Abstract
Approximately 5% of all gynecologic cancers are of the vulva, of which 90% are squamous cell in origin. Adenocarcinomas of the vulva are extremely infrequent with most relating to epithelial glands in the vulvar region. A 53 year old African American female presented to the emergency department complaining of a lesion on her left labia that had been present for the past 6 months. In the operating room, multiple biopsies were taken of the left labial lesion as well as the right, and sufficient tissue was sent to pathology for analysis. The pathology report demonstrated adenocarcinoma of the vulva with intestinal type features, CD20+, CK7-, mCEA+, vimentin -, p53+. These findings were consistent with a colorectal primary; however, no colorectal primary was discovered. The intestinal type of primary adenocarcinoma of the vulva is a rare variant, and only a few cases have been reported to date. It histologically resembles mucinous colonic carcinomas, but immunohistochemical workups with various tumor markers are needed before confirmation.
Keywords: Adenocarcinoma, Oncology, Vulvar carcinoma, Vulvar cancer, Gynecology
Highlights
-
•
Adenocarcinoma of the vulva is rare.
-
•
A rare variant of adenocarcinoma of the vulva is intestinal-type that resembles colorectal
-
•
CK7-, mCEA+, CK20+, p53+ IHC profile favors intestinal primary
-
•
Treatment for this cancer should be individualized
1. Introduction
The vulvar region of the female reproductive system represents an area that encompasses all structures from the mons pubis to the perineal body. This region is exposed to many different environmental insults such as UV radiation, opportunistic infections, sexually transmitted diseases, and physical trauma. Specific risk factors related to these insults, which can contribute to the development of vulvar carcinoma, are HPV infection, radiation exposure, and smoking. Other risk factors include vulvar dystrophies, such as lichen sclerosus, age, and Northern European descent. Approximately 5% of all gynecologic cancers are of the vulva, of which 90% are squamous cell in origin (Beckman et al., 2014). Other frequent malignancies occurring in the vulva include basal cell carcinomas, melanomas, and sarcomas. Adenocarcinomas of the vulva are extremely uncommon, and most relate to epithelial glands in the vulvar region (i.e. Bartholin glands). Of the subtypes of adenocarcinomas, the intestinal type is an even more rare variant of vulvar adenocarcinoma. In the 4th edition of WHO classification of tumors of female reproductive organs, primary villoglandular mucinous adenocarcinoma is defined as a primary invasive glandular epithelial tumor of intestinal type. Synonyms are cloacogenic carcinoma or cloacogenic adenocarcinoma (Kurman et al., 2011). Currently, this type of vulvar cancer is vastly understudied. However, presentation and treatment can differ significantly from the majority of squamous cell vulvar carcinomas. We will present a case of intestinal-type adenocarcinoma of the vulva in order to demonstrate the presentation and treatment of such neoplastic variants.
2. The case
A 53 year old African American female presented to the emergency department complaining of a lesion on her left labia that had been present for the past 6 months. Our service was consulted to examine and evaluate the patient. Upon questioning, the patient noted that the lesion was initially itchy and smaller in size. The patient tried over the counter creams and ointments with no relief. The lesion continued to grow in size and became more painful. The patient noted yellowish, white discharge from the area as well. The patient had scheduled an appointment with a primary care physician to assess the lesion but when the pain became too great, she decided to come to the emergency department. The patient's review of systems was significant for vaginal discharge; however, the remaining systems were negative. The patient denied any vaginal bleeding or odor. The patient's medical history was significant for asthma and hypertension, both of which were controlled medically. The patient's surgical history was unremarkable. Her social history demonstrated a long history of cigarette smoking and occasional alcohol use. Patient denied currently being sexually active, and she admitted to only one partner. Patient denied any significant gynecologic or obstetrical history. Her last menstrual period was 2–3 years ago. During our physical exam a 6 cm purple left labial lesion was noted that was tender to the touch and had white discharge oozing from it. A bimanual exam was performed, and the mass was felt to extend posteriorly toward the rectum. There was also a 1–2 cm mass noted on the right labial area. A speculum exam noted no lesions in the vaginal canal or on the cervix. Cultures were taken of the areas and sent for analysis. Routine labs were drawn, and a urine culture and KOH wet prep were within normal limits. The patient was started on empiric antibiotic therapy and scheduled for a possible incision and drainage and biopsy.
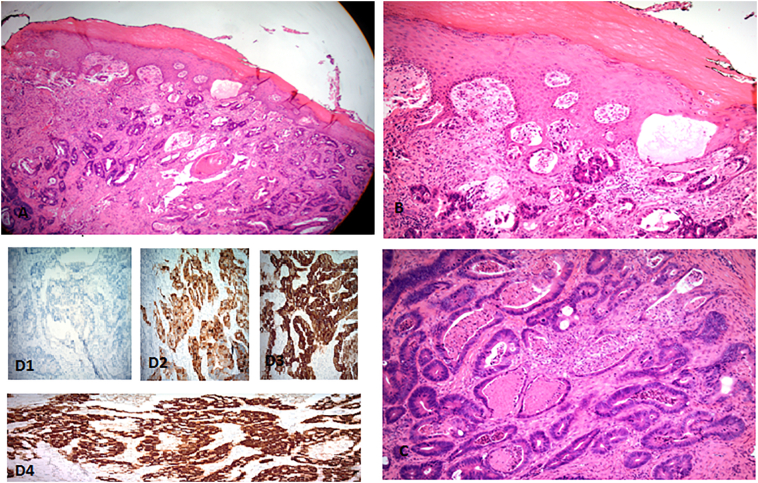
In the operating room, multiple biopsies were taken of the left labial lesion as well as the right, and sufficient tissue was sent to pathology for analysis. The pathology report came back demonstrating adenocarcinoma of the vulva with intestinal type features, CD20+, CK7-, mCEA+, vimentin-, p53+. These findings were consistent with a colorectal primary (See Fig. 1). General surgery was consulted, and they performed an occult stool sample which came back negative. The patient gave a subjective history of an up-to-date colonoscopy which did not demonstrate any precancerous or cancerous lesions; however, no official report was obtained. A CT scan was ordered. It did not show any mass in the colon, and after examination, a colorectal primary was ruled out. The patient was then instructed to follow-up at a facility with a gynecologic oncologist. After examination with the gynecologic oncologist, the patient was referred to an oncologist at a facility closer to her home to manage her chemotherapy and radiation. During that workup, the patient was found to have multiple lung nodules that suggested metastasis.
Fig. 1.
A–B Overlying normal squamous epithelium underlying adenocarcinoma. C- adenocarcinoma with dirty tumor necrosis. D1–4, Immunohistochemical staining of the tumor cells demonstrates diffuse positive reaction for CEA, CK20, and CK7.
Upon seeing an oncologist, it was determined that the patient would begin on a chemotherapy regime of mitomycin-c and 5-fluorouracil. Radiation to the vulva, inguinal nodes, and lung nodules were scheduled. The radiation would be 4500 cGy in 25 fractions using IMRT with a sequential boost to the gross disease at 6480 cGy in 11 additional fractions. Medical oncology decided to change her chemotherapy to weekly carboplatin and taxol. The patient completed multiple rounds of chemotherapy and radiation. However, the patient's treatment was complicated by multiple hospital admissions for pain control. Examinations of the lesions and repeat CT scans demonstrated progression of lesions in the vulvar area and lungs. The patient opted to be placed on hospice care, and died soon after. Patient disease discovery and death occurred in a little over a year's time.
3. Discussion
A patient with a rare diagnosis of primary villoglandular mucinous adenocarcinoma of the vulva is described in this report. The clinical behavior of this neoplasm is indolent, and patients typically present with a vulvar lesion that can be painful or pruritic in nature. Malignancies of the female genital tract are less commonly seen in the vulva than other reproductive tissues, such as the uterus, ovary, or cervix (Beckman et al., 2014). Of the multiple subtypes of vulvar cancer, a primary villoglandular mucinous adenocarcinoma of intestinal type is a rare occurrence, and only a few cases have previously been reported. Villoglandular adenocarcinomas are more commonly noted to be found in the colon and rectum; the possibility of this patient having a primary tumor of the gastrointestinal tract was ruled out by a negative workup, thereby classifying her lesion as intestinal type and not a colorectal primary. This patient displayed the classic immunohistochemical profile of a colorectal carcinoma: CK7- and CK20+.
There is currently no known precise mechanism defined into the development of cloacogenic adenocarcinoma. Some have hypothesized that, since the urethra, lower vagina, and rectum originate from the cloaca prior to their division in embryologic development, a loci of gastrointestinal tissue remains in the lower vagina (Tiltman and Knutzen, 1978). During embryogenesis, the labia majora develops from the labioscrotal folds, and the labia minora develops from the urethral folds. These structures are closely involved in cloacal development, and it is believed they can experience malignant transformation similar to a primary adenocarcinoma of the colon (Lee et al., 2017).This remnant tissue develops enteric neoplasia and, subsequently, cloacogenic adenocarcinoma in this group of patients. Other possible mechanisms of a colorectal cancer developing in a non-colorectal site include ectopic intestinal epithelium or sites of intestinal metaplasia within tissues of Mullerian ductal origin (Kennedy and Majmudar, 1993). The definitive origin of this type of tumor is vastly unknown and is still subject to debate.
Immunohistochemical techniques were utilized to determine the origin of villoglandular adenocarcinoma of the vulva. Our patient displayed the immunohistochemical profile of a CK7- and CK20+. Keratin 20, or CK20, is a type I cytokeratin protein of enterocytes and goblet cells, and is specifically found in the gastric and intestinal mucosa. CK7, or cytokeratin-7, is a type II cytoskeletal protein in humans that is specifically expressed in the simple epithelia lining the cavities of the internal organs, and in the gland ducts and blood vessels. This data suggests that the type of this villoglandular mucinous adenocarcinoma is colorectal in origin.
Treatment of these cancers is understudied and involve local resection as curative. Radical local excision with tumor-free margin is viewed as a safe surgical option (Hacker et al., 1984). Our patient, however, presented with an inoperable advanced form of vulvar carcinoma necessitating a more systemic treatment. The best treatment option for our patient involved using chemotherapeutics that are typically used for colorectal primaries, 5-fluorouracil and mitomycin-c and radiation therapy. Mitomycin-c is commonly used to radiosensitize the tumor and 5-fluorouracil is used as an anti-metabolite, principally by inhibiting thymidylate synthase. The patient's treatment was changed by her oncologist due to similar efficacy of the carboplatin with a taxol based treatment.
In summary, the intestinal type of primary adenocarcinoma of the vulva is a rare variant and only a few cases have been reported to date. It histologically resembles mucinous colonic carcinomas and immunohistochemical workups with various tumor markers are needed before confirmation. There are currently no standardized guidelines for the diagnosis, clinical course, and treatment of intestinal type mucinous adenocarcinoma of the vulva. Most treatments today are personalized toward the patient. This case study provides useful information to help establish the diagnosis and treatment modalities for intestinal type mucinous adenocarcinoma of the vulva.
Author contributions
BK and RM wrote and provided all relevant data for manuscript. PS and MM provided editorial services. All authors reviewed the final manuscript.
References
- Beckman, Charles R., Frank Ling, Wiliam Herbert, Douglas Laube, and Roger Smith. Obstetrics and Gynecology, American College of Obstetricians and Gynecologists, 7e. 7th ed., Baltimore, Lippincott, Wlliams & Wilkins, 2014. Accessed 21 Nov. 2017.
- Hacker N.F., Berek J.S., Lagasse L.D., Nieberg R.K., Leuchter R.S. Individualization of treatment for stage I squamous cell vulvar carcinoma. Obstet. Gynecol. 1984;63:155–162. [PubMed] [Google Scholar]
- Kennedy J.C., Majmudar B. Primary adenocarcinoma of the vulva, possibly cloacogenic: a report of two cases. J. Reprod. Med. 1993;38:113–116. [PubMed] [Google Scholar]
- Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H. IARC Press; France: 2011. WHO Classification of Tumours of Female Reproductive Organs. Lyon. [Google Scholar]
- Lee I.H., Kim M.K., Lee Y.K., Hong S.R., Lee K.H. Primary mucinous adenocarcinoma of the vulva, intestinal type. Obstet. Gynecol. Sci. 2017;60(4):369–373. doi: 10.5468/ogs.2017.60.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiltman A.J., Knutzen V.K. Primary adenocarcinoma of the vulva originating in misplaced cloacal tissue. Obstet. Gynecol. 1978;51:30s–33s. [PubMed] [Google Scholar]