Abstract
Sigmoid metastasis of renal cell carcinoma (RCC) is very rare. Herein we report a case of pathologically proven asynchronous abdominal wall and sigmoid metastases after a right nephrectomy. An 84-year-old man underwent right radical nephrectomy for clear cell renal cell carcinoma (ccRCC) 13 years ago. Solitary contralateral abdominal wall metastasis was found for left abdominal mass 9 years after nephrectomy. The man experienced melena underwent resection of sigmoid colon tumor in February, 2016. The postoperative pathological examinations revealed that the tumors were metastases of ccRCC. Recurrence more than 5 years after nephrectomy has been accepted as late recurrence by the majority of urologists now. Late recurrence is one of the specific biological behaviors of RCC. Asynchronous late recurrence of abdominal wall and sigmoid metastases in ccRCC has not been reported before. When patients have sigmoid mass after nephrectomy for RCC, doctors may consider the possibility of late recurrence.
Keywords: Sigmoid metastasis, Late recurrence, Renal cell carcinoma, Asynchronous metastases, Abdominal wall metastasis
1. Introduction
Renal cell carcinoma (RCC) is the third most frequent malignanies of the urinary tract and represents approximately 2%–3% of all adult malignancies [1], [2], [3]. The most common RCC subtype is clear cell RCC (ccRCC), accounting for about 80% [3], [4]. The biological behavior of RCC is variable. The characteristic prolonged course and ability to metastasize to unusual sites which called late recurrence are not observed in other cancers, although there is no standard definition for late recurrence. It is arbitrarily defined as recurrence more than 10 years after nephrectomy in the early times, and the majority of publications define this specific biological behavior as recurrence more than 5 years after initial treatment as time goes on, and some urologists even identify 48 months as the best threshold for defining late and early recurrences [5], [6], [7], [8].
RCC is notorious for the ability to metastasize to unusual sites. Although there is statistically difference in different studies, the most common locations are the lung, bone, liver, brain and lymph nodes [9], [10]. Unusual sites such as pancreas, breast, thyroid, stomach and skin have also been reported more frequently in patients with late recurrence than patients with an early metastatic tumor [10], [11], [12], [13]. Here, we reported a case of asynchronous late recurrence of abdominal wall and sigmoid metastases of ccRCC.
2. Case report
An 84-year-old man was admitted for a 1-month history of intermittent hematochezia who had undergone intraperitoneal open radical nephrectomy 13 years ago and had been found abdominal wall metastasis for 9 years after nephrectomy.
The primary tumor was 4.5 cm × 3.0 cm × 3.0 cm in the right kidney, which was confined in renal capsule, and no lymph node metastasis. Histologically, the tumor was clear cell type. Immunohistochemically, the tumor cells were positive for cytokeratins 1-3(CK 1-3), B-cell lymphoma-2 (Bcl-2), and cluster of differentiation (CD34), but negative for P-glycoprotein (PGP), p53, topoisomerase (Topo-Ⅱ) and vimentin. He received regular follow-up in the first 3 years and was detected no metastasis. As is shown in Fig. 1, there was a mass in the obliquus externus abdominis of left abdominal wall following nephrectomy after 9 years. The left abdominal wall mass underwent a resection procedure. The frozen samples confirmed to be ccRCC metastasis, grade 2 by the World Health Organization (WHO) grade system. Metastatic carcinoma can be seen among striated muscle and fibrous connective tissue. Then, regular follow-up was also conducted and no abnormality was observed in the following 3 years.
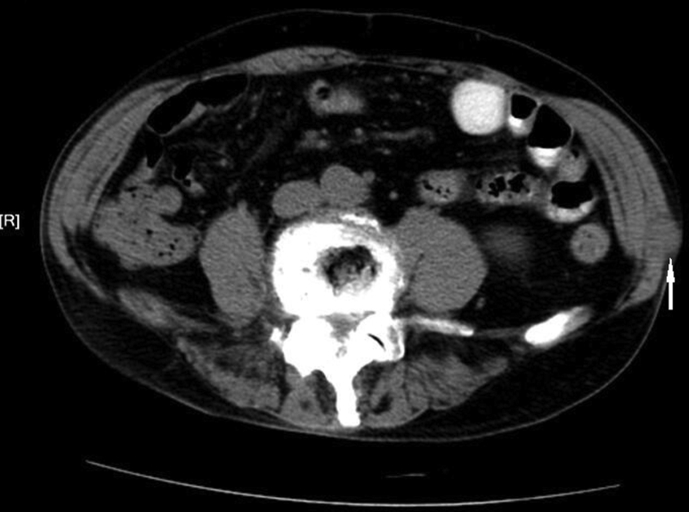
Figure 1.
Abdominal computed tomography (CT) shows a 2.5 cm × 1.8 cm similar round low density image in the obliquus externus abdominis of left abdominal wall (arrow: The mass of abdominal metastasis).
In February 2016, the patient was hospitalized for hematochezia. Physical examination was irrelevant. The following laboratory data were recorded: Hemoglobin 92 g/L, hematocrit 28.5%, white blood cell count 5.31 × 109/L, albumin 38 g/L, aspartate aminotransferase (AST) 29 U/L, alanine aminotransferase (ALT) 17 U/L, alkaline phosphatase 57 U/L, and total bilirubin 9.1 μmol/L. The serum level of alphafetoprotein (AFP) was 4 ng/mL, and carcinoembryonic antigen (CEA) was 3.8 ng/mL. However, high signal was seen on diffusion-weighted imaging (DWI) and T2-weighted imaging (T2WI, Fig. 2), and isointense signal was seen on T1-weighted imaging (T1WI) of pelvic magnetic resonance, which showed a mass at sigmoid. The imaging diagnosis highly suspected it as sigmoid colon carcinoma. Colonoscopy also revealed the mass in the same area. But the pathological findings of biopsy specimen taken from the mass showed it to be merely necrotic tissue. After multidisciplinary team discussion, we performed a resection of sigmoid colon tumor. After the surgery we can see that the solid mass with gray section protruding into the lumen occupied half of the sigmoid cavity. Histopathologic examination demonstrated ccRCC metastasis, grade 3 by Fuhrman grading system (Fig. 3). Carcinoma tissues infiltrated the whole layer but without lymph node metastasis. Immunohistochemically, the tumor cells were positive for Ki67, epidermal growth factor receptor (EGFR), cytokeratins 8 (CK8), cluster of differentiation 10 (CD10), but negative for, cytokeratins 7 (CK7), cytokeratins 20 (CK20), p53 and caudal-related home-odomain transcription-2 (CDX-2). The old man denied targeted therapy and the postoperative course was uneventful after 9 months' follow-up.
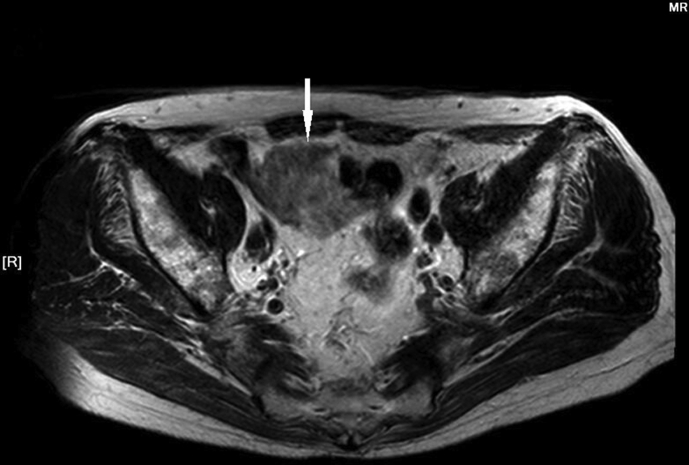
Figure 2.
Pelvic magnetic resonance imaging (MRI) shows a 4.3 cm × 5.8 cm × 4.3 cm mass of high signal (compared to muscle) on T2WI at sigmoid, the intestinal cavity becomes narrow (arrow: The mass of sigmoid metastasis).
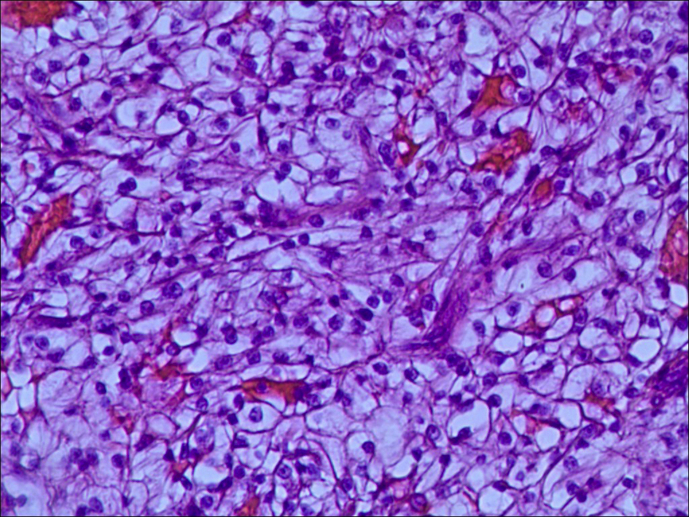
Figure 3.
Microscopic study of sigmoid: Metastasis of clear cell renal cell carcinoma (40×magnification; Haematoxylin and Eosin stain).
3. Discussion
According to an international multicenter study, 20%–40% of RCC patients develop recurrence after curative surgery [14]. Recurrence may occur after nephrectomy at any time. Most recurrent cases tend to occur within the first 5 years after primary surgery, and approximately 5%–10% patients develop late recurrence after the initial postoperative 5 years [14], [15]. RCC has the potential to metastasize to various sites. Metastases locate differently between cases of early and late recurrence. Interestingly, Santoni et al. [16] found that metastases to lymph nodes, liver and brain were associated with worst overall survival (OS), while pancreatic metastases were associated with longer survival. And the presence of bone and liver metastases was associated with the worst OS of all metastatic RCC (mRCC) patients [7].
The mechanism of late recurrence has not been clarified. In general, a secondary tumor may be caused by direct extension, peritoneal implantation, lymphatic metastasis or hematogenous spread. RCC cells disseminate early and reside as single cell or as micrometastatic clusters in host organs. The reason of late recurrence has not been elucidated, and the most popular hypothesis is “seed and soil”, holding that the low growth rate and long-time inactivity are caused by characteristics of the metastasis tumor cells and the microenvironment of metastasis [17]. These disseminated metastatic tumor cells may be in a state like dormancy initially, because intrinsically lack the ability to colonize temporarily or prevented from colonizing by the microenvironment, or maybe both. Since immunity is an important factor for the growth of tumor cells, we may suppose that tumor cells begin to grow when immunity decreased. There is a predominance of female among patients with late recurrence, and women have a better survival rate than men, suggesting that the activity of tumor cells may be influenced by endocrine [5], [18]. Although several possible hypotheses have been proposed to clarify the reason of late recurrence, the real cause is still unknown and fascinates urologists.
Different sites of late recurrence may show various clinical symptoms, so diagnostic evaluation should be adapted according to the specific metastatic site. Sigmoid masses may remain asymptomatic till the late disease stages [19]. The common symptoms include intermittent abdominal pain, hematochezia and abdominal mass. Physical examination aiming to find palpable abdominal mass has a limited role in diagnosis. There are no special laboratory parameters or diagnostic biomarkers indicating sigmoid metastasis until now. With solid sigmoid masses, imaging investigations of CT or MRI especially enhanced scan are quite important diagnostic examinations. Colonoscopy can confirm and reveal histology of imaging indeterminate sigmoid masses and should be considered for active surveillance in selecting patients.
Late recurrence of RCC is relatively infrequent, and there is no standard treatment guideline for us to follow. After confirming the metastatic deposits coming from RCC originally, urologists usually select therapeutic regimen by experience. Surgical treatment for isolated recurrence is considered to be the optimal choice in a series of metastasectomy. Tumor resection is curative only if all tumor deposits are excised. For patients with oligo-metastatic disease, surgery is palliative to alleviate the sufferings of patients. In cases where complete surgical removal is not feasible, palliative treatments and systemic treatments can be considered. Chemotherapy shows no more curative effect in patients with clear cell mRCC, which is not recommended for systemic therapy for mRCC [20]. The clinical effect of cytokine is not satisfactory with a low response rate [21], [22]. Since the multikinase inhibitor sorafenib was approved by FDA as first-line therapy for advanced renal carcinoma in December 2005, targeted drugs have changed the therapeutic landscape of RCC. There are tyrosine kinase inhibitors (TKI) including sorafenib, axitinib and cabozantinib, monoclonal antibody against circulating VEGF like bevacizumab and mammalian target of rapamycin (mTOR) inhibitor like everolimus currently [23], [24], [25], [26], [27], [28]. Messina et al.[29] reported a case of patient who developed bone metastasis recurrence of RCC 16 years after primary surgery. The patient showed a surprisingly long term response to sunitinib, which is maintained after 74 months of treatment. As Programmed Death-1 (PD-1) inhibitors are approved in Japan and the USA, immune checkpoint blockade shows great prospect in the treatment of RCC. That pembrolizumab and nivolumab target the PD-1 receptor, atezolizumab blocks the ligand PD-L1, and ipilimumab targets the cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) are the main mechanisms. Nivolumab is strongly recommended after one or two lines of VEGF-targeted therapy in mRCC [30]. The clinical effectiveness of some drugs or different pharmacotherapeutic schemes for mRCC is currently in trials.
There are several retrospective studies in Canada, Japan, Korean and Italy [7], [16], [19], [31]. However, some viewpoints are still in dispute, such as prognosis and the predictive factors for late recurrence. In a multicenter Canadian study published recently, Kroeger et al. [7] retrospectively evaluated survival outcomes and treatment responses to targeted therapies for a series of 1210 patients with recurrence after surgery for localized RCC. Between 2003 and 2013, 313 patients (26%) experienced late recurrence (more than 5 years). Patients who experienced late recurrence were generally young, with fewer sarcomatoid features, more clear cell histology, and lower Fuhrman grade. They found that later recurrence had significantly longer progression-free survival (PFS) and OS. The current study showed a better response to first-line targeted therapies, a longer PFS and better OS in late recurrence when compared with early recurrence. Bozkurt et al. [31] also demonstrated the better prognostic value of late recurrence in terms of PFS and OS. In an Italian multicenter study researchers retrospectively investigated the clinicopathological features and the outcome of patients with late recurrent RCC. Santoni et al. [16] found that there was no significant difference in terms of PFS among sorafenib, sunitinib and pazopanib treatments. Another database from CORONA/SATURN-Project came to the conclusion that time to recurrence is a significant predictor of cancer-specific survival. The earlier after surgery recurrence occurs, the more reduced is survival after recurrence [19].
Previous studies showed that age at initial surgery, stage ≥pT2 and preoperative high-sensitivity C-reactive protein levels were independent predictive factors of late recurrence [10], [32]. Bozkurt et al. [31] found that there was significant difference in Fuhrman grade between the early and late recurrence patients. However, there was no statistically significant difference in variables such as age, sex, tumor necrosis and histological subtypes. Brookman-May et al. [14] found that lymphovascular invasion, Fuhrman grade 3/4 and tumor stage greater than pT1 were independent predictors of late recurrence. In Santoni's study of lymphovascular invasion, Fuhrman grade 3/4 and tumor stage were not significantly associated with OS and PFS in patients with late recurrence of RCC [16]. Further large cohort studies involving more patients to determine risk factors of late recurrence and to predict those who may develop recurrence in the future are demanded.
4. Conclusion
Late recurrence is the specific biological behavior of RCC, although the phenomenon of late recurrence is rare after initial curative treatment for most malignancies. Unusual metastatic sites have been reported more frequently in patients with late recurrence than patients with an early metastatic tumor. When patients with more than 5-year history of RCC are found clinically suspicious tumors, doctors may consider the possibility of late recurrence.
Author contributions
Study concept and design: Fangyuan Zhang, Gang Zhao, Dong Wei.
Data acquisition: Fangyuan Zhang, Yang Yang, Qi An, Xin Chen.
Drafting of manuscript: Fangyuan Zhang, Pengjie Wu.
Critical revision of the manuscript: Fangyuan Zhang, Dong Wei.
Final approval of manuscript: Jianye Wang, Gang Zhao, Dong Wei.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful to the patients who participated in this study.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Cohen H., McGovern F. Renal cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Rini B.I., Campbell S.C., Escudier B. Renal cell carcinoma. The Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 4.Thibodeau B.J., Fulton M., Fortier L.E., Geddes T.J., Pruetz B.L., Ahmed S. Characterization of clear cell renal cell carcinoma by gene expression profiling. Urol Oncol. 2016;34 doi: 10.1016/j.urolonc.2015.11.001. 168.e1–9. [DOI] [PubMed] [Google Scholar]
- 5.McNichols D.W., Segura J.W., DeWeerd J.H. Renal cell carcinoma: long-term survival and late recurrence. J Urol. 1981:12617–12623. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 6.Tapper H., Klein H., Rubenstein W., Intriere L., Choi Y., Kazam E. Recurrent renal cell carcinoma after 45 years. Clin Imaging. 1997:21273–21275. doi: 10.1016/s0899-7071(96)00042-3. [DOI] [PubMed] [Google Scholar]
- 7.Kroeger N., Choueiri T.K., Lee J.L., Bjarnason G.A., Knox J.J., MacKenzie M.J. Survival outcome and treatment response of patients with late relapse from renal cell carcinoma in the era of targeted therapy. Eur Urol. 2014:651086–651092. doi: 10.1016/j.eururo.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Grüllich C., Vallet S., Hecht C., Duensing S., Hadaschik B., Jager D. Local salvage therapy for late (≥2 years) metastatic and local relapse of renal cell cancer is a potentially curative treatment irrespective of the site of recurrence. Urol Oncol. 2016;34 doi: 10.1016/j.urolonc.2015.11.022. 238.e9–17. [DOI] [PubMed] [Google Scholar]
- 9.Ljungberg B., Alamdari F.I., Rasmuson T., Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999:84405–84411. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 10.Fujii Y., Ikeda M., Kurosawa K., Tabata M., Kamigaito T., Hosoda C. Different clinicopathological features between patients who developed early and late recurrence following surgery for renal cell carcinoma. Int J Clin Oncol. 2015;20:802–807. doi: 10.1007/s10147-014-0775-2. [DOI] [PubMed] [Google Scholar]
- 11.Kassabian A., Stein J., Jabbour N., Parsa K., Skinner D., Parekh D. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000;56:211–215. doi: 10.1016/s0090-4295(00)00639-7. [DOI] [PubMed] [Google Scholar]
- 12.Pathe N., Raymond J., Cintra A.U. Metastatic renal cell cancer presenting as a breast mass. Clin Adv Hematol Oncol. 2012;10:124–126. [PubMed] [Google Scholar]
- 13.Onorati M., Petracco G., Uboldi P., Redaelli D.G., Romagnoli S., Albertoni M. A solitary polypoid gastric metastasis 20 years after renal cell carcinoma: an event to be considered, and a brief review of the literature. Pathologica. 2013;105:132–136. [PubMed] [Google Scholar]
- 14.Brookman-May S.D., May M., Shariat S.F., Novara G., Zigeuner R., Cindolo L. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma — results from a comprehensive multi-centre database (CORONA/SATURN-Project) BJU Int. 2013;112:909–916. doi: 10.1111/bju.12246. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.P., Weight C.J., Leibovich B.C., Thompson R.H., Costello B.A., Cheyille J.C. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011;78:1101–1106. doi: 10.1016/j.urology.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Santoni M., Conti A., Porta C., Procopio G., Sternberg C.N., Basso U. Sunitinib, pazopanib or sorafenib for the treatment of patients with late relapsing metastatic renal cell carcinoma. J Urol. 2015;193:41–47. doi: 10.1016/j.juro.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Fidler I.J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 18.Nakano E., Fujioka H., Matsuda M., Osafune M., Takaha M., Sonoda T. Late recurrence of renal-cell carcinoma after nephrectomy. Eur Urol. 1984;10:347–349. doi: 10.1159/000463826. [DOI] [PubMed] [Google Scholar]
- 19.Adamy A., Chong K.T., Chade D., Costaras J., Russo G., Kaag M.G. Clinical characteristics and outcomes of patients with recurrence 5 years after nephrectomy for localized renal cell carcinoma. J Urol. 2011;185:433–438. doi: 10.1016/j.juro.2010.09.100. [DOI] [PubMed] [Google Scholar]
- 20.Ljungberg B., Bensalah K., Canfield S., Dabestani S., Hofmann F., Hora M. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Motzer R.J., Bacik J., Murphy B.A., Russo P., Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 22.McDermott D.F., Regan M.M., Clark J.I., Flaherty L.E., Weiss G.R., Logan T.F. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 23.Bellmunt J., Négrier S., Escudier B., Awada A., Aapro M., SIOG T The medical treatment of metastatic renal cell cancer in the elderly: position paper of a SIOG Taskforce. Crit Rev Oncol Hematol. 2009;69:64–72. doi: 10.1016/j.critrevonc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg C.N., Davis I.D., Mardiak J., Szczylik C., Lee E., Wagstass J. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 25.Motzer R.J., Escudier B., Tomczak P., Hutson T.E., Michaelson M.D., Negrier S. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri T.K., Escudier B., Powles T., Tannic N.M., Mainwaring P.N., Rini B.I. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 28.Coppin C. Everolimus: the first approved product for patients with advanced renal cell cancer after sunitinib and/or sorafenib. Biologics. 2010;4:91–101. doi: 10.2147/btt.s6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messina C., Di Meglio A., Nuzzo P.V., Boccardo F., Ricci F. Very late recurrence of renal cell carcinoma experiencing long-term response to sunitinib: a case report. Tumori. 2015;101:e79–e81. doi: 10.5301/tj.5000268. [DOI] [PubMed] [Google Scholar]
- 30.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozkurt O., Hacıbekiroglu I., Kaplan M.A., Duzkopru Y., Uysal M., Karaca H. Is late recurrence a predictive clinical marker for better sunitinib response in metastatic renal cell carcinoma patients? Clin Genitourin Cancer. 2015;13:548–554. doi: 10.1016/j.clgc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Park Y.H., Baik K.D., Lee Y.J., Ku J.H., Kim H.H., Kwak C. Late recurrence of renal cell carcinoma >5 years after surgery: clinicopathological characteristics and prognosis. BJU Int. 2012;110:E553–E558. doi: 10.1111/j.1464-410X.2012.11246.x. [DOI] [PubMed] [Google Scholar]