Abstract
Sugar-sweetened beverages (SSBs) increase risk of cardiometabolic disease. Young people consume the largest amounts of SSBs and have experienced the greatest relative gains in obesity in the past several decades. There is evidence of addictive properties of both caffeine and sugar, the primary ingredients in SSBs, but little research into such properties of SSBs in naturally occurring consumption patterns. Thus, in this exploratory study, we sought to examine potentially addictive properties of SSBs during a 3-day SSB cessation intervention in overweight and obese adolescents who typically consume ≥3 SSBs daily. Participants (n=25) were aged 13-18 years, mostly female (72%), and African American (56%) or Hispanic (16%) with a BMI≥95th %tile (76%). Withdrawal symptoms and SSB craving were assessed approximately 1-week apart, during both regular SSB consumption and a 3-day period of SSB cessation in which participants were instructed to drink only plain milk and water. During SSB cessation, adolescents reported increased SSB cravings and headache and decreased motivation, contentment, ability to concentrate, and overall well-being (uncorrected Ps<0.05). After controlling the false discovery rate, changes in motivation, craving, and well-being remained significant (corrected Ps<0.05). Using 24-hr recalls and drink journals, participants reported lower total daily consumption of sugar (−80 g) and added sugar (−16 g) (Ps<0.001) during cessation. This study provides preliminary evidence of withdrawal symptoms and increased SSB cravings during cessation in a diverse population of overweight or obese adolescents.
Keywords: obesity, sugar-sweetened beverages, addiction, withdrawal, craving, adolescence
Introduction
Reducing consumption of sugar-sweetened beverages (SSBs) has become a global public health priority (Lobstein, 2017). This is due to strong evidence that SSB consumption increases risk of obesity and cardiometabolic disease (Hu, 2013; Malik, Popkin, Bray, Despres, & Hu, 2010) and the fact that SSBs are the largest contributor to added sugar in the diet across multiple countries (Block, 2004; Brisbois, Marsden, Anderson, & Sievenpiper, 2014; Reedy & Krebs-Smith, 2010; Sanchez-Pimienta, Batis, Lutter, & Rivera, 2016). Excessive SSB consumption is a particular concern among adolescents, who consume more SSBs than any other age group in the U.S. (Kit, Fakhouri, Park, Nielsen, & Ogden, 2013). Adolescents also experienced the greatest relative increase in obesity prevalence, which quadrupled over the past several decades (U.S. Department of Health and Human Services, 2014).
Compared to other foods, SSBs have unique attributes that may promote overconsumption of calories. First, compared to calories from solid food, liquid calories appear to be less satiating and incompletely compensated for by eating fewer calories later in the day (Almiron-Roig et al., 2013; Mattes, 1996). Second, SSBs contain primary ingredients—caffeine and sugar—with evidence of addictive potential. Caffeine withdrawal is an established disorder recognized by the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) and the International Statistical Classification of Diseases and Related Health Problems (ICD-10) (American Psychiatric Association, 2013; Meredith, Juliano, Hughes, & Griffiths, 2013; World Health Organization, 1992). The continued use of caffeine despite harm (e.g., insomnia, hypertension) has been documented, and caffeine dependence syndrome is recognized by ICD-10 (Meredith et al., 2013; World Health Organization, 1992).
In contrast, research on the addictive potential of sugar is a relatively new but burgeoning area. The majority of such studies have used animal models, which reveal parallels between added sugars and substances of abuse in bingeing, craving, tolerance, and withdrawal (DiNicolantonio, O'Keefe, & Wilson, 2017). Also, human neuroimaging studies show that high sugar intake activates similar neural circuitry and reward systems as substances of abuse (Smith & Robbins, 2013). Although there is an evolutionary rationale for liking sweetness to encourage consumption of nutrient- and energy-containing foods (e.g., mother’s milk or fruits), added sugars are distinct in that they are extracted, concentrated, and separated from the nutrients in their original forms (e.g., beets or corn). This extraction and concentration process is akin to that of addictive substances like opium from poppies or ethanol from fruit (Davis, 2014).
Adolescence is a particularly susceptible period for addiction, when still-developing brains are highly sensitive to substances and when risk-taking is more likely due to a faster developing limbic system (which supports emotion) than prefrontal cortex (site of executive control) (IOM (Institute of Medicine) and NRC (National Research Council), 2011). Although still minors, the transition to the teenage years is marked by greater autonomy in food purchasing and more frequent exposure to advertising for SSBs (Powell, Szczypka, & Chaloupka, 2010). In 2009, carbonated beverages were the food category with the highest marketing expenditures for teens, totaling nearly $400 million (Federal Trade Commission, 2012).
Although there is evidence of addictive potential of ingredients in SSBs, there is a dearth of research on such properties of SSBs themselves, particularly among adolescents. However, the five-fold increase in SSB consumption since the 1950’s (Economic Research Service, 2017) coupled with still high levels of consumption (Rosinger, Herrick, Gahche, & Park, 2017) despite increased publicity of the associated health consequences, is consistent with the “use despite harm” manifestation of addiction. Given the heavy marketing, consumption, and health consequences of SSBs among youth, determining the extent to which SSBs exhibit addictive properties could have important regulatory, communications, and obesity treatment implications. Thus, in this exploratory study, we sought to examine the extent to which SSBs exhibit potentially addictive properties, namely symptoms of withdrawal and craving, among overweight and obese adolescents who consume high amounts of SSBs.
Methods
Study Design
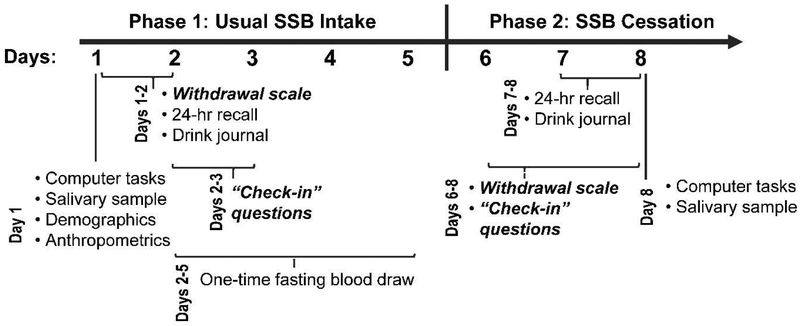
We employed a single arm intervention trial in which pre-post measurements were taken approximately 1-week apart. During the first 5 days, participants were instructed to continue their usual beverage consumption, followed by a 3-day period of SSB cessation (Figure 1). A 3-day period was selected to overlap with peak severity for symptoms of caffeine, alcohol, and nicotine withdrawal (Hughes, 2007; Juliano & Griffiths, 2004; Kosten & O'Connor, 2003; Shiffman et al., 2006). This study was approved by the institutional review boards at the UC San Francisco, UC Berkeley, and Children’s Hospital and Research Center Oakland.
Figure 1.
Study timeline
Participants and Recruitment
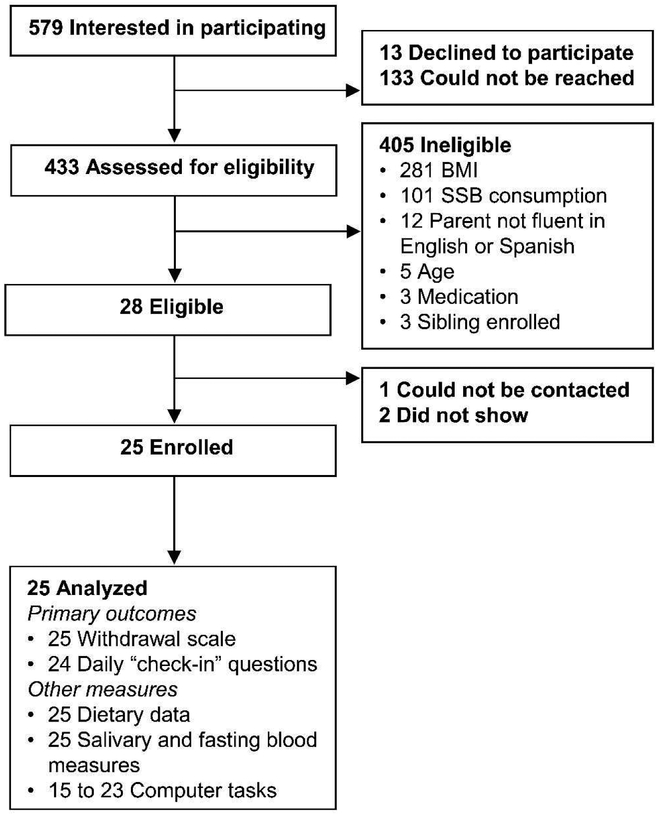
Eligible participants were aged 13-18 years who consumed ≥3 SSBs daily, had a BMI≥85th percentile for age and sex, spoke English, and had a parent/guardian who spoke English or Spanish. Eligibility criteria included BMI≥85th percentile due to evidence that those with high BMIs may be more susceptible than their normal BMI peers to the potentially addictive properties of foods/beverages (Epel et al., 2014; Schulte & Gearhardt, 2017). Teens were ineligible if they were pregnant, nursing, or undergoing pharmacological treatment for mental health. The study was advertised through parent listservs and by flyers at local hospitals, clinics, health fairs, and high schools. Participants were screened for eligibility and recruited over the phone. Figure 2 shows participant flow. Of 28 eligible participants identified, 25 enrolled in the study. For all participants, we obtained assent and informed parental consent.
Figure 2.
Participant flow
Intervention
The purpose of the study was described to participants as exploring “how soda affects teenagers’ health” and learning “how teenagers react when they stop drinking sugary drinks.” Participants were instructed to drink their usual beverages over a 5-day period (Phase 1: Usual SSB intake) and then to drink only plain water or plain milk over the next 3-day period (Phase 2: SSB cessation). Participants received these instructions verbally, in writing, and graphically through a calendar. Researchers reminded participants of these instructions during data collection visits at a local children’s hospital. On the last day of usual intake and the first day of cessation (days 5 and 6), a researcher called and/or texted participants to remind them to drink only plain milk and water on days 6-8. Participants were reimbursed for travel and received up to $160 for participation.
Measures
The primary outcomes were symptoms of withdrawal, measured by (1) a modified scale of mood, behavior, and physical symptoms (Griffiths et al., 1990) for identifying symptoms of caffeine withdrawal, and (2) dimensions of mood, including SSB craving, assessed five times per day via check-in questions. We focused on withdrawal and craving, common manifestations of addiction / substance use disorders, because (1) withdrawal perpetuates continued use of a substance to alleviate symptoms, and (2) craving predicts frequency of substance use and relapse (American Psychiatric Association, 2013; D. H. Epstein, Marrone, Heishman, Schmittner, & Preston, 2010; Oslin, Cary, Slaymaker, Colleran, & Blow, 2009). Figure 1 shows the timing of all measures. Although we drew upon an assessment tool for caffeine withdrawal and craving, many of these symptoms may also occur in response to cessation from high sugar intake, as evidenced by animal models (DiNicolantonio et al., 2017).
We used 11 items sensitive to caffeine cessation from a scale by Griffiths and colleagues (1990). This scale asked, “How do you feel right now?” for each dimension: having a headache, feeling energetic, awake/alert, tired, good overall, drowsy/sleepy, content/satisfied, motivated to do work, outgoing, self-confident, and able to concentrate. Wording was modified for some items to use terminology more familiar to adolescents (i.e., “outgoing” instead of “social disposition,” “tired” instead of “lethargy/fatigue,” “feel good overall” instead of “well-being,” and “awake/alert” instead of “alert/attentive”). We used a 5- (instead of 4-) point response scale to capture greater variability: 0 (not at all), 1 (a little), 2 (somewhat), 3 (moderately), and 4 (very much). Participants completed the scale on the first two days of usual SSB intake and one-week later, during each cessation day. We calculated total scale score by reverse coding positive items (e.g., “I feel good overall”) before summing, such that higher scores indicated greater severity of withdrawal. Responses on each day and responses averaged across days of the cessation phase were compared with responses averaged across days of the usual intake phase.
To assess craving and the times at which certain withdrawal symptoms may be most severe, participants completed four check-in questions five times daily, either by mobile text message or a check-in diary. These questions assessed SSB cravings as well as perceptions of overall well-being, tiredness, and irritability. Griffiths et al. (1990) identified craving and irritability as also sensitive to caffeine withdrawal(Griffiths et al., 1990). Check-in questions were completed at 10am, 12pm, 4pm, 6pm, and 9pm on days 2-3 of the usual SSB phase and on each day of SSB cessation. Each of the following questions used a 10-point response scale: How do you feel? 1 (really bad) – 10 (really good); How much do you want [favorite SSB] right now? 1 (I don’t want at all) – (I really want); How tired are you? 1 (not tired at all) – 10 (really tired); and How irritable are you? 1 (not at all irritable) – 10 (really irritable). During the usual SSB phase, responses at each time point were averaged across days (e.g., response at 10am on day 2 was averaged with the response at 10am on day 3) and served as the comparison for each day of SSB cessation responses.
Additional outcomes assessed (see Figure 1 for timing) included dietary intake, salivary caffeine, and secondary outcomes of attention, impulsivity, and the relative reinforcing value of SSBs. Through the Automated Self-Administered 24-Hour (ASA24®) dietary assessment tool, participants recalled the types and amounts of foods and beverages consumed the previous day. We additionally assessed beverage intake using a self-administered daily beverage recall (i.e., “drink journal”), which probed for how often and in what quantities the following were consumed: plain milk (whole, 2%, 1%, and skim); flavored milk; plain non-dairy milk; flavored non-dairy milk; horchata; smoothies; 100% fruit juice; tamarindo, jamaica, or aguas frescas; fruit-flavored drinks; sports drinks; energy drinks; tap water; bottled water; and caffeinated and uncaffeinated regular soda, diet soda, unsweetened coffee or tea, and coffee or tea with added sugar. Drink journal beverages that were not reported on ASA24, or that were reported in larger quantities, were coded for nutrients using the USDA’s Food and Nutrient Database for Dietary Studies and the Food Patterns Equivalents Database (U.S. Department of Agriculture, 2016). The corresponding differences in nutrients and quantities were added to those from ASA24 and averaged across days for each study phase.
Saliva samples were used to assess caffeine intake and provided an indicator of adherence among caffeinated SSB consumers, which was communicated to participants. Participants provided 5mL samples after abstaining from food and drink for 30 minutes, rinsing their mouth, and removing lipstick or balm. Samples were stored at −20°C and analyzed by the UCSF Clinical Pharmacology Laboratory (San Francisco, CA).
Computer tasks assessed attention via the Stroop tasks for color and word naming (MacLeod, 1991; Stroop, 1935) (Inquisit 3, Millisecond Software, LLC); impulsivity via delay and probabilistic discounting (Millisecond Software, LLC) (Mazur, 1987; Rachlin, Raineri, & Cross, 1991); and the relative reinforcing value of SSBs (RRVSSBs) via the Behavioral Choice Computer Task (L. H. Epstein, Carr, Lin, & Fletcher, 2011; Goldfield, Epstein, Davidson, & Saad, 2005).
Other measures taken at the start of the study included age, sex, race/ethnicity, anthropometrics, parental education, living arrangement, and fasting serum glucose, insulin, hemoglobin A1c, lipids, adiponectin, and leptin. The fasting blood draw occurred during scheduled morning appointments on days 2-5 of the usual intake phase.
Analytic sample
All 25 participants provided responses to the 11-item withdrawal scale and dietary data. Three had missing values for one withdrawal scale item, which was imputed from the mean of the individual’s other scale items. Twenty-four participants provided responses to check-in questions during both phases, which was 83% non-missing at each time point through 12 pm on day 8 (last day of cessation). Thereafter, check-in data had missingness ≥40% and thus were not analyzed. Computer task data were available from 23 participants, but for delay/probability discounting, only 15 participants had non-outlying data that could be fit using the hyperbolic equations (Mazur, 1987; Rachlin et al., 1991). Complete case analyses were performed on these secondary outcomes.
Analysis
Internal consistency of the withdrawal scale was assessed by Cronbach’s alpha. Change in individual item scores and total scale scores from the withdrawal scale were examined using paired t-tests. Daily check-in responses at each time of day during the cessation phase were compared to responses at the same times during the usual intake phase using post hoc contrasts after fitting linear regression models with indicators for each time point and robust standard errors (Huber-White sandwich). The remaining outcomes were compared between phases using paired t-tests or Wilcoxon signed-rank tests in the event of non-normality. In this exploratory study, results of the above statistical tests are presented both uncorrected for multiplicity and corrected after applying the Benjamini-Hochberg procedure to control the false discovery rate to <5% (Benjamini & Hochberg, 1995). Although the sample size limited our ability to test for separate or interactive effects of caffeine and sugar, in additional exploratory analyses uncorrected for multiplicity, we restricted analyses to those who reported consuming low levels of caffeine from beverages (<55mg/day) at baseline, less than the amount in a 20 fl oz bottle of cola. Analyses were conducted in STATA13 (StataCorp LP, College Station, TX).
Results
Table 1 shows participant baseline characteristics assessed during the usual SSB intake phase. The majority of teens were female (72%), non-Hispanic African American (56%), with a BMI≥95th %tile (76%), and a mother with greater than a high school education (64%). Fewer than half lived with both parents (44%) and had a father with greater than a high school education (45%). Mean consumption of SSBs was 32 ± 22 fl oz/day with soda accounting for the largest share (42%) of SSBs, followed by fruit-flavored drinks (35%). However, the majority of participants (n=19, 76%) reported consuming low amounts of daily caffeine (<55 mg). Only five participants reported consuming no caffeine from beverages at baseline. Mean fasting hemoglobin A1c level was within the diagnostic range for pre-diabetes.
Table 1.
Characteristics of 25 participants
| Mean±SD or n (%) | |
|---|---|
| Age (years) | 15.3 ± 1.4 |
| Female | 18 (72) |
| Race/ethnicity | |
| Non-Hispanic | |
| African American | 14 (56) |
| More than one race | 3 (12) |
| White | 2 (8) |
| Asian/Pacific Islander | 1 (4) |
| Hispanic African American | 1 (4) |
| Hispanic/Latino white | 4 (16) |
| BMI | 33 ± 7 |
| BMI%tile | 96.1 ± 0.04 |
| BMI≥95th %tile | 19 (76) |
| Waist circumference, cm | 102 ± 24 |
| Socioeconomic characteristics | |
| Mother’s education >high school degree | 16 (64) |
| Father’s education >high school degree | 9 (45) |
| Lived with both parents | 11 (44) |
| Daily self-reported beverage intake, fl oz/day | |
| Sugar-sweetened beverages | |
| Soda with added sugar | 14 ± 17 |
| Fruit drinks and smoothies | 11 ± 13 |
| Sports drinks | 4 ± 8 |
| Sweetened coffees or teas | 2 ± 6 |
| Energy drinks | 1 ± 5 |
| Flavored milks | 0.4 ± 1.2 |
| Total sugar-sweetened beverages | 32 ± 22 |
| Non-sugar-sweetened beverages, fl oz/day | |
| Water | 18 ± 26 |
| Juice, 100% | 5 ± 8 |
| Unflavored milk, dairy or non-dairy | 2 ± 4 |
| Diet soda | 1 ± 2 |
| Unsweetened coffee or tea | 1 ± 2 |
| Fasting serum measures | |
| Glucose, mg/dl | 96.2 ± 7.8 |
| Insulin, μg/ml | 23.6 ± 9.9 |
| Hemoglobin A1c, % | 5.9 ± 0.6 |
| Triglycerides, mg/dl | 75.5 ± 36.5 |
| Total cholesterol, mg/dl | 150.3 ± 30.1 |
| HDL, mg/dl | 42.9 ± 12.5 |
| LDL, mg/dl | 92.3 ± 23.1 |
| Adiponectin, μg/ml | 7.4 ± 3.2 |
| Leptin, μg/ml | 42.1 ± 29.1 |
Note: Observations with missing data were excluded from calculations of means, SD, and percentages.
Primary Outcome
Cronbach’s alpha for the modified withdrawal scale (Griffiths et al., 1990) ranged from 0.82 to 0.91 across days of administration, indicating good to excellent internal consistency. Reported symptoms from this scale (Table 2) included increased ratings of headache and decreased feelings of contentment/satisfaction, motivation to do work, and ability to concentrate during the SSB cessation phase, which were most pronounced on the first two days of cessation (days 6 and 7). After applying the Benjamini–Hochberg procedure, only feeling motivated to do work significantly decreased during cessation on day 2 (corrected P<0.05). Supplementary Table S1 contains fully reported statistics. Before correction, there was also a borderline significant decrease in reported wellbeing and increase in the total 11-item scale score on the first and second day of cessation (Table S1).
Table 2.
Symptoms of Withdrawal from 11-item modified Scale of Caffeine Withdrawal
|
Phase 1 (Usual SSB Intake) average[a] (n=25) M ± SD |
Phase 2 (SSB Cessation) average[a] (n=25) M ± SD |
Average change (n=25) M (%) ± SD |
Phase 2 Day 1 of cessation M ± SD |
Phase 2 Day 2 of cessation M ± SD |
Phase 2 Day 3 of cessation M ± SD |
|
|---|---|---|---|---|---|---|
| Total scale scoreb | 16.6 ± 7.6 | 19.2 ± 7.0 | +2.6 (+16%) ± 7.6 | 19.8 ± 9.2 | 20.0 ± 8.3 | 17.6 ± 7.0 |
| Individual itemsc | ||||||
| I have a headache | 0.5 ± 0.8 | 0.9 ± 0.9* | +0.4 (+86%) ± 0.8 | 1.0 ± 1.2* | 1.0 ± 1.3* | 0.6 ± 1.0 |
| I feel energetic/have energyd | 2.0 ± 1.1 | 1.8 ± 0.9 | −0.2 (−11%) ± 1.1 | 1.8 ± 1.1 | 1.6 ± 1.1 | 1.9 ± 1.0 |
| I feel awake/alertd | 2.2 ± 1.1 | 2.0 ± 0.9 | −0.2 (−9%) ± 1.4 | 2.0 ± 1.3 | 2.0 ± 1.1 | 2.0 ± 1.1 |
| I feel tired | 1.9 ± 1.2 | 1.9 ± 1.0 | 0.0 (−1%) ± 1.7 | 1.8 ± 1.4 | 1.8 ± 1.4 | 1.9 ± 1.3 |
| I feel good overalld | 2.7 ± 0.9 | 2.4 ± 0.8 | −0.4 (−13%) ± 0.9 | 2.5 ± 1.2 | 2.1 ± 1.2* | 2.5 ± 1.0 |
| I feel drowsy/sleepy | 1.5 ± 1.1 | 1.2 ± 1.0 | −0.2 (−16%) ± 1.3 | 1.2 ± 1.3 | 1.2 ± 1.4 | 1.2 ± 1.1 |
| I feel content/satisfiedd | 2.3 ± 0.9 | 2.0 ± 0.9 | −0.3 (−11%) ± 0.8 | 1.8 ± 1.2* | 1.9 ± 1.0* | 2.4 ± 0.9 |
| I feel motivated to do workd | 2.3 ± 0.9 | 1.7 ± 1.0** | −0.6 (−25%) ± 0.9 | 1.7 ± 1.2* | 1.5 ± 1.1***¥ | 1.9 ± 1.0 |
| I feel outgoingd | 2.2 ± 0.9 | 2.1 ± 1.0 | −0.1 (−6%) ± 1.0 | 2.0 ± 1.3 | 2.1 ± 1.1 | 2.2 ± 0.9 |
| I feel self-confidentd | 2.8 ± 0.9 | 2.5 ± 1.0 | −0.4 (−13%) ± 1.1 | 2.2 ± 1.4 | 2.4 ± 1.1 | 2.8 ± 1.1 |
| I am able to concentrated | 2.7 ± 0.9 | 2.3 ± 1.0* | −0.4 (−15%) ± 1.0 | 2.2 ± 1.3* | 2.4 ± 1.0 | 2.3 ± 1.3 |
P<0.05
P<0.01, and
P<0.01 uncorrected for multiplicity from paired t-tests comparing cessation (phase 2: average across days and values for each day 1-3) to usual intake (phase 1: average across days).
Benjamini-Hochberg corrected P-value<0.05.
Note: Complete data (n=25) were available on day 1 of usual intake and day 3 of cessation. Last value carried forward or next value carried backward were used to impute missing data on day 1 of phase 2 (n=3) and day 2 of phase 2 (n=5).
Averaged across days of the phase without missing data. Higher scores indicate greater severity of symptoms.
Possible range: 0-44 by summing responses to 11 items.
Individual items from the Modified Griffith Withdrawal Scale. Possible range: 0 (not at all) - 4 (Very much).
In the calculation of the total scale score, items were reverse coded.
In additional exploratory analyses examining these symptoms among low-caffeine consumers (n=19), the magnitude of average change was similar for headache (+0.4 ± 0.9) and motivation (−0.5 ± 1.0). Change in ability to concentrate in this subgroup was attenuated (−0.3 ± 1.0).
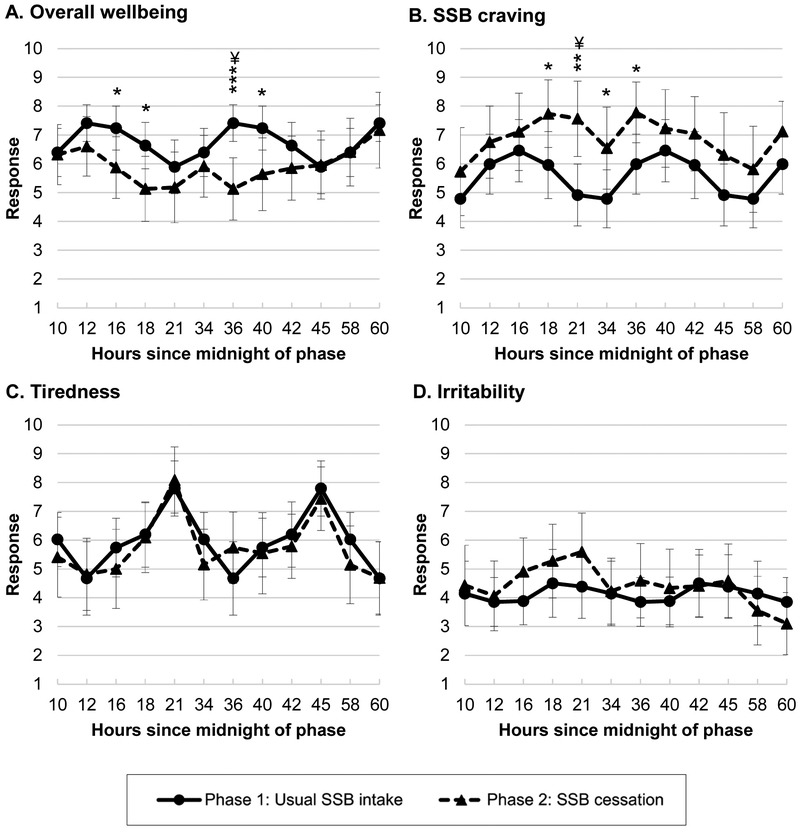
Symptoms of withdrawal assessed by the daily check-in questions five times daily are shown in Figure 3 (full statistics reported in Supplemental Table S2). When uncorrected for multiplicity, at several time points during the SSB cessation phase, ratings of overall wellbeing appeared significantly lower, and ratings of SSB craving appeared significantly higher. After the Benjamini–Hochberg procedure, overall wellbeing at 12pm the second day of cessation and SSB cravings at 9pm the first day of cessation differed significantly from those times during the usual intake phase (corrected Ps<0.05). There were no significant differences between phases for feeling tired or irritable. Results were similar when restricted to those who reported consuming low levels of caffeine from beverages at baseline (not shown).
Figure 3. Daily check-in questions assessing symptoms of withdrawal at regular intervals during usual intake and SSB cessation phases (n=24).
*P<0.05, **P<0.01, and ***P<0.01 uncorrected for multiplicity for differences between the usual intake and SSB cessation phases.
¥ Benjamini-Hochberg corrected P-value<0.05.
Plots show average responses at each time point as well as error bars for 95% CIs. CIs and post hoc pairwise contrasts between each time point were estimated using the margins and pwcompare commands in STATA for postestimation after fitting generalized linear regression models containing indicators for each time point with robust (Huber-White sandwich) standard errors. Usual intake phase responses at each time were averaged across days to serve as the comparisons for each time/day of cessation. Full results are reported in Table S2. Questions and 10-point response scale anchors: (A) How do you feel? 1 (really bad) – 10 (really good); (B) How much do you want your favorite sugar-sweetened beverage right now? 1 (I don’t want at all) – (I really want); (C) How tired are you? 1 (not tired at all) – 10 (really tired); (D) How irritable are you? 1 (not at all irritable) – 10 (really irritable).
Other Outcomes
Table 3 shows changes in dietary outcomes reported on 24-hr recalls and drink journals. Compared to the usual SSB intake phase, during the cessation phase, participants reported decreased daily consumption of SSBs and sugar, energy, and caffeine from beverages without compensatory increases in sugar from food. Consumption of total daily sugar and added sugar from all sources significantly decreased.
Table 3.
Daily dietary intake and salivary caffeine
| Phase 1: Usual SSB intake (n=25) M ± SD |
Phase 2: SSB Cessation (n=25) M ± SD |
Change (n=25) M ± SD |
Za | Pa | |
|---|---|---|---|---|---|
| Daily intakes | |||||
| Sugar sweetened beverages | |||||
| Amount, fl oz | 32 ± 22 | 5 ± 9 | −27 ± 25 | 4.00 | <0.001 |
| Added sugar, g | 20 ± 13 | 2 ± 3 | −18 ± 15 | 4.21 | <0.001 |
| Sugar, g | 80 ± 56 | 11 ± 23 | −69 ± 65 | 4.00 | <0.001 |
| Energy, kcals | 344 ± 238 | 69 ± 151 | −275 ± 303 | 3.62 | <0.001 |
| Caffeine, mg | 40 ± 59 | 1 ± 2 | −39 ± 59 | 3.94 | <0.001 |
| Total beverages | |||||
| Amount, fl oz | 59 ± 32 | 53 ± 36 | −6 ± 26 | 1.06 | 0.29 |
| Sugar, g | 100 ± 58 | 18 ± 26 | −82 ± 59 | 4.21 | <0.001 |
| Energy, kcals | 458 ± 262 | 150 ± 197 | −308 ± 272 | 4.27 | <0.001 |
| Caffeine, mg | 50 ± 60 | 4 ± 14 | −46 ± 57 | 4.12 | <0.001 |
| Caffeine from food, mg | 1.5 ± 0.6 | 1.5 ± 0.5 | 0.0 ± 0.8 | 0.38 | 0.70 |
| Sugar from food, g | 54 ± 39 | 47 ± 33 | −7 ± 45 | −0.63 | 0.53 |
| Total daily foods and beverages | |||||
| Added sugar, g | 26 ± 18 | 10 ± 9 | −16 ± 19 | 3.40 | <0.001 |
| Sugar, g | 144 ± 83 | 64 ± 43 | −80 ± 78 | 3.89 | <0.001 |
| Protein, g | 71 ± 67 | 64 ± 37 | −6 ± 63 | 0.18 | 0.86 |
| Solid fat, g | 25 ± 20 | 25 ± 14 | 0 ± 19 | −0.42 | 0.68 |
| Fiber, g | 11 ± 6 | 10 ± 5 | −1 ± 7 | 0.66 | 0.51 |
| Energy, kcals | 1945 ± 1129 | 1610 ± 761 | −334 ± 1021 | 1.31 | 0.19 |
| Salivary caffeine (μg/ml)b | 0.35 ± 0.62 | 0.13 ± 0.24 | −0.22 ± 0.58 | 2.30 | 0.02 |
Z-statistics and P-values from Wilcoxon Signed Rank Tests comparing usual intake to SSB cessation phases.
Amounts below the quantifiable limit of 0.100 μg/ml were coded as 0 μg/ml.
Most (n=16) reported only consuming water and plain milk during the cessation phase, but 9 participants consumed some SSBs, ranging from 1-8 fl oz among five participants, 14-15 fl oz among three participants, and 42 fl oz in one participant. Flavored milk accounted for 67% of non-adherent SSB consumption. Salivary caffeine, another indicator of adherence, significantly decreased by 63% during the SSB cessation phase (−0.22 μg/ml; P=0.02) (Table 3) to levels similar to those in other studies among participants undergoing caffeine cessation (James & Gregg, 2004; James, Gregg, Kane, & Harte, 2005). Non-adherence was likely in two participants, whose salivary caffeine levels increased from the usual intake phase to 0.51 and 0.96 μg/ml during cessation. The individuals described here were included in all analyses.
Lastly, there were no significant changes in Stroop tasks, delay/probabilistic discounting, or RRVssb (all P-values>0.05).
Discussion
In this exploratory study of the potentially addictive properties of SSBs, we detected an increase in symptoms of craving for SSBs and withdrawal among overweight and obese teens during a 3-day period of SSB cessation. The teens, who were regular consumers of SSBs before cessation, reported the following specific withdrawal symptoms during SSB cessation: increased headache; decreased motivation to do work, contentment, and ability to concentrate; and lower ratings of overall wellbeing. After controlling the false discover rate, increased SSB craving and decreased feelings of motivation and overall wellbeing remained significant. Withdrawal and craving are key manifestations in response to other legal addictive substances with large public impacts, like tobacco and alcohol, and like these substances, SSBs are consumed excessively despite health harms (Bray & Popkin, 2014). This is the first study we are aware of to examine potentially addictive properties of SSBs during a cessation intervention in free-living participants.
In this study, decreased well-being and increased cravings were most pronounced in the afternoon and evening, perhaps because this is when youth typically consume SSBs or are exposed to SSB cues through peer consumption or advertising. This timing could also result from circadian rhythm or timing of meals. Other withdrawal symptoms that were measured only once per day—headache and decreased contentment, motivation, and concentration—were more pronounced on days 1 and 2 of cessation than on day 3 of cessation. Other findings included clinically meaningful reductions in total sugar and sugar from beverages, the latter of which was not compensated for by increased sugar from foods. This finding is consistent with trials demonstrating decreased total sugar intake and/or weight loss from SSB replacement (de Ruyter, Olthof, Seidell, & Katan, 2012; Ebbeling et al., 2012).
The symptoms we observed in this study overlap with those observed in studies of withdrawal from caffeine (De Biasi & Salas, 2008; Juliano & Griffiths, 2004), the only psychoactive substance that can be legally purchased by children (Bernstein et al., 1998). However, in the current study, mean daily caffeine consumption was low (50 mg/d from beverages) compared to studies of caffeine cessation (100-900 mg/d) (De Biasi & Salas, 2008; Juliano & Griffiths, 2004). Yet, even low levels of caffeine may promote SSB consumption. A recent randomized controlled trial blindly assigned 99 adults to caffeinated SSBs (66 mg / 110ml, similar to common cola brands) or non-caffeinated SSBs, ad libitum for 6-weeks (Keast, Swinburn, Sayompark, Whitelock, & Riddell, 2015). The caffeinated group consumed significantly more SSBs and reported greater “liking” of SSBs than the non-caffeinated group. Therefore, caffeine, which is added to soda by manufacturers, may be contributing to high SSB consumption though its psychoactive properties. Manufacturers claim caffeine is added as a flavor enhancer, yet multiple studies have found evidence against flavor activity at concentrations common to SSBs (Keast & Riddell, 2007; Keast et al., 2015).
Although this study was not powered to compare withdrawal symptoms between caffeinated and non-caffeinated SSBs consumers, when we restricted to those usually consuming no/low caffeine from beverages, results were similar for headache, motivation, overall wellbeing, and craving. Therefore, we hypothesize that beyond caffeine, sugar content may have contributed to the observed symptoms, consistent with human neuorimaging studies of sugar consumption (Jastreboff et al., 2016; Smith & Robbins, 2013). Also, in a recent fMRI study examining adolescent brain response to SSBs, overweight teens were assigned to consume 1 ml of water and 1 ml of their preferred soda (Feldstein Ewing, 2016). Compared to water, soda resulted in brain activation in “…the regions that have been implicated in the neurobiological phenotype of substance use severity” and mapped onto “….existing addiction cue exposure literature” (Feldstein Ewing, 2016). Because only 25% of participants selected a caffeinated soda, it is unlikely that caffeine accounted for the results. In another fMRI study, in which adolescents consumed 75 g of glucose and 75 g fructose dissolved in cherry-flavored water (without caffeine), obese but not lean adolescents exhibited both attenuated prefrontal executive control responses and heightened homeostatic and hedonic responses to glucose and fructose (Jastreboff et al., 2016). Although this provides further evidence of the addictive potential of liquid sugar without caffeine in obese youth, the cross-sectional design makes it unclear if this neural response led to or was a consequence of obesity.
Questions have arisen as to whether certain foods/beverages like SSBs are addictive in vulnerable individuals (substance-based “food addiction” framework), or if instead, some individuals are addicted to eating (behavioral addiction framework) (Hebebrand, 2014). To explore this issue, college students and diverse adult populations were surveyed to determine if particular foods played a role in addictive eating (Schulte, Avena, & Gearhardt, 2015; Schulte, Smeal, & Gearhardt, 2017). In these studies, soda was ranked high on indicators of addictive eating, such as loss of control (Schulte et al., 2015; Schulte, Smeal, et al., 2017). Other high-ranking foods, like pizza and cookies, were highly processed with refined carbohydrates and/or added fat. Foods that ranked low were those that were less/not processed like fruits, vegetables, salmon, and beans. These, and other studies (Schulte, Potenza, & Gearhardt, 2017), provide support for the food addiction framework wherein only particular foods or beverages, such as SSBs, trigger addictive behavior. However, not all studies using survey-based approaches have found that soda (Lemeshow et al., 2018) and/or high sugar (mainly without fat or protein) foods were related to food addiction (Markus, 2017).
The current study, with its prospective design, both complements and addresses some limitations of survey-based approaches for identifying potentially addictive foods, which have been mostly cross-sectional and reliant on participants’ cognizance of the harms caused by particular foods and beverages. However, this study’s small sample size limited our ability to examine differences in symptoms by participant characteristics, such as caffeine consumption, and likely resulted in insufficient power to detect effects of SSB cessation on secondary and even primary outcomes. Generalizability is limited to overweight, predominantly female teens. Although the employed design has been used to examine caffeine withdrawal symptoms and has good ecological validity because it involves a naturally occurring pattern of consumption followed by abrupt cessation (De Biasi & Salas, 2008; Juliano & Griffiths, 2004), there are associated limitations. Because participants were free-living, adherence could not be guaranteed, but salivary caffeine and 24-hr recalls provided a degree of reassurance. Also, the use of a single group intervention design, relative to a crossover or two-group design, may be biased by “testing”, or the possibility that taking a baseline survey influenced subsequent responses to the same survey/task during SSB cessation. In particular, testing may have influenced the cognitive and behavioral tasks, for which we did not find significant changes. However, it is also possible that the tasks used were not sensitive enough. Even experimental studies of caffeine cessation do not consistently demonstrate impaired cognitive or behavioral performance (Juliano & Griffiths, 2004). This study was limited to three days of cessation, which overlaps with timing for peak symptoms of withdrawal from caffeine of 20-51 hours (Juliano & Griffiths, 2004) and several symptoms of withdrawal from tobacco and alcohol (Hughes, 2007; Kosten & O'Connor, 2003; Shiffman et al., 2006). However, for other substances, symptoms can peak around 3-5 days, so it is possible our cessation period was not long enough to capture greatest severity for all symptoms. Relatedly, insufficient data for analysis was available from daily check-in questions after 12 pm on day 8. Consequently, we may have missed detecting symptoms that peaked around this time.
Future studies with larger sample sizes should examine possible withdrawal symptoms from SSBs over longer periods of time using a cross-over design, enroll normal and overweight participants and regular consumers and non-consumers of SSBs, examine differences between responses to caffeinated and non-caffeinated SSBs as well as liquid and solid sugar, and examine other potential manifestations of addiction. Neural imaging studies among younger children, coupled with longitudinal assessments of SSB consumption, anthropometrics, and metabolic outcomes, could also help clarify the direction of the association between addictive response to SSBs and weight status.
Conclusion
This exploratory study provides early evidence of potentially addictive properties of SSBs in a diverse population of overweight adolescents. These results, combined with present and future corroborating evidence, could inform clinical practice around helping adolescents reduce SSB intake, have important implications for messaging in public health campaigns, and inform the need for efforts to reduce SSB advertising to youth and SSB availability in and around schools.
Supplementary Material
Acknowledgements
We would like to thank the participants who made this research possible, as well as Ely Niroomand and Elena Barbot-Wheaton for their assistance with this study.
Funding
This work was supported by a University of California Office of the President Multi-Campus Research Initiative grant (PI, Elissa Epel). J. Falbe’s work was supported in part by the American Heart Association (grant #14POST20140055) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant #1K01DK113068-01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. These funders had no role in the study design; collection, analysis or interpretation of data; writing of the report; or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinicaltrials.gov 12-08545
References
- Almiron-Roig E, Palla L, Guest K, Ricchiuti C, Vint N, Jebb SA, & Drewnowski A (2013). Factors that determine energy compensation: a systematic review of preload studies. Nutr Rev, 71(7), 458–473. doi: 10.1111/nure.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological), 289–300. [Google Scholar]
- Bernstein GA, Carroll ME, Dean NW, Crosby RD, Perwien AR, & Benowitz NL (1998). Caffeine Withdrawal in Normal School-Age Children. Journal of the American Academy of Child & Adolescent Psychiatry, 37(8), 858–865. doi: 10.1097/00004583-199808000-00016 [DOI] [PubMed] [Google Scholar]
- Block G (2004). Foods contributing to energy intake in the US: Data from NHANES III and NHANES 1999–2000. J Food Composition Anal, 14, 439–447. [Google Scholar]
- Bray GA, & Popkin BM (2014). Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care, 37(4), 950–956. doi: 10.2337/dc13-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbois TD, Marsden SL, Anderson GH, & Sievenpiper JL (2014). Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients, 6(5), 1899–1912. doi: 10.3390/nu6051899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C (2014). Evolutionary and neuropsychological perspectives on addictive behaviors and addictive substances: relevance to the "food addiction" construct. Subst Abuse Rehabil, 5, 129–137. doi: 10.2147/sar.s56835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, & Salas R (2008). Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med (Maywood), 233(8), 917–929. doi: 10.3181/0712-mr-355 [DOI] [PubMed] [Google Scholar]
- de Ruyter JC, Olthof MR, Seidell JC, & Katan MB (2012). A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med, 367(15), 1397–1406. doi: 10.1056/NEJMoa1203034 [DOI] [PubMed] [Google Scholar]
- DiNicolantonio JJ, O'Keefe JH, & Wilson WL (2017). Sugar addiction: is it real? A narrative review. Br J Sports Med doi: 10.1136/bjsports-2017-097971 [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, & Ludwig DS (2012). A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med, 367(15), 1407–1416. doi: 10.1056/NEJMoa1203388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economic Research Service. (2017). Food Availability (Per Capita) Data System. Retrieved from Washington, DC: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ [Google Scholar]
- Epel ES, Tomiyama AJ, Mason AE, Laraia BA, Hartman W, Ready K, … Kessler D (2014). The reward-based eating drive scale: a self-report index of reward-based eating. PLoS One, 9(6), e101350. doi: 10.1371/journal.pone.0101350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, & Preston KL (2010). Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav, 35(4), 318–324. doi: 10.1016/j.addbeh.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Carr KA, Lin H, & Fletcher KD (2011). Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr, 94(1), 12–18. doi: 10.3945/ajcn.110.010314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. (2012). A Review of Food Marketing to Children and Adolescents: Follow-up Report. Retrieved from Washington DC: http://www.ftc.gov/os/2012/12/121221foodmarketingreport.pdf [Google Scholar]
- Feldstein Ewing SW C. ED,; Hudson KA; Filbey FM; Yakes Jimenez E; Lisdahl KM; Kong AS (2016). Overweight adolescents' brain response to sweetened beverages mirrors addiction pathways. Brain Imaging Behav doi: 10.1007/s11682-016-9564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH, Davidson M, & Saad F (2005). Validation of a questionnaire measure of the relative reinforcing value of food. Eat Behav, 6(3), 283–292. doi: 10.1016/j.eatbeh.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Evans SM, Heishman SJ, Preston KL, Sannerud CA, Wolf B, & Woodson PP (1990). Low-dose caffeine physical dependence in humans. J Pharmacol Exp Ther, 255(3), 1123–1132. [PubMed] [Google Scholar]
- Hebebrand J A. O; Adan R; Antel J; Dieguez C; de Jong J; Leng G; Menzies J; Mercer JG; Murphy M; van der Plasse G; Dickson SL (2014). "Eating addiction", rather than "food addiction", better captures addictive-like eating behavior. Neurosci Biobehav Rev, 47, 295–306. doi: 10.1016/j.neubiorev.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Hu FB (2013). Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev, 14(8), 606–619. doi: 10.1111/obr.12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR (2007). Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res, 9(3), 315–327. doi: 10.1080/14622200701188919 [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) and NRC (National Research Council). (2011). The Science of Adolescent Risk-Taking: Workshop Report. Retrieved from Washington, DC: [Google Scholar]
- James JE, & Gregg ME (2004). Effects of dietary caffeine on mood when rested and sleep restricted. Hum Psychopharmacol, 19(5), 333–341. doi: 10.1002/hup.589 [DOI] [PubMed] [Google Scholar]
- James JE, Gregg ME, Kane M, & Harte F (2005). Dietary caffeine, performance and mood: enhancing and restorative effects after controlling for withdrawal reversal. Neuropsychobiology, 52(1), 1–10. doi: 10.1159/000086172 [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Arora J, Giannini C, Kubat J, Malik S, … Caprio S (2016). Altered Brain Response to Drinking Glucose and Fructose in Obese Adolescents. Diabetes, 65(7), 1929–1939. doi: 10.2337/db15-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, & Griffiths RR (2004). A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl), 176(1), 1–29. doi: 10.1007/s00213-004-2000-x [DOI] [PubMed] [Google Scholar]
- Keast RS, & Riddell LJ (2007). Caffeine as a flavor additive in soft-drinks. Appetite, 49(1), 255–259. doi: 10.1016/j.appet.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Keast RS, Swinburn BA, Sayompark D, Whitelock S, & Riddell LJ (2015). Caffeine increases sugar-sweetened beverage consumption in a free-living population: a randomised controlled trial. Br J Nutr, 113(2), 366–371. doi: 10.1017/s000711451400378x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit BK, Fakhouri TH, Park S, Nielsen SJ, & Ogden CL (2013). Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999-2010. Am J Clin Nutr, 98(1), 180–188. doi: 10.3945/ajcn.112.057943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, & O'Connor PG (2003). Management of drug and alcohol withdrawal. N Engl J Med, 348(18), 1786–1795. doi: 10.1056/NEJMra020617 [DOI] [PubMed] [Google Scholar]
- Lemeshow AR, Rimm EB, Hasin DS, Gearhardt AN, Flint AJ, Field AE, & Genkinger JM (2018). Food and beverage consumption and food addiction among women in the Nurses' Health Studies. Appetite, 121, 186–197. doi: 10.1016/j.appet.2017.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobstein T (2017). Reducing consumption of sugar-sweetened beverages to reduce the risk of childhood overweight and obesity. Retrieved from http://www.who.int/elena/titles/ssbschildhoodobesity/en/ [Google Scholar]
- MacLeod CM (1991). Half a century of research on the Stroop effect: An integrative review. Psychol Bull, 109(2), 163–203. doi: 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- Malik VS, Popkin BM, Bray GA, Despres JP, & Hu FB (2010). Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation, 121(11), 1356–1364. doi: 10.1161/circulationaha.109.876185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus CR R. PJ; Brouns F; Schepers R (2017). Eating dependence and weight gain; no human evidence for a 'sugar-addiction' model of overweight. Appetite, 114, 64–72. doi: 10.1016/j.appet.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Mattes RD (1996). Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav, 59(1), 179–187. [DOI] [PubMed] [Google Scholar]
- Mazur JE (1987). An adjusting procedure for studying delayed reinforcement In Commons JEMML, Nevin JA, & Rachlin H (Ed.), Quantitative analysis of behavior: Vol. 5 The effect of delay and of intervening events of reinforcement value (pp. 55–73). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Meredith SE, Juliano LM, Hughes JR, & Griffiths RR (2013). Caffeine Use Disorder: A Comprehensive Review and Research Agenda. J Caffeine Res, 3(3), 114–130. doi: 10.1089/jcr.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Cary M, Slaymaker V, Colleran C, & Blow FC (2009). Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug Alcohol Depend, 103(3), 131–136. doi: 10.1016/j.drugalcdep.2009.03.009 [DOI] [PubMed] [Google Scholar]
- Powell LM, Szczypka G, & Chaloupka FJ (2010). Trends in exposure to television food advertisements among children and adolescents in the United States. Arch Pediatr Adolesc Med, 164(9), 794–802. doi:2010.139 [pii] 10.1001/archpediatrics.2010.139 [doi] [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, & Cross D (1991). Subjective probability and delay. J Exp Anal Behav, 55(2), 233–244. doi: 10.1901/jeab.1991.55-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy J, & Krebs-Smith SM (2010). Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc, 110(10), 1477–1484. doi: 10.1016/j.jada.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger A, Herrick K, Gahche J, & Park S (2017). Sugar-sweetened beverage consumption among U.S. youth, 2011–2014. NCHS data brief. Retrieved from Hyattsville, MD: [PubMed] [Google Scholar]
- Sanchez-Pimienta TG, Batis C, Lutter CK, & Rivera JA (2016). Sugar-Sweetened Beverages Are the Main Sources of Added Sugar Intake in the Mexican Population. J Nutr, 146(9), 1888S–1896S. doi: 10.3945/jn.115.220301 [DOI] [PubMed] [Google Scholar]
- Schulte EM, Avena NM, & Gearhardt AN (2015). Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One, 10(2), e0117959. doi: 10.1371/journal.pone.0117959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, & Gearhardt AN (2017). Associations of Food Addiction in a Sample Recruited to Be Nationally Representative of the United States. Eur Eat Disord Rev doi: 10.1002/erv.2575 [DOI] [PubMed] [Google Scholar]
- Schulte EM, Potenza MN, & Gearhardt AN (2017). A commentary on the "eating addiction" versus "food addiction" perspectives on addictive-like food consumption. Appetite, 115, 9–15. doi: 10.1016/j.appet.2016.10.033 [DOI] [PubMed] [Google Scholar]
- Schulte EM, Smeal JK, & Gearhardt AN (2017). Foods are differentially associated with subjective effect report questions of abuse liability. PLoS One, 12(8), e0184220. doi: 10.1371/journal.pone.0184220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, … Balabanis M (2006). Natural history of nicotine withdrawal. Addiction, 101(12), 1822–1832. doi: 10.1111/j.1360-0443.2006.01635.X [DOI] [PubMed] [Google Scholar]
- Smith DG, & Robbins TW (2013). The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry, 73(9), 804–810. doi: 10.1016/j.biopsych.2012.08.026 [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. doi: 10.1037/h0054651 [DOI] [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service,. (2016). Food Surveys Research Group. Retrieved from https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/ [Google Scholar]
- U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau. (2014). Child Health USA 2014. Retrieved from https://mchb.hrsa.gov/chusa14/health-status-behaviors/adolescents/adolescent-overweight-obesity.html#source1 [Google Scholar]
- World Health Organization. (1992). The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.