Abstract
Introduction
A considerable part of old people suffer from Chronic Cerebral Hypoperfusion (CCH) in their long lives but have no way to change. The Modified Dioscorea Pills (MDP), a Chinese compound herbal prescription, has good clinical efficacy for CCH related diseases such as Vascular Dementia, whereas, what happened and how MDP works in CCH need to be clarified. Here, we investigate the neural inflammation and gliosis, neuronal apoptosis and regeneration in an animal model of CCH and interfered with MDP to explore some mechanisms of this Chinese herbal medication.
Methods
40 rats were randomly divided into Sham operated Group, Model Group and MDP Group according to a Random Number Table. CCH models were made by the modified 2-VO (two vessels occlusion) operation. The intelligence of rats were measured by Morris Water Maze (MWM) test; H & E staining and transmission electron microscope (TEM) were applied to observe the pathological and ultrastructural changes in hippocampus; The expression of key genes including growth associated protein 43 (GAP-43) and vascular endothelial growth factor (VEGF) and key protein including Bax, Bcl-2, nuclear factor-κB (NF-κB p65), microtubule associated protein-2 (MAP-2), Oligodendrocyte transcription factor 2(Olig-2), glial fibrillary acidic protein (GFAP) of hippocampus were detected.
Results
CCH lead to learning and memorial impairment and MDP can partly restore them; Neural inflammation, Neuronal apoptosis and astrocyte hyperplasia were common in Model Group but they were partly reversed by MDP; The expressions of GAP-43mRAN and VEGF mRNA in Model Group were much higher than those in Sham operated Group, but they reached the highest in MDP Group (P < 0.01 or P < 0.05).
Conclusions
Through regulating the expressions of key genes and proteins, MDP partly restore the intrinsic structure of Neurovascular Unit (NVU) in hippocampus, which revealed one of its therapeutic mechanisms on CCH.
Keywords: Neurology, Pharmaceutical science, Neuroscience
1. Introduction
Chronic cerebral hypoperfusion (CCH) is not rare in clinical. The prolongation of CCH can lead to destruction of blood-brain barrier, loss of neurons, oxidative stress, and finally lead to impaired spatial learning [1]. In some extreme cases, too strong inflammatory response, cell death and hypoxia resulted from CCH can lead to cerebral edema or even result in high mortality and disability [2, 3, 4].
CCH involves in many neurodegenerative diseases such as Alzheimer's disease (AD) and Vascular Dementia (VD). A considerable number of CCH patients bearing intolerable agonies such as cognitive impairment, language dysfunction, apraxia and so on have few efficacious way to combat it. Some therapies have limited efficacy for ameliorating symptoms. Therefore, it is of great theoretical and practical significance to study the pathological changes and treatment strategies of CCH.
Hippocampus is a critical cerebral zone in charge of learning and memory. The hippocampus is easily damaged in CCH evidenced by a massive neuronal loss of CA1 zone accompanied by a dense staining for GFAP indicating astrogliosis [5]. On the other hand, the activated inflammation, angiogenesis and neurogenesis interacts with one another [6], indicating remodeling of NVU which deeply influences multiple neurological functions.
The Modified Dioscorea Pills (MDP) is a traditional Chinese medical preparation composed of 14 kinds of herbs. A series of clinical and experimental researches have verified its good efficacy to treat VD [7, 8], quite a part of which resulted from CCH. Weather and how MDP act on the hippocampus NVU in CCH and furtherly treat VD are still unknown. In this study, modified 2-VO method was used to prepare CCH models. Through the interference of MDP, the rats' intelligence was measured, the expression of key proteins and genes involved in inflammation, apoptosis, neurogenesis, angiogenesis and astrogliosis were tested so as to provide profound understanding of CCH and alternative means to treat it.
2. Methods
2.1. Animals
The animal handling was approved by the Institutional Animal Care and Use Committee (IACUC) of the First Hospital of Guiyang City (Guiyang, 550002, China). The experimental protocol is in agreement with the European Community guidelines [9]. 40 adult male Sprague-Dawley rats were purchased from the Medical Laboratory Animal Centre of Guizhou Medical University (Guiyang, 550002, China). The animals (weighting 200 ± 20 g) were housed in an animal room (12:12 h light/dark circle; 22–24 °C) with free access to food and water.
2.2. Modified dioscorea pills (MDP) preparation
MDP concentrated decoction (provided by Hubei Traditional Chinese Medicine Hospital, lot number: Z20150027, Wuhan, China), composed of 14 kinds of Chinese herbs. The Preparation process is as follows, all herbs were added to about 400 ml of water, including: Dioscorea, 30 g; pared Rehmannia Root, 24 g; prepared Polygonum Multiflorum, 24 g; Codonopsis Pilosula, 20 g; radices Paeoniae Alba, 20 g; Codonopsis Pilosula, 20 g; Polygala Root, 12 g; Angelica Sinensis, 20 g; Acorus Gramineus Soland, 10 g; Atractylodes Macrocephala, 18 g; Poria Cocos, 18 g; the bark of Eucommia, 12 g; Lycium Chinensis, 18 g and Schisandra Chinensis, 10 g. Through the processes of boiling, concentration, filtration and refining, these herbs with the water were condensed to 250 ml decoction (about 1 ml decoction contains compound extract from 1g these raw proportionate herbs) and preserved in bottle in refrigerator at 4 °C for use.
All herbs are collected from the same batch to ensure consistent drug concentrations. The main active substances of this prescription include Yam Polysaccharide, Polygonum Multiflorum Polysaccharide, Rehmannia Glutinosa Polysaccharide, Lyciumbar Barum Polysaccharide, β-asarone, Angelica Small Molecule Polysaccharide, etc., which have the functions of antioxidant, clearing free radicals, anti-aging, anti-apoptosis, and promoting nerve regeneration.
2.3. Preparation of the modified 2 vessels occlusion (2-VO) models
After one week of adaptive inhabiting and feeding, 40 rats were divided into operated group including 28 rats and sham-operated group including 12 rats according to a random number table. Rats in operated group were subjected to CCH by ligating the bilateral common carotid arteries (CCA) under anesthesia with Isoflurane inhaling. The operation process is as follows: The right CCA of rat was isolated and ligated at both ends prior to be cut at the center of the two ligation points, then the skin was sutured and 4000 units of penicillin were instilled locally to prevent infection; the left CCA was treated the same way as the right one one week later. Rats in Sham operated Group were undergone the same treatment except no ligation and no cut of any side of CCA.
2.4. Animal grouping and study design
During and after 2-VO operation, one rat died in Sham operated Group and 5 rats died in the operated group. One week after 2-VO, the rats in operated group were categorized into MDP Group (12 rats) and Model Group (11rats) according to the random number table. All rats were gavaged with different fluid (10 g kg−1·d−1) for 45 days, which calculated according to a conversion coefficient of 0.162 [10], equivalent to an adult dose of 1.62 g·kg−1·d−1. Rats in Model Group and Sham operated Group take saline as gavaged fluid, whereas rats in MDP Group were gavaged with MDP concentrated decoction.
2.5. Neurobehavioral assessment and brain tissue sampling
The spatial learning and memorial capacities of rats was measured by Morris Water Maze (MWM). The equipment was supplied by Chengdu Taimeng Science and Technology Co., Ltd., China. The test was began on the 40th day after the first day of gavaging, including navigation test and probing test as other studies [11]. The day after MWM test finishing, rats were capitalized and brain tissues were obtained through perfusion sampling and fresh sampling. The former is carried out for immunohistochemistry and pathological examination which involved with 6 rats of each group, whereas the latter is treated for RT-PCR test involved with 5 rats of each group. For RT-PCR detection, the frontal and parietal cortex of 5 rats in each group were removed and the hippocampus were dissociated and stored in liquid nitrogen at -80 °C for PCR detection.
2.6. Histopathological, and immunohistochemistry analysis
For histopathological analysis, rats' hippocampus were exposed, isolated and embedded in paraffin. Hippocampus were cut into approximately 4 μm coronal sections and stained with hematoxylin and eosin (H & E). Histopathological changes of hippocampus were observed under an optical microscope (Leica Microsystems Ltd., Wetzlar, Germany).
For immunohistochemical analysis, the steps of preparing hippocampal paraffin sections were the same as H & E staining. After restoring antigen, blocking endogenous peroxidase, sections were added individual primary antibodies with different dilution ratio, including rabbit anti-Bax, 1:200 (abcam, China. Lot:ab32503); rabbit anti-Bcl-2, 1:100 (Proteintech Group, Inc, Wuhan, lot: 12789-1-AP); rabbit anti-NF-kB p65, 1:150(Proteintech Group, Inc, Wuhan, lot: 10745-1-AP); rabbit anti-MAP-2, 1:50 (Proteintech Group, Inc, Wuhan, lot: 17490-1-AP); rabbit anti-Olig-2, 1:50(Proteintech Group, Inc, Wuhan, lot: 13999-1-AP); mice anti-GFAP, 1:15(Santa cruz, China. Lot: sc-58766); Lastly all the sections were added horseradish peroxidase labeled secondary antibodies IgG polymer, freshly prepared DAB coloring solution and Harris hematoxylin for counterstaining and color development. Select three sections of each protein and three 400-fold photographs of each section to analyze. The optical densities of Bax, Bcl-2, NF-kB p65p65, MAP-2, Olig-2, GFAP were analyzed using IPP6.0 software. The average optical density was compared and analyzed.
2.7. Observation of neuronal nuclei by transmission electron microscope (TEM)
Focus on the nuclei of neurons in CA1 Zone, the ultrastructure of nuclei were studied. Take 3 specimens of CA1 zone of the left hippocampus tissue which size was about 1 mm3, by fixation, rinsing, dehydration, infiltration, embedding and other steps to make ultra-thin sections which thickness was about 60–80 nm. Then they were double stained with uranyl acetate and lead citrate for 10 min respectively prior to be dried over a night. Select some representative sections to observe by TEM (Hitachi H-7000FA. Wuhan University, China).
2.8. Quantitative real-time polymerase chain reaction (qRT-PCR)
Trizol method was used to extract RNA (Aidlab Biotechnologies Co., Ltd, Beijing, Lot:252250AX), 2 ul of dissolved RNA was taken, the concentration and purity of the sample were measured with a micro-spectrophotometer. Based on the OD260/OD280 ratio, the RNA quality was estimated and the ratio should be 1.8 ~ 2.0. According to the target gene nucleic acid sequences provided by the American Biological Gene Bank, the standard cDNA sequence of the target gene was found, then in the open reading frame (ORF) conserved region, in combination with DNA star primer analysis software and network BLAST analysis software, Primer5 design software was used to design and synthesize primer (Qinke Biotechnology Co., Ltd. China), the list of primers used in this study is shown in Table 1.
Table 1.
Primers sequences of target genes.
| Name | Primer | Sequence | Size |
|---|---|---|---|
| β-actin | Forward | 5′- CACGATGGAGGGGCCGGACTCATC -3′ | 240 bp |
| Reverse | 5′- TAAAGACCTCTATGCCAACACAGT -3 | ||
| VEGF | Forward | 5′- CGTCTACCAGCGCAGCTATTG -3’ | 145 bp |
| Reverse | 5′- CTCCAGGGCTTCATCATTGC -3 | ||
| GAP43 | Forward | 5′- GGCTCTGCTACTACCGATGC -3’ | 185 bp |
| Reverse | 5′- TTGGAGGACGGCGAGTTAT -3 |
The cDNA was reversely transcribed and the mRNA was amplified and dissolved in the PCR instrument. The data was calculated using the ABI7900/illumina eco (7900/Viia7) analysis system. The final data were analyzed with 2−△△Ct, β-actin was used as an internal reference to calculate GAP-43 and VEGF mRNA content.
2.9. Statistical analyses
All data except pathological and ultrastructural images were analyzed with SPSS 22 software (IBM, Armonk, NY, USA). The quantitative date were expressed as mean ± SEM. Differences among different groups were analyzed by One-way analysis of variance. Differences between groups using post hoc Tukey's test for intergroup and intragroup comparison. P value < 0.05 was considered statistically significant.
3. Results
3.1. CCH model rats have obvious intelligence deficiency and MDP revert it
In navigation test, the escaping latencies of each group hadn't statistically difference in the first two days of the experiment. On the third day, Sham operated Group's escaping latency is shorter than it of Model Group (P < 0.05). On the fourth day, the escaping latency of MDP Group decreased rapidly and was similar to that of Sham operated Group, they were shorter than it in Model Group (P < 0.05, P < 0.05). In probing test, compared with Model Group, three parameters including passing platform frequencies, swimming time and distance in target quadrant in Sham operated Group and MDP Group were more than those of Model Group (P < 0.01 and P < 0.05). There was no statistical difference in total swimming distance between three groups (Table 2).
Table 3.
The parameters of probing test.
| n | Crossing platform frequencies | Swimming time in quadrant I(s) | Swimming distance in quadrant I (cm) | Total swimming distance (cm) | |
|---|---|---|---|---|---|
| Sham operated group | 13 | 11.6±1.7** | 16.7±4.3* | 102.3±24.5** | 2614.6±114.5 |
| Model group | 12 | 3.7±0.8 | 6.2±1.8 | 42.7±11.3 | 2536.7±121.6 |
| MDP group | 11 | 9.4±1.5* | 14.3±2.8* | 98.8±21.4** | 2467.8±153.4 |
Table 2.
Escaping latency among different groups ().
| n | 1st day | 2nd day | 3rd day | 4th day | |
|---|---|---|---|---|---|
| Sham-operated group | 13 | 81.2±20.6 | 61.0±24.9 | 18.2±6.7* | 11.2±4.7* |
| Model group | 12 | 95.2±23.0 | 64.7±18.3 | 32.2±13.8 | 35.4±22.1 |
| MDP group | 11 | 102.3±12.9 | 64.6±23.5 | 38.2±15.2 | 10.5±2.4* |
*Compared to model group on the same day, P < 0.05.
3.2. CCH leads to loss of hippocampal neurons and sparse nerve fibers, MDP partly restored hippocampal constitution
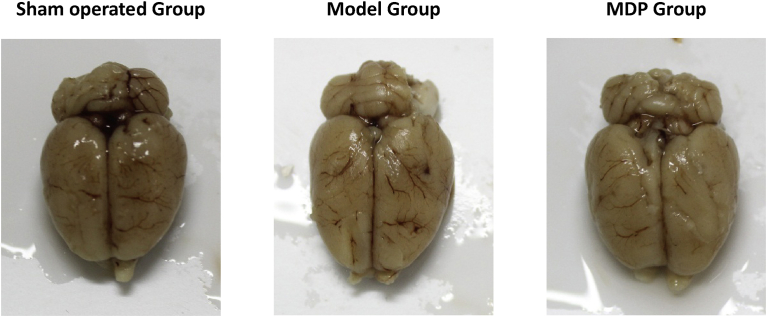
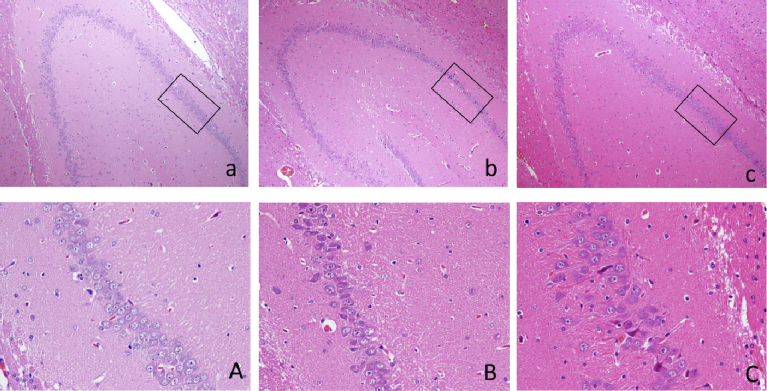
Compared the appearance of the entire brain specimens, the brain specimens in Model Group were looked more pale, drying and atrophy than those in Sham operated Group; while in MDP group the brain specimens were slightly edema and their size and shape were basically normal (Fig. 1). In pathological observation, there were marked differences among three groups. In Model Group, compared to Sham operated Group, the number of neurons in hippocampal CA1 area reduced, pyramidal neurons were distributed in 1–3 layers, the arrangement of cells was disordered and nerve fibers were sparse; From 400 times magnification images, the neurons morphology was abnormal, vacuoles could be seen in cytoplasm; Some nuclei were dark-dyeing, pyknosis or even disappeared. In MDP Group, the neurons in the CA1 region of hippocampus were arranged more neatly, the numbers of pyramidal cells significantly increased and they were arranged in 5–7 layers, the structures of cells were basically normal, nerve fibers were dense and regular (Fig. 2).
Fig. 1.
The representative overall specimen of rats brain among three group. The pictures show representative rats' brains (fresh sampling) in Sham operated Group, Model Group and MDP Group.
Fig. 2.
H & E staining images of hippocampus in each group. Above: Figure a, b, c respectively represent 100-fold images of Sham operated Group, Model Group and MDP Group of Hippocampus, The parts in the box in image a-c are enlarged (400 fold) in the figures below (A, B, C), which represents the pathological changes of CA1 zone in three groups. Below: Figure A, B, C respectively represent 400-fold images of Sham operated Group, Model Group and MDP Group of Hippocampal CA1 Zone.
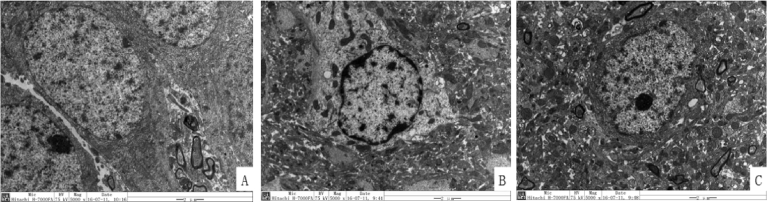
From TEM the ultrastructure of nucleus was observed, the nuclei and organelles of Sham operated Group were basically normal. The nuclear membrane of Model Group were shrinking and damaged, chromatin concentrated along the nuclear membrane (the so-called nuclear heterochromatin edge set), chromatin formed blocks of different shapes and sizes, some split into fragments or even penetrated into the cytoplasm. In cytoplasm, organelles were destroyed and amorphous substances were distributed everywhere. In MDP Group, although the organelle destruction was observed, the nuclear membrane and the nucleus were relatively intact, indicating that the degree of apoptosis was relatively little (Fig. 3).
Fig. 3.
Changes of neuronal nucleus among different group. Figure A, B, C are the representative images of the ultrastructure of nucleus of Sham operated Group, Model Group and MDP Group.
3.3. CCH results in neuroinflammation, apoptosis, remodeling of hippocampal CA1 zone
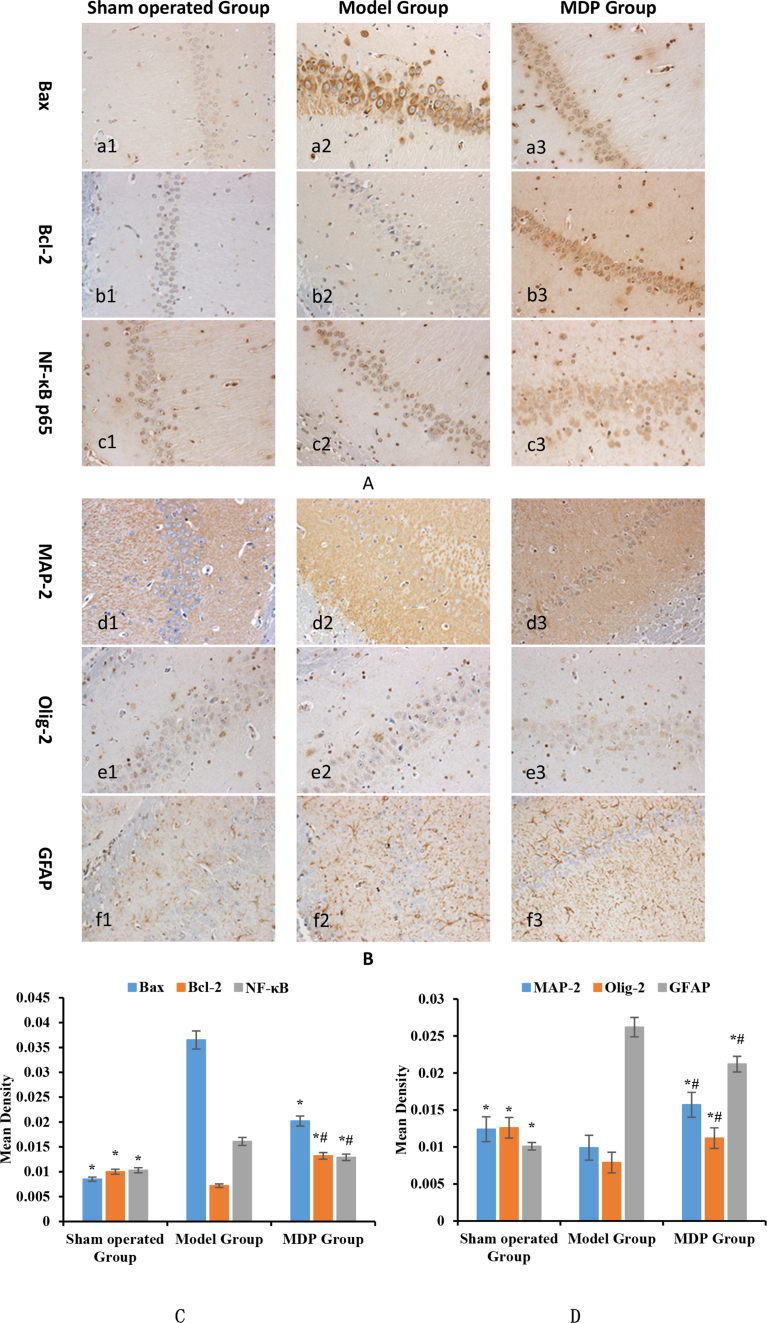
Through immunohistochemical analysis the expressions of key proteins in the Hippocampal CA1 Zone were detected. Among these key proteins, NF-κB p65, Bax, GFAP were obviously up-regulated in Model Group compared to Sham operated Group, but the tendency was inverted by MDP. The expression of MAP-2, Olig-2, and Bcl-2 significantly down-regulated in Model Group and were all inverted in MDP Group, the expressions of MAP-2 and Bcl-2 were even higher than those in Sham operated Group (Fig. 4).
Fig. 4.
Expression of key protein associated apoptosis, inflammation and cell-marker among different group. Note: (1) Meanings of the combination of letters and numbers in the icon: a, b, c, d, e, f are respectively represented as proteins of Bax, Bcl-2, NF-Κb, MAP-2, Olig-2 and GFAP; 1, 2, 3 are respectively represented as Sham operated Group, Model Group and MDP Group. (2)*: Compared to Model Group, P < 0.05; #: Compared to Sham operated Group, P < 0.05. Part A, C described the expression of key proteins associated apoptosis and inflammation among three groups. Expression of Bax was up-regulated in Model Group (a2), but it was inhibited by MDP (a3). For the expression of Bcl-2, the trend is completely an opposite, it was down-regulated in Model Group (b2) but boosted by MDP (b3). The trend of NF-κB is similar to that of Bax (c2, c3). Part B, D delineate the expression of cell-specific marker proteins among three groups. MAP-2 was inhibited in Model Group (d2) but encouraged by MDP (d3); For the expression of Olig-2, the trend was roughly same to MAP-2 (e2, e3); For the expression of GFAP, the trend is completely an opposite (f2, f3).
3.4. CCH boosts the expression of GAP-43 mRNA and VEGF mRNA, MDP furtherly facilitates it
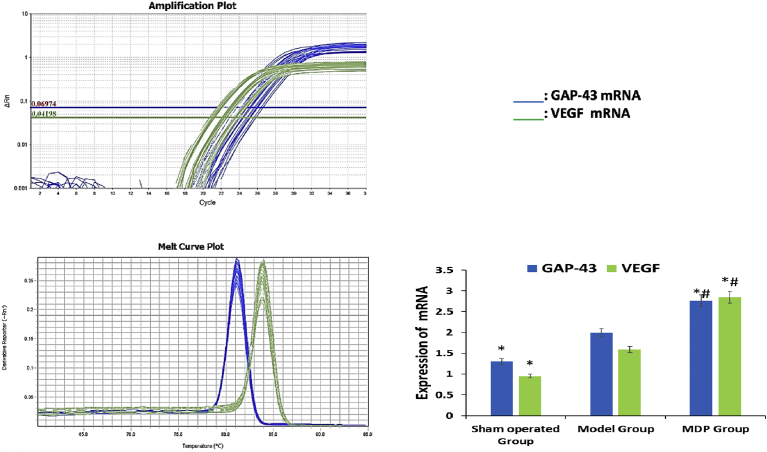
In PCR test, compared to those in Sham operated Group, the expressions of GAP-43 mRNA and VEGF mRNA significantly up-regulated in Model Group and furtherly up-regulated in MDP Group. The results were significant (P < 0.01 or P < 0.05) (Fig. 5).
Fig. 5.
Expression of GAP-43 mRNA and VEGF mRNA among three groups. Part A, B respectively demonstrates the amplification and melt plot of two target mRNA. The histogram of Part C illustrates the comparison of two target mRNA expressions among three groups. A consistency change can be seen that the expressions of GAP-43 mRNA and VEGF mRNA are all up-regulated in Model Group and further up-regulated in MDP Group.
4. Discussion
Similar to humans [5], rats have a complete set of Willis rings connecting the carotid and vertebral arteries. 2-VO operation results in rats' cerebral blood flow decreased by 70% [12], so it results in chronic global cerebral ischemia including hippocampal hypoperfusion, which is in line with the purpose of this study. From Morris Water Maze test, it can be seen that the spatial learning and referential memorial capacities of rats in Model Group were significantly impaired, which is consistent with previous studies [13, 14], indicating the reliability of this model-making method. What's more, the modified 2-VO operation has advantages of low mortality rate and high success rate. From MWM test, we know that MDP can effectively improve learning and memorial abilities of CCH rats.
It is well known that hippocampus is in charge of memorial forming and extracting. A normal hippocampus is the basis of good learning and memorial ability. The abnormal hippocampal constitution should shed light on the poor behavioral perform of CCH models. From H & E staining and TEM images among three groups, we concluded that CCH lead to considerable number of neurons loss. The mechanisms involved in neuronal loss include apoptosis, autophagy and necrosis, here we focus on the role of apoptosis in CCH and whether MDP has the function to relieve it. Apoptosis contributes to programmed cell death [15], Inflammation plays a very important role in the occurrence, development of apoptosis. To demonstrate inflammation and apoptosis in CCH, immunohistochemistry of key proteins associated to inflammation and apoptosis was carried out, the results give our tentative idea a powerful support. Compared to Sham operated Group, the overexpression of Bax and reduced expression of Bcl-2 in Model Group concurred, the former is a classic apoptosis marker and the latter is a known anti-apoptosis marker [16], these results indicate that apoptosis contributes to neuronal loss in CCH. Additionally, Compared to Sham operated Group, NF-κB p65 is up-regulated in Model Group. NF-κB p65 is a classic inflammation marker, for instance, IKK/IκB/NF-κB p65 inflammatory cascades involves in many inflammatory physiological actions [17]. The suppression of IKK/IκB/NF-κB p65 inflammatory cascades can prevent their translocation into nucleus so as to inhibit IL-6 production. NF-κB p65 is highly expressed under hypoxemia, which can protect neurons against oxidative stress and improve learning and memory function to a certain extent [18], but the excess expressions of various inflammatory factors promoted by NF-κB p65 facilitate apoptosis and gliosis. The over activation of NF-κB p65, up-regulated pro-apoptotic protein and down-regulated anti-apoptotic protein in neurons of CCH models demonstrated that inflammation and apoptosis contribute to the neuronal loss in CCH. It has been confirmed that inflammation, apoptosis and neural remodeling coexist and interplay in CCH. They can be aroused through various ways. Firstly, oxidative stress, free radical damage and inflammatory activation are the main causes of neuronal loss and astrocyte activation [19]. Moreover, Ca2+ homeostasis disruptions, Endoplasmic stress, autophagic dysfunction, excitotoxicity and so on could lead to apoptosis and neuro-inflammation [20]. Lastly, excessive NOX2 in CCH can directly activate the NF-κB p65 pathway in microglia/macrophages [21]. These theories are convincingly in favor of our results. Whereas, in MDP Group, the more survival neurons, down-regulated expression of Bax and NF-κB p65, up-regulated Bcl-2 demonstrate that MDP has the functions to anti-apoptosis and anti-inflammation to protect the neurons and recover the inherent neural construction in CCH. Given the complexity of the MDP component, the concrete mechanism should involve multiple aspects, and should be uncovered in the future.
Another phenomenon we should notice from immunohistochemistry is that in CCH cells constitution of NVU changed. Here we focus on three main kinds of cells in hippocampus, including neurons, astrocytes and oligodendrocyte, which markers are respectively MAP-2 [22], GFAP [23]and Olig-2 [24]. MAP-2 is a dendritic protein important for stabilizing microtubule and maintaining dendritic plasticity. MAP-2 is required for dendrite elongation [25]. It was reported that companied with the brain volumetric losses for alcoholic patients and the elderly, microtubule-associated proteins including MAP-2 and MAP-tau were also reduced [26]. Then we can deduce that expression of MAP-2 reflect the rough number of neurons. GFAP is a marker of glial activation and gliosis [5], it is not difficult to understand that the more higher GFAP express, the more Irreversible glial formation occurs. Oligodendrocytes (OLs) are myelin-forming cells in CNS that ensure rapid conduction of action potentials in neurons. In the adult brain, the transformation and myelination of OLs continues to ensure the continued formation of myelin. In addition, both oligodendrocytes and astrocytes originate from neural progenitor cells and they compete to differentiate and mutually constrain the counterpart's generation after ischemic injury [27], more oligodendrocytes denotes intelligence restore and more astrocytes hints the trend of cognitive deficit. From immunohistochemistry, compared to the Sham operated Group, Map-2 and Olig-2 down-regulated and GFAP up-regulated in Model Group, these changes signify that in CCH the “functional cells” including neurons and myeloid cells (oligodendrocytes) suffered more loss, the supported cells such as astrocytes got more proliferation. In MDP Group, This trend has reversed, which points out that MDP restore the original construction in hippocampus through protecting neurons, oligodendrocytes and preventing gliosis. What is more, the higher expression of MAP-2 and the more neurons in MDP Group than those in Sham operated Group hints the possibility of neuronal regeneration. This should be elaborated in the following paragraphs. The results of immunohistochemistry is highly consistent with those of H & E staining and TEM.
In order to furtherly explore the molecular regulatory process in CCH and interfered by MDP, the expression of key genes including GAP-43 mRNA and VEGF mRNA were detected with qRT-PCR. GAP-43 is a neuronal-specific protein that regulates multiple aspects of neuronal development including promoting neural progenitor cell differentiation and neuronal regeneration, promoting axon sprouting and regeneration after injury [28], promoting the formation and extension of neurites, promoting formation and reconstruction of synapses [29], therefore, the overexpression of GAP-43 mRNA should be found in an regenerating nerve tissue. VEGF is a cytokine mainly expressed by astrocytes in human brain and plays an important role in hypoxia-induced angiogenesis [30]. Hypoxia can induce monocytes to express HIF-1α, which stimulates glial cells to express VEGF [31]. Moreover VEGF prompts the hippocampal neuronal regeneration [32]. In an injured brain tissue, for the purpose of restoring the constituents of neurons and vessels, these two genes are usually simultaneously highly expressed. From the results of PCR, the simultaneously up-regulated genes of GAP-43 mRNA and VEGF mRNA in Model Group indicate the urge need of nerve regeneration in CCH, but hypoxia-induced inflammation, apoptosis of neurons and oligodendrocytes, gliosis are overwhelming, which results in neuronal loss and glial hyperplasia; In MDP Group, things are reversed, companied with the furtherly up-regulated of these two key genes and inhibition of apoptosis and inflammation by MDP, the number of neurons and oligodendrocytes increased and gliosis inhibited to a large extent. The highest expression of GAP-43 mRNA and VEGF mRNA, more neurons and more expression of MAP-2 than those in Sham operated Group both hints the neuronal regeneration which correspond with the results of H & E staining.
Although there are 14 kinds of Chinese herbs and countless elements in this Chinese prescription, previous studies have confirmed some herbs have various mechanisms to anti-ischemia-reperfusion injury in CNS. Through prompt the biosynthesis of enzymes relating to mitochondrial energy conversion, Dioscorea optimizes energy metabolism under hypoxia and resists apoptosis to keep cell “young” [33]. Component of Polygonum multiflorum has many pharmacological activities including antioxidant, anti-inflammation, and anti-aging effects [34]. Codonopsis pilosula can acts as an immunoregulator which inhibits too strong inflammation to weaken apoptosis and gliosis [35]. Paeoniflorin significantly reduces BP, increases microcirculation, improves vascular function and pathological changes, and up-regulates eNOS expression [36]. Those studies on monomer effective active ingredients in this Chinese medicine preparation may help to explain that superposition of multiple target effects of MDP underlie its good efficacy.
In summary, CCH results in apoptosis of neurons and oligodendrocytes, neuro-inflammation and glia hyperplasia which underlie the deficiency of learning and memorial capacities of CCH rats. The up-regulated gene of GAP-43 and VEGF have failed to reverse these pathological change in CCH. Through both dampening inflammation, apoptosis and gliosis, and simultaneously further up-regulating expressions of GAP-43 mRNA and VEGF mRNA to promote regeneration, MDP facilitates restoring the original constituents of NVU in hippocampus to recover the intelligence of CCH rats to a large extent.
5. Conclusions
Complex changes take place in CCH characterized by activation of inflammation, loss of neurons and glialization. MDP facilitates mental recovery in CCH rats by inhibiting neural inflammation, reducing glial formation, maintaining neuronal survival, and promoting nerve regeneration to restore the intrinsic structure of NVU.
Declarations
Author contribution statement
Hongbing Li: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was supported by the Applied Basic Research Programs of Science and Technology Department Foundation of Guizhou Province. No: 黔科合基础[2019]1005.
Competing interest statement
The author declares that he has no competing interests.
Additional information
Data associated with this study is available from the author on reasonable request.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Román G.C. Brain hypoperfusion: a critical factor in vascular dementia. Neurol. Res. 2004;26:454–458. doi: 10.1179/016164104225017686. [DOI] [PubMed] [Google Scholar]
- 2.Blixt J., Gunnarson E., Wanecek M. Erythropoietin attenuates the brain edema response following experimental traumatic brain injury. J. Neurotrauma. 2018;35:671–680. doi: 10.1089/neu.2017.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu Y., Chen J., Wang T., Zhou C., Liu Z., Ma L. Hsp70 inducer, 17-allylamino-demethoxygeldanamycin, provides neuroprotection via anti-inflammatory effects in a rat model of traumatic brain injury. Exp. Ther. Med. 2016;12:3767–3772. doi: 10.3892/etm.2016.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y., Zhang M.Y., Wang T., Fan Y.Y., Yu L.S., Ye G.H. Il-33/st2l signaling provides neuroprotection through inhibiting autophagy, endoplasmic reticulum stress, and apoptosis in a mouse model of traumatic brain injury. Front. Cell. Neurosci. 2018;12:95. doi: 10.3389/fncel.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkas E., Luiten P.G., Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Oh Tae Woo, Jung Hyo Won, Park Yong-Ki. Effect of modified Bo-yang-Hwan-o-Tang, a polyherbal medicine on the hippocampal neuronal damage in a rat model of global ischemia. Pharmacogn. Mag. 2015 Jul–Sep;11(43):665–673. doi: 10.4103/0973-1296.160445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Z.H., Lan H.C., Yang Q., Chen J., Mao S.P., Zha Y.F., Xiao S.J. Clinical research of early intervention of modified Shuyu Pill in vascular cognitive impairment No dementia. Chin. J. Integr. Tradit. West. Med. 2013;33(1):27–30. [PubMed] [Google Scholar]
- 8.Tan Z.H., LiuY Li N. Clinical observation of modified Shuyu Pill combined with acupuncture on aphasia after stroke. J. Emerg. Tradit. Chin. Med. 2013;22(1):34–35. [Google Scholar]
- 9.Guillén Javier, Prins J.B., Smith D., Degryse A.D. Chapter 5 – the European framework on research animal welfare regulations and guidelines. Lab. Anim. 2014:117–188. [Google Scholar]
- 10.Huang Ji-Han, Xiao-Hui Huang, Zhi-Yang Chen, Qing-Shan Zheng, Rui-Yuan Sun. Dose conversion among different animals and healthy volunteers in pharmacological study. Chin. J. Clin. Pharmacol. Therapeut. 2004;9(9):1069–1072. [Google Scholar]
- 11.Tabrizian K., Hashemzaei M., Nasiri A.A., Najafi S., Amelinia F., Sanati M., Shamshirgaran F., Fanoudi S. Folia interactive involvement of hippocampal cAMP/PKA and cyclooxygenase-2 signaling pathways in spatial learning in the Morris water maze. Neuropathology. 2018;56(1):58–66. doi: 10.5114/fn.2018.74660. [DOI] [PubMed] [Google Scholar]
- 12.Choy M., Ganesan V., Thomas D.L. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. Cereb. Blood Flow Metab. 2006;26(8):1066–1075. doi: 10.1038/sj.jcbfm.9600259. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Wang Y., Xie Y., Yang Z., Zhang T. Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem. Res. 2011;36:1840–1849. doi: 10.1007/s11064-011-0502-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L.M., Jiang C.D. Hydrogen sulfide attenuates neuronal injury induced by vascular dementia via inhibiting apoptosis in rats. Neurochem. Res. 2009;34:1984–1992. doi: 10.1007/s11064-009-0006-9. [DOI] [PubMed] [Google Scholar]
- 15.Luo C.L., Li B.X., Li Q.Q., Chen X.P., Sun Y.X., Bao H.J. Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience. 2011;184:54–63. doi: 10.1016/j.neuroscience.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A.1, Kaur G.2. Tinospora cordifolia as a potential neuroregenerative candidate against glutamate induced excitotoxicity: an in vitro perspective. BMC Complement Altern. Med. 2018 Oct 1;18(1):268. doi: 10.1186/s12906-018-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razali Nurul Atika, Nazarudin Nur Amiza, Lai Kok Song, Abas Faridah, Ahmad &Syahida. Curcumin derivative, 2,6-bis(2-fluorobenzylidene)cyclohexanone (ms65) inhibits interleukin-6 production through suppression of nf-κb and mapk pathways in histamine-induced human keratinocytes cell (hacat) BMC Complement Altern. Med. 2018;18(1):217. doi: 10.1186/s12906-018-2223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brambilla R., Bracchi-Ricard V., Hu W.H., Frydel B., Bramwell A., Karmally S. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng L.F., Ghirnikar R.S., Lee Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem. Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 20.Pearn M.L., Niesman I.R., Egawa J., Sawada A., Almenar-Queralt A., Shah S.B. Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell. Mol. Neurobiol. 2017;37:571–585. doi: 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Ma M.W., Dhandapani K.M., Brann D.W. Regulatory role of NADPH oxidase 2 in the polarization dynamics and neurotoxicity of microglia/macrophages after traumatic brain injury. Free Radic. Biol. Med. 2017;113:119–131. doi: 10.1016/j.freeradbiomed.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Serra S.C., Costa J.C., Assunção-Silva R.C., Teixeira F.G., Silva N.A., Anjo S.I., Manadas B., Gimble J., Behie L.A., Salgado A.J. Influence of passage number on the impact of the secretome of adipose tissue stem cells on neural survival, neurodifferentiation and axonal growth. Biochimie. 2018 Oct 17 doi: 10.1016/j.biochi.2018.09.012. pii: S0300-9084(18)30287-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Oh J., Kim Y., Baek D., Ha Y. Malignant gliomas can be converted to non-proliferating glial cells by treatment with a combination of small molecules. Oncol. Rep. 2018 Oct 25 doi: 10.3892/or.2018.6824. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Ghasemi N. The evaluation of astaxanthin effects on differentiation of human adipose derived stem cells into oligodendrocyte precursor cells. Avicenna J. Med. Biotechnol. 2018;10(2):69–74. [PMC free article] [PubMed] [Google Scholar]
- 25.Raber J., Ers T., Akinyeke T., Lee J., Weber Boutros S.J., Turker M.S. Detrimental effects of helium ion irradiation on cognitive performance and cortical levels of map-2 in b6d2f1 mice. Int. J. Mol. Sci. 2018;19(4):1247. doi: 10.3390/ijms19041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labisso Wajana, Raulin Ana Caroline, Nwidu Lucky, Kocon Artur, Wayne Declan. The loss of α- and β-tubulin proteins are a pathological hallmark of chronic alcohol consumption and natural brain ageing. Brain Sci. 2018 Sep 11;8(9):175. doi: 10.3390/brainsci8090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeal D.W., Brandner D.D., Gong X. Unbiased stereological analysis of reactive astrogliosis to estimate age-associated cerebral white matter injury. Neuropathol. Exp. Neurol. 2016 Jun;75(6):539–554. doi: 10.1093/jnen/nlw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams K.R., Mcaninch D.S., Snezana S., Xing L., Megan A., Li W. Hnrnp-q1 represses nascent axon growth in cortical neurons by inhibiting gap-43mrna translation. Mol. Biol. Cell. 2016;27(3):518–534. doi: 10.1091/mbc.E15-07-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R., Zhao J., Ju L. The influence of GAP-43 on orientation of cell division through G proteins. Int. J. Dev. Neurosci. 2015 Dec;47(Pt B):333–339. doi: 10.1016/j.ijdevneu.2015.07.013. Epub 2015 Sep 14. [DOI] [PubMed] [Google Scholar]
- 30.Licht T., Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell. Mol. Life Sci. 2013;70:1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoritsune Erina, Furuse Motomasa, Kuwabara Hiroko, Miyata Tomo, Nonoguchi Naosuke, Kawabata Shinji, Hayasaki Hana, Kuroiwa Toshihiko, Ono Koji, Shibayama Yuro, Miyatake Shin-Ichi. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J. Radiat. Res. 2014 Jul;55(4):803–811. doi: 10.1093/jrr/rru017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarkowski E., Issa R., Sjögren M., Wallin A., Blennow K., Tarkowski A., Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-β in Alzheimer's disease and vascular dementia. Neurobiol. Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 33.Ma J., Kang S.Y., Meng X., Kang A.N., Park J.H., Park Y.K. Effects of rhizome extract of dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in c2c12 myotubes. Molecules. 2018;23(8):2023. doi: 10.3390/molecules23082023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S.J., Hwang Y.H., Mun S.K., Hong S.G., Kim K.J., Kang K.Y., Son Y.J., Yee S.T. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside on ovariectomy induced osteoporosis mouse model. Int. J. Mol. Sci. 2018;19(9):2554. doi: 10.3390/ijms19092554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y.P., Feng B., Zhu Z.K., Feng X., Chen S.F., Li L.X. The polysaccharides from codonopsispilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice. Molecules. 2018;23(7):1801. doi: 10.3390/molecules23071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B., Yang Z.B., Lei S.S., Su J., Jin Z.W., Chen S.H. Combined antihypertensive effect of paeoniflorin enriched extract and metoprolol in spontaneously hypertensive rats. Phcog. Mag. 2018;14(53):44. doi: 10.4103/pm.pm_483_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.