Abstract
Therapeutic targeting of phosphatidyl-inositol 3-kinase (PI3K) is considered as a possible strategy in several types of cancer, including gastrointestinal ones. In vitro and in vivo studies indicated the significance of proapoptotic and antiproliferative inhibition of PI3K. Although there are many phase 1 and 2 clinical trials on PI3K inhibitors in patients with gastrointestinal cancer, the molecular mechanism of PI3K targeting PI3K/ mTOR pathway is not clear. Panclass I, isoformselective, and dual PI3K/mTOR inhibitors are under investigation. This review aimed to indicate PI3K-dependent targeting mechanisms in gastrointestinal cancer and the evaluation of related clinical data.
Keywords: Gastrointestinal cancer, PI3K pharmacological inhibitors, Clinical trials
INTRODUCTION
Phosphatidyl-inositol 3-kinase (PI3K), an intracellular lipid kinase, plays an important role in cell function and cancer development.1 PI3K phosphorylates phosphatidylinositol 4,5 biphosphate (PIP2) to phosphatidylinositol 3,4,5 triphosphate (PIP3) and subsequently PIP3 leads to phosphorylation of Protein Kinase B (AKT) by Phosphoinositide-dependent kinase 1 (PDK1) and Mammalian target of rapamycin complex 2 (mTORC2).2 Phosphatase and tensin homologue protein (PTEN), a tumor suppressor molecule, dephosphorylates PIP3 to PIP2.3 PI3Ks has three classes based on structure and function. Class Ia, the most important PI3K in human cancer, comprised of regulatory (p85) and catalytic (p110) subunits.4 Phosphoinositide-3-Kinase Regulatory Subunits 1,2,3 (PIK3R1,2,3) genes encode different isoforms of p85 regulatory subunit, whereas the catalytic subunits p110α, p110β, and p110γ are products of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), beta (PIK3CB) and delta (PIK3CD), respectively.5 Common somatic mutations of PIK3CA and PIK3CB in cancer cells induce activation of PI3K.6 Many clinical trials are done on PI3K inhibitors in solid tumors, including gastrointestinal (GI) cancer (table 1), but it is not known how various forms of PI3K targeting alter the molecular mechanisms of PI3K/mTOR pathway. This review aimed at indicating the targeting PI3K-dependent mechanisms in GI cancer and evaluating the correlated clinical data.
Table 1. PI3K inhibitors in clinical trials for gastrointestinal cancer treatment .
| Compound name | Cancer type | Mechanism of action | Phase | Arm | Status | Reference |
| BKM120 (Buparlisib) | Advanced solid tumors | Pan-PI3K inhibitor | I | BKM120 | Completed | Bendell et al.20 |
| Advanced solid tumors | Pan-PI3K inhibitor | I | BKM120 | Completed | Baselga et al.21 | |
| Gastrointestinal stromal tumor | Pan-PI3K inhibitor + TK inhibitor | Ib | BKM120 + imatinib | Completed | NCT0146868823 | |
| Advanced colorectal cancer |
Pan-PI3K inhibitor + Topoisomerase 1 inhibitor |
I | Irinotecan + BKM120 | Completed | NCT0130460224 | |
| Metastatic or advanced colorectal cancer |
Pan-PI3K inhibitor + EGFR inhibitor |
I/II |
BKM120 + panitumumab |
Recruiting | NCT0159142125 | |
| Recurrent or metastatic advanced solid tumors | Pan-PI3K inhibitor + DNA synthesis inhibitor + microtubule polymer stabilizer | I |
BKM120 + paclitaxel + Carboplatin |
Recruiting | NCT0129745226 | |
| Metastatic adenocarcinoma of the colon or rectum |
Pan-PI3K inhibitor + Topoisomerase 1 inhibitor |
I |
BKM120 + irinotecan |
Recruiting | NCT0154025327 | |
| Metastatic or advanced solid tumors | mTOR/PI3K inhibitor + Pan-PI3K inhibitor + microtubule polymer stabilizer | Ib | BEZ235-paclitaxel + BKM120-paclitaxel | Ongoing (no longer recruiting) | NCT0128546628 | |
| Advanced solid tumors | Pan-PI3K inhibitor + hedgehog pathway inhibitor | Ib |
BKM120 + erismodegib |
Recruiting | NCT0157666629 | |
|
Advanced solid malignancies |
Pan-PI3K inhibitor + m-TORC1 inhibitor | I/II |
BKM120 + everolimus |
Recruiting | NCT0147020930 | |
| PI3K activated tumors | Pan-PI3K inhibitor | II | BKM120 | Recruiting | NCT0183316931 | |
| Esophageal cancer | pan-PI3K inhibitor | II | BKM120 | Recruiting | NCT0180664932 | |
| Advanced solid tumors |
Pan-PI3K inhibitor + MEK inhibitor |
Ib |
BKM120 + binimetinib |
Recruiting | NCT0136323233 | |
| Advanced solid tumors |
Pan-PI3K inhibitor + MEK inhibitor |
I | BKM120 + GSK1120212 | Completed | NCT0115545334 | |
| Advanced solid tumors |
Pan-PI3K inhibitor + topoisomerase 1 inhibitor |
I |
BKM120 + irinotecan |
Completed | NCT0106848335 | |
| PX-866 | Advanced solid tumors | Pan-PI3K inhibitor | I | PX-866 | Completed | Hong et al.37 |
| Advanced solid tumors | Pan-PI3K inhibitor | I | PX-866 | Completed | NCT0072658338 | |
| ZSTK474 |
Advanced solid malignancies |
Pan-PI3K inhibitor | I | ZSTK474 | Completed | NCT0168247343 |
|
Advanced solid malignancies |
Pan-PI3K inhibitor | I | ZSTK474 | Completed | NCT0128048744 | |
| GDC-0941 (Pictilisib) | Advanced solid tumors | Pan-PI3K inhibitor | I | GDC-0941 | Completed | Wagner et al.46 |
| Advanced or metastatic solid tumors | Pan-PI3K inhibitor | I | GDC-0941 | Completed | NCT0087610947 | |
| Advanced or metastatic solid tumors | MEK inhibitor + Pan-PI3K inhibitor | Ib | Cobimetinib + GDC-0941 | Active, not recruiting | NCT0099689248 | |
| Advanced or metastatic solid tumors | Pan-PI3K inhibitor + EGFR inhibitor | I | GDC-0941 + erlotinib | Active, not recruiting | NCT0097518249 | |
| Advanced solid tumors | PI3K inhibitor + MEK inhibitor | Ib | GDC-0941 + GDC-0973 | Completed | Shapiro et al.50 | |
|
BAY80-6946 (Copanlisib) |
Advanced solid malignancy |
pan-PI3K inhibitor + microtubule polymer stabilizer | I | BAY80-6946 + paclitaxel | Completed | NCT0141141051 |
|
Advanced solid malignancy |
pan-PI3K inhibitor + nucleic acid synthesis inhibitor | I |
BAY80-6946 + gemcitabine or cisplatin + gemcitabine |
Completed | NCT0146053752 | |
|
Advanced solid malignancy |
pan-PI3K inhibitor | I | BAY80-6946 | Completed | NCT0096261153 | |
| Compound name | Cancer type | Mechanism of action | Phase | Arm | Status | Reference |
| BYL719 (Alpelisib) | Gastrointestinal stromal tumor | PI3K inhibitor + TK inhibitor | Ib |
BYL719 + imatinib |
Ongoing | NCT0173596861 |
| Advanced solid tumors | PI3K inhibitor | I | BYL719 | Completed | Juric et al.62 | |
| Stomach and esophageal neoplasms | PI3K inhibitor + HSP90 inhibitor | Ib | BYL719 + AUY922 | Completed | NCT0161395063 | |
| Metastatic colorectal cancer | PI3K inhibitor + RAF inhibitor + EGFR inhibitor | Ib/II |
BYL719 + LGX818 + cetuximab |
Ongoing | NCT0171938064 | |
| Advanced solid tumors | PI3K inhibitor + mTOR inhibitor | Ib | BYL719 + everolimus or BYL719 + everolimus + exemestane | Ongoing | NCT0207793365 | |
| INK1117 (Serabelisib) |
Advanced gastric adenocarcinoma |
PI3K inhibitor | I | INK1117 | Recruiting | NCT0261573067 |
| WX-037 | Solid malignancy | PI3K inhibitor | I | WX-037 | Terminated | NCT0185935169 |
|
XL147 (Pilaralisib) |
Advanced or metastatic solid tumors | PI3K inhibitor + MEK inhibitor | I |
XL147 + primasertib |
Completed | NCT0135733070 |
| Advanced or metastatic solid tumors | PI3K inhibitor + EGFR inhibitor | I | XL147 + erlotinib | Completed | NCT0069264071 | |
| Advanced solid tumors | PI3K inhibitor | I | XL147 | Completed | Shapiro et al.72 | |
| BEZ235 (Dactolisib) | Solid tumor malignancy | mTOR/PI3K inhibitor + MEK1/2 inhibitor | I |
BEZ235 + binimetinib |
Ongoing, not recruiting |
NCT0133776584 |
|
Advanced solid malignancies |
mTOR/PI3K + m-TORC1 inhibitor |
I |
BEZ235 + everolimus |
Not provided |
NCT0150810485 | |
| Solid tumor malignancy | mTOR/PI3K inhibitor | I | BEZ235 |
Ongoing, not recruiting |
NCT0134349886 | |
|
Advanced solid malignancies |
mTOR/PI3K + m-TORC1 inhibitor |
Ib |
BEZ235 + everolimus |
Completed |
Wise-Draper et al.87 |
|
| XL765 (Voxtalisib) | Colorectal carcinoma | PI3K/mTOR inhibitor | I | XL765 | Completed |
Papadopoulos et al.89 |
| LY3023414 (Prexasertib) |
Advanced solid malignancy |
PI3K/mTOR inhibitor | I | LY3023414 | Recruiting | NCT0212414891 |
Mechanism of PI3K inhibitors
Based on the pharmacokinetic properties and selectivity for the ATPbinding cleft on PI3K, PI3K inhibitors can be divided into three classes: panclass I, isoformselective, and dual PI3K/mTOR inhibitors.7 These molecules mostly demonstrate cytostatic effects, resulting in desirable anti-cancer effects in vivo as well as G1 phase arrest in vitro.8 Figure 1 depicts the PI3K pharmacological inhibitors’ mechanism in GI cancer.
Fig. 1.
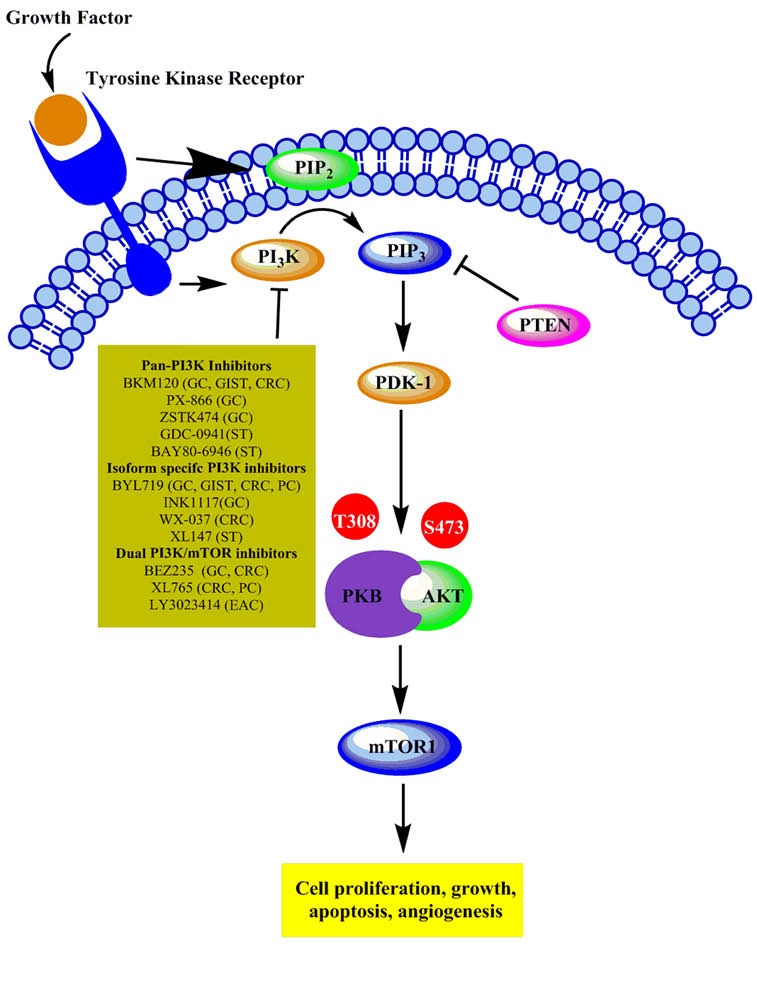
Schematic representation of PI3K pharmacological inhibitors in gastrointestinal cancer. GC: Gastric cancer, GIST: Gastrointestinal stromal tumors, CRC: Colorectal cancer, ST: Solid tumor, PC: Pancreatic cancer, EAC: Esophageal adenocarcinoma
Pan-Class I inhibitors
Panclass I inhibitors have inhibitory effects against each isoform of p110 (PIK3CA).9 Based on early phase trial data it is unlikely that pan-class I PI3K inhibitors have significant activity as single agents. Rational combinations with either cytotoxics or other molecularly targeted agents are therefore being explored and are likely to be more effective in GI cancer. Of the main limitations of pan-class I inhibitors are the wide range of side effects including hyperglycemia, rash, and fatigue, which potentially limit dose escalation leading to sub-optimal inhibition of PI3K. The first report of a molecular agent inhibiting PI3K was about quercetin, which was a nonspecific kinase inhibitor.10 Eventually, more specific panclass I inhibitors were identified, such as Wortmannin and a quercetin analogue, LY294002. Both LY294002 and Wortmannin showed strong PI3Kinhibitory properties. Nevertheless, they had considerable limitations for application in clinical trials.11,12 LY294002 exhibited toxicity, a short halflife, and nonspecific targeting in vivo,13,14 whereas the limitations of Wortmannin included a short halflife and lack of biological stability.15 Preclinical and clinical studies have extensively assessed pan-class I inhibitors, including NVP-BKM120, PX-866, ZSTK474, GDC-0941, and BAY80-6946.
a- NVP-BKM120 (buparlisib): NVPBKM120 (buparlisib) is a potent panclass I PI3K inhibitor with its activity in nano-molar ranges for all the isoforms of class I PI3K. Preclinical investigations have revealed its effectiveness in a diverse range of cancer cell lines, with increased sensitivity in tumors harboring PIK3CA mutations.16 Similar results were also observed in a panel of GI cancer cell lines.17 In the study, the synergistic efficacy of NVP-BKM120, demonstrated that dual PI3K and STAT3 blockade showed a synergism in Kirsten rat sarcoma virus (KRAS) mutant gastric cancer cells. The synergistic effect was not seen in KRAS wild-type cells. Together, these findings suggest that the dual inhibition of PI3K and STAT3 signaling may be an effective therapeutic strategy for KRAS mutant gastric cancer patients.18 Although preclinical studies on BKM120 in GI cancer are still ongoing, it has reached phase I and II clinical trials for other solid cancers such as gastric and colorectal carcinomas.19 A phase 1 clinical trial showed that BKM120 was a safe and well tolerated drug, with a favorable pharmacokinetic profile, evidence of target inhibition, and preliminary antitumor activity.20 In phase 1 trial, BKM120 broadly activated class I PI3K inhibitor and lack of anti-mTOR activity was tolerable at doses to inhibit enzyme activity in blood, skin, or tumor cells.21 Buparlisib in combination with imatinib has antitumor and antiapoptotic activity in imatinib-resistant KIT exon 11+17 mutant tumors,22 and is being evaluated as third-line therapy in a phase 1/2 dose-escalation and dose-expansion clinical trial.23 Two studies of BKM120 in patients with colorectal cancer are underway, one in combination with irinotecan and another in combination with panitumumab.24,25 In other studies, BKM120 was administrated with paclitaxel, docetaxel, LDE225 and everolimus to treat patients with advanced solid tumor or esophageal squamous cell carcinoma.26-32 Some studies have shown the safety, pharmacokinetics, and pharmacodynamics of BKM120 in patients with solid tumors.33-35
b- PX-866: PX866 is a semisynthetic panclass 1, Wortmannin analogue with inhibitory concentrations in nano-molar ranges and better efficacy and a safer pharmacokinetic profile than Wortmannin. Preclinical studies have shown its anticancer effect against several xenograft models of various cancers.36 PX866 has also recently come under limelight for a multicenter trial for advanced solid tumors including gastric tumors. Data from the trial show that PX866 can be administered with endurable toxicity for patients with advanced solid tumors.37,38
c- ZSTK474: ZSTK474, a panclass 1 PI3K inhibitor inhibits all the four isoforms of PI3K and exhibits antitumor activity in vivo against human tumor xenograft models.39-41 In vitro studies in gastric cancer cell lines suggest combination therapy of ZST474 and Insulin-like growth factor receptor (IGFR) inhibitors for treating IGFRpositive cancers to overcome any intrinsic resistance to inhibition of PI3K/Akt/mTOR signaling, since overexpression of IGFR correlated with increased tyrosine phosphorylation on insulin receptor substrate, leading to increased PI3K activation. Hence, combination therapy with both ZST474 and IGFR inhibitors on gastric cells with high IGFR expression exerted a superior therapeutic response.42 Studies have shown safety of ZSTK474 in patients with advanced solid malignancies.43,44
GDC-0941(pictilisib): Pictilisib (GDC-0941) is a potent inhibitor of PI3Kα/δ in cell-free assays, with modest selectivity against p110β and p110γ. In a study, the combination of NU6102 (CDK2 inhibitor) and pictilisib (pan-PI3K inhibitor) resulted in synergistic growth inhibition, and enhanced cytotoxicity in HT-29 cells in vitro and in vivo. These studies identified a novel series of mixed CDK2/PI3K inhibitors and demonstrated that dual targeting of CDK2 and PI3K can result in enhanced anti-tumor activity.45 A phase1 dose-escalation trial of pan-PI3K inhibitor, GDC-0941, is in progress.46 In some studies, GDC-0941was administrated with cobimetinib (Mitogen-activated protein kinase kinase [MEK] inhibitor) and erlotinib (Epidermal growth factor receptor [EGFR] inhibitor) in treating patients with advanced or metastatic solid tumors.47-49 In a recent phase1 trial, the combination of the PI3K and MEK inhibitors GDC-0941 and GDC-0973 was effective against advanced solid tumors at doses that were tolerable for patients.50
d- BAY80-6946 (copanlisib): Copanlisib (BAY 80-6946) is a potent pan-class I PI3K for PI3Kα/β/γ/δ in cell-free assays. In some studies, BAY80-6946 was administrated with paclitaxel and gemcitabine in treating patients with advanced solid tumor.51-53
Isoform specific PI3K inhibitors
PI3K isoform specific inhibitors were designed with an aim to provide comparable or superior efficacy than panclass I inhibitors. One potential advantage of isoform-specific antagonists over broad-spectrum inhibitors is that they can be matched more closely to their single target, yielding increased potency, fewer off-target effects, and improved tolerability. Specific inhibitors of p110α are expected to inhibit PI3K-AKT signaling in tumors with PIK3CA mutations and might also be effective against tumors that express activated Receptor tyrosine kinases (RTKs) or other oncogenes, such as KRAS. P110β plays a key role in PI3K signaling in some Phosphatase and tensin homolog (PTEN)-deficient tumors, so p110β-specific inhibitors might be effective in patients with these particular tumors. One limitation for the use of PI3K inhibitors is that they may fail to block AKT activation in tumors that have gene amplifications, AKT2 mutations, or the AKT1 E17K mutation. Consequently, isoform-specific PI3K inhibitors are currently studied for an enhanced toxicity profile and more complete target inhibition.54,55 As noted below, preclinical and clinical studies have extensively examined isoform-specific PI3K inhibitors such as BYL719, INK1117, WX-037, and XL147.
a- BYL719 (alpelisib): BYL719 (alpelisib) is an α-isoform specific PI3K inhibitor working at nano-molar concentrations with minimal activity against other PI3K isoforms.56 BYL719 exhibited its inhibitory effects in synergy with another inhibitor LJM716, a ligand dependent as well as independent HER3 inhibitor, in gastric cancer xenograft models.57 Interestingly, a combination study was done on Human epidermal growth factor receptor 2 (HER2) positive gastric cancer cell lines, suggesting the sensitivity of this drug towards HER amplifications. BYL719 also recently completed phase1b clinical trial for advanced stage gastric cancer in a combinational study with the HSP90 inhibitor AUY922 in patients whose tumors either harbour molecular alterations of PIK3CA or HER2 amplification.58 Another study showed pharmacological inhibition of PI3K pathway in tumor-bearing KitV558Δ/+ mice with the dual PI3K/mTOR inhibitor voxtalisib, the pan-PI3K inhibitor pilaralisib, and the PI3Kα inhibitor alpelisib each diminished tumor proliferation. Moreover, it has been shown that PI3K inhibition was effective against imatinib-resistant KitV558Δ;T669I/+ tumors.59 The highly selective PI3Kα inhibitor (Wild type and mutant forms) alpelisib (BYL719) showed strong single-agent activity in PIK3CA mutant tumor models and greater antitumor activity in Gastrointestinal stromal tumors (GIST)-specific models when combined with imatinib.60 The antitumor effect of the combination therapy was dependent on the tumor genotype, with imatinib-sensitive KIT exon 11 mutant tumors being the most responsive. A dose-escalation and dose-expansion, multinational, phase 1/2 clinical trial is currently underway for the combination treatment of alpelisib and imatinib as third-line treatment for patients with GIST.61 A phase 1 trial including patients with solid malignancies bearing PIK3CA mutations showed an acceptable side effect profile and efficacy that justified its further development in phase 2 trials.62 Also, a phase I study of isoform specific PI3K inhibitor (p110α) BYL719 and HSP90 inhibitor AUY922 in patients with gastric cancer is ongoing.63 Given the preclinical data suggesting the activation of PI3K as a mechanism of primary resistance to drugs acting on the MAPK pathway, a phase 1 trial of BYL719 in combination with cetuximab and LGX818, a selective protein kinase B-Raf inhibitor, is underway in patients with BRAF mutant metastatic colorectal cancer.64 A phase Ib study of safety and efficacy alpelisib with everolimus in patients with pancreatic tumors is ongoing.65
b- INK1117 (serabelisib, MLN-1117 or TAK-117): INK1117 is another novel, selective p110α inhibitor. It is particularly more effective and sensitive to tumors bearing PIK3CA mutations. With good oral bioavailability in preclinical xenograft studies, it has entered a phase I study for advanced solid tumors including gastric cancer to evaluate its safety, tolerability, pharmacokinetic, and pharmacodynamic properties.66,67
c- WX-037: A study evaluated the novel PI3K inhibitor WX-037 and the MEK inhibitor WX-554, as single agents and in combination, in colorectal carcinoma (HCT116 and HT29) cell lines and tumor xenograft-bearing mice. Pharmacokinetic analyses indicated that there was no interaction between the two drugs at low doses, but at higher doses, WX-037 might delay tumor uptake of WX-554. These studies showed that combined treatment with novel MEK inhibitor WX-554 and novel PI3K inhibitor WX-037 could induce synergistic growth inhibition in vitro, which translates into enhanced anti-tumor efficacy in vivo.68,69
d- XL147 (pilaralisib): Pilaralisib (XL147) is a selective and reversible class I PI3K inhibitor for PI3Kα/δ/γ in cell-free assays, less potent than PI3Kβ. In phase 1 trial, combination XL147 with primasertib (MEK inhibitor) and erlotinib (EGFR inhibitor) in patients with advanced solid tumors.70,71 In phase 1 trial, XL147 broadly active class I PI3K inhibitor that lack anti-mTOR activity, was tolerable at doses shown to inhibit enzyme activity in blood, skin, or tumor cells.72
Dual PI3K/mTOR inhibitors
PI3K/mTOR dual inhibitors inhibit PI3K and downstream mTOR kinase activity by binding to ATPbinding cleft of these enzymes. Regarding the single inhibitors, these drugs have the benefit of inhibiting mTORC1 and mTOCR2, as well as all the isoforms of PI3K. Evidence has suggested that mTORC1/S6K axis has a “twoedge sword”like function in activation of PI3K/mTOR pathway by promoting growth signals downstream of Akt serine/threonine kinase, as well as mediating a potent negative feedback loop that restrains signaling via insulin/IGFR and other RTKs. Dysregulation of this negative feedback loop has been reported to contribute towards resistance in cancers subjected to single inhibitors.73 As a result, the concern for the clinical application of dual inhibition is that it may result in unacceptable toxicity when utilizing doses needed for desirable target inhibition. The theoretical advantage of dual PI3K/m-TOR inhibitors is that they attempt to fully shut down the PI3K pathway, thereby preventing AKT activation, which is the result of the negative feedback of allosteric mTORC1 inhibitors, including everolimus. The structures of the catalytic domains of p110 subunits and mTOR are similar. Therefore, numerous PI3K inhibitors currently under development also inhibit mTOR complexes. These inhibitors were among the first to be developed, and it is anticipated that they will block PI3K-AKT-mTOR signaling in tumors having RTK activation, PIK3R1 mutations, PIK3CA mutations, or PTEN loss.74 Preclinical studies mentioned below have widely evaluated dual PI3K-mTOR inhibitors, including NVP-BEZ235, XL765, and LY3023414.
NVP-BEZ235 (dactolisib or BEZ235): NVPBEZ235 is a novel dual ATPcompetitive PI3K and mTOR inhibitor for p110α/β/γ/δ and mTOR kinase, with inhibitory doses at nano-molar ranges. It first entered phase trials for breast cancer.75 The effectiveness of BEZ235 has been investigated in both PIK3CA mutated and wild type cell lines in vitro and in xenograft models in vivo. The first group reporting an effect of BEZ235 on gastric xenografts showed reduced tumor growth for NCIN87 but not MKN45 or MKN28 xenografts. Interestingly, the reduction in tumor growth correlated with thymidine kinase expression and not PI3K/mTOR pathway inhibition.76 Another group demonstrated in vitro increased sensitivity of AGS, PIK3CA mutated cells than for NCIN87 and MKN45, wild type PIK3CA GC cells.17 Clinically, the response rate for BEZ235 was the highest for patients with PIK3CA mutations compared with those without this mutation.77 Zhang and colleagues investigated the effects of NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, alone and in combination with nanoparticle albumin-bound (nab)-paclitaxel in experimental gastric cancer. BEZ235 effectively inhibited cell proliferation in vitro and provided additive effects in combination with nab-paclitaxel. Net local tumor growth inhibition for the BEZ235, nab-paclitaxel and BEZ235 + nab-paclitaxel groups was 45.1, 77.9, and 97% compared with controls. The effects of treatment on intra tumoral proliferation and apoptosis corresponded with tumor growth inhibition data. Median animal survival was 26.5 days after BEZ235, 90.5 days after nab-paclitaxel and 97 days in the BEZ235+nab-paclitaxel combination treatment group. The findings suggested that BEZ235 exerted some antitumor effects against gastric cancer and enhanced the effects of nab-paclitaxel through inhibition of cell proliferation and modulation of PI3K/mTOR pathway.78 The study investigated in vitro and in vivo efficacy of NVP-BEZ235 in PIK3CA mutant and wild-type colorectal cancer (CRC). In vitro treatment of CRC cell lines with NVP-BEZ235 decreased cell viability. In vivo treatment of colonic tumor-bearing mice with NVP-BEZ235 resulted in transient PI3K inhibition. Longitudinal tumor surveillance by optical colonoscopy demonstrated a 97% increase in tumor size in control mice vs. a 43% decrease in treated mice. These studies provide the rationale for examining the efficacy of dual PI3K/mTOR inhibitor NVP-BEZ235 in treatment of PIK3CA wild-type CRC.79 In mice with xenograft tumors, NVP-BEZ235 inhibited the development of tumor vasculature by inhibiting PI3K and Akt but not mTORC1.80 In a study, it has been demonstrated that paclitaxel (PTX) together with BEZ235 exhibited a synergetic inhibitory effect on colon cancer cell growth. Furthermore, nano-emulsion (NE)-loaded PTX and BEZ235 were more effective than the free drug, and a combination treatment of both NE drugs increased the efficiency of the treatments. Combined treatment with NE-BEZ235 and NE-PTX could kill 50% of HCT-116 and HT-29 cells. The data indicated that the combination therapy of PTX with BEZ235 using NE delivery might hold promise for a more effective approach for colon cancer treatment.81 Yu and co-workers showed that NVP-BEZ235 and cis-diammine dichloroplatinum (DDP) had synergic effects in inhibiting cell proliferation and migration of HT-29 human colorectal adenocarcinoma cells. The expression of protein involved in apoptosis (cleaved caspase-3) was higher in drug combination group compared with NVP-BEZ235 single treatment group .82 In patients with RAS-mutant colorectal carcinoma, the combination of BEZ235 with selumetinib, a MEK inhibitor, produced disease stabilization in 70% of the cases. This is in concordance with the data suggesting that these drugs are cytostatic and highlights the prospect of being tested in combination with chemotherapy drugs.83 BEZ235 is currently being assessed in early clinical trials for safety, pharmacokinetics, and pharmacodynamics in solid tumors.84-87
a- XL765 (voxtalisib or SAR245409): XL765, a dual-target PI3K/mTOR inhibitor, inhibits cell growth and apoptosis in many more cell lines and at lower concentrations as compared with PI3K-selective inhibitors XL147 and PIK90. The effect can be recapitulated by using combinations of single-targeted compounds. XL765 significantly reduces phosphorylation of the mTOR targets S6, S6K, and 4EBP1, which is associated with greater apoptosis induction rather than to PI3K inhibition alone.88 In a phase 1 trial of XL765, disease stabilization was reported in seven (46%) patients with CRC. This high stabilization rate is difficult to interpret outside a randomized trial as it might represent patients with slow growing tumors. Further trials are needed to assess the activity of XL765 in patients with CRC.89
b- LY3023414 (prexasertib): LY3023414 is an oral ATP competitive inhibitor of the class I PI3K isoforms and mTOR. In a study done by Zaidi and colleagues, LY3023414 was used intraperitoneally for rats with esophageal adenocarcinoma (EAC) during 40 weeks. Magnetic resonance imaging (MRI), histology, immunohistochemistry, immunofluorescence, and western blot were used to determine clinical response, apoptosis, and proliferation, respectively. Results showed that LY3023414 was downregulated PI3K-α in the treatment group compared with the controls and this established the rational for clinical testin.90,91
Concluding Remarks
PI3K pathway is significantly implicated in GC carcinogenesis and is currently one of the most important areas in anticancer drug development. Figure 1 summarizes PI3K inhibitors in clinical trials for GC treatment. We suggest key issues for successful development of PI3K inhibitors in patients with GC: 1) Increasing the use of tumor genotype analysis in clinics, which helps to identify patients most likely to respond to drugs targeting PI3K pathway, 2) Development of rational combinations of PI3K inhibitor drugs in order to whether inhibitors of this pathway will be more effective than single agents, 3) Development of new PI3K inhibitor drugs and also more understanding of their pharmacodynamic to evaluate the magnitude of target inhibition required for efficacy, and 4) Further clinical trials on compounds acting on PI3K axis to evaluated optimal results in patients with heterogenous malignancy of gastrointestinal tract.
Please cite this paper as:
Hashemzadeh K, Jokar MH, Sedighi S, Moradzadeh M. Therapeutic Potency of PI3K Pharmacological Inhibitors of Gastrointestinal Cancer. Middle East J Dig Dis 2019:11:5-16. doi: 10.15171/mejdd.2018.122.
Footnotes
ETHICAL APPROVAL There is nothing to be declared.
CONFLICT OF INTEREST The author declares no conflict of interest related to this work.
References
- 1.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–9. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–75. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 6.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell cycle. 2004;3:1221–4. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 7.Martini M, Ciraolo E, Gulluni F, Hirsch E. Targeting PI3K in cancer: any good news? Front Oncol. 2013;3:108. doi: 10.3389/fonc.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith A, Blois J, Yuan H, Aikawa E, Ellson C, Figueiredo JL. et al. The antiproliferative cytostatic effects of a self-activating viridin prodrug. Mol Cancer Ther. 2009;8:1666–75. doi: 10.1158/1535-7163.MCT-08-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 10.Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992;186:624–31. doi: 10.1016/0006-291X(92)90792-J. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T. et al. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992;267:2157–63. [PubMed] [Google Scholar]
- 12.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 13.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H. et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF. et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ihle NT, Paine-Murrieta G, Berggren MI, Baker A, Tate WR, Wipf P. et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non–small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–57. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maira S-M, Pecchi S, Huang A, Burger M, Knapp M, Sterker D. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–28. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 17.Mueller A, Bachmann E, Linnig M, Khillimberger K, Schimanski CC, Galle PR. et al. Selective PI3K inhibition by BKM120 and BEZ235 alone or in combination with chemotherapy in wild-type and mutated human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 2012;69:1601–15. doi: 10.1007/s00280-012-1869-z. [DOI] [PubMed] [Google Scholar]
- 18.Park E, Park J, Han SW, Im SA, Kim TY, Oh DY. et al. NVP-BKM120, a novel PI3K inhibitor, shows synergism with a STAT3 inhibitor in human gastric cancer cells harboring KRAS mutations. Int J Oncol. 2012;40:1259–66. doi: 10.3892/ijo.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJ, Kazic-Legueux M. et al. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Mol Cancer Ther. 2012;11:1747–57. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
- 20.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D. et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, De Jonge MJ, Rodon J, Burris III HA, Birle DC, De Buck SS. et al. A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28:3003. doi: 10.1200/jco.2010.28.15_suppl.3003. [DOI] [PubMed] [Google Scholar]
- 22.Van Looy T, Wozniak A, Floris G, Sciot R, Li H, Wellens J. et al. Phosphoinositide 3-kinase inhibitors combined with imatinib in patient-derived xenograft models of gastrointestinal stromal tumors: rationale and efficacy. Clin Cancer Res. 2014;20:6071–82. doi: 10.1158/1078-0432.CCR-14-1823. [DOI] [PubMed] [Google Scholar]
- 23. Novartis Pharm. A Dose-finding Study of a Combination of Imatinib and BKM120 in the Treatment of 3rd Line GIST Patients [NCT01468688], 2011. https://clinicaltrials.gov/ct2/show/NCT01468688?cond=NCT01468688&rank=1.
- 24. Baranda J. A Trial of Irinotecan and BKM120 in Previously Treated Advanced Colorectal Cancer [NCT01304602], 2011. https://clinicaltrials.gov/ct2/show/NCT01304602?cond=NCT01304602&rank=1.
- 25. Jonker D. P13Kinase Inhibitor BKM120 in Combination With Panitumumab in Patients With Metastatic or Advanced RAS-Wild Type Colorectal Cancer [NCT01591421], 2012. https://clinicaltrials.gov/ct2/show/NCT01591421?cond=NCT01591421&rank=1.
- 26.Smyth LM, Monson KR, Jhaveri K, Drilon A, Li BT, Abida W. et al. A phase 1b dose expansion study of the pan-class I PI3K inhibitor buparlisib (BKM120) plus carboplatin and paclitaxel in PTEN deficient tumors and with dose intensified carboplatin and paclitaxel. Invest New Drugs. 2017;35:742–750. doi: 10.1007/s10637-017-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adjei A. PI3K Inhibitor BKM120 and Docetaxel in Treating Patients with Advanced Solid Tumor That is Locally Advanced, Cannot Be Removed by Surgery, or Metastatic [NCT01540253], 2012. https://clinicaltrials.gov/ct2/show/NCT01540253?cond=NCT01540253&rank=1.
- 28. Novartis Pharm. A Trial of Oral BEZ235 and BKM120 in Combination with Paclitaxel with or Without Trastuzumab [NCT01285466], 2011. https://clinicaltrials.gov/ct2/show/NCT01285466?cond=NCT01285466&rank=1.
- 29. Novartis Pharm. Phase Ib, Dose Escalation Study of Oral LDE225 in Combination With BKM120 in Patients With Advanced Solid Tumors [NCT01576666], 2012. https://clinicaltrials.gov/ct2/show/NCT01576666?cond=NCT01576666&rank=1.
- 30. Owonikoko T. A Phase I Study of BKM120 and Everolimus in Advanced Solid Malignancies [NCT01470209], 2011. https://clinicaltrials.gov/ct2/show/NCT01470209?cond=NCT01470209&rank=1.
- 31. Novartis Pharm. BKM120 for Patients With PI3K-activated Tumors (SIGNATURE) [NCT01833169], 2013. https://clinicaltrials.gov/ct2/show/NCT01833169?cond=NCT01833169&rank=1.
- 32.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D. et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 33. Array BioPharma. Safety, Pharmacokinetics and Pharmacodynamics of BKM120 Plus MEK162 in Selected Advanced Solid Tumor Patients [NCT01363232], 2011. https://clinicaltrials.gov/ct2/show/NCT01363232?cond=NCT01363232&rank=1.
- 34. Novartis Pharm. A Study to Investigate Safety, Pharmacokinetics (PK) and Pharmacodynamics (PD) of BKM120 plus GSK1120212 in Selected Advanced Solid Tumor Patients [NCT01155453], 2010. https://clinicaltrials.gov/ct2/show/NCT01155453?cond=NCT01155453&rank=1.
- 35. Novartis Pharm. Safety of BKM120 Monotherapy in Advanced Solid Tumor Patients [NCT01068483], 2010. https://clinicaltrials.gov/ct2/show/NCT01068483?cond=NCT01068483&rank=1.
- 36.Ihle NT, Williams R, Chow S, Chew W, Berggren MI, Paine-Murrieta G. et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–72. [PubMed] [Google Scholar]
- 37.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O’Bryant CL. et al. A multicenter phase 1 trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–82. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 38. Cascadian Therapeutics Inc. A Phase I Trial of Oral PX-866 (a PI-3K Inhibitor) in Patients with Advanced Solid Tumors [NCT00726583], 2008. https://clinicaltrials.gov/ct2/show/NCT00726583?cond=NCT00726583&rank=1.
- 39.Kong D, Yamori T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci. 2007;98:1638–42. doi: 10.1111/j.1349-7006.2007.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong Dx, Yamori T. ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor identified using the JFCR39 drug discovery system. Acta pharmacol Sin. 2010;31:1189–97. doi: 10.1038/aps.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaguchi S, Fukui Y, Koshimizu I, Yoshimi H, Matsuno T, Gouda H. et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98:545–56. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 42.Isoyama S, Kajiwara G, Tamaki N, Okamura M, Yoshimi H, Nakamura N. et al. Basal expression of insulin-like growth factor 1 receptor determines intrinsic resistance of cancer cells to a phosphatidylinositol 3-kinase inhibitor ZSTK474. Cancer Sci. 2015;106:171–8. doi: 10.1111/cas.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doi T. A Study of ZSTK474 in Japanese Patients with Advanced Solid Malignancies [NCT01682473], 2012. https://clinicaltrials.gov/ct2/show/NCT01682473?cond=NCT01682473&rank=1.
- 44. Lockhart C. A Safety Study of Oral ZSTK474 in Patients with Cancer [NCT01280487], 2011. https://clinicaltrials.gov/ct2/show/NCT01280487?cond=NCT01280487&rank=1.
- 45.Beale G, Haagensen EJ, Thomas HD, Wang LZ, Revill CH, Payne SL. et al. Combined PI3K and CDK2 inhibition induces cell death and enhances in vivo antitumour activity in colorectal cancer. Br J Cancer. 2016;115:682–90. doi: 10.1038/bjc.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Von Hoff DH, LoRusso PM, Tibes R, Shapiro G, Weiss J, Ware JA. et al. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol. 2009;27(15_suppl):3501. doi: 10.1200/jco.2010.28.15_suppl.2541. [DOI] [Google Scholar]
- 47. Hsu J. A Study of GDC-0941 in Participants With Locally Advanced or Metastatic Solid Tumors for Which Standard Therapy Either Does Not Exist or Has Proven Ineffective or Intolerable [NCT00876109], 2009. https://clinicaltrials.gov/ct2/show/NCT00876109?cond=NCT00876109&rank=1.
- 48. Chan I. Safety, Tolerability and Pharmacokinetics of Cobimetinib in Combination with Pictilisib in Patients with Locally Advanced or Metastatic Solid Tumors [NCT00996892], 2009. https://clinicaltrials.gov/ct2/show/NCT00996892?cond=NCT00996892&rank=1.
- 49. Genentech, Inc. A Study of the Safety and Pharmacology of GDC-0941 in Combination with Erlotinib in Patients with Advanced Solid Tumors [NCT00975182], 2009. https://clinicaltrials.gov/ct2/show/NCT00975182?cond=NCT00975182&rank=1.
- 50.Shapiro G, LoRusso P, Kwak EL, Cleary JM, Musib L, Jones C. et al. Clinical combination of the MEK inhibitor GDC-0973 and the PI3K inhibitor GDC-0941: A first-in-human phase Ib study testing daily and intermittent dosing schedules in patients with advanced solid tumors. J Clin Oncol. 2011;29(15_suppl):3005. doi: 10.1200/jco.2011.29.15_suppl.3005. [DOI] [Google Scholar]
- 51. Bayer Inc. Phase I Study of PI3(Phosphoinositol 3)-Kinase Inhibitor BAY80-6946 With Paclitaxel in Patients With Advanced Cancer [NCT01411410],2011.https://clinicaltrials.gov/ct2/show/NCT01411410?cond=NCT01411410&rank=1.
- 52. Bayer Inc. Phase 1 Study of PI3 (Phosphatidylinositol-3)-Kinase Inhibitor Copanlisib With Gemcitabine or Cisplatin Plus Gemcitabine in Patients With Advanced Cancer [NCT01460537], 2011. https://clinicaltrials.gov/ct2/show/NCT01460537?cond=NCT01460537&rank=1.
- 53.Patnaik A, Appleman LJ, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW. et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann Oncol. 2016;27:1928–40. doi: 10.1093/annonc/mdw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edelman G, Bedell C, Shapiro G, Pandya SS, Kwak EL, Scheffold C. et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients with advanced malignancies. J Clin Oncol. 2010;28:A3004. doi: 10.1200/jco.2010.28.15_suppl.3004. [DOI] [Google Scholar]
- 55.Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V. et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan–class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M. et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett. 2013;23:3741–8. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Garrett JT, Sutton CR, Kurupi R, Bialucha CU, Ettenberg SA, Collins SD. et al. Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers. Cancer Res. 2013;73:6013–23. doi: 10.1158/0008-5472.CAN-13-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wainberg ZA, Anghel A, Rogers AM, Desai AJ, Kalous O, Conklin D. et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol Cancer Ther. 2013;12:509–19. doi: 10.1158/1535-7163.MCT-12-0507. [DOI] [PubMed] [Google Scholar]
- 59.Bosbach B, Rossi F, Yozgat Y, Loo J, Zhang JQ, Berrozpe G. et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc Natl Acad Sci U S A. 2017;114:E8448–57. doi: 10.1073/pnas.1711449114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M. et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther. 2014;13:1117–29. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 61. Novartis Pharm. A Dose-finding Study of a Combination of Imatinib and BYL719 in the Treatment of 3rd Line GIST Patients [NCT01735968], 2012. https://clinicaltrials.gov/ct2/show/NCT01735968?cond=NCT01735968&rank=1.
- 62. Novartis Pharm. A Phase Ib/II Study of BYL719 and Cetuximab in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma [NCT01602315], 2012. https://clinicaltrials.gov/ct2/show/NCT01602315?term=Phase+I+study+of+BYL719%2C+an+alphaspecific+PI3K+inhibitor%2C+in+patients+with&rank=1.
- 63. Novartis Pharm. PI3K Inhibitor BYL719 in Combination with the HSP90 Inhibitor AUY922 in Patients with Advanced or Metastatic Gastric Cancer [NCT01613950], 2012. https://clinicaltrials.gov/ct2/show/NCT01613950?cond=NCT01613950&rank=1.
- 64. BioPharma. Study of LGX818 and Cetuximab or LGX818, BYL719, and Cetuximab in BRAF Mutant Metastatic Colorectal Cancer [NCT01719380], 2012. https://clinicaltrials.gov/ct2/show/NCT01719380?cond=NCT01719380&rank=1.
- 65. Novartis Pharmaceuticals. Study of Safety and Efficacy of Alpelisib with Everolimus or Alpelisib With Everolimus and Exemestane in Advanced Breast Cancer Patients, Renal Cell Cancer and Pancreatic Tumors [NCT02077933],2014.https://clinicaltrials.gov/ct2/show/NCT02077933?cond=NCT02077933&rank=1.
- 66.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation, and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rha SY. PI3Kβ Selective Inhibitor With paclitaxel, Advanced Gastric Adenocarcinoma [NCT02615730], 2015. https://clinicaltrials.gov/ct2/show/NCT02615730?cond=NCT02615730&rank=1.
- 68.Haagensen EJ, Thomas HD, Schmalix WA, Payne AC, Kevorkian L, Allen RA. et al. Enhanced anti-tumour activity of the combination of the novel MEK inhibitor WX-554 and the novel PI3K inhibitor WX-037. Cancer Chemother Pharmacol. 2016;78:1269–81. doi: 10.1007/s00280-016-3186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Banerji U. Phase I Study of WX-037 Alone and in Combination with WX-554 in Solid Tumors [NCT01859351], 2013. https://clinicaltrials.gov/ct2/show/NCT01859351?cond=NCT01859351&rank=1.
- 70. Sanofi Company. Oral SAR245408 (XL147) and Oral MSC1936369B in Patients with Locally Advanced or Metastatic Solid Tumors [NCT01357330],2011.https://clinicaltrials.gov/ct2/show/NCT01357330?cond=NCT01357330&rank=1.
- 71.Soria JC, LoRusso P, Bahleda R, Lager J, Liu L, Jiang J. et al. Phase I dose-escalation study of pilaralisib (SAR245408, XL147), a pan-class I PI3K inhibitor, in combination with erlotinib in patients with solid tumors. Oncologist. 2015;20:245–6. doi: 10.1634/theoncologist.2014-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shapiro G, Kwak E, Baselga J, Rodon J, Scheffold C, Laird A. et al. Phase I dose-escalation study of XL147, a PI3K inhibitor administered orally to patients with solid tumors. J Clin Oncol. 2009;27(15 suppl):3500. [Google Scholar]
- 73.Burris HA. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013;71:829–42. doi: 10.1007/s00280-012-2043-3. [DOI] [PubMed] [Google Scholar]
- 74.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuger S, Cörek E, Polat B, Kämmerer U, Flentje M, Djuzenova CS. Novel PI3K and mTOR inhibitor NVP-BEZ235 radiosensitizes breast cancer cell lines under normoxic and hypoxic conditions. Breast Cancer (Auckl) 2014;8:39–49. doi: 10.4137/BCBCR.S13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuereder T, Wanek T, Pflegerl P, Jaeger-Lansky A, Hoeflmayer D, Strommer S. et al. Gastric cancer growth control by BEZ235 in vivo does not correlate with PI3K/mTOR target inhibition but with [18F] FLT uptake. Clin Cancer Res. 2011;17:5322–32. doi: 10.1158/1078-0432.CCR-10-1659. [DOI] [PubMed] [Google Scholar]
- 77.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS. et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitor. Mol Cancer Ther. 2011;10:558–65. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C-Η, Awasthi N, Schwarz MA, Schwarz RE. The dual PI3K/mTOR inhibitor NVP-BEZ235 enhances nab-paclitaxel antitumor response in experimental gastric cancer. Int J Oncol. 2013;43:1627–35. doi: 10.3892/ijo.2013.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roper J, Richardson MP, Wang WV, Richard LG, Chen W, Coffee EM. et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model of PIK3CA wild-type colorectal cancer. PloS one. 2011;6:e25132. doi: 10.1371/journal.pone.0025132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnell CR, Stauffer F, Allegrini PR, O’Reilly T, McSheehy PM, Dartois C. et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 81.Zou H, Li L, Carcedo IG, Xu ZP, Monteiro M, Gu W. Synergistic inhibition of colon cancer cell growth with nanoemulsion-loaded paclitaxel and PI3K/mTOR dual inhibitor BEZ235 through apoptosis. Int J Nanomedicine. 2016;11:1947–58. doi: 10.2147/IJN.S100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu Y, Yu X, Ma J, Tong Y, Yao J. Effects of NVP-BEZ235 on the proliferation, migration, apoptosis and autophagy in HT-29 human colorectal adenocarcinoma cells. Int J Oncol. 2016;49:285–93. doi: 10.3892/ijo.2016.3507. [DOI] [PubMed] [Google Scholar]
- 83.Migliardi G, Sassi F, Torti D, Galimi F, Zanella ER, Buscarino M. et al. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clin Cancer Res. 2012;18:2515–25. doi: 10.1158/1078-0432.CCR-11-2683. [DOI] [PubMed] [Google Scholar]
- 84. BioPharma Company. Safety, pharmacokinetics and Pharmacodynamics of BEZ235 plus MEK162 in Selected Advanced Solid Tumor Patients [NCT01337765], 2011. https://clinicaltrials.gov/ct2/show/NCT01337765?cond=NCT01337765&rank=1.
- 85.Wise-Draper TM, Moorthy G, Salkeni MA, Karim NA, Thomas HE, Mercer CA. et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target Oncol. 2017;12:323–32. doi: 10.1007/s11523-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bendell JC. Study of PI3 Kinase/mTOR Inhibitor BEZ235 Twice Daily for Advanced Solid Tumors [NCT01343498], 2011. https://clinicaltrials.gov/ct2/show/NCT01343498?cond=NCT01343498&rank=1.
- 87.Wise-Draper TM, Moorthy G, Salkeni MA Karim NA, Thomas HE, Mercer CA. et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target Oncol. 2017;12:323–32. doi: 10.1007/s11523-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K. et al. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med (Berl) 2011;89:877–89. doi: 10.1007/s00109-011-0774-y. [DOI] [PubMed] [Google Scholar]
- 89.Papadopoulos K, Tabernero J, Markman B, Patnaik A, Tolcher AW, Baselga J. et al. Phase I safety, pharmacokinetic and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2445–56. doi: 10.1158/1078-0432.CCR-13-2403. [DOI] [PubMed] [Google Scholar]
- 90.Zaidi AH, Kosovec JE, Matsui D, Omstead AN, Raj M, Rao RR. et al. PI3K/mTOR dual inhibitor, LY3023414, demonstrates potent antitumor efficacy against esophageal adenocarcinoma in a rat model. Ann Surg. 2017;266:91–8. doi: 10.1097/SLA.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 91. Patel MR. A Study of prexasertib (LY2606368) With Chemotherapy or Targeted Agents in Participants with Advanced Cancer [NCT02124148], 2014.https://clinicaltrials.gov/ct2/show/NCT02124148?cond=A+Study+of+Prexasertib+%28LY2606368%29+With+Chemotherapy+or+Targeted+Agents+in+Participants+With+Advanced+Cancer&rank=1.


