ABSTRACT
The ketogenic diet (KD) is a nonpharmacologic treatment to reduce seizures with moderate to high success in pediatric patients with intractable epilepsy. Initiated in hospital, parents continue the treatment at home ensuring the ratio of high fat to low carbohydrate/protein is maintained to achieve metabolic ketosis. We conducted a systematic review to examine the quality of life (QoL) for families with a child using the KD for the reduction in epileptic seizures. A systematic review of the literature was conducted from 2007–2014 using key terms and combinations of: “epilepsy,” “ketogenic diet,” “children,” “family,” and “quality of life.” We accessed CINAHL, Medline, PubMed, and PsycINFO. After removing duplicates, we screened 598 papers by title and abstract. Articles comparing alternate diets such as the Atkins diet to the KD, or those focusing on the KD and societal costs, were excluded. Eighteen articles remained, including 7 intervention studies (randomized controlled trial and quasiexperimental), 7 descriptive studies (retrospective), 2 observational studies, and 2 case studies. Most participants were diagnosed with epilepsy at the age of 5 y, and had a trial of antiepileptic drugs (AEDs) and had been using the KD after discharge from the hospital. QoL was infrequently reported as a primary variable and was defined in a variety of ways. We found recurring themes that could affect QoL: efficacy of seizure reduction, nutritional status, child growth and development, and child and family psychosocial impact. The dominant psychological factor was the need for counseling for parents and clear expectations on expected outcomes. Nonadherence and dropout rates were frequent, but unfortunately the reasons and timing were not well documented, and some of these could be associated with QoL. The success of the KD in seizure reduction addressed a primary parental concern. Further research should address KD adherence and dropout rates, and investigate factors of quality of life.
Keywords: epilepsy, ketogenic diet, seizure control, children, quality of life
Introduction
Refractory or unmanageable seizures occur in 20–30% of pediatric patients diagnosed with epilepsy (1). The ketogenic diet (KD) is a dietary therapy originating in the 1920s before the widespread use of antiepileptic drugs (AEDs). Over the years, growing resistance to AEDs has renewed clinical interest in the KD as a viable treatment to reduce seizure activity (2, 3). The KD places a sizable burden on families due to the strict dietary requirements and the side effects experienced by the child.
The efficacy of the classic KD in reducing seizures is heavily dependent upon strict adherence to a macronutrient ratio of 4 g of fat to 1 g of carbohydrate/protein intake. Given the family effort and daily changes required to maintain the diet, we questioned the quality of life (QoL) of families who used the KD for their child as a second or last resort to drug therapy. We conducted a systematic literature review to examine the evidence of the impact of the KD on children with epilepsy and the effect on QoL for their families.
The classic therapeutic KD was developed by Dr. Russell Wilder at the Mayo Clinic in the 1920s. It comprises long-chain fatty acids with a 4:1 fat-to-carbohydrate/protein ratio. It was widely used until the 1940s when AEDs were introduced. Given that AEDs have not been effective for some patients, clinical interest in the KD has re-emerged.
The KD induces metabolic ketosis in the consumer, resulting in the production of ketones that are then used as metabolic fuel (4). It is thought that ketones confer neurologic protection because of their antioxidant and anti-inflammatory effects (5). These neuroprotective properties can result in a reduction in epileptic seizures, although the process is not clearly delineated (6). Most children experience a decrease in seizures from baseline measures while consuming and after ceasing the KD (7). The efficacy of the KD has been reported to reduce seizure activity in children by 50–60%, and ≥10% of children are seizure-free (8).
The KD is highly restrictive in terms of food selection and preparation to maintain the fat-to-carbohydrate/protein ratio. In an effort to improve the palatability and broaden food selection, Dr. Peter Huttenlocher introduced the modified KD composed of medium-chain triglycerides (MCTs) in a 3:1 ratio of fat to carbohydrate/protein (9). The MCT diet allowed for more dietary carbohydrates while maintaining ketosis (10). This was an important development, because it increased the number of food items permitted, thus easing the food-preparation burden on the parents and creating a wider assortment of palatable food for the children.
Side effects of both variants of the KD include gastrointestinal disturbances, constipation, kidney stones, bone fractures, dyslipidemia, and micronutrient deficiencies (11). Both diets are often discontinued earlier than recommended because they are difficult to maintain. The MCT diet is expensive, cannot be used with some medications, and takes longer to reduce seizures (12, 13).
Families preparing the KD require a food scale, a glucometer, and reagent sticks to check blood sugars and daily ketonuria (14). These are added expenses. Calories must be meticulously counted and food carefully prepared to maintain this low carbohydrate and protein intake, because blood glucose elevation from the metabolism of carbohydrates would stimulate insulin release and gluconeogenesis, and the replacement of fat by-products would decrease ketone blood concentrations. Adequate fluids are encouraged but any carbohydrate sources, such as toothpaste or vitamin supplements with lactose fillers, are to be avoided. Wavering adherence to either diet has been traced to food unpalatability, lack of food variety, and the rule to finish everything on the plate. Many activities involving meals or snacks must be modified or eliminated for the child on a KD. The control and monitoring of dietary intake fall on parents; shopping, meal preparation, and those occasions when food is central to social gatherings, including birthdays and holiday celebrations.
An understanding of how this therapeutic dietary treatment affects the family unit allows allied healthcare personnel to provide greater support to patients and their families managing difficult home treatments. We used the WHO definition of “QoL” as “the functional impact of a medical condition as well as the condition's resulting therapy and associated effects on a patient” (15). QoL has multiple interpretations of families but can include family interaction, parenting, emotional well-being, disease and treatment effects, and daily life. Measurements of QoL for families vary. The KD has known benefits in reducing seizures but is a rigorous prescriptive diet that becomes a key component of family life. This review aimed to examine the QoL for families with a child consuming the KD as a treatment for intractable epilepsy.
Methods
Literature search
We conducted the systematic review process on the basis of the guidelines outlined in Magarey (16). We searched the literature in the 7-y span, flanking the FDA's 2008 Alert that AEDs were associated with suicidal ideation and increased risk of suicide. This alert resulted in increased interest by clinicians and families to use nonpharmacologic treatments such as the KD as a means of managing seizures.
Information sources and search
Our literature review included the research databases CINAHL, Medline, and PubMed (human only). PsycINFO was also utilized to inform us on the psychosocial impact on maintaining a KD because it specializes in areas of behavioral science and mental health. The search terms used were KD (ketogenic, KD), the pediatric population (pediatric*, adolescen*, child*), family and treatment support (famil*, adapt*, therapy, compliance), and patient QoL (QoL, lifestyle, socioeconomic status, social class, life satisfaction, emotional adjustment, life changes, life experiences, quality of work, psycholog*).
Inclusion and exclusion criteria
Our inclusion/exclusion criteria for QoL met the World Health recommendations for measuring the quality of life (15). We focused on psychosocial impacts and treatment support for families. Inclusion criteria included the age at which the KD was initially implemented for the patient as that impacts the success or failure of the KD or when enrollment occurred in the respective study. Also, all included articles focused not only on the KD and biometrics but on how they related to the physical functionality of the child and reported degree of dependence on the family.
Following duplicate removal (n = 31), 598 articles were screened by title and abstract according to the inclusion/exclusion criteria. Studies were excluded if they focused on a comparison of diets, such as the Atkins diet compared with KD; or the impact of the KD on societal costs, as well as studies exhibiting a research focus on the population of society compared with that of families.
Quality appraisal
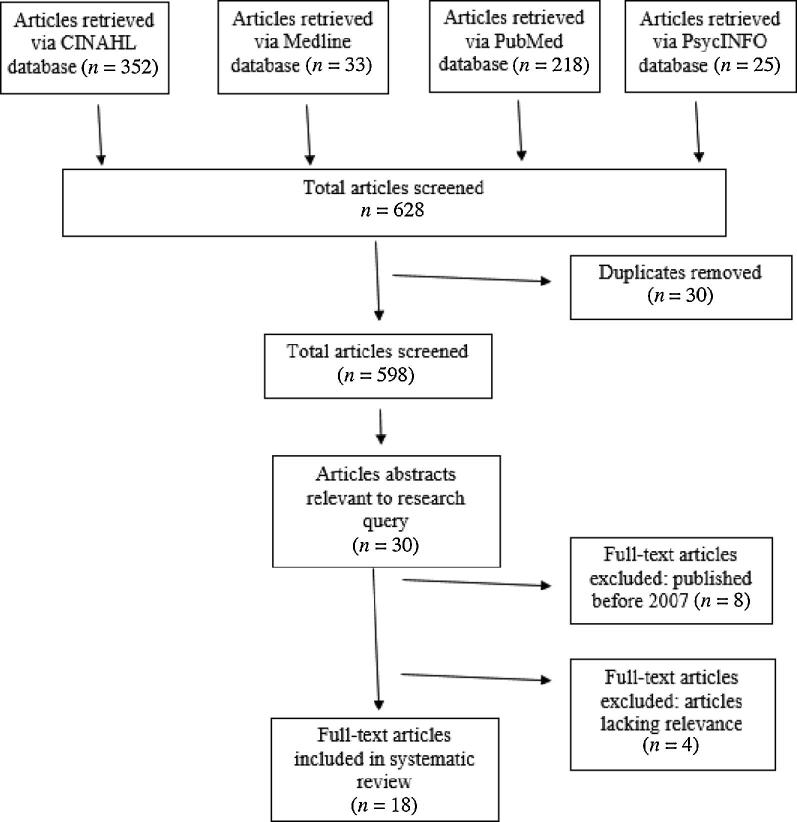
The articles were screened, and the data were extracted from reports by RB, MO, KP, and PW. We used the Critical Appraisal Skills Programme tool to assess the journal articles. Those receiving a score of ≥13 were included in our analysis (Table 1). Reliability was established among readers collaborating on the review of the articles. Given disagreements, the reviewers discussed the qualities of the article content and its relevance to the research question, and then came to an agreement. Thirty-one articles were read entirely, and 18 articles were chosen (Figure 1).
TABLE 1.
Assessment of articles from the literature search
| Critical Appraisal Skills Programme criteria | Clear statement of aims | Appropriate methodology | Appropriate research design | Appropriate recruitment strategy | Appropriate data collection method | Research relations considered | Considered ethical issues | Rigorous analysis | Clear findings | Valued research | Total score/20 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berqvist et al. (26) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 20 |
| Berqvist et al. (2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 20 |
| Carabello et al. (20) | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 17 |
| Christodoulides et al. (22) | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 16 |
| Coppola et al. (27) | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 17 |
| Dressler et al. (21) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 13 |
| Hallböök et al. (33) | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 17 |
| Hawkes and Levine (23) | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 15 |
| Kim et al. (30) | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 16 |
| Kossof et al. (11) | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 13 |
| Lambrechts et al. (32) | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 16 |
| McNamara et al. (29) | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 19 |
| Raju et al. (25) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 15 |
| Selter et al. (12) | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 19 |
| Sharma et al. (28) | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 14 |
| Tagliabue et al. (31) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 14 |
| Taub et al. (7) | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 16 |
FIGURE 1.
Flow diagram of the selection process of literature included in this review (35).
Seven of the 18 studies examined were intervention studies (randomized controlled trial and quasi-experimental), 7 were descriptive studies (retrospective and comparative), 2 were observational studies, and 2 were case studies. Studies were conducted in Europe (United Kingdom, Austria, Italy, Sweden, Netherlands), North America (Canada, United States of America), South America (Argentina), Asia (India, Korea), and Australia. Four key themes emerged: 1) the effectiveness of the KD as a treatment for intractable seizures in pediatric epilepsy, 2) nutrition, 3) child growth and development, and 4) child and family psychosocial impact.
Results
Synthesis
QoL for families of children undertaking the KD was a primary focus in the literature we reviewed. The efficacy of the diet has been established, but contextual effects are not as well known. The studies included in this review are of varied research designs. The quantitative studies were conducted for monitoring efficacy of the KD, growth/development and nutritional deficiencies, and some primary concerns of parents of children with intractable epilepsy. The qualitative studies revealed more cognitive and behavioral effects attributed to the diet. QoL was measured in 2 articles. The implementation of the KD was used internationally as indicated by the numerous countries represented; despite this, the sample sizes in most of the studies were small because of the low number of children on the diet (Supplemental Figure 1). Given the discrepancies in design and the small sample size, the review did not warrant an aggregate data analysis.
Efficacy of the KD
Treatment effectiveness plays an important role in improving healthcare-related QoL (17). This is especially true in pediatric epilepsy when parents and caregivers report lower psychosocial health, and higher stress due to perceived QoL for the patient, interruptions of day-to-day family life, and lack of palatable meals (18, 19). Camfield et al. (18) found the most significant stressors for parents to be seizure frequency and the number of medications prescribed. The KD as a drug-replacement therapy is relatively successful in reducing and even eradicating seizure occurrence, ultimately alleviating the stress associated with recurring seizures and the administration of multiple medications.
We report biophysical metrics as a functional impact of the KD treatment therapy and its effects on the physical functioning of children. Caraballo et al. (20) found in a multicenter study of pediatric patients (n = 216) with intractable epilepsy that 90% of patients experienced a 75% increase in seizure control, and 20.5% became seizure-free. However, seizure recurrence occurred in one-quarter of patients shortly after discontinuation of the KD (20). Dressler et al. (21) had similar findings using KD as a therapy for drug-resistant epilepsy. More than 50% of pediatric patients [n = 50, age 0–16 y (4.5 ± 3.55 y)] displayed significantly lower rates of epileptic electroencephalogram readings (P = 0.009) (21).
Kossoff et al. (11) reported on the effects of long-term use of the KD in a case study that suggested effective control of seizures with minimal adverse effects. They studied a man who was 26 y old with epilepsy who had been on the MCT KD therapy continuously since the age of 5 y. He had no history of kidney stones, constipation, acidosis, or bone issues and averaged 1 epileptic seizure annually (11).
Selter et al. (12) found that tailoring the therapy to suit individual needs while consuming the KD may help to provide improved therapeutic benefits while reducing adverse effects. The retrospective chart review gathered patients consuming the KD therapy for 0.1–4.5 y with a mean duration of 1.1 y (12). They identified 10 dietary and supplement changes (71.6%) and tracked medication adjustments (28.4%) that were implemented to address seizure control and side effects. Dietary changes addressed tastelessness and food monotony, and medication changes were dose increases and the introduction of new AEDs. These adjustments resulted in >50% further seizure reduction in 36 (18%) patients, but only 6 (3%) became seizure-free (12).
Compromised nutrition
Children introduced to the KD experienced rapid alterations in nutritional status, often presenting as micro- and macronutrient deficiencies (22). Christodoulides et al. (22) identified micronutrient deficiencies in children (n = 91, aged 2–16 y), vitamin A, magnesium, and selenium, when using the classic KD and the modified MCT KD. In the group consuming the classic KD (n = 46; age = 2–16 y), mean plasma vitamin A and magnesium decreased (from 1.41 to 1.13 µmol/L, P < 0.001, and from 0.87 to 0.83 µmol/L, P < 0.001, respectively), and mean plasma selenium decreased in the entire group (from 0.95 to 0.88 µmol/L, P < 0.05) (22). Deficiencies such as these can result in decreased growth and compromised immunity as well as hypercalcemia and vitamin D insufficiency. Hawkes and Levine (23) and Sirikonda et al. (24) also reported a positive correlation of selenium deficiency with time on the KD and detailed the rare finding of cardiac decompensation of a male child aged 5 y.
Raju et al. (25) compared the efficacy of the classic KD to a modified 2.5:1 KD and found comparable efficacy and tolerability of both diets in an unblinded comparative study. However, in the 2.5:1 group, there was a trend (P = 0.06) for higher HDL and total cholesterol, but this was not statistically significant perhaps because the study may have lacked power owing to the small sample size (n = 38).
Bergqvist et al. (26) found changes in bone mineral content in 25 children of varying age (7.3 ± 1.9 y) who were treated with the KD for >15 mo. There were decreases in overall growth, bone health, bone density, and serum 25-hydroxyvitamin D [25(OH)D] (26). These changes in vitamin D status were also found in an earlier study in children who were switching from pharmacologic seizure management to a 15-mo trial on the KD (2). The study enrolled 45 school-aged children (aged 5.1 ± 2.7 y), of whom 4% had deficient and 51% had insufficient serum 25(OH)D at baseline. After an initial increase in serum 25(OH)D, concentrations steadily declined to just above baseline; 2% had deficient and 30% had insufficient concentrations after 15 mo (P < 0.05) (2). Relatedly, Hawkes and Levine (23) found similar imbalances in 3 case studies, in which ketotic hypercalcemia was found to occur in 3 different children (aged 2.6–5.5 y) consuming the KD for 6–12 mo; hypercalcemia occurred in tandem with reduced alkaline phosphatase, parathyroid hormone, and 1,25-dihydroxyvitamin D. Low bone mineral accrual during growth is a key determinant for risk of osteoporosis later in life.
Coppola et al. (27) found an increase in total cholesterol (224 ± 88 mg/dL; 175 ± 55 mg/dL; P = 0.038) and triglycerides (267 ± 329 mg/dL; 115 ± 25 mg/dL; P = 0.046) when comparing the KD group (n = 43) with the control group identifying an effect on the cardiovascular system. Sharma et al. (28) similarly found increases from baseline in triglyceride and total cholesterol concentrations, as well as a significant decrease in serum albumin (P = 0.0453). The KD is difficult for families to adhere to given common adverse side effects: constipation, vomiting, diarrhea, and perceived allergies, as well as the constant vigil of dietary restrictions (28).
The KD impacts family culture because meal time becomes treatment time. Meals are different for 1 family member and are often accompanied by the omission of culturally significant foods. Sharma et al. (28) conducted a study in India where vegetarianism is common, which limits sources of proteins and fats for the KD creating additional strain on family finances and lifestyles (26, 28).
Impact of the KD on growth and development
The age at which children started and maintained the KD varied widely in the literature reviewed. Many of the effects of the KD on children's physiology and growth were time-dependent, such as meeting growth standards and the onset of puberty. Coppola et al. (27) evaluated the impact that KD has on endothelial function, arterial morphology, and cardiac diastolic function 6 mo post-KD initiation. When matched for age and gender, and compared with a control group with treatment-resistant seizures, Coppola et al. found that patients consuming the KD (male = 11, female = 12, age = 11 ± 8 y, mean duration 24 ± 29 mo) had a significant increase (P < 0.001) in systemic arterial stiffness, which is positively correlated to increased serum cholesterol and triglyceride concentrations. This demonstrates the necessity of cardiovascular health education for families and patients receiving long-term KD treatment, plus the need for close monitoring of patient cholesterol, triglyceride, and activity levels. Early detection of arterial stiffness in KD patients may allow for prophylactic treatment for cardiovascular disease with angiotensin-converting enzyme inhibitors or calcium-channel blockers (27).
Growth delays are a concern for many parents. Kim et al. (30) conducted a quantitative retrospective descriptive study to examine growth delays and subsequent catch-up growth in epileptic children following discontinuation of long-term (≥2 y) KD therapy. Eighteen children (male = 9, female = 9) aged 2–15 y (mean age = 7.5 y) participated in the study. Significant weight (P = 0.02) and height delays (P = 0.004) occurred if the diet started between 3 and 6 y of age; however, adequate catch-up growth was observed after discontinuing KD therapy. Although this study was conducted with a small sample, it presents valuable information to aid in the development of proactive long-term care planning for young patients placed on KD for periods exceeding 2 y and initiated at a younger age (30).
Tagliabue et al. (31) enrolled 18 patients (males = 8, females = 10, age = 12.4 ± 5.6 y) and compared nutritional status at baseline and 6 mo after the KD was implemented. Nutritional status measures included: BMI, weight, fat mass/free fat mass via dual energy X-ray absorptiometry and total body scans, substrate oxidation rates, and resting energy expenditure. Tagliabue et al. reported that fat oxidation rates significantly increased and were the primary predictor of seizure reduction (β = −0.97, t = −6.3, P < .05). Findings from Tagliabue et al. (31) and Kim et al. (30) indicate that KD effects on growth and development are much more deleterious when the diet is started at a younger age. Parents must be reassured that catch-up growth occurs following growth delay in children consuming the KD.
Psychosocial impacts of the KD
Lambrechts et al. (32) found that despite a slight trend of improved cognition and seizure reduction, mood problems increased, and psychosocial adjustment worsened in children consuming the KD. The study lacked power (n = 15) to support statistically significant findings, but clinically these results do inform. Although Lambrechts et al. compared each participant to their pretest baseline, they did not account for comorbidities of mood disorders such as attention deficit and hyperactivity disorder, oppositional defiant disorder, or pervasive development disorder.
Hallböök et al. (33) evaluated sleep patterns in children with therapy-resistant epilepsy consuming the KD and examined alterations in seizure severity, seizure frequency, QoL, attention, and behavior. QoL was based on the parents’ perception of general attention and behavior. Hallböök et al. found a decrease in total sleep from baseline data (P = 0.05) and total night sleep (P = 0.006). There was an improvement in the National Health Seizure Severity Scale scores (P < 0.001) and QoL (P = 0.001) in the 18 participants after 3 mo consuming the KD. The 11 participants who stayed on the diet for 12 mo showed a decrease in daytime sleep (P = 0.01), decrease in National Health Seizure Severity Scale score (P = 0.005), and increase in QoL (P = 0.005) (33).
Hallböök et al. (33) indicated a correlation between increased rapid-eye movement sleep and improvement in QoL (Spearman r = 0.6, P = 0.01) at 3 mo. However, the study's small sample size results in low statistical power. It also has several confounding variables; not all participants had the same diet ratio, and it included multiple P values for each analysis component, resulting in difficult interpretation of findings.
Taub et al. (7) focused on the risk of seizure recurrence after achieving initial seizure reduction in children treated with the KD. In this retrospective cohort study, Taub et al. used caregiver-reported diaries noting changes in seizure frequency to baseline. Family counseling was recommended for families with children who started to experience breakthrough seizures, to be assured that these seizures did not indicate a decline toward baseline seizure frequency (7). This concurs with Selter et al. (12), who also recommended family counseling if a medication is added with KD treatment, to reassure families that this does not mean the KD is not working but both may be more effective in reducing seizure. Families also reported that having someone to call, such as another family with KD experience, would have made the KD easier for them (29). Parents also indicated that they wish they knew before starting the KD how much it would impact their family, KD side effects, and supplies and time needed to prepare the KD (29).
Discussion
This systematic review suggests that the KD is effective for seizure reduction but is difficult to maintain given low adherence rates, high dropout numbers for those enrolled in studies, and an obvious burden for families. The KD is challenging for families to sustain and has nutritional and psychological effects on children that increase the longer the diet is maintained. Seizure reduction is possible, and parents may trade off QoL for their primary concerns of seizure control and decreased medication. The KD results in a number of metabolic changes that can influence height, bone mass density and fractures, cardiac issues, constipation, and kidney stones. Nurses and dietitians could collaborate on long-term planning goals involving simultaneous nutrition supplementation and frequent, regular monitoring for patients on KD to offset malnutrition, vitamin deficiencies, and excessively high lipid profiles. This support would alleviate family concerns and their expressed wish for being apprised of the impact of the diet. Patient's growth and development during KD therapy can also be supported via long-term planning with the multidisciplinary healthcare team. Healthcare practitioners informed of the impact on the QoL of families maintaining the KD for expected but not guaranteed gains would be better able to support families adhering to this challenging therapy. Provision of nursing education on the importance of proper nutrition and exercise, especially with KD therapy, can also help patients and families.
Limitations
The studies included in this review used varying or no definitions of QoL. Attention to and consistent measures of QoL among studies would give us more reliable information on the effect of the KD on families. We are unaware of a current measurement for QoL in children with epilepsy or those using the KD, but there are a number of family QoL instruments.
Our search was limited to full-text accessibility, and we may have missed relevant articles with less than full text. The keywords may have failed to capture all papers addressing QoL. The limited research found on QoL for families prompted questions throughout this paper.
Methodological issues
Many of the studies had small sample sizes limiting the generalizability of findings, specifically Bergqvist et al. (2) (n = 45), Bergqvist et al. (26) (n = 25), Coppola et al. (27) (n = 43), and Hawkes and Levine (23) (n = 3), as well as case reports (11, 24). Overall, dropout rates were sporadically reported, and estimates were 1–88% (34) most often with no explanation.
Retrospective studies (39%) were over-represented in our review, but we believe that renewed clinical interest in the KD at this time prompted researchers to examine the efficacy of the KD used previously (7, 12, 20, 21, 23, 29, 30). The earliest year included in a study was 1990 (20) and the latest date 2013 (12), spanning 23 y. Disadvantages of retrospective studies include key statistics that cannot be measured, and researchers relying on the accuracy of others for well-documented outcome assessments. The temporal relation is difficult to assess in retrospective studies, and this is important in assessing the efficacy of the KD, growth, and development parameters and family response. The adherence to the KD, dropout rates, and corresponding reasons were omitted.
There were a limited number of institutions represented in 3 of the studies that may introduce institutional bias (2, 26, 27). The recruitment took place from single hospitals reducing variation in the explanation of treatment and support received by the parents, factors that may affect adherence to the protocol. Confounding variables were not accounted for in the study by Bergqvist et al. (2), including comorbidities such as cerebral palsy, seizure types, and washout period when antiepileptic drugs were ceased, and KD initiation occurred.
Recommendations
Researchers focused on the efficacy of the KD and less frequently on QoL factors related to psychosocial well-being of patient or family. We found that parents had information needs and required a greater understanding of the KD before starting the diet and more support from others with the experience. Support groups or telephone chains for parents might be a welcome resource. For children consuming the KD, meeting growth and developmental milestones pose a stressor for parents, and create daily concern at mealtimes. The child's diet becomes a key component of family life. We recommend that future research explore the impact of KD on QoL factors, especially the psychological and psychosocial health of children with epilepsy and their families. We recommend cardiovascular health education for families and patients receiving long-term KD treatment, plus the need for close monitoring of patient blood cholesterol, triglycerides, and physical activity levels. Parents must clearly understand the importance of dietary adherence and meticulous preparation of foods. In addition to this, further research on the impacts of physical activity and play activities for children using the KD is recommended because these provide enjoyment.
Supplementary Material
Acknowledgments
Many thanks to Twyla Ens for her early work and guidance to students. The authors’ responsibilities were as follows—RB, MMO, KP, and PW: conducted the research; KP and CM: had primary responsibility for final content; and all authors: designed the research, analyzed the data, wrote the manuscript, and read and approved the final manuscript.
Notes
Author disclosures: KP, CM, MMO, RB, and PW: no conflicts of interest.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AED, antiepileptic drugs; KD, ketogenic diet; MCT, medium-chain triglycerides; QoL, quality of life; 25(OH)D, 25 hydroxyvitamin D.
References
- 1. Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, Shinnar S, Shuman R, Trevathan E, Wheless JW. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol 1998;55(11):1433–7. [DOI] [PubMed] [Google Scholar]
- 2. Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. AJCN 2008;88:1678–84. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt D, Löscher W.. Drug resistance in epilepsy: Putative neurobiologic and clinical mechanisms. Epilepsia 2005;46(6):858–77. [DOI] [PubMed] [Google Scholar]
- 4. Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007;48(1):43–58. [DOI] [PubMed] [Google Scholar]
- 5. Lima PA de, Brito Sampaio LPde, Damasceno NRT.. Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics 2014;69(10):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol 2006;17(5-6):431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taub KS, Kessler SK, Bergqvist AG. Risk of seizure recurrence after achieving seizure freedom on the ketogenic diet. Epilepsia 2014;55(4):579–83. [DOI] [PubMed] [Google Scholar]
- 8. Kossoff EH, Wang HS. Dietary therapies for epilepsy. Biomed J 2013;36(1):2–8. [DOI] [PubMed] [Google Scholar]
- 9. Huttenlocher P. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res 1976;10:536–40. [DOI] [PubMed] [Google Scholar]
- 10. Yeou-mei CL. Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 2008;49:33–36. [DOI] [PubMed] [Google Scholar]
- 11. Kossoff EH, Turner Z, Bergey GK. Homeguided use of the ketogenic diet in a patient for more than 20 years. Pediatr Neurol 2007;3(6):424–5. [DOI] [PubMed] [Google Scholar]
- 12. Selter JH, Turner Z, Doerrer SC, Kossoff EH. Dietary and medication adjustments to improve seizure control in patients treated with the ketogenic diet. J Child Neurol 2014;30(1):53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu YM, Wang HS. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed J 2013;36(1):9–15. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz RH, Eaton J, Bower BD, Aynsley-Green A.. Ketogenic diets in the treatment of epilepsy: Short-term clinical effects. Dev Med Child Neurol 1989;31(2):145–51. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization (WHO) World Health Organization Quality of Life (WHOQOL): Measuring Quality of Life. Programme on Mental Health. Geneva, Switzerland: World Health Organization; Retrieved fromhttp://www.who.int/mental_health/media/68.pdf/1997. [Google Scholar]
- 16. Magarey JM. Elements of a systematic review. Int J Nurs Pract 2001;7(6):376–82. [DOI] [PubMed] [Google Scholar]
- 17. International Society for Quality of Life Research (ISOQOL) What is health-related quality of life research? Milwaukee, WI: International Society for Quality of Life Research; Retrieved fromhttp://www.isoqol.org/about-isoqol/what-is-health-related-quality-of-life-research/2014. [Google Scholar]
- 18. Camfield C, Breau L, Camfield P.. Impact of pediatric epilepsy on the family: A new scale for clinical and research use. Epilepsia 2008;42(1):104–12. [DOI] [PubMed] [Google Scholar]
- 19. Westphal-Guitti AC, Alonso NB, Vaz Pedroso Migliorini RC, da Silva TI, Azevedo AM, Caboclo LOSF, Sakamoto AC, Yacubian EMT. Quality of life and burden in caregivers of patients with epilepsy. J Neurosci Nurs 2007;39(6):354–60. [DOI] [PubMed] [Google Scholar]
- 20. Caraballo R, Vaccarezza M, Cersósimo R, Rios V, Soraru A, Arroyo H, Agosta G, Escobal N, Demartini M, Maxit C et al.. Long-term follow-up of the ketogenic diet for refractory epilepsy: Multicenter Argentinean experience in 216 pediatric patients. Seizure 2011;20:640–5. [DOI] [PubMed] [Google Scholar]
- 21. Dressler A, Stocklin B, Reithofer E, Benninger F, Freilinger M, Hauser E, Reiter-Fink E, Seidl R, Trimmel-Schwahofer P, Feucht M. Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy—the Austrian experience. Seizure 2010;19:404–8. [DOI] [PubMed] [Google Scholar]
- 22. Christodoulides SS, Neal EG, Fitzsimmons G, Chaffe HM, Jeanes M, Aitkenhead H, Cross JH. The effect of the classical and medium chain triglyceride ketogenic diet on vitamin and mineral levels. Clin Nutr 2011;25(1):16–26. [DOI] [PubMed] [Google Scholar]
- 23. Hawkes CP, Levine MA. Ketotic hypercalcemia: a case series and description of a novel entity. J Clin Endocrinol Metab 2014;99(5):1531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sirikonda NS, Patten WD, Phillips JR, Mullett CJ.. Ketogenic diet: Rapid onset of selenium deficiency-induced cardiac decompensation. Pediatr Cardiol 2012;33:834–8. [DOI] [PubMed] [Google Scholar]
- 25. Raju KNV, Gulati S, Kabra M, Agarwala A, Sharma S, Pandey RM, Kalra V.. Efficacy of 4:1 (classic) versus 2:5:1 ketogenic ratio diets in refractory epilepsy in young children: A randomized open labeled study. Epilepsy Res 2011;96:96–100. [DOI] [PubMed] [Google Scholar]
- 26. Bergqvist AG, Schall JT, Stallings VA. Vitamin D status in children with intractable epilepsy, and impact of the ketogenic diet. Epilepsia 2007;48(1):66–71. [DOI] [PubMed] [Google Scholar]
- 27. Coppola G, Natale F, Torino A, Capasso R, D'Anniello A, Pironti E, Santoro E, Calabrò R, Verrotti A.. The impact of the ketogenic diet on arterial morphology and endothelial function in children and young adults with epilepsy: A case control study. Seizure 2013;23(4):260–5. [DOI] [PubMed] [Google Scholar]
- 28. Sharma S, Gulati S, Kalra V, Agarwala A, Kabra M. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy—Indian experience. Seizure 2009;18(6):446–9. [DOI] [PubMed] [Google Scholar]
- 29. McNamara NA, Carbone LA, Shellhaas RA. Epilepsy characteristics and psychosocial factors associated with ketogenic diet success. J Child Neurol 2012;28(10):1233–7. [DOI] [PubMed] [Google Scholar]
- 30. Kim JT, Kang H-C, Song J-E, Lung MJ, Lee YJ, Lee EJ, Lee JS, Kim HD. Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin Nutr 2013;32(1):98–103. [DOI] [PubMed] [Google Scholar]
- 31. Tagliabue A, Bertoli S, Trentani C, Borrelli P, Veggiotti P.. Effects of the ketogenic diet on nutritional status, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: A 6-month prospective observational study. Clin Nutr 2012;31(2):246–9. [DOI] [PubMed] [Google Scholar]
- 32. Lambrechts DAJE, Bovens MJM, de la Parra NM, Hendriksen JGM, Aldenkamp AP, Majoie MJM. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta Neurol Scand 2013;127(2):103–8. [DOI] [PubMed] [Google Scholar]
- 33. Hallböök T, Lundgren J, Rosén I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia 2007;48(1):59–65. [DOI] [PubMed] [Google Scholar]
- 34. Payne NE, Cross JH, Sander JW, Siswodiya SM. The ketogenic and related diets in adolescence and adults – a review. Epilepsia 2011;52(11):1941–8. [DOI] [PubMed] [Google Scholar]
- 35. Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.