Abstract
The effect of genotype and timing of salicylic acid (SA) application on response of maize to salinity stress has been investigated. Single and triple hybrids (SH and TH, respectively) of maize were grown hydroponically and sprayed with 1 mM SA one week either before or after application of 150 mM NaCl. The effect of salinity on maize performance was stronger than that of SA regime or maize hybrid. The effect of treatments was most evident on root biomass but least evident on number of leaves. The genotypic difference in shoot biomass was vague in non-amended plants but emerged, in favor of the SH, in SA-amended plants. The more vigorous SH exhibited less pigment content (particularly the post-amended plants) and less salt resistance, with preferential native allocation of plant biomass to root compared with the TH. Salicylic acid, particularly the post amendment under salinity stress, was stressful to maize foliage but beneficial to roots. Salinity reduced root growth to a greater extent than shoot growth, with the production of wider and shorter blades; but SA led to the opposite effect. The effect of salinity on leaf chlorophyll a concentration was non-significant in the SH, versus an increase (in the post-amended plants) or a decrease (in the non-amended and pre-amended plants) of the TH. Sub-stomatal CO2 concentration (Ci) was higher in the SH than the TH, particularly in the post-amended plants. Both salinity and SA induced stomatal closure, reduced rates of transpiration and photosynthesis but increased Ci, with variable magnitudes in the two hybrids. Salinity increased concentrations of soluble sugars, proline and Na+ in the leaves, decreased K+ and phenolics concentrations but marginally affected protein concentration with limited effect of SA, which varied according to time of application. The further stressing effect of SA post-amendment to salt-stressed maize can be related to reduced stomatal conductance and concentrations of phenolics, proline and K+ but increased Na+ concentration of the shoot, particularly of the TH.
Keywords: Agriculture, Biochemistry, Physiology, Plant biology
1. Introduction
Salt stress, arising either from primary or secondary salinization of the soil, plays a major role in deterioration of plant productivity, particularly in the arid and semi-arid regions of the world. In the arid zones, limited rainfall, high evapotranspiration rate and poor water quality further aggravate the problem, leading to accumulation of soluble salts in the soil surface. The adverse effect of salinity on plant growth can be attributed either to an osmotic effect, specific ion effect, nutritional imbalance or induction of oxidative stress. These effects are manifested as impaired membrane stability and deterioration of plant performance. The first threat that plants might encounter under salt stress is the lowering of water potential of the medium, resulting in tissue dehydration (Mansour et al., 2005), followed by specific ion effects and nutritional imbalance; where high uptake rates of salt ions will disturb the plant K/Na selectivity and induce deficiency of several nutrients such as K+, Ca2+ and NO3- (George et al., 2012). In addition, salt stress can induce stomatal closure, which limits CO2 fixation and increases the generation of reactive oxygen species (ROS) (Gunes et al., 2007; Murata et al., 2007), leading to oxidative damage of cellular constituents, manifested as enzyme deactivation, lipid peroxidation, protein degradation and DNA damage (Banu et al., 2010).
Although salt resistance in plants is usually quantified in terms of survival rates and/or growth potentiality; it, in fact, involves a complex array of subtle changes at the physiological, biochemical and molecular levels that will ultimately appear as morphological and developmental changes. Plant cells contain a range of protective and repair systems, both enzymatic and non-enzymatic, to protect cells from oxidative damage. These systems might act as scavengers of ROS, e.g. superoxide dismutase, catalase and peroxidases or can regenerate antioxidants e.g. glutathione reductase and dehydroascorbate reductase (Demiral and Türkan, 2005).
The deleterious effects of ROS on plant performance could be mitigated by manipulating several amendments such as growth-stimulating hormones, polyamines and antioxidants (George et al., 2012). Salicylic acid (SA) has been recognized as an endogenous natural signal molecule involved in defense mechanisms by regulating diverse physiological and biochemical processes (Khoshbakht and Asgharei, 2015). Studies have shown that SA can act as a modulator for stresses arising from salinity (Khoshbakht and Asgharei, 2015), drought (Shao et al., 2018), and heavy metals (Zhou et al. 2009) via regulating proline and other osmolytes production (Chandrakar et al., 2016). Exogenous application of SA can enhance the activities of antioxidant enzymes such as peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT) in drought-stressed (Hayat et al., 2008) and salt-stressed (Szepesi, 2008) tomato.
Maize (Zea mays L.) is the third most important cereal crop after rice and wheat. The plant is moderately salt-sensitive C4 species, with wide genotypic variability which allows its cultivation in a wide range of soils and under various climatic conditions (Mansour et al., 2005). In addition to their high nutritive value, maize grains are a good source of bioactive phytochemicals such as carotenoids, phenolics, phytosterols and the unique constituent of potential anti-HIV activity (GNA-maize) (Rouf Shah et al., 2016). As a C4 crop, with high growth rate and relatively short life span, maize cultivation can be considered an efficient way for land utilization in the arid zones. In Egypt, both single and triple hybrids of maize have been widely cultivated. The single hybrid of maize has been reported to exhibit greater yield under the Egyptian conditions with greater water use efficiency compared with the triple hybrid (Al-Naggar et al., 2011), which evaluates the single hybrid as an efficient option for cropping in arid lands.
To alleviate the impact of abiotic stress on plant performance, several plant growth promoters as well as beneficial amendments such as SA and glycinebetaine have been examined in different modes of application, ranging from seed presoaking to foliar spray at different stages of plant development (Chen and Murata, 2008; Banu et al., 2010). It is likely that the effect of exogenous SA depends on several factors, including the dose, plant species, developmental stage and the mode of application (Horváth et al., 2015; Poór et al., 2019). The present work was conducted to resolve the role of timing of SA application to maize in alleviation of salt stress during the vegetative stage. Salicylic acid was applied to maize, as foliar spray, either before or after imposing salt stress to test the hypothesis that application of SA before imposing salt stress triggers some physiological responses necessary for salt resistance, or alternatively application of SA alleviates the consequences of salt stress after its incidence.
2. Materials and methods
2.1. Plant material and growth conditions
Seeds of the single hybrid (SH) and triple hybrid (TH) of maize (Zea mays L.) were obtained from the Institute of Crop Production, Agricultural Research Center at Giza, Egypt. Seeds, selected for uniformity, were surface-sterilized with 5% sodium hypochlorite for five minutes and washed thoroughly with distilled water. Seeds were, then, sown on washed sand in 19 cm diameter plastic pots, three seeds per pot and watered with 0.5 mM CaSO4 for 4 days. Emerged seedlings were successively thinned to one per pot before application of nutrient solution. At this stage, seedlings produced the second foliage leaf and were adequately supplied with a complete nutrient solution for 8 days. The nutrient solution contained the following macronutrients: (mM) N 10.5 (9.5 NO3-, 1 NH4+), K 5, Ca 2, P 0.5, Mg 1, S 1 and the micronutrients (μM) Fe 50, Mn 5, Cu 0.5, Zn 0.5, B 25, Mo 0.25 and Co 0.1.
After 12 days from sowing (8 days from irrigation with the nutrient solution), the salinity × SA treatments started; by that time plants produced the fourth foliage leaf. Plants of each cultivar were divided into three groups: one sprayed with distilled water containing the chemical background of the SA solution (Non-amended), the second group received 1 mM SA as foliar spray one week before imposing salinity stress (Pre-amended) and the third group was intended to receive 1 mM SA one week after imposing salinity stress (Post-amended). Salinity treatment started one week after spraying of the pre-amended plants, where plants of each SA treatment were further divided into two sub-groups: one receiving the nutrient solution (control) and the other received 150 mM NaCl superimposed on the nutrient solution (salinized). One week after imposing of salinity stress the Post-amended plants were sprayed with 1 mM SA and the plants were subjected to salinity treatment for one week more; the salinity treatment, thus, lasted 14 days. The non-amended plants were subjected to salinity stress in the same way as the amended plants. Salicylic acid solution (1 mM) was prepared by dissolving the powder in few drops of dimethyl sulfoxide, followed by the addition of distilled water and adjustment of pH at 5.5 with 0.1 N KOH. Spraying with SA was done once in the early morning. Plants were grown in a greenhouse at the Faculty of Science, Damietta University, Egypt, with irradiance of 1200 μmol m-2 s-1 from natural sunlight in a 14/10 h photoperiod, temperature of 27–35 °C and 80% relative humidity in average.
2.2. Plant harvest and analysis
Gas exchange parameters were estimated just before harvest. Plants were harvested after 33 days from sowing (21 days from beginning of treatments). Plants were gently washed from sand, blotted and separated into roots and shoots. Fresh weights were recorded and pieces of the third youngest leaf were dipped in liquid N2 and kept at −80 °C until used. Dry weights of shoots and roots were estimated after drying at 80 °C in an air-forced oven for 48 hours; and the shoot dry weight was corrected for the leaf portion kept frozen by adding the equivalent dry weight of the frozen portion to the measured shoot dry weight. Dry plant material was ground into a fine powder before analysis. The frozen plant material was used for estimation of leaf pigments and antioxidants, while the powdered dry matter was used for estimation of minerals and carbohydrates. Leaf area of the third youngest leaf (Al) was calculated according to Bonhomme et al. (1982) as follows: Al = 0.5 × L × W, where L is the length and W the width of blade.
2.2.1. Estimation of gas exchange parameters
Photosynthetic rate (A), stomatal conductance (Gs), substomatal CO2 concentration (Ci), transpiration rate (E) and leaf surface temperature (Tl) of the third youngest leaf were measured at 10:00 a.m. using an LCA-4 portable gas exchange system (Analytical Development Company Ltd, England). Measurements were conducted with leaf area of 6.25 cm2, leaf chamber CO2 concentration of 390 ppm at chamber temperature of 37 °C and PPFD of 1800 μmol photons m−2 s−1.
2.2.2. Estimation of photosynthetic pigments
An aliquot of the frozen leaf was macerated in 80% acetone in dim light. The slurry was centrifuged and the clear extract was brought up to volume with 80% acetone and absorbance was read at 452.5, 644 and 663 nm using a UNICO 7200 series spectrophotometer. The concentrations of chlorophyll a, chlorophyll b and carotenoids were calculated (μg ml−1) using the equations of Metzner et al. (1965).
2.2.3. Estimation of carbohydrates
About 0.05 g of the powdered dry leaves was extracted overnight with 5 ml of 80% ethanol. The mixture was centrifuged at 10,000 × g for 10 minutes and extraction was repeated using fresh ethanol. The extracts were combined, dried in a water bath, and the residue was re-dissolved in 5 ml of distilled water before determination of total soluble sugar (TSS). Aliquots (300 μl) of the aqueous extract were made to 1 ml by distilled water, carefully mixed with 4 ml of the anthrone reagent (8.6 mM anthrone in 80% v/v H2SO4) and heated for 10 minutes at 80 °C in a water bath, then cooled for 30 minutes on ice. Absorbance was read at 623 nm and soluble sugars were estimated from a glucose calibration curve in the range of 0–100 μg ml−1 (Schlüter and Crawford, 2001). Starch was assayed in the debris left after extraction of soluble sugars according to Buysse and Merckx (1993). The debris was re-suspended in 3% HCl in a water bath at 70 °C for 2 hours. The mixture was centrifuged at 8000 × g for 10 minutes and starch was determined in the supernatant using the anthrone method.
2.2.4. Estimation of proline
A known weight of the frozen leaf was extracted in 3% sulfosalicylic acid and the clear extract was reacted with the acid ninhydrin reagent according to Bates et al. (1973). Proline concentration was calculated from a standard curve in the range of 0–100 μg proline ml−1.
2.2.5. Estimation of protein
A known weight of the frozen leaf was grinded in liquid nitrogen using pestle and mortar, and the slurry was extracted in 600 μl of 50 mM HEPES (pH 7.4) containing 1 mM EDTA and 6 μl of 500 mM PMSF in methanol. Protein concentration of the clear extract was estimated using the Coomassie brilliant blue reagent according to Bradford (1976) in reference to a standard curve of bovine serum albumin (BSA) in the range 0–100 μg ml−1.
2.2.6. Estimation of free phenolics
Free phenolic content of leaves was estimated using the Folin-Ciocalteau reagent described by Singleton and Rossi (1965). A known weight of the frozen leaf was extracted in 50% methanol for 2 h at 80 °C in a water bath, and the mixture was centrifuged at 10, 000 × g for 5 minutes. Aliquots of the supernatant were reacted with the Folin-Ciocalteau reagent. Absorbance of the resulting blue color was measured at 725 nm, and phenolics content was assayed with reference to a standard curve of gallic acid in the range of 0–20 μg ml−1.
2.2.7. Estimation of minerals
The powdered plant material was extracted in 1.5 ml of distilled water in Eppendorf tubes by heating at 95 °C for 2 hours (Hansen and Munns, 1988). The debris was removed by centrifugation at 8,000 × g for 10 minutes, and the clear extract was used for determination of K+ and Na+ using a Jenway PFP7 flame photometer.
2.3. Statistical analysis
The experiment was factorial with three main factors and 4 replications in a completely randomized design. The main factors were 1) maize hybrid with two levels: SH and TH, 2) SA amendment with three levels: no amendment, pre-amendment and post-amendment and 3) salinity with two levels: control (0 mM NaCl) and salinized (150 mM NaCl). Data analysis was performed by using the SPSS software version 22. Three-way ANOVA evaluated the effects of the main factors and their interactions on plant growth and performance. Mean separation was performed according to the Duncan's multiple range test at P ≤ 0.05. Correlation analysis was estimated as the Pearson correlation coefficient (R) at different levels of significance.
3. Results
3.1. Plant growth
The F ratios and the associated P values reveal that the strongest factor controlling maize growth was salinity, which invariably exerted significant effects on all growth measurements, followed by timing of SA application; while the least effective factor was maize hybrid (data not shown). Likewise, the most sensitive measurement of maize growth was root dry weight while the least-sensitive measure was number of leaves.
The genotypic difference in shoot biomass was vague in non-amended plants but emerged, with appreciable superiority (although non-significant) of the SH over the TH, in amended plants. The adverse effect of salinity on shoot dry weight was mild and more evident in the SH than the TH for non-amended and pre-amended plants but was relatively severe and comparable in the two hybrids for post-amended plants. The effect of SA on shoot dry weight of the two hybrids was marginal and inconsistent but was only pronounced due to post-amendment of the salinized plants where the reduction amounted to 50% in the TH (Fig. 1A). The genotypic variability in root dry weight was marked in favor of the SH, particularly the non-treated and the post-amended plants. The reduction in root DW of the SH due to salinity averaged around 43%, irrespective of the amendment regime; whereas in the TH, the effect of salinity was particularly evident in post-amended plants. In the SH, root dry weight was generally improved by SA amendment, particularly due to post-amendment. In the TH, the beneficial effect of SA was marked in the non-salinized plants, particularly due to post-amendment, versus mild reductions in the salinized plants (Fig. 1B). The R/Sh dry weight ratio was comparable in the two hybrids, except the marked superiority of the SH only in the non-treated plants. The effect of salinity on R/Sh ratio was mild but emerged as marked reduction in the non-amended SH and post-amended plants of both hybrids. By contrast, SA amendment, particularly the pre-amendment, increased R/Sh ratio (Fig. 1C).
Fig. 1.
Shoot dry weight (A), root dry weight (B) and the R/Sh DW ratio (C) of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
The effect of treatments on number of leaves was negligible except for the 10% reduction due to salinity in the non-amended TH (Table 1). The advantage of the SH over TH in leaf dimensions was not evident in non-amended plants; but it emerged for blade length in the amended plants, irrespective of salinity treatment and for blade width in salinized plants, irrespective of timing of SA application. The reduction in leaf dimensions due to salinity was more evident in the amended than in non-amended plants, in blade length than in blade width (for the SH) but was comparable in both dimensions of the TH. The effect of SA amendment on leaf dimensions varied according to the hybrid × salinity combination. SA increased blade length of the SH, particularly in absence of salinity but reduced that of the TH particularly due to post-amendment of salinized plants. Blade width of the SH was non-significantly affected by SA application, but that of the TH was either increased (in non-salinized plants) or reduced (in salinized plants) only due to post-amendment. In non-amended plants, the SH produced slightly larger leaves with greater blade width/length ratio, compared with the TH. This trend was modified by SA amendment, with production of markedly larger leaves of the SH than those of the TH but of comparable width/length ratio or even lower ratio in the SH as was the case in in the post-amended plants. Salinity led to mild increase in the blade width/length ratio of the SH, with non-significant effect in the TH. The effect of amendment regime on blade width/length ratio of the SH was negligible in contrast to a limited increase in the TH (Table 1).
Table 1.
Number of leaves, leaf dimensions and leaf area of the third youngest leaf of the single and triple hybrids of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each value is the mean of 4 replicates ±SE.
| Amendment and salinity level | Number of leaves |
Blade length (cm) |
Blade width (cm) |
Blade area (cm2) |
Blade width/length ratio |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single hybrid | Triple hybrid | Single hybrid | Triple hybrid | Single hybrid | Triple hybrid | Single hybrid | Triple hybrid | Single hybrid | Triple hybrid | |
| No amendment | ||||||||||
| 0 mM NaCl | 7.3 ± 0.23abc | 7.8 ± 0.23a | 59 ± 1.7bc | 62 ± 2.0ab | 4.8 ± 0.17ab | 4.0 ± 0.15cd | 143 ± 9bcd | 124 ± 8cde | 0.08 ± 0.00bc | 0.07 ± 0.00c |
| 150 mM NaCl | 7.0 ± 0.00bc | 7.0 ± 0.00bc | 52 ± 1.7de | 58 ± 1.8bc | 5.2 ± 0.13a | 4.5 ± 0.06bc | 135 ± 6cd | 132 ± 6cde | 0.10 ± 0.00a | 0.08 ± 0.00bc |
| Pre-amendment | ||||||||||
| 0 mM NaCl | 7.0 ± 0.38bc | 7.5 ± 0.27ab | 67 ± 1.8a | 57 ± 0.3bcd | 5.1 ± 0.18a | 5.1 ± 0.10a | 172 ± 9a | 146 ± 2bc | 0.08 ± 0.00bc | 0.09 ± 0.00ab |
| 150 mM NaCl | 7.0 ± 0.00bc | 7.0 ± 0.00bc | 55 ± 1.1cd | 48 ± 1.8e | 5.1 ± 0.26a | 4.5 ± 0.35bc | 139 ± 7cd | 110 ± 9e | 0.09 ± 0.01ab | 0.09 ± 0.01ab |
| Post-amendment | ||||||||||
| 0 mM NaCl | 7.3 ± 0.23abc | 7.3 ± 0.45abc | 67 ± 2.2a | 49 ± 1.3e | 4.8 ± 0.21ab | 4.9 ± 0.12ab | 163 ± 2ab | 122 ± 5de | 0.07 ± 0.01c | 0.10 ± 0.00a |
| 150 mM NaCl | 7.0 ± 0.00bc | 6.8 ± 0.23c | 57 ± 2.6bcd | 37 ± 1.0f | 4.8 ± 0.10ab | 3.7 ± 0.13d | 136 ± 9cd | 69.4 ± 4f | 0.08 ± 0.00bc | 0.10 ± 0.00a |
Means with common letters are non-significantly different at P ≤ 0.05.
3.2. Photosynthetic pigments
The concentrations of Chl a and carotenoids of maize leaves were significantly affected by the hybrid × salinity × SA interaction but Chl b concentration was less evidently affected (data not shown). Generally, the concentrations of Chl a and carotenoids were lower in the SH than the TH (particularly in the post-amended plants); with the exception of the salinized non-amended plants where the reverse was true. Salinity had a non-significant effect on Chl a concentration of the SH but led to either a significant increase (in the post-amended plants) or a significant decrease (in the non-amended and pre-amended plants) of the TH. Salinity non-significantly affected carotenoid concentration of the non-amended SH and pre-amended plants of both hybrids but led to either an increase (in the post-amended plants of both hybrids) or a decrease (in the non-amended TH). Application of SA lowered concentrations of Chl a and carotenoids in the control and salinized SH as well as the control TH but increased them in the salinized TH (Fig. 2).
Fig. 2.
Concentrations of Chl a (A), Chl b (B) and carotenoids (C) in the third youngest leaf of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.0.
Chl b concentration was generally comparable in the two hybrids, except in the non-amended and post-amended salinized plants, where it was significantly higher and lower, respectively in the SH than the TH. Salinity non-significantly affected Chl b concentration of the non-amended and pre-amended SH and TH but led to either a significant increase (in the post-amended TH) or a significant decrease (in the post-amended SH and the non-amended TH). Application of SA generally led to non-significant changes in Chl b concentration; only a significant effect was found in the salinized plants, with a reduction due to post-amendment of the SH versus an increase due to pre- and post-amendment of the TH (Fig. 2).
3.3. Gas exchange
Gas exchange parameters were significantly (P < 0.05) affected by treatments but with non-significant genotypic difference in stomatal conductance and transpiration rate. Sub-stomatal CO2 concentration (Ci) was significantly higher in the SH than the TH, particularly in the post-amended plants. Salinity significantly increased Ci of the non-amended and pre-amended SH but led to non-significant increase in the TH (in general) and the post-amended SH. Both pre-and post-amendment with SA increased Ci of the SH, particularly in absence of salinity but with marked increase in the TH only due to pre-amendment (Fig. 3A). Stomatal conductance (gs) and rate of transpiration (E) were comparable in the two hybrids. Salinity significantly reduced gs and E, particularly in the post-amended TH. Likewise, SA amendment lowered gs and E; and the reduction in E was particularly evident in the salinized SH, irrespective of timing of application; but that in gs was most evident due to post-amendment of the salinized SH (Figs. 3B and 4A). Rate of photosynthesis (A) was comparable in the two hybrids, except the superiority of the TH in the pre-amended non-salinized plants. Salinity significantly reduced A, particularly in the pre-amended TH. Likewise, SA amendment led to general lowering of A but with non-significant reduction in the non-salinized TH (Fig. 4B). Leaf surface temperature (Tl) was slightly lower in the SH than the TH, and the genotypic variability was most evident in non-amended plants. Salinity led to mild increase in Tl, particularly in the non-amended and pre-amended SH. Whereas SA amendment significantly raised Tl of the SH, it mildly reduced it in the TH (Fig. 4C).
Fig. 3.
Sub-stomatal CO2 concentration (A) and stomatal conductance (B) of the third youngest leaf of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
Fig. 4.
Transpiration rate (A), photosynthetic rate (B) and surface temperature (C) of the third youngest leaf of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
3.4. Leaf metabolites
The effect of treatments on most of the leaf metabolites ranged from significant (P < 0.05) to highly significant (P < 0.01). However, proline was the most sensitive variable, being highly significantly affected by the three main factors and their interactions; whereas starch was the least sensitive one with non-significant effect of the main factors and their interactions (data not shown).
There was a general trend of lower soluble sugar (SS) in leaves of the SH compared with the TH, and this pattern was most evident in the non-treated plants but was weakened either by salinity stress or SA amendment. Salinity increased leaf SS, particularly in the non-amended and pre-amended plants. Application of SA to non-salinized plants non-significantly affected leaf SS; whereas in salinized plants, only post-amendment led to an average 40% reduction for both hybrids (Fig. 5A). Although starch content of leaves was non-significantly affected by the treatments, there was a general tendency of lower levels in the SH than the TH in the treated plants with the reverse being evident in the non-treated plants (Fig. 5B). Protein concentration of leaves was, in the overall, higher in the TH than the SH, with non-significant effect of timing of SA amendment. Salinity led to small increase in leaf protein, and the increase was more evident in the TH than the SH, particularly in the pre-amended plants (Fig. 5C). The concentration of leaf phenolics was in favor of the TH in non-amended and pre-amended plants but in favor of the SH in post-amended plants. Salinity decreased leaf phenolics of the amended plants, particularly the pre-amended ones but with inconsistent effect in the non-amended plants (no effect in the SH versus an increase in the TH). The effect of SA amendment on leaf phenolics of the SH was in general non-significant; but in the TH, leaf phenolics were subjected to a decrease, particularly due to post-amendment of the salinized plants (Fig. 6A). Leaf proline was, in the overall, higher in the TH than the SH, and this pattern was either very weak (in the non-treated plants) or even reversed (in the post-amended salinized plants). Salinity significantly increased proline concentration in the non-amended and pre-amended plants but led to marginal effect in the post-amended plants. In absence of salinity, the effect of SA on proline concentration of the SH varied from lowering due to pre-amendment to an increase due to post-amendment, with non-significant effect in the TH. But, under salinity stress, SA amendment markedly reduced leaf proline content of the two hybrids, particularly the TH (Fig. 6B).
Fig. 5.
TSS (A), starch (B) and protein (C) concentrations of the third youngest leaf of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
Fig. 6.
Concentrations of phenolics (A), and proline (B) in the third youngest leaf of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
3.5. Mineral content
Mineral content of maize was significantly (P < 0.05) to highly significantly (P < 0.01) affected by the treatments, except with the non-significant genotypic variability in K+ concentration (data not shown). Although shoot K+ concentration was generally comparable in the two hybrids, it was higher in SH than the TH only in the post-amended salinized plants. The reduction in shoot K+ concentration, in response to salinity, was relatively severe in the pre-amended SH and post-amended TH but was least expressed in the non-amended TH. SA application, particularly the post-amendment, to non-salinized plants, increased shoot K+ concentration of the two hybrids, with negligible effect in salinized plants. Root K+ concentration was comparable in the two hybrids, except in the pre-amended salinized plants, where it was higher in the SH than the TH. The reduction in root K+ concentration in response to salinity was particularly severe in the non-amended plants. SA amendment increased root K+ concentration in the SH, particularly under salinity stress, with inconsistent small effect in the TH (Fig. 7).
Fig. 7.
Concentration of K in the shoot (A) and root (B) of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
Sodium concentration of plant tissue was comparable in the two hybrids; but it was higher in the SH over TH in the shoots of non-treated plants and in the roots of non-treated as well as post-amended salinized plants. Salinity substantially increased shoot Na+ concentration but with a relatively moderate effect in the non-amended TH. In spite of the higher levels of Na in the root, the salinity-induced increase in Na+ concentration was less marked in the root than in the shoot. SA amendment had non-significant effect on shoot Na+ concentration in absence of salinity; but in salinized plants, only post-amendment led to an average 35% increase above non-amended plants of the two hybrids. The effect of SA on root Na+ was more evident in the TH than the SH. In the SH, a significant reduction in root Na+ was found only due to post-amendment of salinized plants, whereas in the TH both pre- and post-amendment lowered root Na+, particularly in non-salinized plants (Fig. 8).
Fig. 8.
Concentration of Na in the shoot (A) and root (B) of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
K/Na ratio of shoot and root was, in the overall, higher in the SH than the TH, particularly in the non-treated plants. Salinity sharply reduced K/Na ratio of shoot and root, with a relatively moderate effect in the non-amended TH. The effect of SA on shoot K/Na ratio was marked only in the non-salinized plants, with a moderate decrease in the SH versus a substantial increase in the TH. In the root, SA amendment slightly affected K/Na ratio of the non-salinized SH but increased it in the salinized plants; whereas in the TH, a general increase was found, particularly due to post-amendment of non-salinized plants (Fig. 9).
Fig. 9.
K/Na ratio of the shoot (A) and root (B) of the SH and TH of Zea mays grown under the impact of 150 mM NaCl and sprayed with 1 mM SA either one week before or one week after application of salinity. Each column represents the mean of 4 replicates ±SE. Columns with common letters are non-significantly different at P ≤ 0.05.
4. Discussion
Among the experimental factors investigated in the present work, salinity was the major factor determining maize growth, followed by timing of SA application; while maize genotype was the least affecting factor. Likewise, among the different growth measures of maize, root dry weight was the most sensitive measure, but number of leaves was the least sensitive one. Salinity negatively impacted leaf area and shoot dry weight of a single maize hybrid (Hussein et al., 2007). The differential response of sesame growth measurements to drought stress has been reported by Khatiby et al. (2016), with significant effect on plant height, number of branches and number of capsules per plant but not on number of seeds per capsule, seed weight and leaf area index. The beneficial effect of SA on growth of maize and soybean was greater in leaf area and dry mass than in plant height and root length (Khan et al., 2003; Vazirimehr et al., 2014). Likewise, spraying with salicylic acid improved growth measurements of maize differentially; with a marked beneficial effect on stem dry weight rather than on stem diameter (Hussein et al., 2007). Although the SH of maize exhibited superior growth over the TH, particularly in terms of biomass production and leaf area; yet, the SH seems to be more salt-sensitive than the TH. For example, the salinity-induced reduction in shoot growth was distinct in the SH independent of SA regime versus a marked reduction only in the post-amended TH. The genotypic variability among maize hybrids in salt tolerance has been reported by Tufail et al. (2013); with differential performance in terms of biomass production, gas exchange characteristics and K/Na selectivity.
The beneficial effect of SA has been reported for chickpea (Farjam et al., 2015) and maize (Hussein et al., 2007; Gunes et al., 2007). Presoaking of cucumber seeds with SA improved growth and alleviated the toxic effect of salinity on seedlings (Wahid et al., 2017). Exogenous SA has been manipulated to alleviate the adverse effect of salt stress (Khoshbakht and Asgharei, 2015; Jini and Joseph, 2017) and water stress (Shao et al., 2018) on plant performance. In contradiction with the above reports, the present findings suggest that the beneficial effect of SA on maize growth (if any) was marginal; instead, SA by itself seems to impose stress on maize foliage, particularly if applied after incidence of salinity to the salt-resistant TH. Late application of SA to salt-stressed maize might thus impose further stress, particularly to the TH; and this can be partially related to reduced stomatal conductance and levels of phenolics, proline and K+ but increased Na concentration of the shoot in the post-amended compared with either non-amended or pre-amended plants. Nevertheless, in spite of its inhibitory effect on shoot growth, SA might be beneficial for root growth, particularly in the salinized SH and the control TH. Regarding the allocation of plant biomass among shoot and root, the relatively vigorous and salt-sensitive SH exhibited preferential native allocation of plant biomass to root at the expense of shoot compared with the TH; a trait which diminished in the treated plants. But, whereas salinity decreased the R/Sh ratio particularly in the SH, SA led to the reverse effect. The differential effect of salinity on root and shoot growth, manifested as increased R/Sh ratio, is of widespread occurrence. It has been postulated that although root is the organ directly exposed to soil salinity, yet it exhibits less salt damage than shoot (Munns and Sharp, 1993).
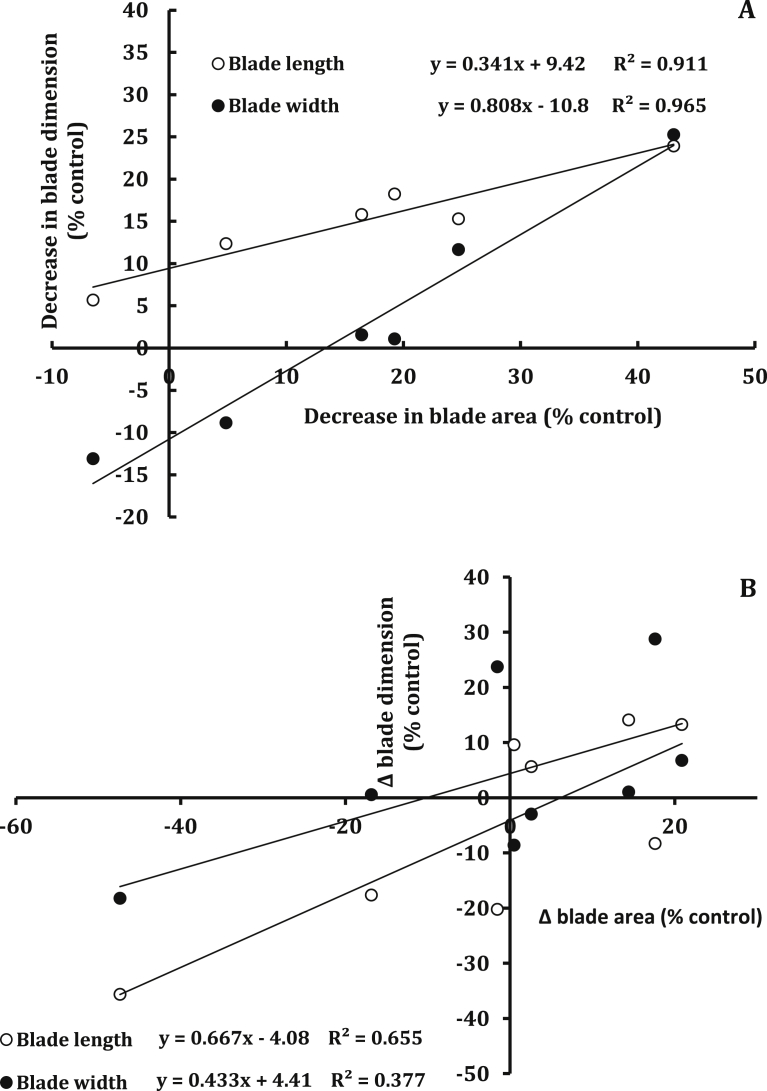
The correlation between blade area in one hand and blade dimensions (blade length and blade width) in the other hand, via considering the coefficient of determination (R2) and the regression coefficient (b) suggests that the adverse effect of salinity on leaf area of maize seems to be primarily determined by the changes in blade width (R2 = 0.965, b = 808) rather than blade length (R2 = 0.911, b = 0.341). By contrast, SA seems to target specifically blade length; as evidenced from the stronger dependence of the SA-induced changes in blade area on the changes in blade length (R2 = 0.655, b = 667) than those in blade width (R2 = 0.377, b = 0. 433) (Fig. 10). Consequently, the two stress factors (salinity and SA) seem to affect leaf morphology differentially, with a stronger effect of salinity, manifested as production of wider and shorter blades versus a moderate and inconsistent effect of SA (production of longer and narrower blades only in the SH).
Fig. 10.
Relative effect of salinity (A) and SA amendment (B) on leaf extension of maize. R2 values followed by (***) are very highly significant (P < 0.001), by (**) are highly significant (P < 0.01) and by (ns) are non-significant (P > 0.05).
In spite of the weak genotypic variability in the photosynthetic pigments, there was a general trend of higher pigment levels in the TH than in the SH. The effects of either salinity or SA amendment on pigment content of maize leaves were mild and varied according to the other factor, in marked interaction with the maize genotype. For example, the advantage of the TH in Chl a and carotenoids was most pronounce in the post-amended plants, in which their concentrations were consistently raised by salinity versus a non-significant effect or even reduction in the non-amended and pre-amended plants. Similarly the effect of SA on pigment concentration was particularly evident when applied after incidence of salinity stress and manifested as a reduction in the non-salinized plants versus an increase under the impact of salinity. An inhibitory effect of salt stress on photosynthetic pigments of maize has been reported (Hichem et al., 2009; Farooq et al., 2015), versus a stimulatory effect of SA Khodary (2004); Khoshbakht and Asgharei (2015). Application of SA, either by presoaking (cucumber seeds) or addition to the rooting medium (maize seedlings) increased pigment content of plants (Wahid et al., 2017; Tufail et al., 2013).
It has been postulated that environmental stress, including salinity stress, inhibit the fixation of CO2 with the generation of ROS, and that the impact of stress does not arise from the photo damage per se but can be attributed to inhibition of the repair of PSII through suppression of the synthesis of PSII proteins (Murata et al (2007); Takahashi and Murata, 2008). The present findings suggest that abiotic stress, either the aggressive salinity stress or the mild SA stress, can induce stomatal closure, manifested as reduced gs, E and A but increased Ci and Tl, and that the effect of salinity can be augmented further by SA application. The greater salinity-induced reduction in gs, along with a lesser increase in Ci of the TH compared with the SH, concomitant with the comparable reduction in A of the two hybrids suggest that salinity can enhance respiration in the TH to a lesser extent than in the SH. Alternatively, this can be explained as reduced CO2 uptake at the chloroplast level, determined by the enzymatic machinery, rather than a mere decrease in stomatal opening (increased resistance to entry of CO2 into the leaves) as proposed by Khan et al. (2003). Another postulation provided by Hichem et al. (2009) is that the reduction in photosynthesis under stress can be attributed mainly to stomatal closure and partially to PSII photoinhibition.
As a consequence of stomatal closure and reduced transpiration under stress conditions, Tl is expected to rise. However, the greater salinity-induced reduction in E with a lesser increase in Tl in the TH than in the SH might signify greater cuticular transpiration, probably due to thinner cuticle of the TH relative to the SH. In contrast to the stressing effect of SA on gas exchange of the two maize hybrids presented in the present work, foliar application of SA has been reported to increase A but to decrease Ci without affecting both gs and E (Khan et al., 2003) and to counteract the NaCl deleterious effects on Rubisco activity and photosynthetic efficiency of maize (Khan et al., 2003; Khodary, 2004). Application of SA to the rooting medium increased A, E, gs, Ci and yield of two maize genotypes under salt stress in a dose-dependent manner, with marked genotypic variability (Tufail et al., 2013; Vazirimehr et al., 2014). In general, the influence of SA on tomato seedlings has been demonstrated to be dose-dependent; with moderate levels (0.1 mM) can be beneficial to photosynthetic efficiency but high levels (1 mM) may be toxic (Poór et al., 2019).
The increased leaf soluble sugars (SS), along with the negligible changes in starch content under the impact of salinity, signify that maize employs SS for osmoregulation under salinity stress; a behavior that was more evident in the SH than the TH and in non-amended than amended plants. The effect of SA on leaf SS of maize was, however, limited and variable; either non-significant in the control plants or inhibitory in the salinized plants. An increase in SS along with reduction in polysaccharides of maize leaves by salinity, with SA exerting the opposite effect has been demonstrated by Khodary (2004). In this regard, the effect of SA has been explained either as an enhanced consumption of soluble sugars, including incorporation into polysaccharides or inhibition of polysaccharide-hydrolyzing enzymes. In addition to soluble sugars, proline might participate in osmoregulation of maize leaves under salinity stress, with marked role in the pre-amended SH and non-amended TH. By contrast, the effect of SA on leaf proline was not consistent and varied according to the hybrid × salinity combination. Exogenous application of SA has been reported to mitigate the adverse effect of salinity on maize growth by increasing production of sugars (Fahad and Bano, 2012) and proline (Hussein et al., 2007). The contribution of proline but not soluble sugars (Esan and Olaiya, 2016) and of proline and Na+ but not K+ in osmoregulation of okra and maize, respectively under salinity stress has been reported by Cicek and Cakirlar (2002). The limited alterations in protein and phenolics contents of maize leaves in response to hybrid, salinity and SA regime point to limited genotypic variability in these two components and suggests that neither protein nor phenolics can be considered as markers for salt injury/resistance in the two used maize hybrids.
Whereas salinity led to marked reduction in K+ concentration of maize tissues, SA led to mild increase, with marked genotype intervention. The less severe reduction in shoot K+, along with the more severe reduction in root K+ in the TH than in the SH under salinity stress signifies that salt resistance of the TH is related to its ability to save shoot K+ at the expense of root K+. In addition, the limited effect of SA in augmentation of the adverse effect of salinity on shoot K+ versus a relieving effect on root K+ might signify that SA can induce limited mobilization of K+ from shoot to root under salinity stress. Inhibition of K+ uptake by salinity has been reported in maize (Farooq et al., 2015); and the role of SA in alleviation of the impact of salt stress has been attributed to increased uptake of nutrients, particularly K+ and reduced uptake of salt ions with reduced K/Na ratio of plant tissues (Fahad and Bano, 2012). These ionic relationships can affect plant performance. The genotypic differences between two maize cultivars in the extent of salt damage to photosynthetic efficiency can be related also to the difference in maintenance of ionic homeostasis (Hichem et al., 2009).
The salinity-induced increase in tissue Na+ concentration was, generally, more pronounced in the salt-sensitive SH than in the TH. Thus, restriction of Na+ uptake and transport might represent a mechanism of avoidance of salt injury in the salt-resistant TH. Salinity has been reported to increase Na+ concentration, Na/K ratio and leaf osmolality of two maize cultivars, without affecting K+ concentration (Cicek and Cakirlar, 2002). Mansour et al. (2005) reported that a salt-sensitive maize cultivar had higher leaf Na+ than the salt-tolerant one. The protective effect of SA against rising of tissue Na+ concentration of salinized maize was generally mild, but it was more evident in the root than the shoot and in the TH than the SH. It seems that SA participates in protection of root of the salt-resistant TH via limitation of the increase in tissue Na+ concentration under salinity stress; but it can, at the same time, augment the effect of salinity in shoot Na+. Presoaking of cucumber seeds with SA alleviated the toxic effect of salinity on cucumber seedlings via decreasing Na+ concentrations and increasing K+ concentrations of plant tissues (Wahid et al., 2017). Amendment of plants with SA reduced Na+ uptake but increased the uptake of other minerals under salt stress (Vazirimehr et al., 2014; Jini and Joseph, 2017). Thus, SA can modify the response of plants to environmental stress and can be used as a plant growth regulator to improve plant growth mineral nutrient uptake under stress conditions. Tufail et al. (2013) demonstrated genotypic variability in efficiency of SA amendment to alleviate the impact of salinity stress on maize growth and maintenance of K/Na and Ca/Na ratios.
5. Conclusions
The single hybrid (SH) of maize, with vigorous growth potentiality, is more salt-sensitive than the less vigorous triple hybrid (TH). Salicylic acid (SA) was stressful to maize foliage, particularly if applied to the TH after incidence of salinity but was beneficial for root growth. Therefore, prior application of SA before incidence of salinity stress is recommended to avoid the combined stress of salinity and SA post-amendment. Whereas salinity decreased the R/Sh ratio particularly in the SH, SA led to the reverse effect. Both salinity and SA induced stomatal closure, leading to low gs, E and A but high Ci and Tl. Maize employs soluble sugars and proline for osmoregulation under salinity stress. The TH has profound ability to save shoot K+ at the expense of root K+. Restriction of Na+ uptake and transport to the shoot might represent a mechanism to avoid salt injury in the salt-resistant TH. Salicylic acid seems to limit the salinity-induced increase in root Na+ concentration but to augment the effect of salinity on shoot Na+.
Declarations
Author contribution statement
Taha Mohamed El-Katony: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zeinab Mahmoud El-Bastawisy: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sama Soliman El-Ghareeb: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by an M.Sc. Grant from Damietta University, Egypt.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors express their deep thanks to Prof. Gaber M. Abogadallah, Professor of Plant Molecular Biology, Damietta University for his appreciated help in gas exchange measurements.
References
- Al-Naggar A.M.M., Soliman S.M., Hashimi M.N. Tolerance to drought at flowering stage of 28 maize hybrids and populations. Egypt. J. Plant Breed. 2011;15(1):69–87. [Google Scholar]
- Banu N.A., Hoque A., Watanabe-Sugimoto M., Islam M.M., Uraji M., Matsuoka K., Nakamura Y., Murata Y. Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Biosci. Biotechnol. Biochem. 2010;74(10):2043–2049. doi: 10.1271/bbb.100334. [DOI] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Bonhomme R., Ruget F., Derieux M., Vincourt P. Relations entre production de matie`re se`che et énergie interceptée chez différents génotypes de mäıs. Comptes Rendus de l’Académie des Sciences, Paris, série III. 1982;294:393–398. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buysse J.A.N., Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993;44(10):1627–1629. [Google Scholar]
- Chandrakar V., Dubey A., Keshavkant S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016;16(3):662–676. [Google Scholar]
- Chen T.H., Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13(9):499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Cicek N., Cakirlar H. The effect of salinity on some physiological parameters in two maize cultivars. Bulg. J. Plant Physiol. 2002;28(1-2):66–74. [Google Scholar]
- Demiral T., Türkan I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005;53(3):247–257. [Google Scholar]
- Esan A.M., Olaiya C.O. Effect of salicylic acid (SA) seeds soaking on the NaCl salt stress induced changes in soluble sugar and protein accumulation in organs of two genotypes of okra plants. Afr. J. Plant Sci. 2016;10(6):105–110. [Google Scholar]
- Fahad S., Bano A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pakistan J. Bot. 2012;44(4):1433–1438. [Google Scholar]
- Farjam S., Kazemi-Arbat H., Siosemardeh A., Yarnia M., Rokhzadi A. Effects of salicylic and ascorbic acid applications on growth, yield, water use efficiency and some physiological traits of chickpea (Cicer arietinum L.) under reduced irrigation. Legume Res. Int. J. 2015;38(1):66–71. [Google Scholar]
- Farooq M., Hussain M., Wakeel A., Siddique K.H. Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015;35(2):461–481. [Google Scholar]
- George E., Horst W.J., Neumann E. Adaptation of plants to adverse chemical soil conditions. In: Marschner P., editor. Marschner’s Mineral Nutrition of Higher Plants. third ed. Academic Press; London: 2012. pp. 409–472. [Google Scholar]
- Gunes A., Inal A., Alpaslan M., Eraslan F., Bagci E.G., Cicek N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007;164(6):728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hansen E.H., Munns D.N. Effect of CaSO4 and NaCl on mineral content of Leucaena leucocephala. Plant Soil. 1988;107(1):101–105. [Google Scholar]
- Hayat S., Hasan S.A., Fariduddin Q., Ahmad A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 2008;3(4):297–304. [Google Scholar]
- Hichem H., El Naceur A., Mounir D. Effects of salt stress on photosynthesis, PSII photochemistry and thermal energy dissipation in leaves of two corn (Zea mays L.) varieties. Photosynthetica. 2009;47(4):517–526. [Google Scholar]
- Horváth E., Brunner S., Bela K., Papdi C., Szabados L., Tari I., Csiszár J. Exogenous salicylic acid-triggered changes in the glutathione transferases and peroxidases are key factors in the successful salt stress acclimation of Arabidopsis thaliana. Funct. Plant Biol. 2015;42(12):1129–1140. doi: 10.1071/FP15119. [DOI] [PubMed] [Google Scholar]
- Hussein M.M., Balbaa L.K., Gaballah M.S. Salicylic acid and salinity effects on growth of maize plants. Res. J. Agric. Biol. Sci. 2007;3(4):321–328. [Google Scholar]
- Jini D., Joseph B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017;24(2):97–108. [Google Scholar]
- Khan W., Prithiviraj B., Smith D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003;160(5):485–492. doi: 10.1078/0176-1617-00865. [DOI] [PubMed] [Google Scholar]
- Khatiby A., Vazin F., Hassanzadeh M., Shadmehri A.A. Effect of foliar application with salicylic acid on some morphological and physiological characteristics of sesame (Sesamum indicum L.) under drought stress. Cercetari Agronomice in Moldova. 2016;49(4):35–42. [Google Scholar]
- Khodary S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Int. J. Agric. Biol. 2004;6(1):5–8. [Google Scholar]
- Khoshbakht D., Asgharei M.R. Influence of foliar-applied salicylic acid on growth, gas-exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica. 2015;53(3):410–418. [Google Scholar]
- Mansour M.M.F., Salama K.H.A., Ali F.Z.M., Abou Hadid A.F. Cell and plant responses to NaCl in Zea mays L. cultivars differing in salt tolerance. Gen. Appl. Plant Physiol. 2005;31(1-2):29–41. [Google Scholar]
- Metzner H., Rau H., Senger H. Untersuchungen zur Synchronisierbarkeit einzelner Pigmentmangel-Mutanten von Chlorella. Planta. 1965;65:186–194. [Google Scholar]
- Munns R., Sharp R.E. Involvement of abscisic acid in controlling plant growth in soils of low water potential. Aust. J. Plant Physiol. 1993;20:425–437. [Google Scholar]
- Murata N., Takahashi S., Nishiyama Y., Allakhverdiev S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007;1767(6):414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Poór P., Borbély P., Bódi N., Bagyánszki M., Görgényi, Tari I. Effects of salicylic acid on photosynthetic activity and chloroplast morphology under light and prolonged darkness. Photosynthetica. 2019;57:367–376. [Google Scholar]
- Rouf Shah T., Prasad K., Kumar P. Maize-A potential source of human nutrition and health: a review. Cogent Food and Agriculture. 2016;2(1):1166995. [Google Scholar]
- Schlüter U., Crawford R.M. Long-term anoxia tolerance in leaves of Acorus calamus L. and Iris pseudacorus L. J. Exp. Bot. 2001;52(364):2213–2225. doi: 10.1093/jexbot/52.364.2213. [DOI] [PubMed] [Google Scholar]
- Shao R.X., Xin L.F., Guo J.M., Zheng H.F., Mao J., Han X.P., Jia L., Jia S.J., Du C.G., Song R., Yang Q.H., Elmore R.W. Salicylic acid-induced photosynthetic adaptability of Zea mays L. to polyethylene glycol-simulated water deficit is associated with nitric oxide signaling. Photosynthetica. 2018;56(4):1370–1377. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-posphotungustic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. [Google Scholar]
- Szepesi Á. Influence of exogenous salicylic acid on antioxidant enzyme activities in the roots of salt stressed tomato plants. Acta Biol. Szeged. 2008;52(1):199–200. [Google Scholar]
- Takahashi S., Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13(4):178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Tufail A., Arfan M., Gurmani A.R., Khan A., Bano A. Salicylic acid induced salinity tolerance in maize (Zea mays) Pakistan J. Bot. 2013;45(S1):75–82. [Google Scholar]
- Vazirimehr M., Rigi K., Branch Z. Effect of salicylic acid in agriculture. International Journal of Plant, Animal and Environmental Sciences. 2014;4:291–296. [Google Scholar]
- Wahid F., Alam M., Ahmad I., Gurmani A.R., Sajid M., Amin N.U., Ali A. Effect of pre-soaking agents on salinity stressed cucumber seedlings. Pakistan J. Agric. Sci. 2017;54(4):781–790. [Google Scholar]
- Zhou Z.S., Guo K., Elbaz A.A., Yang Z.M. Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ. Exp. Bot. 2009;65(1):27–34. [Google Scholar]