Abstract
Prostate multi-parametric magnetic resonance imaging (mpMRI) has shown excellent sensitivity for Gleason ≥7 cancers, especially when their volume is ≥0.5 mL. As a result, performing an mpMRI before prostate biopsy could improve the detection of clinically significant prostate cancer (csPCa) by adding targeted biopsies to systematic biopsies. Currently, there is a consensus that targeted biopsies improve the detection of csPCa in the repeat biopsy setting and at confirmatory biopsy in patients considering active surveillance. Several prospective multicentric controlled trials recently showed that targeted biopsy also improved csPCa detection in biopsy-naïve patients. The role of mpMRI and targeted biopsy during the follow-up of active surveillance remains unclear. Whether systematic biopsy could be omitted in case of negative mpMRI is also a matter of controversy. mpMRI did show excellent negative predictive values (NPV) in the literature, however, since NPV depends on the prevalence of the disease, negative mpMRI findings should be interpreted in the light of a priori risk for csPCa of the patient. Nomograms combining mpMRI findings and classical risk predictors (age, prostate-specific antigen density, digital rectal examination, etc.) will probably be developed in the future to decide whether a prostate biopsy should be obtained. mpMRI has a good specificity for detecting T3 stage cancers, but its sensitivity is low. It should therefore not be used routinely for staging purposes in low-risk patients. Nomograms combining mpMRI findings and other clinical and biochemical data will also probably be used in the future to better assess the risk of T3 stage disease.
Keywords: Prostate cancer, Magnetic resonance imaging, Prostate biopsy, Active surveillance, Nomograms
1. Introduction
In most organs, when there is a suspicion of cancer, an imaging test is performed first. Then, when appropriate, an image-guided biopsy is obtained on suspicious lesions. Prostate cancer (PCa) is an exception to this rule. When PCa is suspected based on an elevated prostate-specific antigen (PSA) level and/or an abnormal digital rectal examination, 10–12 biopsies systematically distributed in predefined locations are usually obtained. Then, prostate imaging may be used for local staging purposes in selected patients with positive biopsy findings. This attitude is explained by the fact that PCa is barely visible on imaging, hence the search for tumors by systematic sampling of the gland.
This paradigm is changing due to recent progress in imaging, and particularly in magnetic resonance imaging (MRI). The so-called multi-parametric MRI (mpMRI) that combines T2-weighted imaging (T2WI) with functional pulse sequences such as diffusion-weighted imaging (DWI) or dynamic contrast-enhanced (DCE) imaging has shown excellent results in PCa detection [1], [2]. As a consequence, biopsies targeting suspicious lesions seen on mpMRI are increasingly used in addition to systematic biopsy [3], [4], [5]. Some authors even proposed to replace systematic biopsy by targeted biopsy based on mpMRI findings [6], [7], but this attitude remains controversial [8], [9], [10].
The purpose of this paper is to review current evidence on the diagnostic performance of prostate mpMRI in tumor detection and local staging, and to assess its potential role in patient management in the future.
2. Technical requirements for prostate mpMRI
Technical requirements for high-quality prostate mpMRI have been summarized by the European Society of Urogenital Radiology (ESUR) and the American College of Radiology (ACR) [11], [12]. Briefly, mpMRI should combine T2WI, DWI and DCE imaging. The use of magnetic resonance proton spectroscopy (MRS) is optional.
T2WI should be obtained in at least two orthogonal planes including the axial plane, using 3 mm slices without any gap and in-plane spatial resolution ≤0.7 mm (phase) × ≤0.4 mm (frequency). DWI should be obtained in the axial plane, with at least three b-values, a maximal b-value ≥1400 s/mm2, and in plane resolution ≤2.5 mm (phase and frequency). In practice, a b-value of 2000 s/mm2 can easily be obtained on modern imagers, even at 1.5 T, and these high b-value trace images are of considerable help in interpreting mpMRI. The diagnostic value of ultra-high b-values (up to 3000 s/mm2) is under evaluation [13]. DCE imaging should be obtained in the axial plane, with a temporal resolution <7 s and in-plane resolution ≤2 mm (phase and frequency). Axial T2WI, DWI and DCE images must be obtained at the same position and with the same slice center to allow direct comparisons.
The combined use of an endorectal coil and an external pelvic phased-array coil provides excellent signal-to-noise ratio. Similarly, 3 T imaging provides better signal-to-noise ratio than 1.5 T imaging. However, the endorectal coil is expensive and introduces artefacts and patient discomfort. 3 T magnetic resonance scanners are also expensive and not available world-wide. Excellent results in PCa detection have been obtained at 1.5 T and 3 T without the use of an endorectal coil [1]. The use of the endorectal coil and 3 T imaging is therefore optional. Imaging at lower field strength (<1.5 T) is discouraged.
3. Interpretation of mpMRI
3.1. Diagnostic criteria for PCa
In the peripheral zone (PZ), PCa appears typically as a focus of low signal intensity on T2WI, with restriction of diffusion and early and intense enhancement at DCE imaging (Fig. 1). Unfortunately, the appearance of PCa in PZ is highly variable and many benign conditions can mimic cancer, making the interpretation of focal lesions in PZ difficult. Besides typical cancers with unambiguous, marked, signal abnormalities on all magnetic resonance sequences, the radiologist must indeed characterize a large variety of lesions with various shapes, mild-to-moderate signal changes or even discrepant results from one pulse sequence to another (e.g., marked signal abnormality on one sequence and normal appearance on another). Experience is therefore of paramount importance in distinguishing cancer foci from benign conditions.
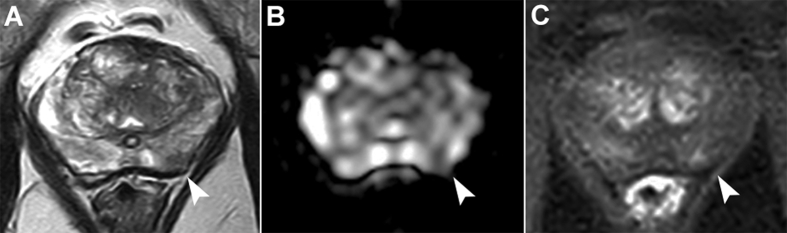
Figure 1.
Multiparametric magnetic resonance images obtained at 3 T in a 70-year-old man with a prostate-specific antigen level of 6 ng/mL. A prior systematic biopsy had shown a Gleason score 6 cancer in the left lobe and the patient was referred for multiparametric magnetic resonance imaging before confirmatory biopsy. Images showed a 5 mm lesion in the left peripheral zone showing low signal intensity at T2-weighted imaging (A, arrowhead), marked restriction of diffusion on the apparent diffusion coefficient map (B, arrowhead) and moderate focal enhancement at dynamic contrast-enhanced imaging (C, arrowhead). Targeted biopsy showed a Gleason score 7 (3 + 4, 10% of grade 4) with a maximum cancer core length of 4 mm.
In the transition zone (TZ), the diagnostic of PCa is made difficult by the presence of nodules of benign prostate hyperplasia (BPH). Typically, PCa appears as an area with low signal intensity on T2WI, lenticular shape, indistinct boundaries, no cyst, no capsule and a restriction of diffusion (Fig. 2) [14], [15]. Areas of (benign) stromal hyperplasia may show the same appearance [14]. Topography is then an important diagnostic criterion. Stromal hyperplasia is indeed scattered within encapsulated BPH nodules, while cancers are mostly located in the antero-apical third of the TZ [15]. The enhancement pattern of TZ cancers is highly variable and DCE imaging is not reliable to differentiate TZ cancers from BPH nodules [14].
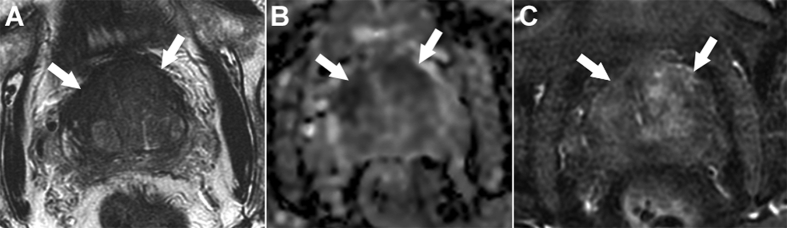
Figure 2.
Multiparametric magnetic resonance images obtained at 3 T in a 75-year-old man with a prostate-specific antigen level of 31 ng/mL and history of two prior negative systematic biopsies. T2-weighted imaging (A) showed an ill-defined lenticular area with marked low-signal intensity in the anterior third of both transition zones (arrows). This area showed a marked restriction of diffusion on the apparent diffusion coefficient map (B, arrows), and almost no enhancement at dynamic contrast-enhanced imaging (C, arrows). Targeted biopsies showed a Gleason score 7 (4 + 3, 60% of grade 4) cancer with a maximum cancer core length of 15 mm.
In 2011, a European consensus meeting recommended to assess the likelihood of malignancy of any lesion described in the prostate using a 5-level subjective score (1. definitely benign; 2. probably benign; 3. uncertain; 4. probably malignant; 5. definitely malignant) [16]. This so-called Likert score is a highly significant predictor of malignancy and efficiently stratifies the risk of malignancy of prostate lesions, at least with experienced radiologists [1], [17], [18], [19]. However, no clear diagnostic criteria are assigned to the five categories of the score that relies only on the radiologist's experience.
Several works showed that simple combinations of image features from the three pulse-sequences could stratify the likelihood of malignancy of prostate lesions, holding promise of more standardized interpretation between radiologists [20], [21]. In 2012, the ESUR endorsed the Prostate Imaging Reporting and Data System (PI-RADS) scoring system that defined semi-objective lesion features for T2WI, DWI, DCE imaging and MRS. The lesion features were assessed using a 5-level scale for each pulse sequence, resulting in a score range of 3–15 without MRS and 4–20 with MRS [11]. Unfortunately, this PI-RADS v1 score did not improve inter-reader variability [22], [23], [24]. The PI-RADS was revised in 2015 by the ESUR and the ACR. The resulting 5-level PI-RADS v2 score introduced the concept of a dominant pulse sequence (T2WI for TZ, DWI for PZ) holding most of the diagnostic value, the role of DCE imaging being reduced to characterizing lesions found indeterminate on DWI in PZ [12]. Even if some aspects of this scoring system have been criticized [25], a recent meta-analysis found that the PI-RADS v2 score was a good predictor for malignancy, with higher pooled sensitivity than the PI-RADS v1 score (0.95 [95% CI: 0.85–0.98] vs. 0.88 [95% CI: 0.80–0.93], p = 0.04) and similar specificity (0.73 [95% CI: 0.47–0.89] vs. 0.75 [95% CI: 0.36–0.94], p = 0.90) [26]. However, the inter-reader reproducibility of the PI-RADS v2 is moderate at best [27], [28], [29], [30]. Consequently, the good results reported in specialized institutions may not be generalized. Given the increasing use of prostate mpMRI over the world [5], [31], [32], this lack of reproducibility will become a major concern in the future.
Interestingly, the scores (Likert or PI-RADS) stratify not only the likelihood of malignancy, but also predict the aggressiveness of the tumor. A lesion scored 5 has indeed a probability of 70%–91% of being malignant, and of 50%–75% of being a Gleason score ≥7 cancer at targeted biopsy. Similarly, the probabilities of overall malignancy and Gleason score ≥7 cancer are 30%–60% and 14%–33% for lesions scored 4, and 7%–35% and 0–17% for lesions scored 3 [17], [18], [33], [34], [35], [36].
Using quantitative thresholds to predict the nature and aggressiveness of prostate focal lesions seen on MRI could improve the interpretation standardization. Many quantitative parameters are significant predictors of malignancy in prostate, either as stand-alone or in combination. As a result, some researchers have developed computer-aided diagnosis (CAD) systems aimed at improving visual diagnosis by measuring quantitative magnetic resonance parameters [19], [37], [38], [39]. Unfortunately, quantitative approaches in MRI are limited by substantial variability across imagers from different manufacturers. Therefore, it may be difficult to define reliable thresholds that could be applied to all imagers. Of the many CAD systems published in recent literatures, only a few have been proven robust enough to be used on data from different manufacturers [40], [41]. Thus, it remains unclear whether quantitative imaging will play a major role in PCa characterization in the future.
3.2. Diagnostic criteria for local extraprostatic extension (ECE)
ECE is assessed on T2WI. Fat-suppressed DCE imaging may also be helpful. Imaging features suggestive of ECE include broad tumor contact with the capsule, asymmetry or invasion of the neurovascular bundles, a bulging prostatic contour, an irregular or spiculated margin, obliteration of the rectoprostatic angle and breach of the capsule with evidence of direct tumor extension or bladder wall invasion [12]. Not all these features have the same diagnostic weight, and it is advised to pay particular attention to “direct” signs of ECE such as tumor signal intensity within the periprostatic fat or the bladder wall, or obliteration of the rectoprostatic angle (Fig. 3). “Indirect” signs such as tumor bulging or broad tumor contact with the capsule must be interpreted with care since they may induce a large number of false positive findings.
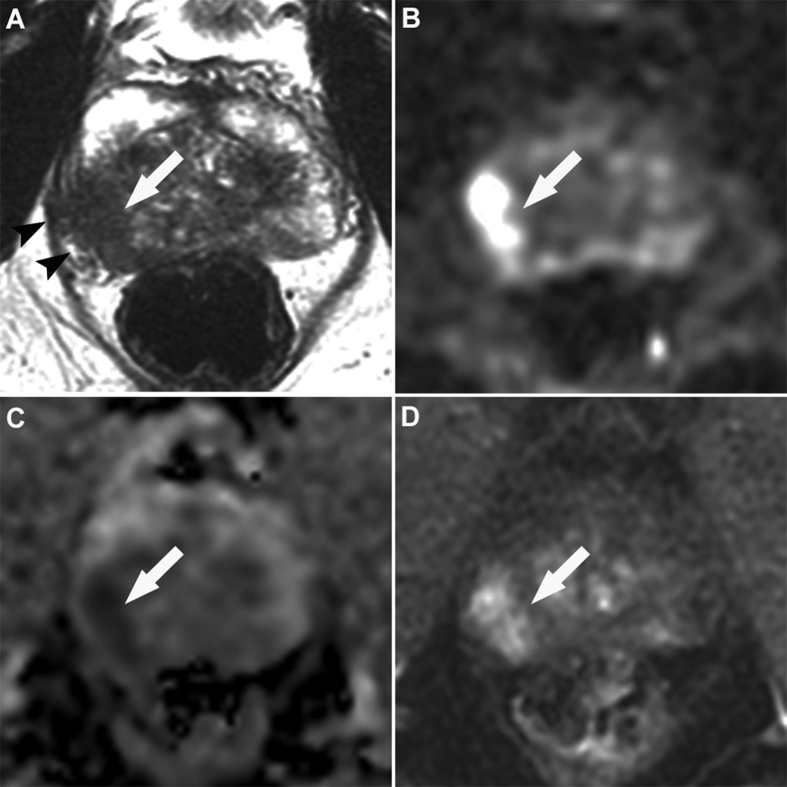
Figure 3.
Multiparametric magnetic resonance images obtained at 3 T in a 54-year-old man with a prostate-specific antigen level of 5.1 ng/mL and positive digital rectal examination of the right prostate lobe. Images showed a typical cancer of the right base with low signal intensity at T2-weighted imaging (A, white arrow), marked restriction of diffusion on trace images obtained with a b-value of 2000 s/mm2 (B, white arrow) and on the corresponding apparent diffusion coefficient map (C, white arrow), and marked focal enhancement at dynamic contrast-enhanced imaging (D, white arrow). T2-weighted imaging also showed a marked extracapsular extension (A, black arrowheads). Targeted and systematic biopsy showed Gleason score 7 (3 + 4, 20% of grade 4) cancer in the right base and right midgland, with a maximum cancer core length of 12 mm.
The features of seminal vesicle invasion (SVI) include focal or diffuse low T2-weighted signal intensity and/or abnormal contrast enhancement within the seminal vesicle, restricted diffusion, obliteration of the angle between the base of the prostate and the seminal vesicle, and demonstration of direct tumor extension from the base of the prostate into and around the seminal vesicle [12]. As for ECE features, it is recommended to have a rather specific interpretation, and to take into account only direct signs of SVI from the prostate base, with obliteration of the prostatoseminal angle. Isolated low T2-weighted signal intensity and/or enhancement with no direct connection with a tumor in the prostate base must be interpreted with care. In our experience, they usually correspond to benign conditions.
Every focal lesion seen in the prostate should receive not only a score of likelihood of malignancy (using the PI-RADS v2 score), but also a score of likelihood of extraprostatic extension. The PI-RADS v1 document provides some guidelines on how to score this extraprostatic extension [11].
As mentioned above, mpMRI specificity in evaluating ECE and SVI tends to be good. Its sensitivity is low. Pooled data from a meta-analysis for ECE, SVI and overall stage T3 showed a sensitivity and specificity of 0.57 (95% CI: 0.49–0.64) and 0.91 (95% CI: 0.88–0.93), 0.58 (95% CI: 0.47–0.68) and 0.96 (95% CI: 0.95–0.97), and 0.61 (95% CI: 0.54–0.67) and 0.88 (95% CI: 0.85–0.91), respectively [42]. Low sensitivity is mostly explained by the fact that mpMRI cannot detect microscopic invasion [43], [44].
The use of high field strength (3 T) or functional imaging in addition to T2WI improves sensitivity for EPE or SVI detection [42], but the experience of the reader remains of paramount importance [45] and the inter-reader agreement is moderate with kappa values ranging from 0.41 to 0.68 [46].
4. The role of mpMRI in the management of clinically localized PCa
4.1. Prebiopsy mpMRI in patients with no history of PCa
Correlation with radical prostatectomy specimens showed that mpMRI detection rates depended on the tumor Gleason score and volume [1], [2]. mpMRI sensitivity is excellent for Gleason score ≥7 cancers with a volume ≥0.5 mL. In a series of 175 pre-prostatectomy mpMRI assessed by two independent readers, detection rates were 21%–29% and 49%–60% for Gleason score ≤6 tumors of less and more than 0.5 mL respectively, 56%–63% and 88%–92% for Gleason score 7 tumors of less and more than 0.5 mL respectively, and 96% for Gleason score ≥8 tumors [1].
Because of this high sensitivity for aggressive tumors, many groups evaluated whether mpMRI could be used to increase the detection of csPCa by showing suspicious lesions that could be targeted at biopsy. A systematic review showed that magnetic resonance/ultrasound fusion targeted biopsies detected significantly more csPCa than standard systematic biopsy. The median difference in detection rate between the two approaches was 6.8% (range, 0.9%–41.4%) and always in favor of targeted biopsy [4]. However, another systematic review showed that the increased detection of csPCa due to targeted biopsy was significant only in the repeat biopsy setting. In patients with history of negative prior biopsy, the relative sensitivity between targeted and systematic biopsy was 1.54 (95% CI: 1.05–2.57). It was only 1.10 (95% CI: 1.00–1.22) in biopsy-naïve patients [3]. This can be explained by the fact that the repeat biopsy group is more favorable for mpMRI, with an increased prevalence of anterior tumors that are well detected by mpMRI [47], but tend to be missed by systematic biopsy.
In biopsy-naïve patients, several prospective monocenter trials gave conflicting results on the added value of targeted biopsy as compared to standard systematic biopsy [48], [49], [50], [51], [52]. However, three recent large prospective multicenter studies gave concordant results that may change guidelines on this matter. The PROMIS study evaluated mpMRI and transrectal ultrasound (TRUS)-guided systematic biopsy against template prostate mapping biopsy in 576 biopsy-naïve men [53]. mpMRI was more sensitive for detection of csPCa (Gleason score ≥4 + 3 or cancer core length ≥6 mm) than TRUS-guided systematic biopsy (93% [95% CI: 88%–96%] vs. 48% [95% CI: 42%–55%], p < 0.0001). However, the analysis was made at the patient level, and did not assess the spatial correlation between areas positive on mpMRI and on template mapping biopsy. The fact that mpMRI had a poor specificity (41% [95% CI: 36%–46%]) may have generated some false positive findings interpreted as true positive at per-patient analysis. Thus, mpMRI sensitivity may have been overestimated and the added value of targeted biopsy cannot be inferred from these results. The PRECISION study randomized 500 patients to either 10–12 core systematic biopsy or to mpMRI with no biopsy if mpMRI was negative (PI-RADS v2 score ≤2) or targeted biopsy without systematic biopsy if mpMRI was positive (up to three lesions targeted and up to four cores per target) [54]. Targeted biopsy detected significantly more men with Gleason score ≥7 cancer (38% vs. 26%, adjusted difference of 12 points [95% CI: 4–20]; p = 0.005), and significantly fewer men with non-significant cancers (defined as Gleason score 6 cancers; 9% vs. 22%, adjusted difference of 13 points [95% CI: 7–19]; p < 0.001). Based on these excellent results, one may think that using mpMRI as a triage test (i.e. performing no biopsy at all when mpMRI is negative and performing only targeted biopsy when mpMRI is positive) could become a new standard of care. However, the MRI-FIRST trial brings nuance to the PRECISION results. In MRI-FIRST, 275 patients underwent 12–14 core systematic biopsy and 3–6 core targeted biopsy by two different operators [55]. The operator performing systematic biopsy was blinded to mpMRI results. Targeted biopsy diagnosed more men with Gleason score ≥7 cancer than systematic biopsy, but the difference was not statistically significant (32.3% [95% CI: 26.5%–38.4%] vs. 29.9% [95% CI: 24.3%–36.0%], p = 0.38). The percentage of patients detected by combined systematic and targeted biopsy (37.5% [95% CI: 31.4%–43.8%]) was substantially higher than that detected by targeted or systematic biopsy alone. Gleason score ≥7 cancers would have been missed in 5.2% (95% CI: 2.8%–8.7%) of patients, had systematic biopsy not been performed, and in 7.6% (95% CI: 4.6%–11.6%) of patients, had targeted biopsy not been performed.
Thus, it seems clear that obtaining a pre-biopsy mpMRI improves the detection of csPCa. However, whether systematic biopsy can be omitted remains controversial. Many groups reported mpMRI had excellent negative predictive value for ruling out csPCa [10]. Yet, analysis of several prospective monocenter trials showed that combined systematic and targeted biopsy performed substantially better than targeted biopsy alone [52], [56], [57], [58], [59], which is in line with the MRI-FIRST results. Interestingly, one retrospective study of 211 patients with unilateral MRI lesions reported that systematic biopsy was useful in addition to targeted biopsy in the lobe containing the MRI lesion; in contrast, the added value of the systematic biopsy of the contralateral lobe was marginal [60]. This suggests that systematic biopsy may play a role in detecting csPCa identified by mpMRI but missed by targeted biopsy. This is in line with MRI-FIRST findings. Indeed, in the MRI-FIRST cohort, systematic biopsy found Gleason score ≥7 cancer missed by targeted biopsy in 13 patients. Five of these 13 patients had negative mpMRI; of the eight remaining patients, five had an MRI lesion in the same sextant in which systematic biopsy found Gleason score ≥7 cancer. Thus, at least in these five patients, targeted biopsy might have missed its target [55]. The accuracy of guiding methods for targeted biopsy has been seldom evaluated in routine conditions [61]. It becomes mandatory to carefully evaluate this accuracy in order to define the minimal number of targeted cores required as a function of the size of the MRI lesion and of the size of the prostate.
Nonetheless, using negative and positive predictive values may be misleading since they are dependent on the prevalence of the disease in the population [62]. A recent systematic review showed that the reported prevalence for overall cancer and csPCa in patients undergoing pre-biopsy mpMRI was highly variable across institutions (13%–74.7% and 13.7%–50.9%, respectively) [10]. Thus, it is necessary to standardize, or at least risk-stratify, the population referred for pre-biopsy mpMRI before defining the need for systematic or targeted biopsy. High-risk patients with negative mpMRI findings may still need systematic biopsy, and low-risk patients with positive mpMRI may not all need biopsy depending on the PI-RADS scores of the MRI lesions. Interestingly, several recent studies suggested that combining mpMRI findings and clinical data such as age, PSA level, prostate volume, history of prior biopsy and/or digital rectal examination findings could improve the prediction of the results of subsequent prostate biopsy [63], [64], [65], [66]. Such nomograms may therefore be used in the future to decide whether the patient needs to undergo biopsy or not, based on his clinical background and mpMRI findings.
4.2. The role of mpMRI in active surveillance (AS)
AS is a strategy aimed at delaying the active treatment of cancers of low aggressiveness, until they show signs of aggressiveness or progression. Since it is a strategy with curative intent, it is essential not to miss the window of opportunity for cure while following up the patient.
There is currently no consensus on the exact criteria that should indicate AS, nor on the criteria that should trigger active treatment. Most groups include only Gleason score 6 cancers with low PSA levels, low PSA density and a small number of positive biopsy cores. Some authors also include patients with Gleason score 7 (3 + 4) cancers, a least when their age is over 70 years. Most authors agree that when AS is considered, the patient should undergo another biopsy. This so-called confirmatory biopsy is intended to identify higher-grade cancer that could have been missed on the original biopsy. The frequency of repeat biopsy during the course of follow-up is not consensual [67].
mpMRI is useful to improve the detection of csPCa at confirmatory biopsy, i.e. at initial assessment of men considering AS [68]. In a recent review of literature, Schoots et al. [69] pooled the results of eight series comprising a total of 931 patients who underwent mpMRI before confirmatory biopsy. mpMRI was positive in 73% of patients. A total of 32% of patients (297/931) were reclassified at confirmatory biopsy due to the discovery of Gleason score ≥7 (3 + 4) cancer. Cancer upgrading occurred in 13% (121/931) on both systematic and targeted biopsies, in 11% (105/931) on systematic biopsy alone and in 8% (71/931) on MRI-targeted biopsy alone. This suggests that mpMRI is useful before confirmatory biopsy, but that systematic biopsy should be performed in addition to targeted biopsies. Performing an mpMRI before confirmatory biopsy is now recommended by the European Association of Urology Guidelines [70].
The role of mpMRI during the course of AS (i.e. after the confirmatory biopsy) is unclear. There is no consensus whether or not serial mpMRIs should be obtained during AS and what would be their optimal frequency. It remains controversial whether negative or stable mpMRI findings during follow-up could safely obviate subsequent biopsy. Small series reported reclassification rates of 35%–70% at follow-up biopsies when mpMRI findings suggest progression, versus 16%–32% in case of negative or stable mpMRI [69].
Before the use of mpMRI could be further assessed during AS, there is a need for standardizing the interpretation of mpMRI in AS patients and for defining precise imaging criteria suggesting progression. A group of experts has recently made propositions, with the hope that this fosters research on that matter [71].
4.3. The role of mpMRI in local staging
Because of its low sensitivity for microscopic ECE and SVI, mpMRI is not recommended for local staging in low-risk patients. However, mpMRI can still be useful for treatment planning in selected low-risk patients (e.g. candidates for brachytherapy) [72].
In the future, other tools may be used in conjunction with mpMRI findings to better predict T3 stage diseases. Several clinical parameters including PSA level, PSA density, age, digital rectal examination, percentage of cancer in biopsy cores, biopsy Gleason score, MRI lesion size have been shown to improve the staging performances when combined with mpMRI features of ECE and SVI [73], [74], [75], [76]. Therefore, it is likely that local staging will be assessed in the future not by mpMRI findings alone but by nomograms combining clinical, biochemical and imaging features.
5. Future perspectives
Because mpMRI will be increasingly used before biopsy, it seems necessary to address three main issues in the near future.
First, the moderate inter-reader reproducibility of prostate mpMRI becomes a critical issue. If the excellent results obtained with mpMRI in referral hospitals are not reproduced in community centers, the broad use of prostate mpMRI will lead to patient mismanagement in a substantial proportion of cases. A large effort of standardization of technical protocols and image interpretation has been undertaken during the last few years. This effort will be continued through future refinements of the PI-RADS score. A major effort is also necessary in radiologists' specialization, improved multidisciplinary discussions, and, probably, in developing certification procedures and effective quality controls in centers performing prostate mpMRI. Quantitative imaging may also help standardizing mpMRI interpretation. Interestingly, one CAD, cross-validated on images from two different manufacturers, has been recently shown to outperform Likert scoring in predicting the presence of Gleason ≥7 cancer at targeted biopsy [77].
Avoiding unnecessary biopsies will also be a major research topic in the future. As discussed above, combining clinical data, PSA-based features and mpMRI findings helps defining patients who could safely avoid prostate biopsy. A prospective and multicentric validation of such a multivariable nomogram is now necessary.
Lastly, evaluating the accuracy of targeted biopsies (accuracy of guiding methods, minimal number of cores needed, influence of the training of the operator, etc.) becomes mandatory since targeted biopsies are likely to be increasingly used alone, without systematic biopsies in the future.
6. Conclusion
Because of its excellent sensitivity for aggressive cancer, mpMRI is increasingly used to localize csPCa before biopsy, and obtaining prostate mpMRI before biopsy will probably become the standard of care in the future. However, it remains necessary to improve mpMRI inter-reader reproducibility, to define the best way to select the patients that could safely avoid biopsy and to evaluate (and improve) the accuracy of the methods guiding the biopsies that target suspicious lesions identified by mpMRI.
Author contributions
Study concept and design: Olivier Rouviere.
Drafting of manuscript: Olivier Rouviere.
Critical revision of the manuscript: Olivier Rouviere, Paul Cezar Moldovan.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Bratan F., Niaf E., Melodelima C., Chesnais A.L., Souchon R., Mege-Lechevallier F. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol. 2013;23:2019–2029. doi: 10.1007/s00330-013-2795-0. [DOI] [PubMed] [Google Scholar]
- 2.Le J.D., Tan N., Shkolyar E., Lu D.Y., Kwan L., Marks L.S. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67:569–576. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 3.Schoots I.G., Roobol M.J., Nieboer D., Bangma C.H., Steyerberg E.W., Hunink M.G. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–450. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Valerio M., Donaldson I., Emberton M., Ehdaie B., Hadaschik B.A., Marks L.S. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68:8–19. doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Renard-Penna R., Rouviere O., Puech P., Borgogno C., Abbas L., Roy C. Current practice and access to prostate MR imaging in France. Diagn Interv Imaging. 2016;97:1125–1129. doi: 10.1016/j.diii.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Venderink W., van Luijtelaar A., Bomers J.G., van der Leest M., Hulsbergen-van de Kaa C., Barentsz J.O. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Arsov C., Rabenalt R., Blondin D., Quentin M., Hiester A., Godehardt E. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68:713–720. doi: 10.1016/j.eururo.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Schoots I.G. Omission of systematic transrectal ultrasound guided biopsy from the MRI targeted approach in men with previous negative prostate biopsy might still be premature. Ann Transl Med. 2016;4:205. doi: 10.21037/atm.2016.03.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouviere O. Will all patients with suspicion of prostate cancer undergo multiparametric MRI before biopsy in the future? Diagn Interv Imaging. 2016;97:389–391. doi: 10.1016/j.diii.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Moldovan P.C., Van den Broeck T., Sylvester R., Marconi L., Bellmunt J., van den Bergh R.C.N. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72:250–266. doi: 10.1016/j.eururo.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Barentsz J.O., Richenberg J., Clements R., Choyke P., Verma S., Villeirs G. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J. PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenkrantz A.B., Parikh N., Kierans A.S., Kong M.X., Babb J.S., Taneja S.S. Prostate cancer detection using computed very high b-value diffusion-weighted imaging: how high should we go? Acad Radiol. 2016;23:704–711. doi: 10.1016/j.acra.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Oto A., Kayhan A., Jiang Y., Tretiakova M., Yang C., Antic T. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2010;257:715–723. doi: 10.1148/radiol.10100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesnais A.L., Niaf E., Bratan F., Mege-Lechevallier F., Roche S., Rabilloud M. Differentiation of transitional zone prostate cancer from benign hyperplasia nodules: evaluation of discriminant criteria at multiparametric MRI. Clin Radiol. 2013;68:e323–e330. doi: 10.1016/j.crad.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson L., Ahmed H.U., Allen C., Barentsz J.O., Carey B., Futterer J.J. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Mozer P., Roupret M., Le Cossec C., Granger B., Comperat E., de Gorski A. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015;115:50–57. doi: 10.1111/bju.12690. [DOI] [PubMed] [Google Scholar]
- 18.Costa D.N., Lotan Y., Rofsky N.M., Roehrborn C., Liu A., Hornberger B. Assessment of prospectively assigned likert scores for targeted magnetic resonance imaging-transrectal ultrasound fusion biopsies in patients with suspected prostate cancer. J Urol. 2016;195:80–87. doi: 10.1016/j.juro.2015.07.080. [DOI] [PubMed] [Google Scholar]
- 19.Niaf E., Lartizien C., Bratan F., Roche L., Rabilloud M., Mege-Lechevallier F. Prostate focal peripheral zone lesions: characterization at multiparametric MR imaging--influence of a computer-aided diagnosis system. Radiology. 2014;271:761–769. doi: 10.1148/radiol.14130448. [DOI] [PubMed] [Google Scholar]
- 20.Rouviere O., Papillard M., Girouin N., Boutier R., Rabilloud M., Riche B. Is it possible to model the risk of malignancy of focal abnormalities found at prostate multiparametric MRI? Eur Radiol. 2012;22:1149–1157. doi: 10.1007/s00330-011-2343-8. [DOI] [PubMed] [Google Scholar]
- 21.Rouviere O., Dagonneau T., Cros F., Bratan F., Roche L., Mege-Lechevallier F. Diagnostic value and relative weight of sequence-specific magnetic resonance features in characterizing clinically significant prostate cancers. PLoS One. 2017;12:e0178901. doi: 10.1371/journal.pone.0178901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkrantz A.B., Kim S., Lim R.P., Hindman N., Deng F.M., Babb J.S. Prostate cancer localization using multiparametric MR imaging: comparison of prostate imaging reporting and data system (PI-RADS) and Likert scales. Radiology. 2013;269:482–492. doi: 10.1148/radiol.13122233. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkrantz A.B., Lim R.P., Haghighi M., Somberg M.B., Babb J.S., Taneja S.S. Comparison of interreader reproducibility of the prostate imaging reporting and data system and Likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol. 2013;201:W612–W618. doi: 10.2214/AJR.12.10173. [DOI] [PubMed] [Google Scholar]
- 24.Vache T., Bratan F., Mege-Lechevallier F., Roche S., Rabilloud M., Rouviere O. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology. 2014;272:446–455. doi: 10.1148/radiol.14131584. [DOI] [PubMed] [Google Scholar]
- 25.Rosenkrantz A.B., Oto A., Turkbey B., Westphalen A.C. Prostate imaging reporting and data system (PI-RADS), version 2: a critical look. AJR Am J Roentgenol. 2016;206:1179–1183. doi: 10.2214/AJR.15.15765. [DOI] [PubMed] [Google Scholar]
- 26.Woo S., Suh C.H., Kim S.Y., Cho J.Y., Kim S.H. Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol. 2017;72:177–188. doi: 10.1016/j.eururo.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Muller B.G., Shih J.H., Sankineni S., Marko J., Rais-Bahrami S., George A.K. Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology. 2015;277:741–750. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenkrantz A.B., Ginocchio L.A., Cornfeld D., Froemming A.T., Gupta R.T., Turkbey B. Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology. 2016;280:793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tewes S., Mokov N., Hartung D., Schick V., Peters I., Schedl P. Standardized reporting of prostate MRI: comparison of the prostate imaging reporting and data system (PI-RADS) version 1 and version 2. PLoS One. 2016;11:e0162879. doi: 10.1371/journal.pone.0162879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer M.D., Brown A.M., Shih J.H., Summers R.M., Marko J., Law Y.M. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging. 2017;45:579–585. doi: 10.1002/jmri.25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthigi A., Sidana A., George A.K., Kongnyuy M., Maruf M., Valayil S. Current beliefs and practice patterns among urologists regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol. 2017;35:32.e1–32.e7. doi: 10.1016/j.urolonc.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberlin D.T., Casalino D.D., Miller F.H., Meeks J.J. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom Radiol (NY) 2017;42:1255–1258. doi: 10.1007/s00261-016-0975-5. [DOI] [PubMed] [Google Scholar]
- 33.Venderink W., Govers T.M., de Rooij M., Futterer J.J., Sedelaar J.P.M. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. AJR Am J Roentgenol. 2017;208:1058–1063. doi: 10.2214/AJR.16.17322. [DOI] [PubMed] [Google Scholar]
- 34.Borkowetz A., Platzek I., Toma M., Renner T., Herout R., Baunacke M. Evaluation of prostate imaging reporting and data system classification in the prediction of tumor aggressiveness in targeted magnetic resonance imaging/ultrasound-fusion biopsy. Urol Int. 2017;99:177–185. doi: 10.1159/000477263. [DOI] [PubMed] [Google Scholar]
- 35.Liddell H., Jyoti R., Haxhimolla H.Z. mp-MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer—a retrospective review of 92 biopsied PIRADS 3 lesions. Curr Urol. 2015;8:96–100. doi: 10.1159/000365697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertan F.V., Greer M.D., Shih J.H., George A.K., Kongnyuy M., Muthigi A. Prospective evaluation of the prostate imaging reporting and data system version 2 for prostate cancer detection. J Urol. 2016;196:690–696. doi: 10.1016/j.juro.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 37.Lemaitre G., Marti R., Freixenet J., Vilanova J.C., Walker P.M., Meriaudeau F. Computer-aided detection and diagnosis for prostate cancer based on mono and multi-parametric MRI: a review. Comput Biol Med. 2015;60:8–31. doi: 10.1016/j.compbiomed.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Litjens G.J., Barentsz J.O., Karssemeijer N., Huisman H.J. Clinical evaluation of a computer-aided diagnosis system for determining cancer aggressiveness in prostate MRI. Eur Radiol. 2015;25:3187–3199. doi: 10.1007/s00330-015-3743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hambrock T., Vos P.C., Hulsbergen-van de Kaa C.A., Barentsz J.O., Huisman H.J. Prostate cancer: computer-aided diagnosis with multiparametric 3-T MR maging—effect on observer performance. Radiology. 2013;266:521–530. doi: 10.1148/radiol.12111634. [DOI] [PubMed] [Google Scholar]
- 40.Peng Y., Jiang Y., Antic T., Giger M.L., Eggener S.E., Oto A. Validation of quantitative analysis of multiparametric prostate MR images for prostate cancer detection and aggressiveness assessment: a cross-imager study. Radiology. 2014;271:461–471. doi: 10.1148/radiol.14131320. [DOI] [PubMed] [Google Scholar]
- 41.Hoang Dinh A., Melodelima C., Souchon R., Lehaire J., Bratan F., Mege-Lechevallier F. Quantitative analysis of prostate multiparametric MR images for detection of aggressive prostate cancer in the peripheral zone: a multiple imager study. Radiology. 2016;280:117–127. doi: 10.1148/radiol.2016151406. [DOI] [PubMed] [Google Scholar]
- 42.de Rooij M., Hamoen E.H., Witjes J.A., Barentsz J.O., Rovers M.M. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol. 2016;70:233–245. doi: 10.1016/j.eururo.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Jager G.J., Ruijter E.T., van de Kaa C.A., de la Rosette J.J., Oosterhof G.O., Thornbury J.R. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol. 1996;166:845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 44.Cornud F., Flam T., Chauveinc L., Hamida K., Chretien Y., Vieillefond A. Extraprostatic spread of clinically localized prostate cancer: factors predictive of pT3 tumor and of positive endorectal MR imaging examination results. Radiology. 2002;224:203–210. doi: 10.1148/radiol.2241011001. [DOI] [PubMed] [Google Scholar]
- 45.Heijmink S.W., Futterer J.J., Hambrock T., Takahashi S., Scheenen T.W., Huisman H.J. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–195. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 46.Futterer J.J., Engelbrecht M.R., Huisman H.J., Jager G.J., Hulsbergen-van De Kaa C.A., Witjes J.A. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005;237:541–549. doi: 10.1148/radiol.2372041724. [DOI] [PubMed] [Google Scholar]
- 47.Lemaitre L., Puech P., Poncelet E., Bouye S., Leroy X., Biserte J. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol. 2009;19:470–480. doi: 10.1007/s00330-008-1153-0. [DOI] [PubMed] [Google Scholar]
- 48.Panebianco V., Barchetti F., Sciarra A., Ciardi A., Indino E.L., Papalia R. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33:17.e1–17.e7. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Baco E., Rud E., Eri L.M., Moen G., Vlatkovic L., Svindland A. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016;69:149–156. doi: 10.1016/j.eururo.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 50.Tonttila P.P., Lantto J., Paakko E., Piippo U., Kauppila S., Lammentausta E. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial. Eur Urol. 2016;69:419–425. doi: 10.1016/j.eururo.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 51.Porpiglia F., Manfredi M., Mele F., Cossu M., Bollito E., Veltri A. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur Urol. 2017;72:282–288. doi: 10.1016/j.eururo.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 52.Garcia Bennett J., Vilanova J.C., Guma Padro J., Parada D., Conejero A. Evaluation of MR imaging-targeted biopsies of the prostate in biopsy-naive patients. A single centre study. Diagn Interv Imaging. 2017;98:677–684. doi: 10.1016/j.diii.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed H.U., El-Shater Bosaily A., Brown L.C., Gabe R., Kaplan R., Parmar M.K. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 54.Kasivisvanathan V., Rannikko A.S., Borghi M., Panebianco V., Mynderse L.A., Vaarala M.H. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rouvière O., Puech P., Renard-Penna R., Claudon M., Roy C., Mège-Lechevalier F. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2018 doi: 10.1016/S1470-2045(18)30569-2. [DOI] [PubMed] [Google Scholar]
- 56.Filson C.P., Natarajan S., Margolis D.J., Huang J., Lieu P., Dorey F.J. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer. 2016;122:884–892. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mariotti G.C., Falsarella P.M., Garcia R.G., Queiroz M.R.G., Lemos G.C., Baroni R.H. Incremental diagnostic value of targeted biopsy using mpMRI-TRUS fusion versus 14-fragments prostatic biopsy: a prospective controlled study. Eur Radiol. 2018;28:11–16. doi: 10.1007/s00330-017-4939-0. [DOI] [PubMed] [Google Scholar]
- 58.Hakozaki Y., Matsushima H., Kumagai J., Murata T., Masuda T., Hirai Y. A prospective study of magnetic resonance imaging and ultrasonography (MRI/US)-fusion targeted biopsy and concurrent systematic transperineal biopsy with the average of 18-cores to detect clinically significant prostate cancer. BMC Urol. 2017;17:117. doi: 10.1186/s12894-017-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fourcade A., Payrard C., Tissot V., Perrouin-Verbe M.A., Demany N., Serey-Effeil S. The combination of targeted and systematic prostate biopsies is the best protocol for the detection of clinically significant prostate cancer. Scand J Urol. 2018;52:174–179. doi: 10.1080/21681805.2018.1438509. [DOI] [PubMed] [Google Scholar]
- 60.Bryk D.J., Llukani E., Taneja S.S., Rosenkrantz A.B., Huang W.C., Lepor H. The role of ipsilateral and contralateral transrectal ultrasound-guided systematic prostate biopsy in men with unilateral magnetic resonance imaging lesion undergoing magnetic resonance imaging-ultrasound fusion-targeted prostate biopsy. Urology. 2017;102:178–182. doi: 10.1016/j.urology.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 61.Moldovan P., Udrescu C., Ravier E., Souchon R., Rabilloud M., Bratan F. Accuracy of elastic fusion of prostate magnetic resonance and transrectal ultrasound images under routine conditions: a prospective multi-operator study. PLoS One. 2016;11:e0169120. doi: 10.1371/journal.pone.0169120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouviere O., Souchon R., Melodelima C. Pitfalls in interpreting positive and negative predictive values: application to prostate multiparametric magnetic resonance imaging. Diagn Interv Imaging. 2018;99:515–518. doi: 10.1016/j.diii.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Radtke J.P., Wiesenfarth M., Kesch C., Freitag M.T., Alt C.D., Celik K. Combined clinical parameters and multiparametric magnetic resonance imaging for advanced risk modeling of prostate cancer-patient-tailored risk stratification can reduce unnecessary biopsies. Eur Urol. 2017;72:888–896. doi: 10.1016/j.eururo.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 64.van Leeuwen P.J., Hayen A., Thompson J.E., Moses D., Shnier R., Bohm M. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017;120:774–781. doi: 10.1111/bju.13814. [DOI] [PubMed] [Google Scholar]
- 65.Alberts A.R., Roobol M.J., Verbeek J.F.M., Schoots I.G., Chiu P.K., Osses D.F. Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: improving the Rotterdam European Randomized Study of Screening for Prostate Cancer risk calculators. Eur Urol. 2018 doi: 10.1016/j.eururo.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 66.Mehralivand S., Shih J.H., Rais-Bahrami S., Oto A., Bednarova S., Nix J.W. A magnetic resonance imaging-based prediction model for prostate biopsy risk stratification. JAMA Oncol. 2018;4:678–685. doi: 10.1001/jamaoncol.2017.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klotz L. Active surveillance for low-risk prostate cancer. Curr Opin Urol. 2017;27:225–230. doi: 10.1097/MOU.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 68.Schoots I.G., Petrides N., Giganti F., Bokhorst L.P., Rannikko A., Klotz L. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627–636. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 69.Schoots I.G., Moore C.M., Rouviere O. Role of MRI in low-risk prostate cancer: finding the wolf in sheep's clothing or the sheep in wolf's clothing? Curr Opin Urol. 2017;27:238–245. doi: 10.1097/MOU.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 70.Mottet N., van den Berg R.C.A., Briers E., Bourke L., Cornford P., De Santis M. 2018. EAU-ESTRO-ESUR-SIOG guidelines on prostate cancer. [Accessed 30 December 2018]. https://uroweb.org/guideline/prostate-cancer/ [Google Scholar]
- 71.Moore C.M., Giganti F., Albertsen P., Allen C., Bangma C., Briganti A. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the PRECISE recommendations—a report of a European school of oncology task force. Eur Urol. 2017;71:648–655. doi: 10.1016/j.eururo.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Albert J.M., Swanson D.A., Pugh T.J., Zhang M., Bruno T.L., Kudchadker R.J. Magnetic resonance imaging-based treatment planning for prostate brachytherapy. Brachytherapy. 2013;12:30–37. doi: 10.1016/j.brachy.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Salami S.S., Vira M.A., Turkbey B., Fakhoury M., Yaskiv O., Villani R. Multiparametric magnetic resonance imaging outperforms the Prostate Cancer Prevention Trial risk calculator in predicting clinically significant prostate cancer. Cancer. 2014;120:2876–2882. doi: 10.1002/cncr.28790. [DOI] [PubMed] [Google Scholar]
- 74.Gupta R.T., Faridi K.F., Singh A.A., Passoni N.M., Garcia-Reyes K., Madden J.F. Comparing 3-T multiparametric MRI and the Partin tables to predict organ-confined prostate cancer after radical prostatectomy. Urol Oncol. 2014;32:1292–1299. doi: 10.1016/j.urolonc.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Raskolnikov D., George A.K., Rais-Bahrami S., Turkbey B., Siddiqui M.M., Shakir N.A. The role of magnetic resonance image guided prostate biopsy in stratifying men for risk of extracapsular extension at radical prostatectomy. J Urol. 2015;194:105–111. doi: 10.1016/j.juro.2015.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng T.S., Sharif-Afshar A.R., Wu J., Li Q., Luthringer D., Saouaf R. Multiparametric MRI improves accuracy of clinical nomograms for predicting extracapsular extension of prostate cancer. Urology. 2015;86:332–337. doi: 10.1016/j.urology.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Dinh A.H., Melodelima C., Souchon R., Moldovan P.C., Bratan F., Pagnoux G. Characterization of prostate cancer with Gleason score of at least 7 by using quantitative multiparametric MR imaging: validation of a computer-aided diagnosis system in patients referred for prostate biopsy. Radiology. 2018;287:525–533. doi: 10.1148/radiol.2017171265. [DOI] [PubMed] [Google Scholar]