Abstract
Alzheimer's disease is an irreversible and progressive brain disease that can cause problems with memory and thinking skills. It is characterized by loss of cognitive ability and severe behavioral abnormalities, and could lead to death. Cholinesterases (ChEs) play a crucial role in the control of cholinergic transmission, and subsequently, the acetylcholine level in the brain is upgraded by inhibition of ChEs. Coumarins have been shown to display potential cholinesterase inhibitory action, where the aromatic moiety has led to the design of new candidates that could inhibit Aβ aggregation. Accordingly, the present work is an in vitro activity, along with docking and molecular dynamics (MD) simulation studies of synthesized coumarin derivatives, to explore the plausible binding mode of these compounds inside the cholinesterase enzymes. For this purpose, a series of previously prepared N1-(coumarin-7-yl) derivatives were screened in vitro for acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities. The assayed compounds exhibited moderate inhibitory activity against AChE, with IC50 values ranging from 42.5 ± 2.68 to 442 ± 3.30 μM. On the other hand, the studied compounds showed remarkable activity against BChE with IC50 values ranging from 2.0 ± 1.4 nM to 442 ± 3.30 μM. In order to better understand the ligand binding site interaction of compounds and the stability of protein-ligand complexes, a molecular docking with molecular dynamics simulation of 5000 ps in an explicit solvent system was carried out for both cholinesterases. We concluded that the tested coumarin derivatives are potential candidates as leads for potent and efficacious ChEs inhibitors.
Keywords: Theoretical chemistry, Pharmaceutical chemistry, Organic chemistry
1. Introduction
Alzheimer's disease (AD) is an irreversible, progressive brain disease that is associated with memory loss and problems with thinking skills. It is characterized by loss of cognitive ability and could ultimately lead to death [1]. Additionally, it is a main cause of dementia, and accounts for close to 75% of dementia cases [2]. There are approximately 2.5–4.0 million Alzheimer's disease patients in the United States and 17 to 25 million worldwide [1, 3] and it is most common among the elderly. Recent studies revealed that cholinergic dysfunction can be associated with selective and irreversible deficiency of the neurotransmitter acetylcholine [4, 5] which is controlled by hydrolysis of acetylcholine via acetylcholinestrase (AChE) and butyrylcholinestrase (BChE). Additionally, AChE predominates in healthy brains, whereas BChE plays a minor role in regulating the brain's ACh levels. In this context, the activity of BChE increases in patients with AD, whereas the activity of AChE drops or remains unchanged [6]. Accordingly, these two enzymes represent valid therapeutic targets for improving the cholinergic deficit which is responsible for the decrease in cognitive, behavioural, and functioning characteristics of AD [7]. Moreover, recent findings indicated that AChE is involved not only in the correct functioning of the central and peripheral nervous systems, but also takes part in the regulation of several other processes such as cell growth, locomotion, and apoptosis [5, 6, 8]. According to these findings, AChE is needed to restrain cholinergic neurotransmission by protecting acetylcholine levels and keeping them stable in the brain through the development of specific AChE and BChE inhibitors [4, 6].
Currently available AChE and BChE inhibitors are synthetic drugs, such as donepezil, donepezil, rivastigmine, tacrine, and galanthamine, which were approved for treatment of AD. However, due to their short half-lives or unfavorable side effects, these drugs are known to have some limitations for clinical use [9]. Some coumarin derivatives have been known to possess anticholinesterasic activity [10, 11]. In this context, Hamulakova and colleagues demonstrated that mono- and bis-coumarin derivatives showed excellent acetylcholinesterase inhibitory activity [11]. In addition, ensaculin, a coumarin derivative which incorporates a benzopyran with a piperazine substituted moiety, was found to improve memory and cognitive function and to exert positive effects against progressive neurodegeneration in patients with AD [12, 13]. Therefore, this combination of effects of ensaculin on neurotransmitter systems, and its potential neurotrophic and neuroprotective properties, makes it an interesting and attractive candidate in the search for a drug treat AD. Furthermore, research findings revealed that ensaculin has low target organ toxicity which is an invaluable advantage in the treatment of AD [14].
In a similar fashion, Zhou and coworkers designed and synthesized three series of coumarin analogues (A, B, C) with phenylpiperazine substituents and examined their potential for treating AD [13]. These authors discovered that coumarins with substitution on positions 3 and/or 4 have parallel anti-AChE activities compared with the reference drug (Donepezil). In another study, Fallarero and colleagues demonstrated that a coumarin derivative named coumarin 106 was found to exert promising inhibitory activity against AChE [15]. Moreover, Zhou et. al. (2008) in their work on coumarins as potential AChE inhibitors suggested the following three hypotheses [13]: (1) the coumarin ring which is a heterocyclic moiety encompasses ensaculin with cognitive functions, can be compatible with a high AChE inhibitory activity, and can act as the peripheral anionic site, which can interact with the peripheral binding site, (2) the nitrogen atom from the phenylpiperazine groups acted as the positive charge center present in many potent AChE inhibitors. X-ray crystallographic studies of the AChE/donepezil and AChE/galantamine complexes confirmed that the positive center can interact with the catalytic center of AChE, and (3) the phenyl ring connected with the piperazine ring acted as the choline binding site.
In addition, other researchers assessed the in vitro inhibitory activity of naturally occurring coumarin derivatives [16]. Along this line, scopoletin and scopolin, two coumarins isolated from Scopolia carniolica, were investigated for their AChE inhibitory activity. These compounds were found to exert moderate, but remarkable, dose-dependent and long-lasting inhibitory activities [16, 17]. Interestingly, different research groups showed that 7-amino-4-methylcoumarin offers rapid detection, visualization, and assay of various aminopeptidase enzymes from different/target microorganisms in samples or plant extracts [18, 19, 20, 21, 22, 23]. In addition to the aforementioned studies, there are several other reports on the AChE inhibitory activity of coumarin derivatives which make them promising candidates for the development of new drugs against AD [12, 24, 25, 26, 27, 28, 29].
In view of the biological activity of coumarin derivatives as cholinesterases inhibitors, and in the search of new candidates that may act as leads in designing AChE and BChE inhibitors, we sought in the present paper to examine the in vitro inhibitory activity of a group of N1-(coumarin-7-yl) amidrazones and a series of 1-(coumarin-7-yl)-4,5-dihydro-1,2,4-triazin-6(1H)-ones against the aforementioned cholinesterases [30, 31]. These compounds were synthesized and characterized in our laboratory, and their antiviral (HIV-1, HIV-2) [30] and antitumor activity investigated [31]. To rationalize the molecular basis for observed biological activity, molecular docking studies in combination with molecular dynamics (MD) simulation in an explicit solvent system was also performed to assess the dynamic behaviour, stability, and conformational transitions of the coumarin derivatives within the ChEs binding site.
2. Experimental
2.1. Chemicals and instruments
Each of the following chemicals was obtained from commercial sources and used as received, unless otherwise indicated: Acetylcholineesterase, butyrylcholinesterase from equine serum, acetylthiocholine iodide, S-butyrylthiocholine chloride, 5,5′-dithiobis(2-nitrobenzoicacid), Ellman's reagent (DTNB), and 9-amino-1,2,3,4-tetrahydroacridine hydrochloride (Tacrine). Buffers and other reagents were of analytical grade. Microplate fluorescence spectrometer, μQuant (BioTek Instruments, Winooski, Vermont, USA), CyberScan PH 510, (Eutech Instruments, Singapore). Electronic Multichannel micropipette, Electronic Single Channel micropipette (Eppendorf AG, Hamburg, Germany). Chemicals and reagents employed for the synthesis of target compounds were purchased from different suppliers and were used without further purification. We determined the melting points (uncorrected) of prepared compounds on a Stuart scientific melting point apparatus in open capillary tubes. We recorded the 1H- and 13C-NMR spectra with the aid of a 300 MHz spectrometer (Bruker DPX-300) using TMS as the internal standard. Chemical shifts are expressed in δ units whereas J-values for 1H-1H, 1H-F and 13C-F coupling constants are given in Hertz. High resolution mass spectra (HRMS) were acquired by means of electrospray ion trap (ESI) technique with a Bruker APEX-4 (7-Tesla) instrument. External calibration was conducted using arginine cluster in a mass range m/z 175-871. We employed silica gel for column chromatography (Fluka), whereas progress of reactions and purity of products were checked by thin-layer chromatography (TLC) using glass plates precoated with silica gel (E. Merck Kiesegel 60 F254 layer thickness 0.25 mm).
2.2. Synthesis of compounds 2, 3a-h, 4a-c, and 5
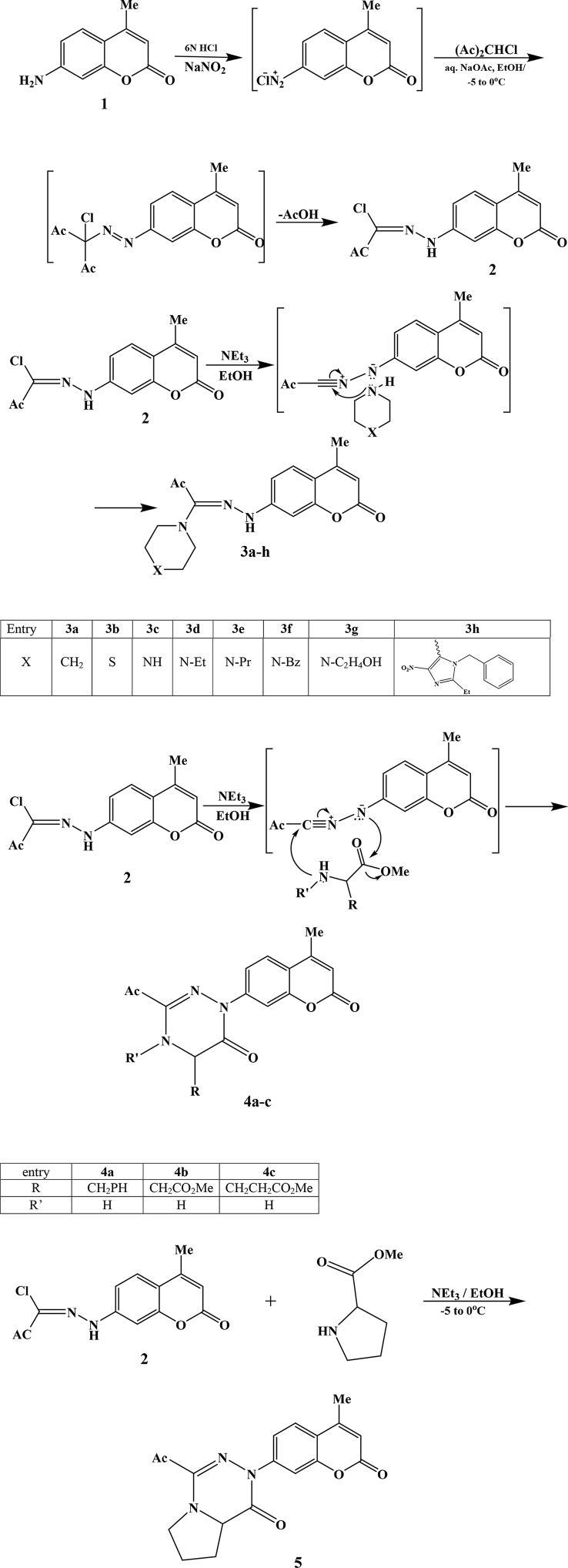
Title compounds were prepared, purified and characterized according to our previously published procedures [30, 31] which involved converting compound 1 to its diazonium salt which upon reaction with 3-chloropentane-2,4-dione afforded compound 2. Reaction of compound 2 with secondary amines gave compounds 3a-h, whereas its reaction with the appropriate amino acid methyl ester led to the formation of compounds 4a-c. On the other hand, reaction of 2 with proline methyl ester yielded compound 5; these synthesis are shown in Fig. 1. For each of the prepared compounds, the melting point and mass and NMR spectra were in agreement with previously published data [30, 31]. For compound 3h: 1H-NMR (300 MHz, CDCl3): δ = 1.28 (t, J = 7.5 Hz, 3H), 2.39 (d, J = 1.0 Hz, 3H), 2.41 (s, 3H), 2.61 (q, J = 7.5 Hz, 2H), 2.68 (m, 4H), 3.36 (m, 4H), 5.17 (s, 2H), 6.12 (d, J = 1.0 Hz), 7.00 (dd, J = 8.7 Hz, 2.0 Hz, 1H), 7.02 (d, J = 7.5 Hz, 2H), 7.30 (d, J = 2.0 Hz, 1H), 7.32–7.37 (m, 3H), 7.51 (d, J = 8.7 Hz), 9.34 (s, 1H). 13C-NMR (75 MHz, CDCl3): δ = 11.3, 18.8, 21.2, 25.9, 46.2, 48.4, 49.6, 101.6, 111.1, 112.2, 114.8, 125.9, 126.0, 128.3, 129.3, 135.4, 139.1, 140.0, 144.5, 145.3, 145.5, 152.5, 155.3, 161.3, 195.4. ESI-HRMS m/z: Calcd. For C29H31N7NaO5 [M + Na]+ 580.22844. Found: 580.22789.
Fig. 1.
Synthesis of target compounds.
2.3. Preparation of solutions
AChE (0.2 mg) was dissolved in 4 mL of sodium phosphate buffer (pH 8.0) to make a 20 μL stock solution. Similarly, BChE (0.2 mg) was dissolved in 1 mL of sodium phosphate buffer (pH 8.0) to make a 300 μL stock solution; these solutions were stored at –90 °C before use. Stock solutions of tested compounds were prepared by dissolving an adequate amount of each compound in 500 μL of methanol. Solutions were then diluted to a series of concentrations with sodium phosphate buffer (pH 8.0) before each experiment. Tacrine was employed as the reference, and was prepared by dissolving 2.35 mg in 500 μL, then 10 μL of this solution was diluted to 1000 μL with sodium phosphate buffer (pH 8.0). In addition, DTNB–sodium carbonate (7.5–16 mg) was dissolved in 1 mL of sodium phosphate buffer (pH 7.0). Likewise, acetylthiocholine iodide (16 mg) was dissolved in 4 mL of deionized water, whereas butyryl thiocholine chloride (4 mg) was dissolved in 8 mL of deionized water.
2.4. In vitro AChE inhibition assay
To evaluate the inhibitory activity against AChE and BChE enzymes, tested compounds were assayed using acetylthiocholine iodide and butyrylthiocholine chloride as a substrate, respectively. Inhibition studies were performed in a 96-well microplate according to the Ellman's method with a final reaction volume of 200 μL [32]. A reaction mixture composed of 130 μL buffer (pH 8), 10 μL of the tested samples, and 20 μL of AChE/ButE. This mixture was incubated for 15 min at 25 °C, followed by addition of 20 μL of DTNB and 20 μL of either acetylthiocholine iodide or butyrylthiocholine chloride. Absorbance dose response for formation of the colored product 5-thio-2-nitrobenzoate anion by the reaction of DTNB and thiocholine, released by hydrolysis of the enzyme, was measured at 412 nm over 10 min by means of a microplate fluorescence spectrometer. Inhibition activity was calculated using enzyme inhibition absorbance dose response using the formula below, with a positive control which gives the absorbance of the enzyme-substrate reaction. The negative control was needed to detect the thiol ester hydrolysis.
Where A0 is the absorbance of the negative control; A1 is the absorbance of test compound; A2 is the absorbance of positive control. Compounds with inhibition activity of more than 50% are considered active with tacrine as a reference; serial dilution of the active tested compounds were conducted according to Ellman's assay [32].
2.5. Data analysis
Experiments were conducted in triplicates. Enzyme inhibition absorbance dose response of the tested compounds was processed using Gene5 ™ Data software analysis (Bio Tek Instruments, Winooski, Vermont, USA). IC50 values were calculated by linear regression analysis using Microsoft Excel 2010.
2.6. Molecular docking
Three-dimensional (3D) structures of coumarin derivatives inhibitors were constructed using the builder module in MOE and were further minimized via MMFF94X force field [33]. Coordinates of X-ray crystallized structures of Torpedo California AChE complex with tacrine (THA/TcAChE) (PDB code: 1ACJ, 2.8 Å) and human BChE complex with 2-(butyrylsulfanyl)-N,N,N-trimethylethanaminium (BCH/hBChE) (PDB code: 1P0P, 2.3 Å) were obtained from the RCSB protein databank. All water molecules and extraneous groups were stripped, and the polar hydrogen atoms were added using MOE. Resulting receptors were then refined and minimized by an Amber99 force field. Molecular docking calculations were carried out by employing the molecular modeling software MOE. All compounds were docked into the binding pocket of both cholinesterases. As a result, 30 conformations for each ligand were generated and ranked on the basis of their docking scores. Top-ranked binding poses of the compounds were selected for further analysis and pose refinement through MD simulations.
2.7. MD simulations protocol
Both ChEs were subjected to classical molecular dynamics simulations to understand the stability and conformational changes in binding patterns obtained from docking calculations. MD simulations were carried out with an AMBER 14 package [33, 34, 35] in which an ff99SBildn force field was used for receptors topology generation [36], whereas generalized AMBER force field (GAFF) parameters were employed in the case of ligands parameterization. After force field implementation, complexes were prepared; all missing atoms and hydrogens were added using xLeap. Receptor charges were neutralized by adding appropriate numbers of sodium ions to AChE and chloride ions to BChE, respectively. After that, receptors were solvated explicitly in a cubic TIP3P water box and the periodic boundary condition set at a margin of 8 Å along each dimension. Before running MD production/long MD simulation, minimization was carried out using the SANDER module of AMBER to relieve bad steric interactions between receptor-ligand complexes and water molecules. Both the steepest descent and conjugate gradient algorithm were utilized to relax the potential energy of the entire system. In total, 1500 steps of minimization were carried out.
At the beginning, 500 steps of minimization with harmonic restraints of 25 kcal/molÅ2 were performed on both receptors and counter ions (the restraints gradually relaxing on both receptors and counter ions). Eventually, 500 steps of unrestrained minimization were carried out. Later, systems were gradually heated from 0–30 K, then restraint was relaxed, and finally equilibration was last up to 70 ps. After the attainment of stability, the NPT ensemble 5000 ps production runs were conducted on each system at constant temperature (300 K) and constant pressure (1 atmosphere) by the Berendsen coupling algorithm. During the entire calculations, general parameters of simulations were kept consistent: integration time step of 2 fs, SHAKE algorithm (constrain bonding involving hydrogen atom), particle mesh Ewald method, a non-bonded interaction cut off the radius of 9 Å. Structural dynamics information was preserved every 1000 steps (2-ps). The resulting 5 ns trajectories of apo protein and ligand-protein complex structures were inspected by plotting root mean square deviation (RMSD), root mean square fluctuation (RMSF) and hydrogen bond distances utilizing the CPPTRAJ module of AMBER package [37] and VMD [38].
3. Results and discussion
3.1. Chemistry and biological activity
Target compounds were synthesized, purified, and characterized according to published procedures [30, 31]; their purity was checked by TLC and by melting point. These compounds were evaluated in vitro for AChE and BChE inhibitory activities by the modified Ellman's method [32] using tacrine, a well-known AChE and BChE inhibitor, as the positive control. Inhibition ratio against AChE and BChE was calculated according to the absorbance dose response of the product in the AChE/BChE catalyzed reaction. Presented in Table 1 are results for the inhibitory activity of the aforementioned coumarin derivatives. AChE and BChE inhibitory activity of compound 1 was very poor, with IC50 values of 875 ± 3.30 and 163.5 ± 4.61 μM, respectively. However, inhibition was found to be influenced by the addition of secondary amines in comparison with the parent compound 1. Synthesized compounds exhibited moderate inhibitory activity against AChE with IC50 values ranging from 42.5 ± 2.68 to 442 ± 3.30 μM; compound 2 was the most active against AChE with an IC50 of 42.5 ± 2.68 μM. On the other hand, the studied compounds exhibited remarkable activity against BChE with IC50 values ranging from 2.0 ± 1.4 nM to 442 ± 3.30 μM, where compound 3h, which contains the 1-benzyl-2-ethyl-4,5-dihydro-4-nitro-1H-imidazole group, was the most potent with an IC50 value of 2.0 ± 1.4 nM. In addition, compounds 4b and 4c showed significant inhibitory activities with IC50 values of 1.1 ± 0.088 and 3.4 ± 0.087 μM, respectively; this could be due to the presence of the acetate group on position 5 of the triazine ring. Similarly, the observed activity of compounds 3a and 3b showed IC50 values of 1.2 ± 0.05 and 7.6 ± 0.15 μM, respectively.
Table 1.
Inhibitory activity of some coumarin derivatives (Fig. 1) against AChE and BChE.
| Compound | AChE (IC50 μM) | BChE (IC50 μM) |
|---|---|---|
| Tacrine | 0.124 ± 0.02 | 7.8 ± 0.06 |
| 1 | 875 ± 3.30 | 163.5 ± 4.61 |
| 2 | 42.5 ± 2.68 | 458 ± 3.30 |
| 3a | 46.25 ± 3.30 | 1.2 ± 0.05 |
| 3b | 81.5 ± 3.30 | 7.6 ± 0.15 |
| 3c | 137 ± 5.61 | 367 ± 5.40 |
| 3d | 216 ± 9.34 | 216 ± 3.05 |
| 3e | 222 ± 2.41 | 222 ± 3.53 |
| 3f | 100 ± 5.53 | 268 ± 3.05 |
| 3g | 116 ± 3.58 | 233 ± 3.30 |
| 3h | 24.25 ± 2.97 | 0.0020 ± 0.0014 |
| 4a | 442 ± 3.68 | 442 ± 3.65 |
| 4b | 135 ± 3.31 | 1.1 ± 0.088 |
| 4c | 129 ± 3.35 | 3.4 ± 0.087 |
| 5 | 163 ± 3.40 | 46.7 ± 0.68 |
3.2. Molecular docking
Molecular docking has been employed as an essential tool in structural molecular biology and computer-aided drug design [39]. The prime objective of ligand-protein docking is to probe the predominant binding modes of a ligand with a protein of known three-dimensional structure [40]. In the present study, we have performed molecular docking of the coumarin derivatives with both cholinesterases using the rigid receptor protocol in MOE [41]. Derivatives showing selective inhibition were docked against their respective enzyme target to determine the binding mode. Hence, compounds 1, 2, 3c, 3d, 3e, 3f, 3g, 4b, and 4c were docked against AChE, whereas the interactions of compounds 1, 2, 5, 3a, 3b, 3d, 3e, 3g, 3h, 4a, 4c, 4c were studied against BchE.
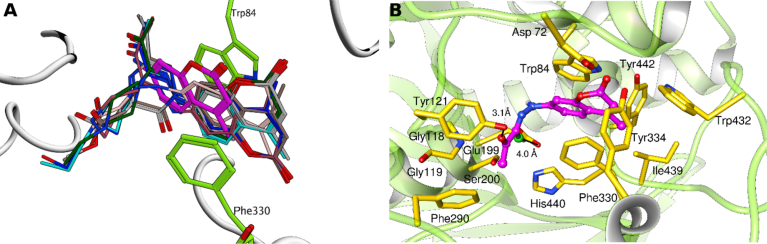
For AChE, results revealed that the coumarin ring of all derivatives, with the exception of 1 and 3f, is stacked against Trp84 and Phe330. As shown in Fig. 2 A, unlike other derivatives, compound 1 could not establish interaction with Phe330. We hypothesize that π–π stacking interaction with Phe330 is the prime factor responsible for the enhanced inhibitory potential of the derivatives. It has been extensively reported that π–π interactions with Tyr84 and Phe330 restrict the entry of substrates into the acyl pocket. Inhibition of AchE from tacrine is also mediated by a similar interaction with Phe330 [42, 43].
Fig. 2.
Molecular docking of coumarin-type inhibitors with function site of AChE A) Alignment of all docked coumarin inhibitors in AChE active site B) interactions of best conformation of compound 2 into the active site pocket of AChE (PDB: 1ACJ).
Compound 2 presents hydrophobic contacts with residues Trp84, Phe290, Phe330, Tyr334, Trp432, Ile439, and Tyr442 in the active site (Fig. 2B). As discussed earlier, the compound exhibits π-stacking interaction with Trp84 and Phe330. We attribute the high affinity of 2 with AChE to an additional halogen bond observed between the propanehydrazonoyl-chloride moiety of the ligand and Glu199. This is in line with the findings of Reddy et al. (2016) who reported a significant increase in AchE inhibitory potential of pyrimidine-ester derivatives following the introduction of a halogen [44]. Substitution of a halogen alters the physicochemical properties of compounds and increases the affinity for AChE through a halogen bond [44].
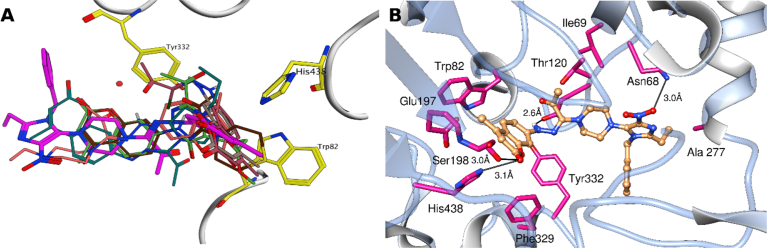
Similarly, docking results showed that all studied compounds are well accommodated in the active site of BChE forming π–π stacking with Trp82 and Tyr332 (Fig. 3A). Top-ranked docking conformation of the most active compound, 3h, depicts the formation of three hydrogen bonds, different hydrophobic contacts, and a salt bridge with the active site residues (Fig. 3B). Potency of 3h can be attributed, in part, to the interaction with residues Asp70 and Tyr332, of the peripheral anionic site (PAS). These two residues are critical for the initial binding of the substrate with the enzyme. Moreover, the ligand displays bonding with Ser198 and His438, two of the three residues of the catalytic triad.
Fig. 3.
Docking of coumarin inhibitors to BChE A) Alignment of best ranked docked pose of coumarin inhibitors in BChE binding pocket B) Binding pose of the most active coumarin derivative (compound 3h) to BChE (PDB: 1P0P).
3.3. Molecular dynamics
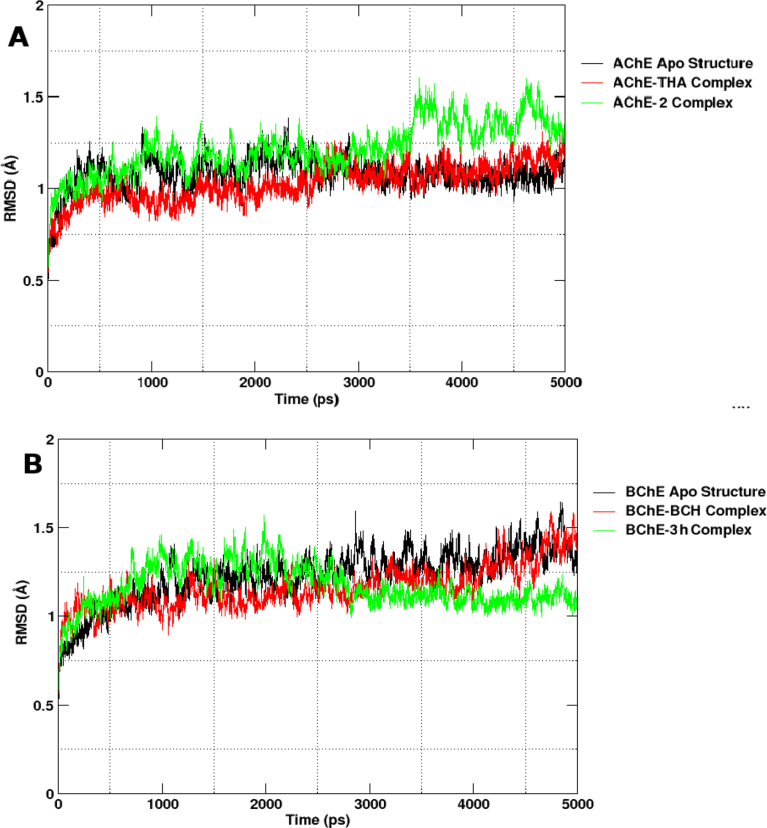
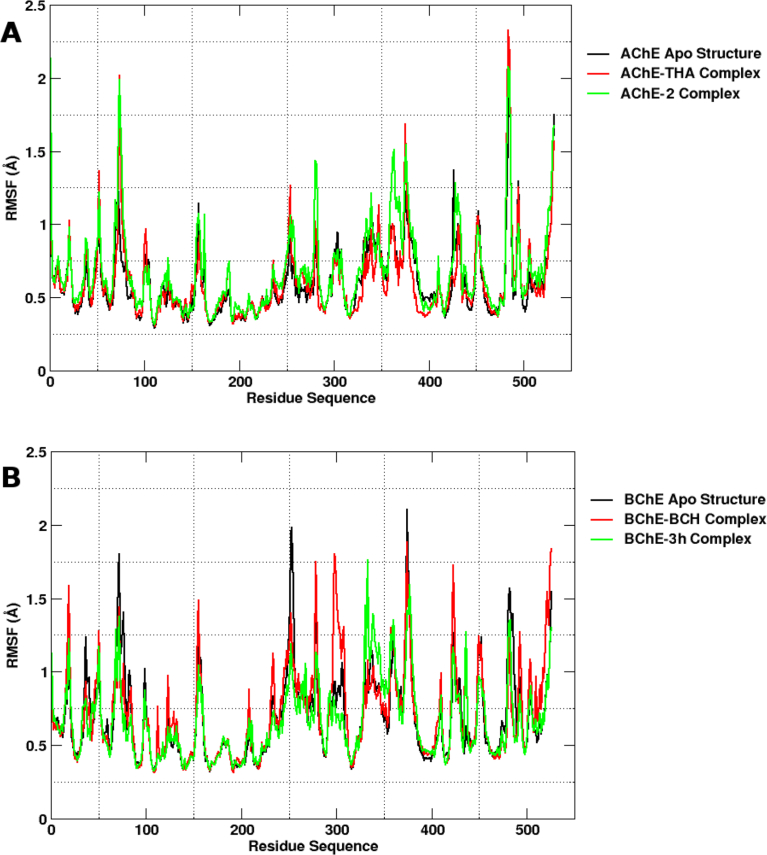
Molecular dynamics (MD) simulation is a valuable computational method that predicts the time-dependent behaviour of a molecular system [45]. MD is also useful in investigating the stability of docked complexes, allowing better insight into the binding mode [46]. In the present study, we have carried out explicit MD simulations of the most potent compounds of the series with their respective enzyme targets. The structural drift and stability were measured by plotting the root-mean-square deviation (RMSD) of protein backbone Cα atoms as a function of time. All systems remained significantly stable over the course of the production run (Fig. 4). Results from Fig. 4 reveal that deviation in each system centres the stability window (3 Å). The observation is further supported by RMSF analysis which highlights the decreased mobility of active site residues in the case of inhibitor–bound complexes (Fig. 5).
Fig. 4.
Root-mean-square deviation (RMSD) plots of protein backbone atoms (C, CA, and N) from the starting structure as a function of time. AChE systems (A, left panel) and BChE systems (B, right panel).
Fig. 5.
Root mean square fluctuation (RMSF) of protein backbone atoms (C, CA, and N) as a function of residue number. AChE systems (A, left panel) and BChE systems (B, right panel).
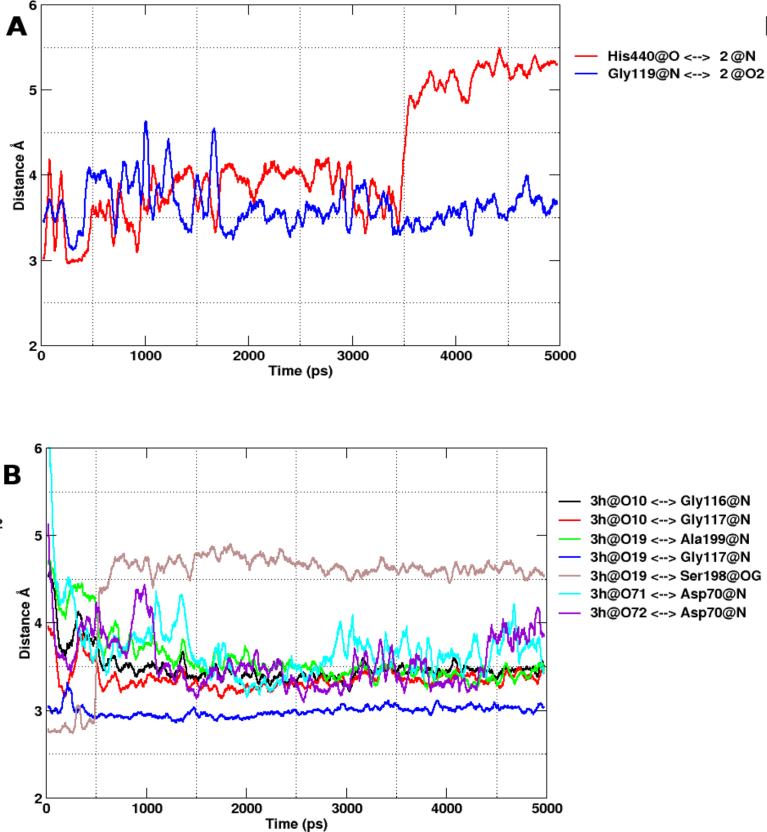
In the case of the AChE-10 complex, the ligand remained anchored to the pocket via stable polar contacts between the ligand and Gly119. However, the bond with His440 was disrupted as the ligand tried to adjust in the cavity (Fig. 6A). The characteristic halogen bond between the propanehydrazonoyl chloride moiety and Glu199 was found to be persistent throughout the production run. The inhibitor remained sandwiched between Trp84 and Phe330 highlighting the contribution of coumarin rings in the binding affinity of the derivatives. Listed in Table 2 are correlations between IC50 and binding energy scores for studied compounds. Data presented in Table 2 show that IC50 values correlate with the calculated binding energies.
Fig. 6.
Hydrogen-bonding distances throughout 5 ns Simulation. AChE with compound 2 (A, left panel) and BChE with compound 3h (B, right panel). The distances were plotted between heavy atoms to heavy atoms.
Table 2.
Correlation between IC50 and binding energy scores.
| Compound ID | AChE (IC50 μM) | Binding energy score | BChE (IC50 μM) | Binding energy score |
|---|---|---|---|---|
| Tacrine (Ref.) | 0.124 ± 0.02 | −7.16 | 7.8 ± 0.06 | −6.83 |
| 1 | 875 ± 3.30 | 5.8 | 163.5 ± 4.61 | −3.12 |
| 2 | 42.5 ± 12.68 | −6.64 | 458 ± 3.30 | 1.3 |
| 3a | 46.25 ± 3.30 | NA | 1.2 ± 0.05 | −6.05 |
| 3b | 81.5 ± 3.30 | NA | 7.6 ± 0.15 | −6.84 |
| 3c | 137 ± 5.61 | −5.2 | 367 ± 3.30 | NA |
| 3d | 216 ± 9.34 | −3.54 | 216 ± 3.05 | −3.51 |
| 3e | 222 ± 2.41 | −3.57 | 222 ± 3.53 | −3.53 |
| 3f | 100 ± 5.53 | −5.12 | 268 ± 3.05 | NA |
| 3h | 116 ± 3.58 | −5.63 | 233 ± 3.30 | −3.86 |
| 3h | 24.25 ± 3.30 | NA | 0.002 ± 0.0014 | −7.64 |
| 4a | 442 ± 3.30 | NA | 442 ± 3.30 | 1.1 |
| 4b | 135 ± 3.31 | −5.2 | 1.1 ± 0.088 | −6.05 |
| 4c | 129 ± 3.30 | −5.3 | 3.4 ± 0.087 | −5.3 |
| 5 | 163 ± 3.40 | NA | 46.7 ± 0.68 | −6.13 |
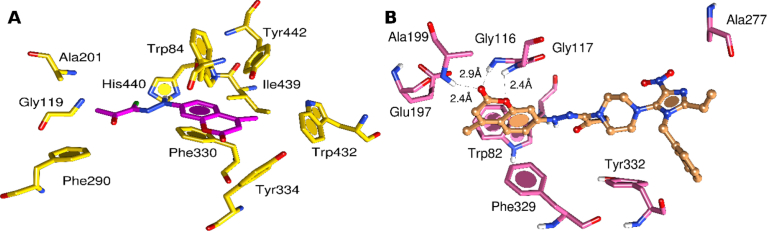
A quick look at Fig. 5B highlights the overall stability of observed hydrogen bonding interactions in BChE-3h complex. We observe stable hydrogen bonds between the acceptor atoms at the coumarin ring and the donor atoms of the oxyanion residues; Gly116, Gly117 and Ala99. MD simulation confirms our docking results, since most of the interaction observed in docking showed notable occupancy in the production run (Fig. 7).
Fig. 7.
Molecular interactions pattern of protein-ligand complexes after MD Simulation. AChE (yellow) complexed with compound 2 (magenta) (A, left panel) and BChE (pink) complexed with compound 3h (brown) (B, right panel).
4. Conclusions
In summary, we have examined the in vitro inhibitory activity of a group coumarin derivatives in the search of new candidates that may act as AChE and BChE inhibitors; these enzymes are associated with Alzheimer's disease. Results revealed that a few of the tested compounds exhibit good activity against BChE. One compound (3h) showed remarkable activity with an IC50 value of 2.0 nM. In addition, in-silico studies rationalize the binding mode of coumarin derivatives in terms of IC50 values, docking score, binding interactions, and stability analysis. The main stabilizing forces were hydrophobic and halogen interactions in the case of AChE, whereas hydrogen bonds were the main determinant of structural stability of BChE–coumarin complexes. Our findings suggest that these compounds might be potent/strong drug candidates against ChEs, and could be leads for new drugs to treat Alzheimer Disease. However, more detailed studies are required to establish the safety and efficacy of these compounds.
Declarations
Author contribution statement
Marwa Abu-Aisheh, Mohammad S. Mustafa: Performed the experiments.
Amal Al-Aboudi: Conceived and designed the experiments; Performed the experiments.
Mustafa M. El-Abadelah: Performed the experiments; Analyzed and interpreted the data.
Saman Y. Ali: Contributed reagents, materials, analysis tools or data.
Zaheer Ul-Haq: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Mohammad S. Mubarak: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Higher Education Commission of Pakistan (HEC Grant ID: NRPU 1509).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Jung M., Park M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules. 2007;12:2130–2139. doi: 10.3390/12092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.dos Santos T.C., Gomes T.M., Pinto B.A.S., Camara A.L., Paes A.M.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer's disease therapy. Front. Pharmacol. 2018;9:1192. doi: 10.3389/fphar.2018.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinton R.D., Yamazaki R.S. Advances and challenges in the prevention and treatment of Alzheimer's disease. Pharm. Res. 1998;15:386–398. doi: 10.1023/a:1011963929012. [DOI] [PubMed] [Google Scholar]
- 4.Al-Aboudi A., Odeh H., Khalid A., Naz Q., Choudhary M.I. Butyrylcholinesterase inhibitory activity of testosterone and some of its metabolites. J. Enzym. Inhib. Med. Chem. 2009;24:553–558. doi: 10.1080/14756360802236393. [DOI] [PubMed] [Google Scholar]
- 5.Kozurkova M., Hamulakova S., Gazova Z., Paulikova H., Kristian P. Neuroactive multifunctional tacrine congeners with cholinesterase, anti-amyloid aggregation and neuroprotective properties. Pharmaceuticals. 2011;4:382–418. [Google Scholar]
- 6.Zhao T., Ding K., Zhang L., Cheng X., Wang C., Wang Z. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of Β-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J. Chem. 2013;2013 [Google Scholar]
- 7.Gordh T., Wahlin Å. Potentiation of the neuromuscular effect of succinylcholine by tetrahydro-amino-acridine. Acta Anaesthesiol. Scand. 1961;5:55–61. doi: 10.1111/j.1399-6576.1961.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen Z.-X. Rationale for diagnosing deficiency of chesond for applying exogenous huches to the treatment of diseases. Med. Hypotheses. 2008;70:43–51. doi: 10.1016/j.mehy.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Shahwar D., Sana U., Ahmad N. Synthesis and evaluation of acetylcholineesterase inhibitory potential and antioxidant activity of benzothiazine derivatives. Turk. J. Chem. 2013;37:262–270. [Google Scholar]
- 10.Loizzo M.R., Tundis R., Menichini F., Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr. Med. Chem. 2008;15:1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- 11.Hamulakova S., Kozurkova M., Kuca K. Coumarin derivatives in pharmacotherapy of Alzheimer's disease. Curr. Org. Chem. 2017;21:602–612. [Google Scholar]
- 12.Shen Q., Peng Q., Shao J., Liu X., Huang Z., Pu X., Ma L., Li Y.-M., Chan A.S., Gu L. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2005;40:1307–1315. doi: 10.1016/j.ejmech.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X., Wang X.-B., Wang T., Kong L.-Y. Design, synthesis, and acetylcholinesterase inhibitory activity of novel coumarin analogues. Bioorg. Med. Chem. 2008;16:8011–8021. doi: 10.1016/j.bmc.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 14.Hoerr R., Noeldner M. Ensaculin (KA-672. HCl): a multitransmitter approach to dementia treatment. CNS Drug Rev. 2002;8:143–158. doi: 10.1111/j.1527-3458.2002.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallarero A., Oinonen P., Gupta S., Blom P., Galkin A., Mohan C.G., Vuorela P.M. Inhibition of acetylcholinesterase by coumarins: the case of coumarin 106. Pharmacol. Res. 2008;58:215–221. doi: 10.1016/j.phrs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Orhan I., Tosun F., Şener B. Coumarin, anthroquinone and stilbene derivatives with anticholinesterase activity. Z. Naturforsch. C. 2008;63:366–370. doi: 10.1515/znc-2008-5-610. [DOI] [PubMed] [Google Scholar]
- 17.Rollinger J.M., Hornick A., Langer T., Stuppner H., Prast H. Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J. Med. Chem. 2004;47:6248–6254. doi: 10.1021/jm049655r. [DOI] [PubMed] [Google Scholar]
- 18.Iwaki S., Nakamura T., Koyama J. Inhibitory effects of various synthetic substrates for aminopeptidases on phagocytosis of immune complexes by macrophages. J. Biochem. 1986;99:1317–1326. doi: 10.1093/oxfordjournals.jbchem.a135599. [DOI] [PubMed] [Google Scholar]
- 19.Acosta D., Cancela M., Piacenza L., Roche L., Carmona C., Tort J.F. Fasciola hepatica leucine aminopeptidase, a promising candidate for vaccination against ruminant fasciolosis. Mol. Biochem. Parasitol. 2008;158:52–64. doi: 10.1016/j.molbiopara.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Budič M., Kidrič M., Meglič V., Cigić B.A. Quantitative technique for determining proteases and their substrate specificities and pH optima in crude enzyme extracts. Anal. Biochem. 2009;388:56–62. doi: 10.1016/j.ab.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 21.Semashko T., Lysogorskaya E., Oksenoit E., Bacheva A., Filippova I.Y. Chemoenzymatic synthesis of new fluorogenous substrates for cysteine proteases of the papain family. Russ. J. Bioorg. Chem. 2008;34:339–343. doi: 10.1134/s1068162008030151. [DOI] [PubMed] [Google Scholar]
- 22.Chung J., Mujeeb A., Jiang Y., Guilbert C., Pendke M., Wu Y., James T.L. A small molecule, lys-ala-7-amido-4-methylcoumarin, facilitates RNA dimer maturation of a stem-loop 1 transcript in vitro: structure-activity relationship of the activator. Biochemistry. 2008;47:8148–8156. doi: 10.1021/bi800230m. [DOI] [PubMed] [Google Scholar]
- 23.Lucas G., Bonhomme N., De Deurwaerdère P., Le Moal M., Spampinato U. 8-OH-DPAT, a 5-HT1A agonist and ritanserin, a 5-HT2A/C antagonist, reverse haloperidol-induced catalepsy in rats independently of striatal dopamine release. Psychopharmacology. 1997;131:57–63. doi: 10.1007/s002130050265. [DOI] [PubMed] [Google Scholar]
- 24.Simeon-Rudolf V., Kovarik Z., Radić Z., Reiner E. Reversible inhibition of acetylcholinesterase and butyrylcholinesterase by 4, 4′-bipyridine and by a coumarin derivative. Chem. Biol. Interact. 1999;119:119–128. doi: 10.1016/s0009-2797(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 25.Brühlmann C., Ooms F., Carrupt P.-A., Testa B., Catto M., Leonetti F., Altomare C., Carotti A. Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J. Med. Chem. 2001;44:3195–3198. doi: 10.1021/jm010894d. [DOI] [PubMed] [Google Scholar]
- 26.Kang S.Y., Lee K.Y., Sung S.H., Park M.J., Kim Y.C. Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: structure–activity relationships. J. Nat. Prod. 2001;64:683–685. doi: 10.1021/np000441w. [DOI] [PubMed] [Google Scholar]
- 27.Urbain A., Marston A., Hostettmann K. Coumarins from Peucedanum ostruthium as inhibitors of acetylcholinesterase. Pharm. Biol. 2005;43:647–650. [Google Scholar]
- 28.Di Giovanni S., Borloz A., Urbain A., Marston A., Hostettmann K., Carrupt P.-A., Reist M. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: thin layer chromatography versus microplate methods. Eur. J. Pharm. Sci. 2008;33:109–119. doi: 10.1016/j.ejps.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Piazzi L., Cavalli A., Colizzi F., Belluti F., Bartolini M., Mancini F., Recanatini M., Andrisano V., Rampa A. Multi-target-directed coumarin derivatives: hache and bace1 inhibitors as potential anti-Alzheimer compounds. Bioorg. Med. Chem. Lett. 2008;18:423–426. doi: 10.1016/j.bmcl.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 30.Mustafa M.S., El-Abadelah M.M., Mubarak M.S., Chibueze I., Shao D., Agu R.U. Synthesis and fluorogenic properties of some 1-(coumarin-7-yl)-4, 5-dihydro-1, 2, 4-triazin-6 (1H)-ones. Int. J. Chem. 2011;3:89–103. [Google Scholar]
- 31.Mustafa M.S., El-Abadelah M.M., Zihlif M.A., Naffa R.G., Mubarak M.S. Synthesis, and antitumor activity of some N1-(coumarin-7-Yl) amidrazones and related congeners. Molecules. 2011;16:4305–4317. doi: 10.3390/molecules16054305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 33.Pearlman D.A., Case D.A., Caldwell J.W., Ross W.S., Cheatham T.E., DeBolt S., Ferguson D., Seibel G., Kollman P. AMBER, A package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput. Phys. Commun. 1995;91:1–41. [Google Scholar]
- 34.Case D.A., Berryman J., Betz R.M., Cai Q.D., Cerutti S., Cheatham T.E., III, Kollman P. University of California; San Francisco: 2014. Amber 14 Reference Manual. [Google Scholar]
- 35.Case D.A., Cheatham T.E., Darden T., Gohlke H., Luo R., Merz K.M., Onufriev A., Simmerling C., Wang B., Woods R. The Amber biomolecular simulation programs. J. Comput. Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheatham T.E., III, Cieplak P., Kollman P.A. A modified version of the Cornell et al. force field with improved sugar pucker phases and helical repeat. J. Biomol. Struct. Dyn. 1999;16:845–862. doi: 10.1080/07391102.1999.10508297. [DOI] [PubMed] [Google Scholar]
- 37.Roe D., Cheatham T.E., III PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9:3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 38.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira L.G., dos Santos R.N., Oliva G., Andricopulo A.D. Molecular docking and structure-based drug design strategies. Molecules. 2015;20:13384–13421. doi: 10.3390/molecules200713384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris G.M., Lim-Wilby M. Molecular docking. Mol. Model. Proteins. 2008;443:365–382. doi: 10.1007/978-1-59745-177-2_19. [DOI] [PubMed] [Google Scholar]
- 41.Molecular Operating Environment (MOE), 2013.08. Chemical Computing Group Inc.; 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7: 2017. [Google Scholar]
- 42.Stoddard S.V., Hamann M.T., Wadkins R.M. Insights and ideas garnered from marine metabolites for development of dual-function acetylcholinesterase and amyloid-β aggregation inhibitors. Mar. Drugs. 2014;12:2114–2131. doi: 10.3390/md12042114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadi-Farani A., Abdi N., Moradi A., Aliabadi A. 2-(2-(4-Benzoylpiperazin-1-yl) ethyl) isoindoline-1, 3-dione derivatives: synthesis, docking and acetylcholinesterase inhibitory evaluation as anti-Alzheimer agents. Iran J. Basic Med. Sci. 2017;20:59–66. doi: 10.22038/ijbms.2017.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy E.K., Remya C., Sajith A.M., Dileep K., Sadasivan C., Anwar S. Functionalised dihydroazo pyrimidine derivatives from Morita–Baylis–Hillman acetates: synthesis and studies against acetylcholinesterase as its inhibitors. RSC Adv. 2016;6:77431–77439. [Google Scholar]
- 45.Ganesan A., Coote M.L., Barakat K. Molecular dynamics-driven drug discovery: leaping forward with confidence. Drug Discov. Today. 2017;22:249–269. doi: 10.1016/j.drudis.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Liu K., Watanabe E., Kokubo H. Exploring the stability of ligand binding modes to proteins by molecular dynamics simulations. J. Comput. Aided Mol. Des. 2017;31:201–211. doi: 10.1007/s10822-016-0005-2. [DOI] [PubMed] [Google Scholar]