Abstract
Objective:
The two primary – seemingly contradictory – strategies for classifying child psychiatric syndromes are categorical and dimensional; conceptual ambiguities appear to be greatest for polythetic syndromes such as autism spectrum disorder (ASD). Recently, a compelling alternative has emerged that integrates both categorical and dimensional approaches (ie, hybrid model) thanks to the increasing sophistication of analytic procedures. This study aimed to quantify the optimal phenotypic structure of ASD by comprehensively comparing categorical, dimensional, and hybrid models.
Method:
The sample comprised 3,825 youth, who were consecutive referrals to a university developmental disabilities or child psychiatric outpatient clinic. Caregivers completed the Child and Adolescent Symptom Inventory-4R (CASI-4R), which includes an ASD symptom rating scale. A series of latent class analyses, exploratory and confirmatory factor analyses, and factor mixture analyses was conducted. Replication analyses were conducted in an independent sample (N=2,503) of children referred for outpatient evaluation.
Results:
Based on comparison of 44 different models, results indicated that the ASD symptom phenotype is best conceptualized as multi-dimensional versus a categorical or categorical-dimensional hybrid construct. ASD symptoms were best characterized as falling along three dimensions (ie, social interaction, communication, and repetitive behavior) on the CASI-4R.
Conclusion:
Findings reveal an optimal structure with which to characterize the ASD phenotype using a single, parent-report measure, supporting presence of multiple correlated symptom dimensions that traverse formal diagnostic boundaries and quantify the heterogeneity of ASD. These findings inform understanding of how neurodevelopmental disorders can extend beyond discrete categories of development and represent continuously-distributed traits across the range of human behaviors.
Keywords: autism spectrum disorder, ASD phenotype, classification, dimensional models, Child and Adolescent Symptom Inventory
INTRODUCTION
Autism spectrum disorder (ASD) is a neurobehavioral syndrome characterized by atypical social interactions and communications, and repetitive or rigid behaviors.1 ASD is traditionally conceptualized as a distinct categorical syndrome that is qualitatively different from typical development; however, there is increasing evidence indicating that ASD traits are widely distributed in the general population (i.e., dimensional),2,3 or even that the disorder is best characterized by a hybrid of the categorical and dimensional models.4,5 Given these diverse conceptualizations of ASD, defining the boundaries and optimal phenotypic structure of ASD is important for research efforts examining the etiology, genetic and neural correlates, assessment, classification, and treatment of ASD.
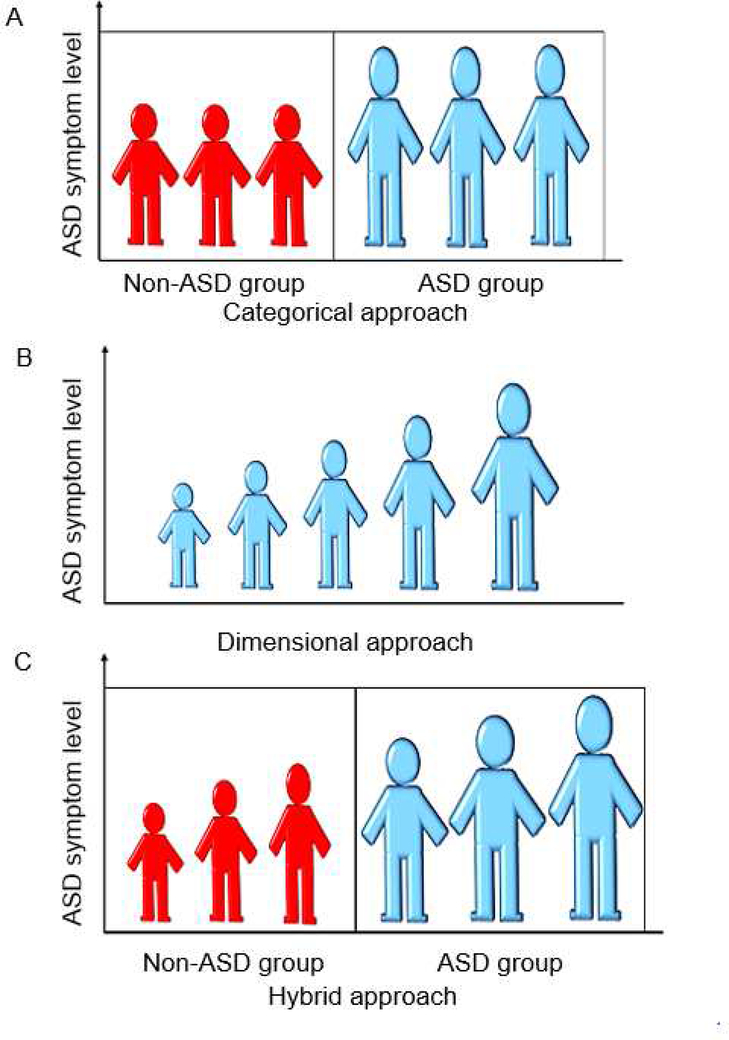
Historically, the Diagnostic Statistical Manual of Mental Disorders has conceptualized ASD as a categorical construct with clear delineation between affected and unaffected individuals (Figure 1A). This conceptualization of ASD has shaped the way researchers probe differences between individuals with and without ASD; namely, examining group differences between affected and unaffected individuals rather than examining whether ASD traits exist along a continuum in a dimensional manner more broadly. Some studies probing the validity of the categorical conceptualization of ASD have found support for a categorical latent structure of ASD symptoms as opposed to a dimensional one.6,7
Figure 1. Various Models of Autism Spectrum Disorder.
Note: Model A depicts a categorical approach to autism spectrum disorder. Model B depicts a dimensional approach to autism spectrum disorder. Model C depicts a categorical-dimensional hybrid approach to autism spectrum disorder.
Currently, there is growing support for a dimensional model of ASD in which ASD traits are continuously distributed in the general population (Figure 1B). In fact, studies investigating the latent factor structure of ASD symptoms have found support for the dimensional nature of ASD symptoms across the diagnostic threshold for ASD2,8 and the stability of ASD traits in the general population.9 Studies of the dimensional approach of ASD have yielded mixed results; whereas some research suggests a single underlying ASD dimension, reflecting severity of symptoms, other studies have found support for multiple dimensions that differ by symptom type.10,11 The dimensional conceptualization of ASD is also concordant with results of some genetic studies, which suggest that the genetic contributions to ASD traits are heterogeneous, and appear to be continuously distributed.12
Recently, a third cogent conceptualization of the structure of ASD proposes a categorical-dimensional hybrid model, which integrates both categorical and dimensional representations of ASD symptoms in that it acknowledges qualitative differences between individuals (Figure 1C). These qualitative differences might represent individuals with and without ASD features, or differences among different ASD subtypes. In addition to these categorical parsings of individuals, hybrid models allow for within-group heterogeneity reflected by standing on one or more dimensional constructs within a given ASD-related group. For instance, categories representing two subtypes of ASD might each contain a severity dimension, allowing individuals within each category to differ from one another to an extent. To date, there has been relatively little investigation of the hybrid model of ASD.4,5 Additionally, most studies pertain to either general population samples or individuals with ASD alone, but not both. This can bias the obtained factor structure, most notably by artificially magnifying group differences, highlighting the importance of examining phenotypic model structure in clinically diverse samples.
Investigating the optimal structure of ASD symptoms can inform conceptual and methodological assumptions regarding autism research ranging from appropriate research designs to pathogenesis. For instance, it is not currently evident if candidate biomarkers of ASD are indicative of the state of having the disorder13 or rather reflect particular traits that vary across the general population9,14 and may confer broader risk of psychopathology.15 If ASD is best characterized as dimensional, it may be more productive to focus on variables that influence a continuum of behavioral traits in the general population, rather than identifying factors specifically associated with the ontogenesis of ASD, and how they confer vulnerability for and manifest into ASD-related symptoms. Delineating the optimal structure of ASD traits can also influence decisions about assessment and treatment by guiding what clinicians evaluate and making decisions about differential diagnosis and referral to appropriate specialists.15 Further, the nosology of ASD influences whether clinicians should evaluate quality of symptoms (as is typically done in categorical structures) versus impairment (which is inherent in a dimensional conceptualization). It also guides whether it is prudent for clinicians to aim to treat symptoms versus the entire disorder. Without empirically identifying an optimal structure of ASD, clinicians may be assessing, diagnosing, and treating ASD traits epiphenomenally.
Thus, our primary aim was to delineate the optimal structure of ASD symptoms by comprehensively comparing categorical, dimensional, and categorical-dimensional hybrid models. We isolated the optimal ASD symptom structure in youth referred to a university-based developmental disabilities clinic or a child and adolescent psychiatry outpatient clinic. We then tested the generalizability of our model (i.e., replication analysis) in an independent, heterogeneous group of clinic referrals from various geographic locations. An important strength of our strategy was to include both ASD and non-ASD clinic referrals to mitigate the risk that inferences about ASD structure would be biased by well-documented co-occurring psychopathology in youth with ASD. Based on the findings of prior studies,2,8–10,14 we hypothesized that the dimensional approach would better characterize the structure of ASD symptoms than the categorical or hybrid approaches. Further, as the instrument we utilized (i.e., Child and Adolescent Symptom Inventory-4R; CASI-4R)16 was originally created under the DSM-IV-TR system, we hypothesized that item content would influence any obtained factor structure, favoring a 3-factor dimensional solution (social interaction, communication, restrictive and repetitive behaviors), which would also be congruent with previous studies.2,17
METHOD
Participants
Primary sample.
The study sample comprised 3,825 youth between 6 and 22 years old (M = 11.35, SD = 3.53). Considering that many individuals with ASD are eligible for services until the age of 22, and they are likely eligible to continue to have their symptoms rated in school settings, this study included participants aged between 6 and 22, in order to maximize the external validity and functional range of the sample. See Table 1 for sample characteristics. Participants were consecutive referrals to a university developmental disabilities clinic (i.e., DD referrals; n=1,319) or a child psychiatric outpatient clinic (i.e., psychiatry referrals; n=2,506) between 1994 and 2012. The DD referrals comprised those who were either (a) diagnosed as having ASD (n=899; 68%) according to DSM-IV-TR criteria or (b) another, non-ASD psychiatric or developmental disorder (n=420; 32%). Among youth with ASD, 300 met criteria for autistic disorder, 225 met criteria for Asperger’s syndrome, and 374 met clinical criteria for pervasive developmental disorder, not otherwise specified (PDD-NOS). Both clinic samples are described in prior publications.17–19 The psychiatry referrals comprised two consecutive cohorts: an initial cohort (n=1,276) and second cohort for whom diagnostic data were not available (n=1,230). Clinical diagnoses were available for most cases (n=917; 72%) in the initial cohort. The prevalence of each clinical diagnosis was as follow: attention-deficit/hyperactivity disorder (77%), learning disabilities (34%), language disorders (23%), oppositional defiant disorder (17%), major depressive disorder (12%), anxiety disorders (12%), social anxiety (11%), and ASD (16%). Among those diagnosed with ASD, 17 received a diagnosis of autistic disorder, 39 Asperger’s syndrome, and 88 PDD-NOS. We merged the two psychiatry referral cohorts in our analyses, because additional statistical analyses (i.e., independent group t-tests and chi-square tests) revealed no significant differences between the cohorts in terms of gender proportions, average age, and average ASD subscale scores measured.
Table 1.
Study Sample Characteristics
| Variable |
Developmental disabilities clinic referrals |
Psychiatry referrals |
|---|---|---|
| N | 1,319 | 2,506 |
| Sex | ||
| Boys | 1,062 | 1,730 |
| Girls | 257 | 775 |
| Age | ||
| Mean | 9.62 | 12.27 |
| SD | 3.04 | 3.42 |
| ASD sub-scale | ||
| SI | ||
| Mean | 5.51 | 2.36 |
| SD | 3.34 | 2.73 |
| COM | ||
| Mean | 4.97 | 1.46 |
| SD | 3.48 | 2.00 |
| RRB | ||
| Mean | 4.77 | 1.95 |
| SD | 3.11 | 2.35 |
Note: ASD = autism spectrum disorder; COM = communication; RRB = restricted repetitive behavior; SI = social interaction.
Replication sample.
In order to investigate the generalizability of our findings, we conducted a full reanalysis using CASI-4R data from an independent, heterogeneous and geographically diverse sample (N=2,503) comprising children (6–12 years) referred for evaluation to a number of outpatient clinics from four previously described studies: (1) a psychology clinic within a large pediatric hospital (n=1,537),20 (2) an RCT for ADHD and co-occurring severe aggression (n=168);21 (3) outpatient mental health clinics (n=462);22 and (4) a psychological testing service within a pediatric hospital (n=336).23,24 See Supplement 1, available online, for more details about each of these studies.
Measures
Child and Adolescent Symptom Inventory-4R (CASI-4R).
The CASI-4R16 contains items that reflect DSM-IV-TR diagnostic criteria for emotional and behavioral problems in children and adolescents. The ASD subscale has 12 items that assess the three core DSM-IV ASD symptom domains (i.e., social deficits, communication deficits, perseverative behaviors; see Table S1, available online). Each domain contains four items each, which are scored on a 4-point Likert-type scale ranging from 0 (never) to 3 (very often). These items were then dichotomized with scores of 0 and 1 recoded to 0 and scores of 2 and 3 recoded to 1. A number of prior studies have documented satisfactory psychometric properties (e.g., internal consistency, test-retest reliability, convergent and divergent validity) of the CASI-4R subscales in both community-based normative and clinical populations.17 Specifically, previous studies have indicated notable clinical utility of the CASI-4R in identifying ASD in both clinically-referred and non-referred youth samples.25,26 The 12 ASD items demonstrated excellent internal consistency (Cronbach’s alpha ranged from .85 to .86 across different subsamples) in our study.
Statistical Analyses
Analysis strategy.
A total of 170 individuals (4.4%) had some missing values in their responses. Those who missed only one item on a sub-scale, an average of the remaining items replaced the missing item. Response was coded as missing if a subscale had more than one missing value. Prior research has shown that this mean substitution procedure had a comparable performance to other methods (e.g., a multiple imputation method), especially in datasets with low levels of missingness.27 We rounded up some non-integer values, which were produced as the result of this mean substitution, to the nearest whole integers for analytical simplicity. We conducted this missing data correction procedure separately for each of three ASD sub-scales.
Additionally, sensitivity analyses were conducted to determine to what extent retaining versus excluding remaining missingness could impact our results. We performed subsequent sets of exploratory factor analysis separately in (a) the full dataset (N=3,825) that contained missing values, and (b) a case-wise deleted dataset (n=3,655) with missing values excluded. This sensitivity analysis revealed no significant statistical difference between the two datasets. Thus, we report results from the full dataset.
All analyses were conducted in Mplus (version 7.42).28 CASI-4R ASD items were treated as categorical variables by use of robust maximum likelihood estimation (MLR). We used the Bayesian information criteria (BIC) to evaluate model fit, which is supported by previous simulation research for comparison of non-nested models, such as those fit here 29. The model with the smallest BIC value best balances fit and parsimony, with BIC differences of 10 between two models indicating 150:1 posterior odds in favor of the model with superior (lower) BIC.
Latent class analysis.
Latent class analysis (LCA) classifies individuals into distinct subgroups based on individuals’ item endorsement profiles. In LCA, the correlations among the dichotomous indicators (i.e., the 12 ASD items) were related to categorical latent factors (i.e., ASD symptom class factors) in a logistic regression framework. We modeled a series of LCA models for 2- through 7-classes in the primary sample to examine how well a class-based approach (i.e., distinct ASD symptom classes) explained correlation patterns among the observed CASI-4R indicator variables.
Exploratory factor analysis.
Exploratory factor analysis (EFA) is used to investigate the underlying structure of a set of variables by determining the number of continuous latent variables that account for the correlations among indicators. In EFA models, all indicator variables are allowed to load on all factors. We extracted 1 through 5 factors to determine the number of factor(s) that best described the structure of ASD symptoms. We used an oblique geomin rotation in EFAs, because oblique rotations provide more flexible modeling solutions than do orthogonal rotations.
Confirmatory factor analysis.
Confirmatory factor analysis (CFA), like EFA, is used to explore the underlying dimensional structure of a set of indicator variables. We investigated two CFA models. First, based on the results of a previous study17 and the best-fitting EFA from our study, we modeled a 3-factor CFA defining social interaction (SI), communication (COM), and restricted and repetitive behavior (RRB) factors, and we allowed the item “difficulty engaging in socially appropriate conversation” to cross-load on the SI and COM factors. Second, to investigate the possible change in model fit without this cross-loading, we modeled a 3-factor CFA without this cross-loading: The item in question loaded solely on the COM factor, assigned there based on the result of EFA. We chose the former as our final CFA model, based on (a) the result from our EFA indicating that item cross-loaded on both domains, (b) the wording of that item, and (c) the result of the comparisons of the two different types of CFA (see Table 2 for more information).
Table 2.
Model Fit Information of Various Statistical Models
| Model | k | LL | BIC | Description |
|---|---|---|---|---|
| EFA | ||||
| 1F | 24 | −17174.77 | 34546.71 | |
| 2F | 35 | −16958.76 | 34205.06 | |
| 3F | 45 | −16825.55 | 34020.80 | Best fitting EFA |
| 4F | 54 | −16798.63 | 34040.90 | |
| 5F | 62 | −16782.74 | 34074.85 | |
| CFA | ||||
| 3Fa | 27 | −16916.50 | 34054.81 | |
| 3Fb | 28 | −16884.96 | 33999.95 | Best fitting overall |
| LCA | ||||
| 1C | 12 | −21925.31 | 43949.21 | |
| 2C | 25 | −17670.96 | 35547.30 | |
| 3C | 38 | −17182.27 | 34676.73 | |
| 4C | 51 | −16999.40 | 34417.80 | |
| 5C | 64 | −16915.11 | 34356.01 | |
| 6C | 77 | −16841.50 | 34315.60 | Best fitting LCA |
| 7C | 90 | −16797.75 | 34334.90 | |
| EFMA | ||||
| 2C-1F | 49 | −16906.44 | 34215.44 | |
| 2C-2F | 71 | −16754.96 | 34093.23 | Best fitting EFMA |
| 2C-3F | 91 | −16708.63 | 34164.86 | |
| 3C-1F | 74 | −16771.07 | 34150.09 | |
| 3C-2F | 107 | −16689.73 | 34258.53 | |
| 3C-3F | 137 | −16650.47 | 34426.47 | |
| 4C-1F | 99 | −16728.75 | 34270.84 | |
| 4C-2F | 143 | −16651.53 | 34477.88 | |
| 4C-3F | 183 | −16609.23 | 34721.91 | |
| 5C-1F | 124 | −16690.41 | 34399.56 | |
| 5C-2F | 179 | −16596.96 | 34664.49 | |
| 5C-3F | 229 | −16571.06 | 35023.48 | |
| 6C-1F | 149 | −16659.55 | 34543.21 | |
| 6C-2F | 215 | −16574.45 | 34915.24 | |
| 6C-3F | 275 | −16533.86 | 35327.00 | |
| LCFA | ||||
| 2C-1F | 25 | −17670.96 | 35547.30 | |
| 2C-2F | 28 | −17670.96 | 35571.95 | |
| 2C-3F | 29 | −17670.96 | 35580.16 | |
| 3C-1F | 27 | −17188.68 | 34599.17 | |
| 3C-2F | 31 | −17188.21 | 34631.09 | |
| 3C-3F | 33 | −17186.86 | 34644.83 | |
| 4C-1F | 29 | −17138.69 | 34515.64 | |
| 4C-2F | 34 | −17031.97 | 34343.26 | |
| 4C-3F | 37 | −17027.62 | 34359.21 | |
| 5C-1F | 31 | −17125.34 | 34505.36 | |
| 5C-2F | 37 | −16984.89 | 34273.76 | |
| 5C-3F | 41 | −16969.44 | 34275.72 | |
| 6C-1F | 33 | −17122.42 | 34515.94 | |
| 6C-2F | 40 | −16953.04 | 34234.70 | |
| 6C-3F | 45 | −16914.32 | 34198.34 | Best fitting LCFA |
Note: BIC = Bayesian information criteria; C = class; CFA = confirmatory factor analysis; EFA = exploratory factor analysis; EFMA = exploratory factor mixture analysis; F = factor; k = number of parameters; LCA = latent class analysis; LCFA = latent class factor analysis. LL = log-likelihood.
Model subscripts:
CFA with no cross-loading
CFA with cross-loading of item “difficulty engaging in socially appropriate conversation”; see the Statistical Analyses subsection for more information about the cross-loading
Exploratory factor mixture analysis.
Exploratory factor mixture analysis (EFMA) takes a class-dimension hybrid approach to modeling the latent variables underlying a set of indicator variables.28 EFMA is used when the underlying factor structure, and the sources of sample heterogeneity, are unknown and thus explores continuous latent variables within classes of individuals in an exploratory fashion; that is, EFMA does not pre-specify factor structure and thus not use explicit model parameterization. Exploratory factor models with increasing number of factors are investigated in increasing number of class solutions. For example, in a 2-class model, researchers can fit one dimensional factor within each class; they can then separately fit two dimensional factors within each class, and so on; then, 3-class models can be investigated with various numbers of dimensional factors within each class. The end point of the combination of classes and dimensional factors can be determined by investigating the optimal number of latent classes indicated by the best-fitting LCA and the optimal number of dimensional factors indicated by the best-fitting EFA.
Latent class factor analysis.
Latent class factor analysis (LCFA) incorporates both categorical and dimensional features in modeling in which within-class variation is allowed through the use of continuous latent factors once individuals are classified into latent classes.30 The major distinction between EFMA and LCFA is that the latter uses explicit model parameterizations to pre-specify the factor structure; that is, the researcher specifies which observed variables load on which factor(s) using model parameterizations in each class solution in the same manner as in CFA. Following the LCFA methodology proposed by Clark and colleagues,30 we began by fitting a LCFA models with 2-classes/1-factor (i.e., a general ASD factor) and then increased the number of classes and factors in subsequent models. Ultimately, we fit a series of LCFA models up to the 6-class/3-factor level, yielding a total of 15 LCFA models.
A subsample analysis including individuals whose ASD diagnostic status was known.
Including only individuals with a confirmed ASD (or a non-ASD) diagnosis (n=1,914; 1,108 individuals were from the DD referrals, and 806 individuals were from the psychiatry referrals), we conducted another subset of analyses where we further specified the three following models and compared them with other best-fitting models: (a) a 2-class confirmatory LCA model (CLCA),31 which identified latent ASD and non-ASD classes (i.e., diagnostically-driven classes) in such a way that each participant’s latent class membership was constrained to be equal to one’s observed group membership (i.e., those with a confirmed ASD diagnosis were assigned to the ASD class, and those with non-ASD diagnoses were assigned to the non-ASD class), (b) a 2-class exploratory LCA model (ELCA) extracting two classes (i.e., empirically-driven classes), where item endorsement probabilities were freely estimated in each class without any model constraints, and (c) a 2-factor/2-class LCFA model that exactly reflected DSM-5-based categorization (i.e., extracting social communication and RRB factors in ASD and non-ASD classes by use a mixture model of CFA and confirmatory LCA).30
These three models were then compared with the best-fitting dimensional and hybrid models that were conducted in the same subsample. The purpose of conducting this reanalysis was to answer to the following question: Does a class-based approach (either empirically-based or diagnostically-based classifications) or a DSM-5 based hybrid approach (i.e., the DSM-5-driven LCFA model) provide a better fit to the data than the other approaches when diagnostic status is known?
Replication analyses.
In order to investigate the replicability and generalizability of our findings, we conducted the same analyses described above using the CASI-4R data from the replication sample described above, who were not included in our primary sample (See Supplement 1, available online, for more details about the replication sample). The same set of EFA, CFA, LCA, EFMA, and LCFA (i.e., a total of 44 models) models was analyzed for the replication sample.
RESULTS
Structure of ASD Symptoms
Table 2 shows model fit information for latent categorical (LCA), dimensional (EFA and CFA), and two hybrid (EFMA and LCFA) models of the structure of ASD symptoms.
Categorical models.
LCA models showed gradual improvement of fit from 2- to 6-class solutions, with the 6-class solution being identified as optimal. Beyond six classes, fit began to deteriorate. In the best-fitting 6-class LCA, distinct latent ASD classes were characterized by (a) high rates of all ASD symptoms, (b) social awkwardness, (c) social amotivation, (d) communication problems, (e) restricted repetitive behavior, and (f) low rates of overall ASD symptoms.
Dimensional models.
EFA model fit improved with the number of factors extracted, with optimality being shown at the 3-factor model, and then decreased with subsequent factors. In the best fitting 3-factor EFA, the 3 factors that emerged were communication (COM), social interaction (SI), and restricted repetitive behavior (RRB; see Table S2, available online). The item “difficulty engaging in socially appropriate conversation” cross-loaded on the SI and COM factors, loading stronger on the COM factor. In CFA, a 3-factor CFA without cross-loadings fit the data poorer than did the best fitting 3-factor CFA with the cross-loading.
Hybrid models.
Guided by results of EFA and LCA, we tested hybrid models with 1-, 2-, and 3-factors solutions in 2- through 6-classes. EFMA models showed wide ranging fit index values across various class and factor number combinations. A 2-class/2-factor model was the best fitting EFMA by a large margin. Similarly, LCFA models showed wide ranging fit index values, with a 6-class/3-factor model was the best fitting LCFA.
Overall model comparison.
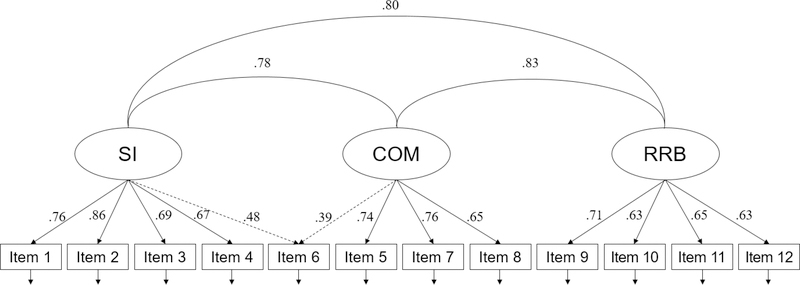
Between LCA, EFA, CFA, EFMA, and LCFA analyses, we compared 44 models in total. Comparison of these models indicated that the best fitting model was a 3-factor CFA, specifying SI, COM, and RRB factors, where the item “difficulty engaging in socially appropriate conversation” cross-loaded on the SI and COM factors (Figure 2). The second-best fitting model was a three-factor EFA, followed by the four-factor EFA, the three-factor CFA without any cross-loading, and then the five-factor EFA. Thus, the five best fitting models were all dimensional models with at least three factors. Of those, models that allowed a cross-loading for the item above were superior to the model that did not.
Figure 2. Best Fitting 3-Factor Oblique Confirmatory Factor Analysis Model With a Cross-Loading Of Item 6.
Note: For the entire list of the 12 items, see Table S1, available online. The parameter estimates for the cross-loaded item are depicted in dashed lines. All parameter estimates are standardized and significant at p < .001. COM = communication factor; Item 6 = “difficulty engaging in socially appropriate conversation”; RRB = restricted and repetitive behavior factor; SI = social interaction factor.
A subsample analysis including individuals whose ASD diagnostic status was known.
We conducted a subsequent set of analyses where we modeled (a) a diagnostically-driven LCA (i.e., confirmatory LCA), (b) an empirically-driven LCA (i.e., exploratory LCA), and (c) a DSM-5-driven LCFA. In the 2-class confirmatory LCA, 47% of the subsample was assigned to the ASD class, and 53% of the subsample was assigned to the non-ASD class. The 2-class exploratory LCA yielded a (a) high ASD class (45% of the subsample), which was characterized by increased probability of endorsing all ASD items, and (b) low ASD class (55%), which demonstrated low endorsement of all ASD items (Table 3). We then compared those three models above with the best fitting models of each approach from the previous analyses. Overall, results indicated that the CFA model provided the optimal fit among those models compared (Table 3).
Table 3.
Comparison of Diagnostically- and Empirically-Driven Latent Class Analysis (LCA) and Other Best-Fitting Models in a Subsample Whose Autism Spectrum Disorder (ASD) Diagnoses Were Available
| Model fit index values | |||||||
|---|---|---|---|---|---|---|---|
| Models | k | LL | BIC | ||||
| ELCA | 25 | −9929.562 | 20046.830 | ||||
| CLCA | 25 | −12014.234 | 24216.173 | ||||
| EFMAa | 71 | −9344.797 | 19222.680 | ||||
| LCFAb | 45 | −9449.843 | 19237.560 | ||||
| LCFAc | 27 | −12014.230 | 24231.190 | ||||
| CFAd | 28 | −9446.573 | 19103.376 | ||||
| Items | LCA item endorsement probabilities |
CFAd factor loadings |
|||||
| ELCA | CLCA | ||||||
| High ASD | Low ASD | ASD | Non-ASD | SI | COM | RRB | |
| (44.71) | (55.29) | (46.96) | (53.04) | ||||
| Relates to others in an unusual way | .652 | .067 | .528 | .149 | .831 | − | - |
| Difficulty playing with and relating to other children | .783 | .089 | .633 | .188 | .941 | - | - |
| Lack of interest in making friends | .447 | .039 | .371 | .087 | .810 | - | - |
| Lacks interest in or awareness of other people’s feelings | .447 | .065 | .349 | .133 | .737 | - | - |
| Language difficulties | .516 | .055 | .404 | .131 | - | .844 | - |
| Difficulty engaging in socially appropriate conversation | .850 | .101 | .696 | .202 | .403 | .551 | - |
| Speaks in an odd way | .411 | .019 | .331 | .070 | - | .873 | - |
| Difficulty engaging in make-believe play | .305 | .008 | .245 | .047 | - | .802 | - |
| Is preoccupied with certain topics | .647 | .110 | .561 | .160 | - | - | .792 |
| Distressed by small changes in routine or environment | .541 | .139 | .462 | .189 | - | - | .633 |
| Engages in odd repetitive movements | .421 | .060 | .368 | .089 | - | - | .756 |
| Has intense interest in parts of objects | .291 | .016 | .254 | .036 | - | - | .816 |
Note: Numbers in parentheses under the LCA item endorsement probabilities section represent the percentage of individuals assigned to a given ASD symptom class. BIC = Bayesian information criteria; CFA = confirmatory factor analysis; CLCA = confirmatory latent class analysis; COM = communication factor; ELCA = exploratory latent class analysis; k = number of parameters; LL = log-likelihood; RRB = restricted repetitive behavior factor. SI = social interaction factor.
Model subscripts:
The 2-class/2-factor model
The 6-class/3-factor model
The DSM-5-based 2-class/2-factor model
CFA with cross-loading of item “difficulty engaging in socially appropriate conversation”.
Replication analyses.
Overall, the results of the replication analyses were highly similar to our original analyses (using the primary sample) in that (a) the 3-factor dimensional model best fit the data (BIC = 20354.332); (b) the same top five models (i.e., 3-factor EFA, 4-factor EFA, 3-factor CFA with cross-loading, 5-factor EFA, and 3-factor CFA with no cross-loading, in order of model parsimony) were supported (see Table S3a, available online, for more information), which were all dimensional models; and (c) the best-fitting models for each approach were almost identical to those in our original analyses (see Table S3b, available online, for more information). We further conducted factor congruence analyses, which examined the similarity of the optimal 3-factor model between two sets of factor loadings (i.e., one from the original analysis and the other from the replication analysis), using Tucker’s congruence coefficients.32 These coefficients ranged from .91 to .97, indicating almost identical factors across the two independent samples.
DISCUSSION
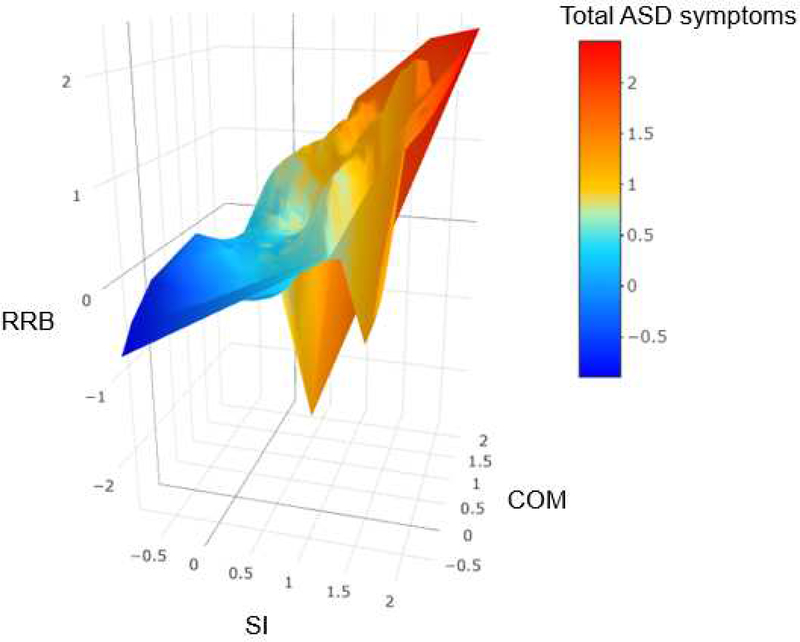
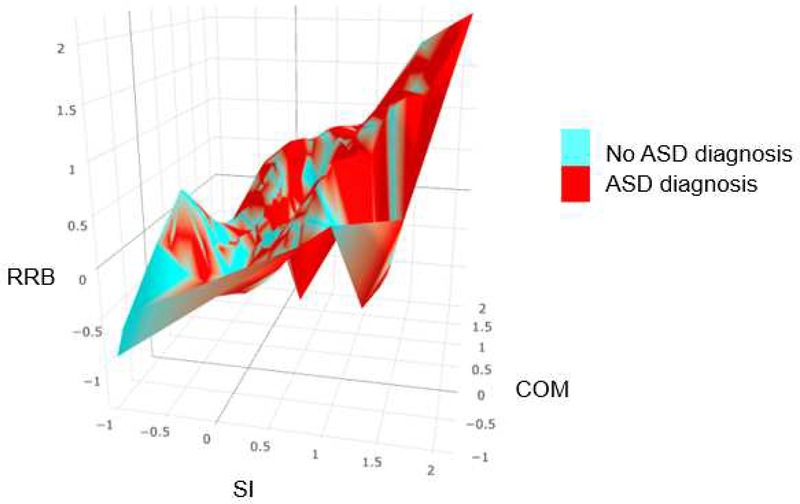
This study was the first to systematically compare various dimensional (i.e., EFA, CFA), categorical (i.e., LCA), and dimensional-hybrid models (i.e., EFMA, LCFA) to understand the structure of observable phenotypic expression of ASD symptoms across independent (non-familial) ASD and psychiatric comparison samples. Our findings indicate that a 3-factor CFA (Figure 2), specifying social interaction, communication, and restrictive and repetitive behaviors factors, was optimal among all analyses and provide additional support for the DSM-IV-TR model of ASD, as least as described in the CASI-4R. Conversely, ASD is defined by two symptom clusters (i.e., social communication and RRB) in DSM-5,1 a position for which there is some support in previous investigations.33 Additionally, both our primary and replication analyses indicate that ASD is best conceptualized as being dimensional rather than categorical (either diagnostically-based categories or empirically-based categories) or a categorical-dimension hybrid (Table 2; see Table S3a, available online ). As depicted in Figure 3A (and Figure S1, available online), ASD symptoms were continuously distributed from one extreme to another along with its three core dimensions. Moreover, the symptoms of individuals with ASD and the symptoms of individuals without ASD did not group into two separate and distinct clusters on the three dimensional space; rather, the distribution of their symptoms appeared highly similar to the continuous symptom distribution of the entire sample, with overlap between the two diagnostic groups. (Figure 3B; see Figure S2, available online). In sum, both our comprehensive comparisons of various statistical models and a graphical depiction of empirical ASD symptom data provide robust evidence that the ASD phenotype is dimensional, composed of multiple related spectra.
Figure 3. Distribution of Empirical Autism Spectrum Disorder (ASD) Symptoms.
Note: Numbers on each axis represent factor scores, which indicate individuals’ symptom level in a given domain. Higher factor scores indicate more severe condition. While factor scores were generated using an oblique rotation in confirmatory factor analysis, those scores were plotted along the orthogonal axes for visualization purpose. We used a smoothing function developed by Wood, Scheipl, and Faraway43 to smooth Figures A and B, in order to leave out noise and better capture important patterns in the data. Figure A depicts the distribution of factor scores of the total sample. The figure is color-coded based on individuals’ total ASD symptoms. The factor scores were computed and saved from the best-fitting three-factor confirmatory factor analysis conducted in the total sample. Figure B depicts the distribution of factor scores of individuals whose ASD diagnostic status was known. The figure is color-coded based on individuals’ ASD diagnoses. The factor scores were computed and saved from the best-fitting three-factor confirmatory factor analysis conducted in that sub-sample (see Figure S2, available on-line, for animated three-dimensional interactive figures, which can be zoomed in/out and oriented by the viewer using their mouse). COM = communication factor; RRB = restricted and repetitive behavior factor (for the entire list of the ASD items, see Table S1, available online); SI = social interaction factor.
To our knowledge, Frazier and colleagues5 conducted the only other published study comparing various structures of the ASD clinical phenotype. They found that a hybrid model with two diagnostic categories (ASD vs. non-ASD) and two symptom dimensions (social communication and RRB) was the optimal structure of ASD. Despite seeming similarities between these two studies, they differ in important ways that likely impacted findings. For example, because Frazier and colleagues used caregiver-designated, non-ASD siblings as a comparison group, this likely enhanced the estimated diagnostic group differences due to (a) higher rates of overall psychopathology in youth with ASD (i.e., youth with ASD may have appeared more severe across symptom domains),34 and (b) reporting biases stemming from using the same caregivers to rate both diagnostic groups. In contrast, sample ascertainment in our study permitted less confounded inference about the potential for group differences (which are inferred by the hybrid model, but absent in our final dimensional model). Furthermore, whereas their categorical model was based solely on empirically-derived ASD classes, we additionally modeled (a) diagnostically-driven ASD classes based on clinical ASD diagnoses (e.g., by using confirmatory latent class analysis) and (b) a 2-factor/2-class factor mixture solution that exactly reflected the DSM-5 based categorization. Our findings suggest that the dimensional approach is the best fit of the data, when comparing to not only any sort of class-based approaches but also DSM-5 based hybrid approach. The three factors that emerged (social interaction, communication, and restrictive and repetitive behaviors) are consistent with the core components of ASD identified in past literature quantitatively7,35,36 and qualitatively37 as well as in the DSM-IV. While some research has suggested that social and communication factors are difficult to disentangle (pace DSM-5),36 results from our large discovery and replication samples indicate that social interaction and communication may best be conceptualized as separate but correlated dimensions, and the inclusion of the separation between the two domains provides for a superior accounting of ASD phenotypic symptom structure.
In addition to providing valuable information regarding ASD nosology and classification, our findings have important implications for assessment and intervention as well as the pursuit of pathogenesis. Although the DSM-51 dictates clinical status based on the presence or absence of a specific set of ASD symptoms, our findings suggest it may be more prudent to focus on impairment levels in core symptom domains to better identify youth in need of support,38 which can be easily achieved with the use of dimensional measures of ASD symptoms. Accordingly, treating individual symptom dimensions, rather than ASD as a unitary disorder, would allow for more individualized and flexible treatment.39 Significant resources are being allocated to the identification of biologic markers for ASD.14,28,40,41 Our finding that ASD traits are dimensional supports a transdiagnostic model of etiology that plays an important role in ASD but is not confined to a categorical syndrome,42 which may guide and influence the scope and specificity of the search for such markers.
The current study has some limitations. First, even though we included non-ASD clinic-referred comparison group, not including typically-developing comparison group may somewhat limit the generalizability of the present findings to typically developing youth. Second, ASD diagnostic status was not available for the majority of the psychiatric clinic referrals, which may have impacted model fit in relevant analyses. However, the successful replication of our results mitigates against this concern. Third, symptom profiles were based on a single measure from one informant, and additional research is necessary to determine whether our findings generalize to other measures (e.g., DSM-5-referenced or other none-DSM-referenced measures) and sources and, in so doing, to the ASD syndrome more generally across contexts.
In sum, our findings are consistent with the notions that ASD traits are widely distributed in the general population of clinic referrals; there are three core domains of symptoms (social, communication, repetitive behavior); and diagnostic models and associated symptom clusters are better conceptualized as dimensional, all of which have important clinical implications. Moving forward, researchers and clinicians should consider further refinements in this multi-dimensional, transdiagnostic model of ASD symptomatology that lead to additional improvements in the measurement and conceptualization of ASD clinically and epidemiologically.
Supplementary Material
Acknowledgments
The authors thank Sin-Ying Lin, B.S., of Stony Brook University, for technical assistance for creating the three-Dimensional figures.
Funding
This study was supported, in part, by the Matt and Debra Cody Center for Autism and Developmental Disabilities. The LAMS study was supported by grant from the National Institute of Mental Health (NIMH) (Dr. R. L. Findling, PI; NIMH R01MH073967). The TOSCA study was supported by grants from National Institute of Mental Health (NIMH) to The Ohio State University (Dr. M. G. Aman PI; R01 MH077907), Case Western Reserve University (Dr. R. L. Findling; R01 MH077750), the University of Pittsburgh (Dr. O. Bukstein, PI; R01 MH077676), and Stony Brook University (Dr. K. D. Gadow, PI; R01 MH 077997), NIH General Clinical Research Center grant M01RR10710 (Stony Brook University), Clinical and Translational Science Awards from the National Center for Advancing Translational Sciences grants 8UL1TR000090-05 (The Ohio State University), and UL1 RR024153 and UL1TR000005 (University of Pittsburgh). Dr. Matthew D. Lerner received support from NIMH grant R01MH110585, the Simons Foundation Autism Research Initiative (SFARI# 381283), and a NARSAD Young Investigator Award (#24890) during the course of this project.
This study was supported, in part, by the Matt and Debra Cody Center for Autism and Developmental Disabilities. The LAMS study was supported by a grant from the National Institute of Mental Health (NIMH) (R.L. Findling, PI; R01MH073967). The TOSCA study was supported by grants from the NIMH to The Ohio State University (M.G. Aman, PI; R01 MH077907), Case Western Reserve University (R.L. Findling; R01 MH077750), the University of Pittsburgh (O. Bukstein, PI; R01 MH077676), Stony Brook University (K.D. Gadow, PI; R01 MH 077997), the National Institutes of Health (NIH) General Clinical Research Center grant M01RR10710 (Stony Brook University), Clinical and Translational Science Awards from the National Center for Advancing Translational Sciences grants 8UL1TR000090-05 (The Ohio State University), and UL1 RR024153 and UL1TR000005 (University of Pittsburgh). Dr. Lerner received support from NIMH grant R01MH110585, the Simons Foundation Autism Research Initiative (SFARI# 381283), and a NARSAD Young Investigator Award (#24890) during the course of this project.
The authors thank Sin-Ying Lin, BS, of Stony Brook University, for technical assistance for creating the three-dimensional figures.
Footnotes
Author Conflicts of Interest/Disclosures
Authors report no conflicts of interest, except Kenneth D. Gadow, PhD, of Stony Brook University, who is a shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-4R.
Disclosure: Dr. Gadow is a shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-4R. Drs. Eaton and Lerner, Mr. Kim, Ms. Keifer, and Mr. Rodriguez-Seijas report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders-DSM-5. Washington, DC: auteur; 2013. [Google Scholar]
- 2.Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. J Child Psychol Psychiatry. 2004;45(4):719–726. [DOI] [PubMed] [Google Scholar]
- 3.Noordhof A, Krueger RF, Ormel J, Oldehinkel AJ, Hartman Ca. Integrating Autism-Related Symptoms into the Dimensional Internalizing and Externalizing Model of Psychopathology. The TRAILS Study. Journal of Abnormal Child Psychology. 2014:577–587. [DOI] [PubMed] [Google Scholar]
- 4.Elton A, Di Martino A, Hazlett HC, Gao W. Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Biological Psychiatry. 2015;80(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frazier TW, Youngstrom Ea, Speer L, et al. Validation of Proposed DSM-5 Criteria for Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazier TW, Youngstrom Ea, Sinclair L, et al. Autism spectrum disorders as a qualitatively distinct category from typical behavior in a large, clinically ascertained sample. Assessment. 2010;17:308–320. [DOI] [PubMed] [Google Scholar]
- 7.James RJ, Dubey I, Smith D, Ropar D, Tunney RJ. The latent structure of autistic traits: a taxometric, latent class and latent profile analysis of the adult autism spectrum quotient. Journal of autism and developmental disorders. 2016;46(12):3712–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantino JN. The quantitative nature of autistic social impairment. Pediatric research. 2011;69(5 Pt 2):55R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson EB, Munir K, Munafò MR, Hughes M, McCormick MC, Koenen KC. Stability of autistic traits in the general population: further evidence for a continuum of impairment. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(4):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiades S, Szatmari P, Zwaigenbaum L, et al. Structure of the autism symptom phenotype: A proposed multidimensional model. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(2):188–196. [DOI] [PubMed] [Google Scholar]
- 11.Kamp-Becker I, Smidt J, Ghahreman M, Heinzel-Gutenbrunner M, Becker K, Remschmidt H. Categorical and dimensional structure of autism spectrum disorders: The nosologic validity of asperger syndrome. Journal of Autism and Developmental Disorders. 2010;40(8):921–929. [DOI] [PubMed] [Google Scholar]
- 12.Lundstrom S, Reichenberg A, Anckarsater H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. The BMJ. 2015;350:h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson EB, St Pourcain B, Anttila V, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nature genetics. 2016;48(5):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10(12):872–878. [DOI] [PubMed] [Google Scholar]
- 16.Gadow K, Sprafkin J. Child and adolescent symptom inventory-4R. Stony Brook, NY: Checkmate Plus; 2005. [Google Scholar]
- 17.Kim H, Keifer CM, Rodriguez Seijas C, Eaton NR, Lerner MD, Gadow KD. Structural hierarchy of autism spectrum disorder symptoms: an integrative framework. Journal of Child Psychology and Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadow K, Sprafkin J. The symptom inventories: An annotated bibliography [On-line]. Stony Brook, NY: Checkmate Plus; 2016. [Google Scholar]
- 19.Lecavalier L, Gadow KD, DeVincent CJ, Edwards MC. Validation of DSM-IV model of psychiatric syndromes in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(2):278–289. [DOI] [PubMed] [Google Scholar]
- 20.Lavigne JV, Cromley T, Sprafkin J, Gadow KD. The Child and Adolescent Symptom Inventory-Progress Monitor: a brief Diagnostic and Statistical Manual of Mental Disorders, -referenced parent-report scale for children and adolescents. Journal of child and adolescent psychopharmacology. 2009;19(3):241–252. [DOI] [PubMed] [Google Scholar]
- 21.Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(9):948–959. e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Findling RL, Youngstrom EA, Fristad MA, et al. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. The Journal of clinical psychiatry. 2010;71(12):1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellanos I, Kronenberger WG, Pisoni DB. Questionnaire-based assessment of executive functioning: Psychometrics. Applied Neuropsychology: Child. 2018;7(2):93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy JD, Kronenberger WG, Dunn DW. Development of a very brief measure of ADHD: The CHAOS scale. Journal of attention disorders. 2017;21(7):575–586. [DOI] [PubMed] [Google Scholar]
- 25.DeVincent C, Gadow KD, Strong G, Schwartz J, Cuva S. Screening for autism spectrum disorder with the Early Childhood Inventory-4. Journal of Developmental & Behavioral Pediatrics. 2008;29(1):1–10. [DOI] [PubMed] [Google Scholar]
- 26.DeVincent CJ, Gadow KD. Relative clinical utility of three child symptom inventory-4 scoring algorithms for differentiating children with autism spectrum disorder vs. attention-deficit hyperactivity disorder. Autism Research. 2009;2(6):312–321. [DOI] [PubMed] [Google Scholar]
- 27.Parent MC. Handling item-level missing data: Simpler is just as good. The Counseling Psychologist. 2012:0011000012445176. [Google Scholar]
- 28.Muthén L, Muthén B. Mplus: Statistical analysis with latent variables (Version 7.11)[Software]. Muthén & Muthén, Los Angeles: 2013. [Google Scholar]
- 29.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling. 2007;14(4):535–569. [Google Scholar]
- 30.Clark S, Muthén B, Kaprio J, et al. Models and strategies for factor mixture analysis: two examples concerning the structure underlying psychological disorders (http://www.statmodel.com/download/FMA%20Paper_v142.pdf ). Accessed; July 23, 2017. [DOI] [PMC free article] [PubMed]
- 31.Alarcón M, Abrahams BS, Stone JL, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. The American Journal of Human Genetics. 2008;82(1):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzo-Seva U, Ten Berge JM. Tucker’s congruence coefficient as a meaningful index of factor similarity. Methodology. 2006;2(2):57–64. [Google Scholar]
- 33.Bishop SL, Havdahl KA, Huerta M, Lord C. Subdimensions of social-communication impairment in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2016;57(8):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanne SM, Abbacchi AM, Constantino JN. Multi-informant ratings of psychiatric symptom severity in children with autism spectrum disorders: The importance of environmental context. Journal of autism and developmental disorders. 2009;39(6):856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin EJ. Personality correlates of the broader autism phenotype as assessed by the Autism Spectrum Quotient (AQ). Personality and Individual Differences. 2005;38(2):451–460. [Google Scholar]
- 36.Beuker KT, Schjølberg S, Lie KK, et al. The structure of autism spectrum disorder symptoms in the general population at 18 months. Journal of autism and developmental disorders. 2013;43(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of autism and developmental disorders. 1979;9(1):11–29. [DOI] [PubMed] [Google Scholar]
- 38.Kaat AJ, Gadow KD, Lecavalier L. Psychiatric symptom impairment in children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2013;41(6):959–969. [DOI] [PubMed] [Google Scholar]
- 39.Lerner M, White S, McPartland J. Mechanisms of change in psychosocial interventions for autism spectrum disorders. Dialogues in clinical neuroscience. 2012;14(3):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batty M, Meaux E, Wittemeyer K, Rogé B, Taylor MJ. Early processing of emotional faces in children with autism: an event-related potential study. Journal of experimental child psychology. 2011;109(4):430–444. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proceedings of the National Academy of Sciences. 2010;107(49):21223–21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damiano CR, Mazefsky CA, White SW, Dichter GS. Future directions for research in autism spectrum disorders. Journal of Clinical Child & Adolescent Psychology. 2014;43(5):828–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood SN, Scheipl F, & Faraway JJ (2013). Straightforward intermediate rank tensor product smoothing in mixed models. Statistics and Computing, 23(3), 341–360. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.