards a stratification of malignancy. PLoS One 7, e46456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brozek-Pluska B, Kopec M, Surmacki J, and Abramczyk H (2015) Raman microspectroscopy of noncancerous and cancerous human breast tissues. Identification and phase transitions of linoleic and oleic acids by Raman low-temperature studies. Analyst 140, 2134–2143 [DOI] [PubMed] [Google Scholar]
- 86.Cotte AK, Aires V, Fredon M, Limagne E, Derangere V, Thibaudin M, Humblin E, Scagliarini A, de Barros JP, Hillon P, Ghiringhelli F, and Delmas D (2018) Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat Commun 9, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montopoli M, Bellanda M, Lonardoni F, Ragazzi E, Dorigo P, Froldi G, Mammi S, and Caparrotta L (2011) "Metabolic reprogramming" in ovarian cancer cells resistant to cisplatin. Curr Cancer Drug Targets 11, 226–235 [DOI] [PubMed] [Google Scholar]
- 88.Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, and Owada Y (2016) Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS One 11, e0147717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kashuba E, Pavan Yenamandra S, Darekar SD, Yurchenko M, Kashuba V, Klein G, and Szekely L (2009) MRPS18-2 protein immortalizes primary rat embryonic fibroblasts and endows them with stem cell-like properties. Proceedings of the National Academy of Sciences of the United States of America 106, 19866–19871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darekar SD, Mushtaq M, Gurrapu S, Kovalevska L, Drummond C, Petruchek M, Tirinato L, Di Fabrizio E, Carbone E, and Kashuba E (2015) Mitochondrial ribosomal protein S18-2 evokes chromosomal instability and transforms primary rat skin fibroblasts. Oncotarget 6, 21016–21028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, Wakabayashi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Fujioka T, Tanji S, Mo YY, Cao D, Wilber AC, and Watabe K (2011) Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat 130, 387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitra R, Chao O, Urasaki Y, Goodman OB, and Le TT (2012) Detection of lipid-rich prostate circulating tumour cells with coherent anti-Stokes Raman scattering microscopy. BMC cancer 12, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, and Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, and Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ravacci GR, Brentani MM, Tortelli TC, Torrinhas RS, Santos JR, Logullo AF, and Waitzberg DL (2015) Docosahexaenoic Acid Modulates a HER2-Associated Lipogenic Phenotype, Induces Apoptosis, and Increases Trastuzumab Action in HER2-Overexpressing Breast Carcinoma Cells. Biomed Res Int 2015, 838652. [DOI] [PMC free article] [PubMed] [Google Scholar]