Abstract
Anxiety and mood disorders affect both men and women. The majority of experimental models of stress, however, are completed using only male animals. For repeated social defeat (RSD), a rodent model, this is due to the inherent difficulty in eliciting male aggression toward female mice. To address this limitation, a recent study showed that a DREADD-based activation of the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) was effective in inducing aggressive behavior in male mice towards females in a social defeat paradigm. Therefore, the goal of this study was to determine if this modified version of RSD in females elicited behavioral, physiological, and immune responses similar to those reported in males. Here, we show that female mice subjected to RSD with the male DREADD aggressor developed anxiety-like behavior and social avoidance. These behavioral alterations coincided with enhanced neuronal and microglial activation in threat-appraisal regions of the brain. Moreover, stressed female mice had an enhanced peripheral immune response characterized by increased myelopoiesis, release of myeloid cells into circulation, and monocyte accumulation in the spleen and brain. These results are consistent with previously reported findings that male mice exposed to RSD exhibited increased fear and threat appraisal responses, enhanced myelopoiesis, myeloid cell release and trafficking, and anxiety-like behavior. These findings validate that RSD is a relevant model to study stress responses in female mice.
Keywords: repeated social defeat, female, anxiety, brain, monocytes
1. Introduction
Chronic psychosocial stress is a well-established precipitant of psychiatric disease (Kendler et al., 1999; Kessler, 1997). Women, in particular, are more likely to suffer from stress-related psychiatric disorders, including anxiety disorders, post-traumatic stress disorder, and major depression (Kessler et al., 1993; Kessler et al., 2012; Kessler et al., 1995). It has been suggested that differences in stress susceptibility between women and men could be responsible for the increased prevalence of mood and anxiety disorders in women (Kajantie and Phillips, 2006; Kendler et al., 2001; Seney and Sibille, 2014; Verma et al., 2011). Despite this, only a small proportion of preclinical studies include female subjects (Beery and Zucker, 2011; Blanchard et al., 1995). Therefore, conclusions drawn from studies using exclusively male subjects may not always be applicable to females.
Repeated Social Defeat (RSD) is a preclinical social stress model that consists of exposing a cohort of resident rodents to an aggressive intruder, who will physically defeat and subordinate the residents. After 6–10 days of defeat, subordinate mice display depressive- and anxiety-like behaviors including social avoidance, anhedonia, reduced body weight, and avoidance of open spaces (Blanchard et al., 2001; Kinsey et al., 2007; Krishnan et al., 2007; McKim et al., 2016b; Reader et al., 2015). RSD is a relevant model of psychosocial stress that has ethologic validity and neuroimmune similarities to human psychiatric disorders (Powell et al., 2013; Reader et al., 2015). Parallel to clinical studies of chronic stressors, socially defeated mice have increased plasma IL-6 and release of glucocorticoid-insensitive and primed/hyperinflammatory Ly6Chi monocytes into circulation (Avitsur et al., 2002a; Avitsur et al., 2001; Stark et al., 2002; Stark et al., 2001). These results are consistent with clinical studies of patients with anxiety and depression, who show elevated pro-inflammatory cytokines such as IL-6 and IL-1β (Maes et al., 1997; Maes et al., 1993; Maier and Watkins, 1998; O’Donovan et al., 2010; Reichenberg et al., 2001). In addition, chronic caregiver stress is associated with an increased CD14+/CD16− monocyte population that shows a blunted glucocorticoid response (Miller et al., 2014). These are the functional analogues to the inflammatory Ly6Chi monocytes detected in mice (Yang et al., 2014). Furthermore, mice exposed to RSD display enhanced microglial reactivity, a phenomenon also seen in depressed suicide victims (Torres-Platas et al., 2014). The RSD paradigm has been used extensively in mice and rats to study the neurobiology of anxiety- and depressive-like behaviors (McKim et al., 2016a; Reader et al., 2015; Wohleb et al., 2014c).
One criticism of RSD has been its exclusion of female subjects due to its dependence on inter-male social hierarchies and aggression (McKim et al., 2016a; Reader et al., 2015; Wohleb et al., 2014c). Eliciting male aggression towards females of reproductive age is difficult, and female mice naturally display very little aggression or territory defense towards conspecific intruders (Palanza and Parmigiani, 2017). Other models of stress used in female rodents, including chronic social instability (Herzog et al., 2009), maternal separation (Tsuda and Ogawa, 2012; Tsuda et al., 2011), and restraint (Zavala et al., 2011), have demonstrated sex-specific differences. Unfortunately, these stressors have been criticized as artificial and not social in nature (Almeida et al., 2002; Bjorkqvist, 2001; Kessler, 1997).
The social defeat model has been successfully used in both male and female animals of other rodent species, including the California mouse (Peromyscus californicus) and Syrian hamster (Mesocricetus auratus). Males and females of both species display territorial behavior and aggression towards conspecifics, allowing for experimental induction of attack behavior and subordination (Huhman et al., 2003; Trainor et al., 2011). These models have discovered sex differences in behavioral responses to social defeat (Huhman et al., 2003; Solomon et al., 2007; Steinman and Trainor, 2017; Trainor et al., 2011), though the nature of the differences varies between species. For example, defeated male Syrian hamsters show longer-lasting submissive behaviors compared to defeated female hamsters. Conversely, defeated female California mice show more robust social avoidance following defeat (Trainor et al., 2011). These studies indicate that mechanisms underlying behavioral responses to stress may differ between sexes and species.
The role of central and peripheral inflammation in the pathophysiology of anxiety-like behavior has not been studied in either of these model species. The predominant species used to study the neuroimmunological mechanisms of stress is Mus musculus owing the ease of genetic manipulation. Relatively few studies have applied the social defeat model successfully to female Mus musculus mice. One such study used application of male urine to female C57BL/6J mice to initiate aggression from a male conspecific, and found that defeated females showed reduced social interaction and sucrose preference (Harris et al., 2017) consistent with previously published studies in male mice (Krishnan et al., 2007). Takahashi et al. (Takahashi et al., 2017) used DREADD-based activation of the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) to generate male ERα-Cre aggressor mice that would attack female mice upon injection of a designer drug. Using this model, they found that females who were susceptible to social defeat showed social avoidance and increased plasma IL-6, indicating that social defeat in female mice also increases peripheral inflammation. Our previous studies in male mice showed that social defeat causes anxiety-like behavior, enhances myelopoiesis, and activates microglia in regions of the brain associated with fear and anxiety (Reader et al., 2015; Weber et al., 2017; Wohleb et al., 2014c). Here, our goal was to determine if peripheral and central immune responses to RSD are similar in female mice.
In this study, we used a modified RSD paradigm incorporating the DREADD-aggressors generated by Takahashi et al. (Takahashi et al., 2017) to elicit aggression towards female C57BL/6 mice. Here, the aim was to use these modified DREADD-aggressors to defeat female mice and investigate the effects of RSD on behavior and neuronal, microglial, and peripheral immune activity in female mice. Social defeat of female mice led to the development of anxiety-like behavior and social avoidance. Furthermore, female mice exposed to RSD had a peripheral immune response characterized by elevated plasma IL-6, enhanced bone marrow myelopoiesis, release of myeloid cells into circulation, and monocyte trafficking to the spleen and brain. Thus, we determined that RSD elicited behavioral, central nervous system, and peripheral immune responses in female mice. Comparisons of male and female responses to social defeat will be the goal of future studies (McKim et al., 2016a; Reader et al., 2015; Wohleb et al., 2014c).
2. Materials and Methods
2.1. Mice:
Female C57BL/6 mice (6–8 weeks old) were obtained from Charles River Breeding Laboratories (Wilmington, MA) and allowed to acclimate to their surroundings for 7–10 d before initiation of experiments. ERα-Cre mice were generously supplied by Dr. Scott Russo at Mount Sinai Hospital, New York. At Mount Sinai, 8-week old ERα-Cre mice received bilateral injections with a Cre-dependent DIO-Gq-DREADD-expressing AAV in the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) as previously described (Takahashi et al., 2017). These mice will be referred to as “DREADD aggressors”. The DREADD aggressors were pre-screened for consistent aggressive behavior prior to arriving at our institution. At the time of experimentation, the DREADD aggressors were 8 months old. Resident C57BL/6 mice were housed in cohorts of three while DREADD aggressors were singly housed. All mice were housed in standard 11.5” × 7.5” × 6” polypropylene cages. Rooms were maintained at 21 °C under a 12-h light–dark cycle (lights on at 0600) with ad libitum access to water and rodent chow. All procedures were in accordance with the NIH Guidelines and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2. Repeated Social Defeat:
Female mice were subjected to modified version of repeated social defeat (RSD) stress, similar to previously published protocols used with male mice (McKim et al., 2017; Sawicki et al., 2017). DREADD aggressors were injected with 3.0 mg/kg clozapine-N-oxide (CNO) intraperitoneal to activate ER+ neurons in the VMHvl 30 min prior to encounter with female mice (Takahashi et al., 2017). Activation of these neurons produces indiscriminate aggressive behavior as previously described (Lee et al., 2014). DREADD aggressors were introduced into cages of established female cohorts for 30 min per night for 6 consecutive nights immediately before the onset of the dark cycle (18:00 h). Consistent aggressive behavior was ensured by experimenter observation. Since these DREADD aggressors attacked more frequently than CD-1 aggressors, the duration of each RSD cycle was reduced to 30 minutes (compared to 2 hours in our standard RSD protocol for male mice). During each cycle, interactions between the DREADD aggressors and female mice were monitored closely to ensure defeat of the resident female mice and consistent aggression towards all cagemates. DREADD aggressors attacked the female mice by biting at the back, hind limbs, and tail with very low latency between attacks (<15 seconds). All three female mice in the cage received similar frequency of attacks from the DREADD aggressors. The female mice displayed submissive posturing, fleeing, and huddling during the 30 minutes of RSD. The health status of the mice was carefully examined throughout all experiments. Female mice were weighed daily prior to RSD and on the final day before sacrifice. Additionally, coat status was checked daily to ensure that the female mice were grooming. Mice that were lethargic, moribund, or lost 20% of their body weight were removed from the study. Less than 5% of female mice met the early removal criteria for wounding, which resembles previous studies using RSD in male mice (McKim et al., 2016a; McKim et al., 2015; Ramirez et al., 2015; Wohleb et al., 2014b). Female mice were group-housed in cohorts of 3 between cycles of RSD. All behavior and biological measures were obtained 14 h after the final cycle of RSD. This time point was selected because the hypothalamic-pituitary-adrenal axis (HPA) activation returned to baseline by 14 hours after the final cycle (Hanke et al., 2012b). Wounding was scored before sacrifice using a scale from 0–5, with 0 correlating to no visible or palpable wounds, and 5 correlating to confluent wounds on the back and hind limbs. In this study, all females had wounding scores between 3 and 4 (covering >33% or >50%, respectively, of the back and hind limbs) on the day of sacrifice.
2.3. Isolation of Cells from Bone Marrow, Blood, and Spleen:
Tissues were collected immediately following CO2 asphyxiation. Whole blood was collected with EDTA-lined syringes by cardiac puncture. Red blood cells (RBCs) in blood samples were lysed using RBC lysis buffer (8.8 g NH4Cl, 1.0 g KHCO3, 0.038 g EDTA dehydrate in 1 L dH2O). Spleens were collected in ice-cold phosphate-buffered saline (PBS) and mechanically disrupted to obtain single cell suspensions. To collect bone marrow, the epiphyses of femurs were cut off and the marrow was flushed out with ice-cold PBS. Cell pellets were washed and total number of cells was determined with a BD Coulter Particle Count and Size Analyzer (Beckman Coulter, Inc.; Pasadena, CA) as previously published (McKim et al., 2016b; Wohleb et al., 2014a).
2.4. Isolation of Brain CD11b+ Cells:
CD11b+ cells were isolated from whole brain homogenates as previously reported (McKim et al., 2017; Sawicki et al., 2017). Brains were mechanically disrupted with Potter homogenizers and centrifuged at 900×g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-Healthcare; Chicago, IL). A discontinuous Percoll density gradient was layered as follows: 50, 35, and 0% isotonic Percoll. The gradient was centrifuged for 20 min at 2000×g and cells were collected from the interphase between the 70 and 50% Percoll layers. These cells were referred to as enriched CD11b+ cells based on previous studies demonstrating that viable cells isolated by Percoll density gradient yields >90% CD11b+ cells (Wohleb et al., 2013).
2.5. IL-6 ELISA:
Whole blood was collected 14 h following the final cycle of RSD via cardiac puncture using EDTA-lined syringes. Blood was then centrifuged to separate plasma from cells. Plasma IL-6 was quantified by standard sandwich ELISA as previously described (Hanke et al., 2012a). For IL-6 determination, rat anti-mouse antibody was used and performed per manufacturer’s instructions (BD Pharmingen Cat # 555240; San Diego, CA).
2.6. Flow Cytometry:
Labeling of cell surface antigens was performed as previously described (McKim et al., 2017; Sawicki et al., 2017). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (BD Biosciences). Cells were washed and then incubated with the appropriate antibodies (CD45, CD11b, Sca-1, eBioscience; Ly6C, Ly6G, Ter119, CD3, B220, BD Biosciences) for 1 h at 4°C. Cells were washed and then re-suspended in PBS for analysis. Cell numbers were estimated using counting beads (Invitrogen; Carlsbad, CA). Non-specific binding was assessed using isotype matched antibodies. Antigen expression was determined using a Becton-Dickinson FACSCalibur cytometer (BD Biosciences) with a nine-color upgrade (Cytek Biosciences; Fremont, CA). Data were analyzed using FlowJo software (Tree Star; Ashland, OR) and positive labeling for each antibody was determined based on isotype stained controls.
2.7. Immunohistochemistry and Digital Imaging Analysis:
At 14 h after the last cycle of social defeat, brains were collected from mice after carbon dioxide asphyxiation and transcardial perfusion with sterile PBS (pH 7.4) followed by 4% paraformaldehyde. Brains were post-fixed in 4% formaldehyde for 24 h, and then incubated in 30% sucrose for an additional 72 h at 4°C. Fixed brains were frozen with isopentane (−78 °C) and sectioned (25 μm) using a Leica CM1950 cryostat (Leica Biosystems; Buffalo Grove, IL). Brain regions were classified based on reference markers used in the stereotaxic mouse brain atlas (Paxinos and Franklin, 2008) and were consistent between animals. Sections were placed free-floating in cryoprotectant until staining. Sections were washed in PBS with 1% bovine serum albumin (BSA), blocked with 5% normal donkey serum and 0.5% Triton-X in PBS, and incubated with primary antibodies: rabbit anti-mouse ΔFosB (1:1000; Abcam; Boston, MA; catalog number ab184938) and goat anti-mouse Iba-1 (1:500; Abcam; catalog number ab5076). Primary incubations were completed overnight at 4°C. Sections were then washed in PBS and incubated with a fluorochrome-conjugated secondary antibody (Alexa Fluor 488 or Alexa Fluor 594). Sections were mounted on slides and cover-slipped with Fluoromount G (Southern Biotech; Birmingham, AL) and stored at room temperature. Fluorescent images were visualized using an epi-fluorescent Leica DM5000B microscope. Images were captured using a Leica DFC300 FX camera and imaging software at 200× magnification. Regions of interest (ROIs) were selected from brain sections using landmarks from the stereotaxic mouse brain atlas. Representative images for each ROI are shown in Supplemental Figure 1. For Iba-1 analysis, a threshold for positive staining was determined by the ImageJ software that included all cell bodies while excluding background staining. Threshold for positive staining was kept consistent between animals within the same brain regions. Data were processed by densitometric scanning of the threshold targets using ImageJ software. All results are expressed as average percent area in the positive threshold for all representative images. For Iba-1+ cell counts and ΔFosB analysis, cells with positive labeling were individually counted in each region. Cell counts and % area of the prelimbic cortex, motor cortex, and CA3 of the hippocampus were measured in one FOV per animal. The basolateral amygdala was identified based on its location compared to the external capsule at bregma −1.22 mm and cells within a 700 um elliptical region were quantified (Supp. Fig.1). Quantification was performed by an experimenter who was not blinded to the groups.
2.8. RNA Isolation and Real-Time PCR:
A 1 mm coronal brain section that includes the cortex, hippocampus, striatum, and hypothalamus was removed and immediately flash frozen in liquid nitrogen. RNA was isolated using tri-reagent/isopropanol precipitation and RNA concentration was determined by NanoDrop (ThermoFisher; Waltham, MA). RNA (1.2μg) was reverse transcribed to cDNA using an RTRETROscript kit (Ambion; Foster City, CA). Quantitative PCR was performed using the Applied Biosystems (Foster City, CA) Assay-on-Demand Gene Expression protocol as previously described (Wohleb et al., 2012; Wohleb et al., 2011; Wohleb et al., 2014a; Wohleb et al., 2013). Briefly, experimental cDNA was amplified by real-time PCR where a target cDNA (e.g., IL-1β, TNFα, IL-6) and a reference cDNA [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (non-fluorescent quencher). Fluorescence was determined on a ThermoFisher Applied Biosystems QuantStudio Real-Time PCR system (ThermoFisher). Relative gene expression was analyzed using the ΔΔCT method and results are expressed as fold difference from GAPDH.
2.9. Anxiety-Like Behavior:
Anxiety-like behavior was determined at the onset of the light cycle (06:00 h) using the open field test as previously described (Kinsey et al., 2007). The open-field test was used to assess anxiety-like behavior in these studies because previous experiments using male mice showed that it was a robust and reproducible behavioral test in the context of social defeat (McKim et al., 2016b; McKim et al., 2017; Wohleb et al., 2012; Wohleb et al., 2011; Wohleb et al., 2014a; Wohleb et al., 2013). Furthermore, measures of thigmotaxis in the open-field test demonstrate high degrees of validity for modeling anxiety-like behaviors [reviewed in (Prut and Belzung, 2003)]. Behavioral tests were conducted in a dedicated room with light levels in the test apparatus averaging 3–5 lux (Lux Light Meter, Elena Polyanskaya). Mice were placed in the corner of the test apparatus (40 × 40 × 25 cm Plexiglas box) and activity was recorded for 5 min. Mice with anxiety-like behavior entered the center of the open-field slower and spent less time in the open field (Prut and Belzung, 2003). Behavior was recorded and analyzed using an automated system (Fusion Open Field, Omnitech; Columbus, OH).
2.10. Social Avoidance:
Social avoidance was determined immediately following open field testing as previously described (Sawicki et al., 2017). In Trial 1 (empty), an experimental (female) mouse was placed into the arena with an empty wire mesh cage and activity was recorded for 2.5 min. In Trial 2 (social), an uninjected DREADD aggressor was placed in the wire mesh cage, the experimental mouse was placed in the arena, and activity was recorded for another 2.5 min. Activity in the social interaction behavior test was recorded and analyzed using an automated system (Fusion Open Field, Omnitech; Columbus, OH)..
2.11. Statistical Analysis:
All data were expressed as treatment means ± standard error of the mean (SEM). Individual data points more than two standard deviations above and below the mean were counted as outliers, and were excluded in the subsequent analyses. Main effects of RSD were evaluated by a two-sample t-test. Correlations were calculated using a two-sided Pearson’s product-moment correlation in R. Threshold for statistical significance was set at p<0.05.
3. Results
3.1. Exposure to RSD Induced Anxiety-like Behavior and Social Avoidance in Female Mice.
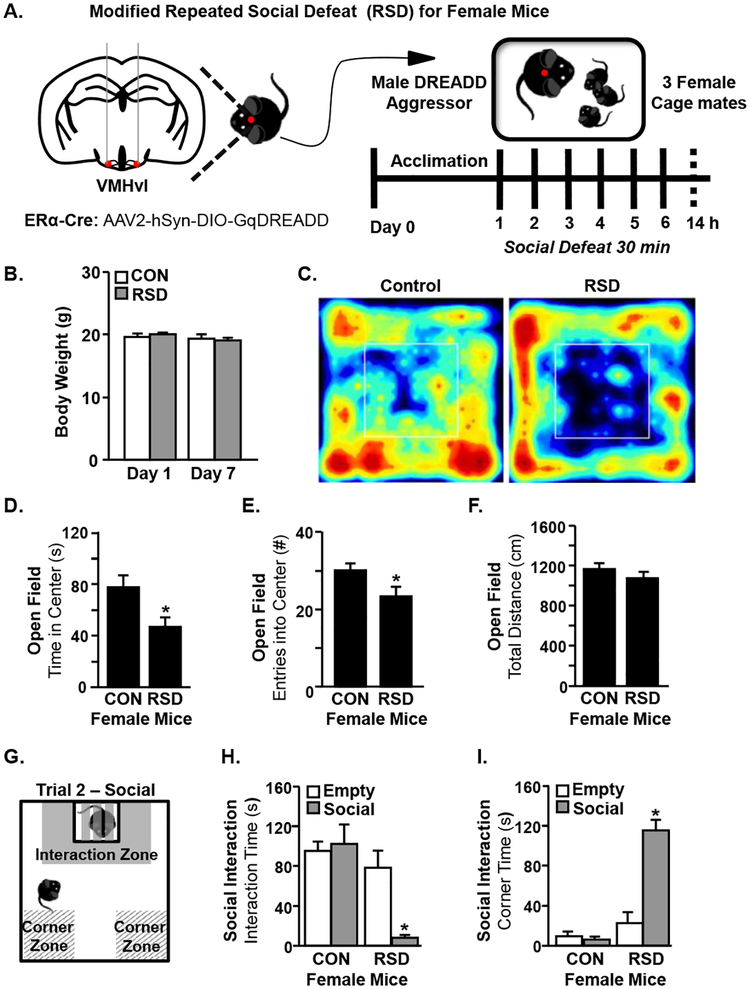
The goal of this study was to determine if repeated social defeat (RSD) elicited behavioral, physiological, and immune responses in female mice similar to those previously reported in male mice (Hanke et al., 2012a; Kinsey et al., 2007; McKim et al., 2017; Wohleb et al., 2011; Wohleb et al., 2013). To address this question, a modified version of RSD was used. This modified RSD used a male DREADD aggressor in which injection of CNO causes aggressive behavior (Takahashi et al., 2017) because male mice will not normally defeat a female (Fig.1A). In the first experiment, female mice were subjected to RSD with the male DREADD aggressor and anxiety-like and social avoidance behaviors were determined 14 h after the last cycle of defeat (Prut and Belzung, 2003; Wohleb et al., 2011). Notably, the health status of the female mice was monitored throughout the experiment and there were no significant changes in body weight between groups (Fig.1B). The representative heat maps of the activity in the open field illustrate anxiety-like behavior (Fig.1C). For example, female mice exposed to RSD spent less time in the center of the open field (Fig.1D, p<0.05) and made fewer entries into the center compared to controls (Fig.1E, p<0.05). Total distance traveled was not significantly different between control and RSD mice indicating that there were no baseline motor deficits between groups (Fig.1F).
Figure 1. Modified RSD in Female Mice Induces Anxiety-Like Behavior and Social Avoidance.
A) Adult female C57BL/6 mice were subjected to six cycles of modified repeated social defeat (RSD) for 30 minutes per day using a male DREADD aggressor and open field and social avoidance behaviors were determined 14 h after the last cycle of social defeat (n=16–18). DREADD aggressors were male ERα-Cre mice that have received AAV-Gq-DREADD injections bilaterally in the VMHvl. Subsequent injection of CNO activates ERα+ neurons at this locus and increases aggressive behavior. B) Female mice were weighed prior to the first cycle of RSD (Day 1) and prior to sacrifice (Day 7). C) Representative heat maps of activity in the open field. D) Time spent in the center of the open field for control and RSD mice. E) Entries into the center of the open field for control and RSD mice. F) Total distance traveled in the open field. G) Schematic of social avoidance testing. H) Time spent in the interaction zone of the social avoidance test for control and RSD mice during the empty and social trials. I) Time spent in the corner zone of the social avoidance test for control and RSD mice during the empty and social trials. Bars represent the mean +/− SEM. Means with asterisks (*) are significantly different than controls (p <0.05).
Next, a two-trial social interaction test was used to determine social avoidance 14 h after RSD in female mice (Fig.1G). The first trial was the “empty trial” where there was no aggressor mouse present in the interaction cage. Notably, both control and RSD groups readily explored the cage in the absence of a partitioned aggressor. When the aggressor was present, however, female mice subjected to RSD had increased social avoidance with reduced time spent in the interaction zone of the social trial compared to controls (Fig.1H, p<0.05). In addition, female mice exposed to RSD spent more time in the corner zones of the social trial compared to controls (Fig.1I, p<0.05). Taken together, female mice had increased anxiety-like behavior and social avoidance following exposure to the modified version of RSD.
3.2. RSD in Female Mice Activated Neurons in Brain Regions Associated with Threat Appraisal and Fear Responses.
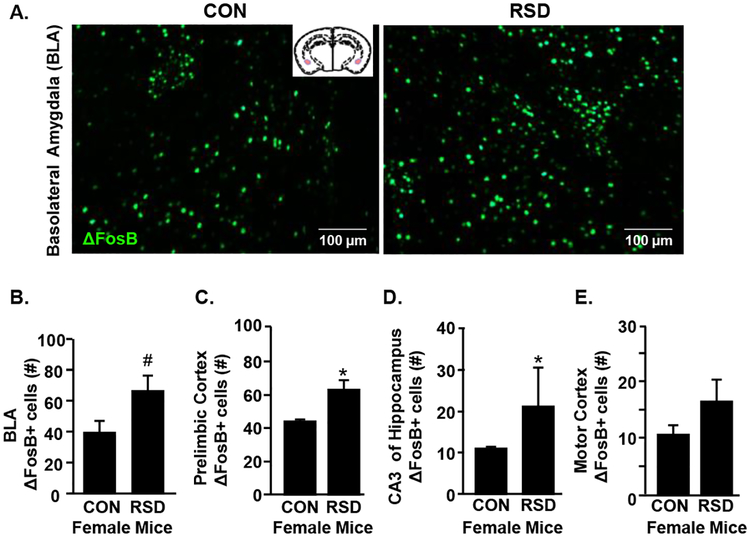
RSD causes a robust increase in neuronal activation within brain regions associated with fear and threat appraisal in male mice (McKim et al., 2017). Thus, we next investigated the level of neuronal activation in amygdala, prelimbic cortex and hippocampus of female mice exposed to RSD. Representative images of neuronal (ΔFosB) labeling are shown in the basolateral amygdala (BLA) (Fig.2A) 14 h after the last cycle of social defeat. Female mice exposed to RSD showed a trend towards increased number of ΔFosB+ neurons in the BLA (Fig.2B, p=0.06). In addition, RSD significantly increased ΔFosB labeling in the prelimbic cortex (Fig.2C, p<0.05) and the CA3 of the hippocampus (Fig.2D, p<0.05). RSD did not significantly increase neuronal activation in the motor cortex, which is not associated with threat appraisal (Fig.2E). Taken together, RSD elicited neuronal activation in threat appraisal and fear brain regions in female mice.
Figure 2. RSD in Female Mice Activates Neurons in Brain Regions Associated with Fear and Threat Appraisal.
Adult female C57BL/6 mice were subjected to six cycles of modified repeated social defeat (RSD) for 30 minutes per day using a male DREADD aggressor. Mice were perfused and PFA-fixed 14 h following the final cycle of RSD. Brains were sectioned and ΔFosB was determined in the Prelimbic Cortex, Basolateral Amygdala (BLA), CA3 of the hippocampus, and the Motor Cortex (n=6 per group). A) Representative images of ΔFosB labeling in the BLAof control and RSD mice. Number of ΔFosB+ cells in the B) BLA, C) prelimbic cortex, D) CA3 region of the hippocampus, and E) motor cortex. Bars represent the mean +/− SEM. Means with asterisks (*) are significantly different than controls (p <0.05). Means with (#) showed a trend (p = 0.06) to be different from controls.
3.3. RSD Enhanced Myelopoiesis and Increased Monocyte Accumulation in the Blood and Spleen of Female Mice.
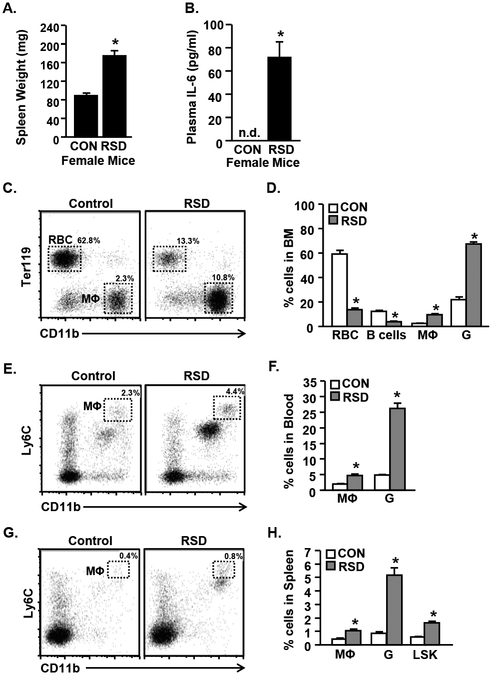
RSD in male mice promotes threat appraisal and activation of the sympathetic nervous system (SNS), which in turn, elicits a robust increase in the mobilization of myeloid cells (monocytes and granulocytes) from the bone marrow (Engler et al., 2004; Powell et al., 2013; Wohleb et al., 2013). Thus, we next sought to determine if RSD in females elicited a similar peripheral immune profile. Female mice were subjected to RSD and bone marrow, blood, and spleen were collected 14 h after the final cycle of RSD. Two hallmarks of RSD stress in male mice are increased spleen weight and plasma IL-6 (Avitsur et al., 2002b; Stark et al., 2002; Stark et al., 2001). Here, spleen weight and IL-6 were increased by RSD in female mice (Fig.3A&B, p<0.01). Plasma IL-6 was not detected in control female mice (Fig.3B). There was no significant correlation between wounding and spleen weight (t(4)=−0.056, p=0.96, r = −0.028) or plasma IL-6 in female mice (t(4)=−2.350, p=0.076, r=−0.761) (data not shown). Moreover, labeling of cells with antibodies to CD11b and Ter119 showed that RSD increased the proportion of monocytes and granulocytes (Fig.3C&D, p<0.001 for each) in the bone marrow. Red blood cell (RBC) and B-cell proportions were decreased in the bone marrow of socially defeated female mice (Fig.3D, p<0.001 for each). Fig.3E shows representative bivariate dot plots of CD11b and Ly6C labeling in the blood of control and socially defeated female mice. Female mice exposed to RSD showed higher percentages of monocytes (CD11b+/Ly6Chi) and granulocytes (CD11b+/Ly6G) in circulation compared to controls (Fig.3F, p<0.001 for each).
Figure 3. RSD Causes Myelopoiesis and Increases Monocyte Accumulation in the Blood and Spleen of Female Mice.
Adult female C57BL/6 mice were subjected to six cycles of modified repeated social defeat (RSD) for 30 minutes per day using a male DREADD aggressor. Spleen, bone marrow, and blood were harvested 14 h following the final cycle of social defeat and A) Spleen weight and B) plasma IL-6 levels were determined (n=6). C) Representative bivariate dot plots of CD11b and Ter119 labeling in the bone marrow are shown. D) Proportion of cells in the bone marrow including red blood cells (RBC; CD11b-/Ter119+), B-cells (CD11b-/B220+) monocytes (MΦ; Cd11b+/Ly6Chi) and granulocytes (G; CD11b+/Ly6G+). E) Representative bivariate dot plot of CD11b and Ly6C labeling in the blood. F) Proportion of monocytes (MΦ; Cd11b+/Ly6Chi) and granulocytes (G; CD11b+/Ly6G+) in blood. G) Representative bivariate dot plot of CD11b and Ly6C labeling in the spleen. H) Proportion of monocytes (MΦ; Cd11b+/Ly6Chi), granulocytes (G; CD11b+/Ly6G+), and LSK cells (Lineage-/Sca-1+/cKit+) cells in the spleen. Bars represent the mean +/− SEM. Means with asterisks (*) are significantly different than controls (p <0.05).
Following defeat stress in male mice, CD11b+ myeloid cells traffic from the circulation to distant organs including the spleen (Wohleb et al., 2014a). In female mice, RSD also increased the percentage of monocytes (CD11b+/Ly6Chi) and granulocytes (CD11b+/Ly6G+) in the spleen (Fig.3G&H, p<0.01 for each). Additionally, RSD increased the proportion of hematopoietic stem/progenitor cells (Lin-/Sca-1+/cKit+; LSK) within the spleen (Fig.3H, p<0.01). Taken together, these data indicate that female mice produced a peripheral immune response to defeat stress consisting of enhanced myelopoiesis, release of myeloid cells and IL-6 into circulation, and accumulation of myeloid cells in the spleen.
3.4. RSD in Female Mice Promoted Microglial Restructuring and Monocyte Accumulation in the Brain.
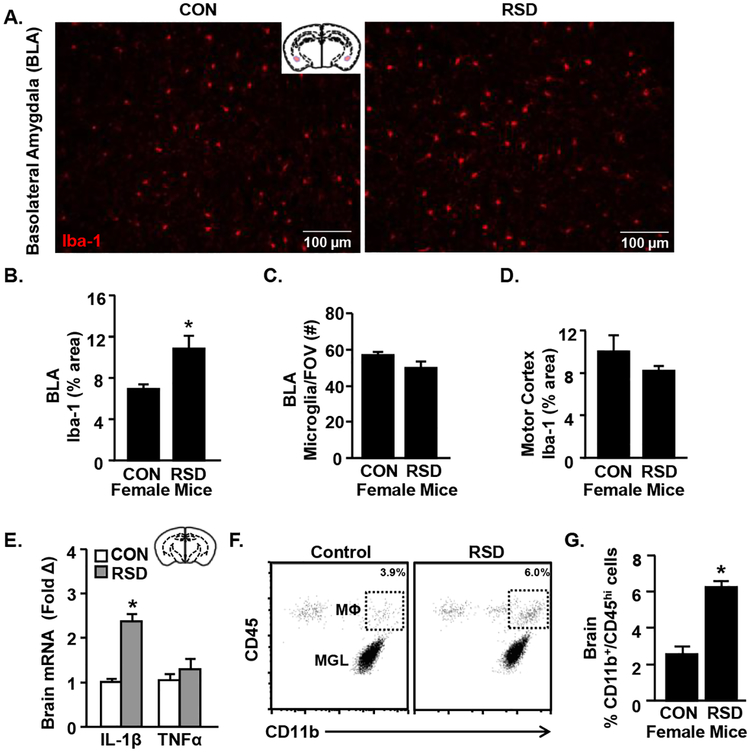
In male mice, RSD caused hypertrophy and de-ramification of microglia within threat appraisal regions, which were associated with an increased inflammatory profile of the microglia (Wohleb et al., 2011). Next, we aimed to determine if RSD in female mice caused microglial morphological restructuring in brain regions associated with fear/anxiety and threat appraisal. Fig.4A shows representative images of Iba-1 labeling the basolateral amygdala (BLA) of control and RSD-exposed mice. There was increased percent area of Iba-1 labeling in the BLA in mice exposed to RSD compared to controls (Fig.4B, p<0.05) with no difference in microglia cell number between groups (Fig.4C). We interpret these data to indicate microglial restructuring with stress (Donnelly et al., 2009). As expected, microglial restructuring was not evident in the motor cortex after RSD (Fig.4D). The motor cortex is not a critical region associated with threat appraisal. In addition, two inflammatory cytokines (IL-1β and TNFα) were assessed in a coronal brain section 14 h after RSD exposure in female mice. IL-1β mRNA expression in the brain was increased by RSD compared to controls (Fig.4E, p<0.001). TNFα mRNA expression, however, was not increased in the coronal brain section.
Figure 4. RSD in Female Mice Promotes Microglial Restructuring and Monocyte Accumulation in the Brain.
Adult female C57BL/6 mice were subjected to six cycles of modified repeated social defeat (RSD) for 30 minutes per day using a male DREADD aggressor. Mice were perfused and PFA-fixed 14 h following the final cycle of RSD. Brains were sectioned and Iba-1 was determined (n=6 per group). A) Representative Iba-1 labeling in the basolateral amygdala (BLA) of control and RSD mice. B) Percent area of Iba-1 labeling and C) microglia cell counts per field of vision in the BLA. D) Percent area of Iba-1 labeling in the motor cortex. E) In a separate study, RNA was isolated from a coronal brain slice that included the hippocampus and amygdala 14 h following the last cycle of RSD and IL-1β and TNFα mRNA levels were determined (n=6). In a separate study, mice were subjected to RSD as above and the cell from the brain were Percoll-enriched (n=6). F) Representative bivariate dot plots of CD11b and CD45 labeling in the brain. G) Percentage of microglia (CD11b+/CD45med) and monocytes (CD11b+/CD45hi) in the brain 14 h after RSD.
Published studies showed that the activity of IL-1β following RSD in male mice was essential for the recruitment of peripheral CD11b+ myeloid cells to the brain vasculature and perivascular spaces (McKim et al., 2017; Wohleb et al., 2014a; Wohleb et al., 2014d; Wohleb et al., 2013). Therefore, in this study, monocyte accumulation in the brain was determined 14 h after RSD in female mice. Representative bivariate dot plots of CD11b and CD45 labeling are shown in Fig.4F. RSD increased the proportion of monocytes (CD11b+/CD45hi) within the brain compared to control mice (Fig.4G, p<0.01). In summary, RSD in female mice promoted microglial restructuring in threat appraisal brain regions, increased IL-1β expression, and enhanced the accumulation of monocytes in the brain.
4. Discussion
Exposure to chronic psychosocial stress increases the risk of developing mood or anxiety disorders in both males and females (Gilman et al., 2013; Kessler, 1997) Although the reported prevalence of anxiety and depression is higher in females than males (Ford and Erlinger, 2004; Kessler et al., 1993; Kessler et al., 2012), the majority of current research studying the neurobiological mechanisms of stress uses male mice. Thus, the aim of this study was to determine if a modified version of repeated social defeat (RSD) in females (Takahashi et al., 2017) elicited behavioral, physiological and immune responses similar to those reported in males. Here, we confirm that DREADD-based activation of the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) is an effective method to elicit male aggressive behavior towards female mice during each social defeat cycle (Takahashi et al., 2017). Moreover, female mice subjected to this modified version of RSD showed similar immune and behavioral responses to those described in male mice. For example, female mice exposed to RSD exhibited increased social avoidance and anxiety-like behavior. In addition, female mice exposed to RSD showed characteristic markers of the stress response observed in male mice, including splenomegaly, increased myelopoiesis, and monocyte accumulation the spleen and brain (Reader et al., 2015; Weber et al., 2017; Wohleb et al., 2014c). These findings indicate an overlap between the sexes in the neurobiological mechanisms in response to RSD.
One important finding was that the modified version of RSD caused anxiety-like behavior and social avoidance in female mice. These behavioral changes in females are consistent with the ones reported in male mice after RSD (Kinsey et al., 2007; Reader et al., 2015; Weber et al., 2017; Wohleb et al., 2014c). We acknowledge that male and female rodents can have variable susceptibility to stress depending on the model used (Haller et al., 1999; Palanza and Parmigiani, 2017; Trainor et al., 2011). For example, it was reported that female rodents are more sensitive to social isolation while male rodents are more susceptible to social defeat when measuring body weight changes and HPA-axis activity (Haller et al., 1999). Notably, we did not make direct male versus female comparisons here, so there may be RSD by sex interactions for certain physiological or behavioral responses to stress. Nonetheless, we observed similar development of anxiety-like behavior and social avoidance in females as reported in male mice after six cycles of RSD (McKim et al., 2016b; McKim et al., 2017; Wohleb et al., 2012; Wohleb et al., 2011; Wohleb et al., 2014a; Wohleb et al., 2014d; Wohleb et al., 2013). For example, defeated females spent significantly less time in the center of an open field compared to controls, and also spent less time interacting with another mouse in the social interaction assay. These findings are consistent with previously published studies using DREADD aggressors to defeat female mice; in those studies defeated females showed increased anxiety-like behavior in an elevated plus maze and social avoidance (Takahashi et al., 2017). Unlike Takahashi et al., we did not find that our female mice separated into susceptible and resilient groups based on social interaction. This may be due to the procedural differences between our study and theirs. For example, we group house the female mice during RSD and use 30 minutes of defeat per day for 6 days, while Takahashi et al. used “paired” aggression for 5 minutes per day for 10 days. Thus, these two differences: 1) disruption of the social hierarchy (in the group housed mice) and 2) the longer exposure to the aggressor limit the occurrence of resilience. Another method of social defeat in females also induced similar behavioral phenotypes, including reduced sucrose preference (a surrogate for anhedonia) and decreased social interaction (Harris et al., 2017). Thus, social defeat of male and female mice induces anxiety-like behavior and social avoidance.
Another key finding was that female mice exhibited neuronal activation that spatially corresponded with regions of the brain involved with threat appraisal. Previous studies in male mice showed that social defeat increased neuronal activation (ΔFosB) in the prelimbic cortex, amygdala, hippocampus, and paraventricular nucleus (McKim et al., 2017), regions associated with fear and threat responses (Calhoon and Tye, 2015; LeDoux, 2003). In the present study, female mice displayed a similar pattern of neuronal activation in these threat appraisal regions following social defeat. In male mice, neuronal activation precedes SNS activity that drives a peripheral immune response (Reader et al., 2015; Weber et al., 2017; Wohleb et al., 2014c). Because both males and females show enhanced neuronal activity in the same regions of the brain associated with threat appraisal, we conclude that both sexes interpret the stressor (RSD) in a similar manner.
Next, we demonstrated that the modified version of RSD in female mice induced a peripheral immune response. For example, RSD in female mice caused splenomegaly and elevated plasma IL-6. One concern with the RSD model is that peripheral immune responses could be due to the wounding that occurs with aggressive encounters. For example, in male mice, we and others have shown that wounding is involved in producing glucocorticoid (GC)-insensitive splenocytes (Avitsur et al., 2007; Foertsch et al., 2017). However, with our more recent data, we show that splenomegaly is driven by increased β-adrenergic signaling and appears to be largely independent of wounding. For example, modulating the SNS response using propranolol or clonazepam prevented myelopoiesis and GC-insensitivity (Hanke et al., 2012a; Ramirez et al., 2016). Neither intervention had any effect on intruder aggression or wounding after RSD. Additionally, we have recently shown that IL-6 production during RSD in male mice is dependent on corticosterone (Niraula et al., 2018). Interventions with adrenalectomy or metyrapone (11β-hydroxylase inhibitor) treatment ablated the increase in plasma IL-6 after RSD without preventing wounding. Thus, we would not expect plasma IL-6 levels after RSD to be solely dependent on wounding. Notably, in the current study, neither spleen weight nor plasma IL-6 levels were correlated with wound score in our female mice. This supports the idea that wounding alone is insufficient to produce the peripheral immune responses seen with RSD in male or female mice.
In this study, defeated female mice showed enhanced myelopoiesis, release of Ly6Chi monocytes into circulation, and trafficking of immune cells to the brain and spleen. As cellularity remains relatively constant in the bone marrow after stress, increased production of myeloid cells results in compensatory decrease in production of red blood cells and lymphocytes (Engler et al., 2004; McKim et al., 2018). This shift towards myelopoiesis is analogous to previous reports in male mice (Engler et al., 2004; Powell et al., 2013; Wohleb et al., 2013). In previous studies, β-adrenergic blockade in male mice prevented elevated plasma IL-6 and increased release of CD11b+ myeloid cells during RSD (Hanke et al., 2012a). These data indicate that the myelopoietic response of RSD is also mediated by sympathetic nervous system activation in the bone marrow. Therefore, it is likely that this response would be conserved across the sexes. Furthermore, splenomegaly following RSD in male mice has previously been attributed to redistribution of leukocytes and progenitor cells from the bone marrow to the spleen (Avitsur et al., 2002b; Engler et al., 2004) and expansion in RBC production which partially ameliorates stress-induced anemia (McKim et al., 2018). Here, we also observed increased myeloid and hematopoietic progenitor cells in the spleens of defeated female mice, indicating a similar pattern of blood cell release and trafficking from the bone marrow to the spleen in female mice. Notably, our recent study showed that a β-adrenergic agonist, isoprenaline, increased the release of stem cells from the bone marrow that migrated to the spleen and resulted in splenomegaly (McKim et al., 2018). Again, this extramedullary hematopoiesis in the spleen after RSD appears to be SNS-dependent and occurs independent of wounding. Finally, clinical studies of chronic stress in humans (including caregiving stress, low socioeconomic status, and PTSD) also show increased circulating IL-6 and pro-inflammatory, GC-insensitive monocytes (reviewed in (Weber et al., 2017)). This parallels the inflammatory profile that we found in the current study independent of wounding. The homogeneity of wounding scores in our defeated mice may have hindered our ability to detect a correlation between wound severity and peripheral inflammatory markers. However, based on our intervention studies in male mice and reports from human studies, we believe that wounding is not the primary factor responsible for peripheral immune responses to RSD in female mice.
In the current study, we also detected an increased presence of hematopoietic stem/progenitor cells (Lin-/Sca-1+/cKit+; LSK) in the spleens of female mice exposed to RSD. This is relevant because these cells develop into monocytes that persist in the spleen 24 days later (McKim et al., 2016b). Previous studies demonstrated that RSD in males induced long-term sensitization that caused mice to have enhanced immune and behavioral responses following subsequent exposure to an acute stressor (Wohleb et al., 2014a). These mice were termed “stress-sensitized” because they displayed exaggerated responses to an otherwise sub-threshold stressor. Further studies showed that the spleens of RSD-exposed mice served as a unique reservoir of primed monocytes that were released following sympathetic outflow in response to an acute stressor (McKim et al., 2016b). For example, sub-threshold acute stress caused these primed monocytes to traffic to the brain and promoted the recurrence of anxiety-like behavior (McKim et al., 2016b). In the current study, the resolution of anxiety-like behavior and neuroimmune responses to stress in female mice was not examined. In Syrian hamsters, females express fewer submissive behaviors and show reduced duration of behavioral changes after social defeat compared to males (Huhman et al., 2003). Therefore, addressing the possibility of sex differences in the temporality of responses to RSD to mice is an important task. This limitation can be addressed in future studies by exposing female mice to RSD and evaluating behavior at additional time points (e.g. 8 and 24 days after the final cycle of RSD) (McKim et al., 2016b; Wohleb et al., 2014a). However, the presence of hematopoietic progenitor cells in the spleens of female mice after RSD indicates strong evidence for a long-term neuroimmune sensitization that can regulate behavioral responses similar to that detected in male mice.
A noteworthy finding in this study was that female mice exposed to the modified version of RSD exhibited microglial activation and recruitment of monocytes to the brain. For instance, RSD in female mice induced microglial restructuring in threat appraisal brain circuitry and increased the number of peripheral Ly6Chi monocytes in the brain. Notably, this was associated with increased IL-1β mRNA expression in the brains of stressed female mice, a key cytokine response that links immune activation to behavioral alterations during RSD (McKim et al., 2017). This is consistent with previous reports that RSD in male mice leads to microglial hyper-reactivity and increased production of chemokines that recruit monocytes from the periphery to the brain (McKim et al., 2017). These monocytes interact with the brain vasculature to propagate an inflammatory signal and influence behavior (McKim et al., 2017; Menard et al., 2017; Sawicki et al., 2015). While we did not confirm the precise location of recruited monocytes (e.g. vascular, perivascular, or parenchymal) by immunohistochemistry, based on our previous studies and flow assessment, we conclude that female mice show robust recruitment of peripheral myeloid cells to the brain after stress.
Studies using RSD in males have demonstrated that neuronal activation precedes the development of microglial activation (McKim et al., 2017; Ramirez et al., 2016; Wohleb et al., 2011). These data are interpreted to indicate that neuronal activation caused by threat appraisal during stress mediates the regional activation of microglia. In support of this idea, several animal models of chronic stress have shown microglial reactivity in specific brain regions associated with threat appraisal, including the prelimbic cortex, hippocampus, and amygdala (Calcia et al., 2016; Hinwood et al., 2013; Tynan et al., 2010). We have found through various intervention studies (including beta blockers, GABA agonists, and microglial inhibitors) that both the neuronal and microglial responses are necessary for the development of anxiety-like behavior following RSD in male mice (Hanke et al., 2012a; McKim et al., 2016a; McKim et al., 2017; Ramirez et al., 2016; Wohleb et al., 2011). We believe the same interventions would prevent anxiety-like behavior in female mice because these neuroimmune responses to RSD were also present in female mice. Therefore, as in male mice, the pathways of communication between neurons, activated microglia, and peripheral monocytes likely drive the behavioral responses induced repeated social stress in female mice.
A limitation of the current study is the absence of male mice exposed to RSD from the DREADD aggressors. Therefore, there were no direct male-to-female comparisons made in this study. This was because of the nature of the social defeat and the modifications of the protocol. For example, the conventional RSD model consists of 2 hours of defeat by a retired CD-1 male breeder. This mouse does not need to be “induced” by a central CNO injection to be aggressive towards a cohort of male mice (Kinsey et al., 2007; Reader et al., 2015; Weber et al., 2017). Moreover, the ERα-Cre DREADD aggressors are more aggressive than a typical retired CD-1 breeder. To account for this and minimize potential wounding, the duration of each social defeat cycle was reduced to 30 minutes in females. Despite these minor changes in protocol, the defeated female mice in this study showed remarkably comparable behavioral, neuronal, microglial, and immune responses to male mice from our previously published studies (Engler et al., 2004; Kinsey et al., 2007; McKim et al., 2017; Sawicki et al., 2015; Wohleb et al., 2011; Wohleb et al., 2014d; Wohleb et al., 2013). However, we do expect to see interactions between sex and RSD in future studies. For example, recent studies on brain transcriptional signatures in human postmortem samples have found markedly different expression profiles between depressed men and women (Labonte et al., 2017; Seney et al., 2018). In particular, microglia-specific genes were upregulated in men and downregulated in women, suggesting sex-specific neuroinflammatory dynamics (Seney et al., 2018). While our data suggests microglial involvement in the pathogenesis of anxiety-like behavior in both male and female mice, we have not characterized the complete transcriptional profile of microglia after RSD. It is likely that we may find divergent pathways in these activated microglia in future studies employing direct male-to-female comparisons.
In summary, the neuroimmune communication that contributes to anxiety-like behavior following RSD is similar in male and female mice. Our current findings indicate that social defeat in female mice leads to anxiety-like behavior, social avoidance, anatomically relevant patterns of neuronal and microglial activation, and a peripheral immune response marked by myelopoiesis and elevated plasma IL-6. We have characterized the mechanisms of microgliamonocyte and brain-bone marrow communication and their role in the development of anxiety-like behavior extensively in male mice (Reader et al., 2015; Weber et al., 2017; Wohleb et al., 2014c). Because the same key components are present in the female response to RSD, we reason that the pathways between appraisal of a chronic stressor (RSD), the peripheral immune response, and onset of anxiety-like behavior are the similar across the sexes. These findings help validate RSD as a model that is relevant to study stress responses in male and female mice.
Supplementary Material
Supplemental Figure 1. Representative ΔFosB staining for each region of interest. Coronal sections were chosen based on landmarks from the stereotaxic mouse brain atlas. Each region was quantified at 200× magnification using one field-of-view per animal with the exception of the basolateral amygdala (BLA), which was quantified as shown by the dashed ellipse. The solid lines represent the external capsule (ec). CA3 = CA3 region of hippocampus; PrL = prelimbic cortex; MTX = motor cortex. % area Iba-1 was quantified in the same regions.
Highlights.
DREADD male mice display aggressive behaviors toward female mice
Behavioral, physiological and immune responses elicited by social defeat in females
Behavioral alterations coincided with enhanced neuronal and microglial activation in threat-appraisal brain regions
Acknowledgements
This study was supported by National Institute of Health (NIMH) grants R01-MH-093473 and R01-MH-093472 to JFS. DBM and CMS were supported by NIDCR Training Grant T32-DE014320. DBM was supported by F31-MH109234 and CMS was supported by F30-DE026075. WY is supported by an OSU Fellowship. We thank The Ohio State University Comprehensive Cancer Center’s (OSUCCC) Campus Microscopy and Imaging Facility, supported in part by National Cancer Institute (NCI) grant P30-CA016058, for the instruments and services to generate confocal images presented in this report. We also thank Drs. Russo and Takahashi (Department of Neuroscience, Icahn School of Medicine at Mount Sinai, One Gustav Levy Place, New York, NY 10029) for the use of the DREAAD mouse model and for their helpful consultation on the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Almeida DM, Wethington E, Kessler RC, 2002. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment 9, 41–55. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kinsey SG, Bidor K, Bailey MT, Padgett DA, Sheridan JF, 2007. Subordinate social status modulates the vulnerability to the immunological effects of social stress. Psychoneuroendocrinology 32, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF, 2002a. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. Journal of neuroimmunology 124, 54–61. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Sheridan JF, 2002b. Social stress alters splenocyte phenotype and function. Journal of neuroimmunology 132, 66–71. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF, 2001. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and behavior 39, 247–257. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews 35, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkqvist K, 2001. Social defeat as a stressor in humans. Physiology & behavior 73, 435–442. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ, 1995. Gender bias in the preclinical psychopharmacology of anxiety: male models for (predominantly) female disorders. Journal of psychopharmacology 9, 79–82. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC, 2001. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiology & behavior 73, 261–271. [DOI] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD, 2016. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 233, 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM, 2015. Resolving the neural circuits of anxiety. Nature neuroscience 18, 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG, 2009. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. Journal of neuroscience methods 181, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF, 2004. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. Journal of neuroimmunology 148, 106–115. [DOI] [PubMed] [Google Scholar]
- Foertsch S, Fuchsl AM, Faller SD, Holzer H, Langgartner D, Messmann J, Strauss G, Reber SO, 2017. Splenic glucocorticoid resistance following psychosocial stress requires physical injury. Scientific reports 7, 15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP, 2004. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Archives of internal medicine 164, 1010–1014. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Trinh NH, Smoller JW, Fava M, Murphy JM, Breslau J, 2013. Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychological medicine 43, 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halasz J, Makara GB, 1999. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain research bulletin 50, 33–39. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF, 2012a. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain, behavior, and immunity 26, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF, 2012b. β-adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, Abbas AI, Leonardo ED, Bolkan SS, Hen R, Gordon JA, 2017. A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, Flugge G, Fuchs E, 2009. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience 159, 982–992. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR, 2013. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cerebral cortex 23, 1784–1797. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM, 2003. Conditioned defeat in male and female Syrian hamsters. Hormones and behavior 44, 293–299. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI, 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA, 2001. Are there sex differences in the reliability of a lifetime history of major depression and its predictors? Psychological medicine 31, 617–625. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal relationship between stressful life events and the onset of major depression. The American journal of psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kessler RC, 1997. The effects of stressful life events on depression. Annual review of psychology 48, 191–214. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB, 1993. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of affective disorders 29, 85–96. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU, 2012. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research 21, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R, 2007. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain, behavior, and immunity 21, 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, Lorsch ZS, Hamilton PJ, Calipari ES, Hodes GE, Issler O, Kronman H, Pfau M, Obradovic ALJ, Dong Y, Neve RL, Russo S, Kazarskis A, Tamminga C, Mechawar N, Turecki G, Zhang B, Shen L, Nestler EJ, 2017. Sex-specific transcriptional signatures in human depression. Nature medicine 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2003. The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology 23, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ, 2014. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H, 1997. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9, 853–858. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpe S, Meltzer HY, Bosmans E, Suy E, Calabrese J, Cosyns P, 1993. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry research 49, 11–27. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, 1998. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological review 105, 83–107. [DOI] [PubMed] [Google Scholar]
- McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP, 2016a. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF, 2015. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF, 2016b. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biological psychiatry 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP, 2017. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, Sheridan JF, 2018. Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis. Cell Rep 25, 2552–2562 e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonte B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ, 2017. Social stress induces neurovascular pathology promoting depression. Nature neuroscience 20, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, Kobor MS, Cole SW, 2014. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, behavior, and immunity 41, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula A, Witcher KG, Sheridan JF, Godbout JP, 2018. Interleukin-6 Induced by Social Stress Promotes a Unique Transcriptional Signature in the Monocytes That Facilitate Anxiety. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM, 2010. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain, behavior, and immunity 24, 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Parmigiani S, 2017. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neuroscience and biobehavioral reviews 76, 134–143. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K, 2008. The mouse brain in stereotaxic coordinates. 3rd edition. [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW, 2013. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Ramirez K, Niraula A, Sheridan JF, 2016. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain, behavior, and immunity 51, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Shea DT, McKim DB, Reader BF, Sheridan JF, 2015. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun 46, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF, 2015. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T, 2001. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry 58, 445–452. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, Kim JK, Weber MD, Jarrett BL, Godbout JP, Sheridan JF, Humeidan M, 2017. Ropivacaine and Bupivacaine prevent increased pain sensitivity without altering neuroimmune activation following repeated social defeat stress. Brain, behavior, and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki CM, McKim DB, Wohleb ES, Jarrett BL, Reader BF, Norden DM, Godbout JP, Sheridan JF, 2015. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience 302, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, Sibille E, 2018. Opposite Molecular Signatures of Depression in Men and Women. Biological psychiatry 84, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Sibille E, 2014. Sex differences in mood disorders: perspectives from humans and rodent models. Biology of sex differences 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL, 2007. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Hormones and behavior 52, 211–219. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF, 2002. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. Journal of neuroimmunology 124, 9–15. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF, 2001. Social stress induces glucocorticoid resistance in macrophages. American journal of physiology. Regulatory, integrative and comparative physiology 280, R1799–1805. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Trainor BC, 2017. Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Seminars in cell & developmental biology 61, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, Flanigan ME, Pfau ML, Menard C, Dumitriu D, Hodes GE, McEwen BS, Nestler EJ, Han MH, Russo SJ, 2017. Establishment of a repeated social defeat stress model in female mice. Scientific reports 7, 12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N, 2014. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain, behavior, and immunity 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK, 2011. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PloS one 6, e17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda MC, Ogawa S, 2012. Long-lasting consequences of neonatal maternal separation on social behaviors in ovariectomized female mice. PloS one 7, e33028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda MC, Yamaguchi N, Ogawa S, 2011. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 22, 259–263. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR, 2010. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, behavior, and immunity 24, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Verma R, Balhara YP, Gupta CS, 2011. Gender differences in stress response: Role of developmental and biological determinants. Industrial psychiatry journal 20, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Godbout JP, Sheridan JF, 2017. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP, 2012. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF, 2011. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP, 2014a. Reestablishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biological psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP, 2014b. Reestablishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP, 2014c. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Frontiers in neuroscience 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF, 2014d. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF, 2013. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang L, Yu C, Yang XF, Wang H, 2014. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker research 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JK, Fernandez AA, Gosselink KL, 2011. Female responses to acute and repeated restraint stress differ from those in males. Physiology & behavior 104, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representative ΔFosB staining for each region of interest. Coronal sections were chosen based on landmarks from the stereotaxic mouse brain atlas. Each region was quantified at 200× magnification using one field-of-view per animal with the exception of the basolateral amygdala (BLA), which was quantified as shown by the dashed ellipse. The solid lines represent the external capsule (ec). CA3 = CA3 region of hippocampus; PrL = prelimbic cortex; MTX = motor cortex. % area Iba-1 was quantified in the same regions.